Abstract
Background:
Multiple agencies have developed health-based toxicity values for exposure to perfluorooctanoic acid (PFOA). Although PFOA exposure occurs in utero and through breastfeeding, current health-based toxicity values have not been derived using fetal or child dosimetry. Therefore, current values may underestimate the potential risks to fetuses and nursing infants.
Objective:
Using fetal and child dosimetry, we aimed to calculate PFOA maternal human equivalent doses (HEDs), corresponding to a developmental mouse study lowest observed adverse effect level (LOAEL, 1 mg/kg/day). Further, we investigated the impact of breastfeeding duration and PFOA half-life on the estimated HEDs.
Methods:
First, a pharmacokinetic model of pregnancy and lactation in mice was used to estimate plasma PFOA levels in pups following a maternal exposure to 1 mg PFOA/kg/day for gestational days 1–17. Four plasma PFOA concentration metrics were estimated in pups: i) average prenatal; ii) average postnatal; iii) average overall (prenatal and postnatal); and iv) maximum. Then, Monte Carlo simulations were performed using a pharmacokinetic model of pregnancy and lactation in humans to generate distributions of maternal HEDs that would result in fetal/child plasma levels equivalent to those estimated in pups using the mouse model. Median (HED50) and 1st percentile (HED01) of calculated HEDs were calculated.
Results:
Estimated PFOA maternal HED50s ranged from 3.0 × 10−4 to 1.1 × 10−3 mg/kg/day and HED01s ranged from 4.7 × 10−5 to 2.1 × 10−4 mg/kg/day. All calculated HEDs were lower than the HED based on adult dosimetry derived by the Environmental Protection Agency (EPA) (5.3 × 10−3 mg/kg/day).
Conclusion:
Our results suggest that fetal/child dosimetry should be considered when deriving health-based toxicity values for potential developmental toxicants.
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of thousands of chemicals of varying carbon-chain lengths, degrees of fluorination, and functional groups (Buck et al., 2011). While humans are usually exposed to a complex mixture of PFAS and their precursors, specific individual species have historically been singled out for consideration of health effects and risk assessment. One such species, per-fluorooctanoic acid (PFOA), has been measured in drinking water, food, and air (Fraser et al., 2012; Mak et al., 2009; Post et al., 2013; Shoeib et al., 2011; Sinclair et al., 2007; Tittlemier et al., 2007). In developmental studies of PFOA exposure in mice, pups born to dams administered PFOA during gestation showed multiple adverse health effects, including elevated leptin and insulin concentrations (Hines et al., 2009), neurotoxicity (Johansson et al., 2008), reduced bodyweight and changes in mammary epithelial branching and growth (White et al., 2007), and reduced ossification and accelerated puberty (Lau et al., 2006). Epidemiological studies of developmental exposure to PFOA have also reported associations between exposure and a variety of health outcomes, including reduced birth weight (Johnson et al., 2014), reduced response to vaccination (Grandjean et al., 2012; Grandjean et al., 2016; Granum et al., 2013), and behavioral problems (Oulhote et al., 2016).
Monitoring programs have shown widespread PFOA contamination in drinking water in North America. In Canada, PFOA is not routinely monitored in drinking water supplies (Health Canada 2016); however, a national survey in 2009–2010 of raw and treated water samples indicated that between 15% and 68% of samples had PFOA levels above the detection limits, depending on the season and year (Health Canada 2016). PFOA measured in five drinking water samples from Niagara-on the-Lake collected between 2006 and 2008 had a mean concentration of 2.1 × 10−3 μg/L (Mak et al., 2009). Numerous instances of PFOA exposure have been documented in the United States through the ingestion of water from public drinking water systems. PFOA exposure through contaminated municipal drinking water systems has occurred near the DuPont PFAS manufacturing facilities in Ohio and West Virginia (Emmett et al., 2006; Steenland et al., 2009), near the 3 M manufacturing facilities in Minnesota (Minnesota Department of Health, 2008) and Alabama (Agency for Toxic Substances and Disease Registry, 2013), at the Warminster and Willow Grove Naval Bases in Pennsylvania (Environmental Protection Agency, 2015), and at the Pease Tradeport in New Hampshire (New Hampshire Department of Health and Human Services, 2015). PFOA drinking water concentrations in these communities ranged from non-detect to 3.55 μg/L. The detection of PFOA in drinking water highlights the need for health-based toxicity values and drinking water guidelines that are protective for the population, including vulnerable individuals such as pregnant women and children.
In its recent evaluation of PFOA, the U.S. Environmental Protection Agency (EPA) calculated a reference dose (RfD) of 2.0 × 10−5 mg/kg/day (Environmental Protection Agency, 2016) based on a developmental toxicity study in mice in which pups born to dams administered 1 mg PFOA/kg/day for gestational days 1–17 showed reduced ossification at birth and accelerated puberty in males as assessed on post-natal day 24 (Lau et al., 2006). This RfD was used to develop a drinking water health advisory value of 0.07 μg/L using a water relative source contribution of 20% (Environmental Protection Agency, 2016). The Agency for Toxic Substances and Disease Registry (ATSDR) has derived a draft intermediate oral Minimum Risk Level (MRL) for PFOA of 2.0 × 10−5 mg/kg/day based on liver effects in monkeys (Agency for Toxic Substances and Disease Registry, 2015). Health Canada has proposed a draft Maximum Acceptable Concentration (MAC) for PFOA in drinking water of 0.2 μg/L based on liver effects in rats (Health Canada 2016). Several states including Maine (Maine Center for Disease Control and Prevention, 2016), Michigan (Michigan Department of Environmental Quality, 2015), Minnesota (Minnesota Department of Health, 2017), New Jersey (New Jersey Drinking Water Quality Institute, 2015), and Vermont (Vermont Department of Health, 2016) have also proposed or adopted drinking water guidelines for PFOA.
In general, health-based toxicity values are by definition (e.g., reference doses) protective of the population at large, including fetuses and nursing infants. However, typical risk assessment approaches are based on adult/maternal dosimetry rather than fetal/child dosimetry. This overlooks the fact that fetal plasma levels may be more toxicologically relevant than adult/maternal levels. It also does not account for the fact that the relationship between maternal exposure and fetal/child exposure may differ across species. Indeed, studies have shown that PFOA levels in mouse pups can be similar to levels in dams (Fenton et al., 2009; Wolf et al., 2007), whereas human infant levels at six months of age were approximately four times higher than maternal levels at delivery (Fromme et al., 2010). Therefore, an approach based solely on adult/maternal dosimetry may lead to an underestimation of risks for potential developmental toxicity.
Risk assessment based on fetal/child dosimetry requires either measuring or estimating chemical levels in the developing organism (Thompson et al., 2008). Lau et al. (2006) reported maternal and developmental toxicity of PFOA in the mouse, leading to early pregnancy loss, compromised postnatal survival, delays in general growth and development, and sex-specific alterations in pubertal maturation. This study was used by the EPA to estimate their RfD value; however, this study did not measure PFOA plasma levels in fetuses or pups. These levels should be estimated in order to assess the potential human risk of fetuses and nursing infants. In general, there is limited PFOA pharmacokinetic data available in animals (Butenhoff et al., 2004; Kemper, 2003). Similarly, human data on plasma PFOA levels in fetuses/children for a given maternal daily dose is limited.
Where relationships between external doses and internal concentrations have not been determined experimentally, pharmacokinetic models can be employed to perform estimations. Pharmacokinetic modeling uses mathematical descriptions of absorption, distribution, metabolism, and excretion of chemicals in the body to estimate plasma or tissue levels for a given organism and a given dose. Recently, several pharmacokinetic models have been used to successfully predict early-life plasma PFOA concentrations in early life mice (Rodriguez et al., 2009), rats (Loccisano et al., 2012), and humans (Loccisano et al., 2013; Verner et al., 2016).
In our study, we used pharmacokinetic models of gestation and lactation in mice (Rodriguez et al., 2009) and humans (Verner et al., 2016). The mouse model was selected because it was the only model available for mice. The human model was selected over other options because it was best suited to simulate lifetime exposures for both mother and child. These models were used to estimate, i) fetal and pup plasma levels resulting from maternal exposure to the lowest observed adverse effect level (LOAEL) reported by Lau et al. (2006) and ii) the lifetime daily human equivalent dose (HED) in women leading to fetal and child levels matching these fetal and pup plasma levels. These estimates can be used as points of departure (PODs) to calculate health-based toxicity values (e.g., RfDs, MRLs, etc.) and corresponding drinking water guidelines for PFOA that may be protective of fetuses and nursing infants.
2. Methods
Two existing pharmacokinetic models of developmental exposure to PFOA were used for our analyses: a mouse model developed by Rodriguez et al. (2009) and a human model developed by Verner et al. (2016). Both models include gestation and lactation, and the human model simulates a woman’s lifetime exposure, including breastfeeding periods up to 30 months. Different terminology is used to describe the offspring in the two published models. In the mouse model the fetus is referred to as concepti and the postnatal life-stage is referred to as pup. In the human model all of the developmental stages are integrated under one term: child. In this paper, we use fetus when referring specifically to the prenatal period for both mice and human. The term pup is used for the postnatal period in mice (age 0 to 42 days), while the term child is used for the postnatal period in the human (age 0 to 2).
2.1. Mouse model
The pharmacokinetic mouse model by Rodriguez et al. (2009) describes the distribution of PFOA in the dam as well as in the fetus and the pups. The model is constructed with several compartments: the dam (including uterus, mammary tissues, fat, liver, and kidney), the fetus, the pups, and milk. PFOA administration to the dam occurs via oral ingestion. Elimination of PFOA from the dam occurs via the kidney compartments, where PFOA transfers to filtrate through glomerular filtration and is either reabsorbed or excreted in urine. Fetal growth is initiated at the onset of placental blood flow on gestational day 6. Placental transfer of PFOA between the dam and fetus is based on placental blood flow and fetal:maternal plasma partition coefficients. After birth, PFOA intake for the pups occurs solely through lactation, where the amount ingested is calculated based on the milk:maternal plasma partition coefficient and milk intake for a litter size of 10 pups. PFOA excretion for pups is through urine, and this output is recirculated to the dam to reflect maternal stimulation of pup urine excretion, which involves urine consumption by the dam. The mouse model is described in detail in Rodriguez et al. (2009).
2.2. Human model
The pharmacokinetic human model by Verner et al. (2016) describes the distribution of PFOA in both the mother and the child. This two-compartmental model presents all maternal features in one maternal compartment, and all the fetus/child features in one child compartment. The volume of these compartments is based on published volumes of distribution and body weight (which fluctuates during the simulation). Both maternal and child compartments have an intake route, an elimination route, as well as placental and lactational transfer between compartments. PFOA intake for both mother and child was assumed to be solely through oral ingestion (Verner et al., 2016). Elimination for both compartments was based on PFOA half-lives derived from published data (Bartell et al., 2010; Olsen et al., 2007). Placental transfer is modeled as a two-directional flow in equilibrium that replicates published cord:maternal plasma ratios. Lactational exposure is defined as the product of breast milk ingestion, the plasma PFOA concentration in the mother, and the milk:plasma partition coefficient of PFOA. The precision of this model was evaluated against levels measured in children participating to two previously published studies (Fromme et al., 2010; Granum et al., 2013). In short, when using individual-specific maternal PFOA levels at delivery, anthropometric measurements and duration of breastfeeding, the model explained 60% of the variability in levels measured in 6-month-olds, and 62% of the variability in levels measured in 36-month-olds. The human model development and evaluation are described in detail in Verner et al. (2016).
2.3. Model simulations
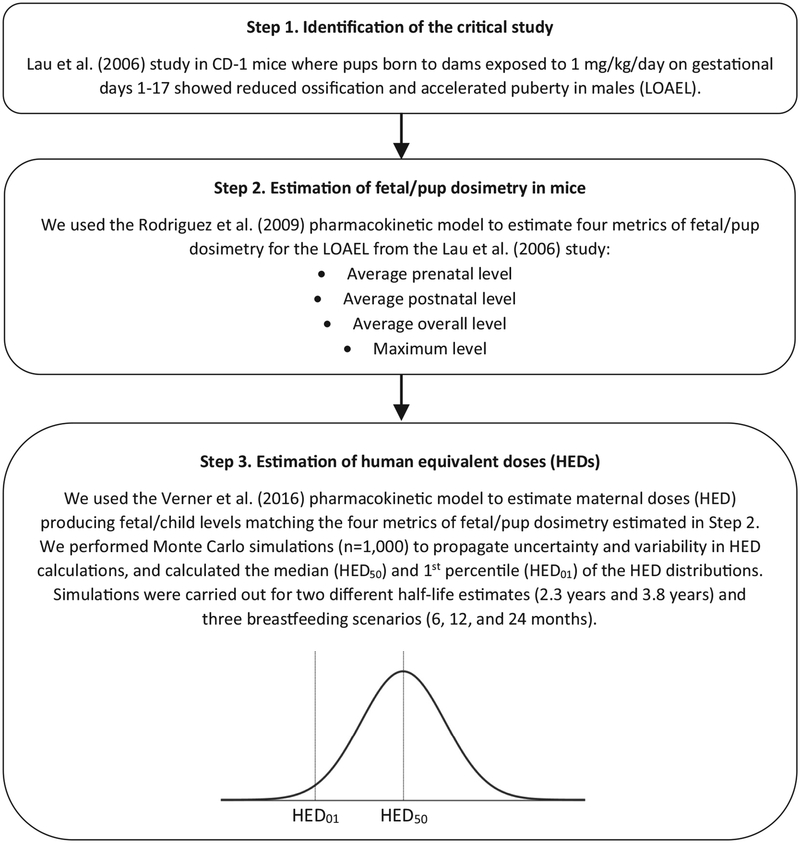
The step-wise method applied for model simulations is described in Fig. 1.
Fig. 1.
Step-wise method applied for model simulations to derive maternal HEDs.
We used the pharmacokinetic model of pregnant and lactating CD-1 mice (Rodriguez et al., 2009) to simulate exposure of dams to 1 mg/kg/day from gestational day 1–17. This dose was obtained from a study by Lau et al. (2006), where pups born to dams exposed to the LOAEL of 1 mg/kg/day PFOA for gestational days 1–17 showed reduced ossification at birth and accelerated puberty in males as assessed on post-natal day 24. Lau et al. (2006) was selected as the critical study for this analysis in order to mirror the critical study selected by EPA for derivation of the RfD for PFOA (Environmental Protection Agency, 2016). Whereas only prenatal exposure could affect ossification as assessed at birth, either prenatal exposure, postnatal exposure or both could influence pubertal development. For this reason, we calculated multiple exposure metrics which could be toxicologically relevant for pubertal development; we predicted average (prenatal, postnatal, and overall) and maximum plasma PFOA concentrations in pups. The simulations were run until postnatal day 42 (1000 h), which included the gestation (18 days) and lactation periods (21 days). As litter size in Lau et al. (2006) ranged from 3 to 10 pups, our simulations were run with litter sizes of 10 pups, as this produces more conservative results. Two renal transport maximum constants were proposed by Rodriguez et al. (2009). We used the 2.9 mg/h/kg value that was calibrated against paired dam and pup serum PFOA levels, and provided a better fit to the pup levels reported more recently (Macon et al., 2011).
We subsequently performed Monte Carlo simulations with the human model to estimate distributions of maternal HEDs that would result in plasma PFOA concentrations in the child equivalent to the average (prenatal, postnatal, and overall) and maximum plasma PFOA concentrations predicted in pups. The goal of these Monte Carlo simulations was to propagate model error and inter-individual variability through HED calculations. We ran 1000 Monte Carlo simulations per dose metric (average prenatal level, average postnatal level, average overall level, and maximum level), for half-lives of 2.3 years (Bartell et al., 2010) and 3.8 years (Olsen et al., 2007), and for breastfeeding durations of 6 months, 12 months, and 24 months. In other words, Monte Carlo simulations were performed for 24 combinations of dose metric, half-life, and breastfeeding duration.
We quantified error in the mouse model by comparing simulated pup levels to pup levels measured on postnatal days 7, 14, and 21 in a study where dams were exposed to 1 mg/kg/d from gestational days 1–17, and which was not used for model calibration (Macon et al., 2011); for each of these three measurements, we calculated measured/simulated level ratios (postnatal day 7: 0.65; postnatal day 14: 1.22; postnatal day 21: 0.42). These ratios indicate that the model overestimated mean experimental levels on days 7 and 21, whereas it underestimated the mean experimental level on day 14 (see Fig. 2). We quantified inter-individual variability using data from Macon et al. (2011) study by calculating the mean coefficient of variation in measured pup PFOA levels over the three time points (mean coefficient of variation: 0.36). Unfortunately, we could not locate studies reporting fetal PFOA levels for this dosing regimen, so only measured postnatal pup levels were used to quantify model error and inter-individual variability for the mouse model. At each Monte Carlo iteration (i), the mouse Fetal/pup PFOA metrici based on which the HED was calculated was varied as follows:
| (1) |
where the Simulated PFOA metrici was the fetal/pup level estimated with the model (i.e., average prenatal level, average postnatal level, average overall level, or maximum level), the Errori was randomly sampled from a uniform distribution based on the range described above (0.42 to 1.22), and the Variabilityi was randomly sampled from a normal distribution with a mean of 1.00 and a standard deviation of 0.36 (coefficient of variation in experimental pup levels). A uniform distribution was used for the Error because only three data points were available, which was insufficient to adequately characterize a normal distribution.
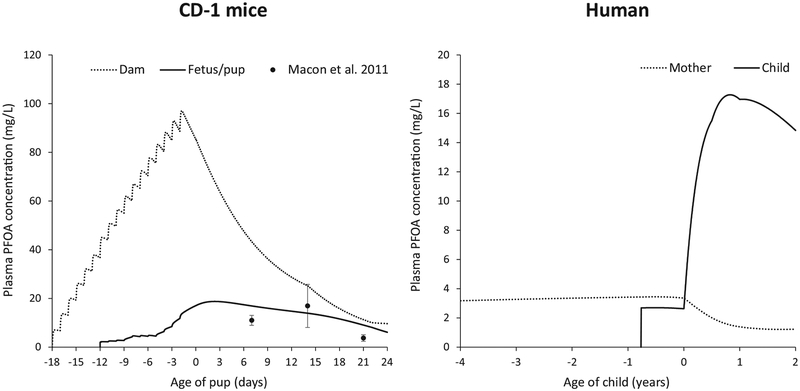
Fig. 2.
Simulated plasma PFOA concentrations in CD-1 dam/pups and human mother/child. For the CD-1 dam and pups, lines represent simulated PFOA levels for a maternal PFOA dose of 1 mg/kg/day for gestational days 1–17(litter size of 10). Mean (•) and standard deviation (error bars) of serum PFOA levels in pups from the Macon et al. (2011) study are also presented. For the human mother and child, lines represent simulated PFOA levels for a maternal daily intake (3.2 × 10−4 mg/kg/d) leading to an average overall concentration in the child matching that from the simulation for CD-1 pups. Age 0 in both graphs indicates delivery. Dashed lines represent plasma PFOA concentrations in the dam/mother, thick solid line represents plasma PFOA concentrations in the pup/child. Simulations for humans start at maternal age 0, but only the four years prior to delivery are presented in the figure.
At each Monte Carlo iteration, the Fetal/pup PFOA metrici calculated in Eq. (1) was used as the basis for HED calculation using the human model. In addition to model error and inter-individual variability from the mouse model/data, we randomly sampled human model parameters (i.e., maternal age at delivery, prepregnancy body weight, sex of child, child’s weight, milk/plasma partition coefficient, volume of distribution, half-life, cord/mother partition coefficient, volume of breast milk) from the same parameter distributions described in Verner et al. (2016). This sampling strategy was assumed to account for inter-individual variability and uncertainty. This assumption was supported by previous Monte Carlo simulations where measured child/mother level ratios fell within the distribution of simulated data (Verner et al., 2016). For each Monte Carlo simulation, the maternal dose (HED) leading to a child PFOA level matching the calculated Fetal/pup PFOA metrici was determined by iterative simulations using the golden section search approach. Model simulations were performed using acslX modeling software (Aegis Technologies Inc., Huntsville, AL).
Monte Carlo simulations yielded distributions of HED values for each combination of dose metric, half-life and breastfeeding duration. For each of these distributions, we extracted the 1st percentile (HED01) and 50th percentile (HED50) of HED values. Confidence intervals around these values were estimated using 1000 bootstrap resamplings. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Selection of target PFOA plasma concentrations
Simulations of dams administered 1 mg PFOA/kg/day from gestational day 1–17, resulted in average (prenatal, postnatal, or overall) or maximum plasma PFOA concentrations in pups ranging from 6.6 to 18.8 mg/L (Fig. 2). PFOA concentrations of 6.6 mg/L (average prenatal concentration), 14.2 mg/L (average postnatal concentration) 11.7 mg/L (average overall concentration), and 18.8 mg/L (maximum concentration) were estimated by the model.
3.2. Estimated maternal exposure doses/HEDs
The estimated maternal HEDs from Monte Carlo simulations for varying breastfeeding durations and PFOA half-lives are presented in Table 1. Median HEDs (HED50s) ranged from 3.0 × 10−4 to 1.1 × 10−3 mg/kg/day, and the 1st percentile of HED distributions (HED01s) ranged from 4.7 × 10−5 to 2.1 × 10−4 mg/kg/day. The lowest predicted HEDs were obtained when simulating average post-natal and overall PFOA concentration with a breastfeeding duration of two years and a PFOA half-life of 3.8 years. The highest predicted HEDs were obtained when simulating average prenatal concentration using a half-life of 2.3 years, regardless of the duration of breastfeeding.
Table 1.
1st percentile (HED01) and 50th percentile (HED50) of estimated maternal Human Equivalent Doses (HEDs) for different PFOA concentration metrics (average prenatal, average postnatal, overall average, and maximum). HEDs were calculated for breastfeeding durations of 6 months, 12 months and 24 months, and for PFOA half-lives of 2.3 years and 3.8 years. 95% confidence intervals were estimated using 1000 bootstrap resamplings.
| Breastfeeding duration | Matching PFOA concentration metric | Human equivalent dose (mg/kg/day) (95% confidence intervals) |
|||
|---|---|---|---|---|---|
| Half-life: 2.3 years | Half-life: 3.8 years | ||||
| HED01 | HED50 | HED01 | HED50 | ||
| 6 months | Prenatal (average) | 2.1 × 10−4 | 1.1 × 10−3 | 1.4 × 10−4 | 7.0 × 10−4 |
| (1.4 × 10−4, 2.4 × 10−4) | (1.0 × 10−3, 1.1 × 10−3) | (1.1 × 10−4, 1.6 × 10−4) | (6.8 × 10−4, 7.3 × 10−4) | ||
| Postnatal (average) | 1.0 × 10−4 | 7.3 × 10−4 | 6.4 × 10−5 | 4.4 × 10−4 | |
| (8.5 × 10−5, 1.3 × 10−4) | (6.9 × 10−4, 7.8 × 10−4) | (5.2 × 10−5, 8.4 × 10−5) | (4.1 × 10−4, 4.6 × 10−4) | ||
| Overall (average) | 9.9 × 10−5 | 7.0 × 10−4 | 6.2 × 10−5 | 4.3 × 10−4 | |
| (7.1 × 10−5, 1.2 × 10−4) | (6.7 × 10−4, 7.5 × 10−4) | (5.0 × 10−5, 7.2 × 10−5) | (4.1 × 10−4, 4.6 × 10−4) | ||
| Maximum | 9.6 × 10−5 | 6.3 × 10−4 | 6.2 × 10−5 | 3.9 × 10−4 | |
| (8.7 × 10−5, 1.1 × 10−4) | (5.9 × 10−4, 6.7 × 10−4) | (5.2 × 10−5, 7.3 × 10−5) | (3.8 × 10−4, 4.2 × 10−4) | ||
| 12 months | Prenatal (average) | 2.1 × 10−4 | 1.1 × 10−3 | 1.4 × 10−4 | 7.0 × 10−4 |
| (1.4 × 10−4, 2.4 × 10−4) | (1.0 × 10−3, 1.1 × 10−3) | (1.1 × 10−4, 1.6 × 10−4) | (6.8 × 10−4, 7.3 × 10−4) | ||
| Postnatal (average) | 8.6 × 10−5 | 5.5 × 10−4 | 5.3 × 10−5 | 3.2 × 10−4 | |
| (7.8 × 10−5, 9.6 × 10−5) | (5.1 × 10−4, 5.7 × 10−4) | (4.3 × 10−5, 6.6 × 10−5) | (3.1 × 10−4, 3.5 × 10−4) | ||
| Overall (average) | 7.8 × 10−5 | 5.4 × 10−4 | 5.0 × 10−5 | 3.3 × 10−4 | |
| (5.9 × 10−5, 9.6 × 10−5) | (5.1 × 10−4, 5.8 × 10−4) | (3.9 × 10−5, 5.8 × 10−5) | (3.2 × 10−4, 3.6 × 10−4) | ||
| Maximum | 8.9 × 10−5 | 5.8 × 10−4 | 5.8 × 10−5 | 3.5 × 10−4 | |
| (8.1 × 10−5, 1.0 × 10−4) | (5.4 × 10−4, 6.1 × 10−4) | (4.8 × 10−5, 6.7 × 10−5) | (3.4 × 10−4, 3.7 × 10−4) | ||
| 24 months | Prenatal (average) | 2.1 × 10−4 | 1.1 × 10−3 | 1.4 × 10−4 | 7.0 × 10−4 |
| (1.6 × 10−4, 2.4 × 10−4) | (1.0 × 10−3, 1.1 × 10−3) | (1.2 × 10−4, 1.6 × 10−4) | (6.8 × 10−4, 7.3 × 10−4) | ||
| Postnatal (average) | 8.0 × 10−5 | 4.9 × 10−4 | 4.9 × 10−5 | 3.0 × 10−4 | |
| (7.4 × 10−5, 8.8 × 10−5) | (4.7 × 10−4, 5.2 × 10−4) | (4.0 × 10−5, 6.1 × 10−5) | (2.8 × 10−4, 3.2 × 10−4) | ||
| Overall (average) | 7.3 × 10−5 | 5.0 × 10−4 | 4.7 × 10−5 | 3.1 × 10−4 | |
| (5.6 × 10−5, 9.2 × 10−5) | (4.7 × 10−4, 5.3 × 10−4) | (4.3 × 10−5, 5.5 × 10−5) | (2.9 × 10−4, 3.3 × 10−4) | ||
| Maximum | 8.9 × 10−5 | 5.8 × 10−4 | 5.8 × 10−5 | 3.5 × 10−4 | |
| (7.8 × 10−5, 9.8 × 10−5) | (5.4 × 10−4, 6.1 × 10−4) | (4.8 × 10−5, 6.6 × 10−5) | (3.4 × 10−4, 3.7 × 10−4) | ||
Estimated HEDs based on metrics including postnatal levels were influenced by breastfeeding duration. Longer durations of breastfeeding were associated with lower HEDs. For example, the HED50 based on the average overall PFOA concentration (prenatal and postnatal) was 1.3 times lower when using a breastfeeding duration of 12 months compared to a duration of 6 months. Predicted HEDs were also influenced by half-life. For all plasma PFOA metrics and exposure scenarios, HEDs obtained using a 2.3-year half-life were approximately 1.5–1.7 times higher than those obtained with a half-life of 3.8 years. Overall, the HEDs derived from average postnatal PFOA plasma concentrations were approximately 1.5–2.9 times lower than HEDs derived from average prenatal PFOA plasma concentrations. HED01 values were approximately 5.0–7.3 times lower than HED50 values.
4. Discussion
PFOA has been detected in drinking water systems across the United States and Canada (Health Canada 2016; Hu et al., 2016), and the development of health-based toxicity values and guidelines for PFOA in drinking water is a crucial component of human health risk assessment. The scientific literature establishes that PFOA crosses the placenta and transfers from mother to child during lactation (Cariou et al., 2015; Hinderliter et al., 2005; Lee et al., 2013). Several epidemiological studies have suggested associations between early life PFOA exposure and health outcomes, including reduced birth weight (Johnson et al., 2014), reduced response to vaccination (Grandjean et al., 2012; Grandjean et al., 2016; Granum et al., 2013), and behavioral problems (Oulhote et al., 2016). As a result, questions related to the risks associated with breastfeeding are asked frequently in communities with identified PFOA exposure pathways. In fact, the Minnesota Department of Health recently released a revised drinking water guideline for PFOA based specifically on exposure to the exclusively breast-fed infant (Minnesota Department of Health, 2017), thus highlighting the importance of consideration of lactational PFOA exposures in human health risk assessment.
To date, health-based toxicity values for PFOA have been based on adult dosimetry, even in cases where the critical effect was developmental toxicity. Not accounting for prenatal or lactational exposure could potentially result in health-based toxicity values that underestimate the potential risk of developing fetus and child, especially when the relationship between maternal levels and levels in the developing organism is known to differ across species. In this context, applying innovative approaches to improve risk assessment of early-life exposures to persistent environmental chemicals during gestation and lactation is essential to better protect vulnerable populations from the potential for adverse health effects (Lehmann et al., 2014). Prior work in this area has demonstrated that persistent environmental chemicals have the potential to accumulate in a women’s body in the years leading up to pregnancy and breastfeeding, and that children’s intake of these chemicals can be much greater than maternal daily intake (Haddad et al., 2015; Lehmann et al., 2014; Verner et al., 2016). It is also well established that breastfeeding is an important source of exposure to PFOA in children (Cariou et al., 2015; Mondal et al., 2014). To incorporate fetal and child exposures into PFOA risk assessment, we used pharmacokinetic models of gestation and lactation in mice and humans to derive potential HEDs for PFOA based on fetal/child dosimetry. Our results suggested that the relationship between maternal exposure and fetal/child exposure is different in mice and humans for PFOA (both prenatally and postnatally), a finding that is supported by studies where a much greater child/mother level ratio was observed in humans (Fromme et al., 2010) than in mice (Fenton et al., 2009; Wolf et al., 2007). These HEDs can be used to develop health-based toxicity values and drinking water equivalent levels (DWELs) based on fetal/child dosimetry.
Pharmacokinetic modeling showed that the duration of breast-feeding has a marked effect on children’s plasma PFOA concentrations (Verner et al., 2016). In our study, the lowest HED values were obtained for durations of breastfeeding of 24 months. Breastfeeding periods of 24 months may overestimate actual breastfeeding practices for most populations. However, breastfeeding periods up to, and sometimes longer than 24 months have been documented (e.g., Mondal et al., 2014). Additionally the World Health Organization (WHO) advises exclusive breastfeeding for 6 months, and a continuance of breast-feeding combined with complementary food up to 24 months of age or even beyond (World Health Organization, 2011). HED estimates based on 24 months of breastfeeding are expected to be protective of most nursing children.
Published estimates of PFOA biological half-life vary, with reported values being as high as 3.8 years (arithmetic mean) estimated in retired workers exposed to PFOA (Olsen et al., 2007), and as low as 2.3 years (median) in adults exposed to PFOA in contaminated drinking water (Bartell et al., 2010). HEDs estimated using a half-life of 2.3 years were approximately 1.5–1.7 times higher than HEDs estimated using a half-life of 3.8 years. This finding highlights the importance of accounting for the uncertainty in half-life estimates in the calculation of health-based toxicity values.
Overall, the HEDs derived from average postnatal and overall PFOA plasma concentrations were the lowest. Both metrics include the period during which nursing children are exposed through breastfeeding, an exposure route that substantially increases plasma PFOA levels. In the absence of toxicity data to confirm the most appropriate dose metric, postnatal and overall PFOA plasma concentrations may be more protective metrics for deriving maternal HEDs for PFOA.
Our findings suggest that current PFOA risk assessment methods could be improved by applying available pharmacokinetics models to consider fetal and child dosimetry. However, our study has a few limitations to be accounted for when interpreting or applying our results. In the case of the Rodriguez et al. (2009) mouse model, some pharmacokinetic parameters were obtained from rats. Although the model could simulate PFOA plasma concentrations, it does not allow simulations of tissue levels of PFOA in the maternal system, fetus, or pup. For both the mouse and the human model milk:plasma partition coefficients were modeled as constant, even though this parameter may change during lactation. Additional pharmacokinetic data in animals and humans would shed light on how PFOA partitions into milk over time and improve estimates of lactational transfer. The elimination rate constant for the pup was assumed to be equal to the elimination rate in the dam, which might not be the case (Rodriguez et al., 2009). Although the mouse model was validated against levels measured in dams from the Lau et al. (2006) study (used to derive the RfDs herein), it was only validated against pup levels from another study (Wolf et al., 2007) with higher doses which required a different calibration of renal resorption parameters. Therefore, there is some uncertainty around estimated pup levels in the Lau et al. (2006) study. While Lau et al. (2006) provides endpoint and exposure data, it does not report internal levels in the fetus or pup.
Additional human pharmacokinetic data and reporting of internal PFOA tissue levels over time will greatly improve the application of the approach proposed in this study, and provide a better understanding of the transfer of PFOA from mother to fetus/child. Indeed, a greater emphasis on collection of pharmacokinetic and toxicity data for environmental emerging contaminants, including PFAS, would allow the approach described here to be applied more broadly to answer important public health questions. Further, tissue-specific PFOA measurements as well as measurements of others chemicals in this class in target organs would allow for derivation of HEDs based on critical effects and potentially more relevant dose metrics.
Our results suggest that fetal/child dosimetry should be considered when deriving PFOA health-based toxicity values. In their recent evaluation, the EPA used the LOAEL (1 mg/kg/day) from the Lau et al. (2006) developmental toxicity study in mice to calculate a HED (5.3 × 10−3 mg/kg/day) based on dosimetry in the dam (estimated using the Wambaugh et al., 2013 pharmacokinetic model) and in the mother (estimated using a simple equation based on PFOA volume of distribution and half-life, assuming that clearance from the body should equal dose to the body at steady-state). When using the Rodriguez et al. (2009) and Verner et al. (2016) models to calculate a HED based on dam/maternal dosimetry, we obtained a similar HED of 5.2 × 10−3 mg/kg/day. On the other hand, all HED50s and HED01s derived based on pup/child dosimetry were below this value. When EPA’s RfD derivation method (Environmental Protection Agency, 2016) is applied to our estimated HEDs, alternative RfDs are also much lower than those previously derived by the EPA and Health Canada. Using our estimated HED50s as PODs and the 300 composite uncertainty factor (a factor of 10 to account for intraspecies variability [UFH], a factor of 3 to account for interspecies variability in pharmacodynamics [UFA], and a factor of 10 to account for LOAEL to no observed adverse effect level (NOAEL) extrapolation [UFL]) our estimated health-based toxicity values range from 1.0 × 10−6 to 3.7 × 10−6 mg/kg/day. When using our estimated HED01s and only using the pharmacodynamics component of the intraspecies variability uncertainty factor (UFH) because using the 1st percentile of the distribution accounts for pharmacokinetic variability (composite factor: 90), estimated health-based toxicity values range from 5.2 × 10−7 to 2.3 × 10−6 mg/kg/day. To derive a lifetime health advisory for PFOA, the EPA divided their RfD by the drinking water intake rate reported for lactating women (0.054 L/kg day) and multiplied by a relative source contribution of 0.2. When this method is applied to our estimated alternative health-based toxicity values (HED01s and HED50s), the resulting drinking water guidelines are also lower than many available drinking water guidelines for PFOA, ranging from 1.9 × 10−6 to 1.4 × 10−5 mg/L, or 1.9 to 14 ppt.
5. Conclusion
This study applied pharmacokinetic modeling approaches to estimate maternal and fetal/child human equivalent doses which correspond to a mouse dam and pup dosimetry to evaluate the impact of in utero and breastfeeding PFOA exposure. Overall, human equivalent doses estimated based on fetal/child dosimetry using pharmacokinetic models were much lower than the current human equivalent doses values based on adult dosimetry used by the EPA and Health Canada in the derivation of their health-based toxicity values for PFOA. This study highlights how future risk assessments, including derivation of health-based toxicity values (e.g., RfDs, MRLs) and drinking water guidelines for PFOA should consider the potential impact of in utero and lactational PFOA exposure on the developing fetal/child. This approach could also be extended to evaluate other potential developmental toxicants (including other PFAS) which are a current challenge to regulatory government agencies.
Acknowledgments
This work was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2016–06101). Marc-André Verner is recipient of a Research Scholars J1 Award from the Fonds de recherche du Québec – Santé (FRQS).
References
- Agency for Toxic Substances and Disease Registry, 2013. Exposure Investigation Report-Perfluorochemical Serum Sampling in the Vicinity of Decatur, AL, Morgan, Lawrence, and Limestone Counties Division of Community Health Investigation Available: http://www.atsdr.cdc.gov/HAC/pha/Decatur/Perfluorochemical_Serum%20Sampling.pdf.
- Agency for Toxic Substances and Disease Registry, 2015. Draft Toxicological Profile for Perfluoroalkyls Available: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. [PubMed]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K, 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Perspect 118 (2), 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. , 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag 7 (4), 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL Jr., Hinderliter PM, Lieder PH, Jung R, Hansen KJ, et al. , 2004. Pharmacokinetics of perfluorooctanoate in cynomolgus monkeys. Toxicol. Sci 82 (2), 394–406. [DOI] [PubMed] [Google Scholar]
- Health Canada, 2016. Perfluorooctanoic Acid (PFOA) in Drinking Water Available: http://www.healthycanadians.gc.ca/health-system-systeme-sante/consultations/acide-perfluorooctanoic-acid/document-eng.php.
- Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, et al. , 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ. Int 84, 71–81. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM, 2006. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J. Occup. Environ. Med 48 (8), 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency, 2015. Perfluorinated Chemicals in Drinking Water, Questions and Answers Available. http://www.epa.gov/reg3hwmd/super/sites/PA6170024545/fs/Warminster-Horsham_Joint_Fact_Sheet_FINAL_2-20-15.pdf.
- Environmental Protection Agency, 2016. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA)
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, et al. , 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol 27 (3–4), 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, et al. , 2012. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ. Sci. Technol 46 (2), 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. , 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol 44 (18), 7123–7129. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, et al. , 2012. Serum vaccine antibody concentrations in children exposed to per-fluorinated compounds. JAMA 307 (4), 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jorgensen E, 2016. Serum vaccine antibody concentrations in adolescents exposed to per-fluorinated compounds. Environ. Health Perspect 125 (7), 077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, et al. , 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol 10 (4), 373–379. [DOI] [PubMed] [Google Scholar]
- Haddad S, Ayotte P, Verner MA, 2015. Derivation of exposure factors for infant lactational exposure to persistent organic pollutants (POPs). Regul. Toxicol. Pharmacol 71 (2), 135–140. [DOI] [PubMed] [Google Scholar]
- Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy GL Jr., 2005. Perfluorooctanoate: placental and lactational transport pharmacokinetics in rats. Toxicology 211 (1–2), 139–148. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE, 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell. Endocrinol 304 (1–2), 97–105. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. , 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett 3 (10), 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P, 2008. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 29 (1), 160–169. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, et al. , 2014. The navigation guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ. Health Perspect 122 (10), 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper RA, 2003. Perfluorooctanoic acid: toxicokinetics in the rat Project ID: DuPont 7473.
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. , 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci 90 (2), 510–518. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim MK, Bae J, Yang JH, 2013. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 90 (5), 1603–1609. [DOI] [PubMed] [Google Scholar]
- Lehmann GM, Verner MA, Luukinen B, Henning C, Assimon SA, LaKind JS, et al. , 2014. Improving the risk assessment of lipophilic persistent environmental chemicals in breast milk. Crit. Rev. Toxicol 44 (7), 600–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loccisano AE, Campbell JL Jr., Butenhoff JL, Andersen ME, Clewell HJ 3rd, 2012. Evaluation of placental and lactational pharmacokinetics of PFOA and PFOS in the pregnant, lactating, fetal and neonatal rat using a physiologically based pharmacokinetic model. Reprod. Toxicol 33 (4), 468–490. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Longnecker MP, Campbell JL Jr., Andersen ME, Clewell HJ 3rd, 2013. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J. Toxicol. Environ. Health A 76 (1), 25–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, et al. , 2011. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol. Sci 122 (1), 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine Center for Disease Control and Prevention, 2016. Maximum Exposure Guidelines (MEGs) for Drinking Water Available: http://www.maine.gov/dhhs/mecdc/environmental-health/eohp/wells/documents/megtable2016.pdf.
- Mak YL, Taniyasu S, Yeung LW, Lu G, Jin L, Yang Y, et al. , 2009. Perfluorinated compounds in tap water from China and several other countries. Environ. Sci. Technol 43 (13), 4824–4829. [DOI] [PubMed] [Google Scholar]
- Michigan Department of Environmental Quality, 2015. Rule 57 Water Quality Values Available: http://www.michigan.gov/deq/0,4561,7-135-3313_3681_3686_3728-11383-,00.html.
- Minnesota Department of Health, 2008. Perfluorochemical Contamination in Lake Elmo and Oakdale, Washington County, Minnesota Available: http://www.health.state.mn.us/divs/eh/hazardous/sites/washington/lakeelmo/phaelmooakdale.pdf.
- Minnesota Department of Health, 2017. Toxicological Summary for: Perfluorooctanoic Acid Available: http://www.health.state.mn.us/divs/eh/risk/guidance/gw/pfoa.pdf.
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, et al. , 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ. Health Perspect 122 (2), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Hampshire Department of Health and Human Services, 2015. Frequently Asked Questions Regarding Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA) Detected in the Pease Tradeport Water System Available: http://www.dhhs.nh.gov/dphs/documents/pease-water-faqs.pdf.
- New Jersey Drinking Water Quality Institute, 2015. Maximum Contaminant Level Recommendations for Perfluorononanoic Acid in Drinking Water. Basis and Background Available: http://www.nj.gov/dep/watersupply/pdf/pfna-recommend-final.pdf.
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. , 2007. Half-life of serum elimination of perfluorooctanesulfonate, per-fluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect 115 (9), 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P, 2016. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ. Int 97, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, Louis JB, Lippincott RL, Procopio NA, 2013. Occurrence of per-fluorinated compounds in raw water from New Jersey public drinking water systems. Environ. Sci. Technol 47 (23), 13266–13275. [DOI] [PubMed] [Google Scholar]
- Rodriguez CE, Setzer RW, Barton HA, 2009. Pharmacokinetic modeling of per-fluorooctanoic acid during gestation and lactation in the mouse. Reprod. Toxicol 27 (3–4), 373–386. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, MW G, Lee SC, 2011. Indoor sources of poly- and per-fluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environ. Sci. Technol 45 (19), 7999–8005. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB, Kannan K, 2007. Quantitation of gas-phase per-fluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ. Sci. Technol 41 (4), 1180–1185. [DOI] [PubMed] [Google Scholar]
- Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, et al. , 2009. Predictors of PFOA levels in a community surrounding a chemical plant. Environ. Health Perspect 117 (7), 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Sonawane B, Barton HA, DeWoskin RS, Lipscomb JC, Schlosser P, et al. , 2008. Approaches for applications of physiologically based pharmacokinetic models in risk assessment. J. Toxicol. Environ. Health B Crit. Rev 11 (7), 519–547. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, et al. , 2007. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem 55 (8), 3203–3210. [DOI] [PubMed] [Google Scholar]
- Vermont Department of Health, 2016. PFOA (Perfluorooctanoic Acid) Available: http://www.healthvermont.gov/health-environment/drinking-water/perfluorooctanoic-acid-pfoa.
- Verner MA, Ngueta G, Jensen ET, Fromme H, Volkel W, Nygaard UC, et al. , 2016. A simple pharmacokinetic model of prenatal and postnatal exposure to per-fluoroalkyl substances (PFASs). Environ. Sci. Technol 50 (2), 978–986. [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Setzer RW, Pitruzzello AM, Liu J, Reif DM, Kleinstreuer NC, et al. , 2013. Dosimetric anchoring of in vivo and in vitro studies for per-fluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci 136 (2), 308–327. [DOI] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. , 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci 96 (1), 133–144. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, et al. , 2007. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol. Sci 95 (2), 462–473. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2011. Exclusive Breastfeeding for Six Months Best for Babies Everywhere Available: http://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/.