Abstract
Cancer metabolism is emerging as a chemotherapeutic target. Enhanced glycolysis and suppression of mitochondrial metabolism characterize the Warburg phenotype in cancer cells. The flux of respiratory substrates, ADP, and Pi into mitochondria and the release of mitochondrial ATP to the cytosol occur through voltage-dependent anion channels (VDACs) located in the mitochondrial outer membrane. Catabolism of respiratory substrates in the Krebs cycle generates NADH and FADH2 that enter the electron transport chain (ETC) to generate a proton motive force that maintains mitochondrial membrane potential (ΔΨ) and is utilized to generate ATP. The ETC is also the major cellular source of mitochondrial reactive oxygen species (ROS). αβ-Tubulin heterodimers decrease VDAC conductance in lipid bilayers. High constitutive levels of cytosolic free tubulin in intact cancer cells close VDAC decreasing mitochondrial ΔΨ and mitochondrial metabolism. The VDAC–tubulin interaction regulates VDAC opening and globally controls mitochondrial metabolism, ROS formation, and the intracellular flow of energy. Erastin, a VDAC-binding molecule lethal to some cancer cell types, and erastin-like compounds identified in a high-throughput screening antagonize the inhibitory effect of tubulin on VDAC. Reversal of tubulin inhibition of VDAC increases VDAC conductance and the flux of metabolites into and out of mitochondria. VDAC opening promotes a higher mitochondrial ΔΨ and a global increase in mitochondrial metabolism leading to high cytosolic ATP/ADP ratios that inhibit glycolysis. VDAC opening also increases ROS production causing oxidative stress that, in turn, leads to mitochondrial dysfunction, bioenergetic failure, and cell death. In summary, antagonism of the VDAC–tubulin interaction promotes cell death by a “double-hit model” characterized by reversion of the proproliferative Warburg phenotype (anti-Warburg) and promotion of oxidative stress.
1. INTRODUCTION
1.1. Mitochondria, Energy Production, and Biosynthesis
Mitochondria, classically described as the “power house” of cells in textbooks, produce about 95% or more of the total ATP in nonproliferating cells. Respiratory substrates including fatty acids, pyruvate, and certain amino acids are fully oxidized in mitochondria. The highly efficient ATP-generating machine requires the entrance of respiratory substrates, ADP, and Pi through the mitochondrial outer membrane (MOM). Metabolites that reach the intermembrane space are further transported into the matrix by numerous different carriers located in the mitochondrial inner membrane (MIM). Respiratory substrates in the matrix, catabolized in the Krebs or tricarboxylic acid cycle, generate NADH and FADH2 which enter the electron transport chain (ETC) comprised of protein Complexes I–IV. The Krebs cycle is fueled by the intermediate acetyl-coenzyme A (AcCoA) which is generated by the oxidation of glucose-derived pyruvate, β-oxidation of fatty acids and from the catabolism of the amino acids leucine, isoleucine, glycine, serine, and tryptophan. In oxidative phosphor-ylation (OXPHOS), the transfer of electrons from NADH and FADH2 to reduce the final acceptor molecular O2 to H2O, drives proton translocation across the MIM by Complexes I, III, and IV. Proton accumulation in the intermembrane space generates a negative transmembrane ΔΨ (mitochondrial ΔΨ) and positive ΔpH, the components of the proton motive force (Δp). Δp then drives ATP synthesis from ADP and Pi by Complex V (F1F0-ATP synthase) (Nicholls & Ferguson, 2013; Fig. 1).
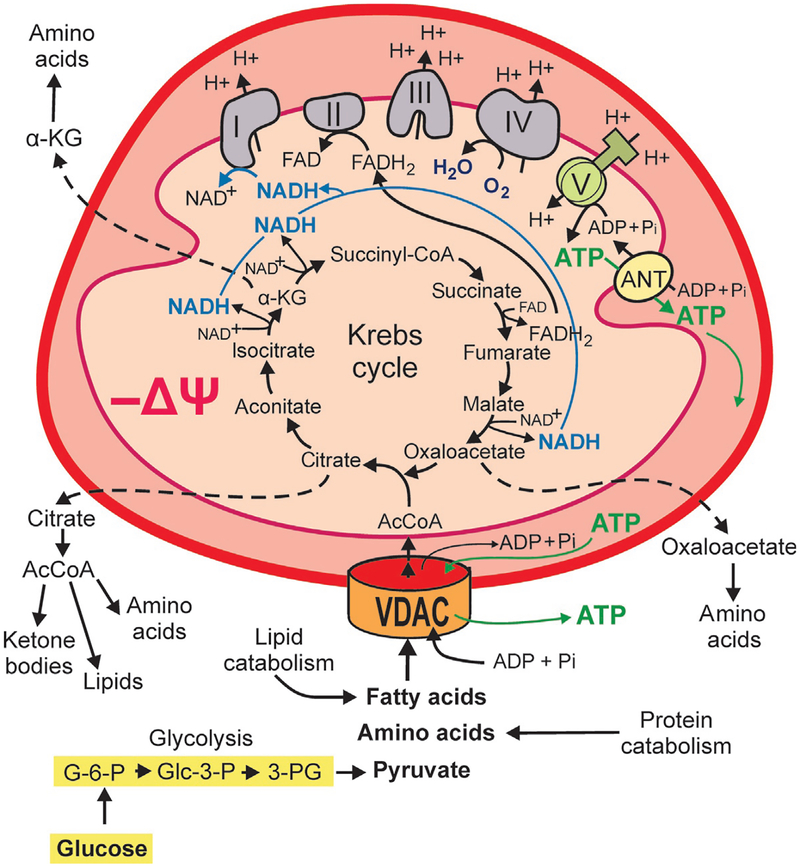
Fig. 1.
Mitochondrial metabolism. Flux of metabolites including fatty acids, certain amino acids, pyruvate, ADP, and Pi across the mitochondrial outer membrane occurs through VDAC. Catabolism of respiratory substrates in the Krebs cycle generates NADH and FADH2, which fuel the electron transport chain (Complexes I–IV) and supports oxidative phosphorylation. The Krebs cycle also produces metabolic intermediaries released to the cytosol for the biosynthesis of proteins and lipids. Proton pumping by the respiratory chain across mitochondrial inner membrane (MIM) generates mitochondrial ΔΨ. Protons moving back across MIM into the matrix drive ATP synthesis from ADP and Pi by the F1-F0-ATP synthase (Complex V). Mitochondrial ATP is exported from the matrix by the adenine nucleotide transporter (ANT) and released to the cytosol through VDAC.
From a biosynthetic perspective, the Krebs cycle does not only contribute NADH and FADH2 to the ETC to produce ATP but also generates metabolites involved in cell signaling and biosynthesis. AcCoA is essential for the synthesis of lipids, ketone bodies, amino acids, and cholesterol; citrate is used for lipid biosynthesis and oxaloacetate and α-ketoglutarate for the synthesis of some nonessential amino acids (Frezza, 2017; Fig. 1).
An important feature of mitochondrial metabolism is that full oxidation of respiratory substrates maximizes the yield of ATP per mole of substrate and leaves no residual carbon backbones. The dynamic range of ATP synthesized depends on the cell type, catabolic, and anabolic activities. In general, in most cell types that do not undergo uncontrolled cell division the biosynthesis of macromolecules depends mostly on the turnover of protein, lipids, and nucleic acids. Moreover, differentiated cells with the possible exception of neurons and cardiac myocytes only proliferate to maintain the population within a defined number of replication cycles. By contrast, the frequent cell division in cancer cells poses a metabolic challenge. Replicating cells adjust the bioenergetics status and biosynthetic machinery to support the high demand of biomass generation for daughter cells.
1.2. Bioenergetics and Biosynthesis in the Warburg Phenotype
Energy production, energy utilization, and supply of substrates for metabolic reactions including biosynthesis are finely regulated in all eukaryotic cells. Cells that do not proliferate operate at a different dynamic equilibrium of supply and demand for energy and substrates than cells that undergo continuous divisions.
One of the differences in the bioenergetics of tumor cells was initially observed in the early 20th century by Warburg who made the seminal observation that tumors produce more lactic acid than differentiated cells even in the presence of oxygen. The tumor metabolic phenotype, termed the Warburg effect after his contribution, is characterized by enhanced glycolysis and suppression of mitochondrial metabolism even in the presence of physiological levels of oxygen (Warburg, 1956; Warburg, Wind, & Negelein, 1927). Warburg also proposed that the cause of cancer was an irreversible but incomplete damage to respiration causing low energy production that was compensated by increased conversion of glucose to lactic acid (fermentation). Cells that could successfully increase glucose utilization through successive divisions to overcome the detrimental effects of defective respiration eventually became cancerous (Warburg, 1956). The provocative idea of failing mitochondria as the cause of cancer was quickly challenged by Weinhouse and others who demonstrated concurrent high glycolysis and oxidative metabolism in cancer tissues (Weinhouse, 1956). After the Warburg–Weinhouse controversy, several investigations confirmed both enhanced glycolysis and active mitochondrial metabolism in cancer cells. Functional mitochondria in tumor cells have been demonstrated by measurements of ATP generation, NADH production, oxygen consumption, and maintenance of mitochondrial ΔΨ, among other parameters (Lim, Ho, Low, Choolani, & Wong, 2011; Maldonado, Patnaik, Mullins, & Lemasters, 2010; Mathupala, Ko, & Pedersen, 2010; Moreno-Sanchez et al., 2014; Nakashima, Paggi, & Pedersen, 1984; Pedersen, 1978; Singleterry, Sreedhar, & Zhao, 2014).
The relative contribution of mitochondria to total cellular ATP generation by OXPHOS is generally lower in cancer cells compared to differentiated cells. Mitochondria produce about 95% of the total ATP by OXPHOS in differentiated cells, and the remaining 5% is generated by the aerobic catabolism of glucose in the cytosol. By contrast in cancer and other proliferating cells, glycolysis contributes 20%–90% of total ATP production (Griguer, Oliva, & Gillespie, 2005; Nakashima et al., 1984). Enhanced glycolysis in cancer cells has been associated with a high rate of cell proliferation (Griguer et al., 2005; Guppy, Leedman, Zu, & Russell, 2002; Moreno-Sanchez, Rodriguez-Enriquez, Marin-Hernandez, & Saavedra, 2007; Scott et al., 2011). The “glucose avidity” of tumors is currently used to diagnose primary tumors, recurrences, and metastases by positron emission tomography of the glucose analogue 18fluorodeoxyglucose (Zhu, Lee, & Shim, 2011). Although enhanced glycolysis is a feature of almost all cancer cells, the bioenergetic profiles of tumors are remarkably heterogeneous not only among different tumor types but also among cells in the same tumor. Subsets of cells predominantly glycolytic or displaying an oxidative metabolism have been shown in gliomas and large B-cell lymphomas (Beckner et al., 2005; Bouzier, Voisin, Goodwin, Canioni, & Merle, 1998; Caro et al., 2012). The metabolic heterogeneity of tumors raises questions about the influence of metabolic differences on cell proliferation and response to chemotherapy.
Incomplete catabolism of glucose in the cytosol by glycolysis generates only 2mol of ATP per mole of glucose sparing carbon backbones in the form of lactate, whereas mitochondrial oxidation of 2mol of pyruvate generated from 1mol of glucose to CO2 and H2O generates about an additional 31mol of ATP. Although the total amount of mitochondrial ATP calculated considers the currently accepted proton stoichiometries for ATP synthesis, ATP/ADP Pi exchange, respiration, and the malate/aspartate shuttle, the actual ATP yield could also be less due to proton leak to the mitochondrial matrix (Brand, 2005; Rich, 2003; Rich & Marechal, 2010; Walker, 2013; Wikstrom, Sharma, Kaila, Hosler, & Hummer, 2015). In cancer cells, lower efficiency of ATP generation by aerobic glycolysis appears to be offset by high glycolytic rates (Locasale & Cantley, 2010). Regardless, it is unlikely that ATP generation be a limiting factor for cell proliferation because the ATP demand for cell division is lower than the energy requirements for basal cellular processes (Kilburn, Lilly, & Webb, 1969).
A long-standing question in cancer metabolism since the initial observations of Warburg has been why would cancer cells switch from an oxidative metabolism to a glycolytic phenotype especially considering that changes in ATP production may or may not be crucial for cell division. Would the dynamic range of energy production be strikingly different in highly proliferating cells compared to the nonproliferating cells or does the switch actually serve other purposes?
A dividing cell must double its biomass (proteins, lipids, and nucleic acids) before mitosis. This high biosynthetic demand requires the generation of simple carbon backbones that can be used as building blocks for the synthesis of new macromolecules. Cells expressing the Warburg phenotype partially break down glucose with a high proportion of pyruvate converted into lactate instead of being fully oxidized in mitochondria to CO2 and H2O (Fig. 2). In addition, a decreased mitochondrial utilization of glutamine and fatty acids provides additional carbon precursors for biomass formation in proliferating cells (Cairns, 2015; DeBerardinis, Sayed, Ditsworth, & Thompson, 2008; Keibler et al., 2016; Liberti & Locasale, 2016; Lunt & Vander Heiden, 2011). Enhanced glycolysis also provides by-products of glucose catabolism and NADPH. Glucose-6-phosphate, glyceraldehyde-3-phosphate, and 3-phosphoglycerate, as metabolic intermediaries, contribute to the synthesis of nucleotides, lipids, and amino acids, respectively (Fig. 1). Increased NADPH production by the pentose phosphate pathway is used for reductive biosynthesis.
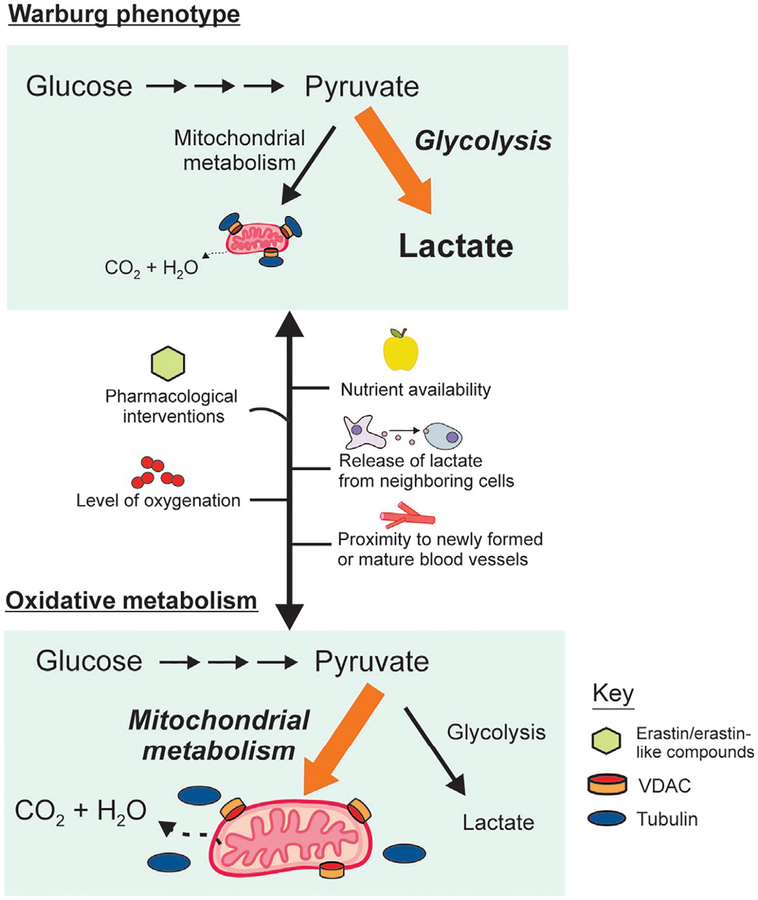
Fig. 2.
Metabolic flexibility of tumors and VDAC opening. Cancer cells switch between oxidative and glycolytic bioenergetic profiles depending on nutrient availability, tissue oxygenation, intratumor localization, and pharmacological treatments to inhibit glycolysis or to promote mitochondrial metabolism. In cancer cells, constitutive high free tubulin blocks VDAC conductance, suppresses mitochondrial metabolism, and decreases cytosolic ATP/ADP to favor glycolysis. Reversal of the inhibitory effect of tubulin by VDAC–tubulin antagonists leads to VDAC opening and reversal of the Warburg phenotype.
In the Warburg phenotype, mitochondria are not just passive generators of ATP but also essential contributors of metabolites required for cell proliferation. Catabolism of glutamine, pyruvate, and other respiratory substrates generates biosynthetic precursors in the Krebs cycle, including α-ketoglutarate and oxaloacetate for synthesis of nonessential amino acids and citrate exported to the cytosol to be converted into AcCoA and utilized for the synthesis of fatty acid, cholesterol, and amino acids (DeBerardinis & Cheng, 2010; Fig. 1). Recently, one-carbon metabolism, a set of reactions that transfer one-carbon units from serine and glycine, has been shown to be important for de novo synthesis of purines and thymidylate in highly proliferative tumors (Meise & Vazquez, 2016). In summary, the prop-roliferative Warburg phenotype is sustained by the differential cytosolic and mitochondrial utilization of glucose and other fuels that determine a bioenergetic profile that favors biosynthesis.
1.3. Mechanisms to Suppress Mitochondrial ATP Production: A Drive on Glycolysis
Mitochondrial oxidation of respiratory substrates by OXPHOS maximizes the yield of ATP per mol of fuels that enter mitochondria. The electrogenic adenine nucleotide translocator (ANT) located in the MIM transport the newly synthesized ATP in the mitochondrial matrix to the cytosol exchanging ATP−4 for ADP−3 (Fig. 1). In differentiated cells with predominantly oxidative metabolism, cytosolic ATP/ADP ratios can be 50–100 times higher than in the mitochondrial matrix (Schwenke, Soboll, Seitz, & Sies, 1981). Oxidative metabolism favors a high cytosolic ATP/ADP ratio which suppresses glycolysis through inhibition of phosphofructokinase-1 (PFK-1), although other mechanisms may be involved. ATP is a strong allosteric inhibitor, whereas ADP and AMP activate PFK-1 (Mor, Cheung, & Vousden, 2011; Moreno-Sanchez et al., 2007). In cancer cells, suppression of mitochondrial metabolism contributes to a low cytosolic ATP/ADP ratio, which releases this brake on glycolysis.
Recently, we demonstrated that closing of the voltage-dependent anion channels (VDACs) by free tubulin limits the influx of metabolites into mitochondria and limits ATP production, whereas replacement of electrogenic ATP/ADP exchange by ANT with a nonelectrogenic exchange mechanism decreases cytosolic ATP/ADP ratios. These two independent mechanisms contribute to suppress mitochondrial metabolism and to maintain a low cytosolic ATP/ADP ratio favoring aerobic glycolysis in cancer cells (Maldonado et al., 2016, 2013; Maldonado & Lemasters, 2014).
2. VDAC CHANNELS AND MITOCHONDRIAL METABOLISM
2.1. The MOM: A VDAC-Containing Interphase to Modulate Cellular Bioenergetics
The MOM is a functional barrier that physically separates mitochondria from the cytosol (Fig. 1). VDAC, the most abundant protein in the MOM, is the gateway through which most respiratory substrates, ADP, and Pi enter mitochondria and ATP exits. Based on its role in metabolite exchange between mitochondria and the cytosol and its subcellular localization, VDAC opening is proposed to be a master regulator to globally modulate mitochondrial bioenergetics and the intracellular flow of energy (Lemasters & Holmuhamedov, 2006; Maldonado & Lemasters, 2012, 2014; Maldonado et al., 2013).
Interactions between VDAC with tubulin and possibly other proteins, such as hexokinase (Pastorino & Hoek, 2003; Wolf et al., 2011) and posttranslational modifications of VDAC especially phosphorylation by protein kinase A (PKA) and glycogen synthase 3β (GSK3β), modulate the open/closed state of VDAC (Sheldon, Maldonado, Lemasters, Rostovtseva, & Bezrukov, 2011). Single and double knockdown of the three different VDAC isoforms support this concept that VDAC serves as a master regulator of mitochondrial metabolism in cancer cells (Maldonado et al., 2013). Thus, VDAC regulation by free tubulin emerges as a mechanism to block or promote OXPHOS and indirectly regulate glycolysis through the cytosolic ATP/ADP ratio. Ultimately, VDAC–tubulin interactions appear as a new pharmacological target to increase mitochondrial metabolism in cancer cells and to reverse Warburg metabolism (Maldonado, 2017).
2.2. VDAC Structure and Regulation of the Conductance
A protein with pore-forming activity first described in extracts of mitochondria from Paramecium tetraurelia (Schein, Colombini, & Finkelstein, 1976) was initially called mitochondrial porin and later renamed VDAC (Colombini, 1979). VDAC present in all eukaryotic cells comprises three isoforms, VDAC1, VDAC2, and VDAC3, encoded by separate genes. VDAC1 and VDAC2 are the main isoforms in most mammalian cells, including cancer cells in which they account for up to 90% of the total. The least abundant isoform, VDAC3, comprising the remaining 10% (De Pinto et al., 2010; Huang, Shah, Bradbury, Li, & White, 2014; Maldonado et al., 2013) is abundant only in testis (Sampson et al., 2001; Sampson, Lovell, & Craigen, 1997).
VDAC in humans and mice is a ~30-kDa protein enclosing an aqueous channel of ~3nm internal diameter that allows the passage of molecules up to ~5kDa (Colombini, 1980, 2012; Song & Colombini, 1996). The influx of polar metabolites through VDAC is determined mostly by their charge and size (Colombini, 1980, 2004). Once in the intermembrane space polar metabolites cross the MIM through several specific carriers that utilize electrical, chemical, or electrochemical potential gradients to transport diverse solutes including pyruvate, Pi, ADP, ATP, acylcarnitine, citrate, oxoglutarate, and glutamate. The activity of mitochondrial carriers is finely regulated to allow a sufficient flux of metabolites to adapt to different physiological demands (Palmieri & Pierri, 2010). The availability of solutes to the carriers in the MIM depends on the metabolites produced in the mitochondrial matrix mainly through the Krebs cycle and the metabolites that access the intermembrane space through VDAC in the MOM. Thus, the probability of VDAC in an open or close conformation has a substantial impact on mitochondrial metabolism and cellular bioenergetics.
In the closed state, only small ions like Na+, K+, or Cl− but not most anionic metabolites including respiratory substrates, ATP, ADP, and Pi permeate through VDAC. Structurally, VDAC1 has a barrel configuration with staves formed by 19 β-strands (Hiller, Abramson, Mannella, Wagner, & Zeth, 2010; Ujwal et al., 2008). An additional N-terminal sequence forms the only α-helical segment that appears to move to the center of the channel, blocking the passage of metabolites. Recently, a similar β barrel structure with 19 β-strands has been shown for VDAC2 from zebrafish (Schredelseker et al., 2014). Gating and selectivity of VDAC1 and VDAC2 are highly conserved among mammals (Blachly-Dyson & Forte, 2001).
After the initial discovery, the consensus was that VDAC was constitutively open to the flux of metabolites between the mitochondrial matrix and the cytosol as an “all-time open gate.” Extensive research in vitro and in intact cells showed instead that VDAC opening is subjected to modulation. Numerous studies have shown regulation by multiple factors, including hexokinase (Al Jamal, 2005; Azoulay-Zohar, Israelson, Abu-Hamad, & Shoshan-Barmatz, 2004; Nakashima, Paggi, Scott, & Pedersen, 1988), Bcl2 family members (Tsujimoto & Shimizu, 2000), glutamate (Gincel, Silberberg, & Shoshan-Barmatz, 2000), ethanol (Holmuhamedov & Lemasters, 2009; Lemasters & Holmuhamedov, 2006), and NADH (Zizi, Forte, Blachly-Dyson, & Colombini, 1994). VDAC phosphorylation by protein kinases, GSK3β, PKA, and protein kinase C epsilon (PKCε), blocks or inhibits association of VDAC with other proteins, such as Bax and tBid, and also regulates VDAC opening (Azoulay-Zohar et al., 2004; Baines et al., 2003; Das, Wong, Rajapakse, Murphy, & Steenbergen, 2008; Lee, Zizi, & Colombini, 1994; Rostovtseva et al., 2004; Vander Heiden et al., 2000, 2001). PKA-dependent VDAC phosphorylation and GSK3β-mediated VDAC2 phosphorylation increase VDAC conductance (Bera, Ghosh, & Das, 1995; Das et al., 2008; Sheldon et al., 2011). Here, we will focus on the inhibitory effect of free tubulin on VDAC in cancer cells as a regulatory mechanism of VDAC opening and as a pharmacological target (Maldonado et al., 2010, 2013).
3. VDAC–TUBULIN INTERACTION
3.1. VDAC Inhibition by Free Tubulin
Mitochondrial ΔΨ in cancer cells is sustained both by the respiratory chain and from hydrolysis of glycolytic ATP by the mitochondrial F1F0-ATPase working in reverse. We previously showed that treatment of cancer cells with the microtubule stabilizer paclitaxel or the microtubule destabilizers nocodazole and colchicine decreased and increased, respectively, cytosolic free tubulin. We also showed that low and high cytosolic free tubulin promotes high and low mitochondrial ΔΨ, respectively (Maldonado et al., 2010). By contrast, in the nonproliferating rat hepatocyte, mitochondrial ΔΨ was relatively insensitive to changes in free tubulin levels. The lack of response to pharmacological interventions to stabilize/destabilize micro-tubules could be explained by the much lower constitutive pool of free tubulin in rat hepatocytes compared to hepatocarcinoma cells. Non-proliferating hepatocytes do not need a reservoir of tubulin for spindle formation at mitosis. Thus, microtubule stabilization with paclitaxel does not increase ΔΨ in hepatocytes, because levels of free tubulin are already low, whereas microtubule destabilization still increases tubulin and, in turn, decreases ΔΨ. These findings imply that VDAC is indeed constitutively open in nonproliferating hepatocytes. By contrast, paclitaxel increases and nocodazole/colchicine decreases ΔΨ in tumor cells, leading to the conclusion that endogenous free tubulin partially closes VDAC in tumor cells (Maldonado et al., 2010). We propose that inhibition of VDAC conductance by free tubulin is a mechanism that contributes to the suppression of mitochondrial metabolism in the Warburg phenotype. Our studies performed in intact cancer cells were in agreement with earlier work showing that heterodimeric αβ-tubulin closes VDAC inserted into lipid bilayers and decreases respiration in isolated brain mitochondria and permeabilized synaptosomes (Rostovtseva et al., 2008; Timohhina et al., 2009).
Knockdown studies of VDAC1, VDAC2, and VDAC3 in HepG2 cells further characterized the role of VDAC in mitochondrial metabolism in cancer cells and showed that VDAC sensitivity to tubulin inhibition is iso-form dependent. Single knockdown of each of the three VDAC isoforms, especially the minor isoform VDAC3, decreased mitochondrial ΔΨ, indicating that all VDAC isoforms contribute to ΔΨ formation. Knockdown of VDAC3 also decreased cellular ATP and ADP and the NAD(P)H/NAD(P)+ ratio, suggesting that the least abundant isoform VDAC3 contributed most to the maintenance of mitochondrial metabolism (Maldonado et al., 2013). The response of each isoform to tubulin inhibition was characterized by double knockdown of VDAC isoforms in all combinations. All single and double knockdowns partially reversed the suppression of ΔΨ induced by free tubulin (Maldonado et al., 2013). Electrophysiology studies of VDAC1 and VDAC2 isolated from double-knockdown HepG2 cells inserted in lipid bilayers showed that both isoforms were almost equally sensitive to tubulin inhibition, whereas VDAC3 was insensitive at tubulin concentrations even five-fold higher than those used to inhibit VDAC1 and VDAC2 (Maldonado et al., 2013). The voltage gating and the response to dimeric αβ-tubulin of constitutive VDAC isolated from wild-type HepG2 cells compared to VDAC from heart and liver mitochondria were almost identical. These similarities suggest that the differential response to tubulin inhibition in cancer vs nonproliferating cells depends on the amount of cytosolic free tubulin and not on a cell type-specific sensitivity of the channels. The knockdown studies supported the conclusion that VDAC3, at least in HepG2 cells, is constitutively open, whereas VDAC1 and VDAC2 are totally or partially closed by free tubulin.
3.2. VDAC–Tubulin Modulation of Cellular Bioenergetics During Cell Cycle
Biosynthesis of new macromolecules to double the biomass before mitosis occurs during G1, S, and G2 phases of the cell cycle. VDAC–tubulin-dependent suppression of mitochondrial metabolism caused by high constitutive free tubulin would favor the probiosynthetic Warburg phenotype during these growth stages. In fact, in HeLa, NIH3T3, NCI-H292, and other cancer cells, most of the cell cycle lasting 20–30h is composed of G1, S, and G2 phases, whereas cell division that occurs during the M or mitotic phase lasts only about 30min (Hahn, Jones, & Meyer, 2009). During mitosis, energy demand increases sharply to support chromosome segregation and cytokinesis. At this point, where null or minimal biosynthesis is expected, a Warburg phenotype would not be beneficial for cell division. Moreover, full oxidation of respiratory substrates with maximum yield of ATP may be required to meet the high energy demands of cell division. An open VDAC would allow maximum OXPHOS activity. A possible sequence of events is that as the spindle forms during prophase, the free tubulin pool decreases abruptly, releasing tubulin inhibition of VDAC. VDAC opening then promotes increased mitochondrial metabolism reverting the Warburg phenotype precisely when the energy demand is maximal. After mitosis, the pool of free tubulin increases again, and cells return to a high glycolytic, proproliferative phenotype during the nonmitotic stages of the cell cycle (Maldonado, 2017; Maldonado & Lemasters, 2012).
4. TUMOR METABOLIC FLEXIBILITY: ADVANTAGES OF TARGETING METABOLISM IN CHEMOTHERAPY
Our work showing constitutive inhibition of VDAC conductance in cancer cells by free tubulin raised questions about the possibility of decreasing cell proliferation by modifying the bioenergetic status of the cell. Cytotoxic chemotherapy is commonly based on drugs that cause cell death or prevent cell growth by inhibiting microtubule function, protein function, or DNA synthesis and replication. Although from a bioenergetics perspective both glycolysis and mitochondria contribute to energy production in cancer cells, most of the attempts to target metabolism as a new approach for cancer therapy have been directed to inhibit glycolysis.
The increasingly recognized heterogeneity of tumor metabolism and the capability of tumor cells to switch bioenergetics profiles from glycolytic to oxidative and vice versa open questions about the feasibility of developing metabolism oriented chemotherapies as sole treatments or as coadjuvants for current chemotherapeutic protocols. The predominance of a glycolytic or oxidative metabolism in cancer cells is determined genetically and by both temporary and long-term epigenetic changes. The relative contribution of glycolysis and OXPHOS can vary substantially over time depending on multiple factors, including availability to different fuels, proximity to newly formed vs mature blood vessels, and the release of soluble factors such as lac-tate from neighboring cells (Fig. 2).
Inadequate blood perfusion in rapidly growing tumors not only exposes cells to hypoxia but also to a lower supply of nutrients including glucose. Hypoxia can decrease the OXPHOS flux depending on the cell type, time of hypoxic exposure, and environmental conditions. When comparing MCF-7 and HeLa cells that predominantly depend on OXPHOS for ATP supply, prolonged hypoxia increases glycolysis only in MCF-7 cells (Rodriguez-Enriquez et al., 2010). In solid tumors the respiratory chain can still be fully functional at oxygen levels as low as 0.5%, indicating that hypoxic tumor cells exposed to <2% oxygen in rapidly growing and heterogeneously perfused tumors still produce ATP by OXPHOS. Under those conditions, even if pyruvate utilization is compromised mitochondria from tumor cells can utilize glutamine as an energy source so actually both glycolysis and OXPHOS can sustain tumor growth (Mullen et al., 2012).
Nutrient availability not only influences tumor growth but can also promote a switch from aerobic glycolysis to OXPHOS in breast cancer cell lines and lymphoma cells cultured in glucose-free media (Robinson, Dinsdale, MacFarlane, & Cain, 2012; Smolkova et al., 2010). The broad adaptability of tumor cells to oxidize other substrates when glucose or glutamine is limited includes the utilization of lactate, methionine, argi-nine, cysteine, asparagine, leucine, acetate, and even proteins and lipids from the microenvironment (Chung et al., 2005; Clavell et al., 1986; Comerford et al., 2014; Commisso et al., 2013; Keenan & Chi, 2015; Kennedy et al., 2013; Kreis, Baker, Ryan, & Bertasso, 1980; Mashimo et al., 2014; Scott, Lamb, Smith, & Wheatley, 2000; Sheen, Zoncu, Kim, & Sabatini, 2011; Sonveaux et al., 2008). Inhibition of Complex III by antimycin and Complex I by piericidin A triggers a compensatory increase in the uptake and consumption of glucose in myoblasts. In these myoblasts total cellular ATP production before and after OXPHOS inhibition was similar, indicating that the loss of ATP generation by OXPHOS was fully compensated by increased glycolytic ATP (Liemburg-Apers, Schirris, Russel, Willems, & Koopman, 2015).
The metabolic heterogeneity and flexibility of tumors and the potential to switch between glycolytic and oxidative metabolisms underscore the relevance of mechanisms that underlie adaptive changes like VDAC regulation. Most research efforts to target tumor metabolism have been directed toward inhibition of glycolysis (Doherty & Cleveland, 2013; Pelicano, Martin, Xu, & Huang, 2006). Only recently mitochondrial metabolism emerged as a chemotherapeutic target with most approaches attempting to inhibit mitochondrial metabolism in cancer cells (Bhat, Kumar, Chaudhary, Yadav, & Chandra, 2015; Weinberg & Chandel, 2015). The observation that the antidiabetic drug metformin decreased the prevalence of certain types of cancer triggered an interest in the role of mitochondrial inhibition as a mechanism to suppress abnormal cell proliferation (Giovannucci et al., 2010; Libby et al., 2009). Although metformin decreases OXPHOS by inhibiting Complex I of the respiratory chain, it also inhibits the mammalian target of rapamycin (mTOR), interferes with folate metabolism, and activates AMP kinase. It is uncertain if the antiproliferative effect of metformin is actually due to OXPHOS inhibition (Jara & Lopez-Munoz, 2015). Other approaches to inhibit mitochondrial metabolism in various cancer cell models include etomoxir to inhibit carnitine O-palmitoyltransferase 1 and subsequently mitochondrial fatty acid oxidation (leukemia); tigecycline to inhibit mitochondrial protein translation (leukemia); glutaminase inhibitors (breast cancer, lymphoma); and the compound VLX600 to inhibit OXPHOS (colon cancer) (Samudio et al., 2010; Skrtic et al., 2011; Wang et al., 2010; Zhang et al., 2014). Instead of inhibiting mitochondrial metabolism, other antiproliferative strategies promote mitochondrial metabolism. For example the pyruvate analog dichloroacetate, which promotes cell killing in several cancer cell lines and in some in vivo models, activates pyruvate dehydrogenase to increase mitochondrial metabolism (Sutendra & Michelakis, 2013).
5. VDAC–TUBULIN ANTAGONISTS: A STRATEGY FOR OPENING VDAC
5.1. Erastin and VDAC–Tubulin Antagonists
Relative closure of VDAC in cancer cells and the broad metabolic consequences of VDAC opening turn the VDAC–tubulin interaction into a novel pharmacological target to increase mitochondrial metabolism. Antagonists of the constitutive inhibition of VDAC by free tubulin would be expected to increase oxidative metabolism and promote an anti-Warburg effect by decreasing glycolysis. In search of VDAC opening drugs we reported the small-molecule erastin as the first described antagonist of the inhibitory effect of free tubulin on VDAC (Maldonado et al., 2013). Erastin selectively induces nonapoptotic cell death in human cells engineered to harbor small T oncoprotein and the oncogenic allele of HRAS, v-Ha-ras Harvey rat sarcoma viral oncogene homologue RASv12 (Dolma, Lessnick, Hahn, & Stockwell, 2003). Erastin-dependent cell death is blocked by antioxidants, such as α-tocopherol, butylated hydroxytoluene, and desferal, but not by pan-caspase inhibitors (Dolma et al., 2003). Other cell lines harboring the v-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue (KRAS) and an activating V600E mutation in v-raf-murine sarcoma viral oncogene homo-logue B1 (BRAF) are moderately sensitive to erastin. Erastin is proposed to bind to VDAC2 and VDAC3, leading to oxidative stress and cell death in cells with activated RAS–RAF–MEK signaling (Yagoda et al., 2007).
We showed that erastin in HepG2 cells and other cell lines increases mitochondrial ΔΨ and prevent depolarization induced by high cytosolic free tubulin, an effect not described previously. In addition, erastin added after microtubule destabilizers also restores mitochondrial ΔΨ, indicating that erastin prevents and reverses the inhibitory effect of free tubulin on VDAC (Maldonado et al., 2013). Also, erastin completely blocks tubulin inhibition of VDAC without modifying the voltage dependence of channels from HepG2 cells in planar lipid bilayers, confirming that the effect of erastin is specific for tubulin-dependent inhibition of VDAC (Maldonado et al., 2013). Following the identification of erastin as a VDAC–tubulin antagonist, we identified a group of erastin-like anti-Warburg compounds using a cell-based high-throughput drug screening. We tested the 50,080 DIVERSet Chembridge compound library to select small molecules that, similar to erastin, hyperpolarized mitochondria in the presence of microtubule destabilizers and high cytosolic free tubulin. The six lead compounds hyperpolarized mitochondria without causing changes in tubulin polymerization in a dose-dependent fashion. Erastin and the most potent X1 not only increased mitochondrial metabolism but had an anti-Warburg effect evidenced by the decreased lactate release in HepG2 and Huh7 hepatocarcinoma cells and HCC4006 lung carcinoma cell lines (DeHart, Lemasters, & Maldonado, 2017).
5.2. VDAC Opening, Glycolysis, and Reactive Oxygen Species Formation
VDAC opening in cancer cells leads to three main biological effects: increased mitochondrial metabolism caused by augmented entry of substrates into mitochondria, subsequent decreased glycolysis due to a higher cytosolic ATP/ADP ratio, and increased formation of reactive oxygen species (ROS) caused by enhanced activity of the ETC (Fig. 3).
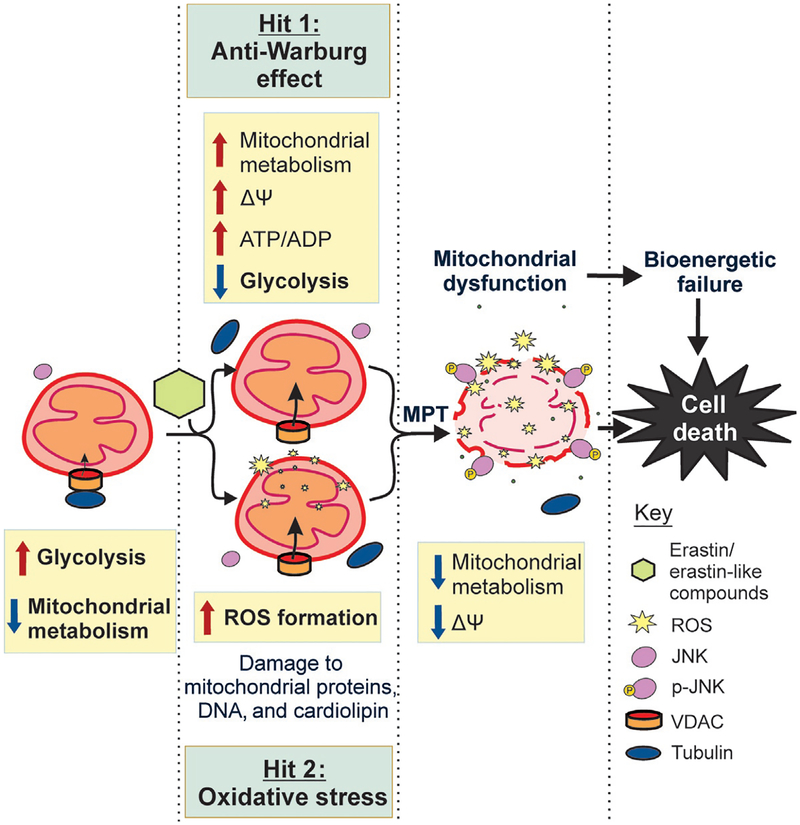
Fig. 3.
Mechanisms to promote cell death after VDAC opening. VDAC–tubulin antagonists open VDAC increasing the flux of metabolites into and out of mitochondria leading to increased mitochondrial ΔΨ, increased mitochondrial metabolism, high cytosolic ATP/ADP ratio, and decreased glycolysis (Hit 1: anti-Warburg effect). VDAC opening also increases ROS formation concurrent with the decrease in glycolysis. Increased ROS damage mitochondrial DNA, cardiolipin, and mitochondrial proteins and activate JNK (Hit 2: oxidative stress). Activated JNK translocates to mitochondria causing mitochondrial dys-function, bioenergetic failure, and cell death.
After VDAC opening, flux of respiratory substrates into mitochondria fuels the Krebs cycle to produce NADH that enters the ETC. Electron pairs from NADH flow down the ETC to the final acceptor O2. Simultaneous with the flow of electrons through the ETC, single electrons leak from Complexes I, II, and III to form the superoxide anion (O2•−) (Chance, Sies, & Boveris, 1979). Although there are other mitochondrial and nonmitochondrial sources of ROS formation, the mitochondrial ETC especially Complex I (site IQ), Complex II (site IIF), and Complex III (site IIIQo) have the highest capacity of ROS production among the seven major mitochondrial sites that produce ROS in mammals (Chen, Vazquez, Moghaddas, Hoppel, & Lesnefsky, 2003; Quinlan et al., 2012; Skulachev, 1996; Tribble, Jones, & Edmondson, 1988). The metabolic fate of O2•− varies depending on the site of origin. O2•− formed at Complexes I and II is released to the matrix, whereas O2•− generated at Complex III is released in large part to the intermembrane space and hence to the cytosol through VDAC (Brand, 2010; Han, Antunes, Canali, Rettori, & Cadenas, 2003; Muller, Liu, & Van, 2004). Superoxide dismutases located in the mitochondrial matrix (manganese-containing enzyme MnSOD or SOD2) and the cytosol (copper- and-zinc-containing enzyme Cu,ZnSOD or SOD1) rapidly convert O2•− to H2O2 (Fridovich, 1997). H2O2, a nonradical and the least reactive of ROS species, diffuses across membranes acting as a cell signaling molecule in proproliferative and prosurvival pathways without necessarily disrupting redox homeostasis (Morgan, Sobotta, & Dick, 2011; Veal, Day, & Morgan, 2007). For example, H2O2 modulates the prosurvival HIF-1 and MAP/ERK, PI3K/akt/mTOR pathways that favor tumorigenesis and metastasis (Clerkin, Naughton, Quiney, & Cotter, 2008; Giles, 2006; Ushio-Fukai & Nakamura, 2008). H2O2 levels depend on the rate of production and degradation by the enzyme catalase or the intercon-version that occurs when H2O2 accept an electron from free and loosely bound Fe2+ to form the highly reactive hydroxyl radical (OH•)by the Fenton reaction.
The effects of mitochondrial ROS on cellular structures depend on the specific ROS. The lifetimes of H2O2 and O2•− allow them to react both with mitochondria and with extramitochondrial structures. By contrast, OH• are so reactive that the effects are almost completely restricted to mitochondria. Both O2•− and OH• inactivate mitochondrial proteins, including ATP synthase, NADH oxidase, and NADH dehydrogenase (Zhang, Marcillat, Giulivi, Ernster, & Davies, 1990). Beyond proteins, ROS damage mitochondrial DNA and lipids in the MIM. ROS-dependent peroxidation of cardiolipin, a MIM phospholipid rich in polyunsaturated fatty acids, is considered an early event in apoptosis (Schenkel & Bakovic, 2014).
Cytosolic ROS activate members of the MAPK family of serine/threonine kinases, especially c-Jun N-terminal kinase (JNK), the extracellular signal-regulated kinase (ERK 1/2), and p38 whose signaling can cause mitochondrial dysfunction (Kamata et al., 2005; Son et al., 2011). In fact, treatment with erastin and X1 in HepG2 and Huh7 hepatocarcinoma cells promoted JNK activation and increased ROS formation that caused mitochondrial dysfunction prevented by antioxidants. Moreover, blocking of the translocation of activated JNK to mitochondria prevented mitochondrial dysfunction induced by VDAC–tubulin antagonists (unpublished). Oxidative stress caused by accumulation of ROS also induces mitochondrial permeability transition (MPT) pores in the inner membrane. Opening of MPT pores results in the MIM becoming highly permeable to ions and solutes up to ~1.5kDa collapsing ΔΨ (Zoratti & Szabo, 1995).
ROS levels are higher in tumor cells from cell lines, in animal models of cancer, and in human tumor tissues compared to nonproliferating cells. Increased H2O2 formation in cell lines and oxidative-induced DNA modifications and 4-hydroxy-2-nonenal-modified proteins in animal models and human tissues support this concept (Kawanishi, Hiraku, Pinlaor, & Ma, 2006; Szatrowski & Nathan, 1991; Toyokuni, Okamoto, Yodoi, & Hiai, 1995; Uchida, 2003). In cancer cells higher levels of ROS are neutralized by a high content of scavenging enzymes and antioxidants including SODs, catalase, and the glutathione system that reduces protein disulfide bonds (Liou & Storz, 2010; Panieri & Santoro, 2016; Sullivan & Chandel, 2014; Venditti, Di, & Di, 2013). Although ROS in cancer cells are proposed to be cytostatic, to favor tumor growth, or to be cytotoxic, the different levels of ROS that would cause each outcome have not been determined experimentally (Marengo et al., 2016; Panieri & Santoro, 2016; Sullivan & Chandel, 2014). Oxidative stress has been reported to induce mitochondrial dysfunction, cell cycle arrest, senescence, apoptosis, or necrosis (Liou & Storz, 2010).
Chemotherapeutic agents including cisplatin, adriamycin, the anthracyclines doxorubicin, epirubicin, and daunorubicin among others promote oxidative stress and depletion of the antioxidant capacity of tumor cells leading to a tumoricidal effect (Conklin, 2004; Faber, Coudray, Hida, Mousseau, & Favier, 1995; Ladner, Ehninger, Gey, & Clemens, 1989; Weijl et al., 1998). VDAC–tubulin antagonists by opening VDAC promote mitochondrial metabolism which increases the activity of the ETC leading to increased ROS formation. Continued enhanced ROS production eventually overcomes the antioxidant capacity of cancer cells leading to cytotoxicity.
5.3. VDAC-Dependent Metabolic Hits: Anti-Warburg Effect and Oxidative Stress
Metabolic heterogeneity is a complicating factor for the success of cancer chemotherapy (Dang, 2012; Eason & Sadanandam, 2016; Gerlinger et al., 2012; Yun, Johnson, Hanigan, & Locasale, 2012). All cancer cells, even with distinct metabolic signatures, display some level of enhanced glycolysis, suggesting different degrees of contribution by VDAC closure to the suppression of mitochondrial metabolism (Griguer et al., 2005; Guppy et al., 2002; Moreno-Sanchez et al., 2007; Scott et al., 2011). Reversal of the inhibitory effect of tubulin on VDAC triggers two distinct and nearly simultaneous effects: (1) increase of mitochondrial metabolism and activation of OXPHOS with consequent decrease of glycolysis (anti-Warburg effect) and (2) an increase in ROS formation leading to oxidative stress (Fig. 3). Oxidative stress is potentially more deleterious in highly glycolytic cells, which presumably could have lower antioxidant capacity since OXPHOS is not very active in these cells. By contrast, the reversal of the Warburg effect could damage more the highly glycolytic cells that survive oxidative stress and continue proliferating or the low glycolytic cells with a presumably constitutively higher basal level of ROS.
The VDAC–tubulin antagonist erastin and erastin-like compounds cause mitochondrial hyperpolarization followed by mitochondrial depolarization indicative of mitochondrial dysfunction in human hepatocarcinoma cells (unpublished). The initial increase in ΔΨ precedes the increase in ROS generation and JNK activation resulting in mitochondrial dysfunction and possibly the onset of MPT. MPT causes nonselective permeabilization of the MIM leading to mitochondrial swelling, loss of ΔΨ and ATP synthesis, rupture of the MOM, and cytochrome c release resulting in cell death (Bonora & Pinton, 2014; Green & Kroemer, 2004). MPT is mediated by the irreversible opening of the permeability transition pore complex (PTPC), a multiprotein pore assembled with proteins from both the MOM and the MIM. VDAC, ANT, cyclophilin D, and the subunit c of the F1F0-ATP synthase among other mitochondrial proteins have been included as PTPC-forming proteins, although the molecular identity of the pore remains debatable (Izzo, Bravo-San Pedro, Sica, Kroemer, & Galluzzi, 2016). VDAC, initially considered a main component of the pore, is dispensable for the onset of MPT. Oxidative stress, a well-known inducer of MPT (Bonora & Pinton, 2014; Kowaltowski, Castilho, & Vercesi, 2001; Takeyama, Matsuo, & Tanaka, 1993) promotes MPT even in knockout cells for all VDAC isoforms (Baines, Kaiser, Sheiko, Craigen, & Molkentin, 2007).
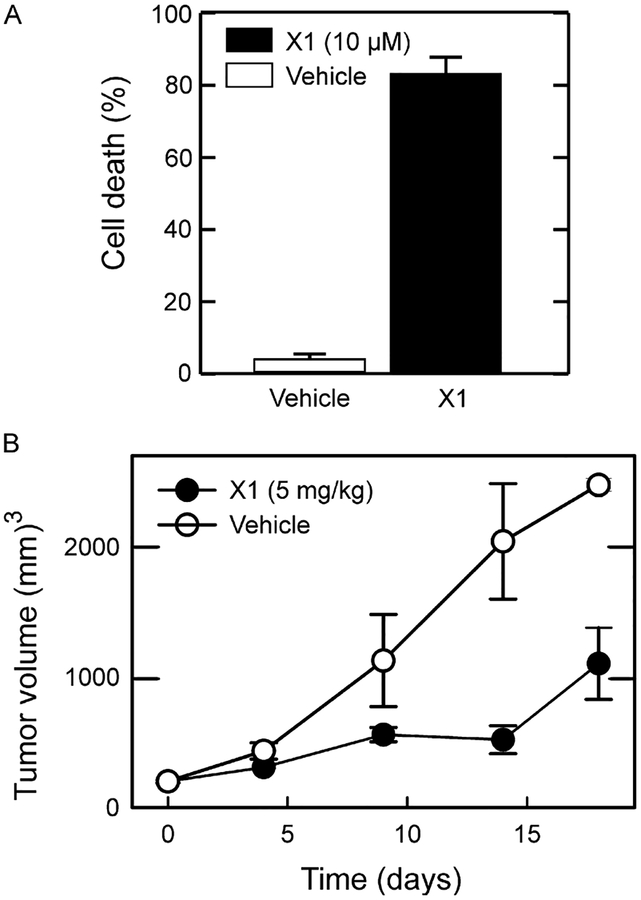
The erastin-like anti-Warburg compound X1 also decreases glycolysis as evidenced by a decrease in lactate release (DeHart et al., 2017). The combination of reversal of Warburg metabolism and oxidative stress by the lead compound caused cell death to human hepatocarcinoma cell lines in culture and to xenografted Huh7 hepatocarcinoma cells (Fig. 4). Thus, erastin and lead erastin-like compounds by causing “two-hits”: an anti-Warburg effect and promotion of oxidative stress, represent a potential new class of cancer chemotherapeutic agents (Fig. 3).
Fig. 4.
Erastin-like compound X1 causes cell death in situ and in vivo. Lead compound X1 caused cell death in Huh7 hepatocarcinoma cells in culture and slowed tumor growth in a xenograft model of Huh7 cells in nude mice.
6. CONCLUDING REMARKS
The VDAC–tubulin interaction in cancer cells operates as a metabolic switch to control cellular bioenergetics and regulate the Warburg phenotype. VDAC opening exerts a global influence on mitochondrial metabolism increasing OXPHOS and ROS production and indirectly modulating glycolysis. Pharmacological inhibition of the VDAC switch triggers two concurrent and complementary “hits”: an anti-Warburg effect that promotes a nonproliferative metabolic phenotype and oxidative stress leading to mitochondrial dysfunction and cell death. VDAC-dependent oxidative stress is expected to promote cell killing in highly glycolytic cells with presumably lower anti-oxidant defenses and to cause nonlethal cell damage in less glycolytic tumor types. The anti-Warburg effect will decrease or stop cell proliferation in those cells in which increase in ROS formation was sublethal. In summary, the VDAC–tubulin interaction represents a new pharmacological target to turn a proproliferative phenotype into a cytotoxic, mitochondrial-dependent, and prooxidant metabolic profile. VDAC–tubulin antagonists could become a new generation of metabolism-oriented cancer chemotherapy.
ACKNOWLEDGMENT
This work was funded by R01CA184456, GM103542, and ACS 13-041-01-IRG to E.N.M.
REFERENCES
- Al Jamal JA (2005). Involvement of porin N,N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane. The Protein Journal, 24, 1–8. [DOI] [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, & Shoshan-Barmatz V (2004). In self-defence: Hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochemistry Journal, 377, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, & Molkentin JD (2007). Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nature Cell Biology, 9, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. (2003). Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circulatory Research, 92, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner ME, Gobbel GT, Abounader R, Burovic F, Agostino NR, Laterra J, et al. (2005). Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of PI3K and gluconeogenesis. Laboratory Investigation, 85, 1457–1470. [DOI] [PubMed] [Google Scholar]
- Bera AK, Ghosh S, & Das S (1995). Mitochondrial VDAC can be phosphorylated by cyclic AMP-dependent protein kinase. Biochemical and Biophysical Research Communications, 209, 213–217. [DOI] [PubMed] [Google Scholar]
- Bhat TA, Kumar S, Chaudhary AK, Yadav N, & Chandra D (2015). Restoration of mitochondria function as a target for cancer therapy. Drug Discovery Today, 20, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachly-Dyson E, & Forte M (2001). VDAC channels. IUBMB Life, 52, 113–118. [DOI] [PubMed] [Google Scholar]
- Bonora M, & Pinton P (2014). The mitochondrial permeability transition pore and cancer: Molecular mechanisms involved in cell death. Frontiers in Oncology, 4, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier AK, Voisin P, Goodwin R, Canioni P, & Merle M (1998). Glucose and lactate metabolism in C6 glioma cells: Evidence for the preferential utilization of lactate for cell oxidative metabolism. Developmental Neuroscience, 20, 331–338. [DOI] [PubMed] [Google Scholar]
- Brand MD (2005). The efficiency and plasticity of mitochondrial energy transduction. Biochemical Society Transactions, 33, 897–904. [DOI] [PubMed] [Google Scholar]
- Brand MD (2010). The sites and topology of mitochondrial superoxide production. Experimental Gerontology, 45, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA (2015). Drivers of the warburg phenotype. Cancer Journal, 21, 56–61. [DOI] [PubMed] [Google Scholar]
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, et al. (2012). Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell, 22, 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, & Boveris A (1979). Hydroperoxide metabolism in mammalian organs. Physiological Reviews, 59, 527–605. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, & Lesnefsky EJ (2003). Production of reactive oxygen species by mitochondria: Central role of complex III. Journal of Biological Chemistry, 278, 36027–36031. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, et al. (2005). Inhibition of cystine uptake disrupts the growth of primary brain tumors. The Journal of Neuroscience, 25, 7101–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell LA, Gelber RD, Cohen HJ, Hitchcock-Bryan S, Cassady JR, Tarbell NJ, et al. (1986). Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. The New England Journal of Medicine, 315, 657–663. [DOI] [PubMed] [Google Scholar]
- Clerkin JS, Naughton R, Quiney C, & Cotter TG (2008). Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Letters, 266, 30–36. [DOI] [PubMed] [Google Scholar]
- Colombini M (1979). A candidate for the permeability pathway of the outer mitochondrial membrane. Nature, 279, 643–645. [DOI] [PubMed] [Google Scholar]
- Colombini M (1980). Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. The Annals of the New York Academy of Sciences, 341, 552–563. [DOI] [PubMed] [Google Scholar]
- Colombini M (2004). VDAC: The channel at the interface between mitochondria and the cytosol. Molecular and Cellular Biochemistry, 256–257, 107–115. [DOI] [PubMed] [Google Scholar]
- Colombini M (2012). VDAC structure, selectivity, and dynamics. Biochimica et Biophysica Acta, 1818, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, et al. (2014). Acetate dependence of tumors. Cell, 159, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature, 497, 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin KA (2004). Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integrative Cancer Therapies, 3, 294–300. [DOI] [PubMed] [Google Scholar]
- Dang CV (2012). Links between metabolism and cancer. Genes & Development, 26, 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Wong R, Rajapakse N, Murphy E, & Steenbergen C (2008). Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circulation Research, 103, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, & Cheng T (2010). Q’s next: The diverse functions of glutamine in metabolism, cell biology, and cancer. Oncogene, 29, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Sayed N, Ditsworth D, & Thompson CB (2008). Brick by brick: Metabolism and tumor cell growth. Current Opinion in Genetics & Development, 18, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHart DN, Lemasters JJ, & Maldonado EN (2017). Erastin-like anti-warburg agents prevent mitochondrial depolarization induced by free tubulin and decrease lactate formation in cancer cells. SLAS Discovery, 23, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pinto V, Guarino F, Guarnera A, Messina A, Reina S, Tomasello FM, et al. (2010). Characterization of human VDAC isoforms: A peculiar function for VDAC3? Biochimica et Biophysica Acta, 1797, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Doherty JR, & Cleveland JL (2013). Targeting lactate metabolism for cancer therapeutics. The Journal of Clinical Investigation, 123, 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S, Lessnick SL, Hahn WC, & Stockwell BR (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell, 3, 285–296. [DOI] [PubMed] [Google Scholar]
- Eason K, & Sadanandam A (2016). Molecular or metabolic reprograming: What triggers tumor subtypes? Cancer Research, 76, 5195–5200. [DOI] [PubMed] [Google Scholar]
- Faber M, Coudray C, Hida H, Mousseau M, & Favier A (1995). Lipid peroxidation products, and vitamin and trace element status in patients with cancer before and after chemotherapy, including adriamycin. A preliminary study. Biological Trace Element Research, 47, 117–123. [DOI] [PubMed] [Google Scholar]
- Frezza C (2017). Mitochondrial metabolites: Undercover signalling molecules. Interface Focus, 7, 20160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I (1997). Superoxide anion radical (O2-), superoxide dismutases, and related matters. Journal of Biological Chemistry, 272, 18515–18517. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England Journal of Medicine, 366, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles GI (2006). The redox regulation of thiol dependent signaling pathways in cancer. Current Pharmaceutical Design, 12, 4427–4443. [DOI] [PubMed] [Google Scholar]
- Gincel D, Silberberg SD, & Shoshan-Barmatz V (2000). Modulation of the voltage-dependent anion channel (VDAC) by glutamate. Journal of Bioenergetics and Biomembranes, 32, 571–583. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. (2010). Diabetes and cancer: A consensus report. CA: A Cancer Journal for Clinicians, 60, 207–221. [DOI] [PubMed] [Google Scholar]
- Green DR, & Kroemer G (2004). The pathophysiology of mitochondrial cell death. Science, 305, 626–629. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR, & Gillespie GY (2005). Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. The Journal of Neuro-Oncology, 74, 123–133. [DOI] [PubMed] [Google Scholar]
- Guppy M, Leedman P, Zu X, & Russell V (2002). Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochemistry Journal, 364, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn AT, Jones JT, & Meyer T (2009). Quantitative analysis of cell cycle phase durations and PC12 differentiation using fluorescent biosensors. Cell Cycle, 8, 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, & Cadenas E (2003). Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. Journal of Biological Chemistry, 278, 5557–5563. [DOI] [PubMed] [Google Scholar]
- Hiller S, Abramson J, Mannella C, Wagner G, & Zeth K (2010). The 3D structures of VDAC represent a native conformation. Trends in Biochemical Sciences, 35, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmuhamedov E, & Lemasters JJ (2009). Ethanol exposure decreases mitochondrial outer membrane permeability in cultured rat hepatocytes. Archives of Biochemistry and Biophysics, 481, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shah K, Bradbury NA, Li C, & White C (2014). Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death & Disease, 5, e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo V, Bravo-San Pedro JM, Sica V, Kroemer G, & Galluzzi L (2016). Mitochondrial permeability transition: New findings and persisting uncertainties. Trends in Cell Biology, 26, 655–667. [DOI] [PubMed] [Google Scholar]
- Jara JA, & Lopez-Munoz R (2015). Metformin and cancer: Between the bioenergetic disturbances and the antifolate activity. Pharmacological Research, 101, 102–108. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, & Karin M (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell, 120, 649–661. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Hiraku Y, Pinlaor S, & Ma N (2006). Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biological Chemistry, 387, 365–372. [DOI] [PubMed] [Google Scholar]
- Keenan MM, & Chi JT (2015). Alternative fuels for cancer cells. Cancer Journal, 21, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keibler MA, Wasylenko TM, Kelleher JK, Iliopoulos O, Vander Heiden MG, & Stephanopoulos G (2016). Metabolic requirements for cancer cell proliferation. Cancer Metabolism, 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Scarbrough PM, Ribeiro A, Richardson R, Yuan H, Sonveaux P, et al. (2013). Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS One, 8, e75154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn DG, Lilly MD, & Webb FC (1969). The energetics of mammalian cell growth. Journal of Cell Science, 4, 645–654. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, & Vercesi AE (2001). Mitochondrial permeability transition and oxidative stress. FEBS Letters, 495, 12–15, %20. [DOI] [PubMed] [Google Scholar]
- Kreis W, Baker A, Ryan V, & Bertasso A (1980). Effect of nutritional and enzymatic methionine deprivation upon human normal and malignant cells in tissue culture. Cancer Research, 40, 634–641. [PubMed] [Google Scholar]
- Ladner C, Ehninger G, Gey KF, & Clemens MR (1989). Effect of etoposide (VP16–213) on lipid peroxidation and antioxidant status in a high-dose radiochemotherapy regimen. Cancer Chemotherapy and Pharmacology, 25, 210–212. [DOI] [PubMed] [Google Scholar]
- Lee AC, Zizi M, & Colombini M (1994). Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. Journal of Biological Chemistry, 269, 30974–30980. [PubMed] [Google Scholar]
- Lemasters JJ, & Holmuhamedov E (2006). Voltage-dependent anion channel (VDAC) as mitochondrial governator-thinking outside the box. Biochimica et Biophysica Acta, 1762, 181–190. [DOI] [PubMed] [Google Scholar]
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, & Evans JM (2009). New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care, 32, 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti MV, & Locasale JW (2016). The warburg effect: How does it benefit cancer cells? Trends in Biochemical Sciences, 41, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg-Apers DC, Schirris TJ, Russel FG, Willems PH, & Koopman WJ (2015). Mitoenergetic dysfunction triggers a rapid compensatory increase in steady-state glucose flux. Biophysical Journal, 109, 1372–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Ho QS, Low J, Choolani M, & Wong KP (2011). Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion, 11, 437–443. [DOI] [PubMed] [Google Scholar]
- Liou GY, & Storz P (2010). Reactive oxygen species in cancer. Free Radical Research, 44, 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, & Cantley LC (2010). Altered metabolism in cancer. BMC Biology, 8, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, & Vander Heiden MG (2011). Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annual Review of Cell and Developmental Biology, 27, 441–464. [DOI] [PubMed] [Google Scholar]
- Maldonado EN (2017). VDAC-tubulin, an anti-warburg pro-oxidant switch. Frontiers in Oncology, 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, DeHart DN, Patnaik J, Klatt SC, Beck GM, & Lemasters JJ (2016). ATP/ADP turnover and import of glycolytic ATP into mitochondria in cancer cells is independent of the adenine nucleotide translocator. Journal of Biological Chemistry, 291, 19642–19650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, & Lemasters JJ (2012). Warburg revisited: Regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. The Journal of Pharmacology and Experimental Therapeutics, 342, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, & Lemasters JJ (2014). ATP/ADP ratio, the missed connection between mitochondria and the warburg effect. Mitochondrion, 19, 78–84, Pt. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Patnaik J, Mullins MR, & Lemasters JJ (2010). Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Research, 70, 10192–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, et al. (2013). Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: Regulation by free tubulin and erastin. Journal of Biological Chemistry, 288, 11920–11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo B, Nitti M, Furfaro AL, Colla R, Ciucis CD, Marinari UM, et al. (2016). Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxidative Medicine and Cellular Longevity, 2016, 6235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, et al. (2014). Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell, 159, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, & Pedersen PL (2010). The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochimica et Biophysica Acta, 1797, 1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, & Vazquez A (2016). Give it or take it: The flux of one-carbon in cancer cells. The FEBS Journal, 283, 3695–3704. [DOI] [PubMed] [Google Scholar]
- Mor I, Cheung EC, & Vousden KH (2011). Control of glycolysis through regulation of PFK1: Old friends and recent additions. Cold Spring Harbor Symposia on Quantitative Biology, 76, 211–216. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Marin-Hernandez A, Saavedra E, Pardo JP, Ralph SJ, & Rodriguez-Enriquez S (2014). Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. The International Journal of Biochemistry & Cell Biology, 50, 10–23. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, & Saavedra E (2007). Energy metabolism in tumor cells. The FEBS Journal, 274, 1393–1418. [DOI] [PubMed] [Google Scholar]
- Morgan B, Sobotta MC, & Dick TP (2011). Measuring E(GSH) and H2O2 with roGFP2-based redox probes. Free Radical Biology & Medicine, 51, 1943–1951. [DOI] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, et al. (2012). Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature, 481, 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Liu Y, & Van RH (2004). Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry, 279, 49064–49073. [DOI] [PubMed] [Google Scholar]
- Nakashima RA, Paggi MG, & Pedersen PL (1984). Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Research, 44, 5702–5706. [PubMed] [Google Scholar]
- Nakashima RA, Paggi MG, Scott LJ, & Pedersen PL (1988). Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Research, 48, 913–919. [PubMed] [Google Scholar]
- Nicholls DG, & Ferguson SJ (2013). Bioenergetics 4 London: Elsevier. [Google Scholar]
- Palmieri F, & Pierri CL (2010). Mitochondrial metabolite transport. Essays in Biochemistry, 47, 37–52. [DOI] [PubMed] [Google Scholar]
- Panieri E, & Santoro MM (2016). ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death & Disease, 7, e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, & Hoek JB (2003). Hexokinase II: The integration of energy metabolism and control of apoptosis. Current Medicinal Chemistry, 10, 1535–1551. [DOI] [PubMed] [Google Scholar]
- Pedersen PL (1978). Tumor mitochondria and the bioenergetics of cancer cells. Progress in Experimental Tumor Research, 22, 190–274. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, & Huang P (2006). Glycolysis inhibition for anti-cancer treatment. Oncogene, 25, 4633–4646. [DOI] [PubMed] [Google Scholar]
- Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, & Brand MD (2012). Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. Journal of Biological Chemistry, 287, 27255–27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PR (2003). The molecular machinery of Keilin’s respiratory chain. Biochemical Society Transactions, 31, 1095–1105. [DOI] [PubMed] [Google Scholar]
- Rich PR, & Marechal A (2010). The mitochondrial respiratory chain. Essays in Biochemistry, 47, 1–23. [DOI] [PubMed] [Google Scholar]
- Robinson GL, Dinsdale D, MacFarlane M, & Cain K (2012). Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene, 31, 4996–5006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Enriquez S, Carreno-Fuentes L, Gallardo-Perez JC, Saavedra E, Quezada H, Vega A, et al. (2010). Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. The International Journal of Biochemistry & Cell Biology, 42, 1744–1751. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, & Bezrukov SM (2004). Bid, but not bax, regulates VDAC channels. The Journal of Biological Chemistry, 279, 13575–13583. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, et al. (2008). Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proceedings of the National Academy of Sciences of the United States of America, 105, 18746–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, et al. (2001). Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. Journal of Biological Chemistry, 276, 39206–39212. [DOI] [PubMed] [Google Scholar]
- Sampson MJ, Lovell RS, & Craigen WJ (1997). The murine voltage-dependent anion channel gene family. Conserved structure and function. Journal of Biological Chemistry, 272, 18966–18973. [DOI] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. (2010). Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. The Journal of Clinical Investigation, 120, 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein SJ, Colombini M, & Finkelstein A (1976). Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. The Journal of Membrane Biology, 30, 99–120. [DOI] [PubMed] [Google Scholar]
- Schenkel LC, & Bakovic M (2014). Formation and regulation of mitochondrial membranes. International Journal of Cell Biology, 2014, 709828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, et al. (2014). High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. Journal of Biological Chemistry, 289, 12566–12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenke WD, Soboll S, Seitz HJ, & Sies H (1981). Mitochondrial and cytosolic ATP/ADP ratios in rat liver in vivo. Biochemistry Journal, 200, 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Lamb J, Smith S, & Wheatley DN (2000). Single amino acid (arginine) deprivation: Rapid and selective death of cultured transformed and malignant cells. British Journal of Cancer, 83, 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, et al. (2011). Comparative metabolic flux profiling of melanoma cell lines: Beyond the Warburg effect. Journal of Biological Chemistry, 286, 42626–42634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen JH, Zoncu R, Kim D, & Sabatini DM (2011). Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell, 19, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, & Bezrukov SM (2011). Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One, 6, e25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleterry J, Sreedhar A, & Zhao Y (2014). Components of cancer metabolism and therapeutic interventions. Mitochondrion, 17C, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, et al. (2011). Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell, 20, 674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP (1996). Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Quarterly Reviews of Biophysics, 29, 169–202. [DOI] [PubMed] [Google Scholar]
- Smolkova K, Bellance N, Scandurra F, Genot E, Gnaiger E, Plecita-Hlavata L, et al. (2010). Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. Journal of Bioenergetics and Biomembranes, 42, 55–67. [DOI] [PubMed] [Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, & Pae HO (2011). Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? Journal of Signal Transduction, 2011, 792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, & Colombini M (1996). Indications of a common folding pattern for VDAC channels from all sources. Journal of Bioenergetics and Biomembranes, 28, 153–161. [DOI] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. (2008). Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. The Journal of Clinical Investigation, 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, & Chandel NS (2014). Mitochondrial reactive oxygen species and cancer. Cancer Metabolism, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, & Michelakis ED (2013). Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Frontiers in Oncology, 3, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski TP, & Nathan CF (1991). Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Research, 51, 794–798. [PubMed] [Google Scholar]
- Takeyama N, Matsuo N, & Tanaka T (1993). Oxidative damage to mitochondria is mediated by the Ca(2+)-dependent inner-membrane permeability transition. Biochemistry Journal, 294(Pt. 3), 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timohhina N, Guzun R, Tepp K, Monge C, Varikmaa M, Vija H, et al. (2009). Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: Some evidence for mitochondrial interactosome. Journal of Bioenergetics and Biomembranes, 41, 259–275. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J, & Hiai H (1995). Persistent oxidative stress in cancer. FEBS Letters, 358, 1–3. [DOI] [PubMed] [Google Scholar]
- Tribble DL, Jones DP, & Edmondson DE (1988). Effect of hypoxia on tertbutylhydroperoxide-induced oxidative injury in hepatocytes. Molecular Pharmacology, 34, 413–420. [PubMed] [Google Scholar]
- Tsujimoto Y, & Shimizu S (2000). VDAC regulation by the bcl-2 family of proteins. Cell Death and Differentiation, 7, 1174–1181. [DOI] [PubMed] [Google Scholar]
- Uchida K (2003). 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Progress in Lipid Research, 42, 318–343. [DOI] [PubMed] [Google Scholar]
- Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, et al. (2008). The crystal structure of mouse VDAC1 at 2.3Å resolution reveals mechanistic insights into metabolite gating. Proceedings of the National Academy of Sciences of the United States of America, 105, 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, & Nakamura Y (2008). Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Letters, 266, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, & Thompson CB (2000). Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proceedings of the National Academy of Sciences of the United States of America, 97, 4666–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, & Colombini M (2001). Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. The Journal of Biological Chemistry, 276, 19414–19419. [DOI] [PubMed] [Google Scholar]
- Veal EA, Day AM, & Morgan BA (2007). Hydrogen peroxide sensing and signaling. Molecular Cell, 26, 1–14. [DOI] [PubMed] [Google Scholar]
- Venditti P, Di SL, & Di MS (2013). Mitochondrial metabolism of reactive oxygen species. Mitochondrion, 13, 71–82. [DOI] [PubMed] [Google Scholar]
- Walker JE (2013). The ATP synthase: The understood, the uncertain and the unknown. Biochemical Society Transactions, 41, 1–16. [DOI] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. (2010). Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell, 18, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1956). On the origin of cancer cells. Science, 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, & Negelein E (1927). The metabolism of tumors in the body. The Journal of General Physiology, 8, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijl NI, Hopman GD, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, et al. (1998). Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Annals of Oncology, 9, 1331–1337. [DOI] [PubMed] [Google Scholar]
- Weinberg SE, & Chandel NS (2015). Targeting mitochondria metabolism for cancer therapy. Nature Chemical Biology, 11, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S (1956). On respiratory impairment in cancer cells. Science, 124, 267–269. [DOI] [PubMed] [Google Scholar]
- Wikstrom M, Sharma V, Kaila VR, Hosler JP, & Hummer G (2015). New perspectives on proton pumping in cellular respiration. Chemical Reviews, 115, 2196–2221. [DOI] [PubMed] [Google Scholar]
- Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, et al. (2011). Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. The Journal of Experimental Medicine, 208, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda N, Von RM, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. (2007). RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature, 447, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Johnson JL, Hanigan CL, & Locasale JW (2012). Interactions between epigenetics and metabolism in cancers. Frontiers in Oncology, 2, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fryknas M, Hernlund E, Fayad W, De MA, Olofsson MH, et al. (2014). Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nature Communications, 5, 3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, & Davies KJ (1990). The oxidative inactivation of mitochondrial electron transport chain components and ATPase. Journal of Biological Chemistry, 265, 16330–16336. [PubMed] [Google Scholar]
- Zhu A, Lee D, & Shim H (2011). Metabolic positron emission tomography imaging in cancer detection and therapy response. Seminars in Oncology, 38, 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizi M, Forte M, Blachly-Dyson E, & Colombini M (1994). NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. The Journal of Biological Chemistry, 269, 1614–1616. [PubMed] [Google Scholar]
- Zoratti M, & Szabo I (1995). The mitochondrial permeability transition. Biochimica et Biophysica Acta, 1241, 139–176. [DOI] [PubMed] [Google Scholar]