Abstract
Background
Laryngeal early glottic tumors can benefit from different treatment modalities, including transoral laser microsurgery, open partial horizontal laryngectomy (OPHL), and radiotherapy. However, the treatment of early glottic tumors with the involvement of the anterior commissure remains controversial. The studies about the role of anterior commissure involvement in oncologic outcomes in patients with early glottic cancer treated with supracricoid laryngectomy (SCL) are very few. For this reason, we conducted a retrospective study to evaluate local recurrence-free survival and specific survival in patients with and without involvement of the anterior commissure who underwent SCL with cricohyoidoepiglottopexy.
Methods
This retrospective study has been carried out on patients with T1b–T2 glottic squamous cell carcinoma submitted to SCL with cricohyoidoepiglottopexy. The patients’ demographic and clinical data were collected, and the histological findings of the surgical specimens were reviewed to identify patients who had involvement of the anterior commissure.
Results
A total of 72 patients were included in the study; two of them were female and 70 were male. The mean age at diagnosis was 61.5±8.0 SD years. In 26 of the 72 (36.2%) patients, anterior commissure was not pathologically involved (group A), while in 46 (63.8%) patients, it was involved (group B). The 5-year local recurrence-free survival rate was 96.1% and 93.48% in groups A and B, respectively, P=0.09. The 5-year disease-specific survival rate was 92.31% and 95.65% in groups A and B, respectively, P=0.057.
Conclusion
SCL with cricohyoidoepiglottopexy seems to be an adequate treatment modality, even for T1b–T2 glottic tumors with anterior commissure involvement.
Keywords: open partial horizontal laryngectomy, laryngeal cancer, supracricoid laryngectomy, cricohyoidoepiglottopexy
Background
Laryngeal early glottic tumors can benefit from different treatment modalities, including transoral laser microsurgery (TLM), open partial horizontal laryngectomy (OPHL) and radiotherapy, with good oncological and functional outcomes.
However, the treatment of T1–T2 glottic tumors with the involvement of the anterior commissure remains controversial.
Some authors argue that the anterior commissure represents a point of decreased resistance to neoplastic diffusion.1,2 This is because Broyles’ ligament penetrates the thyroid cartilage and unlocks the way for tumor invasion. This depends on the lack of perichondrium or periosteum in the area of ligament insertion.3
Other authors believe that the anterior commissure forms a barrier to the tumor extension for the cancer localized in the chordal–commissural region because of the composition of Broyles’ ligament. It is made up of fibrotic connective tissue without vessels and glands, unlike the thyroepiglottic ligament.4,5
The involvement of the anterior commissure, according to some authors, negatively affects free local recurrence survival in patients with early glottic cancer who are treated with CO2 laser or radiotherapy.6–8
Supracricoid laryngectomy (SCL) was introduced by Majer and Rieder9 for the first time, as an organ preservation surgery procedure that removes a portion of larynx while maintaining the physiological functions of speech, swallowing, and respiration, without compromising local control and cure rates. Recently, Succo et al10 have proposed a new classification of open surgery based on the skull-caudal extent of laryngeal structures resected; according to this proposed classification system, SCL is defined as OPHL Type II.
The preservation of at least one cricoarytenoid unit represents the most important functional condition for global functional recovery of the neolarynx, despite the removal of the entire thyroid shield.
Over time, the indications11,12 and surgical techniques have evolved,13–16 which has improved oncological and functional results.17–23
However, there are few studies about the role of anterior commissure involvement in oncologic outcome in patients with early glottic cancer treated with SCL.
For this reason, we conducted a retrospective study to evaluate local recurrence-free survival (LRFS) and disease-specific survival (DSS) in patients with and without involvement of the anterior commissure who underwent supracricoid laryngectomy with cricohyoidoepiglottopexy (SCL-CHEP).
Methods
Patients
This retrospective study was carried out on patients with T1b–T2 glottic squamous cell carcinoma treated with SCL-CHEP between 2004 and 2012 at the Otolaryngology Unit of the Cannizzaro Hospital, Catania, and Otolaryngology – Department of Health Sciences, University of Catanzaro. The study was approved by Institutional Review Boards of University of Catanzaro and Cannizzaro Hospital, and the requirement to obtain written informed consent from patients was waived due to the retrospective nature of the investigation. At the aim to protect patient privacy, patient’s personal information was appropriately anonymized and de-identified prior to analysis. All patients included in the study had to be eligible for SCL-CHEP and with a minimum of 60 months of follow-up time. The patients with cT1a stage cancers were excluded from this study because we preferred to treat them by TLM. All patients were informed of the benefits, risks, possible complications, and alternatives to surgery before giving informed consent. Patients preoperatively underwent videolaryngoscopy with a flexible endoscope and videolaryngostroboscopy. Multiple biopsies were taken by direct microlaryngoscopy under general anesthesia to prove extension of the lesion. The neck was examined by palpation, and ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI)24 were included for staging. The stage was determined in accordance with the seventh edition of the TNM classification established by the American Joint Committee on Cancer. All patients were followed up 1 month after surgery, every 3 months for 3 years, and every 6 months thereafter. Follow-up visits included clinical examination, laryngoscopy, and radiological examinations, including neck ultrasound, chest X-ray every 6 months, and CT or MRI every 1 year or according to clinical evidence. The patients’ demographic and clinical data were collected, and the histological findings of the surgical specimens were reviewed to identify patients who had involvement of the anterior commissure.
Data analysis
The 5-year overall survival time, 5-year local recurrence rate, and 5-year DSS rate were assessed. Larynx preservation index was defined as the percentage of patients maintaining both the larynx and laryngeal function. Statistical analysis was performed using MedCalc software (version 9.0; MedCalc Software bvba, Ghent, Belgium). Data collected included the mean, median, and SD. Pearson’s chi-squared and Fisher’s exact tests were used to identify differences in demographic and clinicopathological data between cohorts. The Kaplan–Meier method was used to study survival, and the log-rank test was used to compare survival curves between groups. A P-value less than 0.005 was considered as statistically significant.
Results
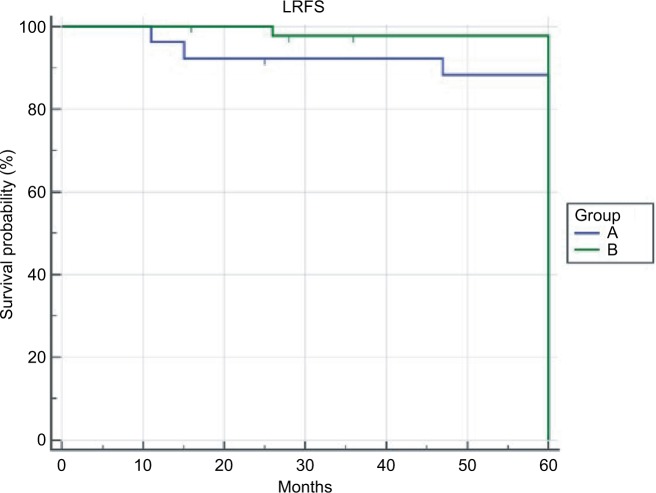
A total of 72 patients were included in the study; two of them were female, and 70 were male. In all, 16 of the 72 patients had previously been treated with TLM and 11 with radiotherapy. The mean age at diagnosis was 61.5±8.0 SD years. Mean follow-up time was 93.3±30.4 SD months. According to cTNM classification, 30 lesions (41.6%) were staged as T1bN0, 40 (55.5%) as T2N0, and two (2.9%) as T2N1. For each patient, additional multiple biopsies were taken from surgical margins and sent for frozen section. When margins were close or positive, the resection was enlarged, and in some cases, the arytenoid was removed. Postoperative pathological staging was as follows: two were pT1a, 25 were pT1b, 40 were pT2, four were pT3, and one was pT4. In eight of the 72 (11.1%) cases, the cT classification was underestimated. In 13 cases (18.8%), one arytenoid was included in the resection. Definitive surgical margins were negative in all cases. In all, 13 of the 72 (18%) patients were submitted to selective neck dissection, two to T2N1, and 11 to T2N0M0 previously treated tumors. None of the patients who underwent neck dissection presented with metastatic lymph nodes. During the follow-up period, four of the 72 (5.55%) patients presented with local recurrence after a mean time of 28.5 months (range: 16–37 months). One of them had pT1b tumor, two had pT3 tumor, and one had pT4 tumor. All of them were treated by salvage total laryngectomy followed by radiation therapy. One of these patients developed a neck metastasis treated by neck dissection, and one developed a local recurrence treated by chemotherapy. Three of the 72 (4.16%) patients developed a pulmonary metastasis after a mean time of 29 months (range 26–34 months) from surgery. The 5-year overall survival rate was 91.67% (six out of 72 patients died). Three patients died because of distant metastasis, two because of non-cancer related diseases (intestinal infarction, liver failure), and one because of local recurrence. The 5-years disease specific survival and specific survival rates were 94.44%. In 26 of the 72 (36.2%) patients, anterior commissure was not pathologically involved (group A), while in 46 (63.8%) patients, it was involved. Comparative analysis of the groups A and B revealed non-statistically significant differences in the patients’ distribution according to age, sex, follow-up time, cT and cN classification, neck dissection, and number of arytenoid cartilages preserved (Table 1). In two (7.6%) cases of group A and in six (13.3%) cases of group B, the clinical classification of T was originally underestimated. The local recurrence rates were 3.5% and 6.5% in groups A and B, respectively, P=1.00. Distant metastasis occurred in 7.6% and 2.2% cases in groups A and B, respectively, while the larynx preservation indexes were 92.4% and 95.6% in groups A and B, respectively (Table 2). The 5-year LRFS rates were 96.1% and 93.48% in groups A and B, respectively, P=0.09 (Figure 1). The 5-year DSS rates were 92.31% and 95.65% in groups A and B, respectively, P=0.057 (Figure 2).
Table 1.
Clinicopathological data of groups A and B
| Data | Group A, n=26 | Group B, n=46 | P-value |
|---|---|---|---|
|
| |||
| Age (years), median, range | 62, 43–72 | 61, 42–75 | 0.75 |
| Sex, n (%) | |||
| Male | 25 (96.1) | 45 (97.8) | 1.00 |
| Female | 1 (3.9) | 1 (2.2) | |
| Follow-up (months), mean±SD | 89.0±34 | 95.7±28.3 | 0.37 |
| cT stage, n (%) | |||
| T1b | 7 (26.9) | 23 (50) | 0.053 |
| T2 | 19 (73.1) | 23 (50) | |
| cN stage, n (%) | |||
| N0 | 25 (96.1) | 45 (97.8) | 1.00 |
| N+ | 1 (3.9) | 1 (2.2) | |
| Neck dissection, n (%) | |||
| No | 21 (80.7) | 38 (82.6) | 0.84 |
| Yes | 5 (19.3) | 8 (17.4) | |
| Arytenoid preserved, n (%) | |||
| Two | 22 (84.6) | 37 (80.4) | 0.075 |
| One | 4 (15.4) | 9 (19.6) | |
Table 2.
Comparison of the oncological results in groups A and B
| Oncological results | Group A, n=26 | Group B, n=46 | P-value |
|---|---|---|---|
|
| |||
| n (%) | n (%) | ||
| Local recurrence | |||
| No | 1 (3.5) | 3 (6.5) | 1.00 |
| Yes | 25 (96.5) | 43 (93.5) | |
| Distant metastasis | |||
| No | 2 (7.6) | 1 (2.2) | 0.29 |
| Yes | 24 (92.4) | 45 (97.8) | |
| Larynx preservation index | |||
| No | 24 (92.4) | 44 (95.6) | 0.61 |
| Yes | 2 (7.6) | 2 (4.4) | |
Figure 1.
The 5-year LRFS.
Abbreviation: LRFS, local recurrence-free survival.
Figure 2.
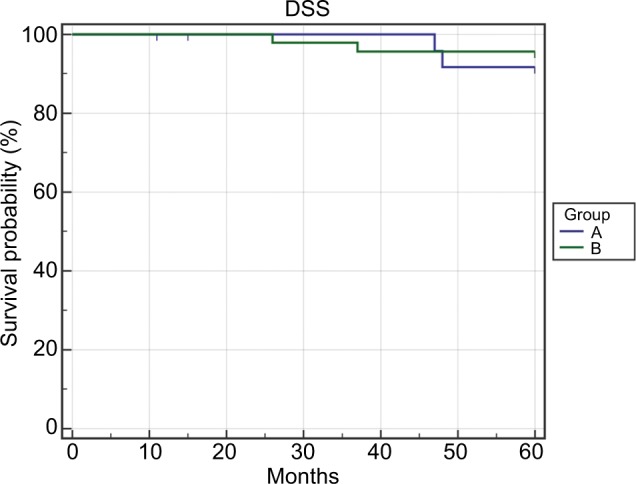
The 5-year DSS.
Abbreviation: DSS, disease-specific survival.
Discussion
Early T1–T2 glottic carcinoma can be treated with radiotherapy or surgery (transoral laser surgery, organ preservation surgery) with good results in terms of 5-year DSS ranging from 87.1% to 91.2%.25
However, the treatment modality of early glottic tumors with involvement of the anterior commissure remains controversial. The data currently available on the role of the involvement of the commissure mainly come from studies on patients treated with TLM or radiotherapy. Most of these studies involve predominantly T1a–T1b patients. According to a recent study by Alkan et al,26 out of 56 patients with T1a–T1b involving anterior commissure, local control was 75% and 87% for those who were treated with TLM and radiotherapy, respectively. However, in patients undergoing radiation therapy, the inability to have a posttreatment pT classification represents a limitation in the comparison of oncological results.
Regarding the surgical treatment, numerous studies have shown that the involvement of the anterior commissure impacts disease-free survival (DFS) and DDS in patients treated with TLM.3,27,28 In Chone et al,3 a study conducted on 62 patients showed a local recurrence rate of 21% and 4% in patients with lesions involving and not involving the anterior commissure, respectively. However, according to other authors,29,30 the involvement of the anterior commissure does not represent a greater risk of local recurrence but of the appearance of more severe recurrences with a lower organ function preservation rate.
Studies concerning the oncological results of SCL in relation to the involvement of the anterior commissure are very few. This is probably because a preference for SCL as a way of treating early glottic tumors is not shared by all; some prefer TLM as a surgical treatment modality. This is because hospitalization and functional recovery times are longer in patients undergoing SCL than in those treated with TLM. However, in the last few decades, there has been a reduction in hospitalization time, a reduction in complications, and a rapid recovery in function in patients undergoing SCL. This is due to an increased prevalence of and experience in the techniques of OPHL and due to the introduction of new modified techniques.15,16 It is therefore important, in the search for the optimal treatment of early glottic carcinoma, to establish whether there is a difference in rates of DFS and DSS between patients with and without involvement of the anterior commissure treated with SCL-CHEP. Laccourreye et al31 in a study conducted on 62 patients, all with glottic carcinoma with involvement of the anterior commissure and treated with SCL-CHEP, reported a 5-year local control rate of 98.2%. Recently, Atallah et al32 in a similar study conducted on 53 patients who underwent SCL-CHEP according to different surgical techniques found a 5-year DFS and DSS of 87% and 95.6%, respectively.
Our study is very similar to that of Atallah et al.32 However, for the first time, as far as we know, we have compared the oncological results of SCL-CHEP in relation to the involvement or not of the anterior commissure. We found that in 11.1% of the cases, the tumors were underestimated, and 75% of these cases showed an extension of the tumor into the anterior commissure. This is in accordance with the findings of Prades et al33 Moreover, Ulusan et al34 demonstrated that glottic tumors with vertical and anterior commissure involvement are more at risk for invasion of the thyroid cartilage.
The oncological results we found, in terms of 5-year LRFS and DFS, were 96.1% and 92.31% in patients without commissure involvement and 93.48% and 95.65% in patients with involvement, respectively. The study thus demonstrated the validity of SCL-CHEP in terms of oncological results in the treatment of tumors with extension into the anterior commissure. However, the study presents limitations represented by the number of patients taken into consideration and by the retrospectivity of the data analysis.
Conclusion
Early glottic tumors with an extension into the anterior commissure represent a clinical subtype of laryngeal tumors at greater risk for local recurrence. The SCL-CHEP seems to be an adequate treatment modality, especially for T1b–T2 glottic tumors with anterior commissure invasion. However, correct clinical and instrumental staging (CT and MRI) and a preoperative multidisciplinary evaluation are necessary to be able to plan and propose the best treatment modality appropriate to the individual case.
Footnotes
Author contributions
EA is the corresponding author and was involved in study design, data acquisition analysis and interpretation, and drafting of the manuscript. VS was involved in data acquisition, interpretation of data, and drafting of the manuscript. AA and DMM were involved in data acquisition and interpretation. MDN and MRB were involved in data acquisition and statistical analysis. AG was the supervisor and assisted with preparation and revision of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rucci L, Gammarota L, Gallo O. Carcinoma of the anterior commissure of the larynx: II. Proposal of a new staging system. Ann Otol Rhinol Laryngol. 1996;105(5):391–396. doi: 10.1177/000348949610500512. [DOI] [PubMed] [Google Scholar]
- 2.Shvero J, Hadar T, Segal K, Levy R, Feinmesser R. Early glottic carcinoma involving the anterior commissure. Clin Otolaryngol Allied Sci. 1994;19(2):105–108. doi: 10.1111/j.1365-2273.1994.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 3.Chone CT, Yonehara E, Martins JE, Altemani A, Crespo AN. Importance of anterior commissure in recurrence of early glottic cancer after laser endoscopic resection. Arch Otolaryngol Head Neck Surg. 2007;133(9):882–887. doi: 10.1001/archotol.133.9.882. [DOI] [PubMed] [Google Scholar]
- 4.Bagatella F, Bignardi L. Morphological study of the laryngeal anterior commissure with regard to the spread of cancer. Acta Otolaryngol. 1981;92(1–2):167–171. doi: 10.3109/00016488109133252. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Zhao J, Wang Z, et al. Study of the Histopathologic Characteristics and Surface Morphologies of Glottic Carcinomas With Anterior Vocal Commissure Involvement. Medicine. 2015;94(29):e1169. doi: 10.1097/MD.0000000000001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitani Y, Kubota A, Furukawa M, Sato K. Prognostic factors for local control in patients receiving radiation therapy for early glottic cancer: anterior commissure involvement and effect of chemoradiotherapy. Eur Arch Otorhinolaryngol. 2016;273(4):1011–1017. doi: 10.1007/s00405-015-3579-8. [DOI] [PubMed] [Google Scholar]
- 7.Peretti G, Nicolai P, Redaelli de Zinis LO, et al. Endoscopic CO2 laser excision for tis, T1, and T2 glottic carcinomas: cure rate and prognostic factors. Otolaryngol Head Neck Surg. 2000;123(1 Pt 1):124–131. doi: 10.1067/mhn.2000.104523. [DOI] [PubMed] [Google Scholar]
- 8.Galli A, Giordano L, Sarandria D, di Santo D, Bussi M. Oncological and complication assessment of CO2 laser-assisted endoscopic surgery for T1-T2 glottic tumours: clinical experience. Acta Otorhinolaryngol Ital. 2016;36(3):167–173. doi: 10.14639/0392-100X-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majer EH, Rieder W. Technic of laryngectomy permitting the conservation of respiratory permeability (cricohyoidopexy) Ann Otolaryngol. 1959;76:677–681. [PubMed] [Google Scholar]
- 10.Succo G, Peretti G, Piazza C, et al. Open partial horizontal laryngectomies: a proposal for classification by the working committee on nomenclature of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2014;271(9):2489–2496. doi: 10.1007/s00405-014-3024-4. [DOI] [PubMed] [Google Scholar]
- 11.Pellini R, Pichi B, Ruscito P, et al. Supracricoid partial laryngectomies after radiation failure: a multi-institutional series. Head Neck. 2008;30(3):372–379. doi: 10.1002/hed.20709. [DOI] [PubMed] [Google Scholar]
- 12.Mercante G, Grammatica A, Battaglia P, Cristalli G, Pellini R, Spriano G. Supracricoid partial laryngectomy in the management of t3 laryngeal cancer. Otolaryngol Head Neck Surg. 2013;149(5):714–720. doi: 10.1177/0194599813500018. [DOI] [PubMed] [Google Scholar]
- 13.Piquet JJ, Desaulty A, Decroix G. Crico-hyoido-epiglotto-pexy. Surgical technic and functional results. Ann Otolaryngol Chir Cervicofac. 1974;91(12):681–686. [PubMed] [Google Scholar]
- 14.Labayle J, Bismuth R. Total laryngectomy with reconstitution. Ann Otolaryngol Chir Cervicofac. 1971;88(4):219–228. [PubMed] [Google Scholar]
- 15.Guerrier B, Lallemant JG, Balmigère G, Bonnet P, Arnoux B. Our experience in reconstructive surgery in glottic cancers. Ann Otolaryngol Chir Cervicofac. 1987;104(3):175–179. [PubMed] [Google Scholar]
- 16.Garozzo A, Allegra E, La Boria A, Lombardo N. Modified supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2010;142(1):137–139. doi: 10.1016/j.otohns.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 17.de Vincentiis M, Minni A, Gallo A, di Nardo A. Supracricoid partial laryngectomies: oncologic and functional results. Head Neck. 1998;20(6):504–509. doi: 10.1002/(sici)1097-0347(199809)20:6<504::aid-hed3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Allegra E, Lombardo N, La Boria A, et al. Quality of voice evaluation in patients treated by supracricoid laryngectomy and modified supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2011;145(5):789–795. doi: 10.1177/0194599811416438. [DOI] [PubMed] [Google Scholar]
- 19.Allegra E, Franco T, Trapasso S, Domanico R, La Boria A, Garozzo A. Modified supracricoid laryngectomy: oncological and functional outcomes in the elderly. Clin Interv Aging. 2012;7:475–480. doi: 10.2147/CIA.S38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito K, Araki K, Ogawa K, Shiotani A. Laryngeal function after supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2009;140(4):487–492. doi: 10.1016/j.otohns.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Schindler A, Favero E, Nudo S, Albera R, Schindler O, Cavalot AL. Long-term voice and swallowing modifications after supracricoid laryngectomy: objective, subjective, and self-assessment data. Am J Otolaryngol. 2006;27(6):378–383. doi: 10.1016/j.amjoto.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Schindler A, Pizzorni N, Mozzanica F, et al. Functional outcomes after supracricoid laryngectomy: what do we not know and what do we need to know? Eur Arch Otorhinolaryngol. 2016;273(11):3459–3475. doi: 10.1007/s00405-015-3822-3. [DOI] [PubMed] [Google Scholar]
- 23.Lips M, Speyer R, Zumach A, Kross KW, Kremer B. Supracricoid laryngectomy and dysphagia: A systematic literature review. Laryngoscope. 2015;125(9):2143–2156. doi: 10.1002/lary.25341. [DOI] [PubMed] [Google Scholar]
- 24.Allegra E, Ferrise P, Trapasso S, et al. Early glottic cancer: role of MRI in the preoperative staging. Biomed Res Int. 2014;2014:890385. doi: 10.1155/2014/890385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady JS, Marchiano E, Kam D, Baredes S, Eloy JA, Park RC. Survival Impact of Initial Therapy in Patients with T1-T2 Glottic Squamous Cell Carcinoma. Otolaryngol Head Neck Surg. 2016;155(2):257–264. doi: 10.1177/0194599816638085. [DOI] [PubMed] [Google Scholar]
- 26.Alkan U, Nachalon Y, Shkedy Y, Yaniv D, Shvero J, Popovtzer A. T1 squamous cell carcinoma of the glottis with anterior commissure involvement: Radiotherapy versus transoral laser microsurgery. Head Neck. 2017;39(6):1101–1105. doi: 10.1002/hed.24723. [DOI] [PubMed] [Google Scholar]
- 27.Galli A, Giordano L, Sarandria D, di Santo D, Bussi M. Oncological and complication assessment of CO2 laser-assisted endoscopic surgery for T1-T2 glottic tumours: clinical experience. Acta Otorhinolaryngol Ital. 2016;36(3):167–173. doi: 10.14639/0392-100X-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachse F, Stoll W, Rudack C. Evaluation of treatment results with regard to initial anterior commissure involvement in early glottic carcinoma treated by external partial surgery or transoral laser microresection. Head Neck. 2009;31(4):531–537. doi: 10.1002/hed.20997. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann C, Cornu N, Hans S, Sadoughi B, Badoual C, Brasnu D. Early glottic cancer involving the anterior commissure treated by transoral laser cordectomy. Laryngoscope. 2016;126(8):1817–1822. doi: 10.1002/lary.25757. [DOI] [PubMed] [Google Scholar]
- 30.Hakeem AH, Tubachi J, Pradhan SA. Significance of anterior commissure involvement in early glottic squamous cell carcinoma treated with trans-oral CO2 laser microsurgery. Laryngoscope. 2013;123(8):1912–1917. doi: 10.1002/lary.24012. [DOI] [PubMed] [Google Scholar]
- 31.Laccourreye O, Muscatello L, Laccourreye L, Naudo P, Brasnu D, Weinstein G. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy for “early” glottic carcinoma classified as T1-T2N0 invading the anterior commissure. Am J Otolaryngol. 1997;18(6):385–390. doi: 10.1016/s0196-0709(97)90058-2. [DOI] [PubMed] [Google Scholar]
- 32.Atallah I, Berta E, Coffre A, Villa J, Reyt E, Righini CA. Supracricoid partial laryngectomy with crico-hyoidoepiglottopexy for glottic carcinoma with anterior commissure involvement. Acta Otorhinolaryngol Ital. 2017;37(3):188–194. doi: 10.14639/0392-100X-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prades JM, Gavid M, Dumollard JM, Timoshenko AT, Karkas A, Peoc’h M. Anterior laryngeal commissure: Histopathologic data from supracricoid partial laryngectomy. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(1):27–30. doi: 10.1016/j.anorl.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Ulusan M, Unsaler S, Basaran B, Yılmazbayhan D, Aslan I. The incidence of thyroid cartilage invasion through the anterior commissure in clinically early-staged laryngeal cancer. Eur Arch Otorhinolaryngol. 2016;273(2):447–453. doi: 10.1007/s00405-015-3503-2. [DOI] [PubMed] [Google Scholar]