Summary
Hematopoietic stem and progenitor cells (HSPCs) undergo self-renewal and differentiation to guarantee a constant supply of short-lived blood cells. Both intrinsic and extrinsic factors determine HSPC fate, but the underlying mechanisms remain elusive. Here, we report that Proteinase 3 (PR3), a serine protease mainly confined to granulocytes, is also expressed in HSPCs. PR3 deficiency intrinsically suppressed cleavage and activation of caspase-3, leading to expansion of the bone marrow (BM) HSPC population due to decreased apoptosis. PR3-deficient HSPCs outcompete the long-term reconstitution potential of wild-type counterparts. Collectively, our results establish PR3 as a physiological regulator of HSPC numbers. PR3 inhibition is a potential therapeutic target to accelerate and increase the efficiency of BM reconstitution during transplantation.
Keywords: Proteinase 3, hematopoiesis, hematopoietic stem cell, hematopoietic progenitor cell, apoptosis
Highlights
-
•
Proteinase 3 (PR3) is expressed in hematopoietic stem and progenitor cells (HSPCs)
-
•
Deficiency of PR3 leads to expansion of HSPCs in murine bone marrow
-
•
PR3 regulates spontaneous HSPC apoptosis by cleaving and activating caspase-3
In this article, Luo and colleagues show that Proteinase 3 (PR3), a member of neutrophil serine proteases, is expressed in HSPCs and regulates HSPC numbers in murine bone marrow. Their results suggest that PR3 deficiency does not affect HSPC proliferation but reduces the rate of spontaneous HSPC apoptosis by cleaving and activating caspase-3.
Introduction
The hematopoietic system is responsible for replenishing short-lived blood cells while regulating the differentiation, self-renewal, and apoptosis of hematopoietic stem cells (HSCs) in the bone marrow (BM). The most primitive HSCs give rise to multilineage hematopoietic progenitor cells (HPCs) capable of producing unilineage progenitors that later differentiate into mature blood cells (Orkin and Zon, 2008). Various aspects of this well-defined hierarchy, such as the relative contribution of HSCs to the peripheral cell pool and the presence of HPCs in adult BM, have recently been challenged (Notta et al., 2016, Sun et al., 2014). Similarly, the nature and regulation of hematopoietic stem and progenitor cell (HSPC) lifespan and apoptosis are not well defined.
HSCs are mostly quiescent with a finite lifespan (Cheshier et al., 1999, Sieburg et al., 2011). Cell cycling and apoptosis in HSCs are dynamically regulated according to context (Takizawa et al., 2011). Deletion of anti-apoptotic Mcl-1 leads to HSC death (Opferman et al., 2005), while overexpression of anti-apoptotic Bcl-2 (Domen et al., 2000) or deficiency of pro-apoptotic Caspase 3 (Janzen et al., 2008) enhances HSC survival. Inhibition of caspase activity facilitates engraftment of donor HSCs and accelerates donor hematopoiesis in a mouse BM transplantation model (Imai et al., 2010). Caspase inhibition in human CD34+ cells results in higher engraftment in NOD/SCID mice, enhanced clonogenicity, and long-term culture-initiating potential in vitro (V et al., 2010). Also, microRNA miR-125a reduces apoptosis of HSPCs and expands the HSPC pool (Guo et al., 2010). However, the mechanisms that regulate apoptosis in HSPCs are not as well understood as those regulating cell cycling.
Proteinase 3 (PR3; encoded by Prtn3) was originally termed myeloblastin due to its ability to induce proliferation and inhibit differentiation of HL-60 cells, a promyelocytic leukemia cell line (Bories et al., 1989). Prtn3 is mainly expressed in granulocytes and granulocyte progenitors. PR3 is a neutrophil serine protease family member whose roles in bacterial killing and post-translational modification of cytokines have been extensively studied in neutrophils (Campanelli et al., 1990, Coeshott et al., 1999). We recently reported that PR3 regulates neutrophil spontaneous death by cleaving and activating pro-caspase-3 (Loison et al., 2014). Surprisingly, here we report that PR3 is also highly expressed in the HSPC compartment and regulates the survival as well as engraftment of HSPCs. PR3 deficiency reduced programmed cell death of HSPCs and expanded their population in the BM. The long-term reconstitution potential of PR3-deficient HSPCs was increased. Collectively, these findings suggest that PR3 limits the number of HSPCs in murine BM.
Results
Prtn3 Is Expressed in Hematopoietic Stem and Progenitor Cells
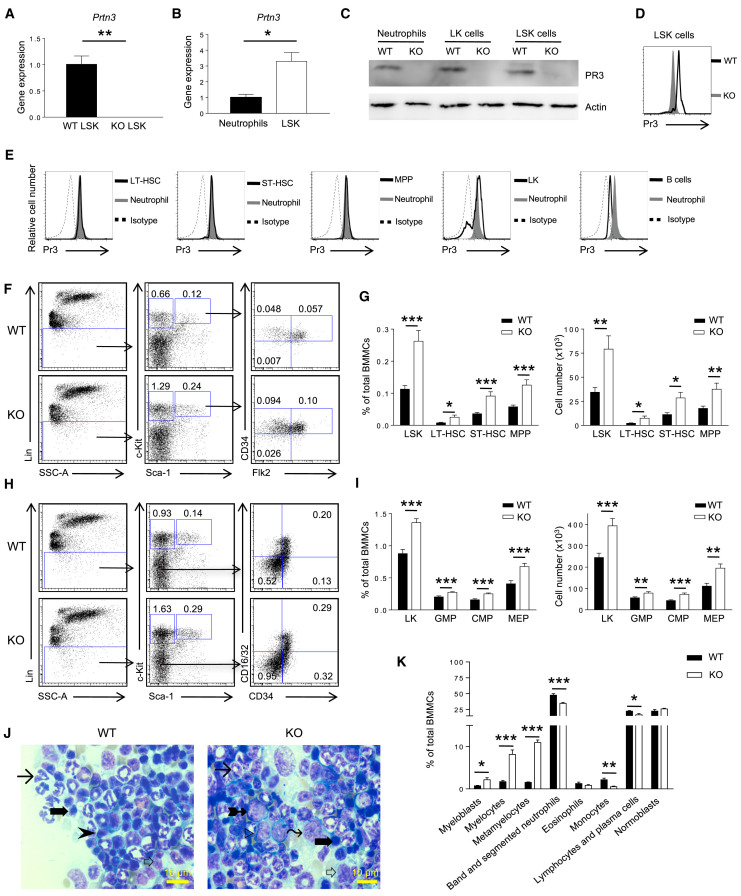
To address whether Prtn3 expression in BM is restricted to neutrophils and myeloid progenitors, we assayed highly purified LSK cells (Lin−c-Kit+Sca1+) and neutrophils (Gr1+CD11b+) from Prtn3-deficient (Prtn3−/−) and control wild-type (WT) mice. High Prtn3 transcript levels were detected in WT but not Prtn3−/− LSK cells (Figures 1A and S1A). Quantification of mRNA revealed 2-fold higher Prtn3 mRNA expression in LSK cells compared with neutrophils (Figure 1B). Examination of two publicly available transcriptome databases of hematopoietic cells revealed the highest Prtn3 expression in primitive HSCs (Figures S1B and S1C) (Chambers et al., 2007, Hyatt et al., 2006). Prtn3 was also detected at the protein level in LSK cells and lineage negative, c-Kit positive, and Sca-1 negative (LK) cells (which include myeloid progenitor cells) as assayed by western blotting and flow cytometry (Figures 1C–1E and S1D). Comparison of PR3 expression among different LSK subsets by conventional flow cytometry revealed that CD34−Flk2− long-term (LT) HSCs, CD34+Flk2− short-term (ST) HSCs, and CD34+Flk2+ multipotent progenitors (MPPs) expressed PR3 at levels comparable with neutrophils (Figure 1E).
Figure 1.
Prtn3 Is Expressed in Hematopoietic Stem/Progenitor Cells and Regulates the Number of Stem and Progenitor Cell Subsets
(A) Prtn3 mRNA expression in sorted BM stem cell-containing populations (LSK cells) in WT and Prtn3−/− mice as determined by quantitative RT-PCR (n = 3 per group).
(B) Comparison of Prtn3 mRNA expression in sorted LSK cells and neutrophils from WT mice. GAPDH was used as a housekeeping control (n = 3 per group).
(C) PR3 protein expression in sorted BM stem (LSK) and progenitor (LK) cell-containing populations and neutrophils as determined by western blotting. Pan-actin was used as a loading control. Results are representative of three independent experiments.
(D) Intracellular PR3 staining in LSK cells from WT and Prtn3−/− BM by conventional flow cytometry. Results are representative of five independent experiments.
(E) Intracellular PR3 staining in different cell populations as determined by conventional flow cytometry. Results are representative of five independent experiments.
(F) Representative flow cytometry plots for LSK subsets in WT and Prtn3−/− mice. Numbers denote the frequency of each population among live singlets.
(G) Quantification of the frequency and number (per one femur and one tibia) of LSK subsets in WT and Prtn3−/− mice (n = 10 per group).
(H) Representative flow cytometry plots for LK subsets in WT and Prtn3−/− mice. Numbers denote the frequency of each population among live singlets.
(I) Quantification of the frequency and number of LK subsets in WT and Prtn3−/− mice (n = 16 per group).
(J) Representative images of histological analysis of BM smears from WT and Prtn3−/− mice. Arrowhead, lymphocyte; long arrow, neutrophil; thick arrow, normoblast; white arrow, monocyte; tailed arrow, myelocyte; right-pointing triangle, myeloblast; wavy arrow, metamyelocyte.
(K) Quantification of cells belonging to different lineages and maturation stages according to histological analysis of BM smears (n = 6 per group).
Scale bar, 10 μm. All values shown are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by unpaired, 2-tailed Student's t test. See also Figure S1.
Prtn3 Deficiency Leads to an Increase in the Number of Stem, Progenitor, and Immature Myeloid Cells in the Murine BM
Due to high Prtn3 expression in HSPCs, we explored whether PR3 modulates hematopoiesis in vivo. Prtn3−/− spleens weighed less than those from WT mice despite no difference in body weight, BM cellularity, and total blood cell count (Figures S1E–S1G). The peripheral blood contained a slightly higher percentage of myeloid cells and a slightly lower percentage of lymphoid cells in Prtn3−/− mice compared with WT mice, while other parameters were unchanged (Figure S1H). We next examined the overall frequency and numbers of HSPCs in the BM of WT and Prtn3−/− mice. The frequency and absolute number of LSK cells in Prtn3−/− mice was twice that of controls. Expansion of the LSK compartment was not restricted to a specific LSK subset: both the proportions and numbers of LT-HSCs, ST-HSCs, and MPPs were higher in Prtn3−/− BM (Figures 1F and 1G). The CD150+CD48− LSK cell compartment was also enhanced in Prtn3−/− mice (Figures S1I and S1J). The proportion and number of LK cells, the HPCs that can give rise to myeloid or erythroid lineages, was 50% higher in Prtn3−/− BM. When the LK population was subfractionated into CD34−CD16/32− megakaryocyte/erythroid progenitors (MEPs), CD34+CD16/32− common myeloid progenitors, and CD34+CD16/32+ granulocyte/monocyte progenitors, the frequency and number of all three subpopulations was increased in Prtn3−/− BM (Figures 1H and 1I).
Given the increased number of stem and progenitor cells in Prtn3−/− mice, we next determined the frequency of cells at different stages of myeloid and lymphoid development. Histological analysis of BM smears from WT and Prtn3−/− mice revealed an increased frequency of immature myeloid cells such as myeloblasts, myelocytes, and metamyelocytes in Prtn3−/− BM and decreased frequencies of band and segmented neutrophils and lymphocytes (Figures 1J and 1K). Collectively, these results indicate that Prtn3 disruption expands HSPCs and enhances hematopoiesis, particularly myelopoiesis.
The Expanded HPC Compartment in Prtn3−/− Bone Marrow Is Functionally Active Both In Vitro and In Vivo
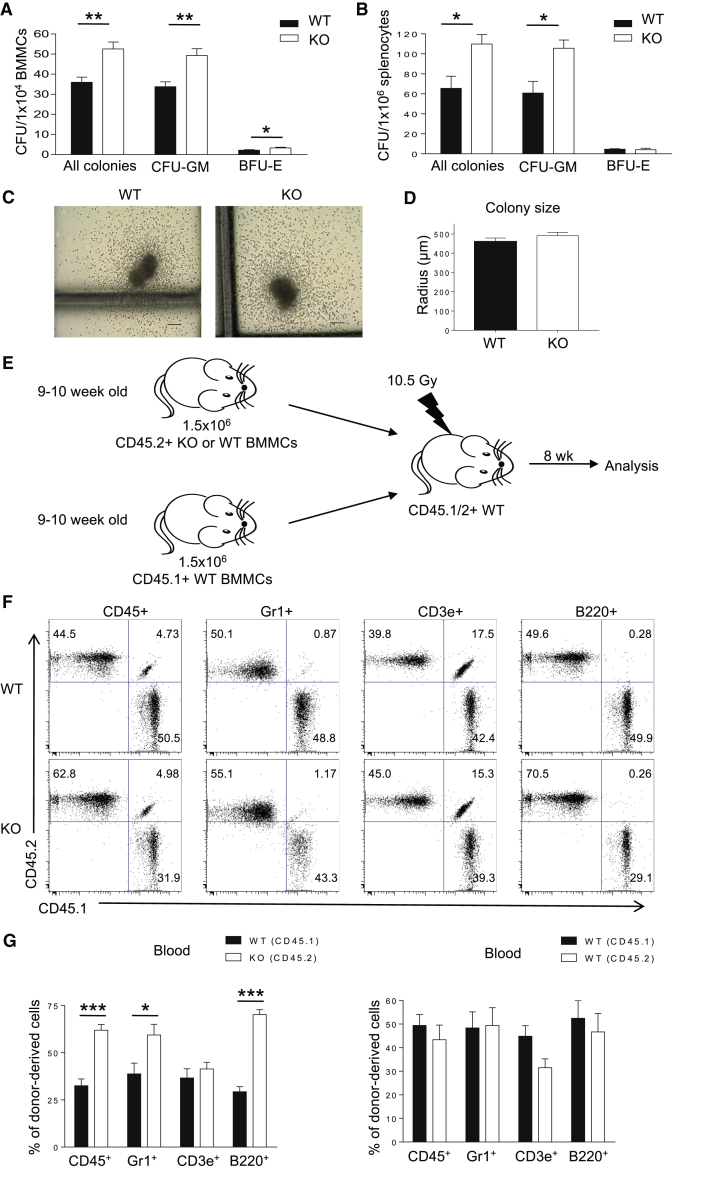
To test if the expanded HPC compartment in Prtn3−/− mice is functional in vitro, we performed colony-forming cell (CFC) assays. Myeloid and erythroid CFC assays with total BM cells confirmed an increase in the number of functional progenitors in Prtn3−/− mice in vitro (Figure 2A). Splenocytes from Prtn3−/− mice also gave rise to more colonies than those from WT mice (Figure 2B), suggesting that the enhanced HPC compartment in Prtn3−/− mice is not BM restricted. Of note, WT and Prtn3−/− total BM-derived colonies were of similar size (Figures 2C and 2D). To examine whether early progenitor cells in Prtn3−/− mice could outcompete WT cells in vivo, we transplanted total BM cells from WT and Prtn3−/− mice into lethally irradiated congenic recipients in a competitive BM transplantation setting (Figure 2E). At 8 weeks post transplant, peripheral blood cells were analyzed for donor contribution by lineage analysis. The Prtn3−/− donor gave rise to more total white blood, myeloid, and B cells, but not T cells, than the WT donor (Figures 2F and 2G). Taken together, these results confirm that Prtn3 disruption expands functionally active HPCs.
Figure 2.
Expanded Hematopoietic Progenitor Cell Compartment in Prtn3−/− Bone Marrow Is Functionally Active.
(A) Quantification of in vitro progenitor cell activity as demonstrated by colony-forming cell assays using BM cells (n = 9 per group).
(B) Quantification of in vitro progenitor cell activity as demonstrated by colony-forming cell assays using splenocytes (n = 3 per group).
(C) Representative images of WT and Prtn3−/− colonies from colony-forming cell assays. Scale bar, 100 μm.
(D) Quantitative analysis of colony sizes from WT and Prtn3−/− colonies. The sizes of at least ten colonies were measured per sample (n = 6 per group).
(E) Scheme of the experimental setup for the competitive bone marrow transplantation.
(F) Representative FACS dot plots for myeloid cells, B cells, and T cells showing the reconstitution capacity of different donors in the recipient mice.
(G) Quantitative analysis of the distribution of cells in the recipient mice at 8 weeks post transplantation (n = 4 per group). Results are representative of two independent experiments.
All values shown are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by unpaired, 2-tailed Student's t test.
Disruption of Prtn3 Accelerates BM Recovery after Irradiation
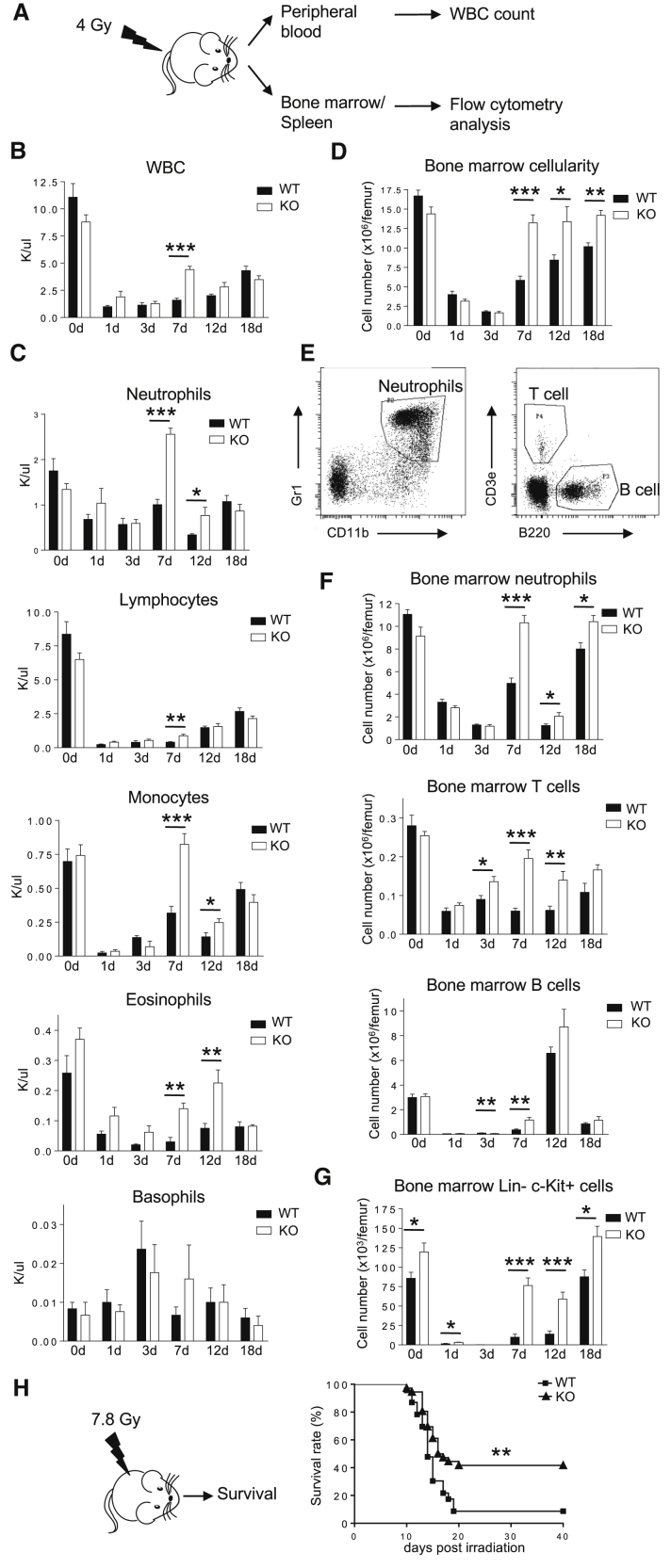
Expansion of HPCs often improves BM recovery after damage, so we investigated whether Prtn3 disruption improves BM recovery in irradiated mice. WT and Prtn3−/− mice were sublethally irradiated (4 Gy), and hematopoietic recovery was assessed by analyzing peripheral blood, BM, and spleen at various time points (Figure 3A). Starting at 7 days post irradiation, Prtn3−/− mice had higher numbers of total white blood cells in the peripheral blood (Figure 3B). Various lineages in the peripheral blood including neutrophils, monocytes, lymphocytes, and eosinophils exhibited faster recovery in Prtn3−/− mice (Figure 3C). Meanwhile, the recovery of red blood cells and hemoglobin was slightly delayed in Prtn3−/− mice (Figure S2A), a phenotype also manifested intriguingly in the aged murine hematopoietic system (Florian et al., 2012). It may suggest myeloid skewing in the myeloid/erythroid differentiation program, with a corresponding higher granulocyte/erythroid ratio. Prtn3−/− mice had higher body weight after irradiation (Figure S2B). While other factors such as reduced intestinal damage may also be responsible for higher body weight after total body irradiation, Prtn3−/− mice also had higher BM cellularity 7 days post irradiation (Figure 3D). BM analysis showed that neutrophil, T cell, B cell, and LK cell recovery was more rapid in the BM of Prtn3−/− mice (Figures 3E–3G). Similarly, splenocytes and the numbers of different cell lineages in the spleen showed a similar recovery pattern (Figures S2C–S2E). An interesting finding that was observed in this study was the extreme fluctuation between day 7 and day 18 with respect to myeloid and B cell recovery (Figures 3C and 3F). We suspect two different progenitor populations are responsible for producing the peripheral blood cells observed on these time points. Nevertheless, these results suggest that Prtn3−/− mice are more resistant to hematopoietic injury at all time points analyzed.
Figure 3.
The Expanded Hematopoietic Progenitor Cell Population in Prtn3−/− Bone Marrow Provides Faster Recovery and Increased Survival after Hematopoietic Injury
(A) The experimental setup used to analyze hematopoietic recovery after challenge with sublethal irradiation (4 Gy).
(B) Total white blood cell counts in WT and Prtn3−/− mice before and after sublethal irradiation (n = 5–9 per group).
(C) Neutrophil, lymphocyte, monocyte, eosinophil, and basophil cell counts in WT and Prtn3−/− mice before and after sublethal irradiation (n = 5–9 per group).
(D) The number of BM mononuclear cells per one femur in WT and Prtn3−/− mice before and after sublethal irradiation (n = 5–9 per group).
(E) Representative FACS dot plots for identifying the frequencies of neutrophils, T cells, and B cells in the BM.
(F) Quantitative analysis of neutrophils, T cells, and B cells per one femur in WT and Prtn3−/− mice before and after sublethal irradiation (n = 5–9 per group).
(G) Quantitative analysis of LK cells per one femur in WT and Prtn3−/− mice before and after sublethal irradiation (n = 5–9 per group).
(H) Scheme of the experimental setup for survival analysis after challenge with 7.8 Gy irradiation and the survival curves of WT and Prtn3−/− mice receiving 7.8 Gy irradiation (n = 23 for WT and n = 36 for Prtn3−/−).
All values shown are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by unpaired, 2-tailed Student's t test (A–G) and log rank (Mantel-Cox) test for survival curve (H). See also Figure S2.
To assess if the quicker hematopoietic recovery in Prtn3−/− mice translated to survival, WT and Prtn3−/− mice were irradiated with a higher dose (7.8 Gy), and survival rates were monitored for 30 days. WT mice started dying around day 10 and had a survival rate of about 10% on day 20 post irradiation. In contrast, Prtn3−/− mice had a survival rate of 50% on day 20 post irradiation (Figure 3H). Collectively, CFC assays, competitive BM reconstitution, and sublethal irradiation experiments all show that the enhanced HPC compartment in Prtn3−/− BM is functional in vitro and in vivo.
Elevated Hematopoiesis Caused by Prtn3 Disruption Is an Intrinsic Feature of HSCs
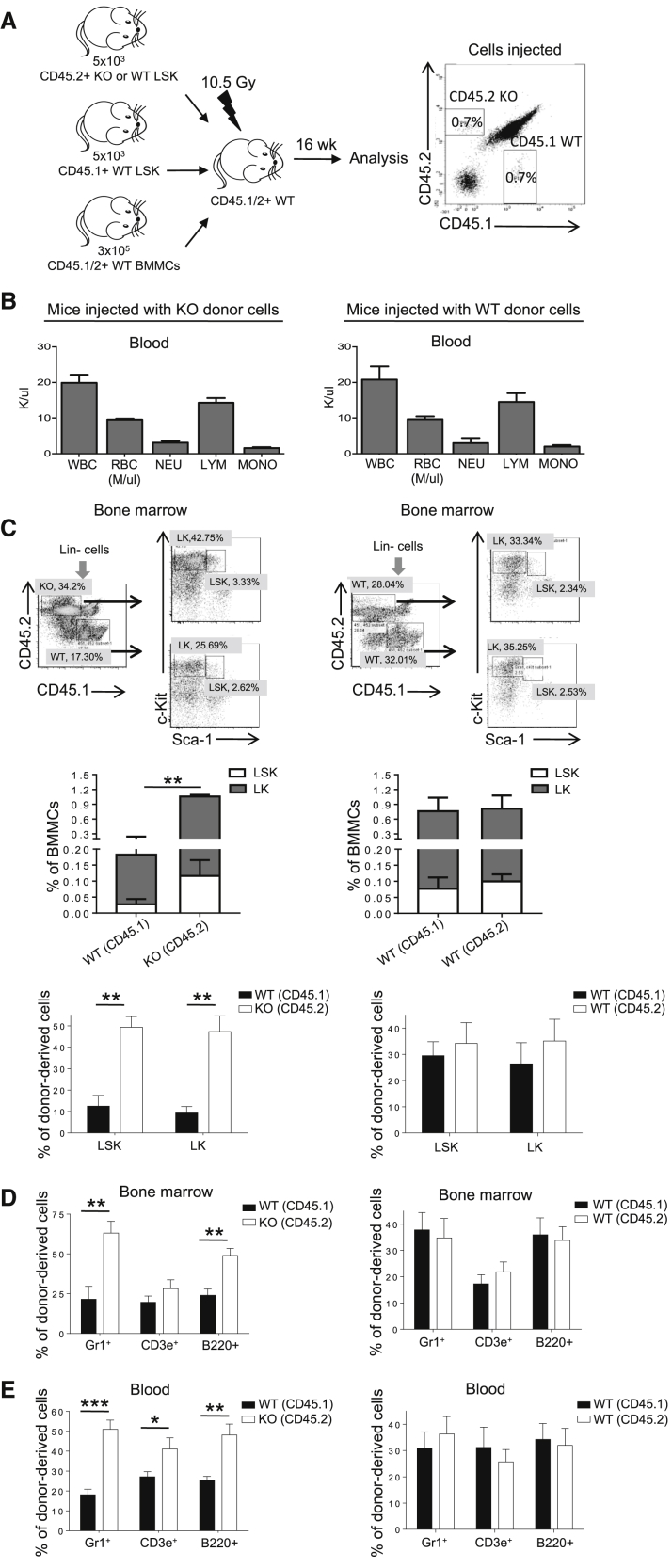
To further delineate whether the enhanced hematopoiesis in Prtn3−/− mice is an intrinsic feature of HSCs, we competitively transplanted LSK cells from WT and Prtn3−/− mice with supporting cells into lethally irradiated WT congenic recipients (Figure 4A). BM and peripheral blood compartments were examined at 16 weeks post transplant to assess the long-term reconstitution efficiency of primitive HSCs (Figure S3).
Figure 4.
Elevated Hematopoiesis Caused by PR3 Deficiency Is an Intrinsic Feature of LSK Cells
(A) Scheme of the experimental setup used to compare WT and Prtn3−/− LSK cells by competitive transplantation.
(B) Peripheral blood cell counts (n = 4 per group).
(C) Representative FACS plots showing WT and Prtn3−/− donor-derived LSK and LK cells in recipient mice are shown in the top panels. Numbers denote the frequency of specific cell populations among the parent gate. The frequency of WT and Prtn3−/− donor-derived LSK and LK cells among total BM cells is shown in the middle panels. The percentage of LSK and LK cells from individual donors is shown in the bottom panels (n = 4 per group).
(D and E) Quantitative analysis of the origin of cells in recipient mice at 16 weeks post transplantation in (D) the BM and (E) peripheral blood (n = 4–7 per group).
All values shown are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by unpaired, 2-tailed Student's t test. See also Figure S3.
Peripheral blood cell counts were similar in mice injected with either WT or Prtn3−/− LSK cells (Figure 4B). However, in mice injected with Prtn3−/− donor LSK cells competing against WT donor LSK cells, we noted a dramatic increase in Prtn3−/− donor-derived chimerism in LSK and LK cells in the BM (Figure 4C). There was also a higher frequency of Prtn3−/− donor-derived myeloid and B cells in the BM (Figure 4D). Similar to BM cells, Prtn3−/− donor-derived chimerism outcompeted that of WT donor in different lineages in peripheral blood (Figure 4E). Control mice reconstituted with competing congenic WT (CD45.2) and WT (CD45.1) LSK cells did not show any difference between the two congenic groups (Figures 4C–4E). In this setup, reconstitution efficiency of WT and Prtn3−/− LSK cells was assessed in the same BM environment at 16 weeks post transplant. Thus, the higher reconstitution efficiency observed in Prtn3−/− LSK recipients suggests that the augmented hematopoiesis induced by Prtn3 disruption is an intrinsic feature of Prtn3-deficient HSCs.
Prtn3 Deficiency in HSPCs Does Not Affect Proliferation but Decreases the Rate of Apoptosis
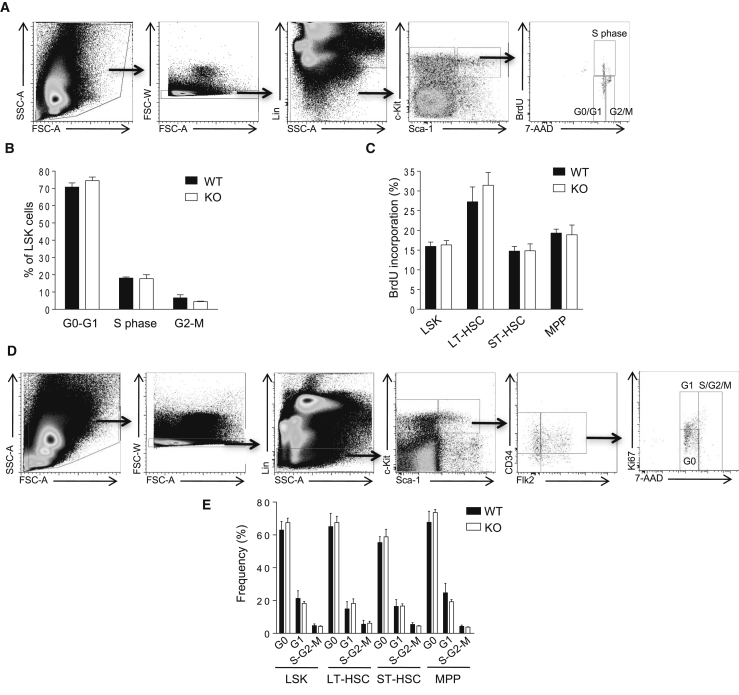
The enhanced stem and progenitor cell compartments in Prtn3−/− mice prompted us to investigate the underlying mechanism. We first explored HSPC proliferation using a bromodeoxyuridine (BrdU) incorporation assay and DNA staining with 7-aminoactinomycin D (7-AAD) to analyze the frequency of LSK cells at different stages of the cell cycle. The percentage of LSK cells at different cell cycle phases (S/G2/M) was similar in WT and Prtn3−/− mice at 24 or 72 hr post BrdU injection (Figures 5A–5C). Although Prtn3−/− mice had a higher number of BrdU+ cells in the BM, this may have been due to increased number of HSPCs. The proportions of BrdU+ cells in the LSK subpopulations were similar in the BM of WT and Prtn3−/− mice (Figure 5C). We also investigated the rate of cell proliferation by Ki67 staining to analyze the frequency of cells in G1 phase. Similarly, the frequencies of LSK cells and LSK subpopulations in G1 phase were also comparable between WT and Prtn3−/− mice (Figures 5D and 5E). These data exclude the possibility that proliferation is responsible for the enhanced stem cell population seen in Prtn3−/− mice.
Figure 5.
PR3 Does Not Affect Proliferation of Hematopoietic Stem and Progenitor Cells
(A) Gating strategy to analyze the cell cycle status of BM LSK cells by BrdU incorporation.
(B) Cell cycle analysis of LSK cells at 24 hr post BrdU injection (n = 4 per group).
(C) The rate of BrdU incorporation in LSK subsets at 72 hr post BrdU injection (n = 4 per group).
(D) Gating strategy to analyze the cell cycle status of BM LSK cells by Ki67 and 7-AAD staining.
(E) Cell cycle analysis of LSK subpopulations as assayed by Ki67 and 7-AAD staining (n = 4 per group).
See also Figure S4.
We recently reported that PR3 regulates neutrophil spontaneous death by cleaving and activating pro-caspase-3 (Loison et al., 2014). We therefore wondered whether PR3 regulated HSPC numbers by modulating their survival. To test this possibility, we first assessed spontaneous HSPC death using annexin V/7-AAD staining with freshly isolated BM cells from WT and Prtn3−/− mice; the frequencies of viable, early apoptotic, and late apoptotic cells in the stem and progenitor cell-containing populations were comparable in both groups (Figure S4A).
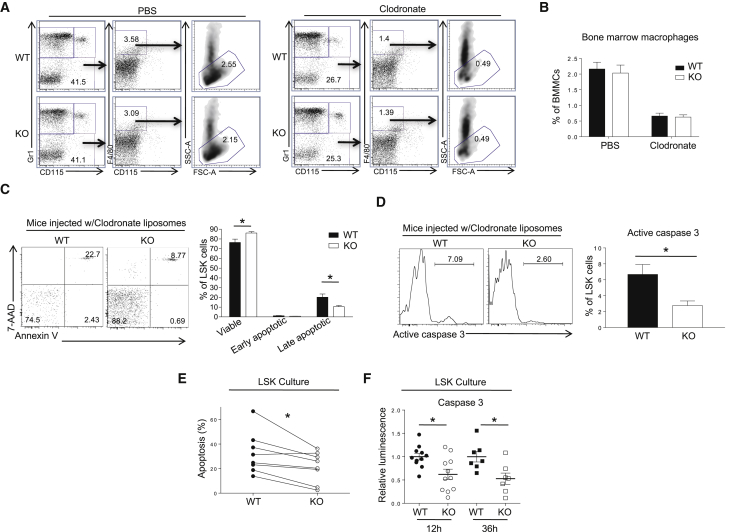
Since macrophages clear apoptotic cells, we decided to deplete BM macrophages using clodronate liposomes as previously described (Giuliani et al., 2001) and then examine LSK cell viability. BM macrophages remain markedly depleted for up to 2 weeks following clodronate liposome injection (Chow et al., 2011). At 9 days after injection, clodronate liposomes depleted 80% of Gr1−CD115−F4/80+ SSC-Anormal BM macrophages in both WT and Prtn3−/− mice, with no difference in the proportion of BM macrophages observed between clodronate or PBS liposome-treated WT and Prtn3−/− mice (Figures 6A and 6B). On day 9 post clodronate liposome injection, the frequency of viable LSK cells (Annexin V−7-AAD−) was higher and the frequency of late apoptotic LSK cells (Annexin V+7-AAD+) was lower in Prtn3−/− BM compared with WT BM (Figure 6C). Of note, both WT and Prtn3−/− BM contained very few early apoptotic LSK cells (Annexin V+7-AAD−), suggesting that early apoptotic LSK cells might be quickly converted to Annexin V+7-AAD+ cells in the BM.
Figure 6.
PR3 Regulates the Apoptosis of Hematopoietic Stem and Progenitor Cells
(A) Gating strategy to identify macrophage frequency in BM after treatment with PBS or clodronate liposomes. Numbers denote the frequency of specific cell subsets among live singlets.
(B) Quantification of BM macrophage frequency in WT and Prtn3−/− mice after liposome injection (n = 13–18 per group).
(C) Frequency of viable (Annexin V− 7-AAD−), early apoptotic (Annexin V+ 7-AAD−), and late apoptotic (Annexin V+ 7-AAD+) LSK cells in WT and Prtn3−/− mice after BM macrophage depletion. Numbers denote the frequency of cells among LSK cells (left panel). Quantification is shown in the right panel (n = 12–13 per group).
(D) Caspase-3 activation in LSK cells using a cell-permeable, fluorescent caspase-3 substrate. Numbers denote the frequency of LSK cells with active caspase-3 (left panel). Quantitative analysis is shown in the right panel (n = 5 per group).
(E) Apoptosis in sorted LSK cell cultures at 36 hr. Rate of apoptosis is calculated relative to the initial number of cells. %Apoptosis = (initial number of cells – total number of Annexin V− 7-AAD− cellular events detected)/initial number of cells (n = 8 per group).
(F) Analysis of caspase-3 activity in sorted LSK cells using a luminescence-based assay at 12 or 36 hr post culture. Data were normalized to the average luminescence value of WT cells for each independent experiment (n = 7–11 per group).
See also Figures S5 and S6.
PR3 regulates neutrophil spontaneous death by cleaving and activating pro-caspase-3 (Loison et al., 2014). We tested if this mechanism also explained the reduced spontaneous death of LSK cells in Prtn3−/− mice. Using a fluorescent probe, we stained for active caspase-3 in LSK cells following macrophage depletion in the BM. Indeed, Prtn3−/− LSK cells exhibited significantly less active caspase-3, suggesting that delayed spontaneous death may well be due to a defect in pro-caspase-3 cleavage by PR3 (Figure 6D). The rates of apoptosis and caspase-3 activation were similar between WT and Prtn3−/− mice when mice were treated with PBS liposomes, confirming that the difference was macrophage dependent (Figures S4B and S4C). Taken together, these results demonstrate that Prtn3−/− LSK cells exhibit a reduced rate of apoptosis compared with WT LSK cells, which is best observed after depletion of BM macrophages.
In another set of experiments, sorted LSK cells from WT or Prtn3−/− mice were cultured in a stem cell maintenance medium containing various recombinant cytokines as described previously (Janzen et al., 2008). Annexin V/7-AAD staining was performed at 36 hr post culture. We observed a higher frequency of viable cells in Prtn3−/− LSK cultures compared with WT LSK cultures (Figure 6E). In addition, we performed a luminescence-based caspase-3 activity assay after a short-term culture of sorted LSK cells (as described in Flach et al., 2014). This assay revealed a 40% and 50% reduction in caspase-3 activity in Prtn3−/− LSK cells at 12 hr and 36 hr post culture, respectively (Figure 6F). Collectively, these ex vivo data further demonstrate that Prtn3−/− LSK cells exhibit a reduced rate of apoptosis, at least partially due to reduced caspase-3 activation.
Next, we tested if HSPCs from Prtn3−/− mice are more resistant to irradiation. Although untreated Prtn3−/− LSK cells had a lower rate of apoptosis than WT counterparts, the rate of irradiation-induced apoptosis was similar between WT and Prtn3−/− LSK cells (Figure S5). In addition, we examined irradiation-induced apoptosis of LSK and LK cells in vivo (Shao et al., 2010, Yu et al., 2010). Similarly, we found that HSPCs from Prtn3−/− mice were not more resistant to irradiation (Figure S6). Collectively, these data suggest that the faster recovery observed in Prtn3−/− mice after sublethal irradiation and enhanced survival after lethal irradiation were likely due to an enlarged HSC pool size, instead of intrinsically increased resistance to irradiation. Thus, PR3 plays a critical role in the spontaneous death but not in irradiation-induced apoptosis of HSPCs. This is consistent with the current understanding that irradiation-induced apoptosis is mainly controlled by canonical caspase-mediated mechanisms.
Discussion
Here, we reveal that Prtn3−/− BM has a higher number of HSPCs. Competitive reconstitution experiments suggest that the expanded HSPC compartment is functional. Previous studies have shown that HSPCs with defective apoptosis display enhanced repopulation ability (Domen et al., 2000, Guo et al., 2010, Janzen et al., 2008). Since PR3 regulates spontaneous HSPC apoptosis by cleaving and activating pro-apoptotic caspase-3, our results identify PR3 as a regulator of HSPC number and function.
Prtn3 is a serine protease mainly expressed in granulocytes and a key player in innate immunity. Our findings suggest that PR3 is also an intrinsic regulator of the HSPC compartment in the BM. High Prtn3 expression levels were detected in HSPCs. To our knowledge, Prtn3 expression in HSPCs has not been reported. PR3 has been shown to play a role in neutrophil spontaneous death (Loison et al., 2014), and we now extend this finding to HSPCs, a population enriched with stem cells. While neutrophil spontaneous apoptosis is dependent on PR3-induced caspase-3 cleavage, caspase-8 and caspase-9 are not essential to this process but instead required for ligand-induced neutrophil apoptosis (Loison et al., 2014). Similarly, spontaneous apoptosis of HSPCs is likely also independent of caspase-8 or caspase-9, due to the absence of stimuli that trigger their activation under unchallenged hemostatic condition.
We previously demonstrated that pro-caspase-3 is a direct PR3 target (Loison et al., 2014). Similar to Prtn3−/− mice, caspase-3-deficient (Caspase 3−/−) mice also have an expanded HSC pool (Janzen et al., 2008). Apoptosis was also decreased in Caspase 3−/− HSCs in vitro but, intriguingly, there was increased proliferation in Caspase 3−/− HSCs as a consequence of hyperactive cytokine signaling. Janzen et al. (2008) attributed the expanded HSC pool in Caspase 3−/− mice to increased proliferation rather than decreased cell death. However, we did not observe any differences in proliferation between WT and Prtn3−/− mice. Of note, non-apoptotic roles for caspase-3 in hematopoiesis such as regulation of lymphocyte proliferation (Woo et al., 2003), differentiation (Yi and Yuan, 2009), and the silencing of type I interferon production in dying cells to render mitochondrial apoptosis immunologically silent (White et al., 2014) have also been described. It remains to be determined whether PR3 contributes to these non-apoptotic caspase-3 functions in hematopoiesis. Importantly, we detected reduced apoptosis in Prtn3−/− LSK cells compared with WT LSK cells both ex vivo after short-term culture and in vivo after depletion of BM macrophages. We also examined the role of PR3 in irradiation-induced HSPC apoptosis. The rate of irradiation-induced apoptosis was similar between WT and Prtn3−/− LSK cells. In addition, HSPCs from Prtn3−/− mice were not more resistant to irradiation compared with HSPCs from WT mice. Collectively, our data indicate that PR3 plays a critical role in the spontaneous death, but not in irradiation-induced apoptosis, of HSPCs. This is consistent with the current understanding that irradiation-induced apoptosis is mainly controlled by canonical caspase-mediated mechanisms. It is likely that irradiation-induced HSPC apoptosis is dependent on caspase-8 and -9, while spontaneous HSPC apoptosis is mediated by PR3. The mechanism that leads to PR3 activation or Prtn3 expression in HSPC needs to be further investigated.
Prtn3 expression in cultured CD34+ cells is regulated by granulocyte colony-stimulating factor and can confer cytokine-independent growth to BM-derived hematopoietic cells (Lutz et al., 2000). While this finding was obtained in vitro with progenitor cells, our in vivo data suggest that PR3 limits the HSPC compartment by driving the spontaneous turnover of HSPCs. In another study, PR3 inhibition arrested growth in a promyelocytic leukemia cell line, HL-60, and promoted granulocytic differentiation (Bories et al., 1989). Similarly, we observed preferential myeloid development in PR3-deficient BM, although a higher proportion of myeloid cells were immature. These discrepancies can be explained by the differential functions of PR3 in primitive HSCs and progenitor cells. Alternatively, the in vitro culture conditions may not closely resemble the in vivo BM environment for HSPC maintenance, self-renewal, and programmed death.
Intrinsic and extrinsic factors determine stem cell fate. HSPC pools undergo self-renewal and differentiation to ensure there are sufficient numbers of blood cells to fight infections and maintain homeostasis and host physiology. However, the number of HSPCs undergoing programmed cell death at a given point in time remains elusive. Various studies suggested a role for pro- and anti-apoptotic proteins in the quantitative and qualitative properties for HSPCs, confirming that cell death also plays a role in fate of HSPCs (Domen et al., 2000, Guo et al., 2010, Imai et al., 2010, Janzen et al., 2008, Opferman et al., 2005, V et al., 2010). Here, we identify PR3 as a regulator of HSPC survival and engraftment. PR3 inhibition might be useful to accelerate and increase the efficiency of myeloid reconstitution during BM transplantation especially when the number of donor cells is limited.
Experimental Procedures
Mice
All experiments, unless otherwise noted, were performed on 8- to 12-week-old sex-matched mice. C57BL/6 WT and Prtn3−/− mice were bred in house in specific pathogen-free animal facilities at Boston Children's Hospital. Prtn3−/− mice were generated as previously described (Loison et al., 2014) and backcrossed to C57BL/6 mice for eight generations. B6.SJL-Ptprca Pepcb/BoyJ mice carrying CD45.1 allele were purchased from Jackson Laboratories and acclimated for 2 weeks. B6.SJL-Ptprca Pepcb/BoyJ mice and C57BL/6 mice were bred in house to generate mice carrying CD45.1 and CD45.2 alleles. In all experiments with knockout mice, we used corresponding littermates as WT controls. All procedures were approved and monitored by the Children's Hospital Animal Care and Use Committee.
Complete Blood Count
Orbital peripheral blood (150 μL) was collected by heparinized capillary tubes (Fisher Scientific, 22-362-566) and transferred into K2-EDTA-coated tubes (Becton Dickinson, 365974). Blood parameters were analyzed by using Hemavet 950FS (Drew Scientific).
Flow Cytometry and Cell Sorting
BM cells from femurs and tibias were flushed into Dulbecco’s PBS (Life Technologies, 14190-250) supplemented with 2% fetal bovine serum (FBS; Atlanta Biologicals, S11150H). Red blood cells were lysed by ACK buffer (Life Technologies, A10492-01). Five million BM cells were incubated with an antibody mixture including antibodies for lineage markers CD3e-APC (Biolegend, 100312), CD4-APC (Biolegend, 100516), CD8a-APC (Biolegend, 100712), CD11b-APC (Biolegend, 101212), CD45R/B220-APC (Biolegend, 103212), Gr1-APC (Biolegend, 108412), Ter119-APC (Biolegend, 116212), as well as Sca1-PE/Cy7 (Biolegend, 108114) and c-Kit-APC/Cy7 (Biolegend, 105826) in DMEM (Life Technologies, 31053-028) supplemented with 2% FBS. For LSK subset analysis, CD34-FITC (eBioscience, 11-0341-85) and CD135-PE (Biolegend, 135306) were used. Alternatively, CD48-PE (Biolegend, 103405) and CD150-PerCp/Cy5.5 (Biolegend, 115921) were used for LSK subsets. For LK subsets, CD34-FITC and CD16/32-PE (Biolegend, 101308) were used. Samples were incubated on ice for 15 min, then washed and filtered before analysis. When CD34 staining was performed, samples were stained for 1 hr on ice. For intracellular PR3 detection, BM cells were fixed and permeabilized using Intracellular Fixation & Permeabilization Buffer Set (eBioscience, 88-8824-00). The PR3 (D-20) antibody was purchased from Santa Cruz. For detection of apoptosis, cells were stained with antibodies against surface markers and then stained with annexin V-FITC (BD Biosciences, 556420) and 7-AAD (BD Biosciences, 559925) in annexin V binding buffer (BD Biosciences, 556454). To detect active caspase-3 by flow cytometry, a Vybrant FAM Caspase Assay Kit (Thermo Fisher Scientific, V35118) was used. Data were collected on FACSCanto II or LSR II flow cytometers (Becton Dickinson) and analyzed by FlowJo software (Tree Star).
For sorting highly purified cells, an immunomagnetic negative selection kit was used to enrich HSPCs (Stem Cell Technologies, 19756). Enriched cells were stained with Streptavidin-FITC (Biolegend, 405202), c-Kit-PE (Biolegend, 105808), and Sca1-APC (Biolegend, 108112). DAPI (BD Biosciences, 564907) was added to exclude dead cells. Cells were sorted on an Aria (Becton Dickinson) or MoFlo (Dako) cell sorter.
Bone Marrow Transplantation
Recipient mice carrying CD45.1 and CD45.2 alleles were γ-irradiated with two doses of 5.25 Gy. For total BM transplantation, recipients were injected with equal numbers of BM cells from each donor. For LSK transplantation, 5 × 103 sorted LSK cells from each donor (CD45.1 or CD45.2) were injected along with 3 × 105 supporting BM cells (CD45.1/2). Retroorbital bleeding was performed every 4 weeks. BM chimerism was analyzed at the end of each experiment. Gr1-FITC (Biolegend, 108406), CD45.1-PE (Biolegend, 110708), CD45.2-APC (Biolegend, 109814), CD3e-PE/Cy7 (Biolegend, 100320), and CD45R/B220-APC/Cy7 (Biolegend, 103224) were used to assess chimerism.
In Vivo Macrophage Depletion
Mice were injected with 250 μL of clodronate or PBS liposomes (ClodLip BV) intravenously at 9 days before tissue collection. BM macrophage depletion was confirmed using F4/80-FITC (Biolegend, 123108), Gr1-PE (Biolegend, 108408), and CD115-APC (Biolegend, 17-1152-82) antibodies.
Analysis of In Vivo Cell Proliferation by BrdU Incorporation
BrdU incorporation was assessed by a commercially available kit (BD Biosciences, 559619). Twenty-four to seventy-two hours before sacrifice, 2 μg of BrdU was injected intraperitoneally in 200 μL of PBS. At indicated time points, the frequencies of LSK, LT-HSC, ST-HSC, and MPP cells at different stages of the cell cycle were determined. In another set of experiments, Ki67-FITC (BD Biosciences, 556026) antibody was used to assess the frequency of cells in G1 phase.
Cell Culture
LSK cells (20,000–30,000) were directly sorted into 96-well round-bottom plates for annexin V/7-AAD staining, and 7,500–10,000 LSK cells in duplicates were sorted into solid white luminescence plates for detection of caspase 3-activity. LSK cells were cultured in Iscove's modified Dulbecco's medium (Life Technologies, 12440-053) supplemented with 5% FBS (Stem Cell Technologies, 06200), 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies, 15140-122), 0.1 mM non-essential amino acids (Life Technologies, 11140-050), 1 mM sodium pyruvate (Life Technologies, 11360-070), 2 mM L-glutamine (Life Technologies, 25030-081), 50 μM β-mercaptoethanol (Life Technologies, 21985-023), recombinant murine stem cell factor (rmSCF; 25 ng/mL), recombinant murine thrombopoietin (rmTPO; 25 ng/mL), recombinant murine Flt-3 ligand (rmFlt-3L; 25 ng/mL), recombinant murine interleukin-3 (rmIL-3; 10 ng/mL), rmIL-11 (25 ng/mL), recombinant murine granulocyte macrophage colony-stimulating factor (rmGM-CSF; 10 ng/mL), and recombinant murine erythropoietin (rmEpo; 4 U/mL) for detection of caspase-3 activity as described previously (Flach et al., 2014). After 12–36 hr culture, caspase-3 activity was detected by using a luminescence-based Caspase-Glo 3/7 assay (Promega, G8090) according to the manufacturer's instructions. For annexin V/7-AAD staining, LSK cells were cultured in the presence of rmSCF (50 ng/mL), rmTPO (50 ng/mL), rmFlt-3L (50 ng/mL), and rmIL-3 (20 ng/mL) as previously described (Janzen et al., 2008). Annexin V/7-AAD staining was performed after 24–36 hr culture. In one set of experiments, cells were irradiated with 2 Gy right after purification. In another set of experiments, cells were cultured for 2 days and medium was replaced to contain rmFlt-3L (10 ng/mL) and rmIL-3 (10 ng/mL) for an additional 3 days as previously described (Janzen et al., 2008). On day 5, cells were irradiated with 2 Gy. Annexin V/7-AAD staining was performed at 24 hr post irradiation. All recombinant cytokines were purchased from Peprotech.
PCR and Gene Expression Analysis
Prtn3 genotyping was done as described previously (Loison et al., 2014). RNA was isolated from fluorescence-activated cell sorted (FACS) BM subpopulations (Gr1+ CD11b+ neutrophils and Lineage− Sca-1+ c-Kit+ cells) using a Picopure RNA isolation kit (Thermo Fisher Scientific, KIT0204). cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad, 1708891), and quantitative RT-PCR was performed using a SYBR Green Quantitative RT-PCR kit (Bio-Rad, 1708880). Primers used are listed below: Prtn3_fwd, 5′-CCCTGATCCACCCGAGATTC-3′; Prtn3_rev, 5′-GGTTCTCCTCGGGGTTGTAA-3′; Gapdh_fwd, 5′-AGAAGACTGTGGATGGCCCCTC-3′; Gapdh_rev, 5′-GATGACCTTGCCCACAGCCTT-3′.
Western Blot Analysis
Western blots were performed as previously described (Loison et al., 2014). Sorted LSK cells were loaded at a 1:1 ratio compared with LK cells and neutrophils. The antibodies against mouse PR3 (P-20) and gp91phox (54.1) were purchased from Santa Cruz. The antibody against mouse pan-actin (AAN01) was purchased from Cytoskeleton and GAPDH (MCA-1D4) was purchased from Encor.
Histological Analysis of Bone Marrow Smears
BM smears were prepared by squeezing the cells from femurs and tibias of mice directly onto slides. Cells were stained by a Diff-Quik Stain Kit (Siemens, B41312-1A) according to the manufacturer's instructions. At least 400 cells were counted to identify the lineage and maturation stage of BM cells. Investigators analyzing the samples were blinded to the identities of samples.
Colony-Forming Cell Assays
BM cells (2 × 104) and splenocytes (1 × 106) from WT and knockout mice were seeded in semisolid Methocult GF M3434 medium containing rmSCF, rmIL-3, recombinant human (rh)IL-6, and rhEpo for detection of colony-forming units-granulocyte, monocyte and burst-forming units-erythroid (Stem Cell Technologies, 03434). Colony numbers were counted on day 7, and images for colony sizes were obtained on day 8.
Statistical Analysis
All values shown are means ± SEM. Statistical significance for indicated datasets was performed by Student's t test on Prism (GraphPad). Unless otherwise indicated, data were analyzed using unpaired Student's t test. Viability and caspase-3 activity in sorted LSK cells as well as myeloid-lymphoid ratios in transplantation experiments were analyzed using paired Student's t test.
Author Contributions
K.K., H.Z., J.M., Y.X., and H.R.L. conceived of the project. K.K., H.Z., X.Z., R.G., H.K., F.L., P.L., Q.R., H.Y., and X.L. designed/performed experiments and analyzed data. K.K. and H.R.L. wrote the manuscript. All authors contributed to the review of the manuscript. H.R.L., Y.X., F.M., and T.C. supervised the project. H.R.L. and Y.X. acquired the funds.
Acknowledgments
The authors thank Joseph Mizgerd, Leslie Silberstein, and Li Chai for helpful discussions. We thank Li Zhao and Giri Buruzula for experimental support. This work was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018RC31002, 2017PT31033); the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2017-I2M-1-015); State Key Laboratory of Experimental Hematology Research Grant (157-Z18-01). Y.X. is supported by grants from National Basic Research Program of China (2015CB964903 and 2012CB966403), National Natural Science Foundation of China (31271484 and 31471116), and Natural Science Foundation of Tianjin City (12JCZDJC24600). H.R.L. is supported by NIH grants (R01AI103142, R01HL092020, and P01 HL095489) and a grant from FAMRI (CIA 123008). Cell sorting was performed at the HSCI/DRC Flow Core (NIH P30DK036836).
Published: November 1, 2018
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.10.004.
Contributor Information
Yuanfu Xu, Email: xuyf@ihcams.ac.cn.
Hongbo R. Luo, Email: hongbo.luo@childrens.harvard.edu.
Supplemental Information
References
- Bories D., Raynal M.C., Solomon D.H., Darzynkiewicz Z., Cayre Y.E. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989;59:959–968. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- Campanelli D., Detmers P.A., Nathan C.F., Gabay J.E. Azurocidin and a homologous serine protease from neutrophils. differential antimicrobial and proteolytic properties. J. Clin. Invest. 1990;85:904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Boles N.C., Lin K.Y., Tierney M.P., Bowman T.V., Bradfute S.B., Chen A.J., Merchant A.A., Sirin O., Weksberg D.C. Hematopoietic fingerprints: an expression database of stem cells and their progeny. Cell Stem Cell. 2007;1:578–591. doi: 10.1016/j.stem.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier S.H., Morrison S.J., Liao X., Weissman I.L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A., Lucas D., Hidalgo A., Mendez-Ferrer S., Hashimoto D., Scheiermann C., Battista M., Leboeuf M., Prophete C., van Rooijen N. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeshott C., Ohnemus C., Pilyavskaya A., Ross S., Wieczorek M., Kroona H., Leimer A.H., Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domen J., Cheshier S.H., Weissman I.L. The role of apoptosis in the regulation of hematopoietic stem cells: overexpression of Bcl-2 increases both their number and repopulation potential. J. Exp. Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach J., Bakker S.T., Mohrin M., Conroy P.C., Pietras E.M., Reynaud D., Alvarez S., Diolaiti M.E., Ugarte F., Forsberg E.C. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M.C., Dorr K., Niebel A., Daria D., Schrezenmeier H., Rojewski M., Filippi M.D., Hasenberg A., Gunzer M., Scharffetter-Kochanek K. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A.L., Wiener E., Lee M.J., Brown I.N., Berti G., Wickramasinghe S.N. Changes in murine bone marrow macrophages and erythroid burst-forming cells following the intravenous injection of liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP) Eur. J. Haematol. 2001;66:221–229. doi: 10.1034/j.1600-0609.2001.066004221.x. [DOI] [PubMed] [Google Scholar]
- Guo S., Lu J., Schlanger R., Zhang H., Wang J.Y., Fox M.C., Purton L.E., Fleming H.H., Cobb B., Merkenschlager M. MicroRNA miR-125a controls hematopoietic stem cell number. Proc. Natl. Acad. Sci. U S A. 2010;107:14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt G., Melamed R., Park R., Seguritan R., Laplace C., Poirot L., Zucchelli S., Obst R., Matos M., Venanzi E. Gene expression microarrays: glimpses of the immunological genome. Nat. Immunol. 2006;7:686–691. doi: 10.1038/ni0706-686. [DOI] [PubMed] [Google Scholar]
- Imai Y., Adachi Y., Shi M., Shima C., Yanai S., Okigaki M., Yamashima T., Kaneko K., Ikehara S. Caspase inhibitor ZVAD-fmk facilitates engraftment of donor hematopoietic stem cells in intra-bone marrow-bone marrow transplantation. Stem Cell Dev. 2010;19:461–468. doi: 10.1089/scd.2009.0251. [DOI] [PubMed] [Google Scholar]
- Janzen V., Fleming H.E., Riedt T., Karlsson G., Riese M.J., Lo Celso C., Reynolds G., Milne C.D., Paige C.J., Karlsson S. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell. 2008;2:584–594. doi: 10.1016/j.stem.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loison F., Zhu H., Karatepe K., Kasorn A., Liu P., Ye K., Zhou J., Cao S., Gong H., Jenne D.E. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J. Clin. Invest. 2014;124:4445–4458. doi: 10.1172/JCI76246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.G., Moog-Lutz C., Coumau-Gatbois E., Kobari L., Di Gioia Y., Cayre Y.E. Myeloblastin is a granulocyte colony-stimulating factor-responsive gene conferring factor-independent growth to hematopoietic cells. Proc. Natl. Acad. Sci. U S A. 2000;97:1601–1606. doi: 10.1073/pnas.97.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notta F., Zandi S., Takayama N., Dobson S., Gan O.I., Wilson G., Kaufmann K.B., McLeod J., Laurenti E., Dunant C.F. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351:aab2116. doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman J.T., Iwasaki H., Ong C.C., Suh H., Mizuno S., Akashi K., Korsmeyer S.J. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Orkin S.H., Zon L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Sun Y., Zhang Z., Feng W., Gao Y., Cai Z., Wang Z.Z., Look A.T., Wu W.S. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115:4707–4714. doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburg H.B., Rezner B.D., Muller-Sieburg C.E. Predicting clonal self-renewal and extinction of hematopoietic stem cells. Proc. Natl. Acad. Sci. U S A. 2011;108:4370–4375. doi: 10.1073/pnas.1011414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Ramos A., Chapman B., Johnnidis J.B., Le L., Ho Y.J., Klein A., Hofmann O., Camargo F.D. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H., Regoes R.R., Boddupalli C.S., Bonhoeffer S., Manz M.G. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J. Exp. Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V M.S., Kale V.P., Limaye L.S. Expansion of cord blood CD34 cells in presence of zVADfmk and zLLYfmk improved their in vitro functionality and in vivo engraftment in NOD/SCID mouse. PLoS One. 2010;5:e12221. doi: 10.1371/journal.pone.0012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.J., McArthur K., Metcalf D., Lane R.M., Cambier J.C., Herold M.J., van Delft M.F., Bedoui S., Lessene G., Ritchie M.E. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M., Hakem R., Furlonger C., Hakem A., Duncan G.S., Sasaki T., Bouchard D., Lu L., Wu G.E., Paige C.J. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat. Immunol. 2003;4:1016–1022. doi: 10.1038/ni976. [DOI] [PubMed] [Google Scholar]
- Yi C.H., Yuan J. The Jekyll and Hyde functions of caspases. Dev. Cell. 2009;16:21–34. doi: 10.1016/j.devcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Shen H., Yuan Y., XuFeng R., Hu X., Garrison S.P., Zhang L., Yu J., Zambetti G.P., Cheng T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.