Abstract
The convergent evolution of the human pygmy phenotype in tropical rainforests is widely assumed to reflect adaptation in response to the distinct ecological challenges of this habitat (e.g. high levels of heat and humidity, high pathogen load, low food availability, and dense forest structure), yet few precise adaptive benefits of this phenotype have been proposed. Here, we describe and test a biomechanical model of how the rainforest environment can alter gait kinematics such that short stature is advantageous in dense habitats. We hypothesized that environmental constraints on step length in rainforests alter walking mechanics such that taller individuals are expected to walk more slowly due to their inability to achieve preferred step lengths in the rainforest. We tested predictions from this model with experimental field data from two short-statured populations that regularly forage in the rainforest: the Batek of Peninsular Malaysia and the Tsimane of the Bolivian Amazon. In accordance with model expectations, we found stature-dependent constraints on step length in the rainforest and concomitant reductions in walking speed that are expected to compromise foraging efficiency. These results provide the first evidence that the human pygmy phenotype is beneficial in terms of locomotor performance and highlight the value of applying laboratory-derived biomechanical models to field settings for testing evolutionary hypotheses.
Keywords: human pygmy phenotype, rainforest, dwarfism, locomotor ecology, animal movement
1. Introduction
The ‘human pygmy phenotype’1 evolved multiple times among hunter–gatherer populations in the equatorial rainforests of Africa, Southeast Asia, and South America [1,2]. Molecular evidence indicates that this phenotype is strongly influenced by genetics and has been shaped by a recent history of positive natural selection on alleles related to growth, development, and metabolism [2–4]. Such findings are consistent with decades of speculation that short stature is an adaptation for coping with the challenges posed by tropical rainforests such as food limitation, warm and humid conditions, and high extrinsic mortality risk due in part to high pathogen load [1].
Another intriguing possibility is that short stature enhances bipedal walking performance amid dense vegetation, a concept first raised by Turnbull [5] and Diamond [6]. Many fieldworkers, including ourselves, are familiar with the struggle to keep pace with rainforest peoples skillfully navigating jungle landscapes. Short stature may indeed confer some advantages during rainforest walking, but, to date, researchers have neither proposed nor empirically tested a locomotor mechanism by which short stature could be selectively beneficial in these environments.
In fact, Turnbull's and Diamond's idea is somewhat counterintuitive in the light of data from laboratory studies on the relationship between human walking performance and stature. During treadmill walking, shorter people expend more mass-specific metabolic energy than taller people in order to walk a unit distance at a given speed (cost of transport (COT); [7]). This is because people with shorter legs need to take more strides to travel a given distance. Since the metabolic cost of a stride is similar across people of different statures, shorter people will have a higher COT at a given speed than taller people [7]. However, walking in a rainforest is different than walking on a treadmill. Rainforests are cluttered with vegetation and unpredictable obstacles which may constrain important features of walking performance.
During fieldwork, we noted one feature of walking that seems especially restricted in rainforests is step length. This impression, if validated, may have important implications for the evolution of the human pygmy phenotype. Two facts are relevant: first, people tend to walk according to a highly predictable speed-step length relationship [8,9]. Second, the relationship between walking speed and COT is U-shaped, and the speed at the minimum of this curve (COTmin) is necessarily higher for taller individuals [7]. Thus, taller individuals would be expected a priori to have higher preferred walking speeds in an open environment. In the presence of natural step length constraints, tall individuals would need to walk more slowly in the rainforest than they would otherwise prefer, while short individuals with smaller absolute step lengths and slower preferred speeds might be less affected by this constraint. We thus hypothesized that taller individuals bear a disproportionate speed cost as they are forced to walk at slower speeds, which would reduce travel distance and encounter rates with prey items. All else equal, if larger-bodied individuals experience reduced foraging efficiency, stature-dependent speed effects could produce, or at least facilitate, directional selection toward shorter stature in forest-dwelling populations.
Here, we briefly discuss the relationship between stature, step length, and walking speed and develop a quantitative gait model, which we refer to as the constrained step length model (CSLM), which hypothesizes that taller individuals incur a larger speed cost (see below for definition) if rainforests restrict step length. We conducted a within-subject design field experiment that tests two key assumptions of the model—that forest environments indeed constrain step length, and that predicted speed-step length relationships are maintained across environments—and test the prediction that there are stature-dependent speed costs in rainforests.
(a). The constrained step length model
Experimental work has shown that in an open, unconstrained environment, the quantitative relationship between preferred speed and step length is highly predictable and follows a simple power function [8,9]:
| 1.1 |
where s is dimensionless step length, v is dimensionless speed, and α and β are empirically derived constant parameters. Dimensionless step length and speed are related to absolute step length (Lstep, m) and speed (V, m s−1) as follows:
| 1.2 |
and
| 1.3 |
where Lleg is leg length (hip height) and g the gravitational constant. Experimental and theoretical work has shown that the preferred speed-step length relationship generally follows the energetically optimal relationship, with deviations from the preferred relationship elevating the energetic cost of walking [10–13]. This finding is derived from laboratory experiments, but the high energetic penalty of deviating from the preferred relationship strongly suggests that it should be maintained even in naturalistic contexts.
The preferred speed-step length relationship of equation (1.1) is the foundation of the CSLM, which relies on two assumptions: (1) absolute step length is environmentally constrained to a constant value in the forest environment (Lstep,C) that is shorter than the preferred step length in an open, unconstrained environment (Lstep,U), and (2) the preferred speed-step length relationship of equation (1.1) is maintained in the constrained environment. The CSLM can be expressed by substituting equation (1.2) into equation (1.1):
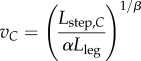 |
1.4 |
The CSLM thus predicts that walking speed in the constrained environment (vC) will be slower than when unconstrained (vU) (figure 1a), leading to a speed cost (vU – vC) that increases with stature (longer Lleg) (figure 1b). If these model predictions are verified, the CSLM could provide an explanation for how short stature might be selectively advantageous for walking in a rainforest. To test the CSLM, we conducted field experiments with two relatively short-statured populations that regularly forage in dense rainforests: the Batek of Peninsular Malaysia and the Tsimane of the Bolivian Amazon.
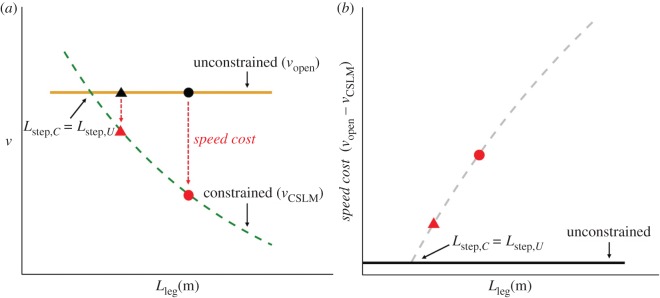
Figure 1.
Depiction of the CSLM and predicted speed costs. (a) The horizontal orange line depicts the preferred dimensionless speed (v)-dimensionless step length (s) relationship from equation (1.1). Note that unconstrained v is independent of Lleg. Unconstrained s and v are depicted for a hypothetical short individual (black triangle) and tall individual (black circle). The green curve shows the relationship between v and Lleg when step length is constrained such that Lstep,C < Lstep,U (equation (1.4)), and the red triangle and circle show s and v for the same hypothetical short and tall individuals. Constraining step length causes a reduction in v, as shown by the red arrows. In the constrained case, v is negatively related to Lleg such that the shorter individual can maintain a faster v than a taller individual. (b) The CSLM-predicted speed cost for the hypothetical short and tall individuals. This quantity is calculated for each individual as vopen − vforest and plotted against the CSLM prediction (grey dashed curve) from panel (a) (vopen – vCSLM). The speed cost is predicted to be greater for taller individuals than for shorter ones. There is no speed cost in the unconstrained environment (black line), and at a small enough stature such that Lstep,C = Lstep,U, the CSLM predicts no speed cost. Note that speed costs may be depicted on either a dimensionless or dimensioned axis.
2. Methods
(a). Study populations
The Batek are one of several indigenous groups living in Peninsular Malaysia, collectively termed Orang Asli (‘original people' in Malay). The Batek live in and around Taman Negara National Park, where they have traditionally hunted, gathered, and fished while maintaining trade relations with outsiders [14]. While today some Batek are semi-nomadic and partially reliant on wild foods, during our study we worked with taller Batek sub-populations who are relatively sedentary, subsist largely on market foods, and experience some genetic admixture through limited marriage with non-Batek. Data on Batek men (n = 21, mean age: 28 years) were collected during summer 2013 at two Batek settlements in Pahang, Malaysia. Batek men averaged 53.8 kg (s.d. = 4.8 kg) in weight and 1.63 m (s.d. = 0.04 m) in height. Average leg length was 0.84 m (s.d. = 0.03 m; range = 0.79–0.89 m).
The Tsimane are semi-sedentary forager-horticulturalists who hunt, gather, fish, and farm in the lowland Amazon of Bolivia [15]. The Tsimane are variably integrated into market economies. The present study occurred in one of the more acculturated villages (Campo Bello). Data on Tsimane men (n = 16, mean age: 30 years) were collected in summer 2017. Tsimane men averaged 63.8 kg (s.d. = 8.7 kg) in weight and 1.63 m (s.d. = 0.08 m) in height. Average leg length was 0.83 cm (s.d. = 0.05 m; range = 0.73–0.93 m). No individuals from either population had any visible locomotor impairment.
The stature variation within the samples is convenient for testing our hypothesis. We assume that variation in stature could derive from differential selection on Lleg, which is supported in part by the strong co-variation between Lleg and stature in both the Batek (Pearson's correlation; r = 0.79) and the Tsimane (Pearson's correlation; r = 0.82).
(b). Walking trials
We performed walking experiments in two conditions: open fields and rainforest understory. For the open trials, subjects walked in a field of short grass (Batek) or dirt (Tsimane). The forest transects in Malaysia were in lowland primary tropical rainforest. The forest transects in Bolivia were in secondary forest but were chosen to be similarly dense and navigable as the transects in Malaysia. All walking transects were on relatively dry and flat terrain that did not vary markedly over the length of the transect.
Subjects walked along transects marked with flagging tape. Despite the cluttered forest environment, walking kinematics appeared generally similar to those in open areas. The transects were longer for the Batek (open, 60 m; forest, 60 m) than for the Tsimane (open, 20 m; forest, 14 m) due to the challenge of finding a suitable longer transect in the secondary forest near Campo Bello. Open trials were conducted before forest trials for both populations due to logistical constraints.
For each condition, subjects walked one to four times along the transects. Subjects were typically unshod or wore light thong sandals according to preference. All subjects from both populations preferred to walk unshod in the forest condition. For each condition, we asked subjects to walk at the speed at which they would comfortably travel while foraging. We consider these walking bouts to reflect ‘preferred walking speeds', although we acknowledge that in the absence of direct respirometry measurements, these speeds do not necessarily reflect COTmin for the study participants.
We counted the number of steps (one-half of stride length) individuals took along transects to the nearest quarter of a step based on the distance of the lead foot past the end of the transect relative to the trailing foot position. Step length was calculated as the length of the transect divided by the number of steps. Step length and speed data were averaged across trials to arrive at a single value for each individual per condition. Nine (out of 92) trials were excluded from analyses because dimensionless walking speed exceeded 0.6, which is just below the typical walk-run transition speed in humans [16].
(c). Data analysis
The CSLM assumes that: (1) forest environments constrain step length and, (2) the preferred speed-step length relationship is maintained between forest and open environments. To test condition (1) we regressed Lstep against Lleg in both the open and forest environments, with the expectation of a positive slope in the open terrain and no relationship in the forest. Accordingly, we used analysis of covariance (ANCOVA) to test for a difference in slopes between conditions and then performed paired-samples t-tests on mean Lstep with the expectation that forest values would be shorter than open values.
To test condition (2) we first derived the preferred speed-step length relationship in each population by fitting equation (1.1) to log-transformed data from the open and forest environments. α and β were derived from the fitted model, as well as R2 values to test model fit. To ensure that it was appropriate to describe data from the open and forest environments by a single curve, we used the residuals of a linear regression of the model to assess differences in fit across the range of observed data.
After determining that the assumptions of the CSLM were satisfied (see below), we tested for stature-dependency in speed cost (within-individual difference in speed between open and forest environments) by regressing the speed cost against Lleg. In order to assess the functional form of the speed cost against Lleg, we compared the fit of predictions derived from the CSLM (predicted speed cost: vopen – vCSLM; corresponding to vU – vC described above) with those of a linear regression by comparing the root mean squared error of these models. All analyses were performed in R v. 3.4.1.
3. Results
(a). Conditions of the constrained step length model
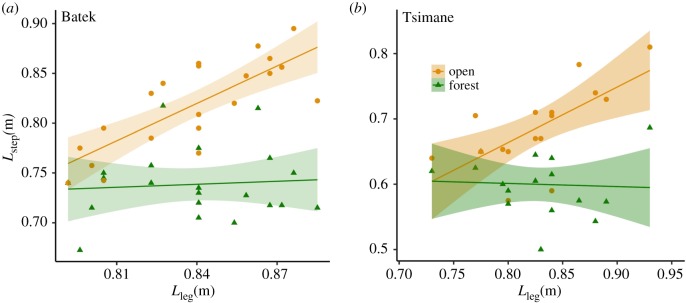
The assumptions of the CSLM were met in both populations. Lstep and Lleg were significantly positively related in the open condition (figure 2; Batek: β(s.e.) = 1.25(0.23), p < 0.001, Tsimane: β(s.e.) = 0.85(0.25), p = 0.004) but not in the forest condition (figure 2; Batek: β(s.e.) = 0.1(0.28), p = 0.73, Tsimane: β(s.e.) = −0.04(0.25), p = 0.85). Thus, the relationship between Lstep and Lleg varied by condition (ANCOVA: p = 0.003 for Batek, p = 0.016 for Tsimane). Mean Lstep was significantly shorter in the forest than in the open environment for both the Batek (Lstep-open = 0.82,, Lstep-forest = 0.74; paired t-test: mean differenceopen-forest = 0.08 m, p < 0.001) and the Tsimane (Lstep-open = 0.69, Lstep-forest = 0.60; paired t-test: mean differenceopen-forest = 0.09 m, p < 0.001).
Figure 2.
Differences in step length between the open and forest environments. Panels (a) and (b) plot the relationships between absolute step length (Lstep) and leg length (Lleg) in the Batek and Tsimane, respectively. Data points represent individual means. Linear regressions for each environment are plotted with 95% confidence intervals. For both populations, the slope of the relationship between Lstep and Lleg was significantly higher in the open (orange lines, circles) than in the forest (green lines, triangles). Paired t-tests indicated significantly shorter Lstep in the forest than in the open in both populations (see main text). Note that, in contrast to the other plots in the manuscript, the y-axis ranges are not the same in panels (a) and (b). (Online version in colour.)
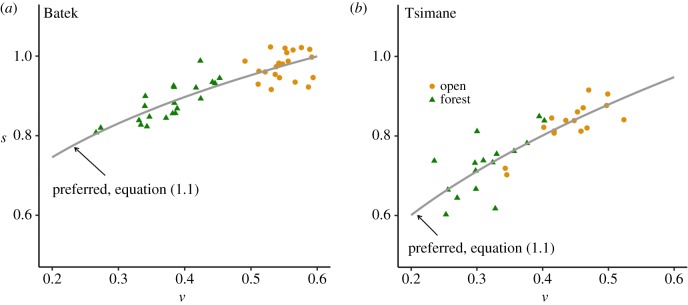
Figure 3 shows the preferred speed-step length relationships for the Batek and Tsimane derived from the data. These relationships are defined by the following equations:
Equations for the preferred relationships exhibited good fit to empirical data for both the Batek (R2 = 0.75) and Tsimane (R2 = 0.68) across the entire range of data with no linear relationships in the residuals.
Figure 3.
The preferred speed-step length relationship in open and forest environments. Panels (a) and (b) plot the preferred dimensionless speed (v)-dimensionless step length (s) relationship (equation (1.1)) in the Batek and Tsimane, respectively. Data points represent individual means. Orange circles indicate the open (unconstrained) environment, while green triangles indicate the forest (constrained) environment. The curves were parameterized using the entire dataset. (Online version in colour.)
(b). Predictions of the constrained step length model
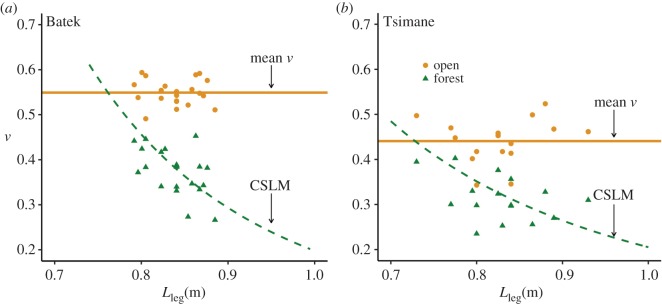
According to the CSLM, we expected that (1) speed would be reduced in the forest compared to the open environment and (2) the magnitude of speed reductions would be stature dependent. Our results support both predictions in the Batek and Tsimane. Regarding prediction (1), we observed significant reductions in mean speed in the forest environment compared to the open environment in both the Batek (paired t-test: mean difference = 0.5 m s−1, p < 0.001) and the Tsimane (paired t-test: mean difference = 0.36 m s−1, p < 0.001).
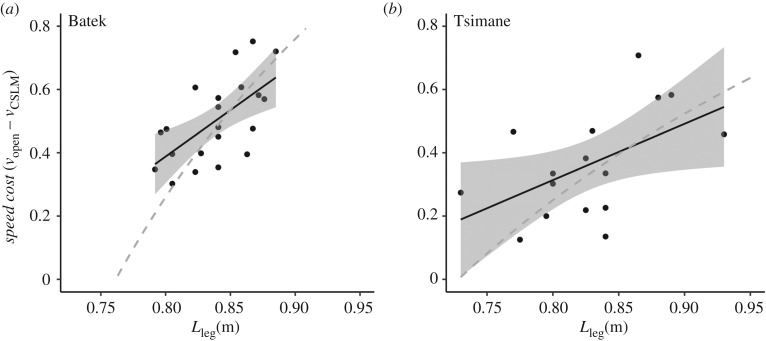
Results of tests of prediction (2) are shown in figures 4 and 5. The difference between the green (equation (1.4) using mean Lstep in forest) and orange (mean vopen) curves in figure 4 shows the expected logarithmic increase in speed cost (i.e. vopen – vCSLM) as a function of Lleg. The empirical data also conform to the expected relationship from the CSLM (figure 4). As shown in figure 5, in which dimensionless values of v were back-transformed into absolute speeds (in m s−1), we observed the expected positive relationship between within-individual speed cost and Lleg in both populations (Batek: β(s.e.) = 2.9(0.83), p = 0.002, Tsimane: β(s.e.) = 1.78(0.78), p = 0.04). The performance of the CSLM for both the Batek and Tsimane was nearly identical to that of the linear fits (Batek: RMSECSLM = 0.12, RMSElinear = 0.10, Tsimane: RMSECSLM = 0.15, RMSElinear = 0.14).
Figure 4.
CSLM predictions in the forest environment. Panels (a) and (b) plot dimensionless speed (v) versus Lleg for the Batek and the Tsimane, respectively. Data points represent individual means (orange circles = open, green triangles = forest). v was essentially constant in the open (horizontal orange lines indicate mean population v in the open). Constrained relationships are plotted using the mean population Lstep for the forest environments (0.74 m for the Batek, 0.60 m for the Tsimane) inserted into equation (1.4) and are represented by the green dashed curves. In contrast to the trend observed in the open, v decreased with Lleg in the forest. Matched-pairs t-tests indicated significantly reduced v in the forest compared to the open (see main text). Note that speed costs predicted by the CSLM can be visualized as the difference between the orange and green curves (figure 5 for depiction on the dimensioned axis). (Online version in colour.)
Figure 5.
Speed cost as a function of Lleg. Panels (a) and (b) plot Lleg versus speed cost for the Batek and Tsimane, respectively. Dashed grey curves represent predicted values from the CSLM, calculated using equation (1.4) (vopen – vCSLM) and illustrated by the difference between the orange and green curves in figure 4. Data points represent individual matched-pairs differences calculated as vopen – vforest. Linear regressions (black lines) with 95% confidence intervals demonstrate a significant positive relationship between speed cost and Lleg for both the Batek and the Tsimane. Linear models and the CSLM exhibited similar fit (see main text). Note that, in contrast to figure 4, speed cost is presented on a dimensioned axis (units: m s−1).
4. Discussion
In this paper, we describe a biomechanical model to explain how locomotion in tropical rainforests can promote or facilitate directional selection for short stature. Using data from a within-subject design field experiment with two rainforest populations, we demonstrate a simple but clear stature-dependent difference in the way people walk in a forest versus open environment. Whereas taller individuals took longer steps in open environments, all individuals were generally constrained to a similar (relatively small) step length in the forest (figure 2). Individuals conformed to the preferred speed-step length relationship across environments (figure 3), highlighting the applicability of previous laboratory-derived biomechanical models to field settings. Most importantly, we show for the first time in ecologically relevant contexts that constraints on step length generate stature-dependent walking speed costs that could make short stature evolutionarily beneficial among forest-dwelling humans (figures 4 and 5).
To illustrate how selection may favour shorter individuals in the rainforest via the CSLM, consider three hypothetical male individuals foraging in the Malaysian rainforest. The individuals differ in stature: one is of typical American stature (Lleg = 0.94 m, mean American male; [17]), another of typical Batek stature (Lleg = 0.84 m, mean value for the Batek in the present study), and the third of typical Efé stature (Lleg = 0.76, male Efé of the Ituri forest in Central Africa, the shortest human population on record; [1]). All three individuals share the same preferred dimensionless walking speed in the open, v = 0.5. In the open, taller stature results in slightly faster absolute walking speeds (American: 1.52 m s−1, Batek: 1.44 m s−1, Efé: 1.37 m s−1), and longer foraging distances covered over the course of two hours (American: 10.9 km, Batek: 10.3 km, Efé: 9.8 km). Taller stature, therefore, is predicted to confer a weak benefit to foraging efficiency in the open landscape, as faster and/or farther travel increases encounter rates with prey. However, the CSLM (Batek equation) shows the opposite is true in the rainforest, where speed (American: 0.61 m s−1, Batek: 0.95 m s−1, Efé: 1.38 m s−1) and distance travelled in two hours (American: 4.4 km, Batek: 6.8 km, Efé: 9.9 km) are both drastically reduced with taller stature. The CSLM suggests that evolving short stature should relax constraints on total energy budgets via increased efficiency of travelling and foraging. Such an evolutionary trajectory may be especially favoured in rainforests, which are notoriously difficult for human survival due to the paucity of edible food items for humans [1]. The hypothesis presented here thus contributes to an integrated model of the evolution of the human pygmy phenotype that includes the additive and potentially interactive effects of multiple selective pressures, including food limitation, heat dissipation, and high pathogen load in warm and humid conditions.
There are good reasons to believe that step length constraints in the form of obstacles (e.g. tree roots or vegetation) are a relevant ecological factor during rainforest locomotion. The CSLM, however, is agnostic with respect to the proximate cause of reduced step lengths, which may derive from more generalized ecological constraints. For instance, maintaining stability during locomotion is a major challenge in the rainforest environment, and reduced step lengths may be an adaptive consequence of dynamic adjustment to uncertain terrain based on visual cues [18], particularly when barefoot.
It is important to note that our use of the phrase ‘constrained,' which connotes extrinsic limitations on step length, differs from the previous use of the term in the literature [19,20] in which ‘constraint' is equated with experimental control on a variable. Our data show that our assumed power relationship fits both the constrained (forest) and unconstrained (open) data, and analysis of the residuals suggests that both conditions fall along the same relationship, in contrast to predictions of the models in refs. [19] and [20]. A recent study comparing speed, step length, and step frequency between rough and open terrain produced results similar to ours [18]. Therefore, the power relationship of our study and previous treadmill-based studies [8,9] is likely the default control strategy in naturalistic conditions.
Although the shortest human populations occur in tropical rainforests, short-statured populations also inhabit dense mountainous terrains and savanna bushlands (e.g. New Guinea highlanders and the San in southern Africa, respectively; [1,6]). These environments differ considerably in climate and vegetation but are similar in having rugged terrain. While we cannot claim to explain why short stature evolves among humans in every instance, the CSLM has the potential to generate stature-dependent costs in any habitat with irregular terrain as long as step length is constrained. It is also probable that aspects of gait other than step length are altered during rainforest locomotion. For instance, twentieth century ethnographers noted that forest people walked with ‘springy steps' and high-stepping gaits [21–23]. Crouched postures are also frequently necessary when walking through dense forest understory [24]. Such altered walking kinematics may also be associated with stature-dependent locomotor costs.
We did not directly measure COT in this study, but the impact of speed reductions on walking economy should be considered in future studies of naturalistic movement. Because COTmin lies at a lower speed (and thus shorter absolute step length) for shorter individuals than for taller individuals, at low speeds tall individuals are necessarily farther from their most economical speed compared to short individuals, even if they are taking preferred step lengths for that speed. Currently, too few naturalistic data exist to address this issue thoroughly. Indeed, actualistic walking speeds vary widely among populations (approx. 0.75–1.75 m s−1) [25] and do not necessarily track COTmin. For example, among Hadza hunter–gatherers living in the open savanna-bushland of Tanzania, average walking speed of individuals during foraging is higher than the speed corresponding to their COTmin [26].
A further limitation of our study is that we did not directly capture the natural range of speeds exhibited during rainforest locomotion. For example, hunting and long-distance travel may require quick movement [24]. Tall individuals may, therefore, negate the expected speed costs in the forest by maintaining speed via increased step frequency, perhaps in order to avoid a loss of foraging performance. However, such a deviation from the preferred speed-step length relationship would incur a metabolic trade-off that is disproportionately large for taller individuals [10–13].
In sum, our model and results show that, when faced with environmental constraints on step length, individuals reduce speed according to the preferred speed-step length relationship. This adaptive adjustment to the forest environment is likely to compromise time allocation and foraging efficiency in a stature-dependent fashion, although this will need to be explored and confirmed in future work. Future field studies of naturalistic foraging that combine accelerometry, inertial measurement units, and GPS will improve the study of locomotion in the field.
Finally, humans are only one of several large mammal taxa that have evolved short stature in association with rainforests. For example, many forest bovids are much smaller than their open-savannah counterparts (e.g. elephant, buffalo, okapi, and hippopotamus; [27]), and at least one human relative, the Late Pleistocene short-statured hominin Homo floresiensis [1], appears to have experienced evolutionary dwarfism in a rainforest context. The ‘manoeuverability hypothesis' has been proposed to explain small body size among forest bovids compared to their open-living counterparts, suggesting dense habitat would ‘hinder access to food items and escape routes, reduce hunting efficiency, and work against hiding' [27]. Reduced body size may also improve human manoeuverability during pursuit or escape. Regardless, the model presented here could further contribute to directional selection for small body-size phenotypes among legged animals whenever environmental constraints cause size-dependent reductions in locomotor performance. As such, locomotor explanations for dwarfing in animal lineages may represent a general phenomenon with broad ecological and taxonomic relevance.
Acknowledgements
We thank the Batek and Tsimane participants, as well as the Malaysian and Bolivian governments for permission to conduct this research. R. Wrangham, D. Lieberman, M. Singh, and two anonymous reviewers provided helpful comments and suggestions.
Endnote
The ‘human pygmy phenotype’ generally refers to short stature in a population, although precise definitions differ among researchers (e.g. mean adult male stature < 150, 155, or 160 cm; ref. [1]). Here, we follow ref. [1] in using the term ‘pygmy’ to refer to the small body-size phenotype, without reference to cultural, geographic, or genetic traits.
Ethics
All subjects in the study gave informed consent and were compensated for their participation. Procedures were approved by the Dartmouth College IRB (protocol no. 22410), UC-Santa Barbara IRB (protocol no. 3-17-0912), and the government of Malaysia (EPU permit no. 40/200/19/2975).
Data accessibility
The data and analyses for the manuscript may be found at https://github.com/ThomasKraft/constrained_step_length_model.
Authors' contributions
V.V.V. and T.S.K. designed the study; V.V.V., T.S.K., and I.T. performed the experiments; V.V.V., T.S.K., and A.Y. performed analyses; all authors wrote and edited the manuscript.
Competing interests
We declare that we have no competing interests.
Funding
Supported by the National Science Foundation Graduate Research Fellowship Program (V.V.V. and T.S.K.), the ANR Labex IAST (V.V.V.), the American School of Prehistoric Research of Harvard University (V.V.V. and I.J.W.), a Wenner-Gren Foundation Dissertation Fieldwork Grant (IT), and National Institute on Aging R01AG024119 (M.G.).
References
- 1.Perry GH, Dominy NJ. 2009. Evolution of the human pygmy phenotype. Trends Ecol. Evol. 24, 218–225. ( 10.1016/j.tree.2008.11.008) [DOI] [PubMed] [Google Scholar]
- 2.Perry GH, et al. 2014. Adaptive, convergent origins of the pygmy phenotype in African rainforest hunter-gatherers. Proc. Natl Acad. Sci. USA 111, E3596–E3603. ( 10.1073/pnas.1402875111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Herráez D, et al. 2009. Genetic variation and recent positive selection in worldwide human populations: evidence from nearly 1 million SNPs. PLoS ONE 4, e7888 ( 10.1371/journal.pone.0007888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amorim CE, Daub JT, Salzano FM, Foll M, Excoffier L. 2015. Detection of convergent genome-wide signals of adaptation to tropical forests in humans. PLoS ONE 10, e0121557 ( 10.1371/journal.pone.0121557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull CM. 1965. Wayward servants: the two worlds of the African pygmies. Garden City, NY: Natural History Press, American Museum of Natural History. [Google Scholar]
- 6.Diamond JM. 1991. Why are pygmies small? Nature 354, 111–112. ( 10.1038/354111a0) [DOI] [PubMed] [Google Scholar]
- 7.Weyand PG, Smith BR, Puyau MR, Butte NF. 2010. The mass-specific energy cost of human walking is set by stature. J. Exp. Biol. 213, 3972–3979. ( 10.1242/jeb.048199) [DOI] [PubMed] [Google Scholar]
- 8.Kuo AD. 2001. A simple model of bipedal walking predicts the preferred speed–step length relationship. J. Biomech. Eng. 123, 264–266. ( 10.1115/1.1372322) [DOI] [PubMed] [Google Scholar]
- 9.Collins SH, Kuo AD. 2013. Two independent contributions to step variability during over-ground human walking. PLoS ONE 8, e73597 ( 10.1371/journal.pone.0073597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt KG, Hamill J, Andres RO. 1991. Predicting the minimal energy costs of human walking. Med. Sci. Sport. Exer. 23, 491–498. [PubMed] [Google Scholar]
- 11.Minetti AE, Capelli C, Zamparo P, Di Prampero PE, Saibene F. 1995. Effects of stride frequency on mechanical power and energy expenditure of walking. Med. Sci. Sport. Exer. 27, 1194–1202. ( 10.1249/00005768-199508000-00014) [DOI] [PubMed] [Google Scholar]
- 12.Umberger BR, Martin PE. 2007. Mechanical power and efficiency of level walking with different stride rates. J. Exp. Biol. 210, 3255–3265. ( 10.1242/jeb.000950) [DOI] [PubMed] [Google Scholar]
- 13.Zarrugh MY, Radcliffe CW. 1978. Predicting metabolic cost of level walking. Eur. J. Appl. Physiol. O. 38, 215–223. ( 10.1007/BF00430080) [DOI] [PubMed] [Google Scholar]
- 14.Endicott KM, Endicott KL. 2008. The headman was a woman: the gender egalitarian Batek of Malaysia. Long Grove, IL: Waveland Press. [Google Scholar]
- 15.Gurven MH, Kaplan H, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Hooper P. 2017. The Tsimane health and life history project: integrating anthropology and biomedicine. Evol. Anthropol. 26, 54–73. ( 10.1002/evan.21515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kram R, Domingo A, Ferris DP. 1997. Effect of reduced gravity on the preferred walk-run transition speed. J. Exp. Biol. 200, 821–826. [DOI] [PubMed] [Google Scholar]
- 17.Marras WS, Kim JY. 1993. Anthropometry of industrial populations. Ergonomics 36, 371–378. ( 10.1080/00140139308967894) [DOI] [Google Scholar]
- 18.Matthis JS, Yates JL, Hayhoe MM. 2018. Gaze and the control of foot placement when walking in natural terrain. Curr. Biol. 28, 1224–1233. ( 10.1016/j.cub.2018.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram JE, Ruina A. 2001. Multiple walking speed-frequency relations are predicted by constrained optimization. J. Theor. Biol. 209, 445–453. ( 10.1006/jtbi.2001.2279) [DOI] [PubMed] [Google Scholar]
- 20.Bertram JE. 2005. Constrained optimization in human walking: cost minimization and gait plasticity. J. Exp. Biol. 208, 979–991. ( 10.1242/jeb.01498) [DOI] [PubMed] [Google Scholar]
- 21.Schebesta P. 1928. Among the Forest Dwarfs of Malaya. London, UK: Hutchinson and Co. [Google Scholar]
- 22.Evans IHN. 1937. The Negritos of Malaya. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Garvan JM. 1964. The Negritos of the Philippines. Horn and Vienna: Verlag Ferdinand Berger. [Google Scholar]
- 24.Turnbull CM. 1986. Survival factors among Mbuti and other hunters of the equatorial African rainforest. In African pygmies (ed. Cavalli-Sforza LL.), pp. 103–123. Orlando, FL: Academic Press. [Google Scholar]
- 25.Bornstein MH, Bornstein HG. 1976. The pace of life. Nature 259, 557–559. ( 10.1038/259557a0) [DOI] [Google Scholar]
- 26.Pontzer H, Raichlen DA, Wood BM, Mabulla AZP, Racette SB, Marlowe FW. 2012. Hunter-gatherer energetics and human obesity. PLoS ONE 7, e40503 ( 10.1371/journal.pone.0040503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bro-Jørgensen J. 2008. Dense habitats selecting for small body size: a comparative study on bovids. Oikos 117, 729–737. ( 10.1111/j.0030-1299.2008.16069.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and analyses for the manuscript may be found at https://github.com/ThomasKraft/constrained_step_length_model.