Abstract
The integrated contributions of climate and macroevolutionary processes to global patterns of species diversity are still controversial in spite of a long history of studies. The niche conservatism hypothesis and the net diversification rate hypothesis have gained wide attention in recent literature. Many studies have tested these two hypotheses for woody species in humid forests; however, the determinants of species diversity patterns for arid-adapted plants remain largely ignored. Here, using a molecular phylogeny and the global distributions of Zygophyllaceae, a typical arid-adapted plant family, we assessed the effects of contemporary climate and net diversification rates on species diversity patterns in drylands. We found the variables representing water availability to be the best predictors for Zygophyllaceae diversity. Specifically, Zygophyllaceae species diversity significantly decreased with the increase in water availability, probably owing to phylogenetic conservatism of water-related niches. The net diversification rates of Zygophyllaceae accelerated sharply in the recent 10 Myr, coinciding roughly with the period of global aridification. The species diversity of Zygophyllaceae significantly increased with the increase in mean net diversification rates per geographical unit, especially in the Old World, supporting the net diversification rate hypothesis. Our study provides a case exploring climatic and evolutionary mechanisms of dryland species diversity patterns, and suggests that the conservatism in water-related niches and elevated net diversification rates in drylands may have jointly determined the global patterns of dryland species diversity.
Keywords: contemporary climate, drylands, historical processes, macroevolutionary processes, species tolerance, water availability
1. Introductions
Large-scale patterns of species diversity and the mechanisms that underlie them have attracted ecologists and evolutionary biologists for centuries [1]. Previous studies have mainly focused on the diversity–contemporary climate relationships. Several climate-based hypotheses have been proposed to explain species diversity patterns [2]. The contemporary climate hypotheses suggest that regions with high energy (ambient energy hypothesis) [3,4], high water availability (water–energy dynamic hypothesis) [5] or low daily range of temperature and low climate seasonality (climatic variability hypothesis) [6], have high carrying capacities, and are able to sustain large population sizes and thus accumulate more species.
The direct roles of evolutionary processes in shaping species diversity patterns also have long been realized [7,8]. In the last two decades, ecologists have refocused on and hence increasingly explored the effects of macroevolutionary processes on spatial patterns of species diversity [9]. In particular, the net diversification rate hypothesis has attracted wide attention from researchers. According to the net diversification rate hypothesis, regions with high net diversification rates (i.e. high speciation rates and/or low extinction rates) have high species diversity [7,10]. However, few studies have quantitatively evaluated the importance of net diversification rates in regulating species diversity patterns [11,12], even though net diversification rates is one of the proximate determinants of species diversity in a region [7,10]. Furthermore, it remains unclear whether the role of net diversification rates in shaping species diversity differs among biogeographic regions. With the increase in the availability of phylogenetic data and the improvement in analytical methods, it is now possible to test the evolutionary hypotheses, such as the net diversification rate hypothesis.
The relationships between geographical patterns in species diversity and contemporary environment could reflect the effects of evolutionary history on species diversity [13,14]. Recently, the proposed niche conservatism hypothesis [15–17] states that most species tend to maintain their ancestral niches and hence have difficulties evolving new physiological tolerances. Therefore, in regions where environmental conditions deviate from the ancestral niche of a clade, species diversity of that clade tends to be low owing to limited colonization as a result of niche conservatism. By contrast, species diversity of a clade tends to be high in regions where environmental conditions are similar to its ancestral niche [15,16]. For example, several studies have shown that the diversity–contemporary climate relationships differ between groups with different evolutionary histories, which supports the niche conservatism hypothesis [17–19]. However, previous studies have mainly focused on animals and woody species, and have paid more attention to the conservatism of thermal niches, whereas relatively few studies have focused on the conservatism of water-related niches.
Drylands cover approximately 40% of land surface on the Earth and are inhabited by about one-third of the global human population [20,21]. Drylands make a significant contribution to the global economy. About 65% of the drylands are used as rangelands, which support approximately 50% of the world's livestock [20]. Moreover, drylands harbour higher biodiversity than previously regarded [22]. Seven of 25 global biodiversity hotspots [23] and almost 30% of the global centres of plant diversity [24] are in drylands. Yet, previous studies have paid less attention to the species diversity patterns in drylands and the mechanisms underlying them, as compared to tropical forests and other biodiversity hotspots in humid regions [25]. In recent years, a growing number of studies have explored the evolutionary history of arid-adapted plants (e.g. Aizoaceae species in Africa [26]; Agave species in North America [27]; succulent plants [28]), contributing greatly to our understanding of the evolution of arid floras. However, studies integrating evolutionary and ecological mechanisms to explain the geographical patterns in species diversity of arid-adapted plants are still scarce.
Zygophyllaceae, including 22 genera and ca 280 species, is a typical arid-adapted plant family and is distributed in drylands of Africa, Eurasia, Australia, North America and South America with a few species extending to mesic regions [29] (figure 1a). Most Zygophyllaceae species are annuals, perennial herbs or small shrubs, but 26 species (belonging to Larrea, Bulnesia, Porliera and Guaiacum) that are mainly distributed in the New World are shrubs or trees with hard wood. These hardwood species have very slow growth rates and a long lifespan [29]. Zygophyllaceae plants are important components or in some cases, dominant species of the vegetation in drylands (e.g. Larrea tridentata in North American deserts) [30]. Moreover, Zygophyllaceae species also include many rare and endangered species (e.g. Tetraena mongolica in western Inner Mongolia, China [31] and Guaiacum species in the New World [32]). Therefore, studying the global pattern of Zygophyllaceae species diversity and its evolutionary mechanisms could be useful in understanding the role of global drylands on the evolution of arid floras.
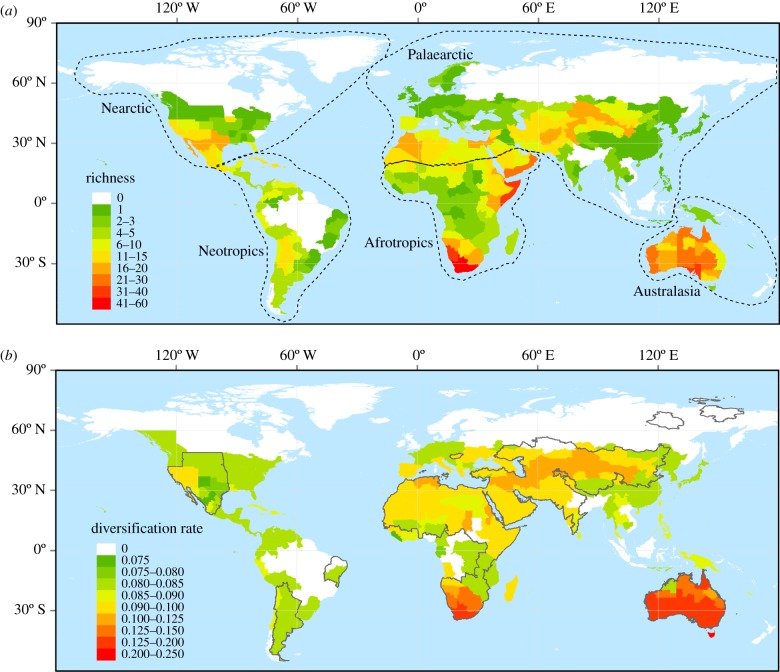
Figure 1.
The global pattern of species diversity (a) and the net diversification rate per geographical unit (b) of Zygophyllaceae species. The dashed lines in (a) represent the boundaries of the five biogeographic regions. See Methods for the division of these biogeographic regions. The black lines in (b) represent the extent of global drylands, which is defined as regions with aridity index less than 0.65.
In this study, we aimed to assess the importance of contemporary climate (through niche conservatism) and evolutionary processes (through net diversification rates) in shaping the present-day diversity patterns of Zygophyllaceae species across the world and across different biogeographic regions. Firstly, we evaluated the effects of net diversification rates on diversity patterns of Zygophyllaceae species. We tested whether there is a positive diversity–net diversification rates relationship as predicted by the net diversification rate hypothesis. Secondly, we evaluated the effects of niche conservatism on Zygophyllaceae diversity. We tested whether there are negative diversity–water availability relationships, positive diversity–energy relationships and positive diversity–climate variability relationships as predicted by the niche conservatism hypothesis [15]. Thirdly, we explored the differences in the primary determinants (net diversification rates and contemporary climate) of species diversity patterns of Zygophyllaceae across different biogeographic regions.
2. Data and methods
(a). Species distribution data
The occurrence data of Zygophyllaceae species were compiled from available published floras, checklists, databases and peer-reviewed journal articles on this family (see the electronic supplementary material, appendix S1 for a complete list of references). Most of the species occurrence data were recorded at the level of administrative divisions (e.g. provinces, states and countries). We geo-referenced these records based on the Global Administrative Areas database (http://www.gadm.org/) by retaining the same geopolitical boundaries (e.g. countries, counties, states, provinces). In order to reduce the effect of area on the estimation of species diversity, we merged small adjacent regions into larger regions so that the areas of different geographical units are close to each other. Finally, the entire land area of the world excluding the Antarctic was divided into 480 geographical units and the average size of these units was ca 270 000 km2 (ca 4° longitude × 4° latitude). This scheme of geographical units has been used in previous studies on the species distributions and species diversity patterns of other groups [19,33]. We combined and standardized the species names from different data sources following The Plant List (http://www.theplantlist.org/, updated to 31 May 2017) which provides accepted Latin names and synonyms for vascular plants and bryophytes. The final distributional database included 3175 occurrence records for 285 Zygophyllaceae species.
(b). Climate data
Climate variables used in this study included three categories, which represent environmental energy (mean annual temperature, MAT; mean annual temperature of coldest quarter, MTCQ; annual potential evapotranspiration, PET), water availability (mean annual precipitation, MAP; water deficit, WD; aridity index, AI) and climate variability (mean diurnal range, MDR; temperature seasonality, TS; precipitation seasonality, PS). See the electronic supplementary material, appendix S2 for more details on the calculation of the climatic variables. In this study, we defined areas with AI of less than 0.65 as drylands following the criterion of World Atlas drylands [34].
The data for MAT, MTCQ, MAP, MDR, TS and PS were obtained from the WorldClim website (www.worldclim.org). The data for PET, AET and AI were obtained from CGIAR-CSI Global PET (www.cgiar-csi.org/data/global-aridity-and-pet-database) and Global Soil Water databases (www.cgiar-csi.org/data/global-high-resolution-soil-water-balance). The spatial resolutions of all these data layers are 30 × 30 arc seconds (ca 1 × 1 km2 at the equator). The value of each climate variable within each geographical unit was calculated as the average of all 1 × 1 km2 grid cells within that unit.
(c). Time calibrated phylogeny
A total of 174 Zygophyllaceae species representing all five subfamilies and 21 out of 22 genera (excepting one monotypic genus Metharme), and two outgroups from Krameriaceae (sister lineage to Zygophyllaceae) were sampled. At the species level, approximately 58% of the currently 285 recognized Zygophyllaceae species were included. We sequenced three chloroplast (rbcL, trnL intron and trnL-F intergenic spacer) and one nuclear (ITS) loci, accounting for a total of 7080 bp of the matrix. Sixteen sequences of the four markers for four species were newly generated in this study, and other sequences are from GenBank (www.ncbi.nlm.nih.gov) (electronic supplementary material, appendix S3). Laboratory procedures, sequence handling and alignments followed Wu et al. [35]. Dated phylogeny of Zygophyllaceae was generated using the Bayesian clock method implemented in BEAST v. 1.8.0 [36]. The best-fit model of nucleotide substitutions for each DNA region was determined by the Akaike information criterion (AIC) in jModelTest v. 2.1.4 [37]: GTR + Γ for ITS and GTR + I + Γ for rbcL, trnL, and trnL-F. Because no appropriate Zygophyllaceae fossil can be used for the calibration of divergence time [38], we adopted the age for the split of Zygophyllaceae and Krameriaceae estimated in broader angiosperm studies as a calibration point. In this study, we used 70 Ma (95% highest posterior density (HPD): 49–88 Ma) estimated by Bell et al. [39] to constrain the stem node of Zygophyllaceae. An uncorrected lognormal relaxed clock model and a birth–death speciation process were used. The Markov chain Monte Carlo (MCMC) searches were run for 50 million generations, sampling every 1000 generations. Effective sample size (ESS) values (greater than 200) were assessed in Tracer v. 1.6 (http://tree.bio.ed.ac.uk/software/tracer). The maximum clade credibility (MCC) tree with mean ages and 95% HPD intervals on nodes was generated using TreeAnnotator v1.8.0.
(d). Estimation of evolutionary rates
Based on the divisions of the global biogeographically realms [40,41] as well as distributions of Zygophyllaceae species, we defined five biogeographic regions: Afrotropics, Australasia, Nearctic, Neotropics, and Palaearctic (including Indo-Malaya) (see figure 1 for the boundaries of these regions). We used the Bayesian Analysis of Macroevolutionary Mixtures (BAMM v. 2.5) [42] to assess the diversification pattern of Zygophyllaceae through time. The parameter globalSamplingFraction was set to be 0.58 following the sampling coverage in the current phylogeny to account for incomplete sampling. This parameter could generate unbiased estimates of evolutionary rates under the assumption that species are missing at random from the phylogeny (BAMM documentation). Total MCMC runs were set to be 10 million generations and parameters were sampled every 1000 generations. The results were analysed using the R package, BAMMtools [42]. ESSs were computed in R package CODA, and the results indicated that all ESS values were greater than 200. In total, 154 Zygophyllaceae species had both distribution information and phylogenetic data. We first plotted the diversification rates through time for all species and for species living in drylands and humid regions separately at the global scale and in the five biogeographic regions. We then extracted the diversification rates of these 154 species and calculated the mean diversification rates within each geographical unit.
At the current stage, it is difficult to sample all Zygophyllaceae species from all over the world. However, such incomplete sampling in the phylogeny may have a weak influence on the temporal and spatial patterns of the mean diversification rates and the subsequent conclusions. Firstly, changes in diversification rates along branches were inferred by BAMM which statistically adds missing species, and to some extent can account for non-random species sampling [43]. Secondly, we also calculated the diversity pattern of the 154 Zygophyllaceae species with phylogenetic information and explored the relationships of the diversity pattern of this reduced dataset with environmental variables and mean net diversification rates per geographical unit. The general trends are consistent with the results based on the full dataset (electronic supplementary material, appendix S4).
(e). Ancestral niche reconstruction and phylogenetic signals of climatic niches
We selected the following dimensions of the climatic niches of Zygophyllaceae species: MAT, MTCQ, PET, MAP, AI, WD, MDR, TS and PS in this study. We used the average value of each climatic variable across all geographical units where a species occurred to represent the climatic niches of this species. We used Blomberg's K [44] to evaluate the phylogenetic signals of the climate niches of Zygophyllaceae species. K values close to zero indicate phylogenetic independence in the evolution of climatic niches. We conducted these analyses using ‘phylosig’ function in the ‘phytools’ package of R [45].
We reconstructed the ancestral climatic niches of Zygophyllaceae species using phylogenetic independent contrast (PIC) and residual maximum likelihood (REML) methods. Both REML and PIC methods are Brownian-motion based estimators. We conducted these analyses using the ‘ace’ function in the ‘ape’ package of R [46].
As the frequency distribution of species diversity per grid cell normally follows Poisson distribution, generalized linear models (GLM) with Poisson residuals were used to test the effects of contemporary climate and evolutionary factors on species diversity pattern of Zygophyllaceae. The proportion of deviance explained by a predictor was used as the R2 of this model and was calculated as: R2 = 1 − residual variance/null variance. We used a modified t-test to test the significance of the GLM coefficients to handle spatial autocorrelations in species diversity which could inflate type I errors and subsequently the significance levels in model tests.
3. Results
(a). Patterns of Zygophyllaceae species diversity
The species diversity of Zygophyllaceae per geographical unit ranged from 1 to 58, with an average of seven species (figure 1a). The Zygophyllaceae species diversity was markedly higher in drylands than in humid regions. The diversity centres were mostly located in the drylands of South Africa and Namibia, eastern and northern Africa, Australia, and Central and Western Asia. By contrast, the species diversity was very low in humid regions such as central Africa, northern America, Europe and the humid parts of South America and Asia (figure 1a). The average species diversity per geographical unit varied significantly among the five biogeographic regions, ranging from 5 to 14. Specifically, Australasia (14 species per unit on average) and the Afrotropics (8) had the highest species diversity per geographical unit on average, followed by the Nearctic (6), while the Neotropics (5) and Palaearctic (5) had the lowest species diversity (figure 1a).
(b). Relationship between Zygophyllaceae diversity and mean net diversification rates
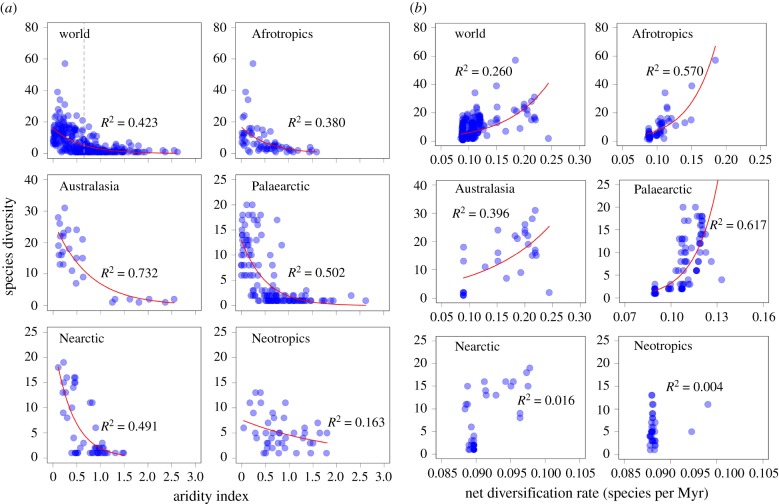
Phylogenetic relationships and divergence times obtained in this study based on four markers (electronic supplementary material, appendix S5) are highly congruent with previous studies [35,47]. The mean net diversification rates per geographical unit significantly varied across the world. The regions with high mean net diversification rates were generally located in drylands (figure 1b). Species diversity per geographical unit significantly increased with the mean net diversification rates at the global scale and in three biogeographic regions (i.e. Palaearctic, Australasia and Afrotropics) (table 1 and figure 2b). Mean net diversification rates per geographical unit explained 19.3–64.6% of the variance in species diversity (table 1 and figure 2a). In the Palaearctic and Afrotropics, mean net diversification rates was the best single predictor of species diversity and its explanatory power was much stronger than that of any contemporary climate variables (table 1).
Table 1.
Explanatory power (R2) of the predictors for the species diversity patterns of Zygophyllaceae evaluated by generalized linear models (GLM) with Poisson residuals. (Regressions were conducted for the world and the five biogeographic regions separately. Symbols in brackets indicate that the relationship between species diversity and the predictor is positive (+) or negative (−). Italics represent the best predictor of species diversity in each region. Asterisks indicate the significance levels of these models. *p < 0.05; **p < 0.01; ***p < 0.001. Variable abbreviations: MAT, mean annual temperature; MTCQ, mean temperature of the coldest quarter; PET, potential evapotranspiration; MAP, mean annual precipitation; WD, water deficit; AI, aridity index; MDR, mean diurnal range of temperature; TS, temperature seasonality; PS, precipitation seasonality; DIV, mean net diversification rate per geographical unit; AGE, mean species age per geographical unit.)
| world | Afrotropics | Australasia | Palaearctic | Nearctic | Neotropics | |
|---|---|---|---|---|---|---|
| MAT (°C) | 3.12 (+) | 16.7 (+) | 0.7 (+) | 1.2 (+) | 33.9 (+) | 14.6 (+) |
| MTCQ (°C) | 1.49 (+) | 33.2 (+)* | 2.7 (+) | 0.0 (+) | 36.1 (+) | 16.8 (+) |
| PET (mm) | 13.6 (+)** | 0.7 (+) | 30.7 (+) | 12.6 (+)* | 59.8 (+)* | 1.4 (+) |
| MAP (mm) | 29.6 (−)*** | 42.7 (−)** | 63.2 (−)* | 46.3 (−)*** | 22.2 (−) | 14.0 (−) |
| WD (mm) | 36.9 (+)*** | 18.7 (+) | 56.8 (+) | 38.1 (+)*** | 67.4 (+)* | 18.9 (+) |
| AI | 42.3 (−)*** | 38.0 (−)* | 73.2 (−) | 50.2 (−)*** | 49.1 (−) | 16.3 (−) |
| MDR (°C) | 29.3 (+)*** | 10.5 (+) | 65.4 (+) | 38.7 (+)*** | 40.7 (+) | 32.4 (+) |
| TS | 1.1 (+) | 12.9 (+) | 61.4 (+) | 8.8 (+) | 13.5 (−) | 20.5 (+) |
| PS | 3.7 (+) | 0.1 (−) | 8.1 (+) | 3.3 (+) | 49.9 (+)* | 13.2 (+) |
| area of geographical units | 7.1 (+)** | 11.3 (+) | 32.4 (+) | 8.2 (+)* | 0.4 (+) | 2.2 (+) |
| DIV | 26.0 (+)*** | 57.0 (+)*** | 39.6 (+) | 61.7 (+)*** | 1.6 (−) | 0.4 (+) |
| AGE | 1.3 (+) | 0.1 (+) | 2.9 (+) | 13.5 (+)** | 37.5 (+)* | 0.9 (+) |
Figure 2.
Zygophyllaceae species diversity as functions of aridity index (a) and mean net diversification rate per geographical unit (b) in the world and the five biogeographic regions. The vertical dashed line in (a) indicates the threshold of aridity index (0.65) for the definition of drylands. Regression lines (red) based on generalized linear models (GLM) with Poisson residuals were drawn when the relationships were significant at p < 0.05.
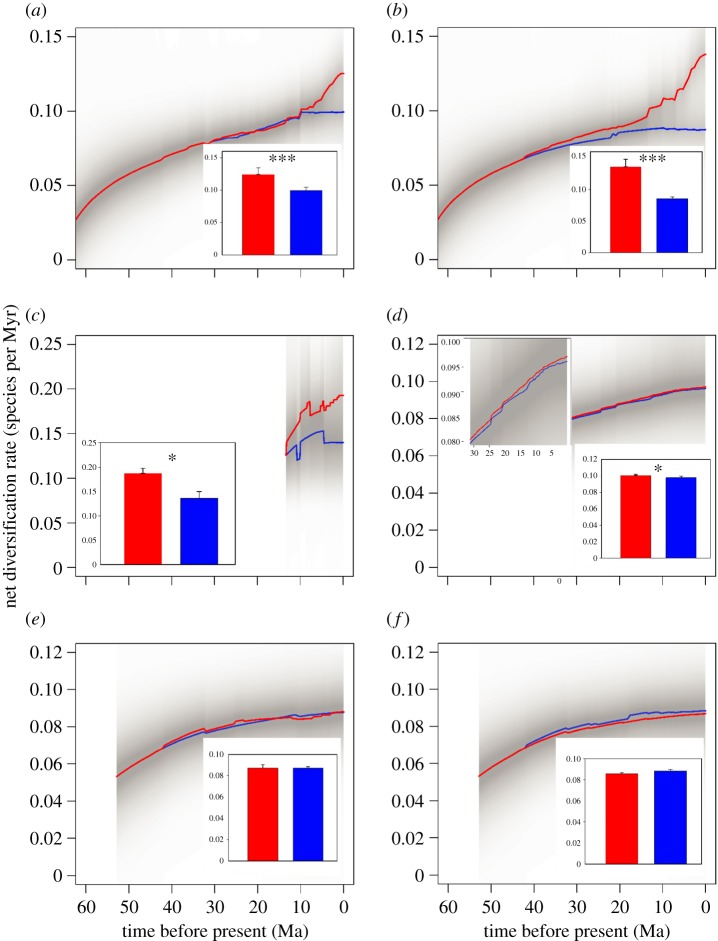
At the global scale and in Australasia and the Afrotropics, the net diversification rates of species living in both drylands and humid regions increased with time. Interestingly, the net diversification rates of dryland species increased abruptly since ca 10–12 Ma, while such increase was not observed for species living in humid regions (figure 3a–c). This led to increasingly higher net diversification rates towards the present for dryland species than for humid-region species. However, in the Palaearctic, Nearctic and Neotropics, the net diversification rates of dryland species was comparable to those in humid regions (figure 3d–f).
Figure 3.
The temporal patterns in the net diversification rate of Zygophyllaceae species living in drylands (red lines) and humid regions (blue lines). The shaded areas surrounding the solid lines represent the 95% confidence intervals of net diversification rate estimations. The inset bar plots show the differences of mean net diversification rate between drylands (red bars) and humid regions (blue bars). The asterisks represent the significance levels of the comparisons: *p < 0.10; ***p < 0.01. (a) World; (b) Afrotropics; (c) Australasia; (d) Palaearctic; (e) Nearctic; (f) Neotropics.
(c). Relationship between Zygophyllaceae diversity and climate
Water-related variables (MAP, WD or AI) were the major climatic determinants of species diversity patterns at the global scale and in four biogeographic regions (except the Neotropics), accounting for 26–77% of the variance in species diversity (table 1). In particular, AI was the primary determinant of Zygophyllaceae species diversity across the world and accounted for 42.3% of the variance in species diversity. The species diversity generally decreased with the increase in water availability at the global scale and in different biogeographic regions (table 1 and figure 2a). By contrast, species diversity increased with the increase in the magnitude of energy-related variables, indicating that the diversity of Zygophyllaceae species is higher in warmer than colder regions. However, the explanatory power of energy-related variables was generally very low except in the Nearctic. The variables representing climate variability (i.e. MDR, TS and PS) were also positively associated with species diversity, indicating that the diversity of Zygophyllaceae species is higher in regions with strong climate variability. MDR explained 10.5–65.4% of the variance in species diversity at the global scale and in the five biogeographic regions. Particularly, MDR was the best single predictor of Zygophyllaceae species diversity in the Neotropics (table 1).
(d). Ancestral climatic niches and niche conservatisms of Zygophyllaceae
The Blomberg's K of three energy related variables (MAT, MTCQ and PET), three water-availability variables (MAP, AI and WD) and three climate variability variables (MDR, TS and PS) ranged between 0.124 and 0.606 and were significantly different from zero (p < 0.001) (table 2), which indicates the presence of significant phylogenetic signals in the climatic niches of extant Zygophyllaceae species. In other words, the phylogenetically more related species tend to have more similar climatic niches.
Table 2.
The reconstructed ancestral climatic niches and the phylogenetic signals in the climatic niches of Zygophyllaceae species. (The ancestral climatic niches were reconstructed by residual maximum likelihood (REML) and phylogenetic independent contrast (PIC) methods, and the phylogenetic signals were evaluated by Blomberg's K [44]. Variable abbreviations: MAT, mean annual temperature; MTCQ, mean temperature of the coldest quarter; PET, potential evapotranspiration; MAP, mean annual precipitation; WD, water deficit; AI, aridity index; MDR, mean diurnal range of temperature; TS, temperature seasonality; PS, precipitation seasonality.)
| ancestral niche |
phylogenetic signal |
|||
|---|---|---|---|---|
| REML | PIC | K | p | |
| MAT (°C) | 12.4 [−9.6, 34.5] | 12.4 [10.9, 13.9] | 0.462 | 1 × 10−4 |
| MTCQ (°C) | 1.8 [−21.7, 25.3] | 1.8 [0.3, 3.3] | 0.606 | 1 × 10−4 |
| PET (mm) | 1268 [1048, 1488] | 1268 [1253, 1283] | 0.471 | 1 × 10−4 |
| MAP (mm) | 326 [106, 546] | 326 [311, 541] | 0.329 | 3 × 10−4 |
| AI | 0.283 [0.006, 0.503] | 0.284 [0.268, 0.298] | 0.243 | 6 × 10−4 |
| WD (mm) | 1192 [979, 1406] | 1192 [1178, 1207] | 0.248 | 1 × 10−4 |
| MDR (°C) | 13.4 [11.1, 15.6] | 13.4 [13.2, 13.5] | 0.233 | 1 × 10−4 |
| TS | 35.8 [13.8, 57.8] | 35.8 [34.3, 37.3] | 0.524 | 1 × 10−4 |
| PS | 0.764 [0.543, 0.984] | 0.764 [0.749, 0.779] | 0.124 | 0.0018 |
The two different methods (i.e. REML and PIC) reconstructed highly consistent ancestral niches of Zygophyllaceae species (table 2). The ancestral thermal niches of Zygophyllaceae reconstructed for MAT, MTCQ, PET, MDR and TS were 12.4°C, 1.8°C, 1268 mm, 13.4°C and 35.8°C, respectively (table 2), which suggest that Zygophyllaceae probably originated in a warm area with a high daily range of temperature and temperature seasonality. The ancestral water-related niches reconstructed for MAP, AI, WD and PS were 326 mm, 0.283, 1192 mm and 0.764 (table 2), respectively, suggesting that Zygophyllaceae probably originated in drylands with high precipitation seasonality. Overall, such climatic condition is closest to the modern ‘hot semi-arid climate’ (BSh) according to the Köppen–Geiger climate classification [48].
4. Discussion
(a). Effects of net diversification rates on spatio-temporal diversity of Zygophyllaceae species
Our analyses showed that net diversification rates of Zygophyllaceae species elevated dramatically after the Mid-Miocene Climatic Optimum (MMCO) (ca 18–14 Ma) [49] when the global climate changed from the MMCO towards cooler temperature [49,50] and higher aridity [51]. The aridification of climate and the expansion of drylands during this period probably facilitated the rapid diversification of Zygophyllaceae (S. D. Wu et al. 2018, unpublished). The expansion of dryland habitats probably provided novel niche space for the colonization and differentiation of Zygophyllaceae species. Ancestral niche reconstruction indicated that the most recent common ancestor of Zygophyllaceae species probably inhabited the semi-arid environment, suggesting that the members of this family were inherently the arid-adapted species. Zygophyllaceae may have already acquired arid-adapted genetic materials before differentiation, which helped them to better survive and adapt to global climatic changes towards more arid climate in the late Miocene.
The fact that dryland species have higher net diversification rates than species living in humid regions could also reflect greater ecological success of Zygophyllaceae species in dry environments. First, Zygophyllaceae species have evolved several functional and morphological traits for adaption to drylands. For example, they usually have: (i) a shallow root system, especially a large number of side roots, which facilitate fast absorption of unpredictable precipitation in drylands [52]; and (ii) small leaves and thick cuticles which reduce the loss of water (e.g. Larrea) [30]. These adaptive traits may facilitate better survival of Zygophyllaceae species in drylands than in humid regions. Beatley [53] found that L. tridentata had lower population density in humid areas than in drylands owing to differences in growing strategies in these two different habitats. Second, the reproduction of Zygophyllaceae species has been found to be significantly limited by water availability. In particular, too much water may be insalubrious to the germination of Zygophyllaceae species. Studies conducted in Northern America [54] and western Erdos, China [55] found that the seed germination rates of L. tridentata and Zygophyllum xanthoxylon peaked at the precipitation of 80–150 mm and at a soil moisture content of 5%, respectively. Moreover, some Zygophyllaceae species (e.g. Z. simplex) are C4 species and succulent species which have large amounts of water storage tissues [56,57]. All these morphological, reproductive and physiological adaptations of Zygophyllaceae species to drought help them better survive the dryland environment, which may lead to increased speciation and/or decreased extinction in drylands.
Geographically, the net diversification rates per geographical unit was higher in drylands than in humid regions (especially in the Afrotropics, the Palaearctic and Australasia). This result differs from previous findings for other groups that net diversification rates is high in humid regions (e.g. the Amazon region [11]; continental margin of Australia [58]; Eastern Asia [33]). More interestingly, we found a positive relationship between the geographical pattern of Zygophyllaceae species diversity and net diversification rates per geographical unit, suggesting that regions with high species diversity are dominated by species with high net diversification rates. This finding is consistent with the prediction of the net diversification rate hypothesis. Similar findings have also been reported for other plant groups (e.g. Arecaceae in the New World [11] and global Rhododendron [33]). Moreover, recent study [12] showed that net diversification rate is also positively correlated with species diversity across clades of salamanders and such relationships are not a statistical artefact. Our results together with previous findings indicate that such positive relationships between species diversity and net diversification rates are consistent across different clades, and provide support for the net diversification rate hypothesis.
(b). The effects of niche conservatism on Zygophyllaceae species diversity
Variables representing water availability were the best predictors for Zygophyllaceae species diversity at the global scale and in most biogeographic regions (except in the Neotropics). Particularly, Zygophyllaceae species diversity significantly decreased with the increase in water availability. A strong negative effect of water availability on Zygophyllaceae species diversity may have been mediated by the evolutionary history of this family. In particular, our results together with previous findings suggest that the negative relationship between species diversity and water availability may be partially owing to the phylogenetic conservatism in the climatic niches of Zygophyllaceae species, especially the water-related niches.
Ancestral niche reconstruction suggests that Zygophyllaceae may have originated in a semi-arid climate (ancestral mean annual precipitation was about 520 mm and AI 0.35). This finding is also supported by previous studies on the origin of Zygophyllaceae. For example, recent biogeographic analyses indicate that Zygophyllaceae may have originated in southern Africa in the early Palaeocene [38]. Moreover, recent palaeoclimate reconstructions showed that the climate in southern Africa was semi-arid during the early Palaeocene and also during the Palaeocene–Eocene thermal maximum when global climate was warm and humid [59]. These findings further support that Zygophyllaceae may have originated in a semi-arid climate.
According to the niche conservatism hypothesis [15,16], species tend to retain their ancestral niche owing to their difficulties in evolving new ecological niches, and hence species diversity would be low in conditions where climate deviates from their ancestral ecological niches. Owing to its origination in a semi-arid climate, Zygophyllaceae species may have evolved fairly strong drought tolerance. The majority of species are, therefore, incapable of surviving in humid regions (e.g. southeast Asia and the Amazon region) where water availability deviates too far from their ancestral dry climate. This may explain why water availability was the best climatic predictor of Zygophyllaceae species diversity. Consistent with our finding, the integrated effects of ancestral niches and niche conservatism on species diversity patterns have also been demonstrated for other plant groups (e.g. Quercus [19]).
MDR explained about 30% (10–65% in different biogeographic regions) of the variance in Zygophyllaceae species diversity (table 1). By contrast, temperature seasonality contributed little to Zygophyllaceae diversity pattern (except in Australasia where temperature seasonality correlated strongly with water availability and MDR). The positive species diversity-MDR relationship may also reflect the effect of niche conservatism. Ancestral climate niche reconstruction showed that Zygophyllaceae species originated in a climate with high MDR (table 2). According to the phenotypic optimality model [60,61], regions with large short-term variations but small long-term variation in temperature favour specialists with narrow thermal ranges. As the diversity pattern of Zygophyllaceae species were more influenced by MDR than by temperature seasonality, we expect Zygophyllaceae species to be thermal specialists. This inference is supported by the findings of the current study, which show that energy-related variables have considerable explanatory power on the variation of Zygophyllaceae species diversity in the Nearctic, the Afrotropics, and Australasia (table 1). The thermal niche of Zygophyllaceae species also showed significant phylogenetic conservatism (table 2), which indicates that the diversity–climate relationships are rooted in their evolutionary histories.
(c). Regional difference in the determinants of species diversity
The relative contributions of net diversification rates and contemporary climate in shaping the species diversity pattern of Zygophyllaceae varied in different biogeographic regions. The mean net diversification rates per geographical unit accounted for more than one third of the variance in the species diversity in the Palaearctic, the Afrotropics and Australasia. Moreover, in the former two regions, the explanatory power of mean net diversification rates was higher than any other contemporary climate variables (table 1). By contrast, the mean net diversification rates did not well explain the variation of Zygophyllaceae species diversity in the New World (i.e. the Nearctic and Neotropics) where contemporary climate dominated the species diversity patterns.
Two reasons may explain the relatively weaker effects of net diversification rates on the New World species diversity than contemporary climate. First, the Nearctic and Neotropics have much less dryland and hence many fewer habitats for Zygophyllaceae species than the Old World. Second, many native Zygophyllaceae species in the New World (e.g. Guaiacum and Larrea species) are slow-growing and long-lived shrubs [62] and have much lower net diversification rates than species in other regions. The low growth rate and long lifespan indicate very low metabolic function and long effective generation time, which may have a depressive effect on both rates of mutation and nucleotide substitution and hence on diversification rates [63,64]. Compared to the species in the New World, most Zygophyllaceae species in the Palaearctic and Afrotropics are herbaceous species with relatively shorter lifespan and tend to have higher speciation and diversification rates. These findings suggest that large areas of drylands and faster diversification in the Old World may have led to stronger effects of net diversification rates on species diversity in these regions.
5. Conclusion
We evaluated the influences of contemporary climate and macroevolutionary processes on the global species diversity pattern of a typical dryland family, Zygophyllaceae. We found that Zygophyllaceae probably originated in a semi-arid climate and the diversification rates of dryland species have accelerated since the mid-Miocene probably owing to global aridification. Owing to the strong phylogenetic conservatism in their water-related niches, water availability dominates the diversity patterns of Zygophyllaceae species at the global scale and in the New World and Australasia. Compared with contemporary climate, net diversification rates dominated the species diversity in the Afrotropics and Palaearctic, supporting the net diversification rate hypothesis. Together these results suggest that the global diversity pattern of Zygophyllaceae species is determined by the integrated effects of both contemporary climate through niche conservatism and macroevolutionary processes. As an important characteristic component of dryland vegetation, the eco-evolutionary mechanisms that underlie the diversity pattern of Zygophyllaceae may, to a large extent, improve our understanding of the effects of global dryland emergence on the evolution of arid floras. Nevertheless, the generality of the conclusions drawn based on this group should be tested using other dryland groups.
Supplementary Material
Acknowledgements
We thank Dr Carsten Rahbek from the University of Copenhagen for providing useful insights.
Data accessibility
The datasets supporting this article are accessible via the electronic supplementary material (electronic supplementary material, appendices S1–S5).
Authors' contributions
Z.W. and Q.W. conceived the idea; Q.W., Z.W., W.W. and S.W. wrote the main text. Q.W. and S.W. performed the analyses. All authors commented on and approved the manuscript.
Competing interests
We declare that we have no competing interests.
Funding
This work was supported by the National Key Research Development Program of China (no. 2017YFA0605101), National Natural Science Foundation of China (no. 31500337, no. 31522012, no. 31470564, no. 31621091, no. 41571499), China Postdoctoral Science Foundation (2016M591008), Chinese Academy of Sciences-Peking University Pioneer Collaboration Team, and the Youth Innovation Promotion Association Foundation of CAS.
References
- 1.Lomolino MV, Riddle BR, Brown JH. 2005. Biogeography, 3rd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Willig M, Kaufman D, Stevens R. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 3.Wright D. 1983. Species-energy theory: an extension of species-area theory. Oikos 41, 496–506. ( 10.2307/3544109) [DOI] [Google Scholar]
- 4.Currie D. 1991. Energy and large-scale patterns of animal- and plant-species richness. Am. Nat. 137, 27–49. ( 10.1086/285144) [DOI] [Google Scholar]
- 5.O'Brien E. 1993. Climatic gradients in woody plant species richness: towards an explanation based on an analysis of southern Africa's woody flora. J. Biogeogr. 20, 181–198. ( 10.2307/2845670) [DOI] [Google Scholar]
- 6.Connell J, Orias E. 1964. The ecological regulation of species diversity. Am. Nat. 98, 399–414. ( 10.1086/282335) [DOI] [Google Scholar]
- 7.Dobzhansky T. 1950. Evolution in the tropics. Am. Sci. 38, 209–221. [Google Scholar]
- 8.Fischer A. 1960. Latitudinal variations in organic diversity. Evolution 14, 64–81. ( 10.1111/j.1558-5646.1960.tb03057.x) [DOI] [Google Scholar]
- 9.Wiens J. 2011. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. 86, 75–96. ( 10.1086/659883) [DOI] [PubMed] [Google Scholar]
- 10.Cardillo M, Orme CD, Owens IP. 2005. Testing for latitudinal bias in diversification rates: an example using new world birds. Ecology 86, 2278–2287. ( 10.1890/05-0112) [DOI] [Google Scholar]
- 11.Svenning J, Borchsenius F, Bjorholm S, Balslev H. 2008. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr. 35, 394–406. ( 10.1111/j.1365-2699.2007.01841.x) [DOI] [Google Scholar]
- 12.Kozak K, Wiens J. 2016. Testing the relationships between diversification, species richness, and trait evolution. Syst. Bot. 65, 975–988. ( 10.1093/sysbio/syw029) [DOI] [PubMed] [Google Scholar]
- 13.Rohde K. 1992. Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65, 514–527. ( 10.2307/3545569) [DOI] [Google Scholar]
- 14.Ricklefs RE. 2006. Evolutionary diversification and the origin of the diversity-environment relationship. Ecology 87, S3–S13. ( 10.1890/0012-9658(2006)87%5B3:EDATOO%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 15.Wiens J, Donoghue M. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644. ( 10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 16.Wiens J, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. ( 10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 17.Harrison S, Grace J. 2007. Biogeographic affinity helps explain productivity-richness relationships at regional and local scales. Am. Nat. 170, 5–15. ( 10.2307/4541087) [DOI] [PubMed] [Google Scholar]
- 18.Buckley L, et al. 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138. ( 10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Wang Z, Rahbek C, Lessard J, Fang J. 2013. Evolutionary history influences the effects of water–energy dynamics on oak diversity in Asia. J. Biogeogr. 40, 2146–2155. ( 10.1111/jbi.12149) [DOI] [Google Scholar]
- 20.Safriel U, et al. 2005. Dryland systems. In Millennium ecosystem assessment (eds Hassan RM, Scholes R, Ash N), pp. 623–662. Washington, DC: Island Press. [Google Scholar]
- 21.Reynolds JF, Smith DM, Lambin EF, Mortimore M, Batterbury SP, Downing TE, Dowlatabadi H, Fernández RJ, Herrick JE. 2007. Global desertification: building a science for dryland development. Science 316, 847–851. ( 10.1126/science.1131634) [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Su X, Shrestha N, Xu X, Wang S, Li Y, Wang Q, Sandanov D, Wang Z. In press. Effects of contemporary environment and Quaternary climate change on drylands plant diversity differ between growth forms. Ecography ( 10.1111/ecog.03698) [DOI] [Google Scholar]
- 23.Myers N, Mittermeier R, Mittermeier C, Fonseca G, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. ( 10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 24.WWF and IUCN (World Wildlife Fund and International Union for Conservation of Nature). 1994. Centers of plant diversity: a guide and strategy for their conservation. Cambridge, UK: IUCN Publications.
- 25.Phillips OL, et al. 2009. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347. ( 10.1126/science.1164033) [DOI] [PubMed] [Google Scholar]
- 26.Klak C, Reeves G, Hedderson T. 2004. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 427, 63–65. ( 10.1038/nature02243) [DOI] [PubMed] [Google Scholar]
- 27.Good-Avila SV, Souza V, Gaut BS, Eguiarte LE. 2006. Timing and rate of speciation in Agave (Agavaceae). Proc. Natl Acad. Sci. USA 103, 9124–9129. ( 10.1073/pnas.0603312103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arakaki M, Christin P, Nyffeler R, Lendel A, Eggli U, Ogburn R, Spriggs E, Moore M, Edwards E. 2011. Contemporaneous and recent radiations of the world's major succulent plant lineages. Proc. Natl Acad. Sci. USA 108, 8379–8384. ( 10.1073/pnas.1100628108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheahan MC. 2007. Zygophyllaceae. In The families and genera of vascular plants (ed. Kubitzki K.), pp. 488–500. Hamburg, Germany: Springer. [Google Scholar]
- 30.Whitford WG. 2002. Ecology of desert systems. San Diego, CA: Academic Press. [Google Scholar]
- 31.Ge X, Hwang C, Liu Z, Huang C, Huang W, Huang K. 2011. Conservation genetics and phylogeography of endangered and endemic shrub Tetraena mongolica (Zygophyllaceae) in Inner Mongolia, China. BMC Genet. 12, 1–12. ( 10.1186/1471-2156-12-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janzen DH. 1988. Tropical dry forest: the most endangered major tropical ecosystem. In Biodiversity (ed. Wilson EO.). pp. 130–137. Washington, DC: National Academy Press. [Google Scholar]
- 33.Shrestha N, et al. 2018. Global patterns of Rhododendron diversity: the role of evolutionary time and diversification rates. Glob. Ecol. Biogeogr. 27, 913–924. ( 10.1111/geb.12750) [DOI] [Google Scholar]
- 34.Middleton NJ, Thomas DSG. 1997. World Atlas of Desertification. New York, NY: United Nations Environment Programme, Edward Arnold.
- 35.Wu SD, Lin L, Li HL, Yu SX, Zhang LJ, Wang W. 2015. Evolution of Asian interior arid-zone biota: evidence from the diversification of Asian Zygophyllum (Zygophyllaceae). PLoS ONE 10, e0138697 ( 10.1371/journal.pone.0138697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973. ( 10.1093/molbev/mss075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256. ( 10.1093/molbev/msn083) [DOI] [PubMed] [Google Scholar]
- 38.Bellstedt D, Galley C, Pirie M, Linder H. 2012. The migration of the palaeotropical arid flora: Zygophylloideae as an example. Syst. Bot. 37, 951–959. ( 10.1600/036364412X656608) [DOI] [Google Scholar]
- 39.Bell C, Soltis D, Soltis P. 2010. The age and diversification of the angiosperms re-revisited. Am. J. Bot. 97, 1296–1303. (doi:10.3732.ajb.0900346) [DOI] [PubMed] [Google Scholar]
- 40.Wallace A. 1877. The geographical distribution of animals: general conclusions. Am. Nat. 11, 157–165. ( 10.1086/271851) [DOI] [Google Scholar]
- 41.Olson D. 2001. Terrestrial ecoregions of the world: a new map of life on earth. Bioscience 51, 933–938. ( 10.1641/0006-3568(2001)051%5B0933:teotwa%5D2.0.co;2) [DOI] [Google Scholar]
- 42.Rabosky DL, Grundler M, Anderson CJ, Title PO, Shi JJ, Brown JW, Huang H, Larson JG. 2014. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707. ( 10.1111/2041-210X.12199) [DOI] [Google Scholar]
- 43.Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 ( 10.1371/journal.pone.0089543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1554/0014-3820(2003)057%5B0717:tfpsic%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 45.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210x.2011.00169.x) [DOI] [Google Scholar]
- 46.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 47.Sheahan M, Chase M. 2000. Phylogenetic relationships within Zygophyllaceae based on DNA sequences of three Plastid regions, with special emphasis on Zygophylloideae. Syst. Bot. 25, 371–384. ( 10.2307/2666648) [DOI] [Google Scholar]
- 48.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. 2006. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 15, 259–263. ( 10.1127/0941-2948/2006/0130) [DOI] [Google Scholar]
- 49.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 50.Holbourn A, Kuhnt W, Clemens S, Prell W, Andersen N. 2013. Middle to late Miocene stepwise climate cooling: evidence from a high-resolution deep water isotope curve spanning 8 million years. Paleoceanography 28, 688–699. ( 10.1002/2013pa002538) [DOI] [Google Scholar]
- 51.Dettman DL, Kohn MJ, Quade J, Ryerson F, Ojha TP, Hamidullah S. 2001. Seasonal stable isotope evidence for a strong Asian monsoon throughout the past 10.7 m.y. Geology 29, 31–34. ( 10.1130/0091-7613(2001)029%3C0031:ssiefa%3E2.0.co;2) [DOI] [Google Scholar]
- 52.Zhou X, Zhou Z, Wu C. 2006. The research of the breeding characters of Zygophyllum xanthoxylum. Pratacult. Sci. 23, 38–41. [Google Scholar]
- 53.Beatley JC. 1974. Effect of rainfall and temperature on the distribution and behavior of Larrea tridentata (Creosote-Bush) in the Mojave Desert of Nevada. Ecology 22, 245–261. ( 10.2307/1935214) [DOI] [Google Scholar]
- 54.Michael G. 1968. Germination requirements of desert shrub Larrea divaricata. Ecology 49, 915–923. ( 10.2307/1936543) [DOI] [Google Scholar]
- 55.Zhang Y, Wang Y. 2010. Reponses of seed germination of four desert species to soil moisture in West Ordos. Bull. Soil Water Conserv. 30, 60–63. (doi:1000-288X(2010)06-0060-04) [Google Scholar]
- 56.Sayed OH. 1996. Adaptational responses of Zygophyllum qatarense Hadidi to stress conditions in a desert environment. J. Arid Environ. 32, 445–452. ( 10.1006/jare.1996.0037) [DOI] [Google Scholar]
- 57.Yang SM, Furukawa I. 2006. Anatomical adaptations of three species of Chinese xerophytes (Zygophyllaceae). J. Forestry Res. 17, 247–251. ( 10.1007/s11676-006-0056-7) [DOI] [Google Scholar]
- 58.Goldie X, Gillman LN, Crisp MD, Wright SD. 2010. Evolutionary speed limited by water in arid Australia. Proc. R. Soc. B 277, 2645–2653. ( 10.1098/rspb.2010.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handley L, Ohalloran A, Pearson P, Hawkins E, Nicholas C, Schouten S, Pancost R. 2012. Changes in the hydrological cycle in tropical East Africa during the Paleocene–Eocene Thermal Maximum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 329, 10–21. ( 10.1016/j.palaeo.2012.02.002) [DOI] [Google Scholar]
- 60.Gilchrist G. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270. ( 10.1086/285797) [DOI] [Google Scholar]
- 61.Chan W, Chen I, Colwell R, Liu W, Huang C, Shen S. 2016. Seasonal and daily climate variation have opposite effects on species elevational range size. Science 351, 1437–1439. ( 10.1126/science.aat9919) [DOI] [PubMed] [Google Scholar]
- 62.Wilson EO, Eisner T. 1968. Lignumvitae—relict island. Nat. Hist. 77, 52–57. [Google Scholar]
- 63.Gillooly JF, Allen AP, West GB, Brown JH. 2005. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc. Natl Acad. Sci. USA 102, 140–145. ( 10.1073/pnas.0407735101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SA, Donoghue MJ. 2008. Rates of molecular evolution are linked to life history in flowering plants. Science 322, 86–89. ( 10.1126/science.1163197) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are accessible via the electronic supplementary material (electronic supplementary material, appendices S1–S5).