Abstract
Variation in life-history strategies has usually been characterized as a single fast–slow continuum of life-history variation, in which mean lifespan increases with age at maturity as reproductive output at each breeding event declines. Analyses of plants and animals suggest that strategies of reproductive timing can vary on an independent axis, with iteroparous species at one extreme and semelparous species at the other. Insectivorous marsupials in the Family Dasyuridae have an unusually wide range of life-history strategies on both purported axes. We test and confirm that reproductive output and degree of iteroparity are independent in females across species. Variation in reproductive output per episode is associated with mean annual rainfall, which predicts food availability. Position on the iteroparity-semelparity axis is not associated with annual rainfall, but species in regions of unpredictable rainfall have longer maximum lifespans, more potential reproductive events per year, and longer breeding seasons. We suggest that these two axes of life-history variation arise because reproductive output is limited by overall food availability, and selection for high offspring survival favours concentrated breeding in seasonal environments. Longer lifespans are favoured when reproductive opportunities are dispersed over longer periods in environments with less predictable food schedules.
Keywords: Dasyuridae, life history, seasonality, fast–slow continuum, iteroparity, semelparity
1. Introduction
Variation between species in schedules of survival, growth, and reproduction is usually considered on one axis of life-history variation from fast to slow [1–3], assuming that trade-offs between age at maturity, fertility, and lifespan constrain life-history strategies, so that species invest most in either reproduction (faster species) or survival (slower species) [4,5]. However, several analyses have suggested that the degree of iteroparity (i.e. breeding repeatedly in a dispersed time period at one end of the spectrum and breeding once in a concentrated time period at the other) is independent of the fast–slow continuum. That is, the degree of iteroparity (number and spacing of reproductive events) is not necessarily traded off with life-history speed (investment in reproduction versus longevity). Stearns [6] found a secondary precociality–altriciality continuum in mammals after accounting for the slow–fast continuum, and Gaillard et al. [7] identified this as part of a semelparity-iteroparity axis that accounts for up to 15% of variation in birds and mammals. In a more recent factor analysis of mammals, Bielby et al. [5] identified a factor that explained up to half of the variance between species, and included maturity, weaning time, and time between reproductive bouts. A second factor explained a further quarter of the variance and described output per reproductive episode. Species at one end produced large litters of small young and species at the other end invested more in large but few young. Bielby et al. [5] interpreted species position on this output axis in terms of the well-known offspring number versus quality trade-off, which is grounded in physiological constraints [8,9]. Salguero-Gomez et al. [10] have recently also demonstrated that the fast–slow continuum in plants includes traits of growth rate and lifespan on one axis and degree of iteroparity on another. Iteroparity is a risk-spreading reproductive tactic and is likely to be an advantage in variable environments. When conditions favouring offspring survival are unpredictable, bet-hedging by repeated breeding increases fitness, given a trade-off between reproduction and survival [11–13]. Bielby et al. [5] called for research to test how the iteroparity and reproductive output axes in mammal life-history variation are associated with environmental variability.
Reproductive output, which includes number and size of offspring, is expected to be constrained by food availability and a female's ability to use energy and nutrients for reproduction [1,14,15]. In marsupial taxa, adoption of higher energy diets has been associated with evolutionary switches to higher reproductive rates. Corresponding trade-offs between reproductive output and other life-history traits have led to the evolution of fast life-history strategies in carnivorous species [16]. Rainfall and temperature seasonality might also influence age-specific survival [17], which determines how species should trade off reproduction and survival [18]. We therefore predict that in carnivorous marsupials from the Family Dasyuridae, which are distributed widely across climate zones, features of climate that increase overall arthropod availability will be correlated with higher reproductive output in terms of litter size per reproductive bout and a faster life history.
Adaptive reproductive timing coincides with events that maximize offspring survival, such as seasonal rainfall and peaks in prey abundance [11,19,20]. For example, the desert chameleon (Furcifer labordi) from Madagascar has evolved semelparity and extended incubation time in an arid, seasonal environment [21]. Similarly, Australian dasyurids with late maturity, monoestry and semelparity occur where there are predictable annual peaks in arthropod abundance, because only one favourable time to wean young per year is possible, given that the marsupial trait of long lactation precludes breeding in the season of an individual's birth [22]. We therefore predict that features of climate that increase food seasonality and the predictability of peaks in prey abundance will be correlated with semelparity in female dasyurids.
Using databases of life-history traits (see the electronic supplementary material for source references), location records in the Australian marsupial Family Dasyuridae, and long-term climate data, we test how female reproductive output, degree of iteroparity, and lifespan covary with food abundance and seasonal predictability of food.
(a). Specific predictions
We hypothesise that reproductive output and degree of iteroparity in females are independent: output will depend on food availability and not food predictability, whereas degree of iteroparity will depend on food predictability and not food availability. We therefore predict that litter size and the number of neonates at birth will covary with the amount of rainfall, and lifespan, length of the annual reproductive season, and the number of reproductive attempts will covary with rainfall predictability.
2. Methods
(a). Study taxa
Dasyurids are predominantly insectivorous, range in size from less than 5 g to 9 kg, and have a maximum lifespan of 1–6 years [23]. The maximum number of young that can be reared is determined by the number of teats, which vary from two to 14. Some groups such as antechinus produce supernumerary young: they give birth to more young than the number of teats, so some inevitably die at birth. Other species produce fewer young than the number of teats [24]. In seasonal breeders, the reproductive season lasts for two weeks to six months, depending on the species. Uniquely in mammals, dasyurid males include the entire spectrum from obligate semelparity to iteroparity. Females can breed multiple times and vary from virtually semelparous to continuous breeding [16,18,25]. Complete male die-off occurs in 20% of dasyurid species (Fisher et al. [22]), including: all in the genera Antechinus, Phascogale, and Dasykaluta [26]; Dasyurus and Parantechinus each contain a single species with facultatively semelparous males [27,28]. Females from some species are monoestrous (i.e. breed once a year), while others are polyoestrous (i.e. can produce multiple litters per season) [29].
(b). Data
We collated published female life-history data on 34 Australian dasyurids taken from 82 studies (table 1; electronic supplementary material). We only included species that are predominantly insectivorous (arthropods are greater than 75% of their diet), because associations between rainfall and arthropod availability have been quantified [22], allowing us to use rainfall as a proxy for food availability [30]. Traits analysed included: body mass at adulthood, maximum lifespan, polyestry versus monoestry, duration of reproductive season (indicating number of possible reproductive attempts), litter size, and number of supernumerary young. Where possible, we used published field studies. Because potential within-breeding episode trade-offs with short-term food supply are likely to be important in dasyurids [30], we used offspring number per reproductive bout rather than a ratio of long-term output over time such as reproductive rate. We used litter sizes recorded within a week of birth, because mothers may progressively lose pouch young during lactation. We calculated mean values for traits when there were multiple studies of the same species. We defined the duration of the reproductive season as the number of weeks with births [31].
Table 1.
Dasyurid species included in this study and the number of published studies data was collated from. PTR (personal trapping records) and PC (personal correspondence).
| Species | No. of studies |
|---|---|
| Antechinomys laniger | 2 |
| Antechinus agilis | 1 and PTR |
| Antechinus bellus | 2 |
| Antechinus flavipes | 3 and PTR |
| Antechinus godmani | 1 and PTR |
| Antechinus leo | 2 |
| Antechinus minimus | 3 |
| Antechinus stuartii | 1 and PTR |
| Antechinus subtropicus | PTR |
| Antechinus mimetes | 2 |
| Dasycercus cristicauda | PC |
| Dasykaluta rosamondae | 3 |
| Dasyuroides byrnei | 2 |
| Dasyurus hallucatus | 3 |
| Dasyurus viverrinus | 2 |
| Ningaui ridei | 3 |
| Parantechinus apicalis | 3 |
| Parantechinus bilarni | 3 |
| Phascogale calura | 2 |
| Phascogale tapoatafa | 4 |
| Planigale gilesi | 3 |
| Planigale ingrami | 3 |
| Planigale maculata | 2 |
| Planigale tenuirostris | 3 |
| Pseudantechinus macdonnellensis | 2 |
| Pseudantechinus ningbing | 1 |
| Sminthopsis crassicaudata | 4 |
| Sminthopsis douglasi | 1 |
| Sminthopsis griseoventer | 1 and PC |
| Sminthopsis leucopus | 3 |
| Sminthopsis macroura | 4 |
| Sminthopsis murina | 2 |
| Sminthopsis ooldea | 2 |
| Sminthopsis virginiae | 2 |
We used rainfall as a proxy for arthropod availability (abundance and activity) [32]. For each species, we used mean annual rainfall at the centroid of geographical range based on all recorded locations [22]. We calculated seasonal predictability of rainfall by collating monthly rainfall from the Bureau of Meteorology [33] at the study sites where life-history information was collected. We gathered these data for the 10 years preceding the end of the study, as Fisher et al. [22] found that 3–8 years of insect and climate data gave repeatable results and clear outcomes in tests of hypotheses at these sites. For each site, we categorized monthly rainfall as ‘high’ if it was in the top 25% of abundances, or ‘low’ if it was in the lower 75% of abundances. We used these categorical data to calculate a Colwell index for rainfall at each site where marsupial life-history data were collected [22]. Colwell's index (P) uses categorical data to measure how tightly an event is linked to a season. P is composed of C (constancy) and M (contingency). Constancy describes how uniform the event is across seasons. Contingency measures the repeatability of seasonal patterns between years. P is maximized when the event occurs constantly throughout the year or if the pattern of occurrence is repeated across years [34].
(c). Statistical analyses
We log-transformed body mass, lifespan, and length of the reproductive season and arcsin-transformed rainfall predictability (P) to normalize the distributions [35]. We used phylogenetic generalized least-squares (PGLS) models in R, using the packages ape [36] and nlme [37] to test the relationships between predictor variables: annual rainfall, rainfall predictability (P), lifespan, length of the reproductive season, and polyestry, and response variables: lifespan, length of the reproductive season, litter size, and supernumerary young, incorporating phylogenetic information and body mass into models. We used a recent marsupial phylogeny [38] to account for interspecific autocorrelation due to phylogeny [39]. We used a multivariate normal prior for the phylogenetic random effects, with unit variances and correlation structure derived from the phylogenetic tree using Grafen's branch lengths [40]. We calculated a pseudo r-squared for each PGLS model [41].
3. Results
(a). Trade-offs and climate predictors of reproductive output
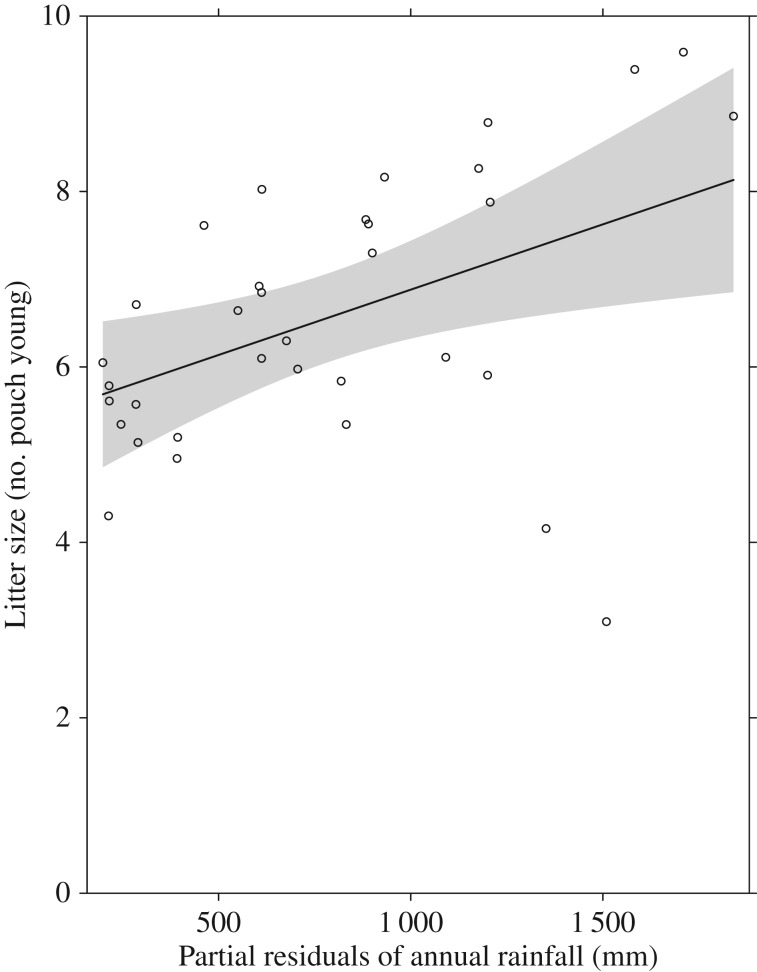
In agreement with our hypothesis that food availability limits reproductive output, species in more arid climates produced fewer young per reproductive bout (litter size versus mean annual rainfall: t = 2.72, p = 0.01, d.f. = 34, slope = 0.001, s.e. = 0.0005; figure 1). Litter size was negatively associated with mass (t = −2.89, p = 0.007, d.f. = 34, slope = −0.68, s.e. = 0.24). Species with larger litters were more likely to have supernumerary young (t = 3.47, p = 0.002, d.f. = 34, slope = 1.64, s.e. = 0.47), and the number of supernumerary young was correlated with annual rainfall (t = 2.14, p = 0.04, d.f. = 34, slope = 358, s.e. = 167), further supporting our prediction that there would be a positive relationship between food availability and reproductive output. Species occurring in Australia's arid and semi-arid zones (less than 350 mm annual rainfall) never had more than seven young, and only one desert species, the kowari (Dasyuroides byrnei), produced any supernumerary young. In agreement with our prediction that reproductive output would not vary with food predictability, litter size was not significantly related to P (rainfall predictability) (t = −0.4, p = 0.69, d.f. = 34, slope = −0.79, s.e. = 2) (pseudo r-squared for model one = 0.32). Litter size was also not associated with traits that indicate the degree of iteroparity in females (litter size versus length of reproductive season: t = −1.85, p = 0.07, d.f. = 34, slope = −0.08, s.e. = 0.04; litter size versus polyestry: t = 1.19, p = 0.24, d.f. = 34, slope = 1.1, s.e. = 0.92) and dasyurids do not trade off litter size against lifespan (litter size versus lifespan: t = −0.04, p = 0.97, d.f. = 34, slope = −0.04, s.e. = 1.03) (pseudo r-squared for model two = 0.25). Litter size of species in our study ranged from four to 10.
Figure 1.
The association between litter size and partial residuals of mean annual rainfall for Australian insectivorous dasyurid species. The line indicates the fitted regression from model one, including 95% confidence intervals.
(b). Trade-offs and climate predictors of the degree of iteroparity
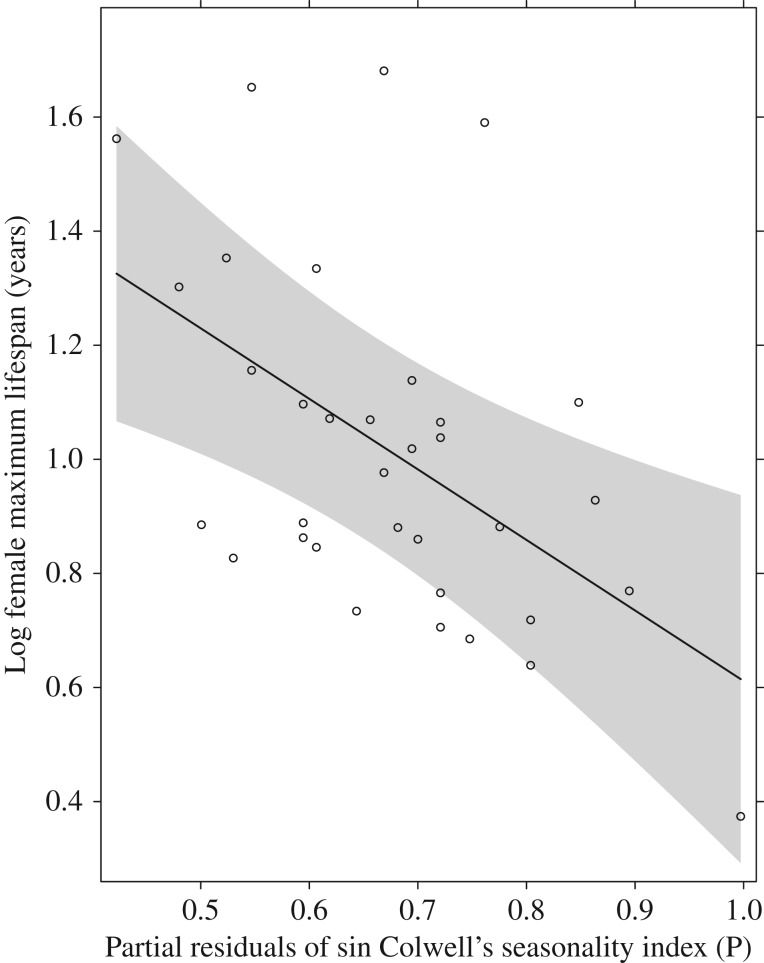
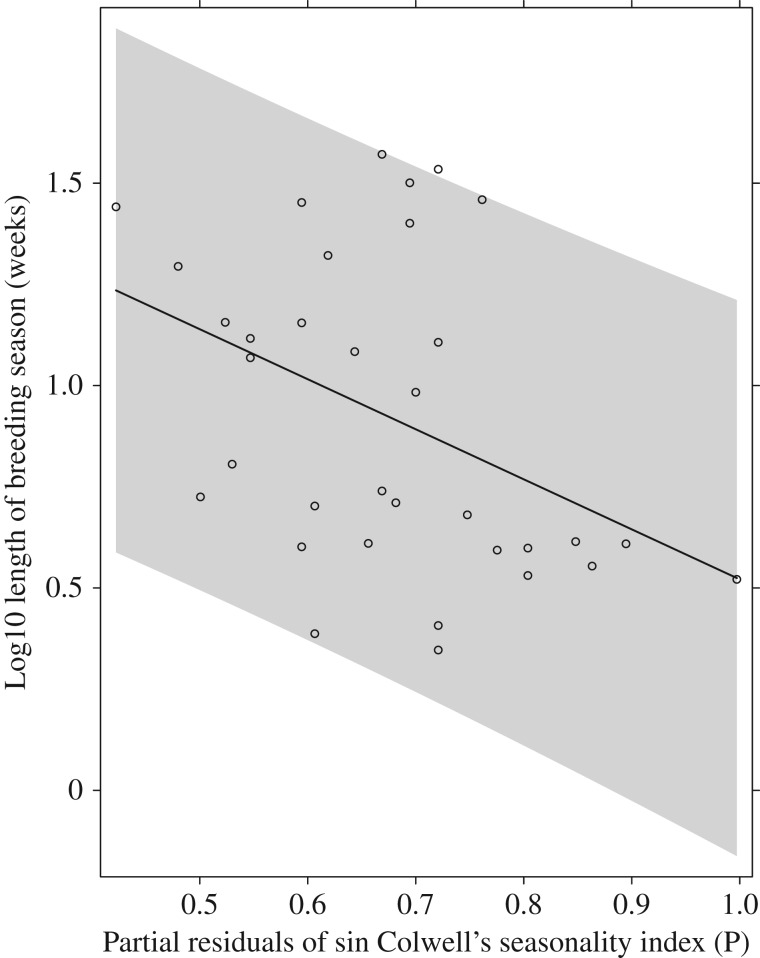
Female maximum lifespan of species in our study ranged from one to six years and were positively associated with mass (t = 2.27, p = 0.03, slope = 0.11, s.e. = 0.05). As predicted, lifespan was longer in areas with more unpredictable food supplies (lifespan versus rainfall predictability index P: t = −3.23, p = 0.003, d.f. = 34, slope = 1.24, s.e. = 0.38, figure 2) (pseudo r-squared for model three = 0.32). Species with long lifespans are more likely to have long reproductive seasons (lifespan versus reproductive season length: t = 4.29, p = 0.0002, d.f. = 34, slope = 0.02, s.e. = 0.005) (pseudo r-squared for model four = 0.38) and to have multiple litters per season (lifespan versus polyestry: t = 2.51, p = 0.02, d.f. = 34, slope = 0.25, s.e. = 0.1) (pseudo r-squared for model five = 0.19). Reproductive season length alone was also strongly associated with rainfall predictability (reproductive season length versus rainfall predictability index P: t = −4.73, p = 0.0001, d.f. = 34, slope = −0.89, s.e. = 0.26, figure 3) (pseudo r-squared for model six = 0.14). This supports our hypothesis that adaptation to a seasonal climate, and therefore predictability of food schedules, favours a short reproductive period in seasonal environments, and a long lifespan with repeat breeding over a long period is more likely to evolve where there is less predictable rainfall. Annual rainfall did not significantly predict rainfall predictability (t = 1.14, p = 0.26, d.f. = 32, slope = 731, s.e. = 643.1), as some regions of arid Australia where dasyurids were sampled have highly predictable rainfall, and some more mesic areas have unpredictable rainfall (figure 4). For example, Ningaui timealeyi (body weight 5.8 g) has a maximum lifespan of one year, and although its Western Australia Pilbara location is a dry environment, summer cyclones are common and most annual rainfalls predictably in February [43]. Planigale gilesi (body weight 6.9 g) in arid western New South Wales is similar in size and ecology but lives for a maximum of five years in a region where low, annual rainfall falls unpredictably across the year [44].
Figure 2.
The association between log female maximum lifespan and partial residuals of sin Colwell's predictability index of rainfall for Australian insectivorous dasyurid species. The line indicates the fitted regression from model three, including 95% confidence intervals.
Figure 3.
The association between log10 length of the breeding season and partial residuals of sin Colwell's predictability index of rainfall for Australian insectivorous dasyurid species. The line indicates the fitted regression from model six, including 95% confidence intervals.
Figure 4.
The centroid point of the geographical range of dasyurid species included in this study and mean annual rainfall throughout Australia. Species are marked with a Δ if Colwell's P is less than 0.7 (less seasonally predictable) and a ○ if Colwell's P is equal to or more than 0.7 (more seasonally predictable). For full species names see table 1. Rainfall raster data were taken from Reside et al. [42].
4. Discussion
Our results agree with several previous analyses, which concluded that aspects of the fast–slow continuum are independent of the semelparity-iteroparity axis in mammals. We focused on offspring number, because previous studies revealing multiple axes of life-history variation in mammals identified reproductive output as a key variable [5,7]. The theory basis of within-bout trade-offs with litter size is well established [15,45]. Experiments and descriptive tests in small eutherian mammals have shown that the trade-off between the number and prenatal growth rate of offspring in a litter is strongly affected by physiological constraints of energy, nutrients, temperature, and tissue capacity. Total investment in reproduction is expected to reduce long-term survival under the disposable soma theory, which states that investment in reproduction reduces individual somatic maintenance [46,47]. In an environment with high extrinsic mortality in adults, organisms should invest in early and high reproductive output rather than long-term maintenance and survival. Position of a species on the fast–slow continuum is therefore expected to depend on aspects of its ecology and environment that affect age-specific mortality risk [48]. Litter size and growth is traded off within each reproductive episode based on maternal investment capacity at the time [9,15]. However, distributing this investment over a longer breeding period does not necessarily change the upper limit on the number of offspring per litter. For example, rate of milk transfer and heat production are mechanisms limiting investment within a reproductive bout [15,45]. Habitats and ecology that cause higher extrinsic mortality risk do not necessarily have higher or lower seasonal predictability of food. If they do, the direction of selection can be reversed. For example, Reznick et al. [49] found that guppies in high predation sites evolved faster reproduction when high predation environments had scarcer food, perhaps because predators indirectly reduced net mortality by reducing density and thus competition for food. In variable environments, organisms that hedge their bets by dispersing reproductive effort over a longer breeding season and have a longer reproductive lifespan have a lower risk of failure [11,45]. Orzack & Tuljapurkar [50] showed that unpredictable environments could favour either high or low reproductive output through their effect on reproductive costs.
In our study, rainfall seasonality was unrelated to annual rainfall. Therefore, aspects of the environment that affect whether iteroparity or semelparity is likely to lead to greater fitness in females are at least partly disconnected from aspects of the environment that affect whether females can invest in large litters and whether mortality risk and reproductive costs are likely to lead to higher fitness in females that increase reproductive effort.
As predicted, a climate variable related to the predictability of peaks in prey abundance (rainfall predictability) was correlated with species position on the semelparity-iteroparity axis, and a variable that alters food availability and reflects energy limitation (annual rainfall) was associated with variation in reproductive output. These findings concord with some previous predictions in mammals and other vertebrates. For example, in the mammal family Leporidae (rabbits and hares) temperature seasonality predicted 71% of the global variation in litter size and body size, and the authors interpreted this in terms of food limitation caused by seasonality. Unpredictable timing of stressful environmental conditions was associated with increased iteroparity, whereas nest predation rate predicted 55% of variation in the timing trait of gestation duration [19]. In endemic mammal families in Madagascar, iteroparity involving short intervals between breeding episodes, a long breeding season, and high adult survival is common, and this has been attributed to the particularly unpredictable timing of rainfall on this island [11]. Comparing desert populations of a ground squirrel on a gradient of increasing seasonal predictability, Whorley [20] also found that more unpredictable rainfall was associated with longer breeding seasons, lower synchrony, and smaller litter size. In Rose's mountain toadlet (Capensibufo rosei), 94% of variation in toad lifespan between years is explained by variation in breeding season rainfall. In dry years, survival is increased and reproductive output is low, and in wet years, toads increase reproduction at the expense of survival [51].
In dasyurids, we found that species with large litters were more likely to occur in high rainfall habitats and to have supernumerary births. Arid zone species rarely had supernumerary young and often failed to have all teats occupied by neonates, suggesting they cannot reliably obtain enough food to produce excess young. We conclude that energy or nutrient availability constrains female reproductive output, consistent with many studies of limitations to reproductive output in small mammals (e.g. [16,52–58]. For example, Sibly & Brown [58] found that mass of mammal neonate tissue was associated with reliable and abundant food. However, seasonality is often also associated with reproductive output, because seasonal environments have a reliable annual pulse of abundant food, especially at high latitudes. For example, offspring number often increases with environmental seasonality in birds [59,60] and mammals, including European lagomorphs [19], boars (Sus scrofa) [61], and ground squirrels (Ammospermophilus leucurus) [20]. Similar trends have not been obvious in carnivorous and Southern Hemisphere mammals at lower latitudes [62,63], with the exception of Antechinus agilis in the relatively low latitude of southern Australia [64]. Unlike seasonal Australian environments, Northern Hemisphere habitats with severe winters have large seasonal peaks in food availability relative to the scarcest season [59].
We found that degree of iteroparity in female dasyurids across the continent was correlated with predictability of rainfall and thus schedules of reliable food availability. Species in environments with seasonally predictable rainfall were more likely to be monoestrous, have shorter lifespans, and shorter reproductive seasons. These species time reproduction so that late lactation, which is energetically costly [56], coincides with the peak in arthropod availability [22]. Female dasyurids in regions of unpredictable rainfall live longer, are more likely to be polyestrous, and have longer reproductive seasons. The opportunity for multiple breeding attempts over several years is likely to be adaptive if survival of young is highly variable [12,65], as a long reproductive period enables bet-hedging, which increases the likelihood of some births during times of high food availability [66]. Bet-hedging strategies occur in plants (desert annuals) [13], bees (Perdita portalis) [67], tortoises (Gopherus agassizii) [68], primates [66], and many other taxa, which spread their reproductive effort over multiple episodes in unpredictable environments [65,69].
Patterns of rainfall explained significant variation in production of young, lifespan, and length of the reproductive season. However, there was still a large proportion of variance unexplained by our models. These effects might be mediated by competition and population density [70], temperature [45], rates of age-specific predation [49,71–73], and torpor [74], which would be promising future avenues for further understanding of the mechanisms.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Simon Blomberg for assistance with R scripting and April Reside for assistance with mapping.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
R.A.C. and D.O.F. created the database. R.A.C. performed the analyses. R.A.C., D.O.F., and A.M.B. contributed to the manuscript. All the authors gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research is supported by the Australian Government's National Environmental Science Program through the Threatened Species Recovery Hub and an Australian Research Council fellowship, Grant/Award no. FTll0100191.
References
- 1.Brown JH, Sibly RM. 2006. Life-history evolution under a production constraint. Proc. Natl Acad. Sci. USA 103, 17 595–17 599. ( 10.1073/pnas.0608522103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey PH. 1989. Life history variation in placental mammals: unifying the data with theory. Oxf. Surv. Evol. Biol. 6, 13–31. [Google Scholar]
- 3.Gaillard J-M, Lemaître J-F, Berger V, Bonenfant C, Devillard S, Douhard M, Gamelon M, Plard F, Lebreton J-D. 2016. Life Histories, Axes of Variation. In Encyclopedia of evolutionary biology, vol. 2 (ed. Kliman RM.), pp. 312–323. Oxford: Academic Press. [Google Scholar]
- 4.Oli MK. 2004. The fast–slow continuum and mammalian life-history patterns: an empirical evaluation. Basic Appl. Ecol. 5, 449–463. ( 10.1016/j.baae.2004.06.002) [DOI] [Google Scholar]
- 5.Bielby J, Mace GM, Bininda-Emonds OR, Cardillo M, Gittleman JL, Jones KE, Orme CD, Purvis A. 2007. The fast-slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 169, 748–757. [DOI] [PubMed] [Google Scholar]
- 6.Stearns SC. 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41, 173–187. ( 10.2307/3544261) [DOI] [Google Scholar]
- 7.Gaillard J-M, Pontier D, Allaine D, Lebreton J, Trouvilliez J, Clobert J. 1989. An analysis of demographic tactics in birds and mammals. Oikos 56, 59–76. ( 10.2307/3566088) [DOI] [Google Scholar]
- 8.Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. ( 10.1086/282929) [DOI] [Google Scholar]
- 9.Rollinson N, Hutchings JA. 2013. Environmental quality predicts optimal egg size in the wild. Am. Nat. 182, 76–90. ( 10.1086/670648) [DOI] [PubMed] [Google Scholar]
- 10.Salguero-Gómez R, Jones OR, Jongejans E, Blomberg SP, Hodgson DJ, Mbeau-Ache C, Zuidema PA, de Kroon H, Buckley YM. 2016. Fast–slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl Acad. Sci. USA 113, 230–235. ( 10.1073/pnas.1506215112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewar RE, Richard AF. 2007. Evolution in the hypervariable environment of Madagascar. Proc. Natl Acad. Sci. USA 104, 13 723–13 727. ( 10.1073/pnas.0704346104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stearns SC. 1992. The evolution of life histories. Oxford: Oxford University Press. [Google Scholar]
- 13.Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88, 1086–1090. ( 10.1890/06-1495) [DOI] [PubMed] [Google Scholar]
- 14.Sibly RM, Brown JH. 2009. Mammal reproductive strategies driven by offspring mortality-size relationships. Am. Nat. 173, E185–EE99. ( 10.1086/598680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Król E, Johnson M, Speakman J. 2003. Limits to sustained energy intake VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 206, 4283–4291. ( 10.1242/jeb.00676) [DOI] [PubMed] [Google Scholar]
- 16.Fisher DO, Owens IP, Johnson CN. 2001. The ecological basis of life history variation in marsupials. Ecology 82, 3531–3540. ( 10.1890/0012-9658(2001)082%5B3531:TEBOLH%5D2.0.CO;2) [DOI] [Google Scholar]
- 17.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. 2009. Fundamental dimensions of environmental risk. Human Nat. 20, 204–268. ( 10.1007/s12110-009-9063-7) [DOI] [PubMed] [Google Scholar]
- 18.Ricklefs RE. 2010. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl Acad. Sci. USA 107, 10 314–10 319. ( 10.1073/pnas.1005862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virgos E, Cabezas-Díaz S, Blanco-Aguiar JA. 2006. Evolution of life history traits in Leporidae: a test of nest predation and seasonality hypotheses. Biol. J. Linnean Soc. 88, 603–610. ( 10.1111/j.1095-8312.2006.00646.x) [DOI] [Google Scholar]
- 20.Whorley JR, Kenagy G. 2007. Variation in reproductive patterns of antelope ground squirrels, Ammospermophilus leucurus, from Oregon to Baja California. J. Mammal. 88, 1404–1411. ( 10.1644/06-MAMM-A-382R.1) [DOI] [Google Scholar]
- 21.Karsten KB, Andriamandimbiarisoa LN, Fox SF, Raxworthy CJ. 2008. A unique life history among tetrapods: an annual chameleon living mostly as an egg. Proc. Natl Acad. Sci. USA 105, 8980–8984. ( 10.1073/pnas.0802468105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher DO, Dickman CR, Jones ME, Blomberg SP. 2013. Sperm competition drives the evolution of suicidal reproduction in mammals. Proc. Natl Acad. Sci. USA 110, 17 910–17 914. ( 10.1073/pnas.1310691110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyck S, Gynther I, Baker A. 2013. Field companion to the mammals of Australia. Sydney, N.S.W: New Holland Publishers. [Google Scholar]
- 24.Ward SJ. 1998. Numbers of teats and pre-and post-natal litter sizes in small diprotodont marsupials. J. Mammal. 79, 999–1008. ( 10.2307/1383108) [DOI] [Google Scholar]
- 25.Charnov EL, Schaffer WM. 1973. Life history consequences of natural selection: Cole's result revisited. Am. Nat. 107, 791–793. ( 10.1086/282877) [DOI] [Google Scholar]
- 26.Krajewski C, Woolley PA, Westerman M. 2000. The evolution of reproductive strategies in dasyurid marsupials: implications of molecular phylogeny. Biol. J. Linnean Soc. 71, 417–435. ( 10.1111/j.1095-8312.2000.tb01267.x) [DOI] [Google Scholar]
- 27.Oakwood M, Bradley AJ, Cockburn A. 2001. Semelparity in a large marsupial. Proc. R. Soc. B 268, 407–411. ( 10.1098/rspb.2000.1369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickman C, Braithwaite R. 1992. Postmating mortality of males in the Dasyurid Marsupials, Dasyurus and Parantechinus. J. Mammal. 73, 143–147. ( 10.2307/1381875) [DOI] [Google Scholar]
- 29.Jones M, Dickman CR, Archer M. 2003. Predators with pouches: the biology of carnivorous marsupials. Collingwood, Melbourne: CSIRO Publishing. [Google Scholar]
- 30.Fisher DO, Blomberg SP. 2011. Costs of reproduction and terminal investment by females in a semelparous marsupial. PLoS ONE 6, e15226 ( 10.1371/journal.pone.0015226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher DO, Nuske S, Green S, Seddon JM, McDonald B. 2011. The evolution of sociality in small, carnivorous marsupials: the lek hypothesis revisited. Behav. Ecol. Sociobiol. 65, 593–605. ( 10.1007/s00265-010-1060-7) [DOI] [Google Scholar]
- 32.Collett RA, Fisher DO. 2017. Time-lapse camera trapping as an alternative to pitfall trapping for estimating activity of leaf litter arthropods. Ecol. Evol. 7, 7527–7533. ( 10.1002/ece3.3275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Climate Data Online: Australian Government. 2016. [cited 2016]. See http://www.bom.gov.au/climate/data/.
- 34.Colwell RK. 1974. Predictability, constancy, and contingency of periodic phenomena. Ecology 55, 1148–1153. ( 10.2307/1940366) [DOI] [Google Scholar]
- 35.Johnson C. 1998. Rarity in the tropics: latitudinal gradients in distribution and abundance in Australian mammals. J. Anim. Ecol. 67, 689–698. ( 10.1046/j.1365-2656.1998.00232.x) [DOI] [Google Scholar]
- 36.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 37.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2018. Linear and Nonlinear Mixed Effects Models.
- 38.May-Collado LJ, Kilpatrick CW, Agnarsson I. 2015. Mammals from ‘down under’: a multi-gene species-level phylogeny of marsupial mammals (Mammalia. Metatheria) . PeerJ 3, e805 ( 10.7717/peerj.805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symonds MR, Blomberg SP. 2014. A primer on phylogenetic generalised least squares. In Modern phylogenetic comparative methods and their application in evolutionary biology. pp. 105–130. Berlin, Germany: Springer. [Google Scholar]
- 40.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. B 326, 119–157. ( 10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 41.Nagelkerke NJ. 1991. A note on a general definition of the coefficient of determination. Biometrika 78, 691–692. ( 10.1093/biomet/78.3.691) [DOI] [Google Scholar]
- 42.Reside AE, VanDerWal J, Kutt AS. 2012. Projected changes in distributions of Australian tropical savanna birds under climate change using three dispersal scenarios. Ecol. Evol. 2, 705–718. ( 10.1002/ece3.197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunlop JN, Saule M. 1982. The habitat and life history of the Pilbara ningaui Ningaui timealeyi. Rec. West. Aust. Mus. 10, 47–52. [Google Scholar]
- 44.Read D. 1995. Gile's planigale. In The Australian museum complete book of Australian mammals (ed. Strahan R.), pp. 107–109. Sydney, Australia: Reed Books. [Google Scholar]
- 45.Speakman JR, Król E. 2010. The heat dissipation limit theory and evolution of life histories in endotherms—time to dispose of the disposable soma theory? Integr. Comp. Biol. 50, 793–807. ( 10.1093/icb/icq049) [DOI] [PubMed] [Google Scholar]
- 46.Selman C, Blount JD, Nussey DH, Speakman JR. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570–577. ( 10.1016/j.tree.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 47.Kirkwood TB, Holliday R. 1979. The evolution of ageing and longevity. Proc. R. Soc. Lond. B 205, 531–546. ( 10.1098/rspb.1979.0083) [DOI] [PubMed] [Google Scholar]
- 48.Healy K, et al. 2014. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B 281, 20140298 ( 10.1098/rspb.2014.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. 2004. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 431, 1095 ( 10.1038/nature02936) [DOI] [PubMed] [Google Scholar]
- 50.Orzack S H, Tuljapurkar S. 2001. Reproductive effort in variable environments, or environmental variation is for the birds. Ecology 82, 2659–2665. ( 10.2307/2679944) [DOI] [Google Scholar]
- 51.Becker FS, Tolley KA, Measey GJ, Altwegg R. 2018. Extreme climate-induced life-history plasticity in an amphibian. Am. Nat. 191, 250–258. ( 10.1086/695315) [DOI] [PubMed] [Google Scholar]
- 52.Nilsen EB, Gaillard JM, Andersen R, Odden J, Delorme D, Van Laere G, Linnell JD. 2009. A slow life in hell or a fast life in heaven: demographic analyses of contrasting roe deer populations. J. Anim. Ecol. 78, 585–594. ( 10.1111/j.1365-2656.2009.01523.x) [DOI] [PubMed] [Google Scholar]
- 53.Clutton-Brock TH. 1984. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 123, 212–229. ( 10.1086/284198) [DOI] [Google Scholar]
- 54.Descamps S, Boutin S, McAdam AG, Berteaux D, Gaillard J-M. 2009. Survival costs of reproduction vary with age in North American red squirrels. Proc. R. Soc. B 276, 1129–1135. ( 10.1098/rspb.2008.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clutton-Brock TH, Guinness FE, Albon SD. 1982. Red deer: behavior and ecology of two sexes. Chicago, IL: University of Chicago press. [Google Scholar]
- 56.Cody ML. 1966. A general theory of clutch size. Evolution 20, 174–184. ( 10.1111/j.1558-5646.1966.tb03353.x) [DOI] [PubMed] [Google Scholar]
- 57.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 58.Sibly RM, Brown JH. 2007. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl Acad. Sci. USA 104, 17 707–17 712. ( 10.1073/pnas.0707725104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103, 458–473. [Google Scholar]
- 60.Badyaev AV, Ghalambor CK. 2001. Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960. ( 10.1890/0012-9658(2001)082%5B2948:EOLHAE%5D2.0.CO;2) [DOI] [Google Scholar]
- 61.Bywater KA, Apollonio M, Cappai N, Stephens PA. 2010. Litter size and latitude in a large mammal: the wild boar Sus scrofa. Mamm. Rev. 40, 212–220. [Google Scholar]
- 62.Lord RD., Jr 1960. Litter size and latitude in North American mammals. Am. Midland Nat. 64, 488–499. ( 10.2307/2422677) [DOI] [Google Scholar]
- 63.Bunnell F, Tait D. 1981. Population dynamics of bears—implications. Dynamics of large mammal populations, pp. 75–98. New York, NY: John Wiley and Sons. [Google Scholar]
- 64.Beckman J, Banks SC, Sunnucks P, Lill A, Taylor AC. 2007. Phylogeography and environmental correlates of a cap on reproduction: teat number in a small marsupial, Antechinus agilis. Mol. Ecol. 16, 1069–1083. ( 10.1111/j.1365-294X.2006.03209.x) [DOI] [PubMed] [Google Scholar]
- 65.Congdon J, Dunham A, Tinkle D. 1982. Energy budgets and life histories of reptiles. In Biology of the Reptilia (eds Gans C, Pough FH), pp. 233–271. New York, NY: Academic Press. [Google Scholar]
- 66.Jones JH. 2011. Primates and the evolution of long, slow life histories. Curr. Biol. 21, R708–RR17. ( 10.1016/j.cub.2011.08.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danforth BN. 1999. Emergence dynamics and bet hedging in a desert bee, Perdita portalis. Proc. R. Soc. B 266, 1985–1994. ( 10.1098/rspb.1999.0876) [DOI] [Google Scholar]
- 68.Lovich JE, Ennen JR, Yackulic CB, Meyer-Wilkins K, Agha M, Loughran C, Bjurlin C, Austin M, Madrak S. 2015. Not putting all their eggs in one basket: bet-hedging despite extraordinary annual reproductive output of desert tortoises. Biol. J. Linnean Soc. 115, 399–410. ( 10.1111/bij.12505) [DOI] [Google Scholar]
- 69.Stearns SC. 1976. Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3–47. ( 10.1086/409052) [DOI] [PubMed] [Google Scholar]
- 70.Gaillard J-M, Festa-Bianchet M, Yoccoz NG. 1998. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 13, 58–63. ( 10.1016/S0169-5347(97)01237-8) [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson GS, South JM. 2002. Life history, ecology and longevity in bats. Aging Cell 1, 124–131. ( 10.1046/j.1474-9728.2002.00020.x) [DOI] [PubMed] [Google Scholar]
- 72.Reznick DA, Bryga H, Endler JA. 1990. Experimentally induced life-history evolution in a natural population. Nature 346, 357 ( 10.1038/346357a0) [DOI] [Google Scholar]
- 73.Ghalambor CK, Martin TE. 2001. Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494–497. ( 10.1126/science.1059379) [DOI] [PubMed] [Google Scholar]
- 74.Turbill C, Bieber C, Ruf T. 2011. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B 278, 3355–3363. ( 10.1098/rspb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.