Abstract
Although the gamete competition theory remains the dominant explanation for the evolution of anisogamy, well-known exceptions to its predictions have raised doubts about the completeness of the theory. One of these exceptions is isogamy in large or complex species of green algae. Here, we show that this exception may be explained in a manner consistent with a game-theoretic extension of the original theory: a constraint on the minimum size of a gamete may prevent the evolution of continuously stable anisogamy. We show that in the volvocine algae, both gametes of isogamous species retain an intact chloroplast, whereas the chloroplast of the microgamete in anisogamous species is invariably degenerate. The chloroplast, which functions in photosynthesis and starch storage, may be necessary to provision a gamete for an extended period when gamete encounter rates are low. The single chloroplast accounts for most of the volume of a typical gamete, and thus may constrain the minimum size of a gamete, preventing the evolution of anisogamy. A prediction from this hypothesis, that isogametes should be larger than the microgametes of similar-size species, is confirmed for the volvocine algae. Our results support the gamete competition theory.
Keywords: anisogamy, isogamy, gamete competition, volvocine algae, chlamydomonadales, chloroplast
1. Introduction
The evolution of dimorphic gametes, or anisogamy, is a topic of intense interest because it includes the evolution of sexes. The dominant theory explaining anisogamy, the gamete competition theory, was proposed by Parker et al. [1] and extended by others [2–5]. The theory posits that with increasing organismal size or complexity there is selection for a larger zygote that is capable of storing sufficient nutrients for rapid postzygotic development. This, in turn, selects for larger gametes, whose combined sizes determine the size of the zygote. The evolution of large gametes provides the opportunity for individuals of one mating type to produce more numerous, smaller gametes and thereby increase their fertility in competition with other members of the same mating type. This opportunity arises because individuals of the other mating type are then forced to produce fewer, even larger, gametes in order to attain a large zygote. Thus, gamete dimorphism evolves as a result of competition among individuals of the same mating type and genetic conflict between mating types.
Predictions from the gamete competition theory have generally been upheld. These have been tested with the green algae (Phylum Chlorophyta), and in particular with the freshwater volvocine algae (Order Chlamydomonadales), a group that exhibits exceptional diversity in adult size and complexity and gamete dimorphism. One prediction, that zygote size (or egg size) should increase with adult size, is upheld for the volvocine algae [6–8]. Another prediction, that the degree of gamete dimorphism should increase with adult size or complexity, is also upheld, but with many exceptions [2,6–10]. The exceptions to this prediction, unicellular species that are anisogamous, even oogamous and colonial and multicellular species that are isogamous, have raised doubts about the completeness of the theory [2,10].
The most complete representation of the theory is a game-theoretic model by Bulmer & Parker [5]. This model extends an earlier game-theoretic implementation of the theory [4] by incorporating gamete viability (survival) as a function of gamete size, in addition to specifying zygote viability as a function of zygote size. The probability of gamete survival, until fusion with another gamete, as a function of gamete size, m, is 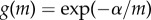 , where α is a positive scaling parameter. Gamete size may affect survival if larger gametes are less at risk of predation or are better provisioned. Similarly, the probability of zygote survival, until development is completed, as a function of zygote size, S, is
, where α is a positive scaling parameter. Gamete size may affect survival if larger gametes are less at risk of predation or are better provisioned. Similarly, the probability of zygote survival, until development is completed, as a function of zygote size, S, is 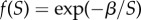 , where
, where 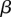 is a positive scaling parameter. These functions are sigmoidal, accelerating from the origin until
is a positive scaling parameter. These functions are sigmoidal, accelerating from the origin until 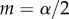 , or
, or 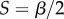 , and then decelerating until reaching the asymptotic value of one. The ancestral condition is likely to have been isogamy with
, and then decelerating until reaching the asymptotic value of one. The ancestral condition is likely to have been isogamy with 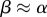 . However,
. However, 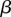 increases with an increase in organismal size or complexity because it takes longer to reach adulthood and therefore the probability of survival to that point decreases for a given zygote size, assuming that larger zygotes are better provisioned and can develop more rapidly. This selects for a larger zygote. The main prediction of the model is that anisogamy is continuously evolutionarily stable when
increases with an increase in organismal size or complexity because it takes longer to reach adulthood and therefore the probability of survival to that point decreases for a given zygote size, assuming that larger zygotes are better provisioned and can develop more rapidly. This selects for a larger zygote. The main prediction of the model is that anisogamy is continuously evolutionarily stable when 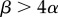 is satisfied. This condition leads to the prediction that for large
is satisfied. This condition leads to the prediction that for large 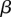 the ratio of the size of the macrogamete, b, to the size of the microgamete, a, the anisogamy ratio, is b/a > 3 [8]. This is upheld for all anisogamous volvocine algae analysed [8].
the ratio of the size of the macrogamete, b, to the size of the microgamete, a, the anisogamy ratio, is b/a > 3 [8]. This is upheld for all anisogamous volvocine algae analysed [8].
The anisogamy ratio also increases with adult size for all anisogamous species of volvocine algae, as predicted by the original theory [7,8]. Interestingly, this pattern includes small, unicellular species that are anisogamous, thus resolving the apparent contradiction to the theory represented by these species. Some unicellular species may benefit from a large zygote because it increases the survival of the dormant zygospore or because it hastens postzygotic growth [2,10]. However, there are many similarly sized unicellular species that are isogamous and, in apparent contradiction to the original theory, larger and more complex species that are also isogamous [7,8]. The game-theoretic model of Bulmer & Parker [5] may explain isogamy in large and complex species by these species requiring large gametes, with large α, such that the condition for stable anisogamy, 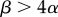 , is violated. The parameter α may be large if, for example, it takes a long time for gametes to encounter one another and fuse, because then larger, better-provisioned gametes may have a higher probability of surviving until fusion.
, is violated. The parameter α may be large if, for example, it takes a long time for gametes to encounter one another and fuse, because then larger, better-provisioned gametes may have a higher probability of surviving until fusion.
Gametes may be large, and thus violate the conditions for stable anisogamy, because of constraints on minimum gamete size, due either to obligate structures that prevent gametes from being smaller or to selection for high viability. Bulmer & Parker [5] suggest that any obligate organelle or structure whose size does not affect viability, such as nuclear chromatin, may play this role. In these cases, the threshold for stable anisogamy will be higher (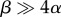 ) because a large proportion of gamete size does not affect viability. Alternatively, the size of the gamete, or some obligate structure, may affect viability such that a gamete must be large to survive long enough to encounter a gamete of the opposite mating type [5]. A clear prediction from this hypothesis is that the gametes of large isogamous species are larger than the microgametes of similarly sized anisogamous species.
) because a large proportion of gamete size does not affect viability. Alternatively, the size of the gamete, or some obligate structure, may affect viability such that a gamete must be large to survive long enough to encounter a gamete of the opposite mating type [5]. A clear prediction from this hypothesis is that the gametes of large isogamous species are larger than the microgametes of similarly sized anisogamous species.
We tested these explanations for isogamy in large and complex volvocine algae. Intact chloroplasts are found in both gametes of isogamous species, but not in the microgametes of anisogamous species. Chloroplasts, or their genomes, which are usually inherited from only the mating-type plus gamete (the macrogamete, or female, in anisogamous species), may be discarded from the mating-type minus gamete (or microgamete). However, chloroplasts may be required for photosynthesis or starch storage in both gametes when gamete encounter rates are low, and thus gametes must survive for extended periods before fusion. This would place a constraint on the minimum size of a gamete and may violate the condition for stable anisogamy in a manner consistent with the gamete competition theory. As predicted, the gametes of large isogamous species are larger than the microgametes of anisogamous species of the same size.
2. Methods
Information was collected on the nature of chloroplasts in gametes of 39 sexual species of volvocine algae (electronic supplementary material, tables S1 and S2). In assigning structural grade, multicellularity (as opposed to coloniality) is defined by the presence of somatic cells. Oogamy is an extreme form of anisogamy, defined by a macrogamete lacking flagella. In some extreme forms of anisogamy, the macrogamete retains flagella but is non-motile. This usually co-occurs with internal fertilization. The presence of a chloroplast in a gamete was either stated in the source text or indicated in a drawing. In the species studied here, there is a single chloroplast in a gamete, if present. Pyrenoids are proteinaceous structures of chloroplasts involved in carbon concentration and are sites of starch storage [11]. Therefore, the presence of pyrenoids was noted. When gametes were described as morphologically indistinguishable from vegetative cells, gametes were assumed to contain the same organelles as vegetative cells.
Data on adult and gamete sizes were also collected for 52 species of sexual volvocine algae (electronic supplementary material, table S3). Protoplasmic volume is the sum of the volumes of cells comprising an adult, excluding extracellular material. Predicted patterns involving gamete and adult volumes were tested using standard statistical methods and phylogenetically independent contrasts based on a composite phylogeny of sexual volvocine algae (electronic supplementary material, figure S1). Details of this phylogeny are given in da Silva [8]. The phylogeny contains 47 species or isolates, 30 of which have data on adult and gamete sizes.
3. Results
(a). Chloroplast
For all 20 isogamous species for which information is available, gametes of both mating types possess an intact chloroplast, typically with at least one pyrenoid (electronic supplementary material, table S1). For all 10 anisogamous species for which information is available for both gametes, the macrogamete possesses an intact, large chloroplast with multiple pyrenoids, while the microgamete possesses a degenerate or reduced chloroplast, often with no pyrenoids (electronic supplementary material, table S2). For an additional eight anisogamous species, information is available for only one of the gametes. For these species, the chloroplast in the macrogamete is intact, while that of the microgamete is degenerate. In the single exception to this pattern, the macrogamete chloroplast of the unicellular Chlamydomonas allensworthii is degraded during gametogenesis to produce a chemoattractant for microgametes [12]. These observations suggest that an intact chloroplast is not necessary for microgamete viability, and may also not be necessary for macrogamete viability. The presence of an intact chloroplast in both gametes of isogamous species suggests that it is necessary for isogamete viability, otherwise the chloroplast would be reduced or degraded as in microgametes. In addition, chloroplasts are large, often taking up most of the volume of a gamete (e.g. [13]). Thus, retaining an intact chloroplast is expected to constrain the minimum size of a gamete.
(b). Anisogamy ratio
Protoplasmic (adult) and gamete volumes are given in the electronic supplementary material, table S3. The ratio of macrogamete volume to microgamete volume, the anisogamy ratio, increases with protoplasmic volume for anisogamous species, as predicted by the gamete competition theory (figure 1). Linear regression of log anisogamy ratio on log protoplasmic volume shows that the slope is significantly greater than zero for anisogamous species (r2 = 0.75; d.f. = 1, 22; F = 65.98; p = 4.58 × 10−8; electronic supplementary material, figure S2). To control for phylogeny, phylogenetically independent contrasts between anisogamous species with the required data were identified from a composite phylogeny of volvocine algae (electronic supplementary material, figure S1). Values for internal nodes were reconstructed by computing means from the two descendent nodes leading to species with the required data. All contrasts were between anisogamous species or clades that were exclusively anisogamous. Thirteen independent contrasts were identified in this way. Eleven of these contrasts have positive slopes of anisogamy ratio on protoplasmic volume, consistent with the prediction and two have slopes of zero (electronic supplementary material, table S4). A sign test shows that the slopes are significantly positive (p = 4.88 × 10−4). This pattern includes unicellular species, indicating that the degree of anisogamy in anisogamous unicellular species is consistent with an increase in anisogamy with body size. However, many large and complex species are isogamous (figure 1), confirming previous reports of isogamy in large and complex species [7,8].
Figure 1.
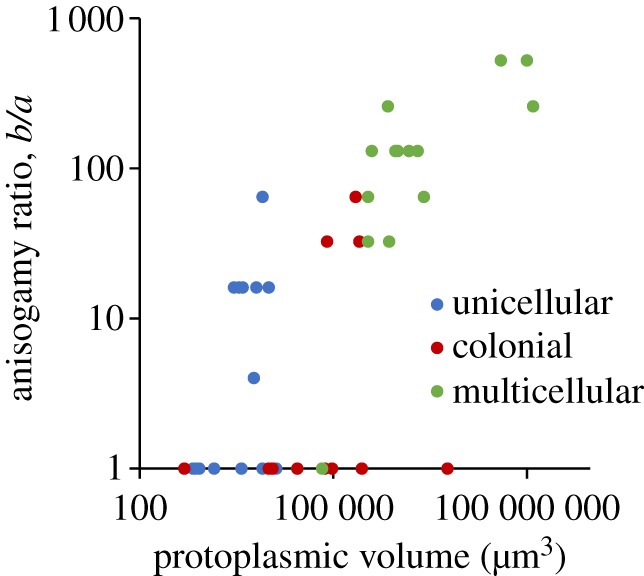
The anisogamy ratio plotted against protoplasmic volume for unicellular, colonial, and multicellular species of volvocine algae.
(c). Isogamete versus microgamete volume
The hypothesis that isogamy in large or complex species is due to a constraint on minimum gamete size predicts that the gametes of isogamous species are larger than the microgametes of anisogamous species of similar size. This prediction was tested by plotting gamete volume against protoplasmic volume separately for unicellular and for colonial or multicellular species (figure 2). Unicellular and colonial or multicellular species were analysed separately because zygote volume increases at a higher rate with protoplasmic volume for unicellular species [7,8]. To control for protoplasmic volume, log gamete volume was regressed on log protoplasmic volume and the residuals were compared between isogametes and microgametes (electronic supplementary material, Results). These residuals were used to compare isogamete and microgamete volumes using phylogenetically independent contrasts. There are only three independent origins of anisogamy in the phylogeny (electronic supplementary material, figure S1), and therefore only three independent contrasts are possible. Each contrast is between residual gamete volumes averaged over at least two species each for an anisogamous clade and for an isogamous clade. Because residuals were calculated independently for unicellular species and colonial and multicellular species, contrasts were made only within each of these two groups. The single contrast for unicellular species is between the anisogamous clade of Oogamochlamys and the isogamous clade of Chlamydomonas in the phylogeny (electronic supplementary material, figure S1; unicellular contrast). The other two contrasts are for colonial and multicellular species. One is between the anisogamous clade containing Colemanosphaera, Platydorina, and closely related Volvox and the isogamous sister clade containing Pandorina and Volvulina (colonial/multicellular contrast 1). The other contrast is between the large anisogamous clade containing Eudorina, Pleodorina, and closely related Volvox and the isogamous clade containing Gonium and Astrephomene (colonial/multicellular contrast 2). Mean residual gamete volumes were compared between isogametes and microgametes for these three contrasts (electronic supplementary material, table S5) using a paired-sample t-test, showing that isogametes (0.0162 ± 0.2516 µm3) are significantly larger than microgametes (–1.4772 ± 0.7779 µm3) (t = 4.8200, d.f. = 2, p = 0.0202).
Figure 2.
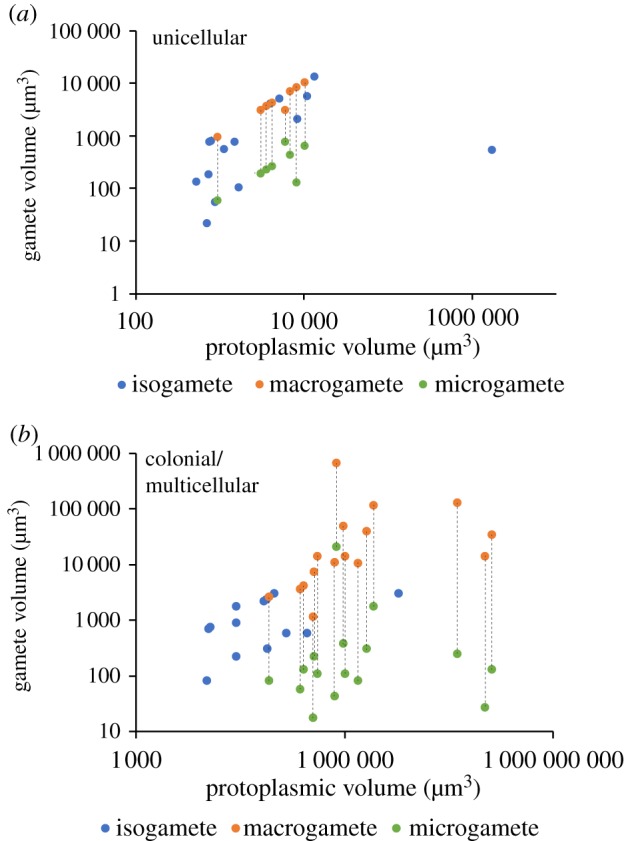
Volumes of isogametes, microgametes, and macrogametes plotted against protoplasmic volume for (a) unicellular species and (b) colonial and multicellular species of volvocine algae. Dashed lines connect the macrogamete and microgamete for each anisogamous species.
4. Discussion
We hypothesize that some large and complex species of volvocine algae retain ancestral isogamy because of a constraint on minimum gamete size. This constraint prevents the evolution of small, numerous gametes by one mating type in response to competition among individuals of that mating type for fusions with gametes of the other mating type. As predicted by this hypothesis, isogametes are larger than microgametes after controlling for adult size. The source of the constraint appears to be the retention of a chloroplast by a gamete. Chloroplasts function in photosynthesis and starch storage and typically occupy most of the volume of a gamete. We reason that a chloroplast is retained when a gamete must be provisioned to survive a long time before encountering a fusion partner. This hypothesis predicts that large and complex species that are isogamous experience low gamete encounter rates. Unfortunately, the ecologies of these species are too poorly known to adequately test this latter hypothesis. Partial support may come from the observation that oogamy is associated with living in pools rather than lakes [10], if smaller pools provide higher gamete encounter rates than larger lakes.
Larger, better-provisioned gametes may have a higher probability of surviving until fusion. For example, the macrogametes of the marine alga Monostroma angicava remain motile for longer than the microgametes [14], presumably because the larger gametes have greater energy reserves. The size of the chloroplast may affect gamete viability through its capacity to store starch or to photosynthesize. This may be the case for the well-studied isogamous unicellular Chlamydomonas reinhardtii, the gamete chloroplast of which contains an abundance of starch, and for which gametes, in their moist soil environment, may need to survive for up to three weeks before fusing [13]. This is an exceptionally long time considering that the vegetative cells may undergo two or three rounds of mitotic cell division every 24 h under optimal conditions [15]. A low gamete encounter rate may increase α in the game-theoretic model of Bulmer & Parker [5] and violate the condition for continuously evolutionarily stable anisogamy, 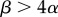 . This condition for stable anisogamy is consistent with anisogamy in the volvocine algae [8].
. This condition for stable anisogamy is consistent with anisogamy in the volvocine algae [8].
A chloroplast, or its genome, is generally inherited only from the mating-type plus gamete, the macrogamete in anisogamous species. Consistent with the maintenance of a functional chloroplast in both gametes of isogamous species, degradation of chloroplast DNA from the mating-type minus gamete occurs only after gamete fusion in the isogamous unicellular C. reinhardtii and Chlamydomonas moewusii [16]. By contrast, in the oogamous multicellular Volvox carteri, digestion of microgamete chloroplast DNA begins before gamete fusion [16]. This pattern is observed in other green algae as well ([17] and references therein).
The uniparental inheritance of chloroplasts and mitochondria has been proposed as an explanation for anisogamy [18]. The argument is that to avoid damaging intracellular conflict between organellar genomes, only one mating type arbitrarily donates the organelle or the organelle's genome. Anisogamy then is a manifestation of such uniparental inheritance, as the gametes of one mating type must be large enough to house the organelles, while the gametes of the other mating type are free to jettison these organelles [19]. The counterargument is that isogamous species also exhibit uniparental inheritance of organelles [20]. Uniparental inheritance may explain why the mating-type minus gamete, which does not contribute organellar genomes to the zygote, evolved to be the microgamete, but it does not explain the evolution of anisogamy. These observations support the view that in order for anisogamy to evolve, the mating-type minus gamete must jettison or reduce the size of, its organelles.
It could be argued that an intact chloroplast, rather than constraining the minimum size of a gamete, is present because the gamete is large enough to house the chloroplast, and isogamy is maintained for some other reason. Then, with isogamy, both gametes would provision the zygote and the chloroplast would be retained for this reason. Consistent with this explanation, the gamete chloroplasts of the unusually isogamous multicellular Astrephomene gubernaculifera and large colonial Volvulina steinii fuse in the newly formed zygote [21]. However, this would also be expected to occur if isogamy is maintained because gametes are selected to be well provisioned, because both gametes must also then provision the zygote.
We consider two alternative hypotheses for isogamy in large or complex volvocine algae that do not require the retention of an intact chloroplast, but neither is well supported. First, there may simply be no selection for anisogamy in these species because there is no gamete competition or gamete limitation [22]. However, this is implausible for microscopic, mostly aquatic algae, with large populations, because gamete competition is expected to occur whenever there is more than one individual of each mating type in a population [22]. Alternatively, large gamete size may be selected because it reduces predation by filter feeders, as has been suggested for adult size in these algae [7]. However, filter feeders can consume cells as large as 10 000 µm3 [7] and nearly all isogametes are below this size (figure 2).
The hypothesis that isogamy is retained in large or complex species when gametes are constrained to be large predicts that isogametes should be larger than microgametes of species of similar adult size. We confirm this prediction for both unicellular species and colonial and multicellular species. This pattern may also be used to refute competing hypotheses that anisogamy evolves in response to gamete limitation rather than competition. These hypotheses argue that anisogamy increases the encounter rate between gametes, when there is gamete limitation, because macrogametes provide a large target and microgametes, being small, and therefore numerous and highly mobile, increase the probability of the target being hit (e.g. [23–25]). As such, these hypotheses generally predict that isogametes should be as small as microgametes in order to maximize the encounter rate [24,25]. Thus these hypotheses explain anisogamy as an evolutionary response to a low gamete encounter rate, whereas we explain the retention of ancestral isogamy as a consequence of a low gamete encounter rate.
It should be noted that a low gamete encounter rate, which we propose to explain the need for well-provisioned gametes, does not preclude gamete competition, because competition occurs within a mating type. That is, regardless of the gamete encounter rate, the relative fitness of an individual of one mating type is the number of fusions to which it contributes compared to others of the same mating type [4,5]. Also, a low gamete encounter rate does not necessarily indicate gamete limitation, as most or all macrogametes may still be fertilized. In any case, if there is gamete competition, anisogamy is expected to evolve in large or complex species regardless of whether there is gamete limitation [22].
Another argument against gamete limitation favouring anisogamy is that the most extreme anisogamy in the volvocine algae is seen in the multicellular Volvox, which are oogamous and have internal fertilization. In these species, numerous microgametes, resulting from multiple gametogenic divisions of a reproductive cell, travel together in a packet and are released from the packet only after entering a coenobium (mature spheroid). The number of microgametes in a packet correlates positively with the number of macrogametes within a coenobium, suggesting that microgamete size is the result of selection to produce enough microgametes to fertilize all of the macrogametes within a coenobium [7], rather than the result of selection to increase the gamete encounter rate. A similar argument applies to internal fertilization in animals, where even weak sperm competition, rather than limitation, explains extreme anisogamy [26]. On its own, gamete limitation appears to be insufficient to explain anisogamy [27], although it may contribute to disruptive selection on gamete size due to gamete competition in highly isolated small spawning groups [22,28].
Previously identified patterns in the green algae that appear to contradict predictions from the gamete competition theory are resolved here. Isogamy in large or complex species may be consistent with the game-theoretic model of Bulmer & Parker [5]. We show that isogametes, in contrast to microgametes, invariably carry an intact chloroplast. We argue that a chloroplast is necessary to provision gametes when gamete encounter rates are low, and that because a chloroplast occupies most of the volume of a gamete, this constrains the minimum gamete size. This minimum size may be large enough to violate the condition for stable anisogamy predicted by the Bulmer & Parker model and shown to be consistent with anisogamy in volvocine algae [8]. As predicted by this hypothesis, isogametes are larger than the microgametes of similar-size species. This pattern also refutes gamete limitation theories, which predict that isogametes should be as small as microgametes. Thus, the gamete competition theory, as extended by Bulmer & Parker [5], is well supported.
Supplementary Material
Acknowledgements
We acknowledge the support of the School of Biological Sciences, University of Adelaide.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
V.L.D. collected the data; J.d.S. conceived, designed, and coordinated the study, analysed the data and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was not funded.
References
- 1.Parker GA, Baker RR, Smith VG. 1972. The origin and evolution of gamete dimorphism and the male-female phenomenon. J. Theor. Biol. 36, 529–553. [DOI] [PubMed] [Google Scholar]
- 2.Bell G. 1978. The evolution of anisogamy. J. Theor. Biol. 73, 247–270. ( 10.1016/0022-5193(78)90189-3) [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth B. 1978. The population genetics of anisogamy. J. Theor. Biol. 73, 347–357. ( 10.1016/0022-5193(78)90195-9) [DOI] [PubMed] [Google Scholar]
- 4.Maynard SJ. 1982. Evolution and the theory of games, viii, p. 224 Cambridge, NY: Cambridge University Press. [Google Scholar]
- 5.Bulmer MG, Parker GA. 2002. The evolution of anisogamy: a game-theoretic approach. Proc. R. Soc. Lond. B 269, 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randerson JP, Hurst LD. 2001. A comparative test of a theory for the evolution of anisogamy. Proc. R. Soc. Lond. B 268, 879–884. ( 10.1098/rspb.2000.1581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell G. 1985. The origin and early evolution of germ cells as illustrated by the Volvocales. In The origin and evolution of Sex (eds Halvarson HO, Monroy A), pp. 221–256. New York, NY: Alan R. Liss, Inc. [Google Scholar]
- 8.da Silva J. 2018. The evolution of sexes: a specific test of the disruptive selection theory. Ecol. Evol. 8, 207–219. ( 10.1002/ece3.3656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowlton N. 1974. A note on the evolution of gamete dimorphism. J. Theor. Biol. 46, 283–285. ( 10.1016/0022-5193(74)90153-2) [DOI] [PubMed] [Google Scholar]
- 10.Madsen JD, Waller DM. 1983. A note on the evolution of gamete dimorphism in algae. Am. Nat. 121, 443–447. ( 10.2307/2461162) [DOI] [Google Scholar]
- 11.Moroney JV, Ynalvez RA. 2007. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot. Cell 6, 1251–1259. ( 10.1128/ec.00064-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenicke L., Starr RC. 1996. The lurlenes, a new class of plastoquinone-related mating pheromones from Chlamydomonas allensworthii (Chlorophyceae). Eur. J. Biochem. 241, 581–585. [DOI] [PubMed] [Google Scholar]
- 13.Martin NC, Goodenough UW. 1975. Gametic differentiation in Chlamydomonas reinhardtii I. Production of gametes and their fine-structure. J. Cell Biol. 67, 587–605. ( 10.1083/jcb.67.3.587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togashi T, Motomura T, Ichimura T. 1997. Production of anisogametes and gamete motility dimorphism in Monostroma angicava. Sex Plant Reprod. 10, 261–268. ( 10.1007/s004970050096) [DOI] [Google Scholar]
- 15.Harris EH. 2009. Introduction to Chlamydomonas and its laboratory use. In The chlamydomonas source book (eds Harris EH, Stern DB, Witman GB), 2nd edn London, UK: Academic Press. [Google Scholar]
- 16.Kuroiwa H, Nozaki H, Kuroiwa T. 1993. Preferential digestion of chloroplast nuclei in sperms before and during fertilization in Volvox carteri. Cytologia 58, 281–291. ( 10.1508/cytologia.58.281) [DOI] [Google Scholar]
- 17.Kagami Y, Mogi Y, Arai T, Yamamoto M, Kuwano K, Kawano S. 2008. Sexuality and uniparental inheritance of chloroplast DNA in the isogamous green alga Ulva compressa (Ulvophyceae). J. Phycol. 44, 691–702. ( 10.1111/j.1529-8817.2008.00527.x) [DOI] [PubMed] [Google Scholar]
- 18.Hoekstra RF. 2011. Nucleo-cytoplasmic conflict and the evolution of gamete dimorphism. In The evolution of anisogamy: A fundamental phenomenon underlying sexual selection (eds Togashi T, Cox PA), pp. 111–130. New York, NY: Cambridge University Press. [Google Scholar]
- 19.Hurst LD, Hamilton WD. 1992. Cytoplasmic fusion and the nature of sexes. Proc. R. Soc. Lond. B 247, 189–194. ( 10.1098/rspb.1992.0027) [DOI] [Google Scholar]
- 20.Maynard SJ, Szathmáry E. 1995. The major transitions in evolution, xiv, p. 346 Oxford, NY: WH. Freeman Spektrum. [Google Scholar]
- 21.Stein JR. 1958. A morphological study of Astrephomene gubernaculifera and Volvulina steinii. Amer. J. Bot. 45, 388–397. ( 10.2307/2439639) [DOI] [Google Scholar]
- 22.Lehtonen J., Kokko H. 2011. Two roads to two sexes: unifying gamete competition and gamete limitation in a single model of anisogamy evolution. Behav. Ecol. Sociobiol. 65, 445–459. ( 10.1007/s00265-010-1116-8) [DOI] [Google Scholar]
- 23.Dusenbery DB. 2011. Gamete encounters. In The evolution of anisogamy: a fundamental phenomenon underlying sexual selection (eds Togashi T, Cox PA), pp. 168–193. Cambridge, MA: Cambridge University Press. [Google Scholar]
- 24.Cox PA, Sethian JA. 1985. Gamete motion, search, and the evolution of anisogamy, oogamy, and chemotaxis. Am. Nat. 125, 74–101. ( 10.2307/2461609) [DOI] [Google Scholar]
- 25.Iyer P, Roughgarden J. 2008. Gametic conflict versus contact in the evolution of anisogamy. Theor. Popul. Biol. 73, 461–472. ( 10.1016/j.tpb.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 26.Parker GA. 1982. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294. [DOI] [PubMed] [Google Scholar]
- 27.Lessells CM, Snook RR, Hosken DJ. 2009. The evolutionary origin and maintenance of sperm: selection for a small, motile gamete mating type. In Sperm biology (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 43–67. London, UK: Academic Press. [Google Scholar]
- 28.Parker GA, Lehtonen J. 2014. Gamete evolution and sperm numbers: sperm competition versus sperm limitation. Proc. R. Soc. B 281, 20140836 ( 10.1098/rspb.2014.0836) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.


