Abstract
The recently extinct Malagasy elephant birds (Palaeognathae, Aepyornithiformes) included the largest birds that ever lived. Elephant bird neuroanatomy is understudied but can shed light on the lifestyle of these enigmatic birds. Palaeoneurological studies can provide clues to the ecologies and behaviours of extinct birds because avian brain shape is correlated with neurological function. We digitally reconstruct endocasts of two elephant bird species, Aepyornis maximus and A. hildebrandti, and compare them with representatives of all major extant and recently extinct palaeognath lineages. Among palaeognaths, we find large olfactory bulbs in taxa generally occupying forested environments where visual cues used in foraging are likely to be limited. We detected variation in olfactory bulb size among elephant bird species, possibly indicating interspecific variation in habitat. Elephant birds exhibited extremely reduced optic lobes, a condition also observed in the nocturnal kiwi. Kiwi, the sister taxon of elephant birds, have effectively replaced their visual systems with hyperdeveloped olfactory, somatosensory and auditory systems useful for foraging. We interpret these results as evidence for nocturnality among elephant birds. Vision was likely deemphasized in the ancestor of elephant birds and kiwi. These results show a previously unreported trend towards decreased visual capacity apparently exclusive to flightless, nocturnal taxa endemic to predator-depauperate islands.
Keywords: computed tomography, Madagascar, recent extinction, Aepyornithidae, fossils
1. Introduction
The recently extinct Malagasy elephant birds (Palaeognathae, Aepyornithiformes) included the largest birds ever discovered. Seven species are recognized across two genera, including the larger, graviportal Aepyornis and the smaller, gracile Mullerornis [1]. The earliest elephant bird fossils are known from the Pleistocene, although recent phylogenetic studies using ancient DNA have estimated that the lineage diverged from its sister taxon, the kiwi, in the mid-Palaeocene [2,3]. Elephant birds have been proposed to be among the dominant terrestrial vertebrates on Madagascar prior to the arrival of humans approximately 2000 years ago and had no known predators [4]. No direct data exist regarding elephant bird foraging or activity patterns but they have generally been considered diurnal herbivores like the New Zealand moa [4–6]. Little else is known about elephant bird biology, representing a crucial gap in our understanding of both the evolutionary history of Palaeognathae and the prehistoric Malagasy ecosystem.
Palaeoneurological investigation can shed light on elephant bird ecology. Relative development of external features of the brain correlates with complexity of associated sensory or cognitive processes, a form–function relationship useful for reconstructing life-history traits of birds (e.g. [7–10]). Palaeognath evolution has been marked by repeated gains of gigantism, flightlessness, island endemism and crepuscularity/nocturnality. Because this group is the sister taxon to all other living birds (Neognathae), investigations of neuroanatomical evolution concomitant with these repeated patterns hold potential to provide insight to early avian brain evolution. Accordingly, the palaeognaths are one of the best-studied bird groups regarding neuroanatomical evolution [6,11–14]. Most attention has been paid to the kiwi, which show extreme neuroanatomical adaptations to nocturnality, including a highly developed olfactory bulb and extremely reduced optic lobes [14–17]. By contrast, little attention has been paid to the sister taxon of kiwi, the highly enigmatic elephant birds.
Wiman & Edinger [18] provided the earliest and only published study of elephant bird brain shape. They described variation in endocranial anatomy across four species, including Aepyornis maximus, A. medius, A. hildebrandti and Mullerornis agilis. The authors noted that the elephant bird olfactory bulbs were large and the optic lobes were greatly reduced relative to overall brain size compared to other ratites. Based on these observations, Wiman & Edinger [18] predicted that vision was deemphasized in elephant birds in favour of enhanced olfactory capacity. The authors further noted that optic lobe development is inversely proportional to body size in elephant birds, with the largest species (A. maximus) exhibiting the smallest lobes relative to overall brain size; Ohashi et al. [19] confirmed this observation. Although Wiman & Edinger [18] provided crucial insights into elephant bird brain morphology, the fidelity of their plaster reconstructions to true brain shape (especially of the olfactory bulbs) likely suffered when the skulls were cut during the construction of their endocranial moulds. Also, recent analyses, some including ancient DNA, provide a new phylogenetic framework within which to compare palaeognath neuroanatomy. Among these new insights is the surprising recovery of elephant birds and kiwi as sister taxa [2,3].
Here, we reinvestigate the neuroanatomy of two elephant bird species, Aepyornis maximus and A. hildebrandti, using high-resolution X-ray computed tomography (CT). CT is a non-destructive source of data useful for digitally constructing casts of the endocranium and associated neurological spaces [8]. First, we redescribe and reinterpret the Aepyornis brain in an evolutionary framework with emphasis on their closest living relatives, the kiwi. We then use phylogenetic comparative methods to investigate the evolution of relative olfactory bulb and optic lobe size across palaeognaths. Finally, we discuss the new insights these data provide to the lifestyle of elephant birds on Madagascar and to palaeognath evolution in general.
2. Methods
(a). Endocast reconstruction and phylogenetic hypothesis
We used high-resolution X-ray computed tomographic data to reconstruct digital endocasts for at least one representative of all major extant and recently extinct palaeognath lineages, including two elephant bird braincases from the National Museum of Natural History, Paris, France, (MNHN F 1910-12 and an uncatalogued MNHN specimen). We also reconstructed the endocasts of a tanager (Passeriformes) and a shorebird (Charadriiformes) as outgroups of analyses of relative optic lobe size. Endocasts were reconstructed in Avizo 9 (FEI) following best practices suggested by Balanoff et al. [20]. Scanning parameters and links to scan data are provided in electronic supplementary material, table S1.
Phylogenetic comparative analyses used the tree and branch lengths from Yonezawa et al. [2] for palaeognaths and from Burleigh et al. [21] for the rest of Aves. Because the latter study did not include elephant birds, we grafted the elephant bird–kiwi–cassowary–emu clade, including branch lengths, from the tree of Yonezawa et al. [2] onto the Burleigh et al. [21] tree. For each analysis, this tree was then pruned of taxa not included in our dataset. All tree files used in this study are provided in electronic supplementary material, tree files 1–4.
(b). Investigating olfactory bulb and optic lobe size among Palaeognathae
To investigate olfactory bulb size evolution across palaeognaths, we compared Bang & Cobb's [22] olfactory ratio (olfactory bulb length relative to cerebral hemisphere length, each measured along the longest axis, regardless of orientation) among our palaeognath sample as well as 88 neognaths taken from Bang & Cobb [22] and the stem palaeognath Lithornis plebius from Zelenitsky et al. [10] using phylogenetic generalized least-squares (GLS) regression and by reconstructing ancestral olfactory ratios. To investigate the relationships between habitat type and olfactory bulb size among palaeognaths, we used one-way ANOVA to test for significant differences in olfactory ratio between open- and forest-dwelling taxa. To investigate optic lobe size evolution, we reconstructed ancestral ratios of surface area of a single optic lobe to total brain surface area among our palaeognath sample with the black-faced grassquit (Tiaris bicolor) and killdeer (Charadrius vociferus, from Smith & Clarke [23]) as neognath outgroups. Measurement data are provided in table 1 and electronic supplementary material, table S2.
Table 1.
Optic lobe, olfactory bulb and brain measurement data for palaeognaths. OLA, optic lobe surface area (cm2); TBA, total brain area (cm2); OptR, ratio of optic lobe surface area to total brain surface area; OBL, olfactory bulb length (longest axis regardless of orientation, cm); CHL, cerebral hemisphere length (longest axis regardless of orientation, cm); OR, olfactory ratio.
| OLA | TBA | OptR | OBL | CHL | OR | ||
|---|---|---|---|---|---|---|---|
| elephant bird | Aepyornis maximus | 1.42 | 119.38 | 0.01 | 1.17 | 4.44 | 0.26 |
| elephant birda | Aepyornis hildebrandti | 1.0 | 4.11 | 0.24 | |||
| southern cassowary | Casuarius casuarius | 2.99 | 81.12 | 0.04 | 1.13 | 4.21 | 0.27 |
| emu | Dromaius novaehollandiae | 2.34 | 52.52 | 0.04 | 0.84 | 3.15 | 0.27 |
| kiwi | Apteryx sp. | 0.24 | 27.01 | 0.01 | 1.2 | 3.5 | 0.34 |
| heavy-footed moa | Pachyornis elephantopus | 1.29 | 65.03 | 0.02 | 0.78 | 3.66 | 0.21 |
| common ostrich | Struthio camelus | 2.58 | 77.09 | 0.03 | 0.69 | 3.91 | 0.18 |
| greater rhea | Rhea americana | 2.08 | 45.06 | 0.05 | 0.74 | 3.34 | 0.22 |
| red-winged tinamou | Rhynchotus rufescens | 1.01 | 12.91 | 0.08 | 0.24 | 1.73 | 0.14 |
| Chilean tinamou | Nothoprocta perdicaria | 0.75 | 8.82 | 0.09 | 0.25 | 1.38 | 0.18 |
| solitary tinamou | Tinamus solitarius | 1.06 | 13.56 | 0.08 | 0.51 | 1.62 | 0.31 |
| brown tinamou | Crypturellus obsoletus | 0.85 | 9.97 | 0.08 | 0.47 | 1.46 | 0.32 |
| lithornithidb | Lithornis plebius | 0.56 | 1.58 | 0.36 |
aOur specimen of A. hildebrandti was missing the dorsal part of the endocranial cavity and so brain area could not be measured.
bData for L. plebius from Zelenitsky et al. [10].
Detailed methods are provided in the electronic supplementary material.
3. Results
(a). Endocast description, comparison and species assignment
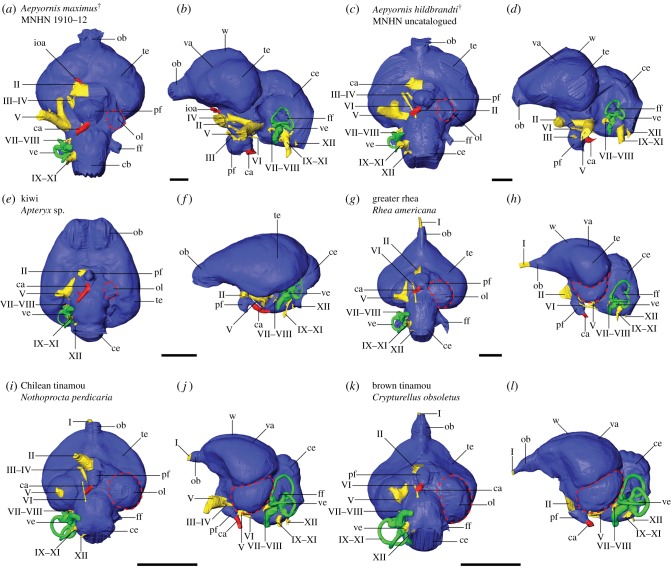
Virtual endocasts of two elephant bird specimens revealed interspecific variation in Aepyornis neuroanatomy. Relative to overall brain size, the larger MNHN F 1910-12 exhibits larger olfactory bulbs, cerebellum and pituitary fossa, as well as smaller optic lobes; they also differ with respect to telencephalon shape, most obviously in dorsal and ventral views (figure 1; electronic supplementary material, figure S1). Taphonomic damage to the skull roof of the uncatalogued MNHN specimen prevents reconstruction of the dorsal part of the endocast and hinders volumetric comparison. The optic lobes in both elephant birds are extremely reduced compared to most other birds (figure 1); in MNHN F 1910-12, they are obsolete as in kiwi, and in the uncatalogued MNHN specimen, they are barely present as in heavy-footed moa (figure 1; electronic supplementary material, figures S1 and S2). MNHN F 1910-12 and the uncatalogued MNHN specimen most resemble Wiman and Edinger's [18] cerebrotypes for A. maximus and A. hildebrandti, respectively, with respect to shape and relative size of the olfactory bulb, cerebral hemispheres, pituitary and optic lobes. These species assignments are used for all subsequent interpretations and discussion.
Figure 1.
Digital endocranial reconstructions of (a,b) elephant bird Aepyornis maximus (MNHN F 1910-12), (c,d) elephant bird Aepyornis hildebrandti, (e,f) kiwi, (g,h) greater rhea, (i,j) the open-dwelling Chilean tinamou and (k,l) the forest-dwelling brown tinamou in (a,c,e,g,i,k) ventral and (b,d,f,h,j,l) left lateral views. Optic lobes are highlighted by dashed lines. Colours: blue, brain; green, inner ear; red, vasculature; yellow, cranial nerves. Abbreviations: I, olfactory nerve; II, optic nerve; III, oculomotor nerve; IV, trochlear nerve; V, trigeminal nerve; VI, abducens nerve; VII–VIII, facial and vestibulocochlear nerves; IX–XI, glossopharyngeal, vagus and accessory nerves; XII, hypoglossal nerve; ca, carotid artery; ce, cerebellum; ff, floccular fossa; ioa, internal ophthalmic artery; ob, olfactory bulb; ol, optic lobe (red dashed outline); pf, pituitary fossa; te, telencephalon; va, vallecula; ve, vestibular organs; w, wulst. Scale bars = 1 cm.
(b). Olfactory bulb size and habitat
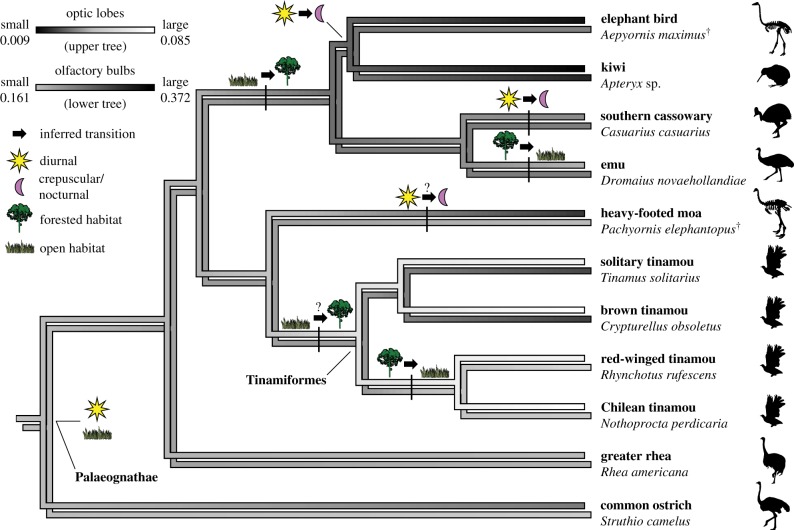
Phylogenetic GLS of olfactory bulb length versus cerebral hemisphere length recovered a positive relationship among both palaeognaths and neognaths, as well as all birds combined (figure 2; electronic supplementary material, results and figure S4). Reconstruction of ancestral olfactory bulb ratios revealed that small olfactory bulbs relative to the cerebral hemispheres are typical among birds and are ancestral for Palaeognathae (figure 3; electronic supplementary material, figures S5–S6 and tables S3–S4). Large olfactory bulbs originated independently in the clade containing elephant birds, kiwi, southern cassowary and emu, as well as among tinamous (figure 3; electronic supplementary material, figures S5–S6). Reconstruction with elephant birds represented by A. maximus rather than A. hildebrandti indicated that the former species retained the large olfactory ratio exhibited by the ancestor it shared with kiwi (figure 3; electronic supplementary material, figure S5). However, reconstruction including A. hildebrandti rather than A. maximus indicated that the former species exhibited a secondarily small olfactory ratio (electronic supplementary material, figure S6). Both phylogenetic and non-phylogenetic ANOVA revealed a significant difference in olfactory ratio between open- and forest-dwelling palaeognaths, regardless of whether A. hildebrandti was treated as occupying grasslands or forests (electronic supplementary material, table S5). Specifically, taxa with large olfactory bulbs occupy forested habitat while those with small bulbs occupy open habitat, except for the emu (figure 2). Emus are diurnal omnivores and occupy open Australian grasslands, despite exhibiting large olfactory bulbs more consistent with a forested habitat. However, large bulbs are exhibited by the closest relatives of emus and are estimated here as ancestral for this group (figure 2; electronic supplementary material, figure S7), suggesting that emus evolved under different foraging conditions than they experience today. The emu lineage is estimated to have first arisen in the Oligocene when Australia was more densely forested than at present [2,24–26]. The most basal divergence within tinamous separates the extant species into generally forest-dwelling and open-area clades [27]. Correspondingly, our sample of tinamous with large bulbs primarily occupies forested habitat and tinamous with small bulbs primarily occupy open habitat (figures 1 and 2). The earliest fossil tinamous are nested within extant clades but occurred at a time when subtropical forests that then characterized southern South America were transitioning into more modern, open environments [27–30]. Thus, it is likely the ancestor of extant tinamous occupied forested rather than open habitat [27]. The heavy-footed moa (Pachyornis elephantopus) exhibits a relatively small olfactory bulb, consistent with an open habitat. Indeed, investigation of DNA recovered from plants in coprolites of four moa species including heavy-footed moa predicted that this species had a diet comprising herbaceous rather than woody plants [31,32]. However, habitat varied across moa species with some occupying primarily forested environments and so it is uncertain which habitat type was ancestral for moa [31–33].
Figure 2.
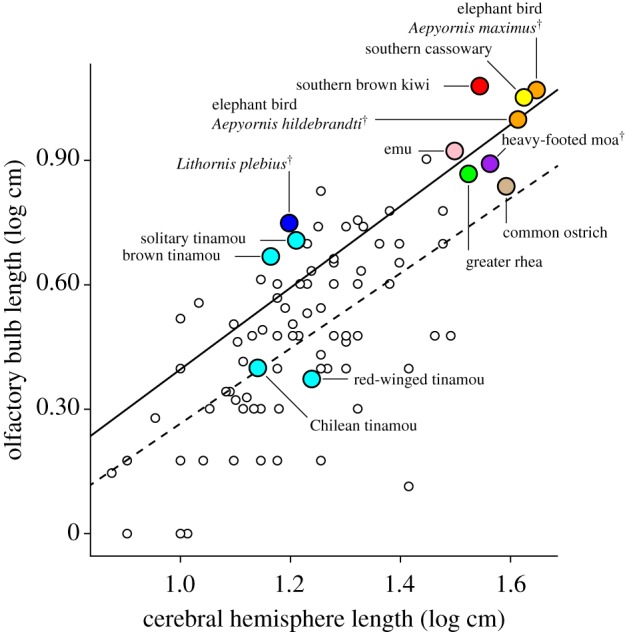
GLS regressions of olfactory ratio (olfactory bulb size versus cerebrum size) for palaeognaths (solid) and neognaths (dashed). Neognath data from Bang and Cobb [22]. Lithornis plebius data from Zelenitsky et al. [10]. Colour legend: orange, elephant birds; red, kiwi; yellow, cassowary; pink, emu; purple, moa; cyan, tinamous; green, rhea; tan, ostrich; blue, lithornithid; white, neognaths.
Figure 3.
Evolution of the sensory ecology of elephant birds and other palaeognaths showing transitions in daily activity patterns (inferred from reconstructions of ancestral relative optic lobe size) and foraging habitat (inferred from reconstructions of ancestral relative olfactory bulb size). Diurnal activity patterns were ancestral for palaeognaths, followed by transition to crepuscularity independently in elephant birds/kiwi, in cassowaries and possibly in some moa. Within the elephant bird–kiwi clade, nocturnality arose independently in the largest elephant birds (Aepyornis) and in kiwi. Open habitat (e.g. grass- and shrubland) was likely ancestral for palaeognaths, followed by transition to forested habitat in the clade including elephant birds, kiwi, cassowaries and emus. Emus likely subsequently transitioned back to open habitat as Australia became deforested leading up to the present. Forested habitat was likely ancestral for tinamous followed by transition back to open habitat in the clade containing the Chilean and red-winged tinamous. Although the heavy-footed moa likely occupied open habitat, other moa taxa occupied forested habitat and it is unclear if open or forested habitat was ancestral for moa. Optic lobe size ancestral state reconstruction (upper tree) is based on electronic supplementary material, figure S7. Olfactory bulb size ancestral state reconstruction (lower tree) is based on electronic supplementary material, figure S5.
(c). Optic lobe size
Reconstruction of ancestral optic lobe/total brain surface area ratios for palaeognaths and two neognath outgroups recovered moderately sized (i.e. emu-sized) optic lobes as ancestral for both the avian crown clade and Palaeognathae (figure 3; electronic supplementary material, figure S7 and table S6). Marked shifts in relative optic lobe size include an increase within tinamous and extreme reduction independently in the elephant bird-kiwi clade (followed by further reduction in each lineage) and in the heavy-footed moa. Because our study sampled only a single moa taxon, it is unclear when reduction occurred among moa.
4. Discussion
Reconstruction of the neuroanatomy of two elephant bird species, Aepyornis maximus and A. hildebrandti, using high-resolution CT, reveals these birds possessed extremely reduced (and apparently obsolete) optic lobes (figure 1), consistent with previous observations based on both physical and digital endocasts [18,19]. Optic lobes are an external feature of the brain corresponding to the optic tectum [9], which is an internal brain region with many sensory and cognitive functions including its role in the tectofugal visual pathway, the dominant of the two major visual pathways in birds (e.g. [34,35]). Among extant birds, extreme reduction of the optic lobes is observed only in nocturnal flightless birds like the parrot kakapo and especially kiwi (figure 1) [14,36]. In kiwi, reduction of the optic lobes corresponds to reduction of the component layers of the tectum and thus reduction of the tectofugal visual pathway [14]. This correlation is consistent with Jerison's [7] principle of proper mass, which predicts that relative development of a brain region corresponds to complexity of associated behaviours. If this correspondence of structural and neurological reduction is also true for elephant birds, the sister taxon to kiwi, then they likely also possessed markedly reduced visual systems and were nocturnal. If additional investigation further supports this hypothesis, it would represent an ironic twist in the evolutionary story of a bird referred to in local Malagasy folklore as vorombazoho, or ‘the bird with keen vision’ [37].
The elephant bird–kiwi clade shows ancestral reduction of the visual system, with extreme reduction of the optic lobes occurring independently in a single clade of elephant birds (Aepyornis) and in kiwi (figure 3). Mullerornis was the other, more gracile clade of elephant birds and also exhibited reduced optic lobes (figure 1) [18], though they were relatively larger than in Aepyornis and kiwi, and closer in size to the kakapo [36]. Although the optic lobes of Aepyornis and kiwi are of similar relative size, kiwi exhibit a suite of additional neuroanatomical and cranial correlates to reduced vision that are not observed in elephant birds, including reduced orbits (and correspondingly reduced eyes) and a wulst that is virtually absent [14]. The wulst is a uniquely avian dorsal projection of the telencephalon and serves as the endpoint of the thalamofugal visual pathway, the lesser of the two major visual pathways in birds [38]. The kiwi visual system has been effectively replaced by sophisticated olfactory [14], somatosensory [39] and auditory [15] systems so completely that blindness has been observed in wild populations of kiwi with no apparent impact on fitness [40]. Elephant birds possessed orbits and wulsts like other palaeognaths, indicating that they may not have been as specialized to nocturnality as kiwi. Despite almost complete loss of their visual systems, the eyes of kiwi are rod-dominated [41], optimizing visual ability in low light, a condition suggested to be remnant of a crepuscular or nocturnal ancestor that relied more heavily on vision [40]; we propose that this crepuscular/nocturnal ancestor was that ancestor shared with elephant birds.
Reduction of vision among birds is an adaptation likely available only to flightless species on predator-depauperate islands. The avian visual system has been proposed to respond to transition to a nocturnal lifestyle either by increased sensitivity or by reduction in favour of other senses [14]. Increased sensitivity characterizes flighted nocturnal birds (e.g. owls, oilbirds, nightjars), which exhibit specializations to manoeuvring and foraging in low-light conditions including rod-dominated retinae, large eyes and hyperdeveloped wulsts [42]. Only flightless nocturnal birds on islands are known to reduce the visual system in favour of other senses, including the elephant birds of Madagascar and the kiwi, kakapo and possibly moa of New Zealand [14,18,36] (figure 3; electronic supplementary material, figures S1–2, S7–8). Reduced visual capacity or blindness has evolved within many non-avian terrestrial vertebrate groups [43–48]; however, only among birds is reduced vision linked to changes in locomotor strategy (i.e. loss of flight). Because birds must navigate a three-dimensional environment when flying, flying ability should greatly influence whether a lineage of nocturnal birds optimizes sensitivity or forgoes vision. It is thus likely that deemphasis of the visual system is an evolutionary pathway available only to flightless taxa, like elephant birds and kiwi. Indeed, the recently extinct Hawaiian waterfowl Talpanas lippa is hypothesized to have been both flightless and nocturnal based on its extremely reduced visual system as well as hindlimb proportions, possibly further supporting this pattern [49]. The rod-dominated eyes of kiwi suggest that reduction of vision followed an initial stage of increased sensitivity [44]. Thus, increased sensitivity to low light and reduced visual capacity may represent an evolutionary sequence rather than alternative nocturnal strategies.
Though sister taxa, the tinamous and moa included in our study show markedly different trajectories with respect to relative optic lobe size (figure 3). The heavy-footed moa exhibited reduced optic lobes, on par with the kakapo and Mullerornis and consistent with nocturnal or crepuscular activity. Ashwell & Scofield [6] investigated the palaeoneuroanatomy of eight moa species, including the heavy-footed moa, and predicted a diurnal activity pattern based on relatively small olfactory bulbs and a well-developed wulst. However, the nocturnal kakapo exhibits a well-developed wulst [36]. Our findings suggest that small olfactory bulbs may be more related to foraging in an open habitat than with activity pattern (figures 2 and 3). Whether optic lobe reduction is unique to the heavy-footed moa or if it is indicative of an ancestral condition is unclear. Ashwell & Scofield [6] reported that moa optic lobes are generally relatively smaller than in other ratites, and so it is possible that reduced visual capacity, and possibly nocturnality, is ancestral for the moa total group or arises within moa. Robust taxonomic sampling within moa is needed to further test this hypothesis. Tinamous, in contrast with moas, exhibit large optic lobes compared with total brain size reconstructed as an increase within the clade relative to outgroups. The biological significance of this apparent increase in relative optic lobe size is unclear, though it is not directly related to habitat type, as both open- and forest-dwelling tinamous exhibit relatively large lobes. Though comparatively little attention has been paid to the sensory systems in tinamous, our results suggest that such investigation may uncover previously unrecognized specialization in the tinamou visual system.
Palaeognaths exhibit a relationship between habitat and relative olfactory bulb size (figures 2 and 3). Like the optic lobes, the olfactory bulbs are known to follow the principle of proper mass, with relative size corresponding to olfactory ability in birds [50,51]. Large olfactory bulbs are observed in birds that forage under conditions where visual cues are limited (e.g. in low light or at sea) [14,52,53]. Except for emus, extant palaeognaths with large olfactory bulbs occupy forested habitats, where visual cues are expected to be limited and emphasis on olfaction would be favoured. Lithornithidae, an extinct clade of flighted stem palaeognaths [54,55], also show markedly well-developed olfactory bulbs [10], consistent with the forested habitats inferred for them in the Palaeocene–Eocene of Europe and North America [56]. Palaeognaths with relatively small olfactory bulbs generally occupy open habitats (e.g. grass- or shrubland). Palaeognaths also exhibit larger olfactory bulbs relative to cerebral hemisphere than neognaths, although neognaths show a similar relationship (figure 2; electronic supplementary material, figures S5 and S6). Zelenitsky et al. [10] observed that olfactory ratios increase relative to body size throughout the early evolution of both palaeognaths and neognaths. Our results suggest that the rate of this increase was higher at the base of palaeognaths than of neognaths, followed by smaller-scale increases in olfactory ratio in those lineages that shifted to forested habitats.
Our study identified species-level variation in olfactory bulb size among elephant birds (figures 1 and 3; electronic supplementary material, figures S1, S5 and S6). Aepyornis maximus retained relatively large olfactory bulbs ancestral of the larger elephant bird–kiwi–cassowary–emu clade, consistent with previous inferences of a forested habitat for elephant birds based on proposed foraging strategy and diet [57,58]. Also, much of the known elephant bird range has been proposed to be densely wooded, with the grasslands replacing woodlands only recently due to human activity [37,59]. The current paucity of elephant bird remains from the forested eastern coast of Madagascar may represent a lack of conditions suitable to subfossil preservation rather than a restriction of elephant bird range. Aepyornis hildebrandti, in contrast with A. maximus, exhibited secondarily reduced olfactory bulbs, more consistent with an open than a forested habitat (electronic supplementary material, figure S6). These species-level differences in olfactory bulb size, combined with differences in optic lobe size observed between species and genera (i.e. Aepyornis versus Mullerornis), may be due to resource/niche partitioning among elephant bird species, with some (e.g. A. maximus) retaining the ancestral nocturnal, forest-dwelling life history while others (e.g. A. hildebrandti, Mullerornis) possibly became better adapted to a grasslands habitat and more crepuscular than nocturnal activity patterns. These results suggest that elephant bird ecology was more complex than previously recognized. Currently, elephant bird species diversity is poorly understood, with many species definitions relying solely on differences in size. Finer understanding of variation in elephant bird ecology will largely depend on reassessment of these species differences.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank R. Allain (MNHN) for specimen access and help; R. David (Max Planck Institute for Evolutionary Anthropology) for assistance CT scanning MNHN F 1910-12 and MNHN 1875-602; M. Colbert and J. Maisano (UTCT) for assistance with CT scanning the uncatalogued Aepyornis specimen, Crypturellus, Tinamus and Rhynchotus; and Z. Li (Institute of Vertebrate Paleontology and Paleoanthropology, China) for access to all remaining CT scans. We thank D. Cannatella (University of Texas at Austin [UT]), N. Crouch (UT), S. Davis (UT), C. Early (University of Ohio), S. English (UT), D. Hillis (UT), S. Hood (UT), D. Ksepka (Bruce Museum), R. MacPhee (American Museum of Natural History), G. Musser (UT), J. Proffitt (UT), T. Worthy (Flinders University) and H. Zakon (UT) for comments and discussion and K. Rosenbach (University of Michigan) for inspiration for the title. We thank two anonymous referees for comments that proved invaluable to improving this report.
Data accessibility
Digital data used in this study are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.7519042 [60]. All other data are available in the electronic supplementary material.
Authors' contributions
C.R.T. participated in design of the study, processed digital data, carried out statistical analyses and drafted the manuscript. J.A.C. conceived of, participated in design of and funded the study. Both authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Funding from the US National Science Foundation supported this research (NSF EAR 1355282 to J.A.C.).
References
- 1.Monnier L. 1913. Paleontologie de Madagascar. VII. Les Aepyornis. Ann. Paleontol. 8, 125–172. [Google Scholar]
- 2.Yonezawa T, et al. 2017. Phylogenomics and morphology of extinct paleognaths reveal the origin and evolution of the ratites. Curr. Biol. 27, 68–77. ( 10.1016/j.cub.2016.10.029) [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KJ, Llamas B, Soubrier J, Rawlence NJ, Worthy TH, Wood J, Lee MSY, Cooper A. 2014. Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 344, 898–900. ( 10.1126/science.1251981) [DOI] [PubMed] [Google Scholar]
- 4.Burney D, Burney L, Godfrey L, Jungers W, Goodman S, Wright H, Jull A. 2004. A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63. ( 10.1016/j.jhevol.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 5.Bond WJ, Silander JA. 2007. Springs and wire plants: anachronistic defences against Madagascar's extinct elephant birds. Proc. R. Soc. B 274, 1985–1992. ( 10.1098/rspb.2007.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashwell KWS, Scofield RP. 2007. Big birds and their brains: paleoneurology of the New Zealand moa. Brain Behav. Evol. 71, 151–166. ( 10.1159/000111461) [DOI] [PubMed] [Google Scholar]
- 7.Jerison H. 1973. Evolution of the brain and intelligence. New York, NY: Academic Press. [Google Scholar]
- 8.Walsh SA, Knoll M. 2011. Directions in palaeoneurology. Palaeontology 86, 263–279. [Google Scholar]
- 9.Walsh S, Milner A. 2011. 11 Evolution of the avian brain and senses. In Living dinosaurs: the evolutionary history of modern birds (eds Dyke G, Kaiser G), pp. 282–305. Oxford: John Wiley & Sons. [Google Scholar]
- 10.Zelenitsky DK, Therrien F, Ridgely RC, McGee AR, Witmer LM. 2011. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B 278, 3625–3634. ( 10.1098/rspb.2011.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corfield JR, Wild JM, Hauber ME, Parsons S, Kubke MF. 2007. Evolution of brain size in the Palaeognath lineage, with an emphasis on New Zealand ratites. Brain Behav. Evol. 71, 87–99. ( 10.1159/000111456) [DOI] [PubMed] [Google Scholar]
- 12.Picasso MBJ, Tambussi CP, Degrange FJ. 2010. Virtual reconstructions of the endocranial cavity of Rhea americana (Aves, Palaeognathae): postnatal anatomical changes. Brain Behav. Evol. 76, 176–184. ( 10.1159/000321173) [DOI] [PubMed] [Google Scholar]
- 13.Romick C. 2013. Ontogeny of the brain endocasts of ostriches (Aves: Struthio camelus) with implications for interpreting extinct dinosaur endocasts. Honors thesis, Ohio University, Athens, OH. [Google Scholar]
- 14.Martin GR, Wilson K-J, Wild JM, Parsons S, Kubke MF, Corfield J. 2007. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2, e198 ( 10.1371/journal.pone.0000198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corfield J, Kubke MF, Parsons S, Wild JM, Köppl C. 2011. Evidence for an auditory fovea in the New Zealand kiwi (Apteryx mantelli). PLoS ONE 6, e23771 ( 10.1371/journal.pone.0023771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corfield JR, Kubke MF, Parsons S, Köppl C. 2012. Inner-ear morphology of the New Zealand kiwi (Apteryx mantelli) suggests high-frequency specialization. J. Assoc. Res. Otolaryngol. 13, 629–639. ( 10.1007/s10162-012-0341-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corfield JR, Eisthen HL, Iwaniuk AN, Parsons S. 2014. Anatomical specializations for enhanced olfactory sensitivity in kiwi, Apteryx mantelli. Brain Behav. Evol. 84, 214–226. ( 10.1159/000365564) [DOI] [PubMed] [Google Scholar]
- 18.Wiman C, Edinger T. 1942. Sur les cranes et les encephales d'Aepyornis et de Mullerornis. Bull. Acadamie Malgache 42, 1–50. [Google Scholar]
- 19.Ohashi T, Manabe M, Yoshida A. 2008. Endocranial anatomy of Aepyornithidae birds. J. Vertebr. Paleontol. 28, 122A–123A. [Google Scholar]
- 20.Balanoff AM, et al. 2016. Best practices for digitally constructing endocranial casts: examples from birds and their dinosaurian relatives. J. Anat. 229, 173–190. ( 10.1111/joa.12378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burleigh JG, Kimball RT, Braun EL. 2015. Building the avian tree of life using a large-scale, sparse supermatrix. Mol. Phylogenet. Evol. 84, 53–63. ( 10.1016/j.ympev.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 22.Bang B, Cobb S. 1968. The size of the olfactory bulb in 108 species of birds. Auk 85, 55–61. ( 10.2307/4083624) [DOI] [Google Scholar]
- 23.Smith NA, Clarke JA. 2012. Endocranial anatomy of the Charadriiformes: sensory system variation and the evolution of wing-propelled diving. PLoS ONE 7, e49584 ( 10.1371/journal.pone.0049584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boles W. 1992. Revision of Dromaius gidju Patterson and Rich 1987 from Riversleigh, Northwestern Queensland, Australia, with a reassessment of its generic position. Nat. Hist. Mus. Los Angel. Cty. Sci. Ser. 36, 195–208. [Google Scholar]
- 25.Boles W. 2001. A new emu (Dromaiinae) from the Late Oligocene Etadunna Formation. Emu 101, 317–321. ( 10.1071/MU00052) [DOI] [Google Scholar]
- 26.White M. 1994. After the greening: the browning of Australia. Kenthurst, New South Wales, Australia: Kangaroo Press. [Google Scholar]
- 27.Bertelli S. 2017. Advances on tinamou phylogeny: an assembled cladistic study of the volant palaeognathous birds. Cladistics 33, 351–374. ( 10.1111/cla.12172) [DOI] [PubMed] [Google Scholar]
- 28.Bown TM, Larriestra CN. 1990. Sedimentary paleoenvironments of fossil platyrrhine localities, Miocene Pinturas Formation, Santa Cruz Province, Argentina. J. Hum. Evol. 19, 87–119. ( 10.1016/0047-2484(90)90013-2) [DOI] [Google Scholar]
- 29.Genise JF, Bown TM. 1994. New Miocene scarabeid and hymenopterous nests and early miocene (santacrucian) paleoenvironments, patagonian Argentina. Ichnos 3, 107–117. ( 10.1080/10420949409386378) [DOI] [Google Scholar]
- 30.Kay RF, Vizcaíno SF, Bargo MS. 2012. A review of the paleoenvironment and paleoecology of the Miocene Santa Cruz Formation. In Early Miocene paleobiology in patagonia (eds Vizcaíno SF, Kay RF, Bargo MS), pp. 331–336. New York, NY: Cambridge University Press. [Google Scholar]
- 31.Wood J, Rawlence N, Rogers G, Austin J, Worthy T, Cooper A. 2008. Coprolite deposits reveal the diet and ecology of the extinct New Zealand megaherbivore moa (Aves, Dinornithiformes). Quat. Sci. Rev. 27, 2593–2602. ( 10.1016/j.quascirev.2008.09.019) [DOI] [Google Scholar]
- 32.Wood JR, Wilmshurst JM, Richardson SJ, Rawlence NJ, Wagstaff SJ, Worthy TH, Cooper A. 2013. Resolving lost herbivore community structure using coprolites of four sympatric moa species (Aves: Dinornithiformes). Proc. Natl Acad. Sci. USA 110, 16 910–16 915. ( 10.1073/pnas.1307700110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worthy TH. 1990. An analysis of the distribution and relative abundance of moa species (Aves: Dinornithiformes). N. Z. J. Zool. 17, 213–241. ( 10.1080/03014223.1990.10422598) [DOI] [Google Scholar]
- 34.Benowitz LI, Karten HJ. 1976. Organization of the tectofugal visual pathway in the pigeon: a retrograde transport study. J. Comp. Neurol. 167, 503–520. ( 10.1002/cne.901670407) [DOI] [PubMed] [Google Scholar]
- 35.Iwaniuk AN, Gutierrez-Ibanez C, Pakan JMP, Wylie DR. 2010. Allometric scaling of the tectofugal pathway in Birds. Brain Behav. Evol. 75, 122–137. ( 10.1159/000311729) [DOI] [PubMed] [Google Scholar]
- 36.Corfield JR, Gsell AC, Brunton D, Heesy CP, Hall MI, Acosta ML, Iwaniuk AN. 2011. Anatomical specializations for nocturnality in a critically endangered parrot, the kakapo (Strigops habroptilus). PLoS ONE 6, e22945 ( 10.1371/journal.pone.0022945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman SM, Jungers WL. 2014. Extinct Madagascar: picturing the island's past. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 38.Güntürkün O, Miceli D, Watanabe M. 1993. Anatomy of the avian thalamofugal pathway. In Vision, brain and behaviour in birds (eds Zeigler H, Bischof H-J), pp. 115–156. Cambridge, MA: MIT Press. [Google Scholar]
- 39.Cunningham S, Castro I, Alley M. 2007. A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. J. Anat. 211, 493–502. ( 10.1111/j.1469-7580.2007.00786.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore BA, Paul-Murphy JR, Tennyson AJD, Murphy CJ. 2017. Blind free-living kiwi offer a unique window into the ecology and evolution of vertebrate vision. BMC Biol. 15, 85 ( 10.1186/s12915-017-0424-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corfield JR, Parsons S, Harimoto Y, Acosta ML. 2015. Retinal anatomy of the New Zealand kiwi: structural traits consistent with their nocturnal behavior. Anat. Rec. 298, 771–779. ( 10.1002/ar.23080) [DOI] [PubMed] [Google Scholar]
- 42.Iwaniuk AN, Wylie DRW. 2006. The evolution of stereopsis and the wulst in caprimulgiform birds: a comparative analysis. J. Comp. Physiol. A 192, 1313–1326. ( 10.1007/s00359-006-0161-2) [DOI] [PubMed] [Google Scholar]
- 43.Bonin J. 1965. The eye of Agamodon angulicpes Peters (Reptilia, Amphisbaenia). Copeia 1965, 324–331. ( 10.2307/1440795) [DOI] [Google Scholar]
- 44.Barr T. 1968. Cave ecology and the evolution of troglobites. In Evolutionary biology (eds Dobzhansky T, Hecht M, Steere W), pp. 35–102. New York, NY: Plenum Press. [Google Scholar]
- 45.Watkins JF, Kroll JC, Gehlbach FR. 1971. Pheromone trail-following studies of typhlopid, leptotyphlopid, and colubrid snakes. Behaviour 40, 282–294. ( 10.1163/156853971X00429) [DOI] [PubMed] [Google Scholar]
- 46.Catania KC. 2000. Cortical organization in Insectivora: the parallel evolution of the sensory periphery and the brain. Brain Behav. Evol. 55, 311–321. ( 10.1159/000006666) [DOI] [PubMed] [Google Scholar]
- 47.Crish SD, Dengler-Crish CM, Catania KC. 2006. Central visual system of the naked mole-rat (Heterocephalus glaber). Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 288A, 205–212. ( 10.1002/ar.a.20288) [DOI] [PubMed] [Google Scholar]
- 48.Němec P, Cveková P, Benada O, Wielkopolska E, Olkowicz S, Turlejski K, Burda H, Bennett NC, Peichl L. 2008. The visual system in subterranean African mole-rats (Rodentia, Bathyergidae): retina, subcortical visual nuclei and primary visual cortex. Brain Res. Bull. 75, 356–364. ( 10.1016/j.brainresbull.2007.10.055) [DOI] [PubMed] [Google Scholar]
- 49.Iwaniuk A, Olson S, James H. 2009. Extraordinary cranial specialization in a new genus of extinct duck (Aves: Anseriformes) from Kauai. Hawaiian Islands 2296, 47–2267. [Google Scholar]
- 50.Bang B. 1971. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anat. Basel 58, 1–76. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel B. 1971. Olfactory sensation in the kiwi and other birds. Ann. NY Acad. Soc. 188, 183–193. ( 10.1111/j.1749-6632.1971.tb13097.x) [DOI] [PubMed] [Google Scholar]
- 52.Grubb TC. 1974. Olfactory navigation to the nesting burrow in Leach's petrel (Oceanodroma Leucorrhoa). Anim. Behav. 22, 192–202. ( 10.1016/S0003-3472(74)80069-2) [DOI] [PubMed] [Google Scholar]
- 53.Nevitt GA, Losekoot M, Weimerskirch H. 2008. Evidence for olfactory search in wandering albatross, Diomedea exulans. Proc. Natl Acad. Sci. USA 105, 4576–4581. ( 10.1073/pnas.0709047105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houde P. 1988. Paleognathous birds from the early tertiary of the northern hemisphere. Cambridge, MA: Nuttall Ornithological Club. [Google Scholar]
- 55.Nesbitt SJ, Clarke JA. 2016. The anatomy and taxonomy of the exquisitely preserved Green River Formation (Early Eocene) lithornithids (Aves) and the relationships of Lithornithidae. Bull. Am. Mus. Nat. Hist. 406, 1–91. ( 10.1206/0003-0090-406.1.1) [DOI] [Google Scholar]
- 56.Grande L, Weinstein J. 2013. The lost world of fossil lake: snapshots from deep time. Chicago, IL: University of Chicago Press. [Google Scholar]
- 57.Lavauden L. 1931. Animaux disparus et légendaires de Madagascar. Rev. Sci. Illus. 69, 297–308. [Google Scholar]
- 58.Clarke SJ, Miller GH, Fogel ML, Chivas AR, Murray-Wallace CV. 2006. The amino acid and stable isotope biogeochemistry of elephant bird (Aepyornis) eggshells from southern Madagascar. Quat. Sci. Rev. 25, 2343–2356. ( 10.1016/j.quascirev.2006.02.001) [DOI] [Google Scholar]
- 59.Burney DA. 1993. Late Holocene environmental changes in Arid Southwestern Madagascar. Quat. Res. 40, 98–106. ( 10.1006/qres.1993.1060) [DOI] [Google Scholar]
- 60.Torres CR, Clarke JA. 2018. Data from: Nocturnal giants: evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions Dryad Digital Repository. ( 10.5061/dryad.7519042) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Torres CR, Clarke JA. 2018. Data from: Nocturnal giants: evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions Dryad Digital Repository. ( 10.5061/dryad.7519042) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Digital data used in this study are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.7519042 [60]. All other data are available in the electronic supplementary material.