Abstract
The mammalian dentition is uniquely characterized by a combination of precise occlusion, permanent adult teeth and a unique tooth attachment system. Unlike the ankylosed teeth in most reptiles, mammal teeth are supported by a ligamentous tissue that suspends each tooth in its socket, providing flexible and compliant tooth attachment that prolongs the life of each tooth and maintains occlusal relationships. Here we investigate dental ontogeny through histological examination of a wide range of extinct synapsid lineages to assess whether the ligamentous tooth attachment system is unique to mammals and to determine how it evolved. This study shows for the first time that the ligamentous tooth attachment system is not unique to crown mammals within Synapsida, having arisen in several non-mammalian therapsid clades as a result of neoteny and progenesis in dental ontogeny. Mammalian tooth attachment is here re-interpreted as a paedomorphic condition relative to the ancestral synapsid form of tooth attachment.
Keywords: pelycosaur, therapsid, dental histology, paedomorphosis, ankylosis
1. Introduction
The origin and evolution of the complex mammalian dentition from the modest heterodonty and continually replaced dentitions of non-mammalian synapsids is a major topic in vertebrate palaeontology [1–7]. In addition to having limited tooth replacement and complex cusp patterns, mammals are unusual among amniotes in having teeth that are not fused to the jaw but suspended in tooth sockets by periodontal ligaments (PDLs) [2,3,8,9]. The dental gomphosis (socketed, ligamentous tooth attachment, sensu [10,11]), unlike dental ankylosis (fused tooth attachment) in most other vertebrates, provides a cushion to resist the compressive and shear forces associated with chewing and a means through which teeth can maintain precise positioning and occlusion [12–15]. Paradoxically, among extant amniotes, the ‘mammalian’ mode of tooth attachment is elsewhere only seen in crocodilians [9,16–19]. The tissues forming the tooth attachment systems in mammals and crocodilians are identical, but the evolution of a gomphosis in the two groups was clearly a case of convergence, given that early synapsids and reptiles had teeth that were fused to the jaws [8,9,20,21].
Hypothetical transitional series have been used to explain how the three-part tooth attachment system in mammals—consisting of cementum, alveolar bone and PDL—might have differentiated from an ancestral ‘bone of attachment’, a single-tissue attachment system that fuses teeth to the jaws in most non-mammalian vertebrates [9–11,22]. These hypotheses treat the mammalian gomphosis as the most complex form of tooth attachment and posit that it has increased in complexity through evolutionary time in association with dental occlusion, culminating in the mammalian condition [10]. This view has been challenged more recently, because even when teeth are fused to the jaws, all amniote teeth possess homologous tooth attachment tissues to those in mammals [19,20,23]. These recent findings are re-framing our understanding of amniote dental evolution, but they still do not provide an evolutionary or developmental process to explain how mammals evolved a gomphosis from the ancestral synapsid condition of ankylosis. Further complicating this question, numerous studies have lauded the excellent fossil record spanning the evolutionary transition towards crown mammals [2,7,10,24], but none have pinpointed when during the 300 Myr evolutionary history of Synapsida this important shift in dental development occurred, and thus it is often assumed that the gomphosis is a mammalian synapomorphy [3,5,9]. Both the evolutionary origins and mechanism explaining how mammals evolved this condition can be empirically tested using histological data from the fossil record of non-mammalian synapsids.
In order to trace and propose a novel mechanism to explain the origins of the mammalian tooth attachment system, we examined histological sections and micro-computed tomography (μCT) scans to document dental ontogeny in a large sample of Permo–Triassic stem-mammalian taxa (figure 1). These data allow us to assess the non-mammalian homologues of cementum, alveolar bone and PDL, and derive a novel method for characterizing dental evolution and ontogeny across synapsid phylogeny. We use this method to test for fundamental shifts in the timing and sequence of dental ontogeny in Palaeozoic and Mesozoic synapsids as a mechanism underlying the transition from ankylosis in early synapsids to the mammalian tooth attachment system.
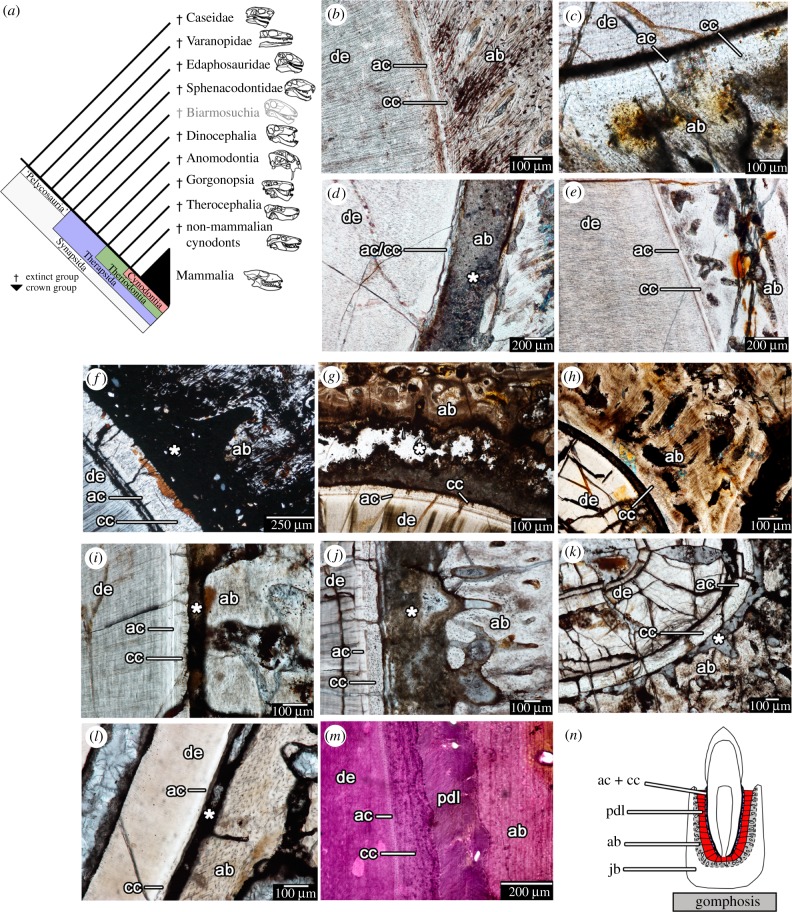
Figure 1.
Tooth attachment in stem and crown mammals (Synapsida). (a) Cladogram of the evolutionary relationships of the major taxa within Synapsida (modified from [5,20,25]). (b–l) thin sections showing tooth attachment tissues in major synapsid groups. (b) The sphenacodontid Dimetrodon (ROM 6039). (c) An indeterminate dinocephalian (BP/1/4851). (d) The anomodont Diictodon (ROM 52624). (e) A second individual of Diictodon (ROM 52624). (f) An indeterminate gorgonopsian (NMT RB404). (g) An indeterminate therocephalian (BP/1/7257). (h) The same therocephalian, but an adjacent tooth position. (i) The derived therocephalian Bauria (BP/1/2523). (j) The cynodont Cynognathus (BP/1/6097). (k) The cynodont Diademodon (BP/1/4652). (l) The extinct ungulate mammal Hyopsodus (USNM 595273). (m) A stained section of the periodontium in an extant badger (Taxidea). (n) Illustrated the arrangement of the attachment tissues of the gomphosis in Synapsida. ab, alveolar bone (grey); ac, acellular cementum (blue); cc, cellular cementum (blue); de, dentine; jb, jawbone; pdl, periodontal ligament (red). Asterisks indicate spaces formerly occupied by periodontal ligament in life.
2. Material and methods
(a). Thin sections
Thirty-six specimens of fossil stem and crown mammals were sectioned at the ROM (Royal Ontario Museum, Toronto, Canada) Palaeohistology Laboratory or at UW (University of Washington, Seattle, WA, USA), including representatives of most of the major lineages of ‘pelycosaur’-grade synapids (Caseidae, Varanopidae, Edaphosauridae, Sphenacodontidae) and therapsids (Dinocephalia, Anomodontia, Gorgonopsia, Therocephalia, Cynodontia) (electronic supplementary material, table S1). Material from the Evolutionary Studies Institute (South Africa) was loaned to A. LeBlanc and R. R. Reisz under SAHRA permit ID 1945 for thin sectioning. Moulds and casts were made of several specimens prior to sectioning. Moulds were made using Blustar Silicones V-SIL 1062 silicone and Hi Pro Green catalyst. Casts were made by pouring Smooth-on-Smooth-Cast 321 or 322 liquid plastic into the silicon moulds and placed under pressure until they had set.
Thin sections were made following standard procedures for sectioning fossil material. Each specimen was embedded in Castolite AC polyester resin and placed under vacuum. The hardened resin blocks were cut using a Buehler Isomet low-speed wafer blade saw (between 225 and 300 rpm) and the cut surfaces were polished using 600-grit silicon carbide powder. The polished surfaces were mounted on frosted plexiglass slides using cyanoacrylate glue and cut again using the Buehler Isomet. The mounted wafer was then ground to approximately 100 µm using a Hillquist grinding machine with a 240-grit grinding cup. Each specimen was then hand ground to the preferred thickness using progressively finer grits (600 and 1000 grit) of silicon carbide powder and polished using 1 µm grit aluminium oxide powder. Thin section images were taken using a Nikon DS-fi2 camera mounted to a Nikon AZ-100 microscope using Nikon NIS-Elements imaging software (Basic Research package).
(b). μCT scans
μCT scans of four skulls of the early cynodonts Galesaurus and Thrinaxodon were also examined, including a juvenile (BP/1/5372) and subadult (BP/1/7199) Thrinaxodon specimen. As per Abdala et al. [6], scans of BP/1/5372 were made at the European Synchrotron Radiation Facility (Grenoble, France) on beamline ID19 in propagation phase-contrast mode (isotropic voxel size of 20.24 µm). BP/1/7199 was scanned on beamline ID19 using the filtered white beam in a half-acquisition mode with a voxel size of 30 µm (see [6] for detailed scanning methodology). The intermediate-sized (BP/1/4602) and large (BP/1/5064) skulls of Galesaurus were μCT scanned at the Evolutionary Studies Institute (University of the Witwatersrand, South Africa) using a Nikon Metrology XTH225/320 LC dual-source CT system [26]. BP/1/4602 was scanned at 130 kV and 185 µA and BP/1/5064 at 170 kV and 95 µA. All scans were segmented in Aviso 6.3 (Visualization Sciences Groups, Merignac, France) and VGStudio MAX 2.2 (Volume Graphics, Heidelberg, Germany). All μCT scan files used for these specimens in this study are available in the Dryad Digital Repository [27].
3. Results
(a). Tooth attachment histology in synapsids
The resulting histological dataset consisted of nearly 120 thin sections and 4 μCT scans spanning at least 20 distinct synapsid taxa. Despite being fossilized specimens, all of the thin sections revealed the presence of the mineralized attachment tissues, cementum and alveolar bone (figure 1b–l) and in comparable arrangements to those in extant mammals (figure 1m). Cementum occurs in two forms: a thin acellular band adjacent to the dentine of the tooth root and a thicker outer layer with abundant cell lacunae (cellular cementum). Alveolar bone is a highly vascularized bone layer forming the tooth socket. Identifying the PDL in fossils is inherently difficult, because the majority of this tissue is uncalcifed and therefore decays shortly after death. The collagen fibre bundles of the PDL span the gap between the cementum and the alveolar bone to suspend the tooth in place (figure 2a,b). Fortunately, these collagen fibre bundles are partially mineralized where they anchor into the alveolar bone and cementum, forming Sharpey's fibres that are visible in extant and fossil material (figure 2). The presence of Sharpey's fibres and a sediment- or mineral-filled gap between the tooth root and the socket bone are therefore strong indicators of the presence of a PDL in a fossil vertebrate [20,23,28].
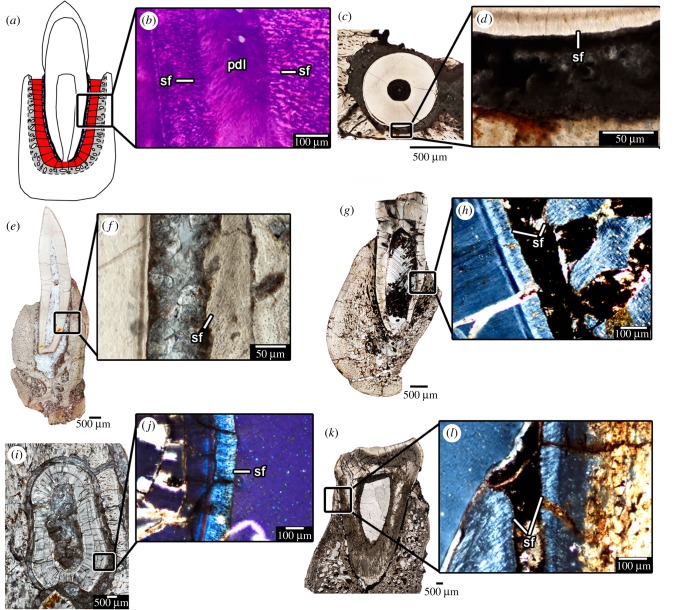
Figure 2.
‘Mammal’-like tooth attachment in non-mammalian therapsids. (a) Illustration of the tooth attachment tissues in a gomphosis. (b) Close-up of the tooth attachment tissues in an extant badger under cross-polarized light, showing the Sharpey's fibres of the PDL in the cementum and alveolar bone. (c) Transverse section of a tooth of a gorgonopsian (BP/1/784). (d) Close-up of the periodontal tissues in (c). (e) Coronal section of a tooth of an indeterminate therocephalian (BP/1/172). (f) Close-up of periodontal tissues in (e). (g) Coronal section of a tooth in the therocephalian Bauria (BP/1/2523). (h) Close-up of periodontal tissues in (g) under cross-polarized light. (i) Transverse section of a tooth in the cynodont Cynognathus (BP/1/6097). (j) Close-up of the periodontal tissues in (i) under cross-polarized light. (k) Coronal section of a tooth in the cynodont Diademodon (BP/1/4652). (l) Close-up of the periodontal tissues in (k) under cross-polarized light. pdl, periodontal ligament; sf, Sharpey's fibres.
All of the ‘pelycosaur’-grade synapsids, exemplified by the sail-backed carnivore Dimetrodon, exhibited dental ankylosis (figure 1b), where the soft tissue comprising the PDL has completely calcified [19,20]. Partially mineralized Sharpey's fibre bundles radiate around the tooth roots within the alveolar bone in these ankylosed teeth and extend to the cellular cementum coating the tooth roots, indicating the presence of a soft tissue attachment prior to complete mineralization [20]. Nearly all of the sectioned teeth were either erupting into the oral cavity (usually preserved only as replacement pits in the jaws), or completely ankylosed to the jaws, with only four teeth in the entire ‘pelycosaur’ sample being incompletely ankylosed to the jaws (figure 1b; electronic supplementary material, information S1).
Unlike the condition in ‘pelycosaurs’, numerous non-mammalian therapsids, including some dinocephalians, therocephalians and the early cynodont Thrinaxodon show evidence of ankylosis and gomphosis-type tooth attachment in the same taxon or even the same individual, indicating prolonged ligamentous tooth attachment prior to complete ankylosis (figure 1c,d,e,g,h; electronic supplementary material, information S1). Some of the teeth in these therapsids show evidence of the centripetal growth of the surrounding alveolar bone, indicating that it is the alveolar bone that extends towards the tooth root through dental ontogeny, eventually encasing the PDL in bone (figure 1d,e,g,h).
(b). ‘Mammal’-like tooth attachment in several non-mammalian synapsids
Several non-mammalian therapsids had teeth that were exclusively attached to the socket by an uncalcified PDL, including tapinocephalid dinocephalians, gorgonopsians, bauriid therocephalians and numerous, but not all cynodont genera (figures 1f,i,j,k; 2; electronic supplementary material, information S1). Among non-mammalian cynodonts, only the early cynodont Thrinaxodon and some tritheledontids show evidence of dental ankylosis in older generations of teeth [6,29,30]. All other cynodonts we and others [31] examined show evidence of well-developed cementum, PDL (indicated by a mineral or sediment filled space between the tooth root and the socket) and alveolar bone without ankylosis. This gomphosis-type of tooth attachment in these therapsid groups is characterized by smooth outer margins of the cementum layers with abundant Sharpey's fibres that radiate around the tooth root (figure 2). The surrounding alveolar bone is also perforated by Sharpey's fibres that have complimentary orientations to those in the cementum. This condition is identical to that in extant mammals (figure 2).
(c). Ancestral character state reconstruction
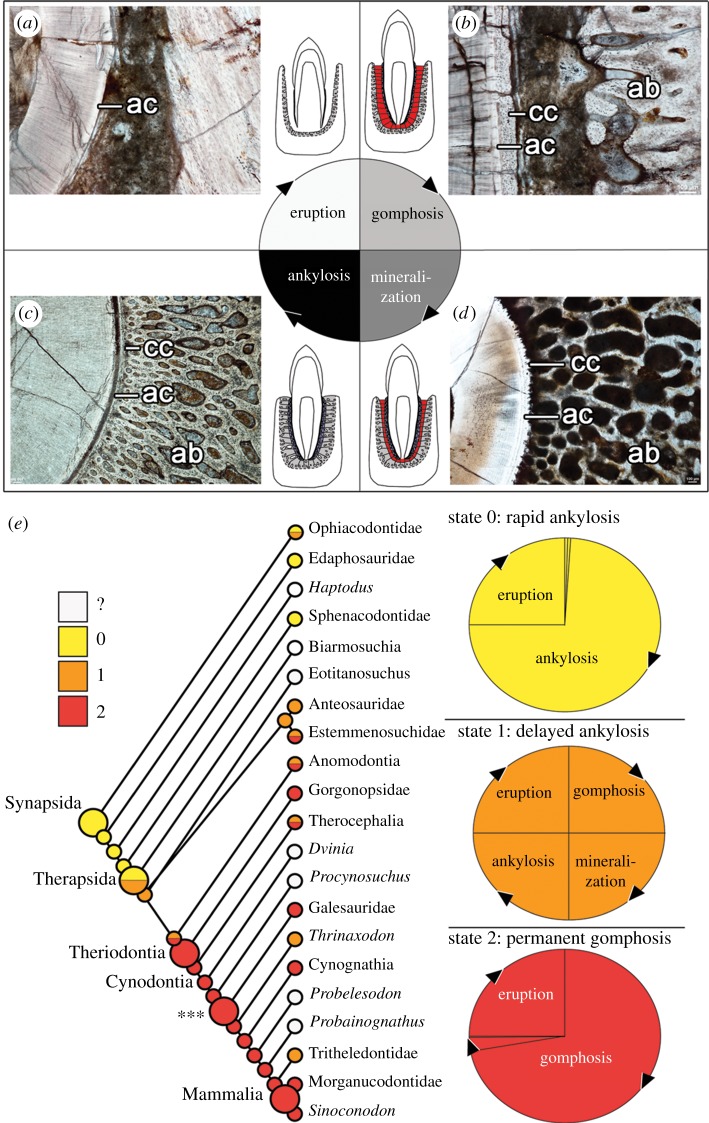
These histological comparisons show that simply mapping the presence of ‘ankylosis’ and ‘gomphosis’ as alternative states across synapsid phylogeny fails the homology test of conjunction [32,33], because some individuals exhibit both states along the same jaw. We did, however, note changes in the relative duration of a standardized sequence of four phases of dental ontogeny (figure 3). Tooth staging schemes are most frequently used to map tooth replacement patterns in vertebrates [34–36]; however, we devised a novel staging scheme to encompass the variation in tooth attachment modes seen in synapsids. After a tooth is shed, all new teeth pass through an eruption stage where the surrounding hard tissues are resorbed and the developing tooth starts to form enamel and dentine (figures 3 and 4a). Upon erupting into the mouth, teeth were attached to alveolar bone by a PDL forming a dental gomphosis (figures 3 and 4b). The gomphosis phase is often followed by gradual inward growth of alveolar bone, which entombed the PDL in bone (figures 3 and 4c). This mineralization phase is characterized by incomplete ankylosis of the tooth root to the socket, with some areas around the tooth exhibiting contact between the cementum and alveolar bone, whereas others are still separated by an unmineralized gap (figures 3 and 4d). The final phase is ankylosis, where the surrounding alveolar bone has completely calcified the PDL and contacts the cementum all around the tooth root.
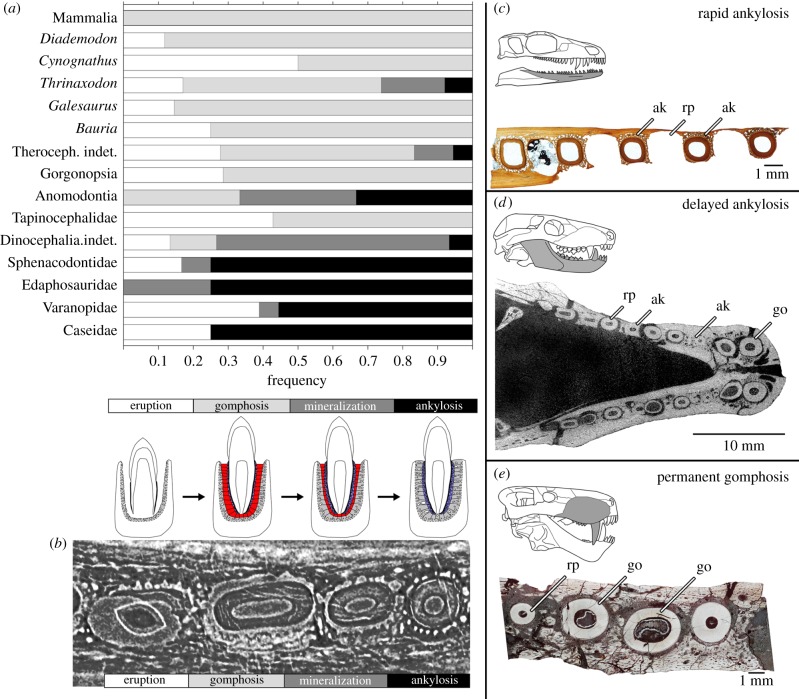
Figure 3.
Tooth attachment and relative frequency of tooth attachment stages. (a) The frequency of teeth at the eruption, gomphosis, mineralization and ankylosis stages in thin sections and CT scans of fossil synapsids, reflecting the proportion of time teeth spend in the respective stages (note: the mammal samples used here all had erupted permanent dentitions). (b) Digital transverse section through four postcanines of Thrinaxodon (BP/1/5372) showing successive stages of eruption, gomphosis, mineralization and ankylosis (from left to right). (c) varanopid dentary transverse section showing teeth at either the eruption stage or completely ankylosed (rapid ankylosis) (ROM 66866). (d) CT image of the lower jaws of the cynodont Thrinaxodon (BP/1/7199) showing teeth at eruption, gomphosis and ankylosis stages (delayed ankylosis). (e) gorgonopsian maxilla (BP/1/2395a) transverse section showing teeth either at the eruption or gomphosis stages (permanent gomphosis). ak, ankylosis; go, gomphosis, rp, resorption pit/erupting tooth. (Online version in colour.)
Figure 4.
Heterochrony and the evolution of synapsid tooth attachment. (a) Eruption stage after the previous tooth is shed involves remodelling of tissues and formation of the tooth crown and early root tissues (image of erupting tooth of the cynodont Cynognathus) (BP/1/6097). (b) Gomphosis stage occurs when all three periodontal tissues are fully formed in a functional tooth (image of an erupted tooth root of Cynognathus) (BP/1/6097). (c) Mineralization stage occurs as alveolar bone begins to calcify centripetally. Minor centrifugal mineralization of cementum may also occur (image of a dinocephalian tooth root) (BP/1/6854). (d) Ankylosis stage occurs when alveolar bone and cementum meet and completely entomb the periodontal ligament (image of the tooth root of Dimetrodon) (ROM 6039). (e) Ancestral character state reconstruction for the major synapsid clades using a three-state character and the phylogeny of Sidor & Hopson [5]. Larger circles indicate the nodes for Synapsida, Therapsida, Theriodonta and Mammalia. Asterisks and associated large circle indicate earliest cynodont node for which histological data was actually observed. Small circles at terminal branches indicate character state codings for each OTU (operational taxonomic unit). ab, alveolar bone; ac, acellular cementum; cc, cellular cementum.
We tabulated the number of teeth represented in each phase along all of our thin sections and CT scans (electronic supplementary material, table S2) and treated these as being proportional to the relative duration of each phase in dental ontogeny in a given taxon (following the rationale of [37]). This method revealed three distinct states of synapsid dental ontogeny, based on the relative duration of the gomphosis and ankylosis phases: (0) teeth pass rapidly to ankylosis after erupting (gomphosis not observed); (1) teeth pass through a gomphosis stage more slowly, followed by ankylosis (gomphosis and ankylosis observed); and (2) teeth retain a permanent gomphosis (ankylosis not observed) (figures 3c–e and 4e). These character states were then mapped across the synapsid phylogeny of Sidor & Hopson [5] using Mesquite's ‘trace character history’ function (electronic supplementary material, Information S2).
The resulting comparisons of dental ontogeny and character state distributions show that ‘pelycosaurs’ have a very short-lived gomphosis phase, because teeth were either in the process of erupting or were ankylosed to the jaws (state 0) (figures 3a and 4e). Therapsids all display proportionally longer-lasting ligamentous phases of tooth attachment relative to ‘pelycosaurs’ (states 1 or 2) (figures 3a and 4e). We also identified several occurrences of a permanent gomphosis (state 2) in tapinocephalids, gorgonopsians, therocephalians and several non-mammalian cynodonts (figures 3a and 4e). Given the relatively coarse resolution of this phylogeny, several of the terminal taxa had to be coded as polymorphic for this character, including anomodonts and therocephalians. Ancestral character state reconstruction suggests that the ancestral state for therocephalians was to have a permanent gomphosis (state 2), with a reversal to delayed ankylosis (state 1) in some therocephalian taxa. The resulting character state distributions also indicate that a permanent gomphosis (state 2) is a symplesiomorphy for mammals, with the transition to a permanent gomphosis characterizing the more inclusive group, Theriodontia (Gorgonopsidae, Therocephalia and Cynodontia). These results also highlight a reversal to delayed ankylosis (state 1) in the early cynodont Thrinaxodon and potentially in tritheledontid cynodonts [29,30] (figure 4e), and the convergent evolution of a permanent gomphosis (state 2) in some herbivorous dinocephalians.
4. Discussion
(a). Heterochrony and the evolution of the mammalian tooth attachment system
Our data do not support the classical hypotheses for the origins of the mammalian tooth attachment system, which invoke evolutionary increases in dental tissue complexity from an ancestral ‘bone of attachment’ to a three-tissue tooth attachment system in crown mammals [9–11]. Whereas finer within-clade comparisons are required to optimize this ontogenetic character, the differences in tooth attachment across Synapsida clearly do not relate to increases in tooth tissue complexity, but to differences in the timing and sequence of dental ontogeny. Teeth are unique organs in that their ontogeny can be examined separately from the ontogeny of the animal itself, because in most vertebrates, the teeth are continually replaced. Sectioning the jaws of non-mammalian synapsids therefore provides a window into synapsid dental development and evolution: each section reveals multiple generations of teeth progressing through a repeating sequence of developmental stages from initiation, tissue differentiation, calcification, to the shedding of a functional tooth [20,38] (figure 3). Comparing dental ontogeny across such a wide range of extinct synapsids reveals that the ancestral condition for Synapsida is for teeth to pass through a gomphosis phase early in dental ontogeny and to rapidly form a stable ankylosis by the extensive alveolar bone formation that fixes the tooth in place (figures 3 and 4) [20].
Using our method for characterizing dental ontogeny and the ‘pelycosaur’ condition as the ancestral synapsid state, we propose that the observed differences in tooth attachment across Synaspida are due to a neotenic (sensu [39]) delay in the onset of ankylosis (a transition from state 0 to 1 in our new dental character) and to progenesis (sensu [39]), or truncation of the mineralization and ankylosis stages in dental ontogeny in the stereotypically mammalian condition of a permanent dental gomphosis (a transition from state 1 to 2). Functional teeth that were attached by a ligament, previously thought to characterize cynodont or strictly mammalian tooth attachment within Synapsida [5,10,22,40,41], appear across all therapsid clades (figures 1c–l and 2). Moreover, the permanent gomphosis in tapinocephalids, gorgonopsians, bauriids and the majority of cynodonts (including mammals) is therefore not the result of repeated, de novo evolution of a PDL from a primordial ‘bone of attachment’, but to delayed or a lack of calcification of the PDL, which is plesiomorphically present in all synapsids [20]. This heterochronic shift in the timing of mineralization and subsequent ankylosis phases results in a paedomorphic (sensu [39]) form of tooth attachment in these taxa. Mammals and several other therapsid groups simply possess functional teeth that remain at an earlier ontogenetic stage compared to ‘pelycosaur’-grade synapsids (figures 3 and 4).
Whereas our understanding of the development and maintenance of the mammalian PDL is steadily improving [42–44], the role that phylogenetic history plays in this process has only recently been explored [20]. Partial mineralization of the PDL in modern caiman led McIntosh et al. [17] to conclude that the crocodilian PDL was intermediate between the ankylosis type attachment in most other reptiles and the gomphosis in mammals. Our results provide a clearer depiction of a phylogenetically ‘intermediate’ condition between the earliest synapsids and crown mammals: the gomphosis in many non-mammalian therapsids is replaced by complete ankylosis, with the PDL eventually becoming entombed in the surrounding alveolar bone and forming a stable ankylosis, a condition exemplified by many non-mammalian therapsids [20] (figures 3 and 4). Where mammals, tapinocephalids, gorgonopsians, many therocephalians and cynodonts differ is in the decrease in an alveolar bone deposition, decreased calcification of the PDL and the maintenance of a non-mineralized region between the tooth root and the alveolus.
(b). Functional implications
The evolution of the gomphosis has been historically linked to complex dental occlusion in stem mammals [3,9], because the widely accepted role of the PDL is to dissipate the forces of occlusion [14,38,45]. However, for the stress-dissipating hypothesis for the origin of the PDL to be supported, it should have arisen in clades that exhibit extensive occlusion or high bite forces and such fossil evidence has been lacking up to this point [10]. Within Synapsida, some non-mammalian clades may have independently evolved a gomphosis in association with comparatively simple dental occlusion (as in tapinocephalids and bauriids), but this could not possibly apply to all groups. For example, the blade-like teeth of gorgonopsians and the shearing teeth of Galesaurus exhibit permanent gomphosis, whereas some of the teeth of the early cynodont Thrinaxodon show evidence of ankylosis [6] (figure 3; electronic supplementary material, information S1). Moreover, all ‘pelycosaurs’ show complete and rapid ankylosis of teeth, irrespective of tooth function (figure 3; electronic supplementary material, information S1). Outside of Synapsida, many archosaurs (including all dinosaurs and extant crocodilians) exhibit a gomphosis, but many do not exhibit dental occlusion [17,28,46], whereas other extinct reptiles exhibit simple occlusion, but have ankylosed teeth [47,48]. The evolution of a permanent ligamentous tooth attachment in synapsids is therefore not uniquely associated with occlusion and may have arisen in association with another factor that many therapsid lineages share to the exclusion of the earlier ‘pelycosaurs’.
We hypothesize that a reduction in the tooth replacement rate across the transition from ‘pelycosaurs’ to therapsids may at least explain why all therapsids show a prolonged (but not always permanent) ligamentous tooth attachment relative to ‘pelycosaurs’ (figures 3 and 4). We found evidence for extensive tooth migration and size discrepancies between functional and replacement teeth, indicating that a significant amount of time had passed between replacement events in several therapsid groups (electronic supplementary material, information S3). A slower tooth replacement rate would mean that teeth spent proportionally more time in the oral cavity. A prolonged ligamentous phase of tooth attachment would mediate the re-positioning of teeth within the jaws through ontogeny, which is a key function of the mammalian PDL [10,13]. This prolonged ligamentous phase may have later been co-opted into its shock-absorbing function in the occluding teeth of mammals and several other therapsid lineages.
5. Conclusion
By comparing tooth tissue formation over a 300 Myr span of synapsid evolution, we can conclude that the mammalian periodontium is not the result of an evolutionary increase in dental complexity, but an example of paedomorphosis: namely a slower calcification of the PDL (neoteny) in some therapsids, and to truncation of the mineralization and ankylosis phases of dental ontogeny (progenesis) in others, including mammals. Heterochrony has played a prominent role in the diversification of mammalian teeth, including evolutionary changes to eruption timing and the evolution of high-crowned (hypsodont) teeth [49,50], but it has played a further role in the evolution of the dental gomphosis that characterizes all mammal teeth. Evolutionary changes to the timing of calcification of the tooth attachment tissues were not restricted to synapsids, however, as similar phenomena have been proposed to explain the occurrences of a ligamentous tooth attachment in some extant and extinct squamates [23]. These similarities between synapsid and squamate dental ontogeny and evolution suggest that heterochrony may be an important driver of dental attachment tissue evolution in amniotes. These findings show that the classical divide between the mammalian gomphosis and reptilian ankylosis is not a distinction between complex and simple teeth, but two ends of a spectrum in the evolution and development of amniote tooth attachment.
Supplementary Material
Acknowledgements
We thank B. Zipfel (Evolutionary Studies Institute), D. Evans (ROM), K. Seymour (ROM), B. Iwama ROM), A. Henrici (CM) and M. Silcox (University of Toronto), for assistance in museum collections and with specimen acquisition. We are also grateful to D. Scott who provided images and preparation of several of the fossils and A. Reid for assistance with the graph in figure 3. We also thank C. Sidor and B. Hammer for providing tapinocephalid and Lystrosaurus material for analysis. We also thank B. Zipfel (ESI) for the loan of the Evolutionary Studies Institute (South Africa) material studied herein. M. Caldwell and D. O. Lamoureux also provided insightful discussions and comments that improved the manuscript.
Ethics
SAHRA (permit ID 1945) authorized the exportation and preparation of the specimens from the Evolutionary Studies Institute in Johannesburg, South Africa. These specimens and resulting thin sections have because been returned to their collections. Thin sections of extant mammals were taken from slide collections at the University of Alberta and did not require ethical approval.
Data accessibility
CT scan data for this study are available on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.v23v5d2 [27].
Authors' contributions
A.R.H.L. conceived the study. A.R.H.L., K.S.B., M.R.W., F.A. and R.R.R. collected and contributed histological and CT scan data. A.R.H.L., K.S.B., M.R.W., F.A. and R.R.R. interpreted the data. A.R.H.L. wrote the manuscript. K.S.B., M.R.W., F.A. and R.R.R. edited the manuscript.
Competing interests
The authors have no competing interests to declare.
Funding
A.R.H.L. was supported by NSERC graduate scholarship (PGSD3-410211-2011) and QEII/Dr F. M. Hill and QEII/Pfizer Graduate Scholarships in Science and Technology. K.S.B. was supported by the Michael Smith Foundation for Health Research. A.R.H.L. and K.S.B. also thank the Killam Trustees for Killam Postdoctoral Fellowships at the University of Alberta and University of British Columbia, respectively. Research support was also provided by NSERC Discovery and SRO Grants (Canada) to R.R.R. and National Research Foundation (South Africa) and Conicet (Argentina) to F.A.
References
- 1.Crompton AW, Jenkins FA. 1968. Minor occlusion in Late Triassic mammals. Biol. Rev. 43, 427–458. ( 10.1111/j.1469-185X.1968.tb00966.x) [DOI] [PubMed] [Google Scholar]
- 2.Weller JM. 1968. Evolution of mammalian teeth. J. Paleontol. 42, 268–290. [Google Scholar]
- 3.Kemp TS. 1982. Mammal-like reptiles and the origin of mammals. London, UK: Academic Press Inc. [Google Scholar]
- 4.Kemp TS. 2006. The origin and early radiation of the therapsid mammal-like reptiles: a palaeobiological hypothesis. J. Evol. Biol. 19, 1231–1247. ( 10.1111/j.1420-9101.2005.01076.x) [DOI] [PubMed] [Google Scholar]
- 5.Sidor CA, Hopson JA. 1998. Ghost lineages and ‘mammalness’: assessing the temporal pattern of character acquisition in the Synapsida. Paleobiol. 24, 254–273. [Google Scholar]
- 6.Abdala F, Jasinoski SC, Fernandez V. 2013. Ontogeny of the early triassic cynodont Thrinaxodon liorhinus (Therapsida): dental morphology and replacement. J. Vertebr. Paleontol. 33, 1408–1431. ( 10.1080/02724634.2013.775140) [DOI] [Google Scholar]
- 7.Osborn JW, Crompton AW. 1973. The evolution of mammalian from reptilian dentitions. Brevoria 399, 1–18. [Google Scholar]
- 8.Owen R. 1840. Odontography; or, a treatise on the comparative anatomy of the teeth; their physiological relations, mode of development, and microscopic structure, in the vertebrate animals. London, UK: Hippolyte Bailliere. [PMC free article] [PubMed] [Google Scholar]
- 9.Peyer B. 1968. Comparative odontology. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 10.Osborn JW. 1984. From reptile to mammal: evolutionary considerations of the dentition with emphasis on tooth attachment. Symp. Zool. Soc. Lond. 52, 549–574. [Google Scholar]
- 11.Gaengler P, Metzler E. 1992. The periodontal differentiation in the phylogeny of teeth – an overview. J. Periodontal Res. 27, 214–225. ( 10.1111/j.1600-0765.1992.tb01671.x) [DOI] [PubMed] [Google Scholar]
- 12.Beertsen W, McCulloch CA, Sodek J. 1997. The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 13, 20–40. ( 10.1111/j.1600-0757.1997.tb00094.x) [DOI] [PubMed] [Google Scholar]
- 13.Saffar J-L, Lasfargues J-J, Cherruau M. 1997. Alveolar bone and the alveolar process: the socket that is never stable. Periodontology 13, 76–90. ( 10.1111/j.1600-0757.1997.tb00096.x) [DOI] [PubMed] [Google Scholar]
- 14.Ho SP, Kurylo MP, Fong TK, Lee SSJ, Wagner HD, Ryder MI, Marshall GW. 2010. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials 31, 6635–6646. ( 10.1016/j.biomaterials.2010.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosshardt DD, Bergomi M, Vaglio G, Wiskott A. 2008. Regional structural characteristics of bovine periodontal ligament samples and their suitability for biomechanical tests. J. Anat. 212, 319–329. ( 10.1111/j.1469-7580.2008.00856.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller WA. 1968. Periodontal attachment apparatus in the young Caiman sclerops. Arch. Oral Biol. 13, 735–743. ( 10.1016/0003-9969(68)90091-5) [DOI] [PubMed] [Google Scholar]
- 17.McIntosh JE, Anderton X, Flores-De-Jacoby L, Carlson DS, Shuler CF, Diekwisch TGH. 2002. Caiman periodontium as an intermediate between basal vertebrate ankylosis-type attachment and mammalian ‘true’ periodontium. Microsc. Res. Tech. 59, 449–459. ( 10.1002/jemt.10222) [DOI] [PubMed] [Google Scholar]
- 18.Kvam T. 1960. The teeth of Alligator mississippiensis (Daud) VI. Periodontium. Acta Odontol. Scand. 18, 67–82. ( 10.3109/00016356009026092) [DOI] [PubMed] [Google Scholar]
- 19.Luan X, Walker C, Dangaria S, Ito Y, Druzinsky R, Jarosius K, Lesot H, Rieppel O. 2009. The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evol. Dev. 11, 247–259. ( 10.1111/j.1525-142X.2009.00327.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBlanc ARH, Reisz RR, Brink KS, Abdala F. 2016. Mineralized periodontia in extinct relatives of mammals shed light on the evolutionary history of mineral homeostasis in periodontal tissue maintenance. J. Clin. Periodontol. 43, 323–332. ( 10.1111/jcpe.12508) [DOI] [PubMed] [Google Scholar]
- 21.de Ricqlès A, Bolt JR. 1983. Jaw growth and tooth replacement in Captorhinus aguti (Reptilia: Captorhinomorpha): a morphological and histological analysis. J. Vertebr. Paleontol. 3, 7–24. ( 10.1080/02724634.1983.10011952) [DOI] [Google Scholar]
- 22.Gaengler P. 2000. Evolution of tooth attachment in lower vertebrates to tetrapods. In Development, function and evolution of teeth (eds Teaford MF, Smith MM, Ferguson MWJ), pp. 173–185. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.LeBlanc ARH, Lamoureux DO, Caldwell MW. 2017. Mosasaurs and snakes have a periodontal ligament: timing and extent of calcification, not tissue complexity, determines tooth attachment mode in reptiles. J. Anat. 231, 869–885. ( 10.1111/joa.12686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopson JA. 1969. The origin and adaptive radiation of mammal-like reptiles and nontherian mammals. Ann. N. Y. Acad. Sci. 167, 199–216. ( 10.1111/j.1749-6632.1969.tb20445.x) [DOI] [Google Scholar]
- 25.Reisz RR. 2006. Origin of dental occlusion in tetrapods: signal for terrestrial vertebrate evolution? J. Exp. Zoolog. B Mol. Dev. Evol. 306B, 261–277. ( 10.1002/jez.b.21115) [DOI] [PubMed] [Google Scholar]
- 26.Jasinoski SC, Abdala F. 2016. Cranial ontogeny of the early triassic basal cynodont Galesaurus planiceps. Anat. Rec. 300, 353–381. ( 10.1002/ar.23473) [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc ARH, Brink KS, Whitney MR, Abdala F, Reisz RR. 2018. Data from: Dental ontogeny in extinct synapsids reveals a complex evolutionary history of the mammalian tooth attachment system Dryad Digital Repository. ( 10.5061/dryad.v23v5d2) [DOI] [PMC free article] [PubMed]
- 28.LeBlanc ARH, Brink KS, Cullen TM, Reisz RR. 2017. Evolutionary implications of tooth attachment versus tooth implantation: a case study using dinosaur, crocodilian, and mammal teeth. J. Vertebr. Paleontol. 37, e1354006 ( 10.1080/02724634.2017.1354006) [DOI] [Google Scholar]
- 29.Gow CE. 1980. The dentitions of the Tritheledontidae (Therapsida: Cynodontia). Proc. R. Soc. Lond. B Biol. Sci. 208, 461–481. ( 10.1098/rspb.1980.0063) [DOI] [PubMed] [Google Scholar]
- 30.Shubin NH, Crompton AW, Sues H-. D, Olsen PE. 1991. New fossil evidence on the sister-group of mammals and early mesozoic faunal distributions. Science 4997, 1063–1065. ( 10.1126/science.251.4997.1063) [DOI] [PubMed] [Google Scholar]
- 31.Jasinoski SC, Chinsamy A. 2012. Mandibular histology and growth of the nonmammaliaform cynodont Tritylodon. J. Anat. 220, 564–579. ( 10.1111/j.1469-7580.2012.01494.x) [DOI] [Google Scholar]
- 32.Patterson C. 1988. Homology in classical and molecular biology. Mol. Biol. Evol. 5, 603–625. [DOI] [PubMed] [Google Scholar]
- 33.Patterson C. 1982. Morphological characters and homology. In Problems of phylogenetic reconstruction (eds Joysey KA, Friday AE), pp. 21–74. New York, NY: Academic Press. [Google Scholar]
- 34.Osborn JW. 1977. The interpretation of patterns in dentitions. Biol. J. Linn. Soc. 9, 217–229. ( 10.1111/j.1095-8312.1977.tb00266.x) [DOI] [Google Scholar]
- 35.Edmund AG. 1960. Tooth replacement phenomena in the lower vertebrates. R. Ont. Mus. Life Sci. Div. Contrib. 52, 1–190. [Google Scholar]
- 36.Bolt JR, Demar RE. 1983. Simultaneous tooth replacement in Euryodus and Cardiocephalus (Amphibia: Microsauria). J. Paleontol. 57, 911–923. [Google Scholar]
- 37.Lawson R, Wake DB, Beck NT. 1971. Tooth replacement in the red-backed salamander, Plethodon cinereus. J. Morphol. 134, 259–270. ( 10.1002/jmor.1051340302) [DOI] [PubMed] [Google Scholar]
- 38.Nanci A. 2013. Ten cate's oral histology: development, structure, and function, 8th edn St Louis, MO: Elsevier Mosby. [Google Scholar]
- 39.McKinney ML, McNamara KJ. 1991. Heterochrony: the evolution of ontogeny. New York, NY: Plenum Press. [Google Scholar]
- 40.Crompton AW. 1989. The evolution of mammalian mastication. In Complex organismal functions: integration and evolution in vertebrates (eds Wake DB, Roth G), pp. 23–40. Washington, DC: John Wiley & Sons Ltd. [Google Scholar]
- 41.Crompton AW. 1995. Masticatory function in nonmammalian cynodonts and early mammals. In Functional morphology in vertebrate paleontology (ed. Thomason JJ.), pp. 55–75. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Diekwisch TG. 2001. The developmental biology of cementum. Int. J. Dev. Biol. 45, 695–706. [PubMed] [Google Scholar]
- 43.Lim WH, Liu B, Mah S-J, Chen S, Helms JA. 2014. The molecular and cellular effects of ageing on the periodontal ligament. J. Clin. Periodontol. 41, 935–942. ( 10.1111/jcpe.12277) [DOI] [PubMed] [Google Scholar]
- 44.Lim WH, Liu B, Mah S, Yin X, Helms JA. 2015. Alveolar bone turnover and periodontal ligament width are controlled by Wnt. J. Periodontol. 86, 319–326. ( 10.1902/jop.2014.140286) [DOI] [PubMed] [Google Scholar]
- 45.Berkovitz B, Shellis P. 2017. The teeth of non-mammalian vertebrates. London, UK: Academic Press. [Google Scholar]
- 46.Fong RKM, LeBlanc ARH, Berman DS, Reisz RR. 2016. Dental histology of Coelophysis bauri and the evolution of tooth attachment tissues in early dinosaurs: dinosaur dental histology. J. Morphol. 277, 916–924. ( 10.1002/jmor.20545) [DOI] [PubMed] [Google Scholar]
- 47.Chatterjee S. 1974. A rhynchosaur from the Upper Triassic Maleri formation of India. Proc. R. Soc. Lond. B 267, 209–261. ( 10.1098/rstb.1974.0001) [DOI] [PubMed] [Google Scholar]
- 48.MacDougall MJ, LeBlanc ARH, Reisz RR. 2014. Plicidentine in the early Permian parareptile Colobomycter pholeter, and its phylogenetic and functional significance among coeval members of the clade. PLoS ONE 9, e96559 ( 10.1371/journal.pone.0096559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forasiepi AM, Sanchez-Villagra MR. 2014. Heterochrony, dental ontogenetic diversity, and the circumvention of constraints in marsupial mammals and extinct relatives. Paleobiology 40, 222–237. ( 10.1666/13034) [DOI] [Google Scholar]
- 50.von Koenigswald W. 2016. Specialized wear facets and late ontogeny in mammalian dentitions. Hist. Biol. 30, 7–29. ( 10.1080/08912963.2016.1256399) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- LeBlanc ARH, Brink KS, Whitney MR, Abdala F, Reisz RR. 2018. Data from: Dental ontogeny in extinct synapsids reveals a complex evolutionary history of the mammalian tooth attachment system Dryad Digital Repository. ( 10.5061/dryad.v23v5d2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
CT scan data for this study are available on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.v23v5d2 [27].