Abstract
Urbanization causes the simplification of natural habitats, resulting in animal communities dominated by exotic species with few top predators. In recent years, however, many predators such as hawks, and in the US coyotes and cougars, have become increasingly common in urban environments. Hawks in the Accipiter genus, especially, are recovering from widespread population declines and are increasingly common in urbanizing landscapes. Our goal was to identify factors that determine the occupancy, colonization and persistence of Accipiter hawks in a major metropolitan area. Through a novel combination of citizen science and advanced remote sensing, we quantified how urban features facilitate the dynamics and long-term establishment of Accipiter hawks. Based on data from Project FeederWatch, we quantified 21 years (1996–2016) of changes in the spatio-temporal dynamics of Accipiter hawks in Chicago, IL, USA. Using a multi-season occupancy model, we estimated Cooper's (Accipiter cooperii) and sharp-shinned (A. striatus) hawk occupancy dynamics as a function of tree canopy cover, impervious surface cover and prey availability. In the late 1990s, hawks occupied 26% of sites around Chicago, but after two decades, their occupancy fluctuated close to 67% of sites and they colonized increasingly urbanized areas. Once established, hawks persisted in areas with high levels of impervious surfaces as long as those areas supported high abundances of prey birds. Urban areas represent increasingly habitable environments for recovering predators, and understanding the precise urban features that drive colonization and persistence is important for wildlife conservation in an urbanizing world.
Keywords: Accipiter hawks, colonization, dynamic occupancy models, Landsat, prey abundance, urbanization
1. Introduction
Close to 4 billion people live in urban areas, and that number will grow to 6.5 billion by 2070 [1,2]. Urbanization is the most irreversible of anthropogenic disturbances [3,4], and as cities expand across the planet, urban ecosystems are becoming an increasingly important environmental setting for shaping ecological processes [5–7]. Although urbanization fundamentally alters biodiversity, urban and peri-urban areas offer novel habitats and food resources for a number of species dependent on characteristics of the urban environment [7]. For example, cities with more green spaces [8,9] or cities close to more intact, natural areas [10,11] generally support a higher abundance and diversity of plants and animals.
In recent decades, many predators recovering from low population sizes due to years of persecution [12] and depressed reproduction due to biomagnification of pesticides in food chains [13] are repatriating natural landscapes and colonizing urban environments. Residential yards and city parks provide suitable habitat for recovering predators such as coyotes (Canis latrans) [14], cougars (Puma concolor) [15] and merlins (Falco columbarius) [16]. Many of these predators not only use urban environments [17] but reach high densities and successfully reproduce [18–22]. For example, peregrine falcons (Falco peregrinus) were historically rare in eastern North America [23] and thought to be extirpated in the mid-1960s [24,25], but after successful captive breeding and reintroduction, they have become common in cities, taking advantage of artificial nesting sites (e.g. window ledges and suspension bridges) and ample prey [26].
Urbanization creates a complex mosaic of impervious surfaces (ground covered by impermeable surfaces such as buildings and roads) interspersed with green spaces (the inverse of imperviousness) of natural and semi-natural habitats [27,28]. Green spaces encompass a diversity of urban open spaces such as city parks, golf courses, cemeteries and abandoned lots [9]. This diversity of both natural and human-modified land cover probably plays a role in how and where predators establish within the urban landscape [29]. For example, in Chicago, IL, USA, mesocarnivores were more likely to colonize and persist in green spaces with higher tree canopy cover and lower housing density than more developed areas [11]. Likewise, the recent colonization of northern goshawks (Accipiter gentilis L.) into Hamburg, Germany was facilitated by afforestation and forest maturation [30]. Thus, despite the presence of built structures and high human density, it is evident that green spaces within cities represent available habitat for recovering predator populations.
In addition to green spaces, urban areas offer food resources that can be highly concentrated and predictable in time and space [31,32], and predators that exploit such resources are rewarded with ample prey [33–36]. For example, after the recovery from a rabies outbreak that swept through central Europe in the late 1960s, red foxes (Vulpes vulpes) shifted to breeding in developed areas and took advantage of anthropogenic food resources, which allowed them to persist in urban environments [34]. Similarly, in Maharashtra, India, leopards (Panthera pardus) persist in urban settings due to the high density of domestic animals that comprise 87% of their diet [37]. Millions of households in cities throughout the world feed wild birds and sustain their populations at elevated densities [38]. This hyperabundance of prey, supported by supplementary feeding, provides an important and predictable food resource for avian predators, such as Cooper's (Accipiter cooperii) and sharp-shinned (A. striatus) hawks that are often attracted to the high levels of bird activity at feeders [39]. Raptors persisting in urban environments that feed primarily on bird species (as opposed to small mammals) have relatively high breeding performance, suggesting that prey availability is an important factor in determining their success [40].
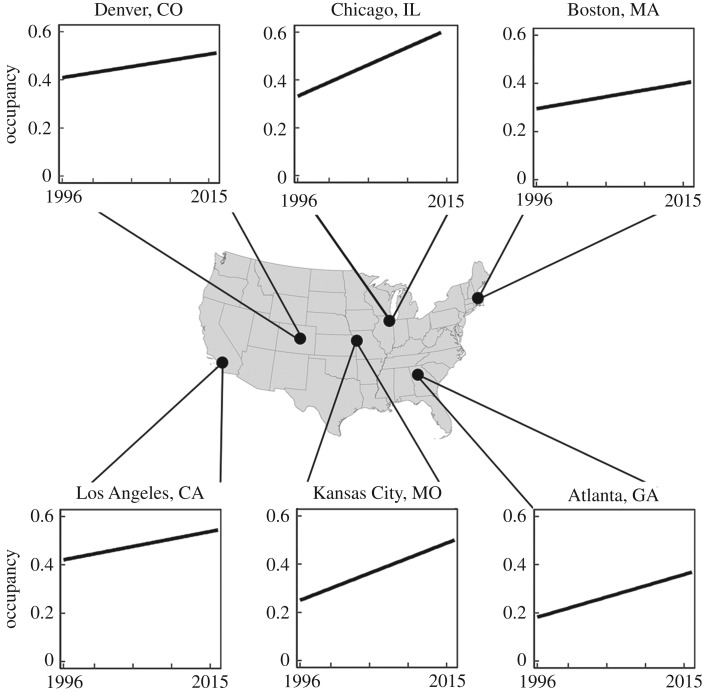
In the mid-twentieth century, Accipiter hawks mirrored the widespread declines of other recovering predators, but their populations are rebounding to the point where they are now increasingly common in urban environments [41]. The increase of hawk populations in cities is a widespread phenomenon across the USA (figure 1), and yet dynamics and mechanisms underlying the colonization and use of these urban landscapes remains unclear [42]. Furthermore, Accipiter hawks are emblematic of the broader suite of recovering and highly adaptable predators colonizing urban environments. They are wide-ranging predators that inhabit a variety of woodland areas and prey on a diversity of bird species [43–45]. Both hawk species share many of the same habitat and prey preferences, but whereas Cooper's hawks are primarily year-round residents in the eastern USA [46], the distribution of sharp-shinned hawks is a mix of winter and year-round residence [43]. Accipiter species are typically ‘perch-and-scan’ hunters that take mostly medium- to large-bodied avian prey [42–44,47], and in urban areas, they have a propensity to forage at bird feeders. Given their ability to prey on a diversity of species and acclimate to different habitats, Accipiter hawks are exemplary species with which to examine the factors facilitating the colonization and persistence of recovering predators in an urban environment.
Figure 1.
Pattern of hawk occupancy (the proportion of sites at which an Accipiter hawk was present during one or more sampling periods) during 1996–2016 in six cities across the United States. Yearly occupancy was calculated using raw Project FeederWatch counts.
Herein, we explore which spatio-temporal features of the urban environment facilitate the colonization and persistence of wintering Accipiter hawks in Chicago. Although previous research has suggested that green spaces and prey resources are important constraints on predators occurring in urban areas [29,30], few studies have explicitly tested the dynamic role of these limitations on predator establishment. We hypothesized that the distribution of tree cover, imperviousness and prey availability would greatly influence long-term trends and patterns of establishment (colonization and persistence) of Accipiter hawks in a major metropolitan area. Specifically, we predicted that areas with higher tree canopy cover would facilitate hawk colonization (i.e. initial arrival) for these woodland predators whereas colonization would be impeded by high amounts of imperviousness. However, their persistence (i.e. ongoing use) of urban areas would require high prey abundance and availability of their preferred prey, with the expectation that higher numbers of backyard birds would promote their persistence in this novel environment.
2. Material and methods
(a). Study area
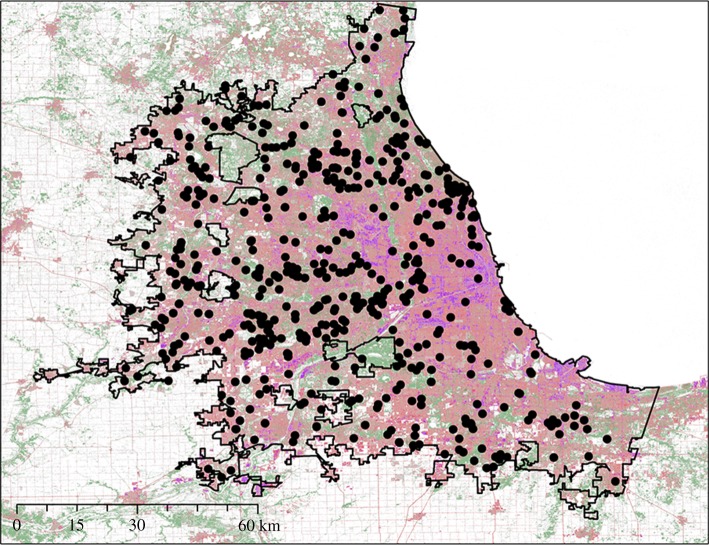
We studied the winter establishment dynamics of Accipiter hawks in Chicago, IL, USA (figure 2), which was well sampled by our citizen science programme (see Project FeederWatch section). The Chicago metropolitan area is expansive and has a gradient from high- to low-density development in which to investigate the effects of the urban environment on hawk establishment. Winters in Chicago are typically cold and variable [48], with daily temperatures ranging from –10.8 to −29.1°C [49]. Chicago has a diversity of green spaces, but only 14% of the city features tree cover. We delineated our study region using the 2016 Census Bureau's TIGER/Line urban areas shapefile [50] (figure 2). Within this dataset, urban areas were represented by densely developed areas encompassing residential, commercial and other non-residential uses supporting more than 50 000 people [50].
Figure 2.
Map depicting the urban study area of Chicago, IL. Black dots represent Project FeederWatch sites from 1996 to 2016. Percentage imperviousness is shown in pink (low) to purple (high) and percentage tree canopy cover is displayed in green (darker green indicates higher percentage tree canopy cover).
(b). Project FeederWatch
Observations of Accipiter hawks and prey species came from Project FeederWatch, a citizen science programme operated by the Cornell Lab of Ornithology and Bird Studies Canada (www.feederwatch.org). The program is designed to study changes in the distribution and abundance of birds in winter across North America. Wells et al. [51] reported complete details of the protocol. We chose this winter-based survey because of its many advantages over other broad-scale programmes such as atlases or the North American Breeding Bird Survey. First, repeated counts are collected at fixed locations, second, counts are dynamic, third, survey effort is recorded, and lastly, the programme adequately samples the urban environment. From early November to late April, programme participants recorded the maximum number of each species seen at their supplemental feeding stations (hereafter sites) during a 2-day count and the number of hours (less than 1, 1–4, 4–8, greater than 8) they spent observing their feeder. Participants reported the geographical location of their feeders using online mapping tools, geographical positioning systems or address information. We excluded any sites where the location information was missing or imprecise. To further reduce potential location error, we also combined feeders that were within 25 m of each other. Counts were repeated throughout the season and separated by a minimum of five days, totalling 22 weeks. We used data collected during the winter seasons (k = 1996, … , 2016), from all feeder sites (i = 1, … , M) within the study region, for a total of 554 sites. To decrease the inclusion of migrating hawks in our sample, we selected only observations recorded during December through February, resulting in 13 weeks of replicate surveys (j = 1, … , J). Sharp-shinned and Cooper's hawks are difficult to differentiate due to their similar size and plumage. Therefore, we combined observations of both species (sharp-shinned = 1082, Cooper's = 4220). We collapsed count observations to identify a site as occupied (yi,j,k = 1) for site i, survey j, year k, if hawk abundance was greater than 0, and as unoccupied (yi,j,k = 0) if no hawks were observed.
(c). Defining urban features
Our model of hawk establishment dynamics included measures of tree canopy cover, imperviousness, prey availability, annual and weekly temperatures and sampling effort at each FeederWatch site. Minimum winter temperatures were included to control for variation in temperature across years as cold winters could facilitate hawk colonization [30]. Temperatures during the week of the survey and participant effort were expected to impact detection of hawks at the feeders [35,45,52].
We considered impervious surface as the inverse of green space and calculated annual imperviousness and tree canopy cover only. We assessed annual imperviousness and tree canopy cover for Chicago using LandSat data. First, we downloaded all available LandSat TM/ETM+/OLI surface reflectance in L1T format covering Chicago (Landsat path/row 023/031). We then masked out cloud, snow and shadow using LandSat QA band, and calculated the mean for each reflectance band for each year [53]. To reduce uncertainties, we included ±1-year imagery around the target years. Next, we obtained ground reference data using a 1 m resolution land cover map provided by Chicago Metropolitan Agency for Planning (CMAP) [54] and calculated per-pixel percentage of impervious surface and tree canopy cover at the Landsat scale to generate a set of temporally consistent training samples [55]. Using a random forest regression tree, we quantified imperviousness and tree canopy cover for each pixel annually from 1996 to 2016. We then averaged annual 30 m imperviousness and tree canopy cover layers to create a time series for each variable at a spatial unit size of 3 km2 (300 hectares). The landscape size approximated the winter home range size of Cooper's hawks [56]. We smoothed annual imperviousness (impi,k) and tree canopy cover (treei,k) time series using a trajectory approach [57]. See electronic supplemental material, figure S1 for accuracy assessment of the layers.
Daily minimum temperatures (°C) were extracted from the PRISM climate dataset, a gridded climate model with a 4 × 4 km resolution [58] for winters (December–February) of 1996–2016. Annual mean minimum temperatures (minTempk) were aggregated across Chicago, while weekly mean minimum temperatures (tmini,j,k) were extracted for each feeder site. Participant effort (efforti,j,k) was included as five categorical levels (less than 1, 1–4, 4–8, greater than 8 h, NA), where the fifth level included observations with missing effort.
We used two annual metrics of prey availability: (1) average prey abundance (preyAbundi,k) calculated as the maximum number of prey recorded each week then averaged across survey weeks each year; and (2) average prey biomass (preyMassi,k) calculated by weighting total prey abundance by species-specific body masses (from: Sibley [59] and iBird [60]).
We tested for collinearity among predictors using Pearson's product correlations coefficient (r). Tree canopy cover and impervious surface were negatively correlated across the years (r = −0.59 to −0.76). Only 30% of the years (1996–2000, 2009) were higher than >±0.7, a commonly used threshold for collinearity [61], however, the overall correlation coefficient was −0.66. Therefore, we included both landscape variables in the global model. Other predictors were not highly correlated (r = ± <0.7).
(i). Hawk establishment model
We evaluated whether urban features influenced colonization and persistence of Accipiter hawks during 1996–2016 using a dynamic occupancy model implemented within a Bayesian framework [62]. Dynamic occupancy models have two main components: (i) an observational sub-model accounting for imperfect detection of hawk site visitation; and (ii) an ecological sub-model estimating true site occupancy/establishment dynamics as the product of two Markovian (i.e. autoregressive) processes: site colonization and persistence [63]. Observed occupancy, γi,j,k, was the result of site i being occupied in year k by a hawk, zi,k, and the probability of a hawk being detected at a feeder, pi,j,k, in a given j replicate survey week,
The probability of detecting a hawk was in turn related to survey effort, efforti,j,k and temperature at the time of the survey, tmini,j,k, through a logit-link function,
Effort was incorporated as categorical intercepts, which represent mean detection probabilities for each level of effort. Vague priors were assumed for the hyperparameters: 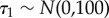 and
and  . True occupancy, zi,k, at site i, year k, was in turn related in the ecological sub-model to the probability of occupancy, ψi,k. In the initial year (k = 1996),
. True occupancy, zi,k, at site i, year k, was in turn related in the ecological sub-model to the probability of occupancy, ψi,k. In the initial year (k = 1996),
with the probability of occupancy in turn having a Uniform prior, 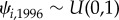 . For the remaining years (k = 1997–2016), occupancy, zi,k, was conditional on occupancy in the previous year,
. For the remaining years (k = 1997–2016), occupancy, zi,k, was conditional on occupancy in the previous year,
The probability of occupancy, ψi,k, in turn depended on the probability that site i persisted as occupied, ϕi,k, given it was occupied in the previous year k − 1, or if it was unoccupied (1 − zi,k−1), on the probability that it was colonized, γi,k [62]:
We related all urban features to the probabilities of colonization and persistence using logit-link functions:
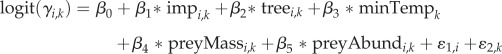 |
and
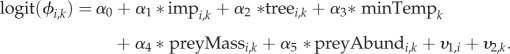 |
Predictors were standardized by dividing their means by one standard deviation to aid convergence and comparison among coefficients [64]. Intercepts were modelled as mean effects, 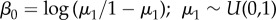 , and
, and 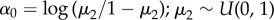 . The n = 1, … , 5 coefficients were given normal priors
. The n = 1, … , 5 coefficients were given normal priors 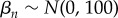 ,
, 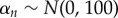 . The repeated-measures design was accounted for using random site ID and year effects:
. The repeated-measures design was accounted for using random site ID and year effects: 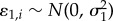 and
and 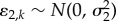 with
with 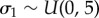 ,
, 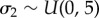 ,
, 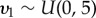 and
and 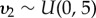 . Missing values were introduced in the measures of prey availability due to the variation in participant retention over the duration of the study. Missing values were estimated by sharing information from the observed values by assigning them common priors [65]. The priors assumed that each prey measure had a Normal distribution with mean and variance values that were common among site and years:
. Missing values were introduced in the measures of prey availability due to the variation in participant retention over the duration of the study. Missing values were estimated by sharing information from the observed values by assigning them common priors [65]. The priors assumed that each prey measure had a Normal distribution with mean and variance values that were common among site and years: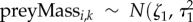 ) and
) and 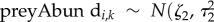 ). The associated standard deviations were given vague priors:
). The associated standard deviations were given vague priors: 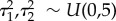 .
.
Posterior distributions of the model parameters were estimated using Markov chain Monte Carlo (MCMC) methods available in program JAGs [66], which we called from R [67] with jagsUI [68]. We ran three parallel chains of 120 000 iterations each, with a thinning rate of 30 and 15 000 burn-in. We tested a range of priors for both alpha and beta parameters (results not shown) as an informal regularization approach and found little variation in the estimates. Therefore, we use 95% credible intervals to assess significant parameters. Model convergence was determined visually using the MCMC trace plots and by ensuring the Gelman–Rubin statistic was less than 1.1 [69]. We used parameter estimates to predict and map the establishment dynamics of hawks at a 3 km grid-cell resolution for other parts of the city that were not sampled. Since we estimated our prey availability metrics at the site level, we held them at the year-specific mean when making spatial predictions. To evaluate whether overlapping landscapes (territory buffers) violates the assumption of independence of residual errors we assessed spatial autocorrelation of model residuals using a Moran's I test separately for each year to account for the different number of sites among years (μ = 108.6, s.d. = 29.2, min = 42, max = 141 sites per year). We found no evidence of spatial autocorrelation (p > 0.05) in either colonization or persistence model residuals.
3. Results
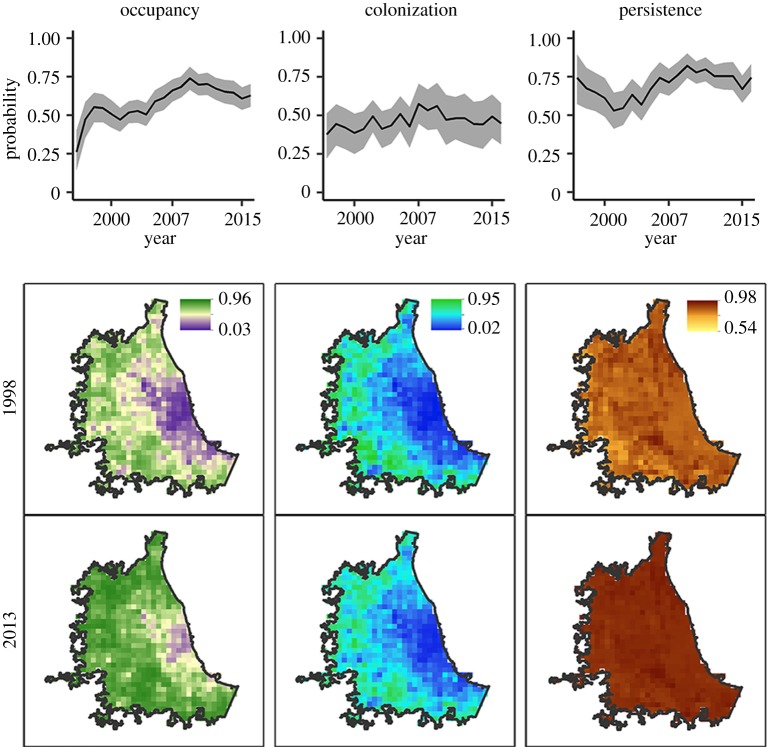
The probability of Accipiter hawk occupancy exhibited a growth phase between 1996 and 2009, increasing from 26% (95% CI = 14–40%) to 74% (95% CI = 67–81%). After 2009, occupancy stabilized around 67% (figure 3). The probability of hawk occupancy was greatest at the periphery of the city and lowest in the city centre (figure 3). During the study, the probability of colonization increased from 38% (22–51%) to a high of 58% (95% CI = 45–71%) in 2007 (figure 3). Similarly, persistence was high during this time, ranging from 53% (95% CI = 41–65%) in 2001 to a high of 82% (95% CI = 74–90%) in 2009 (figure 3). As occupancy stabilized, colonization and persistence also levelled off at 47% and 75%, respectively (figure 3). Colonization was highest outside the city centre, and this pattern remained relatively constant over time, but persistence was more widespread in the later years (figure 3). Detection probability was negatively, but only marginally significantly (CI overlapped 0 by less than or equal to 0.05), related to minimum weekly temperature, indicating hawks were more likely to be detected at feeders during periods of colder temperatures (table 1). As expected, the low effort resulted in low detection probability (electronic supplementary material, table S1).
Figure 3.
Spatio-temporal patterns in the predicted probability of Accipiter occupancy, colonization and persistence in Chicago, IL, USA. The first row depicts the estimated probabilities across the duration of the study (1996–2016), and grey shading represents the 95% credible intervals. Occupancy and colonization probabilities were the highest in the peri-urban areas surrounding the city centre. Persistence was fairly even throughout Chicago, particular in the later years.
Table 1.
Coefficient means and 95% credible intervals (CIs) for the model evaluating the effects of landscape, temperature and prey availability (excluding intercepts) on Accipiter hawk colonization and persistence in Chicago, IL, USA (1996–2016). Colonization was significantly negatively associated with imperviousness and tree canopy cover and positively associated with related to average prey abundance. Persistence was driven primarily by average prey abundance.
| coefficient | parameter | mean | 2.5% CI | 97.5% CI |
|---|---|---|---|---|
| detection parameters | ||||
| τ1 | minimum temperature | −0.04 | −0.08 | 0.01 |
| colonization parameters | ||||
| β1 | imperviousness | −1.16 | −1.71 | −0.69 |
| β2 | tree canopy cover | −0.52 | −1.03 | −0.05 |
| β3 | minimum temperature | −0.02 | −0.46 | 0.42 |
| β4 | prey biomass | 0.19 | −0.20 | 0.54 |
| β5 | average prey | 1.41 | 0.79 | 2.13 |
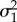 |
error for random year effect | 0.66 | 0.24 | 1.20 |
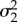 |
error for random site effect | 1.19 | 0.69 | 1.94 |
| persistence parameters | ||||
| α1 | imperviousness | 0.09 | −0.40 | 0.57 |
| α2 | tree canopy cover | 0.30 | −0.11 | 0.74 |
| α3 | minimum temperature | −0.15 | −0.82 | 0.48 |
| α4 | prey biomass | 0.25 | −0.14 | 0.69 |
| α5 | average prey | 3.66 | 2.60 | 4.96 |
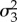 |
error for random year effect | 1.39 | 0.91 | 1.95 |
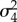 |
error for random site effect | 1.20 | 0.64 | 1.88 |
Urban development expanded throughout the Chicago area during the time of our study, but most of the increase in imperviousness occurred along the periphery of Chicago with the development of low-density peri-urban communities. The increase in imperviousness occurred before 2008 and there was little change in recent years (electronic supplementary material, figure S2). This stabilization in urbanization coincided with the leveling off in the occupancy rates of hawks (figure 3). During the same time, tree canopy cover increased in Chicago, mainly due to tree growth in peri-urban housing communities. However, throughout the whole of Chicago, we found minimal increases in imperviousness (25.9–29.8%) and tree canopy cover (21.5–29.2%) over the duration of the study.
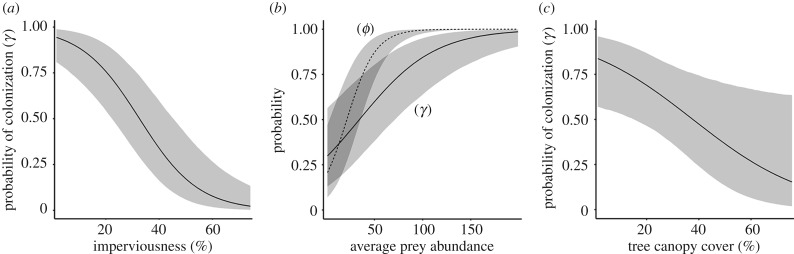
Both tree canopy cover and imperviousness influenced the colonization of Accipiter hawks. Hawks were less likely to colonize sites with the more impervious surface (table 1) and generally did not colonize sites with over 50% impervious surface (figures 4 and 5). Surprisingly, hawks were more likely to colonize landscapes with lower amounts of tree cover, but this effect was less important than imperviousness (table 1; figure 4). Average prey abundance, but not prey biomass, significantly influenced the probability of hawk colonization and persistence, and hawks were more likely to colonize and persist in sites with a higher abundance of feeder birds, regardless of mass (table 1 and figure 4). The effect of prey abundance was much stronger for persistence than it was for colonization (table 1). The probability of persistence increased at a higher rate with prey abundance than colonization, suggesting that hawks persisted at sites with fewer prey than what they needed to colonize (figure 4). Minimum yearly temperature had no effect on colonization or persistence (table 1).
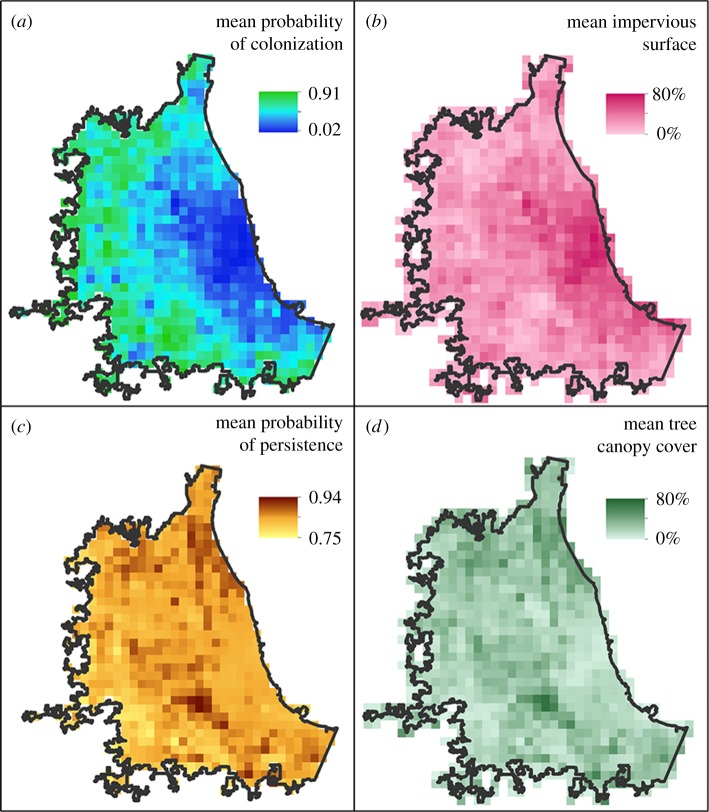
Figure 5.
The mean (a) probability of colonization, (b) impervious surface, (c) probability of persistence and (d) tree canopy cover from 1996 to 2016. Impervious surface and tree canopy cover cell values were aggregated using the mean value from a 30 m to a courser 3 km resolution to match the Accipiter winter territory size.
Figure 4.
Estimated probability of colonization and/or persistence of Accipiter hawks in Chicago as a function of (a) imperviousness, (b) prey abundance and (c) tree canopy cover. As levels of imperviousness and tree canopy cover increased, the probability of colonization decreased significantly. Prey abundance was a strong driver of both hawk colonization and persistence.
4. Discussion
Through a novel combination of citizen science and advanced remote sensing, we quantified the importance of urban features in facilitating the dynamics and long-term establishment of Accipiter hawks in a major metropolitan area. We found that urban features played an important role in the colonization and persistence of Accipiter hawks in Chicago. Hawks initially colonized along the outskirts of the city and were less likely to colonize areas of high imperviousness, avoiding heavily developed areas and landscapes with high tree cover while favouring sites with greater prey abundance (figures 3 and 5). Once hawks colonized a landscape, however, their persistence was dependent only on prey abundance.
During the early years of colonization, Accipiter hawks avoided sites with high imperviousness (less green space). This spatial patterning of occupancy demonstrated a clear avoidance of Chicago city centre and greater sustained rates of colonization around the periphery of the city (figures 3 and 5), similar to the spatial patterns of goshawk colonization in Hamburg, Germany [30]. Over time, hawks spread into highly urbanized areas and persisted across a range of urbanization (imperviousness 0–80%). Imperviousness not only reflects the amount of ground covered by impermeable surfaces, but it represents increased human disturbances (e.g. vehicular traffic, anthropogenic noise, domestic pets). Although human disturbance is known to negatively affect species living in natural [70] rural and urban environments [14,71], our results suggest that the hawks may have been more sensitive to these disturbances during the initial phases of colonization.
Contrary to our predictions, colonization was negatively associated with tree canopy cover; sites with less tree cover showed higher levels of colonization (figure 4). This result was unexpected because Accipiter hawks are generally considered to be forest-dependent species, at least during the breeding season. Little is known about their wintering habitat across their range [44], but wintering Cooper's hawks in Indiana preferred residential and grassy areas and used forested habitat less often than expected [72]. Perhaps the reduced dependence on trees is not surprising given that hawks are not nesting in winter, and their primary concern is survival. Food and their preferred prey species may be more readily available in residential areas where humans are providing subsidies for wild birds [47,72,73].
Prey availability can have lasting effects on demographic rates for predators [74–76] and seems especially important for predators colonizing urban environments [29,30,77]. We predicted hawks would preferentially seek out and colonize areas with high average prey abundance and biomass. Indeed, hawks colonized areas with high average prey abundance (figure 4), but prey biomass was not important suggesting that hawks were not selectively seeking out sites with larger-bodied prey. The lack of a relationship between prey biomass and establishment dynamics could be related to the differences in prey preferences between Cooper's and sharp-shinned hawks, which could not be analysed separately in our study. However, the abundance of potential prey is an important component affecting hawk colonization and persistence in Chicago as well as in other urban [30,73,78] and natural environments [79].
Predation is one of the primary forces shaping the species composition of many urban animal communities, as it is in natural settings [31]. Consequently, recovering predator species colonizing urban areas should have a profound effect on their prey communities. For example, Bell et al. [78] found that the recolonization of Eurasian sparrowhawks (Accipiter nisus) in Britain was correlated with a subsequent decline of house sparrow populations. The long-term association of house sparrows with urban areas caused a release from top-down regulation and a loss of selection favouring anti-predator behaviour, making them vulnerable to the more recent colonization of sparrowhawks [78]. In addition to the house sparrow, the recolonization of urban areas by sparrowhawks in Britain altered the abundance of many common feeder species [80]. Because prey availability was the main factor contributing to the occupancy of Accipiter hawk species in Chicago, it is likely the movement of hawks into the city has altered the prey community, as seen with sparrowhawks in Europe, although this remains to be tested. Many studies of urban-dwelling Accipiter hawks in North America have revealed their diet contained a large percentage of invasive bird species such as pigeon, European starling (Sturnus vulgaris) and house sparrow [22,47,81]. The potential for predators to reduce population sizes of overabundant or invasive species may reduce their competition pressure on native species for resources [29,36].
The USA is adding about 1 million acres of urban development, the equivalent of Los Angeles, Houston and Phoenix combined, each year [82]. As urbanization increases, there is a pressing need for more comprehensive studies of how the colonization of cities by predators is influenced by the spatio-temporal complexities of urban features. Here, we found that prey availability and patterns of imperviousness (the inverse of urban green spaces) were critical factors in Accipiter hawk establishment dynamics in a major metropolitan area. While the factors important in the urban establishment of predators will probably vary by city, determining the relative importance of various urban landscape features provides insight into how recovering predators adapt and colonize novel urban habitats. In addition, urban establishment studies will be important in guiding the management and design of metropolitan environments to facilitate species persistence in these human-modified landscapes.
Supplementary Material
Acknowledgements
This research would not have been possible without the contribution of the many Project FeederWatch participants. We thank J. Clare for statistical guidance. We acknowledge additional support from the Department of Forest and Wildlife Ecology, University of Wisconsin-Madison.
Data accessibility
The model used in this analysis can be found at https://github.com/jmccabe8/hawkDynOccModel. Annual tree canopy cover and impervious surface data can be downloaded at http://silvis.forest.wisc.edu/data/chicago. Project FeederWatch data can be requested at https://feederwatch.org/.
Authors' contributions
B.Z., J.D.M, H.Y., A.P., V.R. and D.N.B. conceived the ideas and designed the research. Avian data were provided by D.N.B. and H.Y. completed all remote sensing analysis. J.D.M. conducted all statistical analyses with input from J.C. and B.Z. J.D.M. and B.Z. wrote the initial draft of the manuscript, and all authors contributed to the editing and approved the final draft.
Competing interests
We declare we have no competing interests.
Funding
Funding for this research was provided through NASA's Citizen Science for Earth Systems programme (grant no. NNX17AI68A).
References
- 1.Acuto M, Parnell S. 2016. Leave no city behind. Science 352, 873 ( 10.1126/science.aag1385) [DOI] [PubMed] [Google Scholar]
- 2.Seto KC, Roberto S, Fragkias M. 2010. The new geography of contemporary urbanization and the environment. Annu. Rev. Environ. Resour. 35, 167–194. ( 10.1146/annurev-environ-100809-125336) [DOI] [Google Scholar]
- 3.Kowarik I. 2011. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 159, 1974–1983. ( 10.1016/j.envpol.2011.02.022) [DOI] [PubMed] [Google Scholar]
- 4.Radeloff VC, et al. 2015. The rise of novelty in ecosystems. Ecol. Appl. 25, 2051–2068. ( 10.1890/14-1781.1) [DOI] [PubMed] [Google Scholar]
- 5.Miller JR, Hobbs RJ. 2002. Conservation where people live and work. Conserv. Biol. 16, 330–337. ( 10.1046/j.1523-1739.2002.00420.x) [DOI] [Google Scholar]
- 6.Martin LJ, Blossey B, Ellis E. 2012. Mapping where ecologists work: biases in the global distribution of terrestrial ecological observations. Front. Ecol. Environ. 10, 195–201. ( 10.1890/110154) [DOI] [Google Scholar]
- 7.Ramalho CE, Hobbs RJ. 2012. Time for a change: dynamic urban ecology. Trends Ecol. Evol. 27, 179–188. ( 10.1016/j.tree.2011.10.008) [DOI] [PubMed] [Google Scholar]
- 8.Hahs AK, et al. 2009. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 12, 1165–1173. ( 10.1111/j.1461-0248.2009.01372.x) [DOI] [PubMed] [Google Scholar]
- 9.Aronson MFJ, et al. 2014. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 281, 20133330 ( 10.1098/rspb.2013.3330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germaine S, Rosenstock SS, Schweinsburg RE, Richardson WS. 1998. Relationships among breeding birds, habitat, and residential development in greater Tucson, Arizona. Ecol. Appl. 8, 680–691. ( 10.1890/1051-0761(1998)008%5B0680:RABBHA%5D2.0.CO;2) [DOI] [Google Scholar]
- 11.Gallo T, Fidino M, Lehrer EW, Magle SB. 2017. Mammal diversity and metacommunity dynamics in urban green spaces: implications for urban wildlife conservation. Ecol. Appl. 27, 2330–2341. ( 10.1002/eap.1611) [DOI] [PubMed] [Google Scholar]
- 12.Chapron G, Kaczensky P, Linnell JDC, von Arx M, Huber D, Andrén H, López-bao JV, Adamec M. 2014. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519. ( 10.1126/science.1257553) [DOI] [PubMed] [Google Scholar]
- 13.Cade TJ, Enderson JH, Thelander CG, White CM.. 1988. Peregrine falcon populations: their management and recovery. Boise, ID: Peregrine Fund.
- 14.Riley SPD, Sauvajot RM, Fuller TK, York EC, Kamradt DA, Bromley C, Wayne RK. 2003. Effects of urbanization and habitat fragmentation on bobcats and coyotes in Southern California. Conserv. Biol. 17, 566–576. ( 10.1046/j.1523-1739.2003.01458.x) [DOI] [Google Scholar]
- 15.Morrison CD, Boyce MS, Nielsen SE, Bacon MM. 2014. Habitat selection of a re-colonized cougar population in response to seasonal fluctuations of human activity. J. Wildl. Manage. 78, 1394–1403. ( 10.1002/jwmg.799) [DOI] [Google Scholar]
- 16.Oliphant LW, Haug E. 1985. Productivity, population density and rate of increase of an expanding Merlin population. Raptor Res. 19, 56–59. [Google Scholar]
- 17.Donihue CM, Lambert MR. 2015. Adaptive evolution in urban ecosystems. Ambio 44, 194–203. ( 10.1007/s13280-014-0547-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley SPD, Hadidian J, Manski DA. 1998. Population density, survival, and rabies in raccoons in an urban national park. Can. J. Zool. 76, 1153–1164. ( 10.1139/z98-042) [DOI] [Google Scholar]
- 19.Rosatte R, Macinnes MJ, Lawson KF. 1990. Rabies control for urban foxes, skunks, and raccoons. In Proceedings of the 14th Vertebrate Pest Conference (eds Davis LR, Marsh RE), pp. 160–167. Davis, CA: University of Califonrnia. [Google Scholar]
- 20.Harris S. 1981. An estimation of the number of foxes (Vulpes vulpes) in the city of Bristol, and some possible factors affecting their distribution. J. Appl. Ecol. 18, 455–465. ( 10.2307/2402406) [DOI] [Google Scholar]
- 21.Crooks KR. 2002. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv. Biol. 16, 488–502. ( 10.1046/j.1523-1739.2002.00386.x) [DOI] [Google Scholar]
- 22.Stout WE, Rosenfield RN. 2010. Colonization, growth, and density of a pioneer Cooper's hawk population in a large metropolitan environment. J. Raptor Res. 44, 255–267. ( 10.3356/JRR-09-26.1) [DOI] [Google Scholar]
- 23.Enderson JH, Heinrich W, Kiff L, White CM. 1995. Population changes in North American peregrines. Trans. North Am. Wildl. Nat. Resour. Conf. 1995, 142–161. [Google Scholar]
- 24.Berger DD, Sindelar CR, Gamble KE. 1969. The status of breeding peregrines in the eastern United States. In Peregrine falcon populations: their biology and decline (ed. Hickey JJ.), pp. 165–173. Madison, WI: University of Wisconsin Press. [Google Scholar]
- 25.Bollengier R. (ed.). 1979. Eastern peregrine falcon recovery plan. Washington, DC: US Department of the Interior, Fish and Wildlife Service. [Google Scholar]
- 26.Gahbauer MA, Box PO, Hx C. 2015. Productivity, mortality, and management of urban peregrine falcons in northeastern North America. J. Wildl. Manage. 79, 10–19. ( 10.1002/jwmg.803) [DOI] [Google Scholar]
- 27.Rottenborn SC. 1999. Predicting the impacts of urbanization on riparian bird communities. Biol. Conserv. 88, 289–299. ( 10.1016/S0006-3207(98)00128-1) [DOI] [Google Scholar]
- 28.Alberti M, Marzluff JM, Shulenberger E, Bradley G, Ryan C, Zumbrunnen C. 2003. Integrating human into ecology: opportunities and challenges for studying urban ecosytems. Bioscience 53, 1169–1179. ( 10.1641/0006-3568(2003)053%5B1169:IHIEOA%5D2.0.CO;2) [DOI] [Google Scholar]
- 29.Magle SB, Lehrer EW, Fidino M. 2016. Urban mesopredator distribution: examining the relative effects of landscape and socioeconomic factors. Anim. Conserv. 19, 163–175. ( 10.1111/acv.12231) [DOI] [Google Scholar]
- 30.Rutz C. 2008. The establishment of an urban bird population. J. Anim. Ecol. 78, 182–190. ( 10.1111/j.1365-2656.2007.0) [DOI] [PubMed] [Google Scholar]
- 31.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. ( 10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 32.Lewis DL, Baruch-Mordo S, Wilson KR, Breck SW, Mao JS, Broderick J. 2015. Foraging ecology of black bears in urban environments: guidance for human–bear conflict mitigation. Ecosphere 6, 1–18. ( 10.1890/ES15-00137.1) [DOI] [Google Scholar]
- 33.Fischer JD, Cleeton SH, Lyons TP, Miller JR, Fischer JD, Cleeton SH, Timothy P. 2012. Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. Bioscience 62, 809–818. ( 10.1525/bio.2012.62.9.6) [DOI] [Google Scholar]
- 34.Contesse P, Hegglin D, Gloor S, Bontadina F, Deplazes P. 2004. The diet of urban foxes (Vulpes vulpes) and the availability of anthropogenic food in the city of. Mamm. Biol. 69, 81–95. ( 10.1078/1616-5047-00123) [DOI] [Google Scholar]
- 35.Chamberlain DE, Vickery JA, Glue DE, Robinson RA, Conway GJ, Woodburn RJW, Cannon AR. 2005. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis (Lond. 1859) 147, 563–575. ( 10.1111/j.1474-919x.2005.00430.x) [DOI] [Google Scholar]
- 36.Bateman PW, Fleming PA. 2012. Big city life: carnivores in urban environments. J. Zool. 287, 1–23. ( 10.1111/j.1469-7998.2011.00887.x) [DOI] [Google Scholar]
- 37.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth KU. 2016. A cat among the dogs: leopard Panthera pardus diet in a human-dominated landscape in Western Maharashtra, India. Oryx 50, 156–162. ( 10.1017/S0030605314000106) [DOI] [Google Scholar]
- 38.Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ. 2008. Garden bird feeding predicts the structure of urban avian assemblages. Divers. Distrib. 14, 131–137. ( 10.1111/j.1472-4642.2007.00439.x) [DOI] [Google Scholar]
- 39.Dunn EH, Tessaglia DL. 1994. Predation of birds at feeders in winter. J. F. Onrithology 65, 8–16. [Google Scholar]
- 40.Kettel EF, Gentle LK, Quinn JL, Yarnell RW. 2017. The breeding performance of raptors in urban landscapes: a review and meta-analysis. J. Ornithol. 159, 1–18. ( 10.1007/s10336-017-1497-9) [DOI] [Google Scholar]
- 41.Rosenfield RN, Bielefeldt J, Affeldt JL, Bechmann DJ. 1995. Nesting density, nest area reoccupancy, and monitoring implications for Cooper's hawks in Wisconsin. J. Raptor Res. 29, 1–4. [Google Scholar]
- 42.Rosenfield RN, Mannan WR, Millsap BA. 2018. Cooper's hawks: the bold backyard hunters. In Urban raptors: ecology and conservation of birds of prey in cities (eds Boal CW, Dykstra CR), pp. 93–109. Washington, DC: Island Press. [Google Scholar]
- 43.Bildstein KL, Meyer KD. 2000. Sharp-shinned hawk (Accipiter striatus), version 2.0. In Birds of North America (ed. Rodewald PG.). Ithaca, NY: Cornell Lab Ornithol. See 10.2173/bna.482 (accessed 1 June 2018). [DOI] [Google Scholar]
- 44.Curtis OE, Rosenfield RN, Bielefeldt J.. 2006. Cooper's hawk (Accipiter cooperii). The birds of North America online. Cornell Lab Ornithol. See http://bna.birds.cornell.edu/bna/species/075. [Google Scholar]
- 45.Wiggers EP, Kritz KJ. 1991. Comparison of nesting habitat of coexisting sharp-shinned and Cooper's hawks in Missouri. Wilson Bull. 103, 568–577. [Google Scholar]
- 46.Boal CW, Dykstra CR. 2018. Urban raptors: ecology and conservation of birds of prey in cities. Washington, DC: Island Press. [Google Scholar]
- 47.Roth TC, Lima SL. 2003. Hunting behavior and diet of Cooper's hawks: an urban view of the small-bird-in-winter paradigm. Condor 105, 474–483. ( 10.1650/7219) [DOI] [Google Scholar]
- 48.Trewartha GT, Horn LH. 1980. Introduction to climate, 5th edn New York, NY: McGraw-Hill. [Google Scholar]
- 49.National Oceanic and Atmospheric Administration. In press. NOAA Online Weather Data. Silver Spring, MD: NOAA.
- 50.US Census Bureau. 2016. TIGER/Line Shapefiles (machine-readable data files). Suitland, MD: US Census Bureau.
- 51.Wells JV, Rosenberg KV, Dunn EH. 1998. Feeder counts as indicators of spatial and temporal variation in winter abundance of resident birds. J. F. Ornithol. 69, 577–586. [Google Scholar]
- 52.Zuckerberg B, Bonter DN, Hochachka WM, Koenig WD, DeGaetano AT, Dickinson JL. 2011. Climatic constraints on wintering bird distributions are modified by urbanization and weather. J. Anim. Ecol. 80, 403–413. ( 10.1111/j.1365-2656.2010.01780.x) [DOI] [PubMed] [Google Scholar]
- 53.Yin H, Khamzina A, Pflugmacher D, Martius C. 2017. Forest cover mapping in post-Soviet Central Asia using multi-resolution remote sensing imagery. Sci. Rep. 7, 1–11. ( 10.1038/s41598-017-01582-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CMAP: Chicago Metropolitan Agency for Planning. 2010. High-resolution land cover, Cook County.
- 55.Gray J, Song C. 2013. Consistent classification of image time series with automatic adaptive signature generalization. Remote Sens. Environ. 134, 333–341. ( 10.1016/j.rse.2013.03.022) [DOI] [Google Scholar]
- 56.Chiang SN, Bloom PH, Bartuszevige AM, Thomas SE. 2012. Home range and habitat use of Cooper's hawks in urban and natural areas. Urban Bird Ecol. Conserv. Stud. Avian Biol. 45, 1–16. [Google Scholar]
- 57.Markwardt CB. 2008. Non-linear least squares fitting in IDL with MPFIT. Astron. Data Anal. Softw. Syst. XVIIXXX, 1–4. (doi:citeulike-article-id:4067445)
- 58.Daly C, Taylor G, Gibson W. 1997. The prism approach to mapping precipitation and temperature. AMS Conf. Appl. Climatol.10, 1–4.
- 59.Sibley D. 2000. The Sibley guide to birds. New York, NY: Alfred A. Knopf. [Google Scholar]
- 60.Mitch Waite Group. 2008. iBird. See http://ibird.com.
- 61.Dormann CF, et al. 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.) 36, 027–046. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 62.MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE. 2006. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Burlington, MA: Academic Press. [Google Scholar]
- 63.Royle JA, Kéry M. 2007. A Bayesian state-space formulation of dynamic occupancy models. Ecology 88, 1813–1823. ( 10.1890/06-0669.1) [DOI] [PubMed] [Google Scholar]
- 64.Royle JA, Nichols JD, Kéry M. 2005. Modelling occurrence and abundance of species when detection is imperfect. Oikos 110, 353–359. ( 10.1111/j.0030-1299.2005.13534.x) [DOI] [Google Scholar]
- 65.Kéry M, Royle JA. 2016. In applied hierarchical modeling in ecology. New York, NY: Academic Press. [Google Scholar]
- 66.Plummer M. 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. See https://r-project.org/conferences/DSC-2003/drafts/Plummer.pdf.
- 67.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 68.Keller K. 2017. jagsUI: a wrapper around ‘rjags’ to streamline ‘JAGS’ analysis. See https://cran.mtu.edu/web/packages/jagsUI/jagsUI.pdf.
- 69.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. ( 10.1214/ss/1177011136) [DOI] [Google Scholar]
- 70.Cruz J, Windels SK, Thogmartin WE, Crimmins SM, Grim LH, Zuckerberg B. 2018. Managing individual nests promotes population recovery of a top predator. J. Appl. Ecol. 55, 1418–1429. ( 10.1111/1365-2664.13062) [DOI] [Google Scholar]
- 71.Hansen AJ, Knight RL, Marzluff JM, Powell S, Gude PH, Jones K. 2005. Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol. Appl. 15, 1893–1905. ( 10.1890/05-5221) [DOI] [Google Scholar]
- 72.Roth TC II, Vetter WE, Lima SL. 2008. Spatial ecology of wintering Accipiter hawks: home range, habitat use, and the influence of bird feeders. Condor 110, 260–268. ( 10.1525/cond.2008.8489) [DOI] [Google Scholar]
- 73.Mannan RW, Boal CW. 2000. Home range characteristics of male Cooper’s hawks in an urban environment. Willson Bull. 112, 21–27. ( 10.1676/0043-5643(2000)112%5B0021:HRCOMC%5D2.0.CO;2) [DOI] [Google Scholar]
- 74.Butler SJ, Mattison EHA, Glithero NJ, Robinson LJ, Atkinson PW, Gillings S, Vickery JA, Norris K. 2010. Resource availability and the persistence of seed-eating bird populations in agricultural landscapes: a mechanistic modelling approach. J. Appl. Ecol. 47, 67–75. ( 10.1111/j.1365-2664.2009.01750.x) [DOI] [Google Scholar]
- 75.Cooper NW, Sherry TW, Marra PP, Inouye BD. 2015. Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 96, 1933–1942. ( 10.1890/14-1365.1) [DOI] [PubMed] [Google Scholar]
- 76.Fuller T, Sievert P. 2001. Carnivore demography and the consequences of changes in prey availability. In Carnivore conservation (eds Gittleman JL, Funk SM, Macdonald D, Wayne RK), pp. 163–179. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 77.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth U. 2013. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS ONE 8, e57872 ( 10.1371/journal.pone.0057872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell CP, Baker SW, Parkes NG, Brooke MDL, Chamberlain DE. 2010. The role of the Eurasian sparrowhawk (Accipiter nisus) in the decline of the house sparrow (Passer domesticus) in Britain. Auk 127, 411–420. ( 10.1525/auk.2009.09108) [DOI] [Google Scholar]
- 79.Drennan JE, Beier P. 2003. Forest structure and prey abundance in winter habitat of Northern Goshawks. J. Wildl. Manage. 67, 177–185. ( 10.2307/3803073) [DOI] [Google Scholar]
- 80.Chamberlain DE, Glue DE, Toms MP. 2009. Sparrowhawk Accipiter nisus presence and winter bird abundance. J. Ornithol. 150, 247–254. ( 10.1007/s10336-008-0344-4) [DOI] [Google Scholar]
- 81.Cava JA, Stewart AC, Rosenfield RN. 2012. Introduced species dominate the diet of breeding urban Cooper's hawks in British Columbia. Wilson J. Ornithol. 124, 775–782. ( 10.1676/1559-4491-124.4.775) [DOI] [Google Scholar]
- 82.Bigelow DP, Borchers A.2017. Major uses of land in the United States, 2012. Washington, DC: Department of Agriculture, Economic Research Service.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The model used in this analysis can be found at https://github.com/jmccabe8/hawkDynOccModel. Annual tree canopy cover and impervious surface data can be downloaded at http://silvis.forest.wisc.edu/data/chicago. Project FeederWatch data can be requested at https://feederwatch.org/.