Abstract
Background
Sensorineural hearing loss occurs as a result of damage to the hair cells in the cochlea, or to the auditory nerve. It negatively affects learning and development in children, and employment and economic attainment in adults. Current policy in Ontario is to provide unilateral cochlear implantation for patients with bilateral severe to profound sensorineural hearing loss. However, hearing with both ears as a result of bilateral cochlear implantation may offer added benefits.
Methods
We completed a health technology assessment, which included an evaluation of clinical benefits and harms, value for money, budget impact, and patient preferences related to bilateral cochlear implantation. We performed a systematic literature search for studies on bilateral cochlear implantation in adults and children from inception to March 2017. We conducted a cost-utility analysis with a lifetime horizon from a public payer perspective and analyzed the budget impact of publicly funding bilateral cochlear implantation in adults and children in Ontario for the next 5 years. Finally, we conducted interviews with adults who have sensorineural hearing loss and unilateral or bilateral cochlear implants, and with parents of children with bilateral cochlear implants.
Results
We included 24 publications (10 in adults, 14 in children) in the clinical evidence review. Compared with unilateral cochlear implantation, bilateral cochlear implantation improved sound localization, speech perception in noise, and subjective benefits of hearing in adults and children with severe to profound sensorineural hearing loss (GRADE: moderate to high). Bilateral cochlear implantation also allowed for better language development and more vocalization in preverbal communication in children (GRADE: moderate). The safety profile was acceptable.
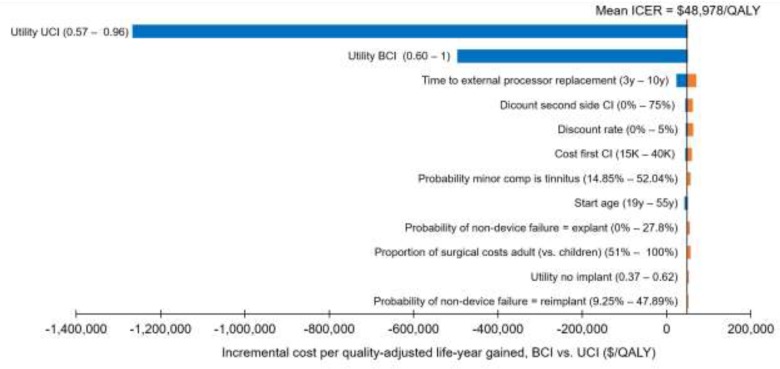
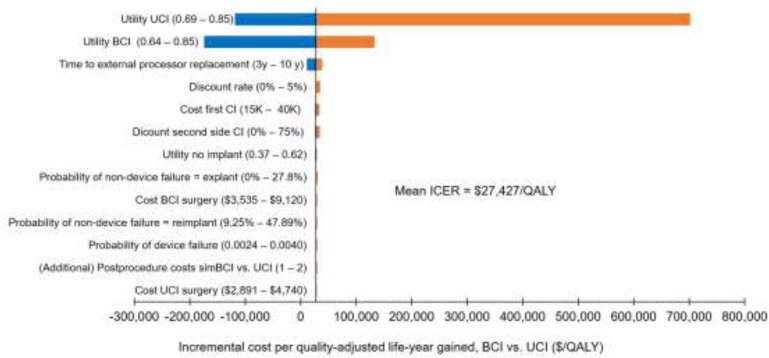
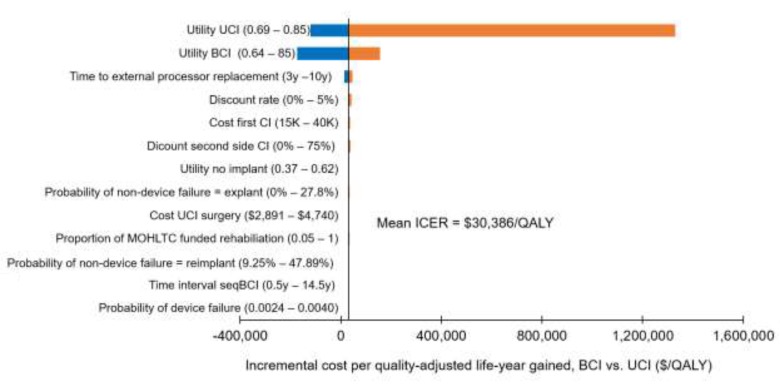
Bilateral cochlear implantation was more expensive and more effective than unilateral cochlear implantation. The incremental cost-effectiveness ratio was $48,978/QALY in adults and between $27,427/QALY and $30,386/QALY in children. Cost-effectiveness was highly dependent on the quality-of-life values used. We estimated that the net budget impact of publicly funding bilateral cochlear implantation for adults in Ontario would be between $510,000 and $780,000 per year for the next 5 years.
Patients described the social and emotional effects of hearing loss, and the benefits and challenges of using cochlear implants.
Conclusions
Based on evidence of moderate to high quality, we found that bilateral cochlear implantation improved hearing in adults and children with severe to profound sensorineural hearing loss. Bilateral cochlear implantation was potentially cost-effective compared to unilateral cochlear implantation in adults and children. Patients with sensorineural hearing loss reported the positive effects of cochlear implants, and patients with unilateral cochlear implants generally expressed a desire for bilateral implants.
OBJECTIVE
This health technology assessment examined the benefits, harms, cost-effectiveness, budget impact, and patient preferences, values, and experiences of bilateral cochlear implantation compared with unilateral cochlear implantation in adults and children with bilateral severe to profound sensorineural hearing loss.
BACKGROUND
Health Condition
The Global Burden of Disease Studies estimated that approximately 6.5% of the world population, or about half a billion people, had disabling hearing loss in 2015.1 In Canada, the 2012/13 Canadian Health Measures Survey showed that about one in five Canadians aged 20 to 79 years (an estimated 4.6 million adults) had some measurable hearing loss.2 Although the prevalence of severe to profound hearing loss in Canada is unknown, the World Health Organization has provided an estimate of 0.8% for high-income countries, including Canada.3
Hearing loss refers to the reduced ability to perceive or understand sounds. It is assessed by pure tone audiometry to test hearing thresholds at frequencies of 0.5 kHz, 1 kHz, 2 kHz, and 4 kHz. The thresholds are measured in decibels (dB) relative to normal hearing, and hearing loss is classified as mild (25–40 dB), moderate (40–55 dB), moderately severe (55–70 dB), severe (70–90 dB), and profound (> 90 dB).4 According to the World Health Organization, disabling hearing loss is defined as hearing loss > 40 dB in adults and > 30 dB in children.5
Otoscopy (examination of the outer ear), immittance audiometry (tests to evaluate middle ear function), and speech perception tests can also be part of a hearing assessment.6 Speech perception can be measured using tests such as the Speech Discrimination Score, the Consonant-Nucleus-Consonant test, the Phonetically Balanced Kindergarten test, the Hearing in Noise test, and AzBio sentences.7,8 Because newborns and babies do not yet have language, their hearing is assessed electronically.9
Severe hearing loss in the early years (before 3 years of age) inhibits or delays the acquisition of spoken language.10 Children with severe hearing loss have lower literacy and lower educational attainments than their peers with normal hearing.11,12 They also experience decreases in quality of life, particularly with respect to school activities and social interactions, negatively affecting their learning and development.13
In adults, disabling hearing loss is associated with economic hardship as a result of low income and/or underemployment.14 Most adults with hearing loss experience profound social isolation and reduced quality of life.15,16 The stigma of hearing loss can deter treatment and further diminish self-esteem and self-efficacy.17 Evidence linking hearing loss in the elderly to increased risk of dementia in various population-based studies18 led the Lancet's International Commission on Dementia Prevention, Intervention and Care to identify the management of hearing loss as an important factor that could prevent or delay the onset of dementia.19
Sensorineural hearing loss refers to damage to the hair cells in the cochlea (the sensory hearing organ) or to the neural pathways of hearing. The causes of sensorineural hearing loss include aging, genetics, noise exposure, Meniere's disease, head trauma, prior ear surgery, medications that are toxic to the ears, and infections, but the cause may also be unknown.20 Tinnitus (also known as “ringing in the ear”) is the perception of sound in the absence of an external sound source.21 There is a strong association between tinnitus and sensorineural hearing loss.22 Of patients with profound hearing loss who were candidates for cochlear implantation, 67% to 86% were reported to have tinnitus.23 Tinnitus can negatively affect quality of life and contribute to emotional distress, clinical depression, and communication problems.24,25
Clinical Need and Target Population
A single cochlear implant offers significant benefits for speech recognition in quiet, and it fulfills a person's basic auditory needs, but without hearing on both sides, patients with hearing loss in two ears are still challenged in daily listening environments that have competing background noise and multiple speakers. The inability to perceive the directionality of sound further compounds their challenge.26
Binaural hearing (hearing with two ears) provides a number of benefits over monaural hearing (hearing with one ear),27 including the following three effects:
Head shadow effect: the head acts as a sound barrier; it shelters the ear closest to the speech source from noise on the other side, and this improves speech comprehension
Binaural summation effect: when both ears receive identical signals, it improves speech perception
Binaural squelch effect: hearing with two ears allows for the spatial separation of signals and noise from competing sources28
The brain's ability to form new neural connections is highest in the first 3.5 years of life,29,30 so access to hearing in early life is critically important for the developing auditory brainstem and for language acquisition.31,32 Optimal auditory development is crucial for language acquisition and integration into mainstream schools, both of which have further implications for education and employment outcomes in later life.33 Early cochlear implantation can help to limit permanent changes in the auditory cortex because of hearing loss.34 However, stimulation from a single implant could cause auditory pathways to mature in the implanted ear, possibly closing a period of development for the auditory pathways and leaving immature pathways in the non-implanted ear.35–37 When bilateral implants are received sequentially (i.e., in two separate surgeries), a long period with a single implant can cause the auditory pathways to develop asymetrically, so that even after the second implant is received, important binaural cues are not processed normally.34,38 Early simultaneous bilateral cochlear implantation for children (i.e., both implants at the same time) could reduce the adverse and potentially irreversible consequences of auditory deprivation in early life.
Certain subgroups of adults could also benefit significantly from bilateral cochlear implantation.26 People with meningitis (because of the risk of cochlear ossification, which would preclude opportunities for future cochlear implantation in the opposite ear), acute bilateral deafness, or concomitant visual loss and deafness have much higher levels of disability. Bilateral cochlear implantation allows these patients to optimize their auditory function, and because they are a small group, second devices have so far been taken from the existing volume to meet this need. The other group that could benefit from bilateral cochlear implantation is young adults. As in older children, the addition of a second implant would provide them with important auditory cues for better hearing in noisy environments and improved sound localization—advantages for educational and employment opportunities. In Ontario, these patients are currently funded for one implant only.
Current Treatment Options
Patients with mild or moderate sensorineural hearing loss can use conventional hearing aids to amplify sounds, but as their hearing loss progresses to severe or profound, hearing aids may no longer be of benefit.
Bilateral cochlear implantation—either simultaneously or sequentially—is the only treatment that can restore binaural hearing for patients with bilateral severe to profound sensorineural hearing loss.
Health Technology Under Review
A cochlear implant replaces the function of the inner ear to help generate sound perception in the brain. It is generally used for patients with severe to profound sensorineural hearing loss in both ears as a result of damage to the basic neural units known as hair cells. Patients with this type of hearing loss usually still have enough neural pathways to be stimulated by electrical signals. The rest of the auditory pathway, which leads to the auditory cortex (the part of the brain that processes sound), can translate and decipher these signals as comprehensible sound, including speech, environmental sounds, noise, and music.
A cochlear implant system consists of two parts. The first is an external (wearable) device that contains a microphone, a speech processor, a battery, and a transmitter. It detects sound, translates it into complex digital information, and transmits it to the internal device. The second is the internal (implantable) device that receives the transmitted signals, converting them into the electrical impulses that stimulate different regions of the cochlea via a series of electrical contacts placed deep inside the inner ear.
Regulatory Information
Cochlear implantation systems are available from at least four manufacturers: MED-EL AG (Austria), Cochlear Corporation (Australia), Advanced Bionics (Switzerland), and Oticon (Denmark). They are licensed by Health Canada as class III devices.
Context
For the management of deafness in both ears, the Ontario Ministry of Health and Long-Term Care provides funding for a single cochlear implant (unilateral cochlear implantation) in the one ear that would benefit most. However, children can and do receive implants in both ears (bilateral cochlear implantation) based on recommendations from the Ontario Cochlear Implant Program and gradual increases in public funding for pediatric programs.
Bilateral Cochlear Implantation in Adults
In Canada, about half of the provincial ministries have created mechanisms to fund bilateral cochlear implantation in children and adults.
Bilateral cochlear implantation in adults is not publicly funded in Ontario. However, it has been done in a small number of adult patients who were thought to be able to perform significantly better with two implants in terms of their educational or employment opportunities, or both. These bilateral implantations have typically been funded by research grants with the following candidacy criteria (personal communication, Ontario Cochlear Implant Program, August 2017):
-
Absolute indications
-
○
Acute hearing loss after meningitis
-
○
Deafness and severe visual impairment (e.g., Usher's Syndrome or congenital conditions)
-
○
Sudden bilateral hearing loss from acquired causes
-
○
-
Relative indications (patients must meet all of the following criteria)
-
○
Age 55 years or less
-
○
Good physical and mental health, with realistic expectations
-
○
No anatomical contraindications
-
○
Preferably employed, in school, or active in the community
-
○
Demonstrated commitment to cochlear implant program goals and rehabilitation
-
○
Audiological status of the second ear being considered for implantation
Hearing loss at least severe (pure tone average ≥ 70 dB); word discrimination scores ≤ 40%
Aided Hearing In Noise Test score in quiet ≤ 60%; AzBio score ≤ 40%; consonant-nucleus-consonant score ≤ 50%
If not using hearing aids, period of nonstimulation is less than 10 years
-
○
The Ontario Cochlear Implant Program has specified that access to a second cochlear implant should be limited to 10% of the total funding target for unilateral cochlear implantation in adults (personal communication, Ontario Cochlear Implant Program, August 2017). Based on 270 funded cases of unilateral cochlear implantation in adults for the 2017/18 fiscal year, 27 additional cochlear implant devices would be needed for sequential bilateral implantation in patients who met the candidacy criteria above.
In the United Kingdom, bilateral cochlear implantation is offered to adults with hearing loss in both ears,39 whereas in Australia, only adults who are unable to benefit from a cochlear implant in one ear and a hearing aid in the other receive bilateral cochlear implantation.40
Bilateral Cochlear Implantation in Children
Funding has been made available for designated pediatric hospitals to provide bilateral cochlear implantation to children. The wait time target for children is 6 weeks. The decision criteria for simultaneous or sequential bilateral cochlear implantation in children are as follows41–43:
Children with bilateral deafness and two ears without contraindications to cochlear implantation would receive simultaneous bilateral cochlear implantation
Children with usable residual hearing—not enough for normal speech and language development but enough for some measurable benefits from two hearing aids—would receive unilateral cochlear implantation. If having a single cochlear implant is better than having two hearing aids, the child would receive a second cochlear implant
Children with developmental delays, auditory neuropathy, or anomalous cochleae (in whom outcomes could be highly variable) would receive unilateral cochlear implantation. Benefits would be measured within 6 to 12 months to decide whether a second cochlear implant is warranted
In the United Kingdom, simultaneous bilateral cochlear implantation is the standard of care for children with profound bilateral sensorineural hearing loss.39 Similarly, the European Bilateral Pediatric Cochlear Implant Forum consensus statement has endorsed bilateral cochlear implantation for children.33
Guideline Recommendations
A number of national and international guidelines on bilateral cochlear implantation have been published. The recommendations from these guidelines are summarized in Appendix 1, Table A1.
CLINICAL EVIDENCE
Research Question
What are the clinical benefits and harms of bilateral versus unilateral cochlear implantation in adults and children with severe to profound bilateral sensorineural hearing loss?
Methods
We developed the research questions in consultation with patients, health care providers, clinical experts, and other health system stakeholders.
Clinical Literature Search
We performed a literature search on March 21, 2017, to retrieve studies published from inception to the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment, National Health Service Economic Evaluation Database (NHS EED), and Database of Abstracts of Reviews of Effects (DARE).
Medical librarians developed the search strategies using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.44 We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment review.
We performed targeted grey literature searching of health technology assessment agency sites and clinical trial registries. See Appendix 2 for the literature search strategies, including all search terms.
The original search strategy included both bilateral cochlear implantation for people with bilateral severe-to-profound hearing loss and unilateral cochlear implantation for people with single-sided deafness. However, the scope of this health technology assessment changed after the search had been run, and unilateral cochlear implantation for people with single-sided hearing loss was excluded; in the review of the evidence, we screened out findings for this intervention. Unilateral cochlear implantation for people with single-sided deafness will be reviewed in a separate health technology assessment, along with other interventions, including bone conduction implantable devices, so that all treatment options can be appropriately compared.
Literature Screening
A single reviewer reviewed the abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies not identified through the search.
Types of Studies
We included:
English-language full-text publications
Health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, prospective comparative observational studies with data for before and after bilateral cochlear implantation
We limited studies to those with a prospective comparative study design and data collected before and after bilateral cochlear implantation to have a baseline measurement against which future gains in performance could be compared.
We excluded:
Animal and in vitro studies
Retrospective studies, cross-sectional studies, non-comparative studies
Case reports, case series, editorials, abstracts or conference proceedings, non-systematic reviews
Studies that reported combined data for children and adults
Types of Participants
Adults and children with severe to profound bilateral sensorineural hearing loss
Types of Interventions
Bilateral cochlear implantation (either simultaneous or sequential) versus unilateral cochlear implantation with or without a conventional hearing aid in the opposite ear for severe to profound bilateral sensorineural hearing loss
Types of Outcomes Measures
Speech perception in quiet
Speech perception in noise
Sound localization
Tinnitus (adults)
Language development (children)
Preverbal communication (children)
Subjective benefits of hearing
Quality of life
Safety
Data Extraction
We extracted relevant data on study characteristics—including study design, sample size, follow-up duration, comparators, reported outcomes, and outcome definition—and summarized them in tables. We contacted authors of the studies to provide clarification as needed.
We considered cochlear implants as a class instead of reviewing individual manufacturers, implant models, or sound processors.
Statistical Analysis
We did not pool the results of the studies, because of differences in testing conditions and outcomes reported. We summarized the results in tables and described them in the text.
Quality of Evidence
We evaluated the level of quality of the body of evidence for each outcome according to the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) Handbook.45,46 The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology. The quality level determination reflects our certainty about the evidence. We assessed risk of bias using the Cochrane Risk of Bias Tool47 for randomized controlled trials and the Risk of Bias in Non-randomized Studies—of Interventions tool (ROBINS-I)48 for nonrandomized studies (Appendix 3).
Expert Consultation
Between January 2017 and October 2017, we consulted with experts in otology, audiology, and neurology about bilateral cochlear implantation in adults and children. Our expert advisors provided advice on research questions, review methods, and review results, and helped place the evidence in clinical context.
Systematic Reviews
A number of health technology assessments and systematic reviews of bilateral cochlear implantation have been conducted in adults and children (Appendix 1, Table A2), but they differed in their inclusion criteria. Most published reviews included case series and retrospective study designs; none restricted their analysis to prospective, comparative studies. In addition, the literature search end dates for these reviews were earlier than June 2015, so they did not capture more recent studies.
In adults, three health technology assessments49–51 and four systematic reviews52–55 collectively showed that bilateral cochlear implantation offered significant gains in speech perception in noise and sound localization compared to unilateral cochlear implantation. However, the benefits for speech perception in quiet and quality of life varied.
In children, three health technology assessments50,51,56 and four systematic reviews57–60 compared bilateral cochlear implantation with unilateral cochlear implantation. They consistently showed that bilateral cochlear implantation improved speech perception in noise and quiet, sound localization, and the subjective benefits of hearing. In a health technology assessment published by the Washington State Health Care Authority in 2013,51 bilateral cochlear implantation showed benefits over unilateral cochlear implantation with respect to complex language skills, hearing function in real-life situations, and disease-specific quality of life; however, this review was based on a small number of low-quality studies.
Because these published reviews (in adults and children) did not fit our specific inclusion criteria (e.g., excluding case series and retrospective studies), or did not include more recent studies, we undertook an evaluation of primary studies.
Results
Literature Search
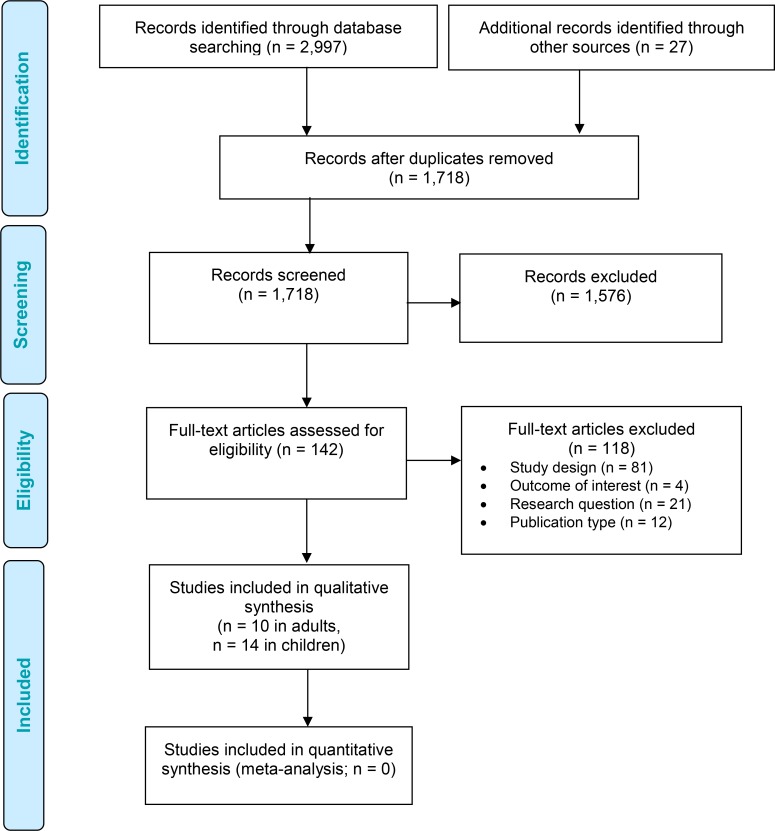
The literature search yielded 1,718 citations published from inception to March 21, 2017, after removing duplicates. We reviewed titles and abstracts to identify potentially relevant articles. We obtained the full text of these articles for further assessment. Twenty-four studies met the inclusion criteria (10 on bilateral cochlear implantation in adults and 14 on bilateral cochlear implantation in children). We reviewed the reference lists of the included studies, but we did not identify any additional relevant studies.
The systematic literature search did not identify any relevant studies that specifically addressed the complications of bilateral cochlear implantation. Multiple databases have been searched with cross-referencing and input from experts to identify studies on the complications of bilateral cochlear implantation and information about the reliability of cochlear implant devices.
Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).61
Figure 1: PRISMA Flow Diagram—Clinical Evidence Review.
Source: Adapted from Moher et al.61
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Bilateral Cochlear Implantation: Adults
Tables 1 and 2 summarize the characteristics of the included studies in adults.
Table 1:
Randomized Controlled Trials of Bilateral Cochlear Implantation in Adults
| Author, Year | Sample Size, n | Implant Type | Sequential or Simultaneous | Comparison | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|
| Smulders et al, 201662 | 19 UCI 19 BCI |
HiRes90K | Simultaneous | BCI vs. UCI (with or without hearing aids in nonimplanted ear) | Speech perception in quiet: CVC words (randomly selected 65 dB, 70 dB, or 75 dB) Speech perception in noise: U-STARR, SISSS (SNR +20 dB) Sound localization Subjective benefits: SSQ, VAS Quality of life: TTO, NCIQ |
12 months |
| Summerfield et al, 200664 | 12 UCI (wait-list control) 12 BCI | Nucleus CI24 |
Sequential | BCI vs. UCI (wait-list control) |
Subjective benefits: SSQ Quality of life: GHSI, HUI-3, VAS, EQ-5D Tinnitus: TAQ |
3, 9 months |
| van Zon et al, 201763 | 19 UCI 19 BCI |
HiRes90K | Simultaneous | BCI vs. UCI (with or without hearing aids in nonimplanted ear) | Speech perception in quiet: CVC words (randomly selected 65 dB, 70 dB, or 75 dB) Speech perception in noise: U-STARR, SISSS (SNR +20 dB) Sound localization Subjective benefits: SSQ Quality of life: TTO, NCIQ, EQ-5D, HUI-3 |
24 months |
Abbreviations: BCI, bilateral cochlear implantation; CVC, consonant-vowel-consonant; EQ-5D, EuroQoL quality-of-life questionnaire; GHSI, Glasgow Health Status Inventory; HUI-3, Health Utilities Index–3; NCIQ, Nijmegen Cochlear Implant Questionnaire; SISSS, speech in spatially separated sources; SNR, signal-to-noise ratio; SSQ, Speech, Spatial, and Qualities of Hearing scale; TAQ, Tinnitus Annoyance Questionnaire; TTO, Time Trade Off; UCI, unilateral cochlear implantation; U-STARR, Utrecht Sentence Test with Adaptive Randomized Roving levels; VAS, visual analog scale.
Table 2:
Observational Studies of Bilateral Cochlear Implantation in Adults
| Author, Year | Sample Size, n | Age at First Implant, y | Duration of Deafness, y | Implant Type | Sequential or Simultaneous | Comparison | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Harkonen et al, 201565 | 15 | NR | NR | CI24M, Concerto | Sequential | BCI vs. UCI ± hearing aids Own control | Speech in noise: phonetically balanced bisyllabic Finnish words (speech 65 dB, noise by +5 dB for fixed SNRs) Sound localization Subjective benefits: SSQ Quality of life: GBI, 15D questionnaire |
6, 12 months |
| Litovsky et al, 200666 | 37 | 27–87 | < 1–15 | Nucleus 24 Contour | Simultaneous | BCI vs. UCI Own control | Speech in quiet: CNC words, HINT sentences (65 dB) Speech in noise: BKB-SIN test (speech 65 dB, noise by +3 dB for fixed SNRs) Subjective benefits: APHAB test |
1, 3, 6 months |
| Mosnier et al, 200967 | 27 | NR | 1–9 | MED-EL Combo 40/40+ | Simultaneous | BCI vs. UCI Own control |
Speech in quiet: Fournier words (70 dB) Speech in noise: Fournier words (SNR +15 dB) Sound localization |
3, 6, 12 months |
| Olze et al, 201268 | 40 | 18–71 | < 1–60 | Nucleus Freedom, Sonata, CI24M, CI40+ Pulsar, CI22M, CI512, Concerto Flex soft, CI513 | Sequential | BCI vs. UCI Own control | Speech in quiet: Freiburger monosyllabic test (65 dB) Speech in noise: HSM sentence test (speech at 70 dB speech, noise at SNR 15 dB), OLSA (65 dB noise, speech adaptive to SNR 50%) Tinnitus: TQ Subjective benefits: OI Quality of life: NCIQ |
0.5–3.4 years |
| Ramsden et al, 200569 | 30 | 33–76 | Left: 1–15 Right: 1–38 |
Nucleus 24CI | Sequential | BCI vs. UCI Own control |
Speech in quiet: CNC words, CUNY sentences (70 dB) Speech in noise: CUNY sentences (+10 dB pre-BCI, +5 to +15 dB SNR post-BCI) |
1 week, 3, 9 months |
| Reeder et al, 201470 | 21 | 36–74 | Implant 1: < 1–45 Implant 2: < 1–55 |
Spectra N22, Freedom N24RE, Esprit 3G N24, Harmony 90K, Harmony CII, Clarion CII, CP810 N512 | Sequential | BCI vs. UCI Own control |
Speech in quiet: CNC words, TIMIT sentences (60 dB) Speech in noise: HINT, TIMIT sentences, BKB-SIN test (+8 dB SNR) Sound localization Subjective benefits: SSQ |
1, 3, 6, 9, 12 months |
| van Zon et al, 201671 | 38 | 50 ± 14 | 19 ± 14 | HiRes90K | Simultaneous | BCI vs. UCI Separate control |
Tinnitus: THI, TQ, VAS | 12 months |
Abbreviations: 15D, 15-dimension; APHAB, Abbreviated Profile of Hearing Aid Benefit; BCI, bilateral cochlear implantation; BKB-SIN, Bamford-Kowal-Bench Speech in Noise; CNC, consonant-nucleus-consonant; CUNY, City University of New York; GBI, Glasgow Benefit Inventory; HINT, Hearing in Noise Test; HSM, Hochmair-Schulz-Moser; NCIQ, Nijmegen Cochlear Implant Questionnaire; NR, not reported; OI, Oldenburg Inventory; OLSA, Oldenburg Sentence Test; SNR, signal-to-noise ratio; SSQ, Speech, Spatial, and Qualities of Hearing scale; THI, Tinnitus Handicap Inventory; TIMIT, Texas Instruments Massachusetts Institute of Technology; TQ, Tinnitus Questionnaire; UCI, unilateral cochlear implantation; VAS, visual analog scale.
Three of the 10 included studies were randomized controlled trials. Of these, two published data from a randomized, controlled trial conducted in the Netherlands on the benefits of simultaneous bilateral cochlear implantation compared with unilateral cochlear implantation in adults with severe bilateral sensorineural hearing loss.62,63 The third trial, by Summerfield et al,64 randomized 24 adults with severe bilateral sensorineural hearing loss into two groups: one receiving bilateral cochlear implantation at the beginning of the trial and the other after a 6-month waiting period (wait-list control).
The other seven included studies were prospective observational studies.65–71 Six compared bilateral cochlear implantation with unilateral cochlear implantation, with or without hearing aids in the nonimplanted ear, using patients as their own controls65–70 The seventh, by van Zon et al,71 was a cohort analysis of a randomized, controlled trial62 that compared simultaneous bilateral cochlear implantation with unilateral cochlear implantation in separate groups. Ramsden et al69 reported different outcomes from the same population as Summerfield et al.64
Speech Perception in Quiet
Table 3 presents the findings for speech perception in quiet.
Table 3:
Speech Perception in Quiet—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized, Controlled Trial | ||||
| Smulders et al, 201662; van Zon et al, 201763 | CVC words, % correct words, median (range) | 12 months: 85 (70–98) 24 months: 89 (52–98) |
12 months: 88 (67–100) 24 months: 88 (55–100) |
NS NS |
| Observational Studies | ||||
| Litovsky et al, 200666 | CNC words, % correct scores | BCI significantly better than either UCI at 1, 3, and 6 monthsa 6 months: 97% of patients performed better |
< .001 NR |
|
| HINT sentences, % correct scores | BCI significantly better than either UCI at 1, 3, and 6 monthsa 6 months: 94% of patients performed better |
< .008 NR |
||
| Mosnier et al, 200967 | Fournier words, % correct words identified | 12 months: 67 ± 5.3% | 12 months: 77 ± 5.0% | < .005 |
| Performance improved significantly over time at 1, 3, and 6 monthsa | UCI: < .002 BCI: < .005 |
|||
| Olze et al, 201268 | FM words, % correct words, mean ± SD | Better ear: 74.4 ± 16.8 Poorer ear: 56.5 ± 24.7 |
81.8 ± 14.2 | < .001 < .001 |
| Ramsden et al, 200569 | CNC words, % correct words | No significant differences between BCI and either UCI at 3 or 9 monthsa | NS | |
| Reeder et al, 201470 | TIMIT sentences, % correct words/sentences | BCI significantly better than either UCIa,b | < .001 | |
Abbreviations: BCI, bilateral cochlear implantation; CNC, consonant-nucleus-consonant; CVC, consonant-vowel-consonant; FM, Freiburger monosyllabic; HINT, Hearing in Noise Test; NR, not reported; NS, not significant; SD, standard deviation; TIMIT, Texas Instruments Massachusetts Institute of Technology; UCI, unilateral cochlear implantation.
Results presented in figures. Numeric data unavailable.
Results reported from the latest follow-up at 1, 3, 6, 9, or 12 months after bilateral cochlear implantation.
The included studies used word and sentence tests to measure speech perception in quiet. The randomized, controlled trial showed no significant difference at 12 and 24 months of follow-up in the percentage of correct consonant-vowel-consonant words presented in quiet among patients randomized to receive either bilateral or unilateral cochlear implantation.62,63 Potential ceiling effects of the test materials may have precluded detection of the benefits of bilateral cochlear implantation.
In contrast, most of the included observational studies reported that patients with bilateral cochlear implantation performed significantly better than those with unilateral cochlear implantation after 1 to 12 months of follow-up.66–68,70
The quality of the evidence was moderate (Appendix 3, Table A7).
Speech Perception in Noise
Table 4 presents the findings for speech perception in noise.
Table 4:
Speech Perception in Noise—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized, Controlled Trial | ||||
| Smulders et al, 201662; van Zon et al, 201763 | U-STARRa SRT, dBb | 12 months: 9.1 (2.2–30.0) 24 months: 9.8 (1.6–22.5) |
12 months: 8.2 (0.3–18.4) 24 months: 7.5 (0.6–19.4) |
NS NS |
| SISSS best,c SRT, dBb | 12 months: 5.0 (−3.1 to 30.0) 24 months: 3.8 (−3.8 to 30.0) |
12 months: 4.1 (−4.7 to 14.1) 24 months: 2.5 (−9.1 to 13.1) |
NS NS |
|
| SISSS worst,d SRT, dBb | 12 months: 14.4 (8.1–30.0) 24 months: 13.3 (5.3–30.0) |
12 months: 5.6 (−2.8 to 22.8) 24 months: 5.9 (−4.7 to 30.0) |
.002 .001 |
|
| Observational Studies | ||||
| Harkonen et al, 201565 | Phonetically balanced bisyllabic Finnish words, % correct (at 12 months) | 0 SNRe: 57 –5 SNRe: 32 |
78 50 |
< .001 .002 |
| Litovsky et al, 200666 | 50% correct keyword speech recognition in BKB-SIN test, SNR,f dB (at 6 months) |
S0N0 Left: 12.97 ± 5.06 Right: 11.42 ± 5.17 |
10.51 ± 5.22 | < .001 |
|
S0N+90° Left: 7.87 ± 6.48 Right: 10.96 ± 6.06 |
6.14 ± 6.29 | < .001 | ||
|
S0N−90° Left: 10.35 ± 6.01 Right: 5.79 ± 6.80 |
3.75 ± 6.29 | < .001 | ||
| Mosnier et al, 200967 | Fournier words, % correct |
+15 SNR dBg 3 months: 42 ± 6.8 6 months: 57 ± 7.0 12 months: 55 ± 6.9 |
49 ± 6.0 62 ± 5.5 63 ± 5.9 |
NS NS < .05 |
|
+10 SNR dBg 3 months: 34 ± 8.0 6 months: 48 ± 9.4 12 months: 55 ± 6.9 |
45 ± 7.4 57 ± 8.0 63 ± 5.9 |
NS NS NS |
||
|
+5 SNR dBg 3 months: 16 ± 5.5 6 months: 24 ± 6.9 12 months: 33 ± 8.0 |
22 ± 6.6 34 ± 8.1 42 ± 8.6 |
NS < .05 < .05 |
||
| Olze et al, 201268 | HSM sentences, % correct |
S0N0 Better ear: 72.1 ± 23.1 Poorer ear: 52.7 ± 28.8 |
81.2 ± 16.1 | < .001 < .001 |
|
SBNP Better ear: 85.4 ± 17.5 Poorer ear: 29.3 ± 25.2 |
87.3 ± 16.5 | NS < .001 |
||
|
SPNB Better ear: 45.0 ± 25.6 Poorer ear: 73.5 ± 33.5 |
82.2 ± 24.7 | < .001 < .001 |
||
| Speech perception of OLSA sentences, dBf |
S0N0 Better ear: 0.74 ± 1.83 Poorer ear: 2.43 ± 3.81 |
−0.26 ± 1.53 | < .001 < .001 |
|
|
SBNP Better ear: −4.76 ± 2.32 Poorer ear: 7.55 ± 3.64 |
−5.29 ± 2.61 | < .05 < .001 |
||
|
SPNB Better ear: 5.42 ± 2.12 Poorer ear: −2.88 ± 4.27 |
−3.78 ± 4.00 | < .001 < .001 |
||
| Ramsden et al, 200569 | CUNY sentences, % of patients who performed better |
S0Nfirst 3 months: BCI 24.6 ± 6.4% better than UCI (first ear) 9 months: BCI 21.0 ± 6.0% better than UCI (first ear) 3 months: BCI 14.3 ± 6.0% better than UCI (second ear) 9 months: BCI 11.7 ± 6.0% better than UCI (second ear) |
< .001 < .001 < .001 < .001 |
|
|
S0Nsecond 3 months/9 months: no difference for BCI vs. UCI (first ear) 3 months: BCI 47.5 ± 6.3% better than UCI (second ear) 9 months: BCI 49.8 ± 5.8% better than UCI (second ear) |
NS < .001 < .001 |
|||
| Reeder et al, 201470 | TIMIT sentences, % correct | Significantly higher scores with BCI than UCIh | < .001 | |
| HINT, % correct | Significantly higher scores with BCI than UCIh | < .01 | ||
| Speech perception of BKB-SIN test, SNR, dB | Significantly better performance with noise presented to either side with BCI than UCIh | < .001 | ||
Abbreviations: BCI, bilateral cochlear implantation; BKB-SIN, Bamford-Kowal-Bench Speech in Noise; CUNY, City University of New York; HINT, Hearing in Noise Test; HSM, Hochmair-Schulz-Moser; NS, not significant; OLSA, Oldenberg Sentence Test; S0N0, speech and noise from the front; S0N+90°, speech from the front and noise from the right side at 90°; S0N−90°, speech from the front and noise from the left side at 90°; S0Nfirst, speech from the front and noise from the first cochlear implant side; S0Nsecond, speech from the front and noise from the second cochlear implant side; SBNP, speech from better ear and noise from poorer ear; SISSS, speech in spatially separated sources; SNR, signal-to-noise ratio; SPNB, speech from poorer ear and noise from better ear; SPL, sound pressure level; SRT, speech reception threshold; TIMIT, Texas Instruments Massachusetts Institute of Technology; UCI, unilateral cochlear implantation; U-STARR, Utrecht Sentence Test with Adaptive Randomized Roving levels; VU-98, Vrije Universiteit 98 sentences.
Dutch VU-98 sentences were presented at a 65, 70, or 75 dB SPL in both speech and noise presented from straight ahead.
Results presented as median (range).
For the UCI group, the best hearing situation was speech presented on the cochlear implant side and noise on the opposite side. For the BCI group, the best hearing situation was speech presented on the best-hearing side and noise on the worst-hearing side.
For the UCI group, the worst hearing situation was noise on the cochlear implant side and speech presented on the opposite side. For the BCI group, the worst hearing situation was speech presented on the worst-hearing side and noise on the best-hearing side.
A negative SNR in test conditions indicates lower sound volume and higher noise volume.
A lower SNR value in dB for speech reception threshold indicates better performance.
A higher SNR in dB indicates higher sound volume.
Results presented in figures. Numeric data unavailable.
The randomized, controlled trial showed that patients with bilateral cochlear implantation performed significantly better in speech perception at 12- and 24-month follow-up than those with unilateral cochlear implantation when noise came from different directions.62,63
The 2011 Minimum Speech Test Battery for adult cochlear implantation users7 provides a standardized assessment protocol to assess the performance of cochlear implants in adults. However, the included observational studies, several of which were published prior to 2011, used different test materials, test configurations, and outcome measurements to evaluate speech perception in noise, with a range of follow-up periods. This heterogeneity in methods and ears implanted precluded direct comparison between studies. Still, almost all reported a significant benefit with bilateral cochlear implantation for speech perception in noise. These effects appeared to be sustained over time.65,66,68–70 In the study by Mosnier et al,67 the advantages of bilateral cochlear implantation were not apparent until 12 months after bilateral cochlear implantation.
The quality of the evidence was moderate (Appendix 3, Table A7).
Sound Localization
Table 5 presents the findings for sound localization.
Table 5:
Sound Localization—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized, Controlled Trial | ||||
| Smulders et al, 201662; van Zon et al, 201763 | Loudspeakers at 60°, % correct responsesa | 12 months: 50.0 (30.0–90.0) | 12 months: 96.7 (73.3–100.0) | < .001 |
| 24 months: 46.7 (30.0–90.0) | 24 months: 96.7 (66.7–100.0) | < .001 | ||
| Loudspeakers at 30°, % correct responsesa | 12 months: 30.0 (16.7–50.0) | 12 months: 76.7 (43.3–96.7) | < .001 | |
| 24 months: 26.7 (6.7–56.7) | 24 months: 63.3 (36.7–100.0) | < .001 | ||
| Loudspeakers at 15°, % correct responsesa | 12 months: 30.0 (20.0–50.0) | 12 months: 53.3 (33.3–90.0) | < .001 | |
| 24 months: 23.3 (13.3–46.7) | 24 months: 53.3 (16.7–90.0) | < .001 | ||
| Observational Studies | ||||
| Harkonen et al, 201565 | Loudspeakers at 45° and 90°, error indexb | 0.73 | 6 months: 0.32 12 months: 0.31 |
< .001 < .001 |
| Mosnier et al, 200967 | Mean % of correct responses per loudspeaker (Setup: 5 loudspeakers in 180° arch at 45° intervals; stimulus: Fournier words with cocktail party background noise) |
12 months: BCI significantly better than UCIc | < .05 | |
| Reeder et al, 201470 | RMS error in degreesd (Setup: 15 loudspeakers in 180° arch at 10° intervals; stimulus: monosyllabic words) |
50° | 6 months: 30° 12 months: 28° |
< .001 < .001 |
Abbreviations: BCI, bilateral cochlear implantation; RMS, root mean square; UCI, unilateral cochlear implantation.
Results were median (range) of the percent of correct responses with 60°, 30°, and 15° angles between loudspeakers.
Error index quantifies the accuracy of sound localization from 0 to 1: 0 corresponds to perfect localization accuracy, and 1 is chance performance. The index is calculated as the sum of all azimuth errors during the test, where azimuth error is the number of loudspeakers between the perceived and presented loudspeaker, divided by the average random error (16 for the setup in Harkonen et al65).
Results presented in figures. Numeric data unavailable.
The smaller the degree of localization error, the better the localization ability.
The randomized, controlled trial showed that at 12- and 24-month follow-up, patients who received bilateral cochlear implantation were better able to locate sounds from various locations than those who received unilateral cochlear implantation.62,63
Consistent with the results from the randomized, controlled trial, all observational studies reported significant benefits from bilateral cochlear implantation versus unilateral cochlear implantation.65,67,70
The quality of the evidence was high (Appendix 3, Table A7).
Tinnitus
Table 6 presents the findings for tinnitus.
Table 6:
Tinnitus—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized, Controlled Trial | ||||
| Summerfield et al, 200664 | TAQa | No significant difference in tinnitus annoyance between BCI and UCI (wait list control) at 3 and 9 monthsb Mean annoyance because of tinnitus increased from UCI (own control) to BCI at 3 monthsb |
NS < .05 |
|
| Observational Studies | ||||
| Olze et al, 201268 | TQ,c mean ± SD | 12.8 ± 12.5 | 8.7 ± 12.2 | < .05 |
| van Zon et al, 201671 | THI,d median (range) | 2 (0–6) | 12 (0–28) | NS |
| TQ,c median (range) | 7 (0–21) | 9 (0–26) | NS | |
| VAS,e median (range) | 1.5 (0–5) | 3 (0–7) | NS | |
Abbreviations: BCI, bilateral cochlear implantation; NS, not significant; SD, standard deviation; TAQ, Tinnitus Annoyance Questionnaire; THI, Tinnitus Handicap Inventory; TQ, Tinnitus Questionnaire; UCI, unilateral cochlear implantation; VAS, visual analog scale.
The Tinnitus Annoyance Questionnaire included 13 questions to measure the annoyance experienced by patients because of tinnitus. Patients responded using a visual analog scale ranging from 0 (never) to 100 (always).64
Results presented in figures. Numeric data unavailable.
The Tinnitus Questionnaire consists of 52 items that incorporate six subscales: emotional distress, cognitive distress, intrusiveness, auditory perceptual difficulties, sleep disturbance, and somatic complaints. The maximum score is 84. The higher the total score, the more distress caused by tinnitus.72
The Tinnitus Handicap Inventory consists of 25 items on the impact of tinnitus on daily life. The maximum score of 100 is divided into five grades that represent the severity of tinnitus: slight, mild, moderate, severe, and catastrophic.73
The visual analog scale measures the loudness of tinnitus; patients marked the strength of tinnitus on a scale of 0 (no tinnitus) to 10 (very loud, disturbing tinnitus).74
The effects of bilateral cochlear implantation on tinnitus were inconsistent. The randomized, controlled trial reported an increase in tinnitus after the second cochlear implantation.64
In contrast, Olze et al68 reported a decrease in tinnitus after the first cochlear implantation, and a further improvement after the second cochlear implantation. A cohort analysis of a randomized, controlled trial showed no significant difference in tinnitus annoyance between bilateral and unilateral cochlear implantation as assessed by several different questionnaires.71
The quality of the evidence was low (Appendix 3, Table A7).
Subjective Benefits of Hearing
Table 7 presents the findings for the subjective benefits of hearing.
Table 7:
Subjective Benefits of Hearing—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized Controlled Trials | ||||
| Smulders et al, 201662; van Zon et al, 201763 | SSQa | 12 months: BCI better than UCIb | < .05 | |
| SSQ speecha | 24 months: 3.1 (1.7–8.3) | 24 months: 5.9 (2.2–8.8) | . 01 | |
| SSQ spatiala | 24 months: 2.4 (5.0–7.3) | 24 months: 6.6 (2.9–8.1) | < .05 | |
| SSQ qualitiesa | 24 months: 4.4 (3.6–10.3) | 24 months: 6.1 (3.7–8.5) | NS | |
| VAS hearingc | 12 months: BCI better than UCIb | < .05 | ||
| 24 months: 65.5 (0.0–94.0) | 75.0 (40.0–90.0) | NS | ||
| Summerfield et al, 200664 | SSQ speecha | Significantly higher scores with BCI than UCI (own control) at 3 and 9 monthsb | < .05 | |
| SSQ spatiala | Significantly higher scores with BCI than UCI (own control, wait list control) at 3 and 9 monthsb | < .01 | ||
| SSQ qualitiesa | Significantly higher scores with BCI than UCI (own control, wait list control) at 9 monthsb | < .01 | ||
| Observational Studies | ||||
| Harkonen et al, 201565 | SSQ,a mean ± SD |
6 months Speech: 5.7 ± 1.3 Spatial: 3.0 ± 1.5 Quality: 6.7 ± 1.3 |
6 months Speech: 6.7 ± 1.5 Spatial: 5.2 ± 1.7 Quality: 7.1 ± 1.0 |
< .01 < .01 < .05 |
|
12 months Speech: 6.3 ± 1.4 Spatial: 5.2 ± 1.7 Quality: 7.1 ± 1.0 |
< .01 < .01 < .01 |
|||
| Litovsky et al, 200666 | APHAB test,d mean | More favourable perceived performance with BCI vs. UCI in ease of communication, background noise, and reverberant listening conditions subscalesb | < .001 | |
| No significant difference in perceived performance between BCI and UCI in aversiveness to sounds subscaleb | NS | |||
| Olze et al, 201268 | OI,e mean ± SD | 3.13 ± 0.84 | 3.70 ± 0.65 | < .001 |
| Reeder et al, 201470 | SSQ speech,a mean | Increased 2.05 from UCI to BCI at 9–12 monthsb | NR | |
| SSQ spatial,a mean | Increased 2.87 from UCI to BCI at 9–12 monthsb | NR | ||
| SSQ quality,a mean | Increased 1.79 from UCI to BCI at 9–12 monthsb | NR | ||
Abbreviations: APHAB, Abbreviated Profile of Hearing Aid Benefit; BCI, bilateral cochlear implantation; NR, not reported; NS, not significant; OI, Oldenburg Inventory; SD, standard deviation; SSQ, Speech, Spatial, and Qualities of Hearing scale; UCI, unilateral cochlear implantation; VAS, visual analog scale.
The Speech, Spatial and Qualities of Hearing questionnaire consists of 40 questions. It subjectively rates hearing disability to reflect the individual's perception of functioning in real-world situations. The speech domain assesses speech recognition in a variety of sound environments. The spatial domain assesses sound direction, distance, and movement. The quality domain assesses segregation of sounds, naturalness, and listening effort. The questionnaire has a scoring system of 0 to 10 for each item: 0 represents minimal hearing ability, and 10 represents complete hearing ability.75
Results presented in figures. Numeric data unavailable.
The visual analog scale consists of two 10 cm scales on which patients rate their hearing and health from 0 (really bad) to 100 (perfect).
The Abbreviated Profile of Hearing Aid Benefit test quantifies the disability associated with hearing loss and the reduction of disability by hearing aids using four subscales: ease of communication, reverberation, background noise, and aversiveness.76
The Oldenburg Inventory consists of 12 questions about different standard listening situations in three domains (hearing in noise, hearing in quiet, and localization). The response choices “always,” “often,” “rare,” “sometimes,” and “never” are scored from 1 to 5. The higher the total score, the better the hearing.68
The Speech, Spatial and Qualities of Hearing questionnaires are commonly used to assess the subjective benefit of hearing following cochlear implantation. Patients with bilateral cochlear implants were consistently rated higher in speech and spatial domains than those with unilateral cochlear implants, suggesting better speech perception under different sound environments and better sound localization with bilateral cochlear implantation.62–65,70 However, the results for the quality domain were inconsistent.
Litovsky et al66 reported the “aided” answers (i.e., with cochlear implants) of the Abbreviated Profile of Hearing Aid Benefit in reference to patients’ real-world listening experiences with either unilateral or bilateral cochlear implantation. Patients with bilateral cochlear implants had more favourable perceived performance in ease of communication, background noise, and reverberant listening conditions than those with unilateral cochlear implantation. There was no significant difference in aversiveness to sounds between unilateral and bilateral cochlear implants.
Olze et al68 measured different listening situations in the hearing in noise, hearing in quiet, and sound localization domains using the Oldenburg Inventory. Patients with bilateral cochlear implants performed significantly better than patients with unilateral cochlear implants.
The quality of the evidence was moderate (Appendix 3, Table A7).
Quality of Life
Table 8 presents the findings for quality of life.
Table 8:
Quality of Life—Adults
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Randomized, Controlled Trials | ||||
| Smulders et al, 201662; van Zon et al, 201763 | VAS-healtha | 12 months: NR | ||
| 24 months: 80 (65–100) | 80 (55–95) | NS | ||
| TTOb | 12 months: BCI better than UCI | < .05 | ||
| 24 months: 100 (50–100) | 24 months: 100 (85–100) | NS | ||
| EQ-5Dc | 12 months: NR | |||
| 24 months: 1.0 (0.8–1.0) | 24 months: 1.0 (0.7–1.0) | NS | ||
| HUI-3d | 12 months: NR | |||
| 24 months: 0.7 (0.4–0.9) | 24 months: 0.8 (0.5–0.9) | NS | ||
| NCIQe | 12 months: BCI better than UCI | NS | ||
| Subscales | ||||
| Basic SP | 24 months: 88.7 (32.5–100.0) | 24 months: 90.0 (60.0–100.0) | NS | |
| Advanced SP | 24 months: 46.5 (17.7–85.0) | 24 months: 62.5 (35.0–95.0) | NS | |
| Speech production | 24 months: 88.7 (32.5–97.5) | 24 months: 91.7 (60.0–100.0) | NS | |
| Self-esteem | 24 months: 62.5 (25.0–92.5) | 24 months: 75.0 (57.2–92.5) | NS | |
| Activity | 24 months: 70.0 (25.0–97.5) | 24 months: 77.5 (43.8–95.0) | NS | |
| Social interaction | 24 months: 62.5 (27.5–77.8) | 24 months: 63.9 (38.9–88.9) | NS | |
| Summerfield et al, 200664 | GHSIf | BCI better than UCI at 9 monthsg | < .05 | |
| HUI-3d | No difference between BCI and UCI at 3 and 9 monthsg | NS | ||
| VAS-healtha | No difference between BCI and UCI at 3 and 9 monthsg | NS | ||
| EQ-5Dc | No difference between BCI and UCI at 3 and 9 monthsg | NS | ||
| Observational Studies | ||||
| Harkonen et al, 201565 | GBI,h mean ± SD | Baseline | 6 months | |
| Total: 43 ± 19 | Total: 35 ± 19 | < .001 | ||
| General: 60 ± 26 | General: 50 ± 25 | < .001 | ||
| Social support: 12 ± 20 | Social support: 1 ± 20 | NS | ||
| Physical health: 8 ± 19 | Physical health: 6 ± 31 | NS | ||
| 12 months | ||||
| Total: 39 ± 17 | < .001 | |||
| General: 56 ± 27 | < .001 | |||
| Social support: 6 ± 12 | NS | |||
| Physical health: 8 ± 23 | NS | |||
| 15D questionnaire,i mean | Baseline | 6 months | ||
| Total: 0.93 | Total: 0.95 | NS | ||
| Depression: 0.84 | Depression: 0.91 | NS | ||
| Distress: 0.91 | Distress: 0.93 | NS | ||
| 12 months | ||||
| Total: 0.96 | .05 | |||
| Depression: 0.94 | .02 | |||
| Distress: 0.98 | .05 | |||
| Olze et al, 201268 | NCIQ,f mean ± SD | 65.4 ± 12.7 | 71.3 ± 12.7 | < .01 |
Abbreviations: BCI, bilateral cochlear implantation; EQ-5D, EuroQoL quality of life questionnaire; GBI, Glasgow Benefit Inventory; GHSI, Glasgow Health Status Inventory; HUI-3, Health Utilities Index–3; NCIQ, Nijmegen Cochlear Implant Questionnaire; NR, not reported; NS, not significant; SD, standard deviation; SP, sound perception; TTO, Time Trade Off; UCI, unilateral cochlear implantation; VAS, visual analog scale.
The visual analog scale consists of two 10 cm scales on which patients rate their hearing and health from 0 (really bad) to 100 (perfect).
The Time Trade Off consists of one question about how many years of the life people are willing to give up to live the rest of their lives with perfect hearing. TTO (%) = (life expectancy – amount of years to give up for perfect hearing/life expectancy) × 100.77
The EQ-5D measures the general quality of life using five subdomains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and three levels (no problems, some problems, serious problems).78
The Health Utilities Index–3 measures general health status. Responses are computed into a composite health utility index score between 0 (dead) and 1 (perfect health).79
The Nijmegen Cochlear Implant Questionnaire assesses health-related quality of life in cochlear implant users in three domains. The physical domain assesses basic sound perception, advanced sound perception, and speech production. The social domain assesses activity and social interaction. The psychological function domain assesses self-esteem. Each question has a three-point response to indicate the degree to which the statement is true. The higher the score, the higher the reported quality of life.80
The Glasgow Health Status Inventory contains 18 questions to measure the social, emotional, and psychological aspects of quality of life affected by impaired hearing and by interventions for impaired hearing. Patients respond to each question on a 5-point Likert scale. The higher the total score, the better the quality of life.64
Results presented in figures. Numeric data unavailable.
The Glasgow Benefit Inventory is a 18-item measure of patient benefit from ear, nose, and throat interventions. It consists of a total score and three subscores (general, social support, and physical health). The total score ranges from −100 (maximal negative benefit), to 0 (no benefit), to +100 (maximal positive benefit).81
The 15D questionnaire is a standardized self-administered instrument to measure health-related quality of life in adults. It consists of 15 dimensions: moving, seeing, hearing, breathing, sleeping, eating, speaking, eliminating, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. The maximum score is 1 (no problems on any dimension), and the minimum score is 0 (equal to being dead).82
The results for quality of life were inconsistent, but the majority of the studies showed no significant difference between bilateral and unilateral cochlear implantation. The heterogeneity of the results could be related to the different questionnaires used. Of the questionnaires, the Glasgow Benefit Inventory was sensitive to different ear, nose, and throat interventions,81 whereas the Nijmegen Cochlear Implant Questionnaire was validated for cochlear implant populations.80 The other questionnaires were not specific to hearing-related quality of life, which may have resulted in null effects.
The quality of the evidence was low (Appendix 3, Table A7).
Bilateral Cochlear Implantation: Children
Table 9 summarizes the characteristics of the included studies in children.
Table 9:
Observational Studies of Bilateral Cochlear Implantation in Children
| Author, Year | Sample Size, n | Age | Age at First Implant | Time Between Implants | Implant Type | Sequential or Simultaneous, n | Comparison | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Cullington et al, 201783 | 1,001 | 0.9–17.9 years (simultaneous) |
0.4–17.2 years (sequential) | 0.1–14.5 years (sequential) | NR | Sequential: 536 Simultaneous: 465 | BCI vs. UCI (own control) | Speech in noise: ATT (noise 55 dB, speech varied adaptively) and adaptive BKB sentences (noise 60 dB, speech varied adaptively) Sound localization |
12, 24, 36 months |
| Galvin et al, 201684 | 20 | 4–15 years | 0.9–14.4 years | 2.4–10.2 years | Nucleus Freedom, Cl24, Cl22 | Sequential | BCI vs. UCI (own control) | Subjective benefits: SSQ-P | 24 months |
| Godar et al, 201085 | 10 | 5–10 years | 1.2–5.2 years | 0.8–6.6 years | Nucleus 24C, 24CA, Nucleus Freedom | Sequential | BCI vs. UCI (own control) | Sound localization: right-left discrimination task | 3, 12 months |
| Peters et al, 200786 | 30 | 3–5 years/> 5–8 years/> 8–13 years | 3–13 years | > 6 months | Nucleus 22, 24, 24C, 24CA | Sequential | BCI vs. UCI (own control) | Speech in quiet: MLNT, LNT, HINT-C (70 dB) Speech in noise: CRISP (fixed SNR) |
3, 6, 9, 12 months |
| Reeder et al, 201787 | 24 | 5–14 years | 2–10 years | 5–15 years | Nucleus 22, Clarion 1.2, Nucleus 24/24RE, Advanced Bionics CII/90K, Nucleus 512 | Sequential | BCI vs. UCI (own control) | Speech in quiet: PBK words, CNC words, BKB sentences (60 dB) Speech in noise: PBK words, CNC words, BKB sentences (+8 dB SNR) Sound localization |
1, 3, 6, 9, 12, 15, 18, 24 months |
| Scherf et al, 200788 | 17 BCI/16 UCI | < 6 years/≥ 6 years | 15 ± 9 monthsa/52 ± 26 monthsa | 7–19 months/22–101 months | NR | Sequential | BCI vs. UCI (own control) | Speech in quiet: Göttinger I and II, NVA (65 dB) Speech in noise: Göttinger I and II, NVA (+10 dB) |
1, 3, 6, 12, 18 months |
| Scherf et al, 200989 | 17 BCI/16 UCI | < 6 years/≥ 6 years | 15 ± 9 monthsa/52 ± 26 monthsa | 7–19 months/22–101 months | NR | Sequential | BCI vs. UCI (own control) | Speech in quiet: Göttinger I and II, NVA (65 dB) Speech in noise: Göttinger I and II, NVA (+10 dB) |
1, 3, 6, 12, 18, 24, 36 months |
| Scherf et al, 200990 | 17 BCI/16 UCI | < 6 years/≥ 6 years | 15 ± 9 monthsa/52 ± 26 monthsa | 7–19 months/22–101 months | NR | Sequential | BCI vs. UCI (own control) | Functional outcomes: CAP, SIR, communication mode, classroom placement, parents’ reports, Würzburg questionnaire | 18 months |
| Scherf et al, 200991 | 17 BCI/16 UCI | < 6 years/≥ 6 years | 15 ± 9 monthsa/52 ± 26 monthsa | 7–19 months/22–101 months | NR | Sequential | BCI vs. UCI (own control) | Functional outcomes: CAP, SIR, communication mode, classroom placement, parents’ reports, Würzburg questionnaire | 1, 3, 6, 12, 18, 24, 36 months |
| Sparreboom et al, 201192 | 29/9 separate UCI control | 2–8 years | 1.1–2.7 years | 1.2–7.2 years | Nucleus 24 | Sequential: 29 | BCI vs. UCI (own control) UCI (separate control) |
Speech in quiet: Dutch ATT (SRT at which 71% of trials lead to correct response) Speech in noise: Dutch ATT (60 dB SPL fixed speech-shaped noise) Sound localization |
6,12, 24 months |
| Sparreboom et al, 201293 | 30/9 separate UCI control |
2–9 years | 0.9–2.7 years | 1.2–7.2 years | Nucleus 24 | Sequential: 30 | BCI vs. UCI (own control) UCI (separate control) | Subjective benefits: SSQ-P Quality of life: VAS health, HUI-3, PedsQL, GCBI, NCIQ |
12, 24 months |
| Sparreboom et al, 201494 | 24 BCI/26 UCI | 8–15 years | 0.9–2.7 years | 1.2–7.2 years | Nucleus Freedom, Nucleus CP810 |
Sequential: 24 | BCI vs. UCI (own control) UCI (separate control) |
Speech in quiet: NVA children's test (65 dB) Speech in noise: NVA children's test (speech-shaped noise at fixed SNR of 0 dB) Language development Sound localization |
5–6 years |
| Strom-Roum et al, 201295 | 73 | 3–15 years | 0.9–6.9 years | 1.0–11.8 years | Nucleus CI22 | Sequential | BCI vs. UCI (own control) | Speech in quiet: Norwegian version of the PBK test (65 dB) | 12, 24 months |
| Tait et al, 201096 | 27 BCI/42 UCI | < 3 years | BCI: 7–33 months UCI: 5–33 months | 1–7 months | NR | Sequential: 9 Simultaneous: 18 | UCI (separate control) | Preverbal communication skills: Tait video analysis | 12 months |
Abbreviation: ATT, Automated Toy Discrimination Test; BCI, bilateral cochlear implantation; BKB, Bench Kowal Bamford; CAP, Categories of Auditory Performance; CNC, consonant-nucleus-consonant words; CRISP, Children's Realistic Intelligibility and Speech Perception test; GCBI, Glasgow Children's Benefit Inventory; HINT-C, Hearing in Noise Test for Children; HUI-3, Health Utilities Index–3; LNT, Lexical Neighborhood Test; MLNT, Multisyllabic Lexical Neighborhood Test; NCIQ, Nijmegen Cochlear Implant Questionnaire; NR, not reported; NVA, Nederlandse Vereniging voor Audiologie; PedsQL, Pediatric Quality of Life inventory; PBK, Phonetically Balanced Kindergarten; SIR, Speech Intelligibility Rating; SPL, sound pressure level; SNR, signal-to-noise ratio; SRT, speech reception threshold; SSQ-P, Speech, Spatial, and Qualities of Hearing scale for parents; UCI, unilateral cochlear implantation; VAS, visual analog scale.
Mean ± standard deviation.
Fourteen prospective observational studies met the inclusion criteria.83–96 Two studies evaluated both sequential and simultaneous bilateral cochlear implantation83,96; the rest evaluated only sequential bilateral cochlear implantation.84–95 The United Kingdom national pediatric bilateral cochlear implantation project consolidated data in 14 centres from 2010 to 2012 on the range of outcomes achieved by children who received bilateral cochlear implantation.83,97,98 One study from this national project reported results on speech perception and sound localization comparing bilateral and unilateral cochlear implantation.83 Eight studies compared bilateral and unilateral cochlear implantation using patients as their own controls.84–91 Sparreboom et al92–94 compared bilateral cochlear implantation with a separate unilateral cochlear implantation control group, and also used patients as their own controls. Tait et al96 used a separate control group for unilateral cochlear implantation.
Scherf et al reported different outcomes at different follow-up periods in four publications.88–91 Similarly, Sparreboom et al published three reports from their cohort.92–94
Speech Perception in Quiet
Table 10 presents the findings for speech perception in quiet.
Table 10:
Speech Perception in Quiet—Children
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Peters et al, 200786 | % correct MLNT, LNT, HINT-C | 12 months | 12 months | |
| 3–5 years (MLNT): 67.3 | 92.3 | .003 | ||
| > 5–8 years (MLNT): 71.0 | 81.1 | .18 | ||
| > 8–13 years (LNT): 69.0 | 86.0 | .004 | ||
| > 8–13 years (HINT-C): 88.0 | 94.0 | .36 | ||
| Reeder et al, 201787 | % correct words/sentences | Higher scores in PBK and CNC words comparing BCI with either UCIa | < .05 | |
| Higher scores in BKB sentences comparing BCI with second cochlear implanta | < .001 | |||
| Scherf et al, 200788; Scherf et al, 200989 | % correct words in Göttinger I and II, NVA | < 6 years old Higher scores comparing BCI with second cochlear implanta |
3 months: .027 | |
| 6 months: .042 | ||||
| 12 months: NS | ||||
| 18 months: .011 | ||||
| 24 months: < .05 | ||||
| 36 months: NS | ||||
| Higher scores comparing BCI with first cochlear implanta | 3 months: NS | |||
| 6 months: .042 | ||||
| 12 months: NS | ||||
| 18 months: NS | ||||
| 24 months: .049 | ||||
| 36 months: NS | ||||
| ≥ 6 years old Higher scores comparing BCI with second cochlear implanta |
3 months: .014 | |||
| 6 months: .003 | ||||
| 12 months: .015 | ||||
| 18 months: .003 | ||||
| 24 months: < .05 | ||||
| 36 months: < .05 | ||||
| Higher scores comparing BCI with first cochlear implanta | 3 months: NS | |||
| 6 months: NS | ||||
| 12 months: NS | ||||
| 18 months: .016 | ||||
| 24 months: .002 | ||||
| 36 months: .001 | ||||
| Sparreboom et al, 201192 | SRT (dB SPL)b |
Own control Significantly lower SRT comparing BCI with second cochlear implanta |
6 months: < .001 | |
| 12 months: < .001 24 months: < .001 |
||||
| Significantly lower SRT comparing BCI with first cochlear implanta | 6 months: < .01 | |||
| 12 months: .054 | ||||
| 24 months: < .001 | ||||
|
Separate UCI control No significant difference in SRT between BCI and UCI |
12 months: .19 | |||
| 24 months: .22 | ||||
| Sparreboom et al, 201494 | Mean % phoneme scores | 3.8% (95% confidence interval 1.2%–6.4%) higher score with BCI than with UCI at 5-year follow-up | NR | |
| Strom-Roum et al, 201295 | Mean % correct words | First cochlear implant | 12 months: 80.9 | < .05 (BCI vs. either cochlear implant) |
| 12 months: 76.3 | 24 months: 82.4 | |||
| 24 months: 78.1 | ||||
| Second cochlear implant | ||||
| 12 months: 58.8 | ||||
| 24 months: 58.8 | ||||
Abbreviations: BCI, bilateral cochlear implantation; BKB, Bench Kowal Bamford; CNC, consonant-nucleus-consonant; HINT-C, Hearing in Noise Test for Children; LNT, Lexical Neighborhood Test; MLNT, Multisyllabic Lexical Neighborhood Test; NR, not reported; NS, not significant; NVA, Nederlandse Vereniging voor Audiologie; PBK, Phonetically Balanced Kindergarten; SPL, sound pressure level; SRT, speech reception threshold; UCI, unilateral cochlear implantation.
Results presented in figures. Numeric data unavailable.
A lower SRT indicates better performance.
Reeder et al87 reported better bilateral performance versus the first implant at all test intervals for correct words in quiet (differences 2.5% to 8.6%).
In the longitudinal study by Scherf et al,88,89 younger children (< 6 years) experienced a bilateral advantage. However, the results for bilateral cochlear implantation were not significantly better than those for unilateral cochlear implantation at all test intervals, possibly because these young children had reached a plateau in speech recognition scores after several months of bilateral cochlear implant use. Also, auditory tests in young children are not very sensitive to small differences in speech perception. In contrast, scores for speech perception in quiet were significantly higher with bilateral cochlear implantation versus the second implant in all test intervals for older children (≥ 6 years). Bilateral advantage did not occur until 18 months of bilateral cochlear implantation use. At 36 months after bilateral cochlear implantation, the median percentage of correct words recognized in quiet was 83% (first implant), 79% (second implant), and 89% (both implants) for younger children, and 60% (first implant), 68% (second implant), and 81% (both implants) for older children. The median percentage of correct words for younger children was higher than that for older children in all conditions.89
Sparreboom et al94 showed that children had significantly better speech perception scores after bilateral cochlear implantation at all test intervals. However, this bilateral advantage was not evident when comparing the bilateral group to a separate unilateral group. Speech perception in quiet improved over time (P < .01) with both bilateral and unilateral cochlear implantation. There was also a significant advantage for bilateral cochlear implantation over the best performance obtained from unilateral cochlear implantation of 3.4 dB at 6 months and 4.8 dB at 24 months (P < .01).
Strom-Roum et al95 reported a mean difference in speech perception in quiet of 4.55% (95% confidence interval 1.29% to 7.81%, P = .07) between bilateral cochlear implantation and the first implant at the 12-month follow-up. The mean difference at the 24-month follow-up was 4.39% (95% confidence interval 1.29% to 7.49%, P = .006).
The studies were not directly comparable because of differences in age at the second cochlear implantation, time interval between cochlear implantations, and test materials; however, the body of evidence suggested an advantage for speech perception in quiet for bilateral cochlear implantation. As well, the bilateral advantage continued to improve as children gained more experience with two implants.
The quality of the evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Speech Perception in Noise
Table 11 presents the findings for speech perception in noise.
Table 11:
Speech Perception in Noise—Children
| Author, Year | Test Measures | UCI and BCI | P Value |
|---|---|---|---|
| Cullington et al, 201783 | S0N0a | No significant difference in combined ATT and BKB results between UCI and BCIb | NS |
| Peters et al, 200786 | S0N0 S0NCI1 |
At 9 months, BCI performed significantly better than UCI on CRISP, regardless of age, as follows: | |
| S0NCI2 | S0N0: 6.8% | .008 | |
| S0NCI1: 13.2% | < .001 | ||
| S0NCI2: 6.8% | < .02 | ||
| Reeder et al, 201787 | SNRc,d (dB) | Significantly lower SNR in BKB-SIN and R-space for BCI vs. either UCIb | < .05 |
| Scherf et al, 200788; Scherf et al, 200989 | % correct wordse | < 6 years old | |
| Higher scores comparing BCI vs. either cochlear implantb | 18 months: .028 (CI1) | ||
| 18 months: .043 (CI2) | |||
| 24 months: NS (Cl1, CI2) | |||
| 36 months: .042 (CI1) | |||
| 36 months: .043 (Cl2) | |||
| ≥ 6 years old No difference in scores comparing BCI vs. either cochlear implantb |
18 months: NS (CI1, CI2) | ||
| 24 months: .001 (Cl1) | |||
| 24 months: .002 (Cl2) | |||
| 36 months: .002 (Cl1) | |||
| 36 months: .002 (Cl2) | |||
| Sparreboom et al, 201192 | S0NCI1 SNR (dB SPL)c,d |
Own control Significantly lower SNR comparing BCI vs. first or second cochlear implantb |
6 months: < .05 |
| 12 months: < .05 | |||
| 24 months: < .05 | |||
|
Separate UCI control group Significantly lower SNR comparing BCI vs. UCI at |
12 months: .42 | ||
| 24 months but not at 12 monthsb | 24 months: < .01 | ||
| Sparreboom et al, 201494 | % phoneme score | BCI obtained an average 9.5% (95% CI 3.5%–15.4%) higher phoneme score than UCI at 5-year follow-up | NR |
Abbreviations: ATT, Automated Toy Discrimination Test; BCI, bilateral cochlear implantation; BKB, Bamford-Kowal Bench; BKB-SIN, Bamford-Kowal Bench Speech in Noise; CI1, first cochlear implant, CI2, second cochlear implant; CRISP, Children's Realistic Intelligibility and Speech Perception test; NR, not reported; NS, not significant; R-space, random-space; S0N0, speech and noise from the front; S0NCI1, speech from the front and noise from first cochlear implant side; S0NCI2, speech from the front and noise from second cochlear implant side; SNR, signal-to-noise ratio; SPL, sound pressure level; SRT, speech reception threshold; UCI, unilateral cochlear implantation.
Only children who scored a speech perception threshold of 55 dB or better in quiet proceeded to noise testing.
Results presented in figures. Numeric data unavailable.
A lower SNR indicates better performance.
Signal-to-noise ratio was calculated by subtracting the fixed noise level of 60 dB SPL from the obtained SRT.
Testing was not done before 18 months of second cochlear implant use because of the difficult nature of the task.
Although various testing configurations and materials were used in the included studies, the evidence suggested that speech perception in noise with bilateral cochlear implantation was better than unilateral cochlear implantation, and that the bilateral advantage sustained over time.86–89,92,94 Reeder et al87 reported better performance with bilateral cochlear implants versus the first cochlear implant at all test intervals for correct words in noise (differences 2.8% to 10.2%) and the Bench-Kowal Bamford speech in noise test (0.5 dB to 1.6 dB) over time. In the longitudinal study by Scherf et al,88,89 younger children (< 6 years) showed a bilateral advantage from 18 months after bilateral cochlear implantation, but older children (≥ 6 years) did not show this advantage until 24 months after bilateral implantation. At 36 months after bilateral cochlear implantation, the median percentage of correct words recognized in noise was 58% (first implant), 55% (second implant), and 68% (both implants) for younger children, and 37% (first implant), 40% (second implant), and 56% (both implants) for older children. The median percentage of correct words was higher for younger children than for older children in all conditions.89
Sparreboom et al92 showed that speech perception in noise was better with bilateral cochlear implantation than with the best unilateral cochlear implantation in 69%, 64%, and 76% of children at 6, 12, and 24 months of follow-up, respectively. The bilateral advantage ranged from 1 dB to 11 dB after 24 months of bilateral cochlear implant use.
The quality of the evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Sound Localization
Table 12 presents the findings for sound localization.
Table 12:
Sound Localization—Children
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Cullington et al, 201783 | Setup: 5 loudspeakers in 180° arch at intervals of 45° | BCI had significant lower localization error at 24 and 36 months’ follow-up than UCIb | 12 months: .090 | |
| 24 months: .008 | ||||
| Stimulus: speech tokens Measures: MAE in degreesa |
36 months: < .001 | |||
| Godar et al, 201085 | Setup: 19 loudspeakers in 180° arch at intervals of 10° Stimulus: Recorded spondee “baseball” Measures: MAA in degreesa |
44.8° | 3 months: 20.4° 12 months: 16.8° | NR |
| Reeder et al, 201787 | Setup: 15 loudspeakers in 140° arch at intervals of 10° Stimulus: monosyllabic words Measures: RMS error in degreesc |
RMS was significantly smaller in BCI and in the first cochlear implant vs. the second cochlear implantb | < .001 | |
| Sparreboom et al, 201192 | Setup: 2 loudspeakers at ± 15°, ± 30°, or ± 90° at 6 and 12 months, adaptive procedure at 24 months Stimulus: common children's songs Measures: % of patients who could lateralize above chance (6 and 12 months),d MAA in degrees (24 months)a |
Own control 0% |
6 months: 57% | NR |
| 12 months: 63% | NR | |||
| 24 months: 83% | NR | |||
| First cochlear implant: 78° | 42° | < .01 | ||
Abbreviations: BCI, bilateral cochlear implantation; MAA, minimum audible angle; MAE, mean absolute error; NR, not reported; RMS, root mean square; UCI, unilateral cochlear implantation.
The smaller the MAE or MAA, the better the sound localization.
Results presented in figures. Numeric data unavailable.
The smaller the RMS, the better the sound localization.
Increased ability to lateralize above chance indicates better sound localization.
Although the included studies used different test setups, stimuli, and outcome measures, the evidence suggested that children with bilateral cochlear implantation showed better sound localization than those with unilateral cochlear implantation. As well, the ability of sound localization appeared to improve over time as children gained experience with both implants.83,85,87,92
The quality of the evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Language Development
Table 13 presents the findings for language development.
Table 13:
Language Development—Children
| Author, Year | Test Measure | UCI | BCI | P Value |
|---|---|---|---|---|
| Sparreboom et al, 201494 | LQa in PPVT-III-NLb | 0.83 (separate UCI control) | 0.95 | NR |
Abbreviations: BCI, bilateral cochlear implantation; LQ, language quotient; NR, not reported; PPVT-III-NL, Peabody Picture Vocabulary test; UCI, unilateral cochlear implantation.
The language quotient is derived by converting the total score to an age-equivalent score, which is then divided by the child's chronological age. It represents a ratio between language age and chronological age. A score of 1.0 indicates an age-appropriate receptive vocabulary.
The Peabody Picture Vocabulary test consists of 204 pictures in series of 12 words. For each word, four pictures are shown and the child points or says the number of the picture that the word describes.99
A cohort of children with sequential bilateral cochlear implantation showed a higher receptive vocabulary (as measured by language quotient) than a matched group of children with unilateral cochlear implantation. The results showed an adjusted mean benefit in language quotient of 0.11 (95% confidence interval 0.01 to 0.21) for children with bilateral cochlear implantation after 5 to 6 years of experience.94
The quality of evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Preverbal Communication Skills
Table 14 presents the findings for preverbal communication skills
Table 14:
Preverbal Communication Skills—Children
| Author, Year | Test Measures | UCI, mean % | BCI, mean % | P Value |
|---|---|---|---|---|
| Tait et al, 201096 | Tait video analysisa | |||
| Vocal turnsb,c | Pre-UCI: 16.4 | Pre-BCI: 20.9 | NS | |
| 12 months: 61.5 | 12 months: 89.0 | < .001 | ||
| Vocal autonomyd | Pre-UCI: 6.6 | Pre-BCI: 4.6 | NS | |
| 12 months: 37.8 | 12 months: 42.3 | NS | ||
| Non-looking vocal turnsb,c | Pre-UCI: 0.5 | Pre-BCI: 0 | NS | |
| 12 months: 37.8 | 12 months: 42.3 | < .001 | ||
| Gestural turnsb,e | Pre-UCI: 53 | Pre-BCI: 59.6 | NS | |
| 12 months: 27.3 | 12 months: 9.2 | < .001 | ||
| Gestural autonomyd | Pre-UCI: 23.1 | Pre-BCI: 8.4 | <.001 | |
| 12 months: 13.4 | 12 months: 1.9 |
Abbreviations: BCI, bilateral cochlear implantation; NS, not significant; UCI, unilateral cochlear implantation.
Tait video analysis is an objective observational method that involves looking at video recordings of children's’ interactions with someone they know well (e.g., a parent or carer). The video recordings can show whether children are becoming more vocal in their communications rather than using silent gestures, and whether there are indications that they are responding to the adults’ speech through hearing rather than vision.100
Turns refer to instances in which the child has an opportunity to communicate. Opportunities occur when the adult has left a pause or where the child interrupts the adult's communication.100
Vocal turns occur where the child has used their voice to communicate, with or without the addition of signs, gestures, or facial expressions.100
Use of autonomy refers to turns in which the child communicates something that cannot be directly predicted from the adult's preceding turn. For example, a child may push away a toy that is offered and point to another toy. This would be classified as gestural autonomy if done silently or as vocal autonomy if vocalization was also used.100
Gestural turns occur when signs, gestures, or facial expressions are used without vocalization. Eye contact is considered gestural communication.100
In two groups of very young children who had not yet acquired oral language, Tait et al96 reported that before cochlear implantation (unilateral or bilateral), both groups were more likely to communicate silently using sign or gesture. At 12 months after implantation, both groups communicated vocally in the majority of their opportunities. Importantly, children with bilateral cochlear implants were more than twice as likely as those with a unilateral cochlear implant to respond vocally to adults through hearing, suggesting that the bilateral implantation group received extra cues from the two implants, allowing them more productive vocal communication without the need to look at adults.
The quality of the evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Subjective Benefits of Hearing
Table 15 presents the findings for the subjective benefits of hearing.
Table 15:
Subjective Benefits of Hearing—Children
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Galvin et al, 201684 | SSQ-Pa | BCI ratings were significantly higher than UCI ratings for speech perception, spatial hearing, and qualities of hearingb | All < .001 | |
| Scherf et al, 200990; Scherf et al, 200991 | CAPc: understand common phrases, % patients | < 6 years old 81% |
< 6 years old 12 months: 100% 18 months: 67% 36 months: 100% |
NR |
| ≥ 6 years old 94% |
≥ 6 years old 12 months: NR 18 months: NRd 36 months: NRd |
NR | ||
| Have a conversation without lip-reading with a familiar talker, % patients | < 6 years old 50% |
< 6 years old 12 months: 69% 18 months: 83% 36 months: 90% | NR | |
| ≥ 6 years old 38% |
≥ 6 years old 12 months: NR 18 months: NRd 36 months: 76% |
NR | ||
| Have a telephone conversation with a familiar talker, % patients | < 6 years old 3% |
< 6 years old 12 months: 16% 18 months: 33% 36 months: 72% |
NR | |
|
≥ 6 years old 7% |
≥ 6 years old 12 months: NR 18 months: 19% 36 months: 35% |
NR | ||
| Oral communication, % patients | < 6 years old 59% |
< 6 years old 12 months: NR 18 months: 92% 36 months: 100% |
NR | |
| ≥ 6 years old 71% |
≥ 6 years old 12 months: NR 18 months: 75% 36 months: 77% |
NR | ||
| Attend mainstream school, % patients |
< 6 years old 59% |
< 6 years old 12 months: NR 18 months: 77% 36 months: 79% |
NR | |
| ≥ 6 years old 47% |
≥ 6 years old 12 months: NR 18 months: 63% 36 months: 69% |
NR | ||
| SIR,e comprehensible speech according to all listeners, % patients |
< 6 years old NR |
< 6 years old 18 months: NR 24 months: 64% 36 months: 80% |
NR | |
| ≥ 6 years old NR |
≥ 6 years old 18 months: NR 24 months: 40% 36 months: 46% |
NR | ||
| Wurzburg questionnaire,f positive experience, median score |
< 6 years old 33.1 |
< 6 years old 12 months: NR 18 months: NRd 36 months: 40 |
NR | |
| ≥ 6 years old 33.1 |
≥ 6 years old 12 months: NR 18 months: NRd 36 months: 33.5 |
NR | ||
| Wurzburg questionnaire,f negative experience, median score |
< 6 years old 20.3 |
< 6 years old 12 months: NR 18 months: 14 36 months: 18.6 |
NR | |
| ≥ 6 years old 23.7 |
≥ 6 years old 12 months: NR 18 months: 24 36 months: 26.7 |
NR | ||
| Sparreboom et al, 201293 | SSQ-Pa |
Own control (pre-BCI) 0.48 (0.39–0.58) |
Own control (pre-BCI) 12 months: 0.60 (0.51–0.66) 24 months: 0.62 (0.56–0.72) |
< .001 < .001 |
|
Separate UCI control 12 months: 0.56 (0.43–0.65) 24 months: 0.50 (0.43–0.65) |
Separate UCI control 12 months: 0.60 (0.51–0.66) 24 months: 0.62 (0.56–0.72) |
.67 .04 |
||
Abbreviations: BCI, bilateral cochlear implantation; CAP, Categories of Auditory Performance; NR, not reported; SIR, Speech Intelligibility Rating; SSQ-P, Speech, Spatial, and Qualities of Hearing scale for parents; UCI, unilateral cochlear implantation.
The Speech, Spatial, and Qualities of Hearing scale for parents was adapted from the adult version of the scale. It is composed of 22 questions. The speech domain assesses speech recognition in a variety of sound environments. The spatial domain assesses sound direction, distance, and movement. The quality domain assesses segregation of sounds, naturalness, and listening effort. The questionnaire has a scoring system of 0 to 10 for each item: 0 represents minimal hearing ability, and 10 represents complete hearing ability. There is an observation period of 1 week during which parents observe their child in the types of listening scenarios described before they are asked to rate their child's performance.101
Results presented in figures. Numeric data unavailable.
Categories of Auditory Performance is an outcome measure of auditory receptive abilities. Parents are asked to rate their child's developing auditory abilities according to eight categories of increasing difficulty. A score of 0 corresponds with “displays no awareness of environmental sounds” and a score of 7 corresponds to “can use the telephone with a familiar talker.”102
Numeric data reported did not allow for direct comparison.
The Speech Intelligibility Rating is a scale consisting of six performance categories arranged in order of increasing difficulty. Audiologists are asked to judge a child's global speech production and to classify their comprehensibility. A score of 0 corresponds to preverbal communication, and a score of 6 corresponds to intelligible speech for all listeners.103
The Wurzburg questionnaire is composed of 11 questions covering different aspects of hearing (e.g., in complex listening situations and directional hearing). Parents are asked to select the category that best describes their child's behaviour and assign a score from 0 (negative experience) to 50 (positive experience). The final question is a yes/no question: “Would you (or would you not) choose a second cochlear implantation for your child if you could? Why?”104
The longitudinal study by Scherf et al91 showed a significant difference in the number of children attending mainstream schools before and 3 years after bilateral cochlear implantation (P = .031). The total number of children who obtained higher scores on the Categories of Auditory Performance test was significantly higher after 3 years of using bilateral cochlear implants (P = .034). A significant number of children had switched to oral communication after 3 years of using bilateral cochlear implants (P = .016). As well, significantly more children obtained a higher Speech Intelligibility Rating 2 to 3 years after bilateral cochlear implantation (P < .001).
In the same study, for the Wurzburg questionnaire, younger children had significantly higher scores than older children for positive experiences 36 months after bilateral cochlear implantation (P = .043). Younger children also had significantly lower scores than older children for negative experiences at 3, 12, and 24 months after bilateral cochlear implantation (P = .015 to .030). No parents reported regretting the decision to proceed with a second implant, although many also recognized that their children still experienced difficulties 3 years after implantation.91 Older children had higher scores for negative experiences with the second cochlear implant over time, suggesting that they had more problems with noise and relied more often on lip-reading.90
Parents also answered three open-ended questions for the Scherf et al study91:
Can you give the main differences between the first and the second cochlear implant?
Is communication with your child easier since activation of the second cochlear implant?
Are there any changes in the behaviours of your child since activation of the second cochlear implant?
Combining the results for younger and older children, the authors reported that the most positive experiences were a more natural way of communicating in real-life situations and spontaneous conversation in background noise. Other positive experiences included improved sound localization, significant receptive and productive language development, the ability to enjoy music and television, positive changes in behaviour (i.e., calmer, happier, and more confident), increased quality of hearing, better balance, and increased safety in traffic or on the street. Although almost all parents and children were content with the second cochlear implant, a few parents of older children reported some disadvantages, including a long adjustment period and the dominance of the first cochlear implant in speech understanding.91 Parents’ reports were similar at 18 and at 36 months after bilateral cochlear implantation.90,91
Sparreboom et al compared results from the Speech, Spatial, and Qualities of Hearing scale before and after bilateral cochlear implantation in the same patients, and all domains showed significant advantages with bilateral implants after 24 months (P < .05 for speech domain, P < .001 for spatial domain, and P < .01 for quality domain). However, only the spatial domain showed a significantly higher rating in the bilateral cochlear implantation group after 24 months (P < .01). The authors observed significant improvements in ratings over time in patients with bilateral cochlear implants (P < .02), but not in those with unilateral cochlear implants (P = .44).93
The quality of the evidence was moderate (Appendix 3, Table A8). We upgraded the quality of the evidence from low to moderate because implantation took place during the critical period for optimal auditory development.
Quality of Life
Table 16 presents the findings for quality of life.
Table 16:
Quality of Life—Children
| Author, Year | Test Measures | UCI | BCI | P Value |
|---|---|---|---|---|
| Sparreboom, et al, 201293 | VAS-healtha | Own control (pre-BCI) | Own control (pre-BCI) | |
| 0.90 (0.80–0.95) | 12 months: 0.90 (0.80–0.97) | NS | ||
| 24 months: 0.90 (0.80–0.96) | NS | |||
| Separate UCI control | Separate UCI control | |||
| 12 months: 0.90 (0.80–0.97) | 12 months: 0.90 (0.80–0.97) | NS | ||
| 24 months: 0.90 (0.80–0.96) | 24 months: 0.90 (0.80–0.96) | NS | ||
| HUI-3b | Own control (pre-BCI) | Own control (pre-BCI) | ||
| 0.58 (0.53–0.78) | 12 months: 0.66 (0.53–0.79) | NS | ||
| 24 months: 0.76 (0.57–0.82) | NS | |||
| Separate UCI control | Separate UCI control | |||
| 12 months: 0.71 (0.58–0.78) | 12 months: 0.66 (0.53–0.78) | NS | ||
| 24 months: 0.71 (0.62–0.82) | 24 months: 0.74 (0.55–0.82) | NS | ||
| PedsQLc | Own control (pre-BCI) | Own control (pre-BCI) | ||
| 0.85 (0.78–0.89) | 12 months: 0.81 (0.72–0.90) | NS | ||
| 24 months: 0.82 (0.67–0.89) | NS | |||
| Separate UCI control | Separate UCI control | |||
| 12 months: 0.84 (0.69–0.94) | 12 months: 0.81 (0.72–0.90) | NS | ||
| 24 months: 0.88 (0.69–0.94) | 24 months: 0.82 (0.67–0.89) | NS | ||
| GCBId | NA | 12 months: NA | NA | |
| 24 months: 10.42 (5.73–32.3) | < .001 | |||
| NCIQe | Own control (pre-BCI) | Own control (pre-BCI) | ||
| 0.74 (0.66–0.82) | 12 months: 0.78 (0.69–0.84) | NS | ||
| 24 months: 0.79 (0.71–0.87) | .02 | |||
| Separate UCI control | Separate UCI control | |||
| 12 months: 0.84 (0.71–0.88) | 12 months: 0.78 (0.69–0.84) | NS | ||
| 24 months: 0.81 (0.71–0.89) | 24 months: 0.79 (0.71–0.87) | NS |
Abbreviations: BCI, bilateral cochlear implantation; GCBI, Glasgow Children's Benefit Inventory; HUI-3, Health Utilities Index–3; NA, not assessed; NCIQ, Nijmegen Cochlear Implant Questionnaire; NS, not significant; PedsQL, Pediatric Quality of Life inventory; UCI, unilateral cochlear implantation; VAS, visual analog scale.
Parents indicated the overall health status of their child using a visual analog scale ranging from 0 (death) to 10 (perfect health). Scores were divided by 10.
The Health Utility Index–3 measures eight domains of general health status.79 The parent proxy version has been validated for children aged 5 years or older.105 The responses are combined into a composite health utility index score between 0 (dead) and 1 (perfect health).
The Pediatric Quality of Life Inventory measures four domains of health-related quality of life.106 Parents complete the parent-proxy version, and children 5 years and older complete the children's version, along with an experienced pediatric clinician. Ratings are given on a Likert scale, ranging from 1 “never a problem” to 5 “almost always a problem,” with total scores ranging from 0 to 100. For children aged 5 to 7 years, ratings are given on a three-point scale. Scores are divided by 100.
The Glasgow Children's Benefit Inventory measures and evaluates a child's health benefit in four domains (emotion, physical health, learning, and vitality) after a hearing intervention. The inventory uses a Likert scale, from 1 “much better” to 5 “much worse”.107 Scores are then converted to range from −100 (maximum harm) to +100 (maximum benefit). The inventory was completed once by the parents, 24 months after bilateral cochlear implantation. Parents of children with a unilateral cochlear implant did not fill out the inventory.
The Nijmegen Cochlear Implant Questionnaire assesses the health-related quality of life in three domains for users of cochlear implants. The physical domain assesses basic sound perception, advanced sound perception, and speech production. The social domain assesses activity and social interaction. The psychological function domain assesses self-esteem. Each question has a three-point response to indicate the degree to which the statement is true. The higher the score, the higher the reported quality of life.80
In the study by Sparreboom et al,93 there were no significant differences in quality of life between bilateral and unilateral cochlear implantation as measured by generic questionnaires including the visual analog scale on health, the Health Utility Index–3, and the Pediatric Quality of Life Inventory. However, the Glasgow Children's Benefit Inventory, a hearing-specific questionnaire, showed significantly higher ratings in all four domains, including emotion (P = .005), physical health (P = .001), learning (P < .001), and vitality (P < .001) 24 months after bilateral cochlear implantation. Significant improvements in quality of life, as measured by the Nijmegen Cochlear Implant Questionnaire, were seen only 24 months after bilateral cochlear implantation use (P = .02), but ratings improved over time (P < .01).
Overall, the results for quality of life were inconclusive. There were no significant improvements as measured by generic questionnaires, but hearing-specific quality of life was significantly improved 24 months after receiving a bilateral cochlear implant. This quality of life improvement was not seen in separate unilateral cochlear implantation control groups.
The quality of the evidence was low (Appendix 3, Table A8).
Bilateral Cochlear Implantation: Harms
None of the included studies for either adults or children reported adverse events. The types of complications were the same for both populations, so we evaluated the safety of bilateral cochlear implantation for adults and children together.
In a retrospective analysis of 500 consecutive cochlear implantations (178 in adults, 322 in children) between 1989 and 2006, the overall complication rate was 16% (18% in adults, 15% in children). Of those complications, 7.2% were reimplantations, 5.6% were major complications, and 3.2% were minor complications. Revision surgery was performed in 10.2%, and the remaining 5.8% were managed medically. The major reasons for revision surgery were device failure, infection, and trauma. Major complications included meningitis and surgery without reimplantation. Minor complications were transient facial palsy, wound hematoma, tinnitus, and infections that resolved with medical treatment.108
In a retrospective analysis of 403 cochlear implantations (168 in adults, 235 in children) between 1993 and 2013, the overall complication rate was 19.9% (27.4% in adults, 14.9% in children). Of those complications, 5% were major complications requiring surgical revision or hospital management (e.g., reimplantation following revision surgery or device failure), and 14.9% were minor complications requiring medical treatment (e.g., acute otitis media in children, and tinnitus and vertigo in adults).109
A retrospective review of 2,827 cochlear implantations performed in 2,311 patients between 1982 and 2011 found 235 cases of revision surgery; device failure accounted for 57.8% of the revision surgeries. Overall rates of revision surgery and device failure were 8.3% and 4.8%, respectively.110
One study reported a very low rate of reimplantation (2.9%) out of 971 devices implanted in 738 children from 1990 to 2010. Among this cohort of children, 20% had meningitis before cochlear implantation.111
These reviews suggest that unilateral cochlear implantation has an acceptable rate of major complications. There is the potential for slightly increased risk with bilateral cochlear implantation, but this risk may be offset with simultaneous implantation, because there would be only one anaesthetic and one opportunity for intraoperative adverse events.26 However, specific risks for the ear itself—such as transient facial nerve injury, device failure, and infection—would be doubled with bilateral cochlear implantation, whether done simultaneously or sequentially. In a recently published United Kingdom multicentre national prospective audit98 of pediatric bilateral cochlear implantation that included 1,397 cochlear implantation procedures in 961 children from 2010 to 2011, the overall major complication rate was 1.6%, including device failure, explantation because of infection, cerebrospinal fluid leak, and meningitis. No permanent facial nerve palsies and no deaths were reported. The rate of minor complications was 6.5%, comprising significant vestibular impairment, wound swelling, and hematoma.
Discussion
We found additional benefits for bilateral over unilateral cochlear implantation in adults and children with severe to profound bilateral sensorineural hearing loss. These benefits appeared to improve over time as patients gained experience with both implants. Bilateral cochlear implantation was crucial for optimizing auditory development and language acquisition in children. It also had the potential for downstream effects on patients’ communication and learning, and on their educational and employment opportunities. The surgical procedure is reasonably safe.
Our findings were consistent with published systematic reviews and health technology assessments (Appendix 1, Table A2), despite heterogeneity in study design, patient characteristics, testing configurations, and outcome measurements.
Limitations
Heterogeneity in study design, testing configurations, and outcome measurements in the included studies
Patient characteristics—including age at second implant, inter-implant interval, and time spent using bilateral devices—could affect auditory development in children and the benefits of bilateral cochlear implantation. These important patient characteristics and their effects may not have been fully captured in the evidence synthesis
Most of the included studies presented their results in figures, making it difficult to obtain specific numbers to compare magnitude of effect/association across studies
Separate unilateral cochlear implantation control groups in observational studies may have had imbalanced patient characteristics at baseline
When patients with bilateral cochlear implantation serve as their own controls, deactivating one cochlear implant does not represent true unilateral hearing; implantation may damage residual hearing in both ears. A separate unilateral cochlear implantation control group would have residual hearing in the nonimplanted ear. In addition, deactivating one cochlear implant would not reflect day-to-day listening conditions
Generic quality-of-life questionnaires are insensitive to hearing benefits, which may underestimate gains from bilateral cochlear implantation
Missing follow-up data in children was inevitable because of their young age and short attention span
Some important patient-reported outcomes (e.g., listening efforts, language development, and oral communication) may not have been captured in the included studies
Potential ceiling effects of some test materials for unilateral cochlear implantation may have precluded demonstration of bilateral benefits
Ongoing Studies
We found one randomized controlled trial and one prospective nonrandomized study on bilateral cochlear implantation through a search of the Nederlands Trial Register and the clinicaltrials.gov website (Appendix 1, Table A3).
Conclusions
Bilateral Cochlear Implantation: Adults
Based on the best evidence available, when comparing bilateral cochlear implantation with unilateral cochlear implantation in adults, we found:
Improved sound localization (GRADE: high)
Improved speech perception in noise (GRADE: moderate) and subjective benefits of hearing (GRADE: moderate)
No difference for speech perception in quiet (randomized, controlled trial; GRADE: moderate), and improved speech perception in quiet favouring bilateral cochlear implantation (observational studies; GRADE: low)
Inconclusive results for tinnitus and quality of life (GRADE: low)
Bilateral Cochlear Implantation: Children
Based on the best evidence available, when comparing bilateral cochlear implantation with unilateral cochlear implantation in children, we found:
Improved speech perception in quiet (GRADE: moderate) and noise (GRADE: moderate)
Improved sound localization (GRADE: moderate) and subjective benefits of hearing (GRADE: moderate)
Better language development (GRADE: moderate)
More vocalization in preverbal communication (GRADE: moderate)
Inconclusive results for quality of life (GRADE: low)
Bilateral Cochlear Implantation: Harms
Based on the best evidence available, bilateral cochlear implantation is reasonably safe.
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of bilateral cochlear implantation compared with unilateral cochlear implantation in adults and children with severe to profound sensorineural hearing loss?
Methods
Economic Literature Search
We performed an economic literature search on March 29, 2017, for studies published from inception to the search date. To retrieve relevant studies, the search was developed using the clinical search strategy with an economic filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment review. We performed a targeted grey literature search of health technology assessment agency sites, clinical trial registries, and Tufts Cost-Effectiveness Analysis Registry. See the Clinical Literature Search section, above, for further details on methods used, and Appendix 2 for literature search strategies, including all search terms.
The original search strategy included both bilateral cochlear implantation for people with bilateral severe-to-profound hearing loss and unilateral cochlear implantation for people with single-sided deafness. However, the scope of this health technology assessment changed after the search had been run, and unilateral cochlear implantation for people with single-sided hearing loss was excluded; in the review of the evidence, we screened out findings for this intervention. Unilateral cochlear implantation for people with single-sided deafness will be reviewed in a separate health technology assessment, along with other interventions, including bone conduction implantable devices, so that all treatment options can be appropriately compared.
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies meeting the eligibility criteria, we obtained full-text articles. We reviewed the reference lists of included studies for any additional studies not identified through the systematic search.
Inclusion Criteria
English-language full-text publications
Cost-utility, cost-effectiveness, cost-benefit, cost-consequence, or cost-minimization analyses
Studies examining bilateral versus unilateral cochlear implantation (with or without hearing aids) in adults or children with bilateral sensorineural hearing loss
Exclusion Criteria
Abstracts, cost-analyses, editorials, case reports, or commentaries
Outcomes of Interest
Incremental cost per quality-adjusted life-year (QALY)
Incremental cost per unit clinical outcome
Costs and incremental costs
Effectiveness (e.g., QALYs) and incremental effectiveness
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Population
Intervention(s) and comparator(s)
Outcomes (i.e., health outcomes, costs, and incremental cost-effectiveness ratios [ICERs])
We contacted authors of the studies to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform development of NICE's clinical guidelines.112 We modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly or partially applicable.
Results
Literature Search
The database search yielded 113 citations published between inception and March 29, 2017, after removing duplicates. We excluded a total of 86 articles based on information in the title and abstract. We then obtained the full texts of 27 potentially relevant articles for further assessment. Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.61
Eight economic evaluations (seven primary research articles and one health technology assessment) met the inclusion criteria. No additional references were identified after hand-searching the reference lists of the included studies.
Review of Included Economic Studies
The eight included publications assessed the cost-effectiveness of bilateral versus unilateral cochlear implantation in individuals with bilateral hearing loss. The studies are summarized in Table 17. The ICERs reported in the literature varied. Two publications113,114 reported on one study that was conducted using the Ontario Ministry of Health and Long-Term Care payer perspective. The study was conducted in adults and compared sequential bilateral cochlear implantation to unilateral cochlear implantation. The ICERs in this study ranged from $16,047/QALY to $55,020/QALY, depending on the tool used to capture health-related quality of life.
Table 17:
Results of Economic Literature Review—Summary
| Results | |||||||
|---|---|---|---|---|---|---|---|
| Name, Year, Location | Analytic Technique, Study Design, Perspective, Time Horizon | Population(s) | Intervention/Comparator(s) | Quality of Life Measurea | Health Outcomes | Costs | Cost-Effectiveness |
| Bichey et al, 2008,115 United States | CUA Pre/post studyb (n = 23) Public health payer perspectivec Lifetime horizon |
Adults and children (age 6–79), postlingually deaf, severe to profound bilateral hearing loss | BCI (sequential)/UCI BCI (sequential)/no intervention |
HUI-3, measured directly in target population (n = 23); measured before cochlear implantation, with UCl, and with BCI | Group means not reported | Group means not reported |
ICER (USD, 5% discount) BCI vs. UCI: $2,187/QALYd BCI vs. no intervention: $23,345/QALYd |
| Bond et al, 2009,50 United Kingdom | CUA Markov cohort model Public health payer perspective Lifetime horizon |
Adults (age 50), postlingually deaf, profound hearing loss Children (mean age 1), prelingually deaf, profound hearing loss |
BCI (sequential)/UCI BCI (simultaneous)/UCI |
HUI-3, obtained from literature |
QALYs (3.5% discount) Adults BCI (sequential): 10.93 BCI (simultaneous): 10.99 UCI: 10.60 Children BCI (sequential): 16.45 BCI (simultaneous): 16.51 UCI: 15.84 |
Cost (GBP, 3.5% discount) Adults BCI (sequential): £53,866 BCI (simultaneous): £53,255 UCI: £34,207 Children BCI (sequential): £93,098 BCI (simultaneous): £87,546 UCI: £60,441 |
ICER (GBP, 3.5% discount) Adults BCI (sequential) vs. UCI: £60,301/QALY BCI (simultaneous) vs. UCI: £49,559/QALY Children BCI (sequential) vs. UCI: £54,098/QALY BCI (simultaneous) vs. UCI: £40,410/QALY |
| Chen et al, 2014113; Kuthubutheen et al, 2015,114 Ontario, Canada | CUA Decision analysis Public health payer perspective 25-year time horizon |
Adults, severe to profound hearing loss, no benefit from hearing aids | BCI (sequential)/UCI BCI (sequential)/ no intervention |
HUI-3, EQ-5D, VAS and TTO, measured indirectly in postlingually deafened adults with severe to profound hearing loss who met criteria for cochlear implantation (n = 30); postlingually deafened adults with at least 1 year of UCI experience (n = 30); postlingually deafened adults with at least 1 year of BCI experience (n = 30); experts (audiologists, surgeons, researchers, therapists; n = 52) |
QALYs (HUI-3; EQ-5D; VAS; TTO) BCI: 20.00; 23.24; 22.00; 23.50 UCI: 19.12; 22.25; 20.25; 20.50 No intervention: 12.38; 18.75; 17.00; 16.25 |
Cost (USD) BCI: $111,764 UCI: $63,622 No intervention: $0 |
ICER (USD: HUI-3; EQ-5D; VAS; TTO)e BCI vs. UCI: $55,020/QALY; $48,142/QALY; $27,510/QALY; $16,047/QALY BCI vs. no intervention: $14,658/QALY; $24,837/QALY; $22,353/QALY; $15,416/QALY |
| Perez-Martin et al, 2017,116 Spain | CUA Markov cohort model Public health payer perspective Lifetime horizon |
Children (age 1), prelingually deaf, severe to profound hearing loss | BCI (simultaneous)/UCI BCI (sequential)/ UCI |
TTO, obtained from literature | Group means not reported | Group means not reported |
ICER (EUR, 3% discount) BCI (simultaneous) vs. UCI: €10,323/QALY BCI (sequential) vs. UCI: €11,733/QALY |
| Smulders et al, 2016,117 Netherlands | CUA RCT (n = 38) Private health payer perspective Lifetime horizon |
Adults (age 18–70), postlingually deaf, severe to profound hearing loss, ability to hear (with hearing aids) until 10 years ago, marginal hearing aid benefit | BCI (simultaneous)/ UCI |
HUI-3, EQ-5D, TTO, VAS, measured directly in target population randomized to BCI (n = 19) or UCI (n = 19) |
Mean 1-year postoperative utility (HUI-3; EQ-5D; TTO; VAS) BCI: 0.71; 0.90; 0.99; 0.75 UCI: 0.68; 0.93; 0.91; 0.79 Mean 2-year postoperative utility (HUI-3; EQ-5D; TTO; VAS) BCI: 0.72; 0.92; 0.99; 0.78 UCI: 0.68; 0.94; 0.90; 0.80 |
Costs (EUR, 0% discount) BCI: €95,290 UCI: €47,972 |
ICER (EUR, 0% discount) BCI reported as cost-effective vs. UCI using HUI-3 and TTO, but not cost-effective using EQ-5D or VAS |
| Summerfield et al, 2002,118 United Kingdom | CUA Decision analysisf Public health payer perspective 30-year time horizon |
Adults, postlingually deaf, profound hearing loss | BCI (simultaneous)/UCI BCI (sequential)/UCI BCI (simultaneous)/hearing aidsg BCI (simultaneous)/no intervention |
TTO measured indirectly in volunteers, including clinicians and staff (n = 70) | Group means not reported | Group means not reported |
ICER (GBP, 6% discount) BCI (simultaneous) vs. UCI: £61,734/QALY BCI (sequential) vs. UCI: £68,916/QALY BCI (simultaneous) vs. hearing aidsg: £35,002/QALY BCI (simultaneous) vs. no intervention: £23,578/QALY |
| Summerfield et al, 2010,119 United Kingdom | CUA Markov modelf Public health payer perspective Lifetime horizon |
Children (age 1), prelingually deaf, severe to profound hearing loss | BCI (simultaneoush)/UCI |
TTO/VAS measured indirectly in clinicians, researchers, undergraduate students, members of the public (n = 180) | Group means not reported | Group means not reported |
ICER (GBP, 3.5% discount, TTO; VAS) BCI vs. UCI: £21,656/QALY; £18,182/QALY |
Abbreviations: BCI, bilateral cochlear implant; CUA, cost-utility analysis; EQ-5D, EuroQoL quality of life questionnaire; HUI-3, Health Utilities Index–3; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; RCT, randomized, controlled trial; TTO, Time Trade Off; UCI, unilateral cochlear implant; VAS, visual analog scale.
Included if study directly measured quality of life.
Case control study stated, but appeared to be a pre/post design.
Societal perspective stated, but appeared to be public health payer perspective.
Unclear how value was calculated; two individuals excluded from analysis.
ICER did not vary for discount rates.
Study design unclear.
Marginal hearing aid users.
Not explicit in text; we assumed they referred to simultaneous implantation.
Applicability and Methodological Quality of Included Studies
The results of the applicability and limitations checklists for economic evaluations applied to the included articles are presented in Appendix 4. All eight studies were deemed directly or partially applicable to the research question. We assessed the methodological quality of these studies. Two studies had minor limitations,50,119 four had potentially serious limitations,64,113,114,116,118 and two had very serious limitations.115,117 Two publications based on a single study were relevant for the Ontario setting.113,114
Discussion
Several studies explored the cost-effectiveness of bilateral cochlear implantation relative to unilateral cochlear implantation in adults and children, and the results (i.e., ICERs) varied. This variation was present in studies assessing adults and children, and in studies assessing simultaneous and sequential bilateral cochlear implantation. Variations could have been due to differences in the utility measures and elicitation methods used, as well as differences in study design, time horizon, payer perspective, and structure of the analysis.
The studies used several direct and indirect preference-based quality-of-life tools. Even within studies, the use of different tools led to wide variations in cost-effectiveness. The Health Utilities Index–3 was a common choice, because it is a generic tool that includes domains for hearing. In some studies, utilities for health states were ascertained indirectly; in others, they were measured directly or by parental proxy. This may also explain some of the variation in the results.
The study designs also differed. Some studies were conducted in conjunction with clinical or observational trials and were unable to fully capture the long-term costs and effects associated with cochlear implantations. Model-based analyses, including decision analytic and Markov cohort models, varied as well. Only Markov models were able to capture the long-term, time-sensitive costs and QALYs associated with cochlear implantation. In some cases, the model structure was unclear.
Conclusions
In the literature, the cost-effectiveness of bilateral versus unilateral cochlear implantation in adults and children varied.
PRIMARY ECONOMIC EVALUATION
Two Ontario publications that were directly applicable to our analysis compared the cost-effectiveness of sequential bilateral cochlear implantation with unilateral cochlear implantation in a single study of adults. Depending on the tool used to assess utilities, the ICERs ranged from $16,047/QALY to $55,020/QALY.113,114 Due to the chronic nature of hearing loss, we decided to conduct an updated Markov model–based primary economic evaluation to capture the long-term, time-sensitive costs and QALYs of bilateral cochlear implantation in the adult population.
Because there were no directly applicable Ontario studies that examined the cost-effectiveness of bilateral cochlear implantation in children, we also conducted a primary economic evaluation for children.
Research Question
What is the cost-effectiveness of bilateral cochlear implantation compared with unilateral cochlear implantation in adults and children with severe to profound sensorineural hearing loss from the perspective of the Ontario Ministry of Health and Long-Term Care?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.120
Type of Analysis
Owing to the availability of preference-based health-related quality-of-life data (i.e., utilities), we performed cost-utility analyses.
We conducted reference case and sensitivity analyses. Our reference case analyses adhered to the Canadian Agency for Drugs and Technologies in Health guidelines121 when appropriate and represent the analyses with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results were affected by varying input parameters and model assumptions.
Intervention and Target Population
The Ontario Ministry of Health and Long-Term Care currently funds unilateral cochlear implantation. We compared unilateral cochlear implantation with bilateral cochlear implantation. Bilateral cochlear implantation surgery can be performed either simultaneously (both implants in one surgery), or sequentially (implants done in two surgeries with a time interval between them, typically for patients with progressive sensorineural hearing loss). We based our target populations and bilateral cochlear implantation specifications on Ontario candidacy criteria from the Ontario Cochlear Implant Program (written communication, Ontario Cochlear Implant Program Provincial Plan, December 2016) and consultation with experts.
The target populations are summarized in Table 18. In each scenario, we based the mean age at first implantation and the proportion of females in the target population on data from the Canadian Institute for Health Information (CIHI) portal.122 We pulled data in September 2017. Where applicable, we obtained data from the National Ambulatory Care Reporting System and the Discharge Abstract Database. We combined all data for Ontario institutions from fiscal years 2012/13 to 2017/18. We used the Canadian Classification Codes for cochlear implantation (1.DM.53.LA-LK and 1.DM.53.LA-LL) to obtain the data. Where applicable, we derived the time interval between sequential bilateral cochlear implantations from average implant times in a systematic review123 and/or expert opinion.
Table 18:
Target Populations Included in the Primary Economic Evaluation
| Type of Analysis | Subpopulation | Type of BCI | Average Age at Implantationa (Range), y | Average Time Between Implants (Range), y |
|---|---|---|---|---|
| Reference cases | Adults (18–55) with progressive severe to profound sensorineural hearing loss (postlingual) | Sequential | 41 (18–55)122 | 3.5 (0.5–19)123 |
| Children ≤ 1 year old who are born with or develop severe to profound sensorineural hearing loss (prelingual) | Simultaneous | 1 (0.5–5), expert opinion | NA | |
| Children with progressive severe to profound sensorineural hearing loss (postlingual) | Sequential | 7 (2–18)122 | 3 (0.5–14.5)123 | |
| Sensitivity analyses | Adults with sudden severe to profound sensorineural hearing loss, or visual impairment and severe to profound sensorineural hearing loss (postlingual) | Simultaneous | 31 (20–40),122 expert opinion | NA |
| Adults with progressive severe to profound sensorineural hearing loss (postlingual) | Sequential | 60 (18–93)122 | 3.5 (0.5–19)123 | |
| Adults with progressive severe to profound sensorineural hearing loss (postlingual) | Sequential | 30 (18–39)122 | 3.5 (0.5–19)123 | |
| Adults with progressive severe to profound sensorineural hearing loss (postlingual) | Sequential | 49 (40–55)122 | 3.5 (0.5–19)123 | |
| Children ≤ 1 year old who are born with or develop severe to profound sensorineural hearing loss (prelingual) | Sequential, delayed because of medical or personal reasons | 1 (0.5–5), expert opinion | 1 (0.5–2) | |
| Children with sudden severe to profound sensorineural hearing loss, or visual impairment and severe to profound sensorineural hearing loss (postlingual) | Simultaneous | 7 (2–17)122 | NA |
Abbreviation: NA, not applicable.
Age at first implant for sequential bilateral cochlear implantation.
We performed three reference case analyses (Table 18). We expected these populations to make up most of the bilateral cochlear implantations in Ontario. We also examined several additional populations in sensitivity analyses due their rarity of occurrence. In these populations, the age of implantation, time between implantations (if applicable), and type of bilateral cochlear implantation were expected to vary.
In our reference case analyses, we did not consider individuals who entered the health care system with one cochlear implant already (i.e., because of immigration, or catch-up programs in those who did not receive bilateral implantation earlier in life).
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care. We also conducted an analysis from a societal perspective.
Discounting, Cycle Length, and Time Horizon
We applied an annual discount rate of 1.5% to both costs and QALYs.121 Because of the chronic nature of hearing loss, we used a lifetime horizon with 6-month cycles in our reference case analyses. We explored the effects of varying discount rates and time horizons in sensitivity analyses.
Main Assumptions
Our main assumptions in the reference case analyses were as follows:
All individuals used their devices; there were no non-users
The number of complications in people who received bilateral cochlear implants were double that of people who received unilateral cochlear implants
All reimplantations done because of severe infection(s) were in the opposite ear. This was consistent with clinical evidence.108,110 Infections in people with bilateral implants resulted in removal without replacement
Individuals experienced a complication only once every 6 months
Minor complications occurred within the first 6 months of implantation
The improvement in quality of life resulting from bilateral cochlear implantation was the same for prelingually and postlingually deafened children
Deafness and cochlear implantation had no effect on life expectancy. Life expectancy was based solely on age- and sex-specific mortality rates
Hearing aids were not used in patients with a unilateral cochlear implant
Utility values remained constant over the patient's lifetime
We assessed these assumptions in the sensitivity analyses.
Model Structure
We developed Markov cohort models to determine the incremental cost per QALY gained of bilateral versus unilateral cochlear implantation in both adults and children. The model structures for unilateral and bilateral cochlear implantation are shown in Figures 3 and 4, respectively.
Figure 3: Model Structure—Unilateral Cochlear Implantation.
aIn the first 6 months.
bThe majority of those who had complications in the “Alive with one implant” state remained in that state. One exception was explantation: those who had an explantation transitioned to the “Alive without implant” state.
Figure 4: Model Structure—Bilateral Cochlear Implantation.
aThose receiving sequential bilateral cochlear implantation began in the “Alive with one implant” stage and progressed to “Alive with two implants” after the specified interval time (years) had elapsed.
bThose receiving simultaneous bilateral cochlear implantation began in the “Alive with two implants” state.
cIn the first 6 months.
dThe dashed lines represent transitions from the “Alive with one implant” state. The solid lines represent transitions from the “Alive with two implants” state.
The model was based on three or five health states, depending on whether a patient received unilateral or bilateral cochlear implantation. In each cycle, patients could remain in a health state, have a complication (event), or progress to a new health state. The health states and transitions between them are described below, along with the complications that may occur.
Unilateral Cochlear Implantation
Alive with one implant: Individuals began in this health state. In the first cycle, they could experience a minor complication. In each cycle, they could experience a major complication (modelled as an event). Individuals could stay in this state; transition to the “alive without implant” state (because of explantation); or transition to the “dead” state
Alive without implant: This state captured individuals who had their devices explanted—a rare complication. In each cycle, they could remain in this state or transition to the “dead” state
Dead
Bilateral Cochlear Implantation
Alive with one implant: Individuals who received sequential bilateral cochlear implantation began in this health state. In the first cycle, they could experience a minor complication. In each cycle, they could experience a major complication (modelled as an event). Individuals could stay in this state; transition to the “alive with two implants” state (after a specified time interval between implants, Table 18); transition to the “alive with one implant, no bilateral” state (because of an infection causing reimplantation in the opposite ear); transition to the “alive without implant” state (because of explantation); or transition to the “dead” state
Alive with two implants: Individuals who received sequential bilateral cochlear implantation progressed to this state after a specified time interval (Table 18). Individuals who received simultaneous bilateral cochlear implantation began in this state. In the first cycle, they could experience a minor complication. In each cycle, they could experience a major complication. Individuals could stay in this state; transition to the “alive with one implant” state (because of an infection causing reimplantation in the opposite ear); or transition to the “dead” state
Alive with one implant, no bilateral implantation: Individuals who had an explantation or infection requiring implantation in the opposite ear could transition to this state. In each cycle, they could experience a major complication. Individuals could stay in this state; transition to the “alive without implant” state (because of an infection causing explantation in the opposite ear); or transition to the “dead” state
Alive without implant: This state captured individuals who had their devices explanted—a rare complication. In each cycle, they could remain in this state or transition to the “dead” state
Dead
Complications
Information about complications (including device failure) was based on three retrospective analyses of cochlear implantation.108–110 We modelled the complications as events associated with one-time costs and disutilities (decreases in quality of life). This was a simplifying assumption, because there was a wide range of potential complications and no literature about ongoing costs and effects after a complication. However, individuals could progress to a different health state after a complication; in that case, their ongoing costs and effects would differ. We considered two main types of complications: minor and major.
Minor Complications
Minor (one-time) complications were those that did not require revision surgery:
Infections: skin infections, otitis media (middle ear inflammation)
Neurological complications: facial palsy, dysgeusia (taste disturbance)
Pain: facial stimulation, facial or neck pain
Tinnitus (ringing in the ear): worsening of previous tinnitus or new occurrence
Vestibular complications: vertigo, dizziness
Other complications: cerebrospinal fluid leaks, hematoma, atlantoaxial subluxation (abnormal neck movement)
Previous studies108,109 have shown that most minor complications occur immediately or shortly after surgical implantation. Therefore, we assumed that all minor complications would occur once per implanted ear within the first 6 months of the procedure. We modelled this as a one-time probability of any minor complication (subsequently divided by type) in the first cycle. In sensitivity analyses, we assessed the effect of minor complications occurring later.
Major Complications
Major complications were events that required revision surgery. We split these into two main categories:
Device failure: Device failure is the most common cause of revision surgery.108,110 We assumed all device failures would require the removal and reimplantation of a new device
Non-device failure: This encompassed all other revision surgeries and included reimplantation, explantation, or minor surgery. Causes could include infection (skin infection or otitis media), cholesteatoma, and displacement or shifting of the device. In our model, revision surgeries were first divided by type, and then by cause (i.e., infection versus noninfection)
We assumed that major complications would occur at a steady rate over the time horizon.
Meningitis is a potentially serious but rare complication in people who undergo cochlear implantation. Only one case in 903 individuals (0.001%) was reported in the complication studies used to inform our model.108,109 The government of Canada recommends that all patients who received cochlear implants be vaccinated against meningitis before implantation.124 Therefore, we assumed that meningitis would not occur.
We acknowledge that the types of complications may vary across populations and with surgical expertise. We chose a conservative model structure based on published literature that provided annual rates of revision, subdivided by type of revision and age group. These rates are presented in Clinical Outcome and Utility Parameters, below. In the sensitivity analyses, we discussed and examined alternative (less conservative) scenarios based on published literature from Ontario, data from manufacturers, and expert opinion.
Clinical Outcome and Utility Parameters
Our main clinical input parameters were mortality rates (transition to the death state), rates of minor and major complications, and the utilities associated with health states and complications used to derive QALYs.
Mortality Rate
We made the conservative assumption that people in all health states had the same mortality rate. We obtained age- and sex-specific mortality rates (2011 to 2013) from Statistics Canada life tables125 and translated them to 6-month probabilities. Based on the literature,50,108–110 we did not expect cochlear implant surgery or the added benefit of a second cochlear implant to affect mortality.
Minor Complications
We based the probability of minor complications (including those in the immediate postoperative period) on the pooled occurrence (weighted average) of two retrospective analyses conducted in France (Table 19).108,109 We assumed a one-time occurrence of minor complications shortly after surgery. This aligned with information about the time of occurrence for most minor complications provided in the retrospective analyses.108,109 We had no information on the timing of minor infections, tinnitus, or pain. We assumed that these would be related to the surgical procedure or wound and would occur within a short time (< 6 months) after implantation. We tested this assumption in the sensitivity analyses.
Table 19:
Pooled Probability of Minor Complications After Cochlear Implantationa
| N (%) | ||
|---|---|---|
| Minor Complication | Adults (N = 346) | Children (N = 557) |
| Infection (skin infections, otitis media) | 7 (2.02) | 24 (4.31) |
| Neurological complications (facial palsy, dysgeusia) | 6 (1.73) | 2 (0.36) |
| Pain (facial stimulation, facial or neck pain) | 7 (2.02) | 2 (0.36) |
| Tinnitus (worsening or new occurrence) | 11 (3.18) | 0 (0.00) |
| Vestibular complications (vertigo, dizziness) | 17 (4.91) | 5 (0.90) |
| Other complications (cerebrospinal fluid leak, hematoma, atlantoaxial subluxation) | 0 (0.00) | 4 (0.72) |
| Total | 48 (13.9) | 37 (6.6) |
We included a final minor complication—external sound processor failure—in our model through costing. We assumed that individuals would need a new sound processor every 5 years. This assumption was consistent with previous cost-effectiveness analyses.113,114,126,127
We assumed that people who had bilateral cochlear implants would experience minor complications at twice the rate of those who had a unilateral cochlear implant.
Major Complications
The annual revision rates for cochlear implantation are shown in Table 20. We obtained values from a retrospective study of 2,827 cochlear implantations110 that highlighted annual revision rates (considering variation in follow-up time) by age and cause of revision. Total revision rates were subdivided into two categories: device failure and non-device failure.110
Table 20:
Annual Revision Rates for Cochlear Implantation by Age Group and Revision Cause
We further subdivided non-device failure by type of surgery (reimplantation, explantation, or minor revision) and indication for surgery (Table 21). We based these rates on values obtained from a retrospective analysis of 500 cochlear implantations.108
Table 21:
Non-device Failure Revision Surgeries by Revision Type and Cause
| Revision Type and Cause | Non-device Failure Revisions, % |
|---|---|
| Reimplantation | 28.57 |
| Infectiona | 50.00 |
| Noninfection | 50.00 |
| Explantation | 19.05 |
| Infection | 100.00 |
| Noninfection | 0.00 |
| Other (minor revision) | 52.38 |
| Cholesteatoma | 27.27 |
| Device shifting or displacement | 54.55 |
| Infection | 18.18 |
We assumed that people who had bilateral cochlear implants would experience major complications at twice the rate of those who had a unilateral cochlear implant.
As discussed above, we examined alternate scenarios for complication rates in the sensitivity analyses.
Utilities
Clinical Effectiveness
The clinical evidence review in this report explored many outcomes related to the clinical effectiveness of bilateral and unilateral cochlear implantation. However, most of the outcomes identified were difficult to translate into outcome measures that would lend themselves well to health economic modelling.
The main measure of clinical effectiveness we used was QALYs, ascertaining quality of life from health utilities. In our reference case analyses, we used measures from the Health Utilities Index–3 (HUI-3) for adult and child populations with severe to profound hearing loss (Table 22). We chose the HUI-3 because it is a validated tool built on the preferences of the Canadian population, including children, and it has domains specific to hearing. We explored utility as measured by other tools in the sensitivity analyses.
Table 22:
Intervention Utilities Included in the Economic Model
| Treatment Group | Corresponding Health State | Utility | Reference |
|---|---|---|---|
| Adults | |||
| Bilateral cochlear implanta | Alive with two implants | 0.800 | Chen et al, 2014,113 and |
| Unilateral cochlear implantb | Alive with one implant | 0.765 | Kuthubutheen et al, 2014114 |
| No cochlear implantc | Alive without implant | 0.495 | |
| Children | |||
| Bilateral cochlear implant | Alive with two implants | 0.830 | Lovett et al, 2010128 |
| Unilateral cochlear implant | Alive with one implant | 0.780 | |
| No cochlear implant | Alive without implant | 0.585 | Barton et al, 2006129 |
Based on a patient with average to above-average performance using a bilateral cochlear implant.
Based on a patient with average to above-average performance using a unilateral cochlear implant.
Based on a patient with severe to profound sensorineural hearing loss who derives no benefit from hearing aids.
In our reference case analysis, we obtained utility values for adults from previous cost-effectiveness analyses conducted in Ontario.113,114 In these analyses, three patient groups (those with severe to profound hearing loss, n = 30; unilateral cochlear implantation users, n = 30; and bilateral cochlear implant users, n = 30) and one expert group (audiologists, researchers, surgeons, and therapists, n = 52) were surveyed to estimate utilities using the HUI-3 and other health utility instruments. The mean ages of respondents in the patient groups and the expert group were 60 and 49 years, respectively. Further details about the assessment and a summary of the clinical vignettes used can be found in Kuthubutheen et al, 2014.114
We did not identify any Ontario- or Canada-specific studies with utility values for children who underwent bilateral cochlear implantation. The clinical evidence review identified only one study in children with preference-based health-related quality of life as an outcome, and this study was conducted in patients with profound sensorineural hearing loss only. As a result, in our reference case analysis, we used bilateral and unilateral cochlear implantation utility values for children from a study in the United Kingdom.128 This study was a cross-sectional analysis of 50 children diagnosed with severe to profound deafness before 2 years of age, and who received their first implant at approximately 3 years of age. This was the only study to evaluate the health utility of bilateral and unilateral cochlear implantation using the HUI-3 (via parental proxy) specifically in children with severe to profound deafness (other studies looked at profound deafness only).
Due to a scarcity of data, we estimated the health utility value for children with severe to profound deafness without intervention using a different source that also used a modified HUI-3 tool. Barton et al129 found that the average gain in health utility (depending on the age and type of hearing loss) from one implantation was +0.13 to +0.26. We took the midpoint, assuming the health utility for a child with no implant was 0.780 – 0.195 = 0.585.
In the absence of available data on postlingually deafened children, we used the above estimates for children who were prelingually or postlingually deafened. Age of onset is associated with better outcomes in implanted and nonimplanted children.130 We assessed the effect of several other utility values in the sensitivity analyses.
We assumed that utility values would remain constant over the time horizon.
Complications
We applied utility decrements to those who incurred complications (Table 23). In the absence of exact durations, we applied the disutility for 1 month for most complications. Only two—tinnitus and vestibular complications in adults—were assumed to persist for longer than 1 month.108,109 For these, we applied the disutility for the patient's lifetime. We were unable to obtain disutilities for neurological, pain, other complications, and surgery, so in our reference case we assumed a disutility of 0.02.
Table 23:
Disutilities Included in the Economic Model
| Complication | Disutility | Duration | Reference |
|---|---|---|---|
| Infection (skin infections, otitis media) | 0.042 | 1 month | Nelson et al, 2015131 (suppl Table 3) |
| Neurological complications (facial palsy, dysgeusia) | 0.020 | 1 month | Assumption |
| Pain (facial stimulation, facial or neck pain) | 0.020 | 1 month | Assumption |
| Tinnitus (worsening or new occurrence) | 0.020 | Ongoing | Stockdale et al, 2017132 |
| Vestibular complications (vertigo, dizziness) | 0.030 | < 1 month (children), ongoing (adults) | Doyle et al, 2011133 |
| Other complications (cerebrospinal fluid leak, hematoma, atlantoaxial subluxation) | 0.020 | 1 month | Assumption |
| Surgery | 0.020 | 1 month | Assumption |
Cost and Resource Use Parameters
We divided costs into four categories: preprocedural, procedural, postprocedural (including rehabilitation), and complication.
Preprocedural Costs and Resource Use
Preprocedural costs included those acquired during assessment (Table 24), when people undergo a series of tests and consultations to determine whether they are eligible for cochlear implantation. We did not consider costs for non-candidates in this analysis; we assumed that they would be identical for both unilateral and bilateral cochlear implantation. However, we did look at the costs for non-candidates in the budget impact analysis. We assumed that if a person was a candidate for unilateral cochlear implantation, they would be a candidate for bilateral cochlear implantation.
Table 24:
Preprocedural Costs Included in the Economic Model
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Preprocedural Assessment Tests, Adults | ||||
| Audiological assessments | 62.67 | 1 | 62.67 | Chen et al, 2014113 |
| Vestibular assessment | 114.90 | 1 | 114.90 | |
| Preprocedural Assessment Tests, Children | ||||
| Audiological assessment | 48.54 | 3 (3 hours) | 145.62 | OPSEU collective agreement134 |
| Language assessment | 48.54 | 1 (1 hour) | 48.54 | |
| Social worker | 48.54 | 1 (1 hour) | 48.54 | |
| Other Preprocedural Costs | ||||
| MRIa | 223.45 | 1 | 223.45 | Schedule of Benefits (X421 + Z430)135,136 |
| CT scanb | 43.15 | 1 | 43.15 | Schedule of Benefits (X001)135,136 |
| Surgical consult | 160.00 | 1 | 160.00 | Schedule of Benefits (A935)135,136 |
| Preoperative general assessment | 65.05 | 1 | 65.05 | Schedule of Benefits (A903)135,136 |
| Total Preprocedural Costs, Adults, $ | ||||
| Unilateral cochlear implantation | 445.77 | |||
| Sequential bilateral cochlear implantation | 891.54 | |||
| Simultaneous bilateral cochlear implantation | 445.77 | |||
| Total Preprocedural Costs, Children, $ | ||||
| Unilateral cochlear implantation | 691.20 | |||
| Sequential bilateral cochlear implantation | 1,382.40 | |||
| Simultaneous bilateral cochlear implantation | 691.20 | |||
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; OPSEU, Ontario Public Service Employees Union.
MRI with anaesthesia used for children.
CT scan used for adults.
We based the assessment process for adults on Chen et al 2014.113 We inflated all costs to 2017 dollars using the Consumer Price Index for Ontario health care goods and services.137 We based the assessment process for children on publicly available information from an Ontario children's hospital.138 We assumed that both adults and children would undergo several hearing and language tests. We also assumed all children would have a 1-hour appointment with a social worker. Additionally, we assumed that all individuals would undergo magnetic resonance imaging (children, expert opinion) or computed tomography scanning (adults), a surgical consult, and a preoperative assessment.
We assumed that people receiving simultaneous bilateral implantation would incur the same assessment costs as people receiving unilateral cochlear implantation. We assumed that people receiving sequential implantation would incur double the costs of those receiving unilateral cochlear implantation, based on a previous cost-effectiveness analysis by Chen et al.113
We included physician fees based on the Ontario Schedule of Benefits.136 We based the costs of audiology, speech language therapy, and social work appointments on the hourly rate of professionals outlined in the Ontario Public Service Employees Union collective agreement (determined by midpoint + 14% Fringe Benefit Pay).134 We examined the costs of appointments in the sensitivity analyses.
Procedural Costs and Resource Use
Procedural costs are presented in Table 25. Based on consultations with manufacturers, we estimated the average device cost of the first cochlear implant (internal component and external sound processor) to be $25,000. Based on previous Ontario cost-effectiveness analyses,113,114 we assumed a 50% discount for a second cochlear implant (for people receiving bilateral implants). We expected a large variation in the cost of devices due to variations in procurement contracts, so we examined a wide range of device costs ($10,000 to $40,000) and second-implant discounts (0% to 75%) in the sensitivity analyses.
Table 25:
Procedural Costs Included in the Economic Model
| Variable | Cost, $ | Reference |
|---|---|---|
| Device cost, first side (internal device + sound processor) | 25,000.00 | Consultation, manufacturers |
| Device cost, second side (internal device + sound processor) | 12,500.00a | Assumption |
| Surgical costs (unilateral) | 4,644.25 | Merdad et al, 2014139,b |
| Surgical costs (bilateral) | 6,199.78 | Merdad et al, 2014139,b |
| Total Procedural Costs, Adults and Children, $ | ||
| Unilateral cochlear implantation | 29,644.25 | |
| Sequential bilateral cochlear implantation | 46,788.50 | |
| Simultaneous bilateral cochlear implantation | 43,699.78 | |
Assumes a 50% discount on the second cochlear implant.
Inflated from 2011 to 2017 Canadian dollars.
Non-device procedural costs included operating room costs, post-anaesthetic care unit costs, operating room supplies, surgeon fees, and inpatient costs. We based the costs for unilateral and simultaneous bilateral cochlear implantation on a costing study conducted at an Ontario children's hospital in fiscal year 2010/11.139 We updated costs to 2017 dollars using the health and personal care values from the Consumer Price Index for Ontario health care goods and services.137 We assumed that the non-device procedural costs for sequential bilateral implantation would be double those of unilateral implantation. Conservatively, we assumed the non-device procedural costs in adults to be the same as for children, but in reality, adults have shorter average lengths of stay for cochlear implantation procedures (13.45 hours for adults and 29.9 hours for children), and therefore potentially lower hospitalization costs.
Postprocedural Costs and Resource Use
Postprocedural costs included the cost of follow-up physician services, programming, evaluation, and rehabilitation (Table 26). We assumed that all individuals would have a follow-up appointment with their surgeon. We assumed that children would have several appointments with an audiologist to receive and turn on their equipment.138 We also assumed that all implant recipients would undergo MAPing (programming the cochlear implant) and evaluation appointments with an audiologist to ensure that their cochlear implant was optimized.
Table 26:
Postprocedural Costs Included in the Economic Model
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Follow-up visit with clinician | 31.00 | 1 | 31.00 | Schedule of Benefits (C002)136 |
| Follow-up Costs, Audiologist Appointments, Adults | ||||
| Year 1 | 48.54 | 5 (5 hours) | 242.70 | OPSEU collective agreement,134 expert opinion |
| Year 2 | 48.54 | 1 (1 hour) | 48.54 | |
| After year 2 | 48.54 | Every other year (1 hour) | 24.27/year | |
| Follow-up Costs, Audiologist Appointments, Children | ||||
| First 6 months | 48.54 | 6 (6 hours) | 291.24 | OPSEU collective agreement,134 expert opinion |
| Second 6 months | 48.54 | 2 (2 hours) | 97.08 | |
| Year 2 | 48.54 | 3 (3 hours) | 145.62 | |
| After year 2 | 48.54 | Every year (1 hour) | 48.54/year | |
| Rehabilitation (Audio-verbal Therapist) | ||||
| Adults | 48.54 | 1 (1 hour) | 48.54 | OPSEU collective agreement,134 expert opinion |
| Children | 48.54 | Weekly (1 hour)a | 2,524/year | |
| Total Postprocedural Costs, Adults | ||||
| (first 2 years, not including rehabilitation, undiscounted, $) | ||||
| Unilateral cochlear implantation | 370.78 | |||
| Sequential bilateral cochlear implantation | 741.56 | |||
| Simultaneous bilateral cochlear implantation | 370.78 | |||
| Total Postprocedural Costs, Children | ||||
| (first 2 years, not including rehabilitation, undiscounted, $) | ||||
| Unilateral cochlear implantation | 564.94 | |||
| Sequential bilateral cochlear implantation | 1,129.88 | |||
| Simultaneous bilateral cochlear implantation | 564.94 | |||
Abbreviation: OPSEU, Ontario Public Service Employees Union.
Occurs in hospital for 5% of children in the reference case analysis.
Based on consultation with experts, we assumed that adults would have five audiologist appointments in the first year after implantation, one in the second year, and one every 2 years after that. We expected children to have six audiologist appointments in the first 6 months (one to receive equipment, three for device turn-on, one for MAPing, and one for evaluation), two in the second 6 months (one for MAPing and one for evaluation), and three in the second year (two for MAPing and one for evaluation). After 2 years, we assumed that one appointment per year for children would be sufficient for MAPing and evaluation.
Rehabilitation, typically in the form of audio-verbal therapy, is crucial for positive outcomes after cochlear implantation, especially in children. In adults, no publicly funded community programs are available. Based on expert consultation, we assumed that each patient would receive one visit with a rehabilitation specialist, and 5% to 10% would pay out of pocket for full rehabilitation in the community (approximately $5,048 per patient for 2 years of weekly rehabilitation therapy). Currently, audio-verbal therapy for children is conducted primarily in the community and publicly funded through the Ministry of Child and Youth Services (Infant Hearing Program) and the Ministry of Education. Some rehabilitation services are offered through hospital-based cochlear implantation programs. In children, these programs are for those who do not have access to community programs. In our reference case, based on communication with clinical experts, we assumed that 5% of children would access rehabilitation through hospital programs (approximately $5,048 per patient for 2 years of weekly rehabilitation therapy). We included only hospital-based rehabilitation in our reference case analysis. We explored community rehabilitation costs further in the sensitivity analysis. We expected those who engaged in rehabilitation to have weekly 1-hour appointments with an audio-verbal therapist, beginning after surgery and lasting for 2 years.
We assumed that people who received simultaneous bilateral implants would have the same postprocedural costs as those who received unilateral implants. We assumed those who received sequential bilateral implants would have double the costs of those who received unilateral implants. We assessed these assumptions in the sensitivity analyses.
Complication Costs and Resource Use
The costs of complications are presented in Table 27. For each, we have specified the included components.
Table 27:
Complication Costs Included in the Economic Model
| Variable | Cost, $ | Components | References |
|---|---|---|---|
| Minor Complications | |||
| Infection (skin infections, otitis media | 91.51 | Emergency department assessment and antibioticsd,e | Schedule of Benefits (H605),136 Gaboury et al, 2010140 |
| Neurological complications (facial palsy, dysgeusia) | 49.23 | Specific assessment and corticosteroidsd,f | Schedule of Benefits (A013),136 Ontario Drug Benefit Formulary141 |
| Pain (facial stimulation, other) | 74.25 | Emergency department assessment | Schedule of Benefits (H605)136 |
| Tinnitus (worsening or new occurrence) | 47.50 | Specific assessment | Schedule of Benefits (A013)136 |
| Vestibular complications (vertigo, dizziness) | 90.65 | Specific assessment and CT scan | Schedule of Benefits (A013),135,136 (X001)136 |
| Other complications (cerebrospinal fluid leak hematoma, atlantoaxial subluxation) | 74.25 | Emergency department assessment | Schedule of Benefits (H605)136 |
| Sound processor replacement | 5,444 | Device | Assistive Devices Program142 |
| Major Complications | |||
| Reimplantation (non-infection) | 29,427.05 | Devicea and surgery | Manufacturers, Merdad et al, 2014139 |
| Reimplantation (infection) | 29,427.05 | Devicea and surgery | Gaboury et al, 2010140 |
| Explantation (infection) | 4,427.05 | Surgery | Gaboury et al, 2010140 |
| Minor revision (infection) | 1,024.70 | Surgical drainage | OCC143b |
| Minor revision (cholesteatoma) | 3,384.44 | Surgery | OCC143c |
| Minor revision (other) | 4,427.05 | Surgery | Merdad et al, 2014139 |
Abbreviations: CT, computed tomography; OCC, Ontario Case Costing.
Cost included only if out of warranty (> 10 years).
Including Canadian Classification of Health Initiatives codes 1DA52, 1DE52, 1DK52, 1DL52, 1DN52, 1DR52.
Including Canadian Classification of Health Initiatives codes 1DK87, 1DL87.
Outpatient drug costs not included for individuals > 24 years or < 65 years.
Drug cost of treating otitis media in Ontario using amoxicillin.140
Assumes prednisolone 50 mg/day × 10 days.144
Minor Complications
We based the costs of minor complications on conservative medical treatment to align with treatment protocols specified in the clinical literature (Table 27).108,109 We assumed that tinnitus and vestibular and neurologic complications would result in a specific assessment with a physician (i.e., primary care provider). We included the cost of a computed tomography scan for people with vestibular complications and the cost of a short course of corticosteroids for people with neurological complications, as in Venail et al.108 We assumed that individuals with an infection, pain, or other minor complication would visit the emergency department but be treated conservatively (without surgical intervention). Infections included the treatment cost of antibiotics (amoxicillin); we assumed that these would be dispensed at a hospital.
Major Complications
For all reimplantations, we assumed that the surgical cost of a unilateral implantation would be incurred in addition to the cost of devices that were no longer under warranty (> 10 years). For explantations (removal and no reimplantation), we assumed that only the surgical cost of a unilateral implantation would be incurred. We obtained the cost of minor revision surgeries because of infection and cholesteatoma from the Ontario Case Costing Tool (2016/17, adults and children).143 We obtained weighted averages based on ambulatory procedures, day surgery, and inpatient procedures. For other minor revision surgeries, we assumed that the surgical cost of a unilateral implantation would be incurred.
The cost of an external sound processor was the maximum paid by the Assistive Devices Program, Ontario Ministry of Health and Long-Term Care, for an external sound processor upgrade/replacement every 3 years, and covers up to 75% of the cost.142
Analysis
We conducted all analyses in TreeAge Pro 2017.
We completed three reference case analyses: one in adults (sequential bilateral cochlear implantation in working-age adults) and two in children (simultaneous bilateral cochlear implantation in prelingually deafened infants and sequential bilateral cochlear implantation in postlingually deafened children).
For each reference case analysis, we ran 5,000 Monte Carlo simulations (probabilistic analysis). These simulations simultaneously captured the uncertainty in all parameters that were expected to vary. When possible, we set distributions around parameters using their mean and standard deviation. If these values were not accessible, we assumed in most cases that the value was fixed. However, for certain key parameters that were expected to vary, we assumed that the mean ± 25% would be representative of the 95% confidence intervals around the mean. The list of model variables and the corresponding distributions are listed in Appendix 5, Table A11.
We calculated mean costs and mean QALYs for each bilateral and unilateral cochlear implantation group. We also presented mean incremental costs, incremental QALYs, and ICERs for bilateral versus unilateral cochlear implantation.
For each reference case target population, we assessed variability and uncertainty in the model using probabilistic and deterministic sensitivity analyses. We presented the results of the probabilistic analyses using cost-effectiveness acceptability curves.
We conducted deterministic one-way sensitivity analyses by varying specific model variables and examining the effect on the results. We presented the results of the one-way sensitivity analyses in tornado diagrams. The variables assessed using one-way sensitivity analysis can be found in Appendix 5, Table A11 (varied to the upper and lower limits of the 95% confidence interval, calculated from the standard error). We also assessed the effect of varying additional parameters, including those related to certain methodological assumptions (Appendix 5, Table A12).
We also ran several scenario analyses relating to methodological and structural uncertainty. All scenario analyses are presented in Table 28, but we have described three in further detail below; these were more likely to affect the results or were important to the Ontario context.
Table 28:
Scenario Analyses
| Scenario | Parameters Used in Reference Case | Parameters Used in Scenario Analysis |
|---|---|---|
| Structure | ||
| Time horizon | Lifetime | 10 years to 30 years |
| Discount | 1.5% costs, 1.5% QALYs | 3% costs, 1.5% QALYs |
| Target Populations | ||
| Adults (20–40 y), sudden severe to profound sensorineural hearing loss (postlingual) | NA | Average age 31,122 simultaneous BCI |
| Adults (18–93 y), severe to profound sensorineural hearing loss (postlingual) | NA | Average age 60,122 sequential BCI, time between implants 3.5 years |
| Adults (18–39 y), severe to profound sensorineural hearing loss (postlingual) | NA | Average age 30,122 sequential BCI, time between implants 3.5 years |
| Adults (40–55 y), severe to profound sensorineural hearing loss (postlingual) | NA | Average age 49,122 sequential BCI, time between implants: 3.5 years |
| Children, severe to profound sensorineural hearing loss (prelingual) | NA | Average age 1 (expert opinion), sequential BCI, time between implants, 1 year |
| Children, sudden severe to profound sensorineural hearing loss (postlingual) | NA | Average age 7,122 simultaneous BCI |
| Reference case 1: BCI type | Sequential | Simultaneous |
| Reference case 2: BCI type | Simultaneous | Sequential |
| Reference case 3: BCI type | Sequential | Simultaneous |
| Clinical Outcomes | ||
| Complications in childhood cochlear implantation | Annual device failure rate 0.64%; annual non-device reimplant rate 0.12% | Annual device failure rate 0.54%;111 annual non-device reimplant rate 0.09%;111 no explantation (expert opinion); all reimplants in same ear (expert opinion) |
| Annual device failure rate | Adults 0.53%; children 0.64% | Cochlear Canada 0.2828%a |
| Ongoing complications | All occur in the first 6 months | All occur in the first 5 years |
| Non-use | Not included | Included 2.2% non-users108b |
| Utilities | ||
| Adults (TTO) | HUI-3 UnoCI 0.495, UUCI 0.765, UBCI 0.800113 |
TTO UnoCI 0.65, UUCI 0.82, UBCI 0.94114 |
| Children (Bond, et al 2007)50 | HUI-3 UnoCI 0.585, UUCI 0.78, UBCI 0.81128,129 |
HUI-3 UBCI = UUCI + 0.0350 |
| Children (Sparreboom et al, 2011)93 | HUI-3 UnoCI 0.585, UUCI 0.78, UBCI 0.81128,129 |
HUI-3 UnoCI 0.395, UUCI 0.58, UBCI 0.7692 |
| Children (adult values) | HUI-3 UnoCI 0.585, UUCI 0.78, UBCI 0.81128,129 |
HUI-3 (adults) UnoCI 0.495, UUCI 0.765, UBCI 0.800113 |
| No utility increase in first 6 months of cochlear implantation | Utility increase applied immediately after cochlear implantation | Utility increase applied 6 months after cochlear implantation |
| Cost and Resource Use | ||
| Perspective | Public health payer perspective | Societal perspective (productivity losses, out-of-pocket costs, costs to other ministries) |
| Use of hearing aids in UCI individuals | No hearing aid costs | Hearing aid costs ($500/5 years, ADP), assuming 80% of patients use119 |
| Postprocedural costs | Table A11 | Based on Fitzpatrick et al, 2006145 (Table A12) |
Abbreviations: ADP, Assistive Devices Program; BCI, bilateral cochlear implantation; HUI-3, health utilities index; NA, not applicable; noCI, no cochlear implantation; QALY, quality-adjusted life-year; TTO, Time Trade Off; U, utility; UCI, unilateral cochlear implantation.
Including all models except CI1500, which was removed from the market because of a high failure rate.
Assume non-user after year 2 (post-rehabilitation).
Scenario: Complications in Childhood Cochlear Implantation
In this scenario, we revised the rate of complications and our assumptions based on a study completed at an Ontario children's hospital111 and consultation with clinical experts. This scenario was based on outcomes at a cochlear implantation centre with many years of expertise. In the study, 971 implants were followed for 5,575 person-years. During this time, 35 reimplantations occurred, including 30 because of device failure.
We assumed the rate of minor revisions would be the same as in the reference case analysis.
Scenario: Utilities
We used several scenarios to examine the effect on choice of utility measure. In adults, we used utility values that were from the same source as our reference case values but obtained using the Time Trade Off method.113 This method showed the greatest increase in utility because of bilateral cochlear implantation, whereas our reference case values (using the HUI-3) were the most conservative.
In children, we conducted three scenario analyses. In the first, we assumed that bilateral cochlear implantation produced a small increase in health utility (+0.03) compared to unilateral cochlear implantation. This was consistent with the value used in the health technology assessment by Bond et al.50 In the second scenario, we used pre- and post–bilateral cochlear implantation HUI-3 values obtained from Sparreboom et al, identified in the clinical evidence review.93 Finally, we used the utility values from the adult reference case, because they may have better reflected older postlingually deafened children or changes in quality of life over time.
Scenario: Societal Perspective
We conducted a scenario analysis incorporating productivity losses, out-of-pocket costs for patients, and costs incurred by other ministries. We calculated productivity losses in dollars for adults who received bilateral cochlear implants and parents of children who received bilateral cochlear implants. We based this on the human capital approach, in which we calculated the expected number of hours a patient or parent would miss from work and multiplied this by the average hourly wage in Ontario. We assumed 1 hour of work would be missed for every 1-hour audiologist or rehabilitation appointment. We also assumed 1 day (8 hours) of work would be missed for every surgical procedure, including the initial surgery and surgical complications. We obtained the average hourly wage in Ontario from Statistics Canada ($26.25, January 2016).146
We included rehabilitation costs in children that would be incurred by other ministries (Infant Hearing Program and Ministry of Education). We also included rehabilitation costs for adults. We assumed 5% of adults would incur rehabilitation costs, based on expert consultations on the number of adults who pay out of pocket for rehabilitation programs. Finally, we included the out-of-pocket costs for external sound processors that would not be covered by the Assistive Devices Program (approximately $1,815).142
Generalizability
The findings of this economic analysis cannot be generalized to all individuals with severe to profound sensorineural hearing loss. They may, however, be used to guide decision-making about the specific patient populations addressed in the analyses conducted by Health Quality Ontario.
Results
Reference Case Analysis
Results for the three target populations assessed in the reference case analysis are shown in Table 29. Generally, bilateral cochlear implantation was more expensive and more effective than unilateral cochlear implantation, but with mean ICER estimates below $50,000/QALY. The mean values were obtained from probabilistic analysis.
Table 29:
Reference Case Analyses—Results
| Strategy | Average Total Cost, $ (95% Confidence Interval) | Incremental Cost, $a | Average Total Effect, QALYs (95% Confidence Interval) | Incremental Effect, QALYsb | ICER, $/QALY |
|---|---|---|---|---|---|
| Adults (Age 18–55 Years), Postlingual Severe to Profound Sensorineural Hearing Loss, Sequential BCI | |||||
| UCI | 65,961 (58,974–73,738) | — | 23.28 (16.67–28.13) | — | — |
| BCI sequential | 113,470 (98,345–127,795) | 47,509 (35,067–54,831) | 24.25 (18.12–28.55) | 0.97 (−6.67 to 8.17) | 48,978 |
| Children, Prelingual Severe to Profound Sensorineural Hearing Loss, Simultaneous BCI | |||||
| UCI | 88,848 (80,810–97,999) | — | 36.07 (31.48–39.88) | — | — |
| BCI simultaneous | 156,319 (143,526–170,580) | 67,471 (61,527–73,799) | 38.53 (29.19–44.39) | 2.46 (−7.50 to 9.91) | 27,427 |
| Children, Postlingual Severe to Profound Sensorineural Hearing Loss, Sequential BCI | |||||
| UCI | 86,376 (78,429–95,389) | — | 34.61 (30.21–38.27) | — | — |
| BCI sequential | 152,010 (137,794–167,120) | 65,634 (58,531–72,511) | 36.77 (28.49–42.09) | 2.16 (−6.69 to 8.84) | 30,386 |
Abbreviations: BCI, bilateral cochlear implant; ICER, incremental cost-effectiveness ratio; QALY quality-adjusted life-year; UCI, unilateral cochlear implant.
Incremental cost = average cost BCI – average cost UCI.
Incremental effect = average effect BCI – average effect UCI.
Sensitivity Analysis
The results of the probabilistic sensitivity analyses for each reference case target population are presented in Table 30 as the 95% confidence intervals for costs and QALYs. The results are also depicted below in Figures 5 to 7.
Table 30:
Scenario Analyses—Results
| ICER, $/QALY | ||||
|---|---|---|---|---|
| Variable | Adults, Sequential | Children, Simultaneous | Children, Sequential | Other |
| Structure | ||||
| Time horizon: 10 years | 103,730 | 39,880 | 72,761 | — |
| Time horizon: 30 years | 55,009 | 30,351 | 37,415 | — |
| Differential discounting | 39,380 | 19,561 | 21,796 | — |
| Target Populations and BCI Type | ||||
| Adults (20–40 y), sudden severe to profound sensorineural hearing loss, simultaneous BCI (postlingual) | — | — | — | 41,180 |
| Adults (18–93 y), severe to profound sensorineural hearing loss, sequential BCI (postlingual) | — | — | — | 59,142 |
| Adults (18–39 y), severe to profound sensorineural hearing loss, sequential BCI (postlingual) | — | — | — | 46,149 |
| Adults (40–55 y), severe to profound sensorineural hearing loss, sequential BCI (postlingual) | — | — | — | 52,283 |
| Children, severe to profound sensorineural hearing loss, sequential BCI (prelingual) | — | — | — | 29,919 |
| Children, severe to profound sudden sensorineural hearing loss, simultaneous BCI (postlingual) | — | — | — | 27,427 |
| BCI type (simultaneous vs. sequential) | 42,644 | 29,875 | 27,784 | — |
| Clinical Outcomes | ||||
| Complications in childhood cochlear implantation (Eskandar, 2011,111 and experts) | — | 28,192 | 30,943 | — |
| Annual device failure rate (Cochlear Canada)a | 47,868 | 25,914 | 28,635 | — |
| Ongoing complications (5 years) | 49,493 | 27,427 | 30,387 | — |
| Non-use (2.2%)b | 49,056 | 27,627 | 30,304 | — |
| Utilities | ||||
| BCI favoured (children, Kuthubutheen et al, 2014,114 TTO) | 15,035 | — | — | — |
| BCI not favoured (children, Bond et al, 200950) | — | 46,213 | 51,680 | — |
| BCI favoured (children, Sparreboom et al, 201192) | — | 8,584 | 9,417 | — |
| Adult utilities for children (Chen et al, 2014113) | — | 37,072 | 41,279 | — |
| No utility increase in first 6 months of cochlear implantation | 49,489 | 28,349 | 20,849 | — |
| Cost and Resource Use | ||||
| Perspective | 58,772 | 33,552 | 39,958 | — |
| Use of hearing aids in UCI individuals | 47,357 | 26,715 | 29,597 | — |
| Postprocedural costs (Fitzpatrick et al, 2006145) | — | 27,420 | 35,763 | — |
| Reference Case | 48,978 | 27,427 | 30,386 | — |
Abbreviations: BCI, bilateral cochlear implantation; TTO, Time Trade Off; UCI, unilateral cochlear implantation.
Including all models except CI1500, which was removed from the market because of a high failure rate.
Assume non-user after year 2 (post-rehabilitation).
Figure 5: Cost-Effectiveness Acceptability Curve—Reference Case 1a,b.
aAdults (age 18 to 55 years), postlingual severe to profound sensorineural hearing loss, sequential bilateral cochlear implantation.
bThe x-axis represents the willingness-to-pay threshold, and the y-axis represents the probability that the intervention is cost-effective. The dotted lines highlight the probability that bilateral cochlear implantation is cost-effective at willingness-to-pay thresholds of $50,000 (51.18%) and $100,000 (56.74%).
Figure 7: Cost-Effectiveness Acceptability Curve—Reference Case 3a,b.
aChildren, postlingual severe to profound sensorineural hearing loss, sequential bilateral cochlear implantation.
bThe x-axis represents the willingness-to-pay threshold, and the y-axis represents the probability that the intervention is cost-effective. The dotted lines highlight the probability that bilateral cochlear implantation is cost-effective at willingness-to-pay thresholds of $50,000 (62.38%) and $100,000 (68.28%).
Figure 6: Cost-Effectiveness Acceptability Curve—Reference Case 2a,b.
aChildren, prelingual severe to profound sensorineural hearing loss, simultaneous bilateral cochlear implantation.
bThe x-axis represents the willingness-to-pay threshold, and the y-axis represents the probability that the intervention is cost-effective. The dotted lines highlight the probability that bilateral cochlear implantation is cost-effective at willingness-to-pay thresholds of $50,000 (63.64%) and $100,000 (68.86%).
The results of the one-way deterministic analyses are presented in Appendix 6, Figures A1 to A3. The most influential parameters were the utilities of bilateral and unilateral cochlear implantation (from a lower ICER for bilateral cochlear implantation versus unilateral cochlear implantation to bilateral cochlear implantation being costlier and less effective than unilateral cochlear implantation), time to external processor replacement (longer time led to lower ICER), discount for a second-side cochlear implant (larger discount led to lower ICER), and cost of the first-side cochlear implant (lower cost led to lower ICER).
The results of the scenario analyses are presented in Table 30.
Longer time horizons led to higher ICERs in each of the populations of the reference case analyses.
We assessed different populations with variations in age and type of bilateral cochlear implantation (sequential or simultaneous). Bilateral cochlear implantation had a higher ICER in older adults. Simultaneous bilateral cochlear implantation also led to lower ICERs than sequential bilateral cochlear implantation.
The tool used to obtain utility values also affected the ICERs (Table 30). In an analysis where we differentially discounted costs (3%) and QALYs (1.5%), the ICERs for all populations were reduced.
Finally, when we included productivity loss, costs to other ministries, and out-of-pocket costs to patients in a societal analysis, ICERs increased for all populations. In adults, the ICER increased to $58,772/QALY. This was because more surgical complications (because of having two implants) and patient time was expected for patients receiving bilateral cochlear implantation. In addition, patients receiving sequential bilateral cochlear implantation would have an additional surgery and more audiologist and physician visits.
Discussion
Our reference case analysis showed that in adults (age 18 to 55 years) with postlingual deafness, sequential bilateral cochlear implantation was cost-effective on average (ICER $48,978/QALY) at commonly used willingness-to-pay thresholds of $50,000/QALY and $100,000/QALY. Simultaneous and sequential bilateral cochlear implantation in prelingually and postlingually deafened children was also on average, cost-effective (ICERs $27,427/QALY and $30,386/QALY, respectively).
Our results were similar to those of Chen et al,113 who showed an ICER of $55,020/QALY when comparing sequential bilateral cochlear implantation to unilateral cochlear implantation in adults. We used utility values for cochlear implantation and some costs from that study, but our study differed in several respects, some of which may have led to lower ICERs. We used a lifetime horizon, allowing the benefits of bilateral cochlear implantation to be realized over a person's life span. We also updated the costs, incorporated disutilities for complications, performed analyses using a Markov model, and conducted probabilistic sensitivity analysis.
Probabilistic analysis indicated that the probability of bilateral cochlear implantation being cost-effective compared to unilateral cochlear implantation was approximately 69%, 68%, and 57% for prelingually deafened children, postlingually deafened children, and postlingually deafened adults, respectively at willingness-to-pay thresholds ≥ $100,000/QALY. This high level of uncertainty was likely due to uncertainty in the utility values for bilateral and unilateral cochlear implantation and associated complications.
Deterministic sensitivity analysis showed that the results were highly sensitive to the utility values used for unilateral and bilateral cochlear implantation. Bilateral cochlear implantation in scenarios with different utility values ranged from highly cost-effective to dominating (less effective and less expensive than unilateral cochlear implantation). In our reference case analysis, the inputs for adults and children had overlapping confidence intervals. This finding aligned with previous cost-effectiveness analyses50,64 and the results from our clinical evidence review. The literature has shown that generic quality-of-life measures may not be sensitive to the difference between hearing with both ears and hearing with one ear. While disease-specific quality-of-life instruments are available (e.g., the Nijmegen Cochlear Implant Questionnaire, the Glasgow Hearing Status Inventory, and the Glasgow Benefit Inventory), converting these results to QALYs is controversial, and mapping algorithms are not currently available. Given the challenges involved in capturing quality-of-life benefits in this population, our results should be considered along with all clinical outcomes (including those we were unable to incorporate into the model).
Our results were also sensitive to time horizon and age at implantation. Logically, the longer a person spends with bilateral cochlear implants, the longer they may accrue clinical benefits and QALYs. Our analyses highlighted that, where possible and clinically indicated, simultaneous implantation in younger patients would result in improved cost-effectiveness.
An analysis from a societal perspective highlighted that when productivity losses, costs to patients, and costs to other ministries were included, the results were less favourable for bilateral cochlear implantation. This was primarily driven by increased numbers of surgeries and health care visits related to bilateral cochlear implantation, leading to lost productivity for patients/caregivers. Another important factor was rehabilitation costs. While rehabilitation plays an important role in the success of cochlear implantation,147 most costs are covered by other ministries (in children) or by the patients themselves (in adults). It is important to highlight the costs that may occur in other sectors if bilateral cochlear implantation is funded and more rehabilitation is required. We expect this would be most likely to occur for patients who receive sequential implants. However, while the societal perspective is important to consider, we were unable to include the societal benefits of bilateral cochlear implantation (such as improvements in education and employment performance) in the model.
Finally, our results were sensitive to the cost of the first cochlear implant and the discount provided on the second-side implant. Currently in Ontario, each cochlear implant centre carries out its own procurement of devices. We provided an average price ($25,000 per implant) and discount (50%) based on consultations with manufacturers and previous analyses,113,114 but we expect the cost of implants to vary. Obtaining cochlear implants at a lower cost, and with a higher second-side discount, will lead to better cost-effectiveness.
Our study had several strengths. We used a lifetime horizon and a Markov cohort structure, which allowed us to capture complications and postprocedural care over time. Hearing loss is a chronic condition, so capturing costs and benefits over time is necessary to understand cost-effectiveness. We also used Ontario-specific cochlear implantation protocols and costs to populate our model. One important example was the incremental surgical cost of simultaneous bilateral cochlear implantation compared with unilateral cochlear implantation, which we obtained from an Ontario costing study.139 This enhanced the generalizability of our results to real-world practice. Finally, we conducted reference case and sensitivity analyses in several subgroups, with variations expected in age, sex, and type of surgery required. This will allow decision-makers to understand the range of cost-effectiveness to be expected.
Our study also had several limitations. While we conducted analyses in several subgroups, we used many of the same parameters throughout the analyses. We were unable to capture the full variation in costs, resource use, and quality of life between subgroups. As well, data specific to subgroups were not available. For example, for quality-of-life data we used two sets of HUI-3 utilities, one for adults and one for children. Within these subgroups, we expected variations to occur. For example, because of an absence of available data, we applied the utility for children with prelingual hearing loss to children with postlingual hearing loss. We also assumed that utilities would remain the same throughout the time horizon, even though children grow into adults. One option was to use or alter the utility value to reflect the adult population. However, children are still developing when they receive their cochlear implants, and the benefits they experience may be different from adults, whose anatomy is fully developed when they receive their implants. The utilities used for children were also obtained using parental proxies, which may have led to overestimation of the health utility gains.148 In the sensitivity analysis, we assessed the effect of using adult utility values in children and found that the ICERs did not change substantially. An additional limitation, previously highlighted, was that we were unable to capture the societal benefits of hearing with two ears (through bilateral cochlear implantation). This remains challenging because of a lack of empirical studies on the topic. Research should be conducted to highlight the real-world educational, professional, and social benefits of bilateral cochlear implantation.
Conclusions
Bilateral cochlear implantation was more expensive but more effective than unilateral cochlear implantation. In adults and children, simultaneous and sequential bilateral cochlear implantation was, on average, cost-effective at commonly used willingness-to-pay thresholds.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis to estimate the cost burden of funding bilateral cochlear implantation in adults and children with severe to profound sensorineural hearing loss. All costs are reported in 2017 Canadian dollars.
Research Question
What is the 5-year budget impact of publicly funding bilateral cochlear implantation for the treatment of severe to profound sensorineural hearing loss in adults and children, within the context of the Ontario Ministry of Health and Long-Term Care?
Methods
Analytic Framework
Currently, four hospitals in Ontario receive (fixed) volume-based funding from the Ontario Ministry of Health and Long-Term Care to perform cochlear implantation. In adults, funding is only for unilateral cochlear implantation. In children, the official position is that only unilateral cochlear implantation is funded, but the Ministry has provided funding enhancements so that pediatric hospital-based cochlear implant programs can perform bilateral cochlear implantation. In children, bilateral cochlear implantation has been the status quo for several years.
We conducted two budget impact analyses: one in adults and one in children. An overview of how we conducted the budget impact analyses is presented in Figure 8. We compared two scenarios: the current scenario, and a new scenario in which bilateral cochlear implantation is publicly funded. The budget impact of funding bilateral cochlear implantation depends on the current volume of implants funded and the change in volume required to reach wait-time targets (from decision-to-treat to implantation), incorporating expected referral patterns and increased demand because of bilateral cochlear implantation. The budget impact also depends on the indication (prelingual, postlingual, progressive, sudden) and the type of cochlear implantation performed (unilateral, simultaneous bilateral, sequential bilateral).
Figure 8: Budget Impact Analysis—Analytic Framework.
*Enhancements required due to wait times, referral increases.
Abbreviation: CI, cochlear implant.
Our data and assumptions are based on the Ontario Cochlear Implant Program 2016–2019 (written communication, Ontario Cochlear Implant Program Provincial Plan, December 2016) and consultation with experts.
Cochlear Implants: Volumes
The expected volumes of cochlear implantations in the current and future scenarios are presented in Table 31. Volumes are presented per implant, not per individual, so someone who received simultaneous bilateral cochlear implantation would count as two implants. We based expected volumes on currently funded volumes (written communication, Ontario Cochlear Implant Provincial Plan, December 2016) and enhancements in funding required due to referral rates, and bilateral cochlear implantations.
Table 31:
Cochlear Implants—5-Year Expected Volume for Funding in Ontario
| Population | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Adults (Assumes an Annual 10% Increase in Referrals and Cochlear Implantations) | |||||
| Current Scenario | |||||
| Unilateral cochlear implantations | 270 | 297 | 327 | 360 | 396 |
| Future Scenario | |||||
| Unilateral cochlear implantations | 270 | 297 | 327 | 360 | 396 |
| Bilateral cochlear implantations | 27 | 30 | 33 | 36 | 40 |
| Total cochlear implantations | 297 | 327 | 360 | 396 | 436 |
| Children (Assumes a Steady Referral Rate) | |||||
| Current Scenario | |||||
| Total cochlear implantations | 146 | 146 | 146 | 146 | 146 |
| Future Scenario | |||||
| Total cochlear implantations | 146 | 146 | 146 | 146 | 146 |
Referral Rates
Referral rates in adult centres have been increasing and are expected to continue increasing due to awareness in the community, population increases and immigration, changes in candidacy, education around candidacy, and the graduation of children with cochlear implants (which may fail over time) into the adult system (written communication, Ontario Cochlear Implant Program Provincial Plan, December 2016). Based on information from the Ontario Cochlear Implant Program and referral patterns in the three adult centres, we assumed a 10% increase in referrals (and therefore, implants) per year.
The pediatric referral rates and corresponding implant rates in Ontario are currently stable (written communication, Ontario Cochlear Implant Program Provincial Plan, December 2016). In our reference case analysis, we assumed that the volume of pediatric implants would remain stable. However, it is difficult to predict an influx in referrals because of external factors (e.g., immigration, immunization). In the sensitivity analysis, we examined a moderate increase in referral rates and subsequent implant volumes.
Bilateral Cochlear Implantation
Bilateral implantation is reserved for candidates who meet the strict candidacy criteria specified in the background section of this health technology assessment. In our reference case analysis, we assumed that an additional 10% of implants would be required to fund bilateral cochlear implantation at the target volume. We assumed that this would increase with referrals over time. In the sensitivity analysis, we examined increased growth in the volumes of bilateral cochlear implantation.
For children, bilateral cochlear implantation is the standard of care and current practice for those with sensorineural hearing loss in Ontario. We assumed no change to this practice in our new funding scenario.
Indication and Type of Bilateral Implantations
The indications for cochlear implantation, target populations, and type of implantation are described in Table 32. The percent of individuals falling into each category are based on expert opinion. As previously stated, bilateral cochlear implantation is the current standard of care for children in Ontario. Based on our consultations with clinical experts, we determined that it is very rare for a child not to receive a second implant. Therefore, in our reference case analysis we assumed that all children would receive bilateral implants.
Table 32:
Indications and Type of Cochlear Implantation Expected in Adults and Children
| Implant Type | Population and Indication | % of Implants |
|---|---|---|
| Adults | ||
| Current Scenario | ||
| Unilateral cochlear implantation | Adults, progressive sensorineural hearing loss (all ages)a | 99 |
| Adults, sudden sensorineural hearing loss (e.g., because of meningitis) or combined hearing and vision lossb | 1 | |
| Future Scenario | ||
| Unilateral cochlear implantation | Adults, progressive sensorineural hearing loss (all ages)a | 91 |
| Bilateral cochlear implantation | Adults, progressive sensorineural hearing loss, meeting the Ontario Cochlear Implant Program candidacy criteria (age 18–55 years)c | 7 |
| Adults, sudden sensorineural hearing loss (e.g., because of meningitis) or combined hearing and vision lossb | 2d | |
| Children | ||
| Current and Future Scenarios | ||
| Unilateral cochlear implantation | NA | — |
| Bilateral cochlear implantation | Children, prelingual sensorineural hearing loss (70%)e | |
| Simultaneous | 65 | |
| Early sequential | 5 | |
| Children, postlingual sensorineural hearing loss (30%)f | ||
| Sequential | 29 | |
| Simultaneous (sudden sensorineural hearing loss) or combined hearing and vision loss | 1 | |
Resource Use and Costs
We obtained most costs from the primary economic evaluation. All costs that were relevant to the Ministry of Health and Long-Term Care were included. These included preprocedural, procedural, postprocedural, and complication costs (see Primary Economic Evaluation, Cost and Resource Use Parameters). We obtained all costs by running the model over a 5-year time horizon. We applied no discounting. The cost per person obtained from the primary economic evaluation can be found in Appendix 7, Table A14. In some sensitivity analyses, we also used the current fixed funding rates for cochlear implantation in Ontario. The Ministry of Health and Long-Term Care currently funds each cochlear implant (bilateral cochlear implantation is two implants) at $32,000 per implant (written communication, Ontario Cochlear Implant Program Provincial Plan, December 2016).
For adults, we assumed that everyone receiving sequential bilateral implants already had one cochlear implant. For children, we assumed that 50% would be receiving their first implant and 50% would be receiving their second.
Analysis
Reference Case
We calculated the net budget impact (cost difference between the current scenario and the future scenario) for adults and children, given the most likely set of input parameters and model assumptions.
In adults, we calculated the cost difference between the future scenario (public funding for bilateral cochlear implantation) and the current scenario (no public funding for bilateral cochlear implantation).
In children, there were no anticipated changes in the future scenario, so the net budget impact was zero.
Sensitivity Analyses
We conducted sensitivity analyses and explored variability in the budget impact by changing key input parameters and model assumptions.
In the first set of sensitivity analyses, we looked at the net budget impact of funding bilateral cochlear implantation compared to the current scenario and current funding structure. We assumed that all cochlear implants funded in the current scenario were funded at the fixed rate of $32,000 per implant. We also included the cost of complications as predicted in the primary economic evaluation. We compared our findings to the future scenario (public funding for bilateral cochlear implantation) using all costs from our model.
We also performed several additional sensitivity analyses in adults and one in children (Table 33).
Table 33:
Sensitivity Analyses
| Parameter | Reference Case Analysis | Sensitivity Analysis |
|---|---|---|
| Adults | ||
| Assessment fees included for all individuals (including those who did not receive cochlear implantation) | NA | Assume 40% (Ontario Cochlear Implant Program) of bilateral cochlear implantation referrals receive implantation; include 100% of referral assessment fees |
| No discount on second-side cochlear implant | 50% | 0% |
| Increased uptake rate of bilateral cochlear implantation | 10% of total implants | + 10% each year (i.e., 20% in year 2, 30% in year 3) |
| No increase in funding because of referral volumes | 10% increase in cochlear implantations because of increased referrals each year | No increase in cochlear implantations each year |
| Funding bilateral cochlear implantation at $32,000 per implant | Model costs | $32,000 per implant |
| Children | ||
| Bilateral cochlear implantation vs. unilateral cochlear implantationa | NA | Compare bilateral cochlear implantation to unilateral cochlear implantation |
Abbreviation: NA, not applicable.
Assuming half of the implants were required for unilateral cochlear implantation.
Results
Reference Case
The results from the reference case analysis are presented in Table 34. The cost per person depended on the indication and type of cochlear implant (Appendix 5, Table A13). Using costs from the primary economic evaluation, the net budget impact of funding bilateral cochlear implantation in adults would be $510,000 to $780,000 per year. In children, cochlear implantation volumes would remain the same, so there would be no budget impact. Cost breakdowns can be found in Appendix 7, Table A15.
Table 34:
Reference Case Analyses—Results
| Budget Impact, Millions | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Adults | ||||||
| Current scenario: no bilateral cochlear implant | 8.23 | 9.08 | 10.01 | 11.04 | 12.16 | 50.53 |
| Future scenario: bilateral cochlear implant | 8.75 | 9.66 | 10.65 | 11.74 | 12.94 | 53.74 |
| Net budget impact | 0.51 | 0.58 | 0.64 | 0.70 | 0.78 | 3.21 |
| Children | ||||||
| Current scenario: bilateral cochlear implant | 3.40 | 3.43 | 3.44 | 3.46 | 3.47 | 17.19 |
| Future scenario: bilateral cochlear implant | 3.40 | 3.43 | 3.44 | 3.46 | 3.47 | 17.19 |
| Net budget impact | 0 | 0 | 0 | 0 | 0 | 0 |
Numbers may appear inexact due to rounding.
Sensitivity Analysis
Assuming that implants in the current scenario would be funded at a fixed cost of $32,000 per implant, the net budget impact of funding bilateral cochlear implantation in adults would be reduced, and in children it would be cost-saving (Table 35).
Table 35:
Sensitivity Analyses—Results, Fixed Funding in Current Scenario
| Budget Impact, Millions | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Adults | ||||||
| Current scenario: no bilateral cochlear implant, $32,00/implant and complications | 8.65 | 9.53 | 10.50 | 11.57 | 12.74 | 52.99 |
| Future scenario: bilateral cochlear implant, model costs | 8.74 | 9.65 | 10.64 | 11.72 | 12.92 | 53.67 |
| Net budget impact | 0.09 | 0.12 | 0.14 | 0.16 | 0.19 | 0.69 |
| Children | ||||||
| Current scenario: bilateral cochlear implant, $32,000/implant and complications | 4.68 | 4.69 | 4.70 | 4.70 | 4.71 | 23.48 |
| Future scenario: bilateral cochlear implant, model costs | 3.40 | 3.43 | 3.44 | 3.46 | 3.47 | 17.19 |
| Net budget impact | −1.28 | −1.26 | −1.25 | −1.25 | −1.24 | −6.29 |
Numbers may appear inexact due to rounding.
The results from the remaining sensitivity analyses are presented in Table 36.
Table 36:
Sensitivity Analyses—Results
| Budget Impact, Millions | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Adults | ||||||
| Reference case | 0.51 | 0.58 | 0.64 | 0.70 | 0.78 | 3.21 |
| Assessment fees included for all individuals (including those who did not receive cochlear implantation) | 0.53 | 0.60 | 0.66 | 0.72 | 0.81 | 3.32 |
| No discount on second-side cochlear implant | 0.81 | 0.90 | 1.00 | 1.09 | 1.22 | 5.03 |
| Increased uptake rate of bilateral cochlear implantation | 0.51 | 1.13 | 1.87 | 2.73 | 3.77 | 10.01 |
| No increase in funding because of referral volumes | 0.51 | 0.52 | 0.52 | 0.52 | 0.52 | 2.60 |
| Funding bilateral cochlear implantation at $32,000 per implant | 0.86 | 0.96 | 1.06 | 1.15 | 1.28 | 5.31 |
| Children | ||||||
| Reference case | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Bilateral cochlear implantation vs. unilateral cochlear implantationa | −1.14 | −1.15 | −1.15 | −1.16 | −1.17 | −5.77 |
Assuming half of the implants were required for unilateral cochlear implantation.
Discussion
To fund bilateral cochlear implantation, little additional budget would be required for children, but an increased budget would be required over the next 5 years for adults. The budget impact would depend highly on the cost per case, as well as on the percentage of adults who received bilateral versus unilateral implants.
This budget impact analysis had several limitations. First, we were unable to predict fluctuations in referral rates because of external factors such as immigration or vaccination. Second, we were unable to fully capture the interactions between the pediatric and adult cochlear implant programs. Children with cochlear implants will eventually graduate into the adult system, and the adult system will then be responsible for the costs of major complications for the rest of the patient's life, including out-of-warranty device costs and surgical costs. Third, we were unable to incorporate complication costs for those currently living with a cochlear implant, because detailed data on the number of cochlear implant patients in Ontario, their age, and their duration with a cochlear implant were unavailable. This means our findings for the budget impact in adults may be an underestimate. Finally, it was difficult to predict the uptake of bilateral cochlear implantation in adults if it were funded. We based our analysis on information from the Ontario Cochlear Implant Program, which incorporated the expertise of individuals from all cochlear implantation centres in Ontario.
Conclusions
The budget impact of publicly funding bilateral cochlear implantation in adults to treat severe to profound sensorineural hearing loss would be $510,000 to $780,000 over the next 5 years.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, impacts, and preferences of those who have lived experience with sensorineural hearing loss. The treatment focus was cochlear implants versus hearing aids or no device.
Background
Patient, caregiver, and public engagement provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the patient, the patient's family and other caregivers, and the patient's personal environment. It also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., sometimes typical outcome measures do not reflect what is important to those with lived experience).149–151 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are important to understand and consider, we contact and speak directly with people who live with a given health condition, including those who may have experience with the intervention we are exploring.
Hearing loss has a significant impact on patients and their families, and it substantially affects their quality of life. The 2012/13 Canadian Health Measures Survey showed that 20% of adults aged 18 to 79 years had at least mild hearing loss in one or both ears, and the prevalence of hearing loss increases with age.152
For this project, we spoke with people who have lived experience with sensorineural hearing loss and its impact. All those interviewed had experience using hearing aids to attempt to manage hearing loss and had used unilateral or bilateral cochlear implants. Gaining an understanding of the day-to-day experience of dealing with hearing loss, including people's experience with cochlear implants, helps us assess the potential value of this technology from the perspective of patients and caregivers.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of patients with hearing loss, including their experience with cochlear implants. Due to the nature of the medical condition and patients’ varied comfort with audio, we offered multiple methods of engagement, including face-to-face, phone, written, and video interviews.
We used qualitative interviews, because this method of engagement allows us to explore the meaning of central themes in the experiences of patients’ hearing loss. Our main task in interviewing is to understand what people tell us and gain an understanding of the story behind their experiences.153 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our primary choice of an interview methodology.
Participant Outreach
We actively reached out to patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, health clinics, hearing loss support associations, and foundations to spread the word about this engagement activity and to make contact with patients, families, and caregivers, including those with experience of hearing loss and cochlear implants.
Inclusion Criteria
We sought to speak with patients with sensorineural hearing loss and their families. Patients did not have to have direct experience with cochlear implants.
We sought broad geographic, cultural, and socioeconomic representations to elicit possible equity issues in accessing and using cochlear implants.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
We conducted interviews and focus groups with 27 people. Of these interviews, 13 were written; the remainder were in-person, phone, or video interviews.
Those interviewed included adults with sensorineural hearing loss and unilateral or bilateral cochlear implants, and parents of children with bilateral cochlear implants. We recruited participants from across Ontario.
All participants had direct experience with cochlear implants. Because no participants received cochlear implants immediately upon diagnosis of sensorineural hearing loss, they were able to compare their experiences of managing hearing loss with various devices, such as hearing aids and cochlear implants.
Approach
At the beginning of the interviews, we explained the role of Health Quality Ontario, the purpose of the health technology assessment, the risks of participation, and how personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 8). We then obtained each participant's verbal consent before starting the interview. With participants’ consent, we audio-recorded the phone interviews and then had the recordings transcribed.
Interviews lasted 20 to 60 minutes. They were loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.154 Questions focused on the impact of hearing loss on patients’ and families’ quality of life, their experiences with treatment options, and their perceptions of the benefits or limitations of using cochlear implants to manage their hearing loss. See Appendix 9 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts and written results. The grounded-theory approach allowed us to organize and compare information across participants. This method consisted of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.155,156 We used the qualitative data analysis software program NVivo (QSR International, Doncaster, Victoria, Australia) to identify and interpret patterns in interview, focus group, and survey data. The patterns we identified then allowed us to highlight the impact of health conditions and treatments on the patients, family members, and caregivers we interviewed.
Results
Lived Experience With Hearing Loss
The patients we interviewed reported progressive hearing loss beginning at a variety of ages. Typically, they first noticed symptoms of hearing loss in a school setting, although a number of patients reported that their hearing loss first became noticeable in later years. Often, patients did not realize the extent of their own hearing loss, unknowingly compensating with lip reading and other coping mechanisms. Most often, hearing loss was more noticeable in one ear, but patients reported progressive hearing loss in both ears at various rates of progression.
It seems that I had more and more difficulty hearing people. I realized I had learned lip reading to help me cope with the hearing loss without even noticing it. I couldn't hear people calling me from behind.
I have not found my hearing loss to be psychologically unsettling, nor do I feel it has held me back from participating in life. I make continuous adjustments that happen quite automatically, and I often don't realize I'm doing so.
I noticed that in places with lots of background noise I maybe had more trouble pulling the voices out of that noise than I had ever had before. That happened so gradually that in retrospect you could see it, but at the time it's difficult to notice that there's any difference.
As the hearing loss continued to progress, patients reported that they began to be forced to compensate in different ways, and the loss of hearing had an increasing impact on their day-to-day life. Examples of this impact could be large or small: from interactions with family to being unable to hear alarms, doorbells, television, or the sound of a phone ringing. Patients who lived in a rural setting reported increased isolation because of a lack of resources.
I couldn't use a phone for about 5 years before my implant. Because of not being able to see people, I couldn't understand what they were saying. It was just a jumble to me. It was not understandable at all.
I was essentially deaf. And it was a very, very trying time, for sure, because I lived out in rural Ontario, so there's no real support there. There's no deaf community to become a part of, you know?
But clarity of sound—in particular in the voice range—those were the issues. I really was in a desperate state by the time we finally got to the implant.
Occasionally, patients reported that hearing loss was accompanied by other symptoms, adding to its effect on their quality of life. One interviewee was also blind, so the progression of hearing loss led to extraordinary challenges in many aspects of life.
I next experienced several vertigo episodes. I noticed that I felt very uncomfortable and disoriented in any room setting where there were more than a dozen people engaged in conversation. It was overwhelming and confusing, and I found the noise level intolerable.
I suffer from severe to profound hearing loss in both ears. With tinnitus, that is classified under catastrophic. The tinnitus itself has reduced my own patience with day-to-day interactions and communication.
Well, I also don't have any vision, so I had to learn a tactile sign language, and then the only way people could talk to me was to know this tactile language that I had to learn.
Patients were not only required to make adjustments in their own life, but also to educate those around them to adjust for their hearing loss. The education extended beyond family to colleagues, co-workers, and social acquaintances.
I have to educate my parents and siblings and spouse on how to communicate with me. They learned to first touch me to get my attention before speaking to me, to not cover their mouth so I can read their lips, and to face me for the same reason.
Social Impact
While several patients spoke positively of the supports and the adjustments they were able to make in their workplace, others felt that their hearing loss affected their ability to do their job effectively.
In terms of my career, it was a setback at a time I should have been making great strides forward. I couldn't do phone calls, had difficulty understanding what was going on in meetings, and could no longer attend trade shows on behalf of my company.
I had lost all ability to communicate with people—or most of my ability to communicate with people—and it was impacting my job.
So probably by the time I was 26, I'm not really sure that I used the phone very much at all. I had to start making concessions at work. So I was a journalist at the time. And obviously that involves a lot of phone calls, and going to conferences, and meeting with people in noisy environments, and cocktails, and stuff like this. It was very, very difficult to do my job, and it was very stressful.
Beyond the workplace, patients also reported that progressive hearing loss significantly affected their social life. As people's hearing deteriorated, simple conversations became more and more frustrating. In a social situation with multiple speakers, it became a huge challenge. A number of those interviewed reported simply withdrawing from social events and avoiding them altogether; they felt it was not worth the frustration and sense of embarrassment. Several patients experienced this social withdrawal as teenagers.
Going out for a meal at a restaurant is something I have to plan carefully: I only choose quiet places, which is a challenge, or choose times that are not the typical lunch or dinner hour so that there are fewer people.
I lost a lot of friends because my personality changed. I couldn't be the same person I was before, when I couldn't follow along with conversations in groups or hear the punch lines of jokes. I had to stop participating in sports, because I couldn't hear the whistle any more. I was afraid to tell people I couldn't hear, because I felt they wouldn't want to be my friend anymore. I wanted to be just like everyone else (doesn't every teen?!).
Yeah, I would do anything I could to avoid social situations, even with people that I knew—especially with people that I didn't know, though. If it was a familiar voice, familiar surroundings, OK. But the anxiety would start to build as I would get to the time that we were supposed to get together. And I hated it. I hated getting together with people, and I sort of mastered the art of the smile and nod.
Emotional Impact
Adjusting to the loss of hearing and the quality-of-life implications often had an emotional impact on patients and their families. A number of those interviewed spoke of negative emotions about themselves and others because of their hearing loss and the challenges it presented. Feelings of isolation, loneliness, and frustration were the most commonly reported. A number of patients reported that others would assume they were mentally challenged, rather than understanding that they had hearing loss.
Other people would yell at me, tease me, laugh at the way I talked, pick on me, and not include me in their activities. Those are the times I would feel hurt, frustrated, and end up in tears. There were times when I hated being deaf and hated myself. Fortunately, my family and friends who cared would step in to comfort and support me.
The most devastating for me as an individual is that people believe and treat you as if you are mentally challenged. When you are struggling to comprehend and communicate, you give up at times, as people just shout the same thing over and over instead of trying to explain in another way so you can get a clue, or they just walk away. This changes your perception of yourself and how you interact with family and friends.
And it's isolating. That's the best way I can describe it. You can be sitting in a room full of people, but you're completely isolated, because you can see their expressions, and maybe you'll be able to read some lips, but you miss the sound of the laughter, you miss the intonation of voice and expression.
I felt like I did not exist. I was an invisible witness to all the things that were happening around me.
This emotional impact could be especially acute when dealing with family members. Several patients spoke of the sense of isolation when family moved away and telephone conversations were not possible because of loss of hearing. It could also affect the raising of children.
But kids grow. And they want to communicate and learn about the world, and not being able to hear them and engage with them the way I want to is awful. It's like feeling locked up. I have all these great ideas I'm so willing to share and so many lessons I want them to get, but the amount of work to just discuss how school was drained me.
Hearing Aids
Beyond compensating for hearing loss through behaviour changes such as lip-reading, the most common tool patients reported using was hearing aids. Depending on when the hearing loss was first diagnosed, hearing aids were introduced at a fairly young age. A number of patients reported using hearing aids in school even as young teenagers. Generally, patients reported that hearing aids were effective at first, allowing a measure of normalcy when facing hearing loss. Patients reported that hearing aids seemed more effective and less cumbersome as the technology advanced.
I've had hearing aids since I was 14. And I was supposed to have them when I was 6 or 7. But I didn't like them. I was too cool for them. Too cool for school. Too cool for the hearing aids. I didn't want to wear them.
Getting used to the hearing aids was not a big issue, since I saw how much it made communication easier in my life and employment.
Two hearing aids were suggested and seemed to bring much improvement to my ability to hear.
And with a succession of increases in the power of the hearing aids, the processors of the hearing aids, I managed fairly well until about 5 years ago.
However, many patients reported that as their hearing loss progressed, hearing aids became less and less effective. Hearing aids amplified the sound, but patients reported that it wasn't the volume that was the challenge, but the comprehension.
And I'd get frustrated with the hearing aids, because they weren't really cutting it for me. Especially near the tail end, for sure.
The hearing aids would provide enough volume, but there was no clarity, even with the volume. So when somebody would test me with the tones in an annual hearing exam or something like that, I could push the little button and say, yes, I heard that tone. But for speech, and particularly for speech in any kind of a noisy environment, it was desperately inadequate.
They really were only amplifying what I could hear, which was a nuisance and not giving me what I couldn't hear.
Cochlear Implants
Almost all patients reported being relatively familiar with cochlear implants even before considering them as a treatment option. With the progressive hearing loss and decreasing effectiveness of hearing aids, patients felt inclined to seek out alternative treatments to compensate for their hearing loss, and cochlear implants were a fairly well-known treatment option.
I've been following cochlear implants since the 80s. And I had a feeling that one day I would have one. I just knew that I would qualify. But I had to wait until I was deaf enough.
And at the end of it all, that was the same day, they said yes, you're a candidate for a cochlear implant. Well, I had to learn a lot very quickly about what a cochlear implant was. This was sort of right out of the blue, as you say.
While many patients felt that the decision to choose to have a cochlear implant was relatively simple, other patients reported initial hesitation for a number of reasons, including fear of the surgery and the loss of any residual hearing, caused by the surgical implantation.
I decided against it initially for two reasons: 1) The type of implant recommended for me, designed to preserve existing hearing, was very new and had not yet been implanted in anyone at that time. I was really worried about being the first. 2) I was also quite fearful of the surgery, and how my life would look afterwards. Since hearing aids had never worked for me, I didn't really feel confident that any device would help me.
So I looked into cochlear implants at some point, and I was like, “No, not for me.” The procedure seemed really invasive, and it was so final, and it was kind of scary. I looked up online one of those sound bites that you can listen to, and it gives you an idea of what it sounds like for a person with a cochlear implant … It was scratchy and echoey and warbly. And I was like, “Oh my God.” I couldn't imagine life like that. So absolutely not.
It was still a difficult decision, as what little sound I heard was still precious. It was very scary to think of losing that in one ear, and success is not guaranteed.
Patients reported seeking out a number of sources of information about cochlear implants to help in their decision-making, including physicians, family members, and support organizations. Often, these sources helped patients decide to try cochlear implants. In other cases, the deterioration of hearing and a feeling of desperation led patients to finally try the surgical option.
You know, it was a very despairing time, and the only thing that pulled me through was knowing that I only had a few more months to go before I was going to get implanted, and the hope that even if it didn't make the buzzing stop, I could hear something.
I was scared. I talked to people around me, and they helped me decide to do it. I was a little bit more confident having their support. I also attended a support group session, and that convinced me even more.
My decision to have a cochlear implant was motivated by a desire to live my life as I had up until I became profoundly deaf. With this new level of hearing, I could not function in the hearing world I always knew.
Once patients had made the decision to pursue a cochlear implant, there were few reported barriers. Patients simply had to meet certain criteria, which included a certain level of hearing loss. A number of patients spoke of having to wait until their hearing deteriorated further before they qualified. In addition, they felt the wait times for cochlear implants were long, although patients did report appreciating that they did not have to pay for the procedure.
Procedure and Activation
Patients generally reported that the cochlear implant procedure was free of complications or different from what they had expected. A few patients reported migraines, nausea, balance issues, or tinnitus as side effects. Patients felt that they were well prepared for the surgery and had reasonable expectations.
The procedure was pretty consistent with what I expected. Time off after surgery was a little bit longer than the hospital had recommended, but everyone is different.
The surgery was very hard. My balance was affected, and I had a hard time standing up. People were saying that the surgery was the easiest part of the process. I don't agree. For me, it was the worst part.
And then when it came time for the actual surgery, I went in there laughing and smiling, and I came out crying. And they asked me if I was in pain, and I said, “No, I'm just happy I'm going to hear.” Literally. I had zero fear about the surgery. Just do it—get on with this.
Patients typically reported having to wait about a month before their cochlear implant was activated. Following activation, they needed extensive auditory rehabilitation to teach the brain how to interpret the impulses from the device. This was unique for each patient, and those interviewed reported a variety of experiences upon activation and during rehabilitation. In general, however, patients reported improvement in sound quality and speech comprehension as rehabilitation progressed and as their brains became accustomed to the device's input. Patients who received their cochlear implant several years ago were able to report more extensively on the improvement in their hearing; those with newer implants felt that there were still improvements to be made.
When I turned on the processor, I got in the car and drove up to my sister's, and it was so loud when I came to a stop light, I just turned it off until I got to my sister's, because I was hearing all these things I hadn't heard for a long time.
It was warbly, distorted, robotic, electronic. It sounded like aliens fighting under water. It was really, really strange.
The cochlear implant is the tool, but if you don't have the training, it's no good to you.
You know, try these different settings. I never did that. You know, try these different exercises at home. Listen to books on tape while reading them. All great ideas, and I didn't do any of them. Honestly, all I needed was time and my brain to pull it all together for me.
Well, it was very frustrating at first, because hearing is a learned behaviour, and your synapses don't connect automatically. So it took a few weeks for me to start hearing things correctly. At the first few days, it was all gibberish, but I was told that's to be expected, and then your brain starts to make sense of the sounds coming in and you can hear properly. And after about 4 to 6 weeks, it was beautiful.
Impact
Patients generally reported a great positive impact with the cochlear implants, especially compared to hearing aids or no device. A number of patients were still able to use hearing aids in their other ear, which they felt complemented the sounds the implant picked up. Patients frequently reported the range of frequencies they could hear because of the cochlear implants: often these were frequencies they had not been able to hear for many years, such as high-frequency bird calls.
My life has changed a lot. When loss is gradual, sometimes you don't know what you are missing until it comes back. I hear birds in trees and wind flapping the rope on a flag pole. I hear high-frequency sounds that there are no records of my ever hearing. My hearing is not perfect, but I can function better than I have in a long time.
Now when I make dinner or cook dinner in the evenings, every day I have my iPad and my little Bose speaker and I play music, which I could never do for years and years and years and years.
Beyond the increased ability to hear sounds at a wide range of frequencies, patients reported that cochlear implants positively affected many areas of their life, greatly increasing their quality of life. These included activities of daily living, such as making phone calls and speaking with others. Social gathering had once been a burden to avoid, but patients now felt that cochlear implants gave them the confidence and ability to navigate those situations.
I'm not afraid to go to the grocery store or anywhere else I might have to communicate with someone. I can work as an auditory-verbal educator and teacher of the deaf/hard of hearing in mainstream settings … I no longer feel like an outsider in my own world.
The cochlear implant has given me back the confidence I had when I was younger. I am more willing to try new adventures, such as participating in group activities and going to a show or movie.
It's no exaggeration to say the cochlear implant changed my life completely.
I would say, at least the first 4 years, it's just something new. Everything still kept improving, and new sounds coming, and trying to figure out what I was hearing. So it was exciting—I didn't find it frustrating. I found it like a kid. I would find it just fascinating, all the noises that happened around me and discovering what they were.
This benefit extended to the workplace as well. Several patients mentioned that the cochlear implant allowed them to progress in their careers, where before they would have been limited with their hearing loss.
But having the implant, I ended up getting a job with the Canadian Hearing Society, and I really put that down to the fact that I had the implant, that it allowed me to then do that job and then eventually become a counsellor with the society. So it gave me my life back, really.
Well, it saved my career. When I retired, I sent an email to the cochlear implant surgery in London with a thank you note, and I said: You implanted me 10 years ago, but you saved my career. You gave me the last 10 years of my career—thank you.
Challenges
Patients reported some challenges associated with cochlear implants. Beyond the procedure and activation, there could be a number of follow-up appointments, which could be difficult for those who lived remotely.
Having to travel 4½ hours to Toronto for follow-up appointments continues to be an issue. I am fortunate to be able to get time off work with pay to attend appointments (as well as the time off to have and recover from the actual surgery). It's still hard to get there, though, working full-time and raising two children.
The access part, being in Northern Ontario, is a bit trickier because only a few specialized hospitals—three in total that I am aware of in the province—have the funding to do the surgeries. Therefore, the travel time and accommodation are also extra expenses that occur when you are living in Northern Ontario.
Patients also reported a varying degree of success with the cochlear implants. While some find themselves able to use a telephone quite easily, others cannot. In addition, as with any technology, there can be technical malfunctions, and patients reported that these do occur occasionally. For patients with a single cochlear implant and poor hearing in the other ear, any time the cochlear implant malfunctions is a challenging, isolating time.
It's not perfect. I can't localize sounds very well, and I still have a hard time when there are multiple speakers in a room, or when there is a speaker at the front of a room. At the end of a long day of hearing, I'm exhausted. I hear better in the early parts of the day than at night.
My challenge was always people's voices (especially women, since their voices are at a higher pitch). My next test will be speaking to my daughter without asking her to repeat everything.
Oh, absolutely. You don't have any direction. Unless you have two of something, then you're not going to have the ability to identify the source or the direction that the sound came from. It's just plain and simple, that's the case.
Patients also reported financial challenges related to replacement and maintenance costs. While a portion of the cost was covered, there were significant out-of-pocket expenses. This also applied to upgrading the technology when available.
The maintenance is costly. Again, I am fortunate to be able to afford the batteries, the cables, the headpieces, the processors … but this is money I could easily spend on other things. OHIP [Ontario Health Insurance Plan] doesn't cover very much.
There are still many cochlear implant users who do not upgrade, simply because they can't afford it.
There are so many families out there that can't get their kids upgraded because it's so expensive, and it made such a difference.
Despite these challenges, all patients interviewed reported being pleased with the cochlear implant; no one regretted the procedure.
Bilateral Cochlear Implants
With the success of one cochlear implant, many patients reported a strong desire for a second. While patients acknowledged that the impact of the second device might not be as drastic as that of the first, they reported strong positive expectations. The ability to sense direction of sound was a common expectation, and patients felt this was significant for safety and confidence. Patients with a unilateral implant perceived that bilateral implants would positively affect their ability to work and increase their quality of life. However, the success of a single implant often leads patients to want bilateral implants, and patients reported frustration at not being able to acquire a second implant in Ontario.
I wish I could have a second implant. If offered, I'd do it in a heartbeat. Any improvement to my hearing has a direct impact on my ability to earn money and contribute more to the economy, and at 44 years old in the prime of my career, hearing well is my top priority and a key item for success.
I can't remember hearing as well as I do today with one ear—with one ear—and yes, I'm still pushing for two, because I want stereo sound. I want to experience that, right?
Adults need to be able to hear out of two ears; we have two ears for a reason. And, you know, I understand that we are blessed to be able to have this surgery performed on the taxpayers’ dime, you know, not having to fork over $100,000 of our own money, which is huge, obviously. But to be denied being able to hear out of two ears, that's kind of a tough pill to swallow.
While the desire for bilateral cochlear implants was common among patients with unilateral devices, it was not universal. Several patients expressed satisfaction with a single device, reporting that with one device and a hearing aid in the other ear, their ability to engage in daily activities was satisfactory. Concerns about the invasiveness of the procedure and the cost of maintenance caused some patients to report hesitation about obtaining a second device, if it were made available. However, these patients also reported that if the hearing loss progressed in their non-implanted ear, they might change their minds about a second implant.
At one point years ago, we put our names on an informal list in case there might be an opportunity to get a second if we paid for it ourselves, but nothing ever came of that. Don't know if I would go for it now at my advanced age, but I might!
I'm satisfied with where I am. If my right ear continues to slip, maybe it would be something I would look at in the future. At the same time, I'm soon to be 74, so how many of these things do I need as I go down [laughs] … as I stumble down the path ahead of me here? I probably wouldn't have a second one. Bilateral implants don't have [the draw] for me at this point, nor do I think they will in the future.
A number of patients had received bilateral cochlear implants. Some were part of research studies, and others had been given bilateral implants because of other medical conditions (e.g., vision loss). We also interviewed parents of children with bilateral implants. All were able to speak to the perceived benefits of bilateral cochlear implants compared to unilateral devices.
Often patients remarked on the comfort and safety of bilateral implants, in case of a mechanical failure or a technical issue with one implant. In such cases, the patient is merely left with reduced, unilateral hearing rather than being completely deaf. Patients felt this was an enormous benefit, avoiding the negative effects of complete hearing loss for an uncertain amount of time while the mechanical issue was resolved.
As well, patients reported the benefits of increased ability to locate the direction of sound with bilateral cochlear implants, a factor they felt increased their safety and comfort navigating physical and social environments. They frequently mentioned hearing the direction of alarms, both at home and in the workplace. Patients also reported the increased richness and quality of sound with bilateral implants. They felt that this often gave them improved comprehension of speech and the ability to filter out background noise.
I'm really happy that I have two of them, because sometimes one breaks. The one time we were on a trip and it broke, we couldn't get a replacement and man, it is so hard to not hear with the two of them, because you can only hear out of the one side, and it sounds quite different only having one on.
So there's the redundancy piece. The other chunk is the safety factor, and although [my bilateral-cochlear-wearing children] are not overly good at direction-finding even with both of them on, at least they're capable of doing some direction-finding.
I didn't really realize … the things that I was missing until I got the second one. It sort of rounded out everything and made it so much better.
I'd say my comprehension went up in terms of being able to understand conversations, either while looking at someone or not looking at them. I was much better that way. I would miss things a lot more in conversations with just the one. Like it was far and away better with one than none. But from one to two, I just had just that bit more of engagement, where I felt confident in conversations, whether on the phone or in person, where I knew that I knew what was being said as opposed to just kind of guessing.
Similar to patients with a single cochlear implant, those with bilateral implants also faced maintenance challenges. Patients reported significant out-of-pocket expenses to replace or repair parts or when facing an upgrade in technology. A few patients had cochlear implants from different manufacturers, causing additional challenges when maintaining or replacing devices.
Discussion
Patient engagement for sensorineural hearing loss and cochlear implants was extensive. We interviewed adults with unilateral cochlear implants and adults and parents of children with bilateral cochlear implants. Because of interviewees’ variable comfort with using a telephone, we conducted interviews using multiple methods, including phone, video, in-person, and written submissions. Patients who had direct experience with cochlear implants were able to compare their experiences with that of other types of treatment, such as hearing aids.
We were able to interview participants from many parts of Ontario, including those who lived in rural or remote areas, to gain their perspectives on equity issues related to hearing loss supports and devices such as cochlear implants.
Those interviewed were highly supportive of cochlear implants and the benefits they provide for patients with hearing loss. Patients reported extensively on the negative impact of hearing loss on their quality of life and the increasing impact of hearing loss progression. Those with unilateral and bilateral cochlear implants emphasized the positive effects of the devices in areas such as social life, work life, and emotional well-being.
While most of those interviewed reported the benefits of cochlear implants, patients did identify challenges with the devices. As with most technologies, patients experienced occasional malfunctions, requiring maintenance or replacement parts, leading to a period of reduced ability to hear and potentially significant out-of-pocket expenses. In addition, the device required time and rehabilitation to teach the brain how to interpret the signals as recognizable sound. Patients reported frustration with this, although most found the experience to be hopeful and exciting after years of progressive hearing loss.
Those interviewed felt that the challenges of cochlear implants were minor compared to the benefits of improved hearing. For adults with unilateral cochlear implants, the success of the device often led to frustration and longing for bilateral implants, so they could reap the benefits of the device in both ears.
Conclusions
Patients with sensorineural hearing loss reported the positive impact of cochlear implants, perceiving that the implants provided social and emotional benefits and improvement in quality of life. Patients with unilateral cochlear implants generally expressed a desire for bilateral implants, despite the out-of-pocket costs associated with device maintenance.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
Based on evidence of moderate to high quality, bilateral cochlear implantation improved objective and subjective benefits of hearing in adults and children with severe to profound sensorineural hearing loss. The implant surgery is generally safe.
Bilateral cochlear implantation was more expensive and effective than unilateral cochlear implantation. The incremental cost-effectiveness ratio was $48,978/QALY in adults and between $27,427/QALY and $30,386/QALY in children. An annual budget increase of $510,000 to $780,000 would be required over the next 5 years to publicly fund bilateral implantation.
Patients with sensorineural hearing loss reported that cochlear implants provided social and emotional benefits and improved their quality of life.
Acknowledgments
The medical editor was Jeanne McKane; others involved in the development and production of this report were Tanveer Singh, Paul Kolodziej, Kellee Kaulback, Ana Laing, Claude Soulodre, Sarah McDowell, Andrée Mitchell, Vivian Ng, Nancy Sikich, and Irfan Dhalla.
We are grateful to Rex Banks, Lisa Caulley, Joseph Chen, Vincent Lin, Vicky Papaioannou, Lorne Parnes, David Schramm, and Wendy Ungar for their expert opinion on bilateral cochlear implantation in adults and children.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Parts of this health technology assessment are based on data and information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed in this assessment are those of the authors and not necessarily those of the Institute.
ABBREVIATIONS
- GRADE
Grading of Recommendation, Assessment, Development and Evaluation
- HUI-3
Health Utilities Index–3
- ICER
Incremental cost-effectiveness ratio
- NICE
National Institute for Health and Care Excellence
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY
Quality-adjusted life-year
GLOSSARY
- Incremental cost-effectiveness ratio (ICER)
Determines “a unit of benefit” for an intervention by dividing the incremental cost by the effectiveness. The incremental cost is the difference between the cost of the treatment under study and an alternative treatment. The effectiveness is usually measured as additional years of life or as “quality-adjusted life years.”
- Markov model
A type of modelling that measures the health state of a patient over the course of treatment. A patient may stay in one health state or move from one health state to another, depending on the effect of the treatment and the progression of the disease.
- Quality-adjusted life-year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (ability to function, freedom from pain, etc.). The QALY is commonly used as an outcome measure in cost–utility analyses.
APPENDICES
Appendix 1: Guideline Recommendations, Health Technology Assessments and Systematic Reviews, and Ongoing Studies
Table A1:
Guideline Recommendations—Bilateral Cochlear Implantation
| Author, Year (Title) | Recommendation Excerpts |
|---|---|
| Schramm D, 201026 (Canadian Position Statement on Bilateral Cochlear Implantation) |
Benefits of bilateral cochlear implantation
Impacts and risks of bilateral cochlear implantation
Indications of bilateral cochlear implantation
Future directions for bilateral cochlear implantation
|
| British Cochlear Implant Group, 201639 (Quality Standards: Cochlear Implant Services for Children and Adults) | Adults who are found to be candidates on completion of assessment are offered a unilateral cochlear implant. In exceptional circumstances (e.g., dual sensory impairment), adults may be offered simultaneous bilateral cochlear implants For children, simultaneous bilateral cochlear implantation is recommended as an option whenever clinically appropriate. Sequential implantation is not supported unless the patient was unilaterally implanted as a child at the time of publication and remains a child at the time of sequential surgery. In exceptional circumstances, sequential bilateral implantation may be required for medical or audiological reasons |
| Ramsden et al, 201233 (European Bilateral Pediatric Cochlear Implant Forum Consensus Statement) | Currently we feel that the infant or child with unambiguous cochlear implant candidacy should receive bilateral cochlear implants simultaneously as soon as possible after definitive diagnosis of deafness to permit optimal auditory development. An atraumatic surgical technique designed to preserve cochlear function, minimize cochlear damage, and allow easy, possibly repeated, reimplantation is recommended |
| National Institute for Health and Care Excellence, 2009157 (Cochlear Implants for Children and Adults With Severe to Profound Deafness) | Simultaneous bilateral cochlear implantation is recommended as an option for the following groups of people with severe to profound deafness who do not receive adequate benefit from acoustic hearing aids:
People who had a unilateral implant before publication of this guidance and who fall into one of the aforementioned categories should have the option of an additional opposite implant only if this is considered to provide sufficient benefit by the responsible clinician after an informed discussion with the individual person and their carers |
| Government of Western Australia, 201340 (Clinical Guidelines for Adult Cochlear Implantation) | Bilateral implantation has become the treatment of choice in people with bilateral severe to profound sensorineural hearing loss who are unable to obtain bimodal benefit (i.e., cochlear implant in one ear and hearing aid in the other). Whenever possible, bilateral implantation should be performed In the case of postlingually deaf adults, simultaneous and sequential implantation appear to provide similar outcomes. Simultaneous bilateral implantation will require funding and surgical considerations, which should be taken into account. Sequential bilateral implantation should be considered if a patient receives limited or no benefit from the opposite hearing aid |
Table A2:
Health Technology Assessments and Systematic Reviews—Bilateral Cochlear Implantation
| Author, Year | Search Period Databases | Included Studiesa | Conclusions |
|---|---|---|---|
| Adults | |||
| Berrettini et al, 201152 | Jan 2000 to May 2010 Pubmed, Medline, Cochrane Database of Systematic Reviews |
13 | Compared with UCI, BCI offered advantages in hearing in noise and sound localization, but less in hearing in a silent environment. There was high inter-individual variability over the benefits of the second cochlear implant |
| Bond et al 200950 | Inception to July 2007 Cochrane Library CENTRAL, Cochrane Database of Systematic Reviews, Medline, Embase, Medline In-Process and Other Non-indexed Citations, Web of Knowledge, DARE, HTA database, Current Controlled Trials, clinicaltrials.gov, National Guidelines Clearinghouse, FDA Centre for Devices and Radiological Health, Medical Healthcare and Regulatory Authority, PsycINFO |
5 | Compared with UCI, BCI increased speech perception in noise and sound localization. BCI may have also improved quality of life in the absence of worsening tinnitus |
| Crathorne et al, 201253 | Inception to Jan 2012 Medline, Embase, Medline In-Process and Other Non-indexed Citations, ISI Science Citation Index, Cochrane Database of Systematic Reviews, NHS CRD databases, EconLit, Biosis Previews, ISI Proceedings, Current Controlled Trials, National Research Register and ClinicalTrials.gov |
19 | All studies reported that BCI improved hearing and speech perception. However, results for quality of life varied and suggested that BCI may have improved quality of life in the absence of worsening tinnitus |
| Gaylor et al, 201354; excerpt from Raman et al, 201149 | Jan 2004 to May 2012 Medline, CENTRAL, Scopus |
15 | Compared with UCI, BCI provided added improvement in speech perception, especially in noisy conditions, and sound localization. The quality-of-life outcomes varied across tests after BCI |
| Raman et al, 201149 | Jan 2004 to Feb 2011 Medline, Scopus, Cochrane Database of Systematic Reviews |
16 | Studies evaluating speech perception in noise found significant gains with BCI vs. UCI (moderate quality of evidence). Results of speech perception in quiet and health-related quality of life varied across studies (low quality of evidence) |
| van Schoonhoven et al, 201355 | Oct 2006 to Mar 2011 Medline, Embase | 14 | Results showed a systematic benefit of BCI over UCI for sound localization, and to a lesser extent for speech perception in noise and quality of life. Most speech perception in quiet outcomes did not show a bilateral benefit |
| Washington State Health Care Authority HTA, 201351 | Inception to Feb 2013 Published systematic reviews, Medline, Embase |
17 | A large number of very small studies on mostly adults with postlingual deafness showed that BCI improved speech perception in noise and sound localization. Based on a moderate level of confidence, BCI improved disease-specific functions and quality of life compared to UCI |
| Children | |||
| Bond et al, 200950 | Inception to July 2007 Cochrane Library CENTRAL, Cochrane Database of Systematic Reviews, Medline, Embase, Medline In-Process and Other Non-indexed Citations, Web of Knowledge, DARE, HTA database, Current Controlled Trials, clinicaltrials.gov, National Guidelines Clearinghouse, FDA Centre for Devices and Radiological Health, Medical Healthcare and Regulatory Authority, PsycINFO |
6 | Despite important limitations in the methodological quality of the existing evidence (e.g., small sample size, poor reporting, and lack of controlling for confounding factors), BCI appeared to provide additional advantages for speech perception in noise, sound localization, and subjective benefits of hearing compared to UCI with or without hearing aids |
| Forli et al, 201157 | Jan 2000 to May 2010 PubMed, Cochrane Database of Systematic Reviews |
20 | Compared with UCI, BCI offered advantages for hearing in noise, sound localization, and hearing in a silent environment. However, there was large heterogeneity between studies |
| Johnston et al, 200958 | Jan 2000 to Jan 2008 Medline, PsycINFO, CINAHL, Web of Science, ERIC |
29 | Sound localization and speech perception in noise appeared to be improved with BCI compared to UCI |
| Lammers et al, 201459 | Inception to July 2013 PubMed, Embase, Web of Science | 21 | Compared with UCI, there was a positive effect of the second implant for especially sound localization and possibly language development |
| Sparreboom et al, 201060 | Oct 2006 to Jun 2009 Medline, Embase | 13 | Based on a low level of evidence, BCI improved speech perception in quiet and noise. However, results on sound localization were less consistent |
| Swedish Council on Technology Assessment in Health Care, 200656a | Unable to extract information from summary | Unable to extract information from summary | Studies using children as their own controls reported improvement in speech perception and sound localization comparing BCI vs. UCI. However, these studies were of low quality because of their study designs |
| Washington State Health Care Authority HTA, 201351 | Inception to Feb 2013 Published systematic reviews, Medline, Embase |
18 | BCI improved speech perception in noise and sound localization compared to UCI. Included studies were predominantly in children with prelingual deafness. Based on a small number of low-quality studies, measures of more complex language skills, hearing function in real-life situations, and disease-specific quality of life were improved by BCI compared to UCI |
Abbreviations: BCI, bilateral cochlear implantation; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index of Nursing and Allied Health Literature; CRD, Centre for Review and Dissemination; DARE, Database of Abstracts and Reviews of Effects; ERIC, Education Resources Information Centre; HTA, health technology assessment; FDA, Food and Drug Administration; ISI, Institute for Scientific Information; NHS, National Health Service; UCI, unilateral cochlear implantation.
The complete report is available only in Swedish.
Table A3:
Ongoing Studies—Bilateral Cochlear Implantation
| ID | Registry | Country | Design | Scientific Title | Comparator |
|---|---|---|---|---|---|
| NTR3232 | Nederlands Trial Register | Netherlands | Randomized controlled trial | Effects and costs of bilateral cochlear implantation in children | Simultaneous vs. sequential BCI BCI vs. UCI |
| NCT00960102 | clinicaltrials.gov | Finland | Prospective nonrandomized study | Children's bilateral cochlear implantation in Finland | BCI vs. UCI + hearing aid |
Abbreviations: BCI, bilateral cochlear implantation; UCI, unilateral cochlear implantation
Appendix 2:Literature Search Strategies
Clinical Evidence Search
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <February 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to March 15, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 12>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
Cochlear Implantation/ (6779)
-
2
Cochlear Implants/ (20275)
-
3
(Cochlea* adj (implant* or device* or prosthes#s or prosthetic*)).ti,ab,kf. (25266)
-
4
or/1–3 (28885)
-
5
((bilateral* or bi lateral* or both sides or two ears or both ears or second or 2nd or sequential* or simultaneous*) adj5 (implant* or device* or prosthes#s or prosthetic* or CI or CIs or user*1)).ti,ab,kf. (38355)
-
6
(BCI or BCIs).ti,ab,kf. (7118)
-
7
5 or 6 (45415)
-
8
4 and 7 (2526)
-
9
Hearing Loss, Unilateral/ (973)
-
10
((single side* or one ear or single ear or unilateral* or uni lateral* or monolateral* or mono lateral* or asymmetric*) adj7 (deaf* or hearing)).ti,ab,kf. (7887)
-
11
9 or 10 (8159)
-
12
4 and 11 (1143)
-
13
8 or 12 (3295)
-
14
exp Animals/ not Humans/ (15947258)
-
15
13 not 14 (2241)
-
16
15 use ppez,coch,cctr,dare,clhta,cleed (1614)
-
17
limit 16 to english language [Limit not valid in CDSR,DARE; records were retained] (1491)
-
18
cochlear implantation/ (6779)
-
19
cochlea prosthesis/ (13032)
-
20
(Cochlea* adj (implant* or device* or prosthes#s or prosthetic*)).tw,kw,dv. (25589)
-
21
or/18–20 (28067)
-
22
((bilateral* or bi lateral* or both sides or two ears or both ears or second or 2nd or sequential* or simultaneous*) adj5 (implant* or device* or prosthes#s or prosthetic* or CI or CIs or user*1)).tw,kw,dv. (38901)
-
23
(BCI or BCIs).tw,kw,dv. (7335)
-
24
22 or 23 (46174)
-
25
21 and 24 (2525)
-
26
bilateral cochlear implant/ (5)
-
27
bilateral cochlear implantation/ (19)
-
28
or/25–27 (2525)
-
29
unilateral hearing loss/ (1536)
-
30
single sided deafness/ (46)
-
31
((single side* or one ear or single ear or unilateral* or uni lateral* or monolateral* or mono lateral* or asymmetric*) adj7 (deaf* or hearing)).tw,kw. (7947)
-
32
or/29–31 (8383)
-
33
21 and 32 (1160)
-
34
28 or 33 (3297)
-
35
(exp animal/ or nonhuman/) not exp human/ (10130516)
-
36
34 not 35 (3191)
-
37
36 use emez (1613)
-
38
limit 37 to english language [Limit not valid in CDSR,DARE; records were retained] (1506)
-
39
17 or 38 (2997)
-
40
39 use ppez (1431)
-
41
39 use coch (0)
-
42
39 use cctr (45)
-
43
39 use dare (8)
-
44
39 use clhta (2)
-
45
39 use cleed (5)
-
46
39 use emez (1506)
-
47
remove duplicates from 39 (1767)
Economic Evidence Search
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <February 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to March 22, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 13>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
Cochlear Implantation/ (6805)
-
2
Cochlear Implants/ (20308)
-
3
(Cochlea* adj (implant* or device* or prosthes#s or prosthetic*)).ti,ab,kf. (25314)
-
4
or/1–3 (28941)
-
5
((bilateral* or bi lateral* or both sides or two ears or both ears or second or 2nd or sequential* or simultaneous*) adj5 (implant* or device* or prosthes#s or prosthetic* or CI or CIs or user*1)).ti,ab,kf. (38413)
-
6
(BCI or BCIs).ti,ab,kf. (7140)
-
7
5 or 6 (45495)
-
8
4 and 7 (2533)
-
9
Hearing Loss, Unilateral/ (974)
-
10
((single side* or one ear or single ear or unilateral* or uni lateral* or monolateral* or mono lateral* or asymmetric*) adj7 (deaf* or hearing)).ti,ab,kf. (7908)
-
11
9 or 10 (8180)
-
12
4 and 11 (1150)
-
13
8 or 12 (3306)
-
14
economics/ (253672)
-
15
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (792112)
-
16
economics.fs. (395995)
-
17
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (744067)
-
18
exp “costs and cost analysis”/ (542525)
-
19
(cost or costs or costing or costly).ti. (230510)
-
20
cost effective*.ti,ab,kf. (264319)
-
21
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (171713)
-
22
models, economic/ (172473)
-
23
markov chains/ or monte carlo method/ (69961)
-
24
(decision adj 1 (tree* or analy* or model*)).ti,ab,kf. (34026)
-
25
(markov or markow or monte carlo).ti,ab,kf. (108309)
-
26
quality-adjusted life years/ (33478)
-
27
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (54615)
-
28
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (87965)
-
29
or/14–28 (2392978)
-
30
13 and 29 (173)
-
31
30 use ppez,coch,cctr,dare,clhta (77)
-
32
limit 31 to english language [Limit not valid in CDSR,DARE; records were retained] (68)
-
33
13 use cleed (5)
-
34
limit 33 to english language [Limit not valid in CDSR,DARE; records were retained] (5)
-
35
32 or 34 (73)
-
36
cochlear implantation/ (6805)
-
37
cochlea prosthesis/ (13051)
-
38
(Cochlea* adj (implant* or device* or prosthes#s or prosthetic*)).tw,kw,dv. (25635)
-
39
or/36–38 (28121)
-
40
((bilateral* or bi lateral* or both sides or two ears or both ears or second or 2nd or sequential* or simultaneous*) adj5 (implant* or device* or prosthes#s or prosthetic* or CI or CIs or user*1)).tw,kw,dv. (38962)
-
41
(BCI or BCIs).tw,kw,dv. (7355)
-
42
40 or 41 (46255)
-
43
39 and 42 (2532)
-
44
bilateral cochlear implant/ (5)
-
45
bilateral cochlear implantation/ (19)
-
46
or/43–45 (2532)
-
47
unilateral hearing loss/ (1543)
-
48
single sided deafness/ (46)
-
49
((single side* or one ear or single ear or unilateral* or uni lateral* or monolateral* or mono lateral* or asymmetric*) adj7 (deaf* or hearing)).tw,kw. (7968)
-
50
or/47–49 (8405)
-
51
39 and 50 (1167)
-
52
46 or 51 (3308)
-
53
Economics/ (253672)
-
54
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (130146)
-
55
Economic Aspect/ or exp Economic Evaluation/ (433597)
-
56
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (769225)
-
57
exp “Cost”/ (542525)
-
58
(cost or costs or costing or costly).ti. (230510)
-
59
cost effective*.tw,kw. (276109)
-
60
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (172781)
-
61
Monte Carlo Method/ (57425)
-
62
(decision adj 1 (tree* or analy* or model*)).tw,kw. (37803)
-
63
(markov or markow or monte carlo).tw,kw. (113343)
-
64
Quality-Adjusted Life Years/ (33478)
-
65
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (58453)
-
66
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (110272)
-
67
or/53–66 (1908443)
-
68
52 and 67 (167)
-
69
68 use emez (80)
-
70
limit 69 to english language [Limit not valid in CDSR,DARE; records were retained] (72)
-
71
35 or 70 (145)
-
72
71 use ppez (63)
-
73
71 use coch (0)
-
74
71 use cctr (4)
-
75
71 use dare (1)
-
76
71 use clhta (0)
-
77
71 use cleed (5)
-
78
71 use emez (72)
-
79
remove duplicates from 71 (93)
Grey Literature Search
Performed on: March 16–21, 2017
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, Tufts Cost-Effectiveness Analysis Registry
Keywords used:
Cochlea, cochlear
Results: 27
Appendix 3:Clinical Evidence Quality Assessment
Table A4:
Risk of Biasa Among Randomized Controlled Trials, Bilateral Cochlear Implantation in Adults (Cochrane Risk of Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Smulders et al, 201662 | Low | Low | Lowb | Low | Highc | Highd,e |
| Summerfield et al, 200664 | Low | Unclearf | Lowb | Low | Unclearg | Highh |
| van Zon et al, 201763 | Low | Low | Lowb | Low | Low | Highd,e,i |
Possible risk of bias levels: low, high, and unclear.
It was impossible to blind either participants or personnel because of the nature of the intervention. Whether a participant had one or two cochlear implants was visible.
The Health Utilities Index–3, the EuroQoL quality of life questionnaire, and the visual analog scale for health were described in the protocol as outcome measures, but their results were not reported.
The self-reported nature of the questionnaires for tinnitus, subjective benefits of hearing, and quality of life increased the potential for bias in favour of bilateral cochlear implantation.
There were potential ceiling effects for the speech in noise test, which may have limited the ability to detect significant differences between unilateral and bilateral cochlear implantation.
No description of allocation concealment.
No report on research protocol.
The tinnitus annoyance questionnaire was not validated.
Generic questionnaires were not sensitive to changes in hearing status, and may have underestimated the gains in quality of life from bilateral cochlear implantation.
Table A5:
Risk of Biasa Among Nonrandomized Trials, Bilateral Cochlear Implantation in Adults (ROBINS-I Tool)
| Pre-intervention | At Intervention | Post-intervention | |||||
|---|---|---|---|---|---|---|---|
| Author, Year | Confounding | Study Participant Selection | Classification of Interventions | Deviations From Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results |
| Harkonen et al, 201565 | Seriousb | Moderatec,d | Low | Low | Low | Moderatee,f | Seriousf |
| Litovsky et al, 200666 | Seriousb | Moderatec,d | Low | Low | Low | Moderatee,g | Low |
| Mosnier et al, 200967 | Seriousb | Moderatec,d | Low | Low | Moderateh | Moderateg | Low |
| Olze et al, 201268 | Seriousb | Lowd | Low | Low | Low | Moderatee,g | Low |
| Ramsden et al, 200569 | Seriousb | Lowd | Low | Low | Low | Moderateg | Low |
| Reeder et al, 201470 | Seriousb | Moderatec,d | Low | Low | Low | Moderatee | Low |
| van Zon et al, 201671 | Moderatei | Low | Low | Low | Low | Moderatee | Low |
Abbreviation: ROBINS-I, Risk of Bias in Non-randomized Studies—of Interventions.
Possible risk of bias levels: low, moderate, serious, critical, and no information.
There were potential differences in patient characteristics at baseline (e.g., age at first and second cochlear implantation, degree and duration of hearing loss, duration of unilateral and bilateral auditory deprivation, history of prior hearing aid experience).
No description of inclusion or exclusion criteria.
In studies in which patients acted as their own controls, those who underwent bilateral cochlear implantation were asked to deactivate one implant to assess the difference between unilateral and bilateral hearing. This did not represent true unilateral hearing, because implantation may cause insertion damage to the cochlea, deteriorating residual hearing. Deactivating one cochlear implant for patients with bilateral cochlear implants would not reflect day-to-day listening conditions.
The self-reported nature of the questionnaires for tinnitus, subjective benefits of hearing, and quality of life increased the potential for bias in favour of bilateral cochlear implantation.
Generic questionnaires were not sensitive to changes in hearing status, and may have underestimated the gains in quality of life from bilateral cochlear implantation.
Test materials for speech perception in quiet were presented at above 60 dB (average conversational level in quiet).7
Number of patients for speech perception in noise at +5 and +10 signal-to-noise ratio dB were lower than +15 signal-to-noise ratio dB.
Cohort analysis of a randomized, controlled trial with balanced patient characteristics; however, information on prior hearing aid use—a potential confounder—was not available.
Table A6:
Risk of Biasa Among Nonrandomized Trials, Bilateral Cochlear Implantation in Children (ROBINS-I Tool)
| Pre-intervention | At Intervention | Post-intervention | |||||
|---|---|---|---|---|---|---|---|
| Author, Year | Confounding | Study Participant Selection | Classification of Interventions | Deviations From Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results |
| Cullington et al, 201783 | Seriousb | Low | Low | Low | Seriousc | Moderated | Low |
| Galvin et al, 201684 | Seriousb | Seriouse,f | Low | Low | Low | Moderateg | Low |
| Godar et al, 201085 | Seriousb | Seriouse,f | Low | Low | Low | Low | Moderateh |
| Peters et al, 200786 | Seriousb | Seriouse,f | Low | Low | Moderatei | Moderatej | Low |
| Reeder et al, 201787 | Seriousb | Moderatef | Low | Low | Moderatei | Low | Low |
| Scherf et al, 200788 | Seriousb | Seriouse,f | Low | Low | Moderatei | Seriousj,k | Low |
| Scherf et al, 200989 | Seriousb | Seriouse,f | Low | Low | Moderatei | Seriousj,k | Low |
| Scherf et al, 200990 | Seriousb | Seriouse,f | Low | Low | Moderatei | Seriousg,l | Low |
| Scherf et al, 200991 | Seriousb | Seriouse,f | Low | Low | Moderatei | Seriousg,l | Low |
| Sparreboom et al, 201192 | Seriousb | Moderatef | Low | Low | Moderatei | Low | Low |
| Sparreboom et al, 201293 | Seriousb | Moderatef | Low | Low | Moderatei | Seriousj,m | Low |
| Sparreboom et al, 201494 | Seriousb | Moderatef | Low | Low | Moderatei | Seriousg,j,n | Low |
| Strom-Roum et al, 201295 | Seriousb | Moderatef | Low | Low | Moderatei | Low | Low |
| Tait et al, 201096 | Seriousb | Moderatee | Low | Low | Low | Low | Low |
Abbreviation: ROBINS-I, Risk of Bias in Non-randomized Studies—of Interventions.
Possible risk of bias levels: low, moderate, serious, critical, and no information.
Potential differences in patient characteristics at baseline (e.g., age at first and second cochlear implantation, degree and duration of hearing loss, duration of unilateral and bilateral auditory deprivation, history of prior hearing aid experience).
Children dropped in and out of the study, suggesting that data were not available for each child at each test interval for patient-related reasons (e.g., attention, fatigue, language) or non-patient-related reasons (e.g., equipment, scheduling).
Children were not included in testing if clinicians considered them unco-operative; results may be skewed toward older children with more advanced development, better hearing performance, better language, and more co-operative behaviour.
There was no description of the selection process (e.g., consecutive or random within a time period).
In studies in which patients acted as their own controls, those who underwent bilateral cochlear implantation were asked to deactivate one implant to assess the difference between unilateral and bilateral hearing. This did not represent true unilateral hearing, because implantation may cause insertion damage to the cochlea, deteriorating residual hearing. Deactivating one cochlear implant for patients with bilateral cochlear implants would not reflect day-to-day listening conditions.
The self-reported nature of the questionnaires for tinnitus, subjective benefits of hearing, and quality of life increased the potential for bias in favour of bilateral cochlear implantation.
Statistical testing on outcomes were not reported.
Not all children returned for all postoperative test intervals. Not all tests could be administered to every child because of young age, limited attention span, etc.
Test materials for speech perception in quiet were presented at above 60 dB (average conversational level in quiet).8
It was unclear which test materials were used at each test interval, although the authors stated that the test materials used were appropriate for the speech development stages of the child.
There were potential ceiling effects, because some children were doing well with their first cochlear implant, making it difficult to demonstrate a bilateral benefit.
Generic questionnaires were not sensitive to changes in hearing status and may have underestimated the gains in quality of life from bilateral cochlear implantation.
Although this study used the same cohort of children with bilateral cochlear implants as Sparreboom et al,92,93 the unilateral cochlear implantation control group had nine children at 2-year follow-up and 26 children at 5- to 6-year follow-up. The results may not be directly comparable within the study series.
Our first consideration was study design; we started with the assumption that randomized controlled trials are high quality, whereas observational studies are low quality. We then took into account five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: the large magnitude of effect, the dose-response gradient, and any residual confounding factors.45 For more detailed information, please refer to the latest series of GRADE articles.45
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | We are very confident that the true prognosis (probability of future events) lies close to that of the estimate |
| Moderate | We are moderately confident that the true prognosis (probability of future events) is likely to be close to the estimate, but there is a possibility that it is substantially different |
| Low | Our confidence in the estimate is limited: the true prognosis (probability of future events) may be substantially different from the estimate |
| Very Low | We have very little confidence in the estimate: the true prognosis (probability of future events) is likely to be substantially different from the estimate |
Table A7:
GRADE Evidence Profile for Comparison of Bilateral Cochlear Implantation With Unilateral Cochlear Implantation in Adults
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Speech Perception in Quiet | |||||||
| 2 (RCTs)62,63 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | None | ⊕ Moderate |
| 5 (observational)66–70 | No serious limitationsb | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| Speech Perception in Noise | |||||||
| 2 (RCTs)62,63 | No serious limitations | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Moderate |
| 6 (observational)65–70 | No serious limitationsb | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| Sound Localization | |||||||
| 2 (RCTs)62,63 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ High |
| 3 (observational)65,67,70 | No serious limitationsb | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| Tinnitus | |||||||
| 1 (RCT)64 | Serious limitations (−1)d | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| 2 (observational)68,71 | No serious limitationsb | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Subjective Benefits of Hearing | |||||||
| 3 (RCTs)62–64 | Serious limitations (−1)d | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Moderate |
| 4 (observational)65,66,68,70 | No serious limitationsb | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| Quality of Life | |||||||
| 3 (RCTs)62–64 | Serious limitations (−1)d | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
| 2 (observational)65,68 | No serious limitationsb | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Low |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development and Evaluation; RCT, randomized, controlled trial.
There were potential ceiling effects in some test conditions, which may have limited the ability to detect significant differences between unilateral and bilateral cochlear implantation.
Observational studies started at a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, risk of selection bias and loss to follow-up). No further downgrade of GRADE was made unless there were more substantial limitations in how the study was conducted.
Inconsistencies in the results of the included studies.
The self-reported nature of the questionnaires for tinnitus, subjective benefits of hearing, and quality of life increased the potential for bias in favour of bilateral cochlear implantation.
Table A8:
GRADE Evidence Profile for Comparison of Bilateral Cochlear Implantation With Unilateral Cochlear Implantation in Children
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Speech Perception in Quiet | |||||||
| 7 (observational)86–89,92,94,95 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Speech Perception in Noise | |||||||
| 7 (observational)83,86–89,92,94 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Sound Localization | |||||||
| 4 (observational)83,85,87,92 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Language Development | |||||||
| 1 (observational)94 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Preverbal Communication | |||||||
| 1 (observational)96 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Subjective Benefits of Hearing | |||||||
| 4 (observational)84,90,91,93 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Moderate |
| Quality of Life | |||||||
| 1 (observational)93 | No serious limitationsa | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | Other considerations (+1)b | ⊕ Low |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
Observational studies started at a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, risk of selection bias and loss to follow-up). No further downgrade of GRADE was made unless there were more substantial limitations in how the study was conducted.
Critical period for optimal auditory development. Bilateral cochlear implantation before the age of 3.5 years takes advantage of plasticity to optimize development of the central auditory nervous system.
Inconsistencies within the included study; five different questionnaires were used to measure quality of life.
Appendix 4: Results of Applicability and Limitation Checklists for Studies Included in Economic Literature Review
Table A9:
Assessment of the Applicability of Studies Assessing the Cost-Effectiveness of Bilateral Versus Unilateral Cochlear Implantation
| Objective: To assess the cost-effectiveness of bilateral cochlear implantation | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Were the perspectives clearly stated, and what were they? | Are estimates of relative treatment effect from the best available source? |
| Bichey et al, 2008115 | Yes | Yes | Partially | Unclear (societal perspective stated, but appeared to be health payer perspective) | Yes |
| Bond et al, 200950 | Yes | Yes | Partially | Yes (health payer perspective in reference case) | Yes |
| Chen et al, 2014113 | Yes | Yes | Yes | Yes (health payer perspective) | Yes |
| Kuthubutheen et al, 2015114 | Yes | Yes | Yes | Yes (health payer perspective) | Yes |
| Perez-Martin et al, 2017116 | Yes | Yes | No | Yes (health payer perspective) | Unclear |
| Smulders et al, 2016117 | Yes | Yes | Partially | Yes (health insurance perspective) | Yes |
| Summerfield et al, 2002118 | Yes | Yes | Partially | Yes (health care perspective) | Yes |
| Summerfield et al, 2010119 | Yes | Yes | Partially | Yes (health care perspective) | Yes |
| Author, Year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Overall judgement (directly applicable/partially applicable/not applicable) |
|---|---|---|---|
| Bichey et al, 2008115 | Yes (5%) | Yes | Partially applicable |
| Bond et al, 200950 | Yes (3.5%) | Yes | Partially applicable |
| Chen et al, 2014113 | Yes, although in a non-traditional manner (0%, 1%, 3%, 5%) | Yes | Directly applicable |
| Kuthubutheen et al, 2015114 | No | Yes | Directly applicable |
| Perez-Martin et al, 2017116 | Yes (3%) | Yes | Partially applicable |
| Smulders et al, 2016117 | No | Yes | Partially applicable |
| Summerfield et al, 2002118 | Yes (6%) | Yes | Partially applicable |
| Summerfield et al, 2010119 | Yes (3.5%) | Yes | Partially applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Table A10:
Assessment of the Limitations of Studies Assessing the Cost-Effectiveness of Bilateral Versus Unilateral Cochlear Implantation
| Objective: To assess the cost-effectiveness of bilateral cochlear implantation | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the estimates of relative treatment effects obtained from best available sources? | Do the estimates of relative treatment effect match the estimates contained in the clinical report? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from best available sources? |
| Bichey et al, 2008115 | NA (not a model) | Yes | No | Yes | Yes | Unclear | Unclear |
| Bond et al, 200950 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Chen et al, 2014113 | Partially | Yes | Yes | Yes | Yes | Yes | Yes |
| Kuthubutheen et al, 2015114 | Partially | Yes | Yes | Yes | Yes | Yes | Yes |
| Perez-Martin et al, 2017116 | Yes | Yes | No | No | Yes | Yes | Yes |
| Smulders et al, 2016117 | NA (not a model) | Yes | No | Yes | Yes | Yes | Yes |
| Summerfield et al, 2002118 | Unclear | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Summerfield et al, 2010119 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Author, Year | Are the unit costs of resources obtained from best available resources? | Is an appropriate incremental analysis presented or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall assessment including applicability to the project (minor limitations/potentially serious limitations/very serious limitations) |
|---|---|---|---|---|---|
| Bichey et al, 2008115 | Unclear | No (unclear how ICER was calculated) | No | No | Very serious limitations |
| Bond et al, 200950 | Yes | Yes | Yes | No | Minor limitations |
| Chen et al, 2014113 | Yes | Yes | Partially (no PSA) | Yes | Potentially serious limitations |
| Kuthubutheen et al, 2015114 | Yes | Yes | Partially (no PSA) | Yes | Potentially serious limitations |
| Perez-Martin et al, 2017116 | Yes | Partially | Yes | Yes | Potentially serious limitations |
| Smulders et al, 2016117 | Yes | No (unclear) | No | Yes | Very serious limitations |
| Summerfield et al, 2002118 | Unclear | Yes | Partially (no PSA) | No | Potentially serious limitations |
| Summerfield et al, 2010119 | Yes | Yes | Yes | No | Minor limitations |
Abbreviations: ICER, incremental cost-effectiveness ratio; PSA, probabilistic sensitivity analysis. Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Appendix 5:Primary Economic Evaluation, Methods—Sensitivity Analysis
Table A11:
Distributions Used in the Probabilistic Sensitivity Analysis
| Parameter | Distribution | Mean | SD | Source |
|---|---|---|---|---|
| Target Population Characteristics | ||||
| Reference case 1, time interval between implants, y | Gamma | 3.44 | 3.37 | CIHI122 |
| Reference case 1, proportion female, % | Beta | 0.5672 | 0.0214 | CIHI122 |
| Reference case 2, proportion female, % | Beta | 0.4398 | 0.0385 | CIHI122 |
| Reference case 3, time interval between implants, y | Gamma | 3.07 | 1.55 | CIHI122 |
| Reference case 3, proportion female, % | Beta | 0.4247 | 0.0236 | CIHI122 |
| Major Complications (Revision Surgeries) | ||||
| 6-month probability of device failure, adults | Beta | 0.0027 | 0.0003a | Wang et al, 2014110 |
| 6-month probability of device failure, children | Beta | 0.0032 | 0.0004a | Wang et al, 2014110 |
| 6-month probability of non-device failure, adults | Beta | 0.0018 | 0.0002a | Wang et al, 2014110 |
| 6-month probability of non-device failure, children | Beta | 0.0021 | 0.0003a | Wang et al, 2014110 |
| % non-device failure = reimplantation | Beta | 0.1176 | 0.2857 | Venail et al, 2008108 |
| % non-device failure = reimplantation, infection | Beta | 0.0588 | 0.1429 | Venail et al, 2008108 |
| % non-device failure = explantation | Beta | 0.0784 | 0.1905 | Venail et al, 2008108 |
| % non-device failure = other | Beta | 0.2157 | 0.5238 | Venail et al, 2008108 |
| % non-device failure = other, infection | Beta | 0.1818 | 0.1163 | Venail et al, 2008108 |
| % non-device failure = other, cholesteatoma | Beta | 0.2727 | 0.1818 | Venail et al, 2008108 |
| % non-device failure = other, manage device | Beta | 0.5455 | 0.1501 | Venail et al, 2008108 |
| Minor Complications | ||||
| 6-month probability of minor complications, adults | Beta | 0.1387 | 0.0186 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = infection, adults | Beta | 0.145833 | 0.05 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = neurological, adults | Beta | 0.125 | 0.0477 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = pain, adults | Beta | 0.1458 | 0.0509 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = tinnitus, adults | Beta | 0.2292 | 0.0607 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = vestibular, adults | Beta | 0.3542 | 0.0690 | Venail et al, 2008108; Farinetti et al, 2014109 |
| 6-month probability of minor complications, adults | Beta | 0.0664 | 0.0106 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = infection, children | Beta | 0.6486 | 0.0785 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = neurological, children | Beta | 0.0541 | 0.0372 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = pain, children | Beta | 0.0541 | 0.0372 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = vestibular, children | Beta | 0.1351 | 0.0562 | Venail et al, 2008108; Farinetti et al, 2014109 |
| % minor complications = other, children | Beta | 0.1081 | 0.0510 | Venail et al, 2008108; Farinetti et al, 2014109 |
| Utilities | ||||
| Bilateral cochlear implant (severe to profound sensorineural hearing loss), adult | Beta | 0.8000 | 0.1020a | Chen et al, 2014113 |
| Unilateral cochlear implant (severe to profound sensorineural hearing loss), adult | Beta | 0.7650 | 0.0976a | Chen et al, 2014113 |
| No cochlear implant (severe to profound sensorineural hearing loss), adult | Beta | 0.4950 | 0.0631a | Chen et al, 2014113 |
| Bilateral cochlear implant (severe to profound sensorineural hearing loss), child | Beta | 0.8300 | 0.0969 | Lovett et al, 2010128 |
| Unilateral cochlear implant (severe to profound sensorineural hearing loss), child | Beta | 0.7800 | 0.0459 | Lovett et al, 2010128 |
| No cochlear implant (severe to profound sensorineural hearing loss), child | Beta | 0.5850 | 0.0332a | Barton et al, 2006129 |
| Disutilities | ||||
| Infection | Gamma | 0.0420 | 0.0097 | Nelson at al, 2015131 |
| Neurological complications | Gamma | 0.0200 | 0.0153b | Assumption |
| Pain | Gamma | 0.0200 | 0.0153b | Assumption |
| Tinnitus | Gamma | 0.0200 | 0.0153b | Stockdale et al, 2017132 |
| Vestibular complications | Gamma | 0.0300 | 0.0038a | Doyle et al, 2011133 |
| Other complications | Gamma | 0.0200 | 0.0153b | Assumption |
| Surgery | Gamma | 0.0200 | 0.0153b | Assumption |
| Costs, $ | ||||
| Cost of first-side cochlear implant | Gamma | 25,000 | 3,188a | Manufacturers |
| Cost of audiologist/speech-language therapist/social worker, per hour | Gamma | 48.54 | 5.66 | OPSEU134 |
| Cost of surgery, unilateral cochlear implant | Gamma | 4,740 | 943 | Merdad et al, 2014139 |
| Cost of surgery, bilateral cochlear implant | Gamma | 6,327 | 1,425 | Merdad et al, 2014139 |
| Cost of cholesteatoma surgery | Gamma | 3,384 | 5,616 | OCC143 |
| Cost of infection (without reimplantation) surgery | Gamma | 1,024 | 1,368 | OCC143 |
Abbreviations: CIHI, Canadian Institute for Health Information; OCC, Ontario Case Costing; OPSEU, Ontario Public Service Employees’ Union; SD, standard deviation.
Used ±25% to obtain 95% confidence intervals and back-calculated standard deviation.
Assumed to range between 0.01 and 0.05, because of uncertainty about the value.
Table A12:
Additional Parameters Varied in One-Way Sensitivity Analyses
| Variable | Reference Case | Range for One-Way Sensitivity Analysis |
|---|---|---|
| Structural | ||
| Discount rate, % | 1.5 | Min: 0; max: 5 |
| Target Populations | ||
| Reference case 1: starting age, y | 47 | Min: 18; max: 64 |
| Reference case 2: starting age, y | 1 | Min: 0.5; max: 5 |
| Reference case 3: starting age, y | 7 | Min: 2; max: 17 |
| Clinical Outcomes | ||
| Time to external processor replacement, y | 5 | Min: 3a (ADP142); max: 10 |
| Cost and Resource Use | ||
| Cost, first-side cochlear implant, $ | 25,000 | Min: 15,000; max: 40,000 |
| Discount, second-side cochlear implant, % | 50 | Min: 0; max: 75 |
| Cost of an audiologist/speech-language therapist/audio-verbal therapist/social worker, per hour, $ | 48.54 | Min: 37.45 (OPSEU134); max: 125.00 (IHP158) |
| Preprocedural cost of second-side implant in sequential BCI relative to UCI, $ | 1 × UCI | Min: 0.12 (Summerfield, 2002118); max: 1 |
| Preprocedural cost of second-side implant in simultaneous BCI relative to UCI, $ | 0 × UCI | Min: 0; max: 1 |
| Surgical costs in adults relative to children, $ | 1 × children | Min: 0.51b (OCC)143; max: 1 |
| Postprocedural cost of second-side implant in sequential BCI relative to UCI, $ | 1 × UCI | Min: 0; max: 1 |
| Postprocedural cost of simultaneous BCI relative to UCI, $ | 1 × UCI | Min: 1; max: 2 |
| Children accessing rehabilitation in hospital, % | 0.05 | Min: 0; max: 1 |
| Adults accessing rehabilitation, % | 0c | Min: 0; max: 0.15 |
Abbreviation: ADP, Assistive Devices Program; BCI, bilateral cochlear implant; IHP, Infant Hearing Program; OCC, Ontario Case Costing; OPSEU, Ontario Public Service Employees Union; UCI, unilateral cochlear implant.
The Assistive Devices Program will replace external cochlear implant processors after 3 years.
Based on the average length of stay in adults compared with children (Canadian Institute for Health Information).122
In the reference case, adults had one rehabilitation visit.
Table A13:
Scenario Analysis, Varying the Cost of Follow-up Carea
| Post-implant Follow-up | Appointment Type | Cost, $ | Mean Visits/Child |
|---|---|---|---|
| Year 1 | Programming and assessment | 3,472.86 | 11.2 |
| Rehabilitation | 9,260.96 | 29.8 | |
| Year 2 | Programming and assessment | 1,390.36 | 4.5 |
| Rehabilitation | 6,803.17 | 21.8 | |
| Year 3 | Programming and assessment | 987.89 | 3.2 |
| Rehabilitation | 5,598.04 | 18.0 |
Appendix 6: Primary Economic Evaluation, Additional Results
Figure A1: Influence of Key Parameters—Reference Case 1a.
aAdults (18 to 55 years), postlingual severe to profound sensorineural hearing loss, sequential bilateral cochlear implantation for bilateral vs. unilateral cochlear implantation.
Abbreviations: BCI, bilateral cochlear implantation; CI, cochlear implant; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; UCI, unilateral cochlear implantation.
Figure A2: Influence of Key Parameters—Reference Case 2a.
bChildren, prelingual severe to profound sensorineural hearing loss, simultaneous bilateral cochlear implantation for bilateral vs. unilateral cochlear implantation.
Abbreviations: BCI, bilateral cochlear implantation; CI, cochlear implant; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; simBCI, simultaneous bilateral cochlear implantation; UCI, unilateral cochlear implantation.
Figure A3: Influence of Key Parameters—Reference Case 3a.
aChildren, postlingual severe to profound sensorineural hearing loss, sequential bilateral implantation for bilateral vs. unilateral cochlear implantation.
Abbreviations: BCI, bilateral cochlear implantation; CI, cochlear implant; ICER, incremental cost-effectiveness ratio; MOHLTC, Ministry of Health and Long-Term Care; QALY, quality-adjusted life-year; seqBCI, sequential bilateral cochlear implantation; UCI, unilateral cochlear implantation.
Appendix 7:Budget Impact Analysis, Additional Results
Table A14:
Budget Impact Analysis, Per-Patient Costs by Indication and Type of Cochlear Implant
| Cost, $ | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Adults (18–93 years), UCI, progressive sensorineural hearing loss | 30,462 | 88 | 63 | 62 | 62 | 30,737 |
| Adults (20–40 years), UCI, sudden sensorineural hearing loss | 30,462 | 88 | 64 | 64 | 64 | 30,742 |
| Adults (18–55 years), sequential BCI (2),a progressive sensorineural hearing loss | 17,962 | 128 | 104 | 103 | 103 | 18,400 |
| Adults (20–40 years), simultaneous BCI, sudden sensorineural hearing loss | 44,568 | 128 | 104 | 103 | 103 | 45,006 |
| Children (≤ 1 year), simultaneous BCI, prelingual sensorineural hearing loss | 45,043 | 366 | 143 | 143 | 143 | 45,838 |
| Children, sequential BCI (1),b prelingual sensorineural hearing loss | 30,934 | 319 | 96 | 96 | 96 | 31,540 |
| Children, sequential BCI (2),a prelingual sensorineural hearing loss | 18,434 | 367 | 143 | 143 | 143 | 19,230 |
| Children, sequential BCI (1),b postlingual sensorineural hearing loss | 30,934 | 319 | 96 | 96 | 96 | 31,540 |
| Children, sequential BCI (2),a postlingual sensorineural hearing loss | 18,434 | 367 | 143 | 143 | 143 | 19,230 |
| Children, simultaneous BCI, sudden sensorineural hearing loss | 45,043 | 366 | 143 | 143 | 143 | 45,838 |
| Children, UCI, prelingual sensorineural hearing loss | 30,934 | 319 | 96 | 96 | 96 | 31,540 |
| Children, UCI, postlingual sensorineural hearing loss | 30,934 | 319 | 96 | 96 | 96 | 31,540 |
| Children, UCI, sudden sensorineural hearing loss | 30,934 | 319 | 96 | 96 | 96 | 31,540 |
Abbreviations: BCI, bilateral cochlear implantation; UCI, unilateral cochlear implantation.
Numbers may appear inexact due to rounding.
Second implant only.
First implant only.
Table A15:
Budget Impact Analysis, Reference Case Cost Breakdowns
| Cost, $ Millions | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Adults | ||||||
| Current Scenario: No Bilateral Cochlear Implantation | ||||||
| Preprocedural | 0.120 | 0.132 | 0.146 | 0.160 | 0.177 | 0.736 |
| Procedural | 8.004 | 8.804 | 9.694 | 10.672 | 11.739 | 48.913 |
| Postprocedural | 0.087 | 0.109 | 0.126 | 0.145 | 0.166 | 0.633 |
| Complications | 0.013 | 0.025 | 0.039 | 0.049 | 0.064 | 0.190 |
| Total | 8.225 | 9.071 | 10.004 | 11.026 | 12.146 | 50.472 |
| Future Scenario: Bilateral Cochlear Implantation | ||||||
| Preprocedural | 0.131 | 0.144 | 0.159 | 0.175 | 0.192 | 0.801 |
| Procedural | 8.498 | 9.357 | 10.301 | 11.331 | 12.475 | 51.962 |
| Postprocedural | 0.095 | 0.118 | 0.137 | 0.158 | 0.181 | 0.689 |
| Complications | 0.015 | 0.029 | 0.044 | 0.061 | 0.079 | 0.228 |
| Total | 8.739 | 9.648 | 10.641 | 11.725 | 12.928 | 53.739 |
| Net budget impact | 0.514 | 0.577 | 0.637 | 0.698 | 0.782 | 3.209 |
| Children | ||||||
| Current and Future Scenarios: Bilateral Cochlear Implantation | ||||||
| Preprocedural | 0.068 | 0.068 | 0.068 | 0.068 | 0.068 | 0.338 |
| Procedural | 3.267 | 3.267 | 3.267 | 3.267 | 3.267 | 16.334 |
| Postprocedural | 0.053 | 0.080 | 0.085 | 0.089 | 0.094 | 0.402 |
| Complications | 0.008 | 0.016 | 0.024 | 0.032 | 0.040 | 0.120 |
| Total | 3.395 | 3.430 | 3.443 | 3.456 | 3.469 | 17.193 |
| Net budget impact | 0 | 0 | 0 | 0 | 0 | 0 |
Numbers may appear inexact due to rounding.
Appendix 8: Letter of Information
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of Cochlear Implants for patients with sensorineural hearing loss. The purpose is to understand whether these devices should be more broadly funded in Ontario.
An important part of this review involves speaking to patients and families of those who have experience with hearing loss, who may or may not have a Cochlear implant. Our goal is to make sure the experiences of patients and caregivers are considered in the funding recommendations for Cochlear implants.
WHAT DO YOU NEED FROM ME?
-
✓
20–40 minutes of your time for a phone or in-person interview to share your story
-
✓
Permission to audio- (not video-) record the interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an Interview with Health Quality Ontario staff. The interview will likely last 20–40 minutes. It will be held in a private location or over the telephone. With your consent, the interview will be audio-taped. The interviewer will ask you questions about you or your loved one's condition and your perspectives about hearing loss and treatment options in Ontario. If you or your loved one have Cochlear implants, the interviewer will ask some additional questions surrounding the device and procedure.
Participation Is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect care you receive.
CONFIDENTIALITY
All Information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying Information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION:
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
If you are interested in participating, please contact Health Quality Ontario staff:
Appendix 9: Interview Guide
Interview for Cochlear Implants
Intro
Explain HQO purpose, HTA process, and purpose of interview History of hearing loss – first symptoms, background (general only)
Lived Experience
Day-to-day routine with hearing loss
What is the impact on quality of life? Has this changed as hearing loss progressed? Impact on family/caregivers, work?
Interventions
What previous therapies/treatments are used and their impact? (hearing aids) How well could you manage your condition with available therapies?
Were there any associated costs/barriers?
Cochlear Implants
Previous information surrounding these devices?
Decision-making for treatment. Was it difficult to weigh potential risks/benefits?
Expectations for cochlear implants Description of the procedure, side effects?
Results, impact, change in quality of life
After implant, do you need any further treatment? Any maintenance costs? Drawback or limitations?
Bilateral Implants
Benefits and/or challenges of bilateral cochlear implantation compared to unilateral implantation
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The clinical epidemiologist was Christine Lee; the health economist was Lindsey Falk; the Patient, Caregiver, and Public Engagement analyst was David Wells; and the medical librarian was Melissa Walter.
KEY MESSAGES
What Is This Health Technology Assessment About?
Sensorineural hearing loss is a condition in which the cochlea (the hearing organ in the ear) or the nerve pathways for hearing are damaged.
A cochlear implant is a device that acts in place of the damaged inner ear to help communicate sound to the brain. At present, the Ontario government funds one cochlear implant for people who have hearing loss in both ears.
This health technology assessment evaluates the potential benefits and harms of having cochlear implants in both ears (bilateral cochlear implantation) as opposed to just one, and whether this is good value for money. We also talked to people with sensorineural hearing loss about their values, preferences, and experiences with cochlear implants.
What Did This Health Technology Assessment Find?
Having cochlear implants in both ears helped people hear better in noisy places and find where sounds came from. The implant surgery was generally safe.
Having cochlear implants in both ears may provide reasonably good value for money compared to having an implant in only one ear. Funding cochlear implants in both ears for people in Ontario would require an extra $510,000 to $780,000 per year.
People with sensorineural hearing loss reported that cochlear implants provided social and emotional benefits and improved their quality of life.
Contributor Information
Health Quality Ontario:
Christine Lee, Lindsey Falk, David Wells, and Melissa Walter
About Health Quality Ontario
Health Quality Ontario is the provincial lead on the quality of health care. We help nurses, doctors and others working hard on the frontlines be more effective in what they do – by providing objective advice and by supporting them and government in improving health care for the people of Ontario.
Our focus is making health care more effective, efficient and affordable which we do through a legislative mandate of:
Reporting to the public, organizations and health care providers on how the health system is performing,
Finding the best evidence of what works, and
Translating this evidence into concrete standards, recommendations and tools that health care providers can easily put into practice to make improvements.
Health Quality Ontario is governed by a 12-member Board of Directors appointed by the Minister of Health and Long-Term Care and with representation from the medical and nursing professions, patients and other segments of health care.
In everything it does, Health Quality Ontario brings together those with first-hand experience – doctors, nurses, other health care providers, patients and families – to hear their experiences and how to make them better. Health Quality Ontario also works collaboratively with organizations across the province to encourage the spread of innovative and proven programs to support high quality, while also saving money and eliminating redundancy. And, we partner with patients to be full participants in designing our programs – another part of our work we take very seriously.
Examples of what we do include providing ways for clinicians to use their collective wisdom and experience to bring about positive change. In 2017, 29 Ontario hospitals participated in a pilot program that reduced infections due to surgery by 18%. This program enabled surgeons to see their surgical data and how they perform in relation to each other and to 700 other hospitals worldwide. We then helped them identify and action improvement practices. Forty-six hospitals across Ontario are now part of this program.
We also develop quality standards that are based on the best evidence, to guide on caring for health conditions where there are gaps in care. Each quality standard provides recommendations to government, organizations and clinicians, and is accompanied by a guide for patients to help them ask informed questions about their care.
In addition, Health Quality Ontario's health technology assessments use evidence to assess the value for money and safety of new technologies and procedures and make recommendations to government on whether or not they should be funded.
And each year, we help organizations across the system create Quality Improvement Plans, for improving health care quality.
Health Quality Ontario is committed to supporting the development of a quality health care system based on six fundamental dimensions: efficient, timely, safe, effective, patient-centred and equitable.
Our goal is to challenge the status quo and to focus on long-lasting pragmatic solutions that improve the health of Ontarians, enhance their experience of care, reduce health care costs, and support the well-being of health care providers – because we believe a quality health system results in Ontarians leading healthier and more productive lives, and a vibrant society in which everyone benefits.
REFERENCES
- (1).Wilson BS, Tucci DL, Merson MH, O'Donoghue GM. Global hearing health care: new findings and perspectives. Lancet. 2017;390(10111):2503–15. [DOI] [PubMed] [Google Scholar]
- (2).Feder K, Michaud D, Ramage-Morin P, McNamee J, Beauregard Y. Prevalence of hearing loss among Canadians aged 20 to 79: audiometric results from the 2012/2013 Canadian Health Measures Survey. Health Rep. 2015;26(7):18–25. [PubMed] [Google Scholar]
- (3).World Health Organization. WHO global estimates on prevalence of hearing loss [Internet]. Geneva, Switzerland: World Health Organization; c2017. [cited 2017 July 31]. Available from: http://www.who.int/pbd/deafness/WHO_GE_HL.pdf?ua=1 [Google Scholar]
- (4).Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23(7):493–500. [PubMed] [Google Scholar]
- (5).World Health Organization. Deafness and hearing loss [Internet]. Geneva, Switzerland: World Health Organization; 2017. [cited 2017 July 31]. Available from: http://www.who.int/mediacentre/factsheets/fs300/en/ [Google Scholar]
- (6).College of Audiologists and Speech-Language Pathologists of Ontario Practice standards and guidelines for hearing assessment of adults by audiologists [Internet]. Toronto (ON): College of Audiologists and Speech-Language Pathologists of Ontario; 2017. [cited 2017 Oct 31]. Available from: http://www.caslpo.com/sites/default/uploads/files/PSG_EN_Hearing_Assessment_of_Adults_by_Audiologists.pdf [Google Scholar]
- (7).Minimum speech test battery for adult cochlear implant users [Internet]. Valencia (CA): Advanced Bionics, Cochlear Americas, Med-El Corporation; 2011. [cited 2017 Sept 19]. Available from: http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf [Google Scholar]
- (8).Uhler K, Warner-Czyz A, Gifford R, Working Group P. Pediatric minimum speech test battery. J Am Acad Audiol. 2017;28(3):232–47. [DOI] [PubMed] [Google Scholar]
- (9).Mason JA, Herrmann KR. Universal infant hearing screening by automated auditory brainstem response measurement. Pediatrics. 1998;101(2):221–8. [DOI] [PubMed] [Google Scholar]
- (10).Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP. Language outcomes in young children with mild to severe hearing loss. Ear Hear. 2015;36 Suppl 1:76S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Qi S, Mitchell RE. Large-scale academic achievement testing of deaf and hard-of-hearing students: past, present, and future. J Deaf Stud Deaf Educ. 2012;17(1):1–18. [DOI] [PubMed] [Google Scholar]
- (12).Lederberg AR, Schick B, Spencer PE. Language and literacy development of deaf and hard-of-hearing children: successes and challenges. Dev Psychol. 2013;49(1):15–30. [DOI] [PubMed] [Google Scholar]
- (13).Roland L, Fischer C, Tran K, Rachakonda T, Kallogjeri D, Lieu JE. Quality of life in children with hearing impairment: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2016;155(2):208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Emmett SD, Francis HW. The socioeconomic impact of hearing loss in U.S. adults. Otol Neurotol. 2015;36(3):545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150(3):378–84. [DOI] [PubMed] [Google Scholar]
- (16).Ciorba A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wallhagen MI. The stigma of hearing loss. Gerontologist. 2010;50(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lin VYW, Black SE. Linking deafness and dementia: challenges and opportunities. Otol Neurotol. 2017;38(8):e237–e9. [DOI] [PubMed] [Google Scholar]
- (19).Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- (20).Hearing Link. What is sensorineural hearing loss? [Internet]. West Sussex, UK: Hearing Link; 2017. [cited 2017 July 31]. Available from: https://www.hearinglink.org/your-hearing/types-causes-of-hearing-loss/what-is-sensorineural-hearing-loss/ [Google Scholar]
- (21).Eggermont JJ, Roberts LE. The neuroscience of tinnitus: understanding abnormal and normal auditory perception. Front Syst Neurosci. 2012;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ahmad N, Seidman M. Tinnitus in the older adult: epidemiology, pathophysiology and treatment options. Drugs Aging. 2004;21(5):297–305. [DOI] [PubMed] [Google Scholar]
- (23).Quaranta N, Wagstaff S, Baguley DM. Tinnitus and cochlear implantation. Int J Audiol. 2004;43(5):245–51. [DOI] [PubMed] [Google Scholar]
- (24).Jakes SC, Hallam RS, Chambers C, Hinchcliffe R. A factor analytical study of tinnitus complaint behaviour. Audiology. 1985;24(3):195–206. [DOI] [PubMed] [Google Scholar]
- (25).Tyler RS, Baker LJ. Difficulties experienced by tinnitus sufferers. J Speech Hear Disord. 1983;48(2):150–4. [DOI] [PubMed] [Google Scholar]
- (26).Schramm D. Canadian position statement on bilateral cochlear implantation. J Otolaryngol Head Neck Surg. 2010;39(5):479–85. [PubMed] [Google Scholar]
- (27).Steven Colburn H, Shinn-Cunningham B, Kidd G, Jr., Durlach N. The perceptual consequences of binaural hearing. Int J Audiol. 2006;45 Suppl 1:S34–44. [DOI] [PubMed] [Google Scholar]
- (28).Akeroyd MA. The psychoacoustics of binaural hearing. Int J Audiol. 2006;45 Suppl 1:S25–33. [DOI] [PubMed] [Google Scholar]
- (29).Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–43. [DOI] [PubMed] [Google Scholar]
- (30).Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–9. [DOI] [PubMed] [Google Scholar]
- (31).Kral A. Auditory critical periods: a review from system's perspective. Neuroscience. 2013;247:117–33. [DOI] [PubMed] [Google Scholar]
- (32).Geers AE, Nicholas JG. Enduring advantages of early cochlear implantation for spoken language development. J Speech Lang Hear Res. 2013;56(2):643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ramsden JD, Gordon K, Aschendorff A, Borucki L, Bunne M, Burdo S, et al. European Bilateral Pediatric Cochlear Implant Forum consensus statement. Otol Neurotol. 2012;33(4):561–5. [DOI] [PubMed] [Google Scholar]
- (34).Gordon KA, Jiwani S, Papsin BC. What is the optimal timing for bilateral cochlear implantation in children? Cochlear Implants Int. 2011;12 Suppl 2:S8–14. [DOI] [PubMed] [Google Scholar]
- (35).Gordon KA, Valero J, Papsin BC. Binaural processing in children using bilateral cochlear implants. Neuroreport. 2007;18(6):613–7. [DOI] [PubMed] [Google Scholar]
- (36).Gordon KA, Valero J, Papsin BC. Auditory brainstem activity in children with 9–30 months of bilateral cochlear implant use. Hear Res. 2007;233(1–2):97–107. [DOI] [PubMed] [Google Scholar]
- (37).Litovsky RY, Gordon K. Bilateral cochlear implants in children: effects of auditory experience and deprivation on auditory perception. Hear Res. 2016;338:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jiwani S, Papsin BC, Gordon KA. Early unilateral cochlear implantation promotes mature cortical asymmetries in adolescents who are deaf. Hum Brain Mapp. 2016;37(1):135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Quality standards: cochlear implant services for children and adults [Internet]. London, UK: British Cochlear Implant Group; 2016. [cited 2017 Jun 28]. Available from: http://www.bcig.org.uk/wp-content/uploads/2016/05/BCIG-Quality-Standard-2016.pdf [Google Scholar]
- (40).Health Networks Branch. Clinical guidelines for adult cochlear implantation [Internet]. Perth, Australia: Department of Health, Western Australia; 2013. [cited 2017 Jun 28]. Available from: http://ww2.health.wa.gov.au/∼/media/Files/Corporate/general%20documents/Health%20Networks/Neurosciences%20and%20the%20Senses/Clinical-Guidelines-for-Adult-Cochlear-Implantation.ashx [Google Scholar]
- (41).Gordon KA, Papsin BC. Benefits of short interimplant delays in children receiving bilateral cochlear implants. Otol Neurotol. 2009;30(3):319–31. [DOI] [PubMed] [Google Scholar]
- (42).Papsin BC, Gordon KA. Cochlear implants for children with severe-to-profound hearing loss. N Engl J Med. 2007;357(23):2380–7. [DOI] [PubMed] [Google Scholar]
- (43).Papsin BC, Gordon KA. Bilateral cochlear implants should be the standard for children with bilateral sensorineural deafness. Curr Opin Otolaryngol Head Neck Surg. 2008;16(1):69–74. [DOI] [PubMed] [Google Scholar]
- (44).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (45).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (46).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. [Internet]. 2013. [cited 2018 Feb 14]. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html
- (47).Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Raman G, Lee J, Chung M, Gaylor JM, Sen S, Rao M, et al. Effectiveness of cochlear implants in adults with sensorineural hearing loss [Internet]. Rockville (MD): AHRQ Technology Assessments; 2011. [cited 2017 Jul 31]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25927131 [PubMed] [Google Scholar]
- (50).Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess. 2009;13(44):1–330. [DOI] [PubMed] [Google Scholar]
- (51).Cochlear implants: bilateral vs. unilateral [Internet]. Olympia (WA): Washington State Health Care Authority; 2013. [cited 2017 Jun 26]. Available from: https://www.hca.wa.gov/assets/program/ci_report_final_041713[1].pdf [Google Scholar]
- (52).Berrettini S, Baggiani A, Bruschini L, Cassandro E, Cuda D, Filipo R, et al. Systematic review of the literature on the clinical effectiveness of the cochlear implant procedure in adult patients. Acta Otorhinolaryngol Ital. 2011;31(5):299–310. [PMC free article] [PubMed] [Google Scholar]
- (53).Crathorne L, Bond M, Cooper C, Elston J, Weiner G, Taylor R, et al. A systematic review of the effectiveness and cost-effectiveness of bilateral multichannel cochlear implants in adults with severe-to-profound hearing loss. Clin Otolaryngol. 2012;37(5):342–54. [DOI] [PubMed] [Google Scholar]
- (54).Gaylor JM, Raman G, Chung M, Lee J, Rao M, Lau J, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(3):265–72. [DOI] [PubMed] [Google Scholar]
- (55).van Schoonhoven J, Sparreboom M, van Zanten BG, Scholten RJ, Mylanus EA, Dreschler WA, et al. The effectiveness of bilateral cochlear implants for severe-to-profound deafness in adults: a systematic review. Otol Neurotol. 2013;34(2):190–8. [DOI] [PubMed] [Google Scholar]
- (56).Maki-Torkko E, Jonsson R. Bilateral cochlear implantation in children [Internet]. Stockholm, Sweden: The Swedish Council on Technology Assessment in Health Care; 2006. [cited 2017 Sept 27]. Available from: http://www.sbu.se/globalassets/publikationer/content0/3/bilateral_cochlear_implantation_ci_children_200601.pdf [Google Scholar]
- (57).Forli F, Arslan E, Bellelli S, Burdo S, Mancini P, Martini A, et al. Systematic review of the literature on the clinical effectiveness of the cochlear implant procedure in paediatric patients. Acta Otorhinolaryngol Ital. 2011;31(5):281–98. [PMC free article] [PubMed] [Google Scholar]
- (58).Johnston JC, Durieux-Smith A, Angus D, O'Connor A, Fitzpatrick E. Bilateral paediatric cochlear implants: a critical review. Int J Audiol. 2009;48(9):601–17. [DOI] [PubMed] [Google Scholar]
- (59).Lammers MJ, van der Heijden GJ, Pourier VE, Grolman W. Bilateral cochlear implantation in children: a systematic review and best-evidence synthesis. Laryngoscope. 2014;124(7):1694–9. [DOI] [PubMed] [Google Scholar]
- (60).Sparreboom M, van Schoonhoven J, van Zanten BG, Scholten RJ, Mylanus EA, Grolman W, et al. The effectiveness of bilateral cochlear implants for severe-to-profound deafness in children: a systematic review. Otol Neurotol. 2010;31(7):1062–71. [DOI] [PubMed] [Google Scholar]
- (61).Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Smulders YE, van Zon A, Stegeman I, Rinia AB, Van Zanten GA, Stokroos RJ, et al. Comparison of bilateral and unilateral cochlear implantation in adults: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2016;142(3):249–56. [DOI] [PubMed] [Google Scholar]
- (63).van Zon A, Smulders YE, Stegeman I, Ramakers GG, Kraaijenga VJ, Koenraads SP, et al. Stable benefits of bilateral over unilateral cochlear implantation after two years: a randomized controlled trial. Laryngoscope. 2017;127(5):1161–8. [DOI] [PubMed] [Google Scholar]
- (64).Summerfield AQ, Barton GR, Toner J, McAnallen C, Proops D, Harries C, et al. Self-reported benefits from successive bilateral cochlear implantation in post-lingually deafened adults: randomised controlled trial. Int J Audiol. 2006;45 Suppl 1:S99–107. [DOI] [PubMed] [Google Scholar]
- (65).Harkonen K, Kivekas I, Rautiainen M, Kotti V, Sivonen V, Vasama JP. Sequential bilateral cochlear implantation improves working performance, quality of life, and quality of hearing. Acta Otolaryngol. 2015;135(5):440–6. [DOI] [PubMed] [Google Scholar]
- (66).Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear. 2006;27(6):714–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Mosnier I, Sterkers O, Bebear JP, Godey B, Robier A, Deguine O, et al. Speech performance and sound localization in a complex noisy environment in bilaterally implanted adult patients. Audiol Neurootol. 2009;14(2):106–14. [DOI] [PubMed] [Google Scholar]
- (68).Olze H, Grabel S, Haupt H, Forster U, Mazurek B. Extra benefit of a second cochlear implant with respect to health-related quality of life and tinnitus. Otol Neurotol. 2012;33(7):1169–75. [DOI] [PubMed] [Google Scholar]
- (69).Ramsden R, Greenham P, O'Driscoll M, Mawman D, Proops D, Craddock L, et al. Evaluation of bilaterally implanted adult subjects with the nucleus 24 cochlear implant system. Otol Neurotol. 2005;26(5):988–98. [DOI] [PubMed] [Google Scholar]
- (70).Reeder RM, Firszt JB, Holden LK, Strube MJ. A longitudinal study in adults with sequential bilateral cochlear implants: time course for individual ear and bilateral performance. J Speech Lang Hear Res. 2014;57(3):1108–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).van Zon A, Smulders YE, Ramakers GG, Stegeman I, Smit AL, Van Zanten GA, et al. Effect of unilateral and simultaneous bilateral cochlear implantation on tinnitus: a prospective study. Laryngoscope. 2016;126(4):956–61. [DOI] [PubMed] [Google Scholar]
- (72).Hiller W, Goebel G. A psychometric study of complaints in chronic tinnitus. J Psychosom Res. 1992;36(4):337–48. [DOI] [PubMed] [Google Scholar]
- (73).Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–8. [DOI] [PubMed] [Google Scholar]
- (74).Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–36. [DOI] [PubMed] [Google Scholar]
- (75).Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43(2):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear. 1995;16(2):176–86. [DOI] [PubMed] [Google Scholar]
- (77).Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5(1):1–30. [DOI] [PubMed] [Google Scholar]
- (78).Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. [DOI] [PubMed] [Google Scholar]
- (79).Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375–84. [DOI] [PubMed] [Google Scholar]
- (80).Hinderink JB, Krabbe PF, Van Den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg. 2000;123(6):756–65. [DOI] [PubMed] [Google Scholar]
- (81).Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415–22. [DOI] [PubMed] [Google Scholar]
- (82).Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–36. [DOI] [PubMed] [Google Scholar]
- (83).Cullington HE, Bele D, Brinton JC, Cooper S, Daft M, Harding J, et al. United Kingdom national paediatric bilateral project: demographics and results of localization and speech perception testing. Cochlear Implants Int. 2017;18(1):2–22. [DOI] [PubMed] [Google Scholar]
- (84).Galvin KL, Mok M. Everyday listening performance of children before and after receiving a second cochlear implant: results using the parent version of the Speech, Spatial, and Qualities of Hearing scale. Ear Hear. 2016;37(1):93–102. [DOI] [PubMed] [Google Scholar]
- (85).Godar SP, Litovsky RY. Experience with bilateral cochlear implants improves sound localization acuity in children. Otol Neurotol. 2010;31(8):1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol. 2007;28(5):649–57. [DOI] [PubMed] [Google Scholar]
- (87).Reeder RM, Firszt JB, Cadieux JH, Strube MJ. A longitudinal study in children with sequential bilateral cochlear implants: time course for the second implanted ear and bilateral performance. J Speech Lang Hear Res. 2017;60(1):276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Scherf F, van Deun L, van Wieringen A, Wouters J, Desloovere C, Dhooge I, et al. Hearing benefits of second-side cochlear implantation in two groups of children. Int J Pediatr Otorhinolaryngol. 2007;71(12):1855–63. [DOI] [PubMed] [Google Scholar]
- (89).Scherf F, Van Deun L, van Wieringen A, Wouters J, Desloovere C, Dhooge I, et al. Three-year postimplantation auditory outcomes in children with sequential bilateral cochlear implantation. Ann Otol Rhinol Laryngol. 2009;118(5):336–44. [DOI] [PubMed] [Google Scholar]
- (90).Scherf F, Van Deun L, van Wieringen A, Wouters J, Desloovere C, Dhooge I, et al. Subjective benefits of sequential bilateral cochlear implantation in young children after 18 months of implant use. ORL J Otorhinolaryngol Relat Spec. 2009;71(2):112–21. [DOI] [PubMed] [Google Scholar]
- (91).Scherf FW, van Deun L, van Wieringen A, Wouters J, Desloovere C, Dhooge I, et al. Functional outcome of sequential bilateral cochlear implantation in young children: 36 months postoperative results. Int J Pediatr Otorhinolaryngol. 2009;73(5):723–30. [DOI] [PubMed] [Google Scholar]
- (92).Sparreboom M, Snik AF, Mylanus EA. Sequential bilateral cochlear implantation in children: development of the primary auditory abilities of bilateral stimulation. Audiol Neurootol. 2011;16(4):203–13. [DOI] [PubMed] [Google Scholar]
- (93).Sparreboom M, Snik AF, Mylanus EA. Sequential bilateral cochlear implantation in children: quality of life. Arch Otolaryngol Head Neck Surg. 2012;138(2):134–41. [DOI] [PubMed] [Google Scholar]
- (94).Sparreboom M, Langereis MC, Snik AF, Mylanus EA. Long-term outcomes on spatial hearing, speech recognition and receptive vocabulary after sequential bilateral cochlear implantation in children. Res Dev Disabil. 2014;36C:328–37. [DOI] [PubMed] [Google Scholar]
- (95).Strom-Roum H, Laurent C, Wie OB. Comparison of bilateral and unilateral cochlear implants in children with sequential surgery. Int J Pediatr Otorhinolaryngol. 2012;76(1):95–9. [DOI] [PubMed] [Google Scholar]
- (96).Tait M, Nikolopoulos TP, De Raeve L, Johnson S, Datta G, Karltorp E, et al. Bilateral versus unilateral cochlear implantation in young children. Int J Pediatr Otorhinolaryngol. 2010;74(2):206–11. [DOI] [PubMed] [Google Scholar]
- (97).Cullington HE, Bele D, Brinton JC, Cooper S, Daft M, Harding J, et al. United Kingdom national paediatric bilateral project: results of professional rating scales and parent questionnaires. Cochlear Implants Int. 2017;18(1):23–35. [DOI] [PubMed] [Google Scholar]
- (98).Broomfield SJ, Murphy J, Wild DC, Emmett SR, O'Donoghue GM, Writing for the UKNPCISAG. Results of a prospective surgical audit of bilateral paediatric cochlear implantation in the UK. Cochlear Implants Int. 2014;15(5):246–53. [DOI] [PubMed] [Google Scholar]
- (99).Dunn LM, Dunn LM. Peabody picture vocabulary test - III. Circle Pines (MN): American Guidance Service; 1997. [Google Scholar]
- (100).Tait ME, Nikolopoulos TP, Wells P, White A. The use and reliability of Tait video analysis in assessing preverbal language skills in profoundly deaf and normally hearing children under 12 months of age. Int J Pediatr Otorhinolaryngol. 2007;71(9):1377–82. [DOI] [PubMed] [Google Scholar]
- (101).Galvin KL, Noble W. Adaptation of the speech, spatial, and qualities of hearing scale for use with children, parents, and teachers. Cochlear Implants Int. 2013;14(3):135–41. [DOI] [PubMed] [Google Scholar]
- (102).Archbold S, Lutman ME, Marshall DH. Categories of auditory performance. Ann Otol Rhinol Laryngol Suppl. 1995;166:312–4. [PubMed] [Google Scholar]
- (103).O'Donoghue GM, Nikolopoulos TP, Archbold SM, Tait M. Cochlear implants in young children: the relationship between speech perception and speech intelligibility. Ear Hear. 1999;20(5):419–25. [DOI] [PubMed] [Google Scholar]
- (104).Winkler F, Schon F, Peklo L, Muller J, Feinen C, Helms J. [The Wurzburg questionnaire for assessing the quality of hearing in CI-children (WH-CIK)]. Laryngorhinootologie. 2002;81(3):211–6. [DOI] [PubMed] [Google Scholar]
- (105).Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Varni JW, Burwinkle TM, Seid M. The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res. 2006;15(2):203–15. [DOI] [PubMed] [Google Scholar]
- (107).Kubba H, Swan IR, Gatehouse S. The Glasgow Children's Benefit Inventory: a new instrument for assessing health-related benefit after an intervention. Ann Otol Rhinol Laryngol. 2004;113(12):980–6. [DOI] [PubMed] [Google Scholar]
- (108).Venail F, Sicard M, Piron JP, Levi A, Artieres F, Uziel A, et al. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg. 2008;134(12):1276–81. [DOI] [PubMed] [Google Scholar]
- (109).Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(3):177–82. [DOI] [PubMed] [Google Scholar]
- (110).Wang JT, Wang AY, Psarros C, Da Cruz M. Rates of revision and device failure in cochlear implant surgery: a 30-year experience. Laryngoscope. 2014;124(10):2393–9. [DOI] [PubMed] [Google Scholar]
- (111).Eskander A, Gordon KA, Kadhim L, Papaioannou V, Cushing SL, James AL, et al. Low pediatric cochlear implant failure rate: contributing factors in large-volume practice. Arch Otolaryngol Head Neck Surg. 2011;137(12):1190–6. [DOI] [PubMed] [Google Scholar]
- (112).National Institute for Health and Care Excellence Process and methods guides. Appendix I quality appraisal checklist–economic evaluations [Internet]. London (UK): National Institute for Health and Care Excellence; 2012. [cited 2017 Sept 1]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations. [Google Scholar]
- (113).Chen JM, Amoodi H, Mittmann N. Cost-utility analysis of bilateral cochlear implantation in adults: a health economic assessment from the perspective of a publicly funded program. Laryngoscope. 2014;124(6):1452–8. [DOI] [PubMed] [Google Scholar]
- (114).Kuthubutheen J, Mittmann N, Amoodi H, Qian W, Chen JM. The effect of different utility measures on the cost-effectiveness of bilateral cochlear implantation. Laryngoscope. 2015;125(2):442–7. [DOI] [PubMed] [Google Scholar]
- (115).Bichey BG, Miyamoto RT. Outcomes in bilateral cochlear implantation. Otolaryngol Head Neck Surg. 2008;138(5):655–61. [DOI] [PubMed] [Google Scholar]
- (116).Perez-Martin J, Artaso MA, Diez FJ. Cost-effectiveness of pediatric bilateral cochlear implantation in Spain. Laryngoscope. 2017;127:2866–72. [DOI] [PubMed] [Google Scholar]
- (117).Smulders YE, van Zon A, Stegeman I, van Zanten GA, Rinia AB, Stokroos RJ, et al. Cost-utility of bilateral versus unilateral cochlear implantation in adults: a randomized controlled trial. Otol Neurotol. 2016;37(1):38–45. [DOI] [PubMed] [Google Scholar]
- (118).Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg. 2002;128(11):1255–62. [DOI] [PubMed] [Google Scholar]
- (119).Summerfield AQ, Lovett RE, Bellenger H, Batten G. Estimates of the cost-effectiveness of pediatric bilateral cochlear implantation. Ear Hear. 2010;31(5):611–24. [DOI] [PubMed] [Google Scholar]
- (120).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR health economic evaluation publication guidelines—CHEERS Good Reporting Practices Task Force. Value Health. 2013. Mar-Apr;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (121).Canadian Agency for Drugs and Technologies in Health Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa (ON): The Agency; 2017. [Google Scholar]
- (122).CIHI portal [Internet]. Ottawa (ON): Canadian Institute for Health Information; 2017. - [cited 2017 Sep]. Available from: https://www.cihi.ca/en/cihi-portal-web-tool [Google Scholar]
- (123).Smulders YE, Rinia AB, Rovers MM, van Zanten GA, Grolman W. What is the effect of time between sequential cochlear implantations on hearing in adults and children? A systematic review of the literature. Laryngoscope. 2011;121(9):1942–9. [DOI] [PubMed] [Google Scholar]
- (124).Government of Canada. Canadian immunization guide: part 3 – vaccination of specific populations [Internet]. Ottawa (ON): Government of Canada; 2016. [cited 2017 Dec]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-7-immunization-persons-with-chronic-diseases.html [Google Scholar]
- (125).Statistics Canada. Life tables (2011-2013), Can Sim Table 053-0003 [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated c2014; cited 2017 Dec]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=0530003&pattern=cpi&tabMode=dataTable&srchLan=-1&p1=1&p2=50 [Google Scholar]
- (126).Foteff C, Kennedy S, Milton AH, Deger M, Payk F, Sanderson G. Cost-utility analysis of cochlear implantation in Australian adults. Otol Neurotol. 2016;37(5):454–61. [DOI] [PubMed] [Google Scholar]
- (127).Foteff C, Kennedy S, Milton AH, Deger M, Payk F, Sanderson G. Economic evaluation of treatments for pediatric bilateral severe to profound sensorineural hearing loss: an Australian perspective. Otol Neurotol. 2016;37(5):462–9. [DOI] [PubMed] [Google Scholar]
- (128).Lovett RE, Kitterick PT, Hewitt CE, Summerfield AQ. Bilateral or unilateral cochlear implantation for deaf children: an observational study. Arch Dis Child. 2010;95(2):107–12. [DOI] [PubMed] [Google Scholar]
- (129).Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom, IV: cost-effectiveness of pediatric cochlear implantation. Ear Hear. 2006;27(5):575–88. [DOI] [PubMed] [Google Scholar]
- (130).Stacey PC, Fortnum HM, Barton GR, Summerfield AQ. Hearing-impaired children in the United Kingdom, I: Auditory performance, communication skills, educational achievements, quality of life, and cochlear implantation. Ear Hear. 2006;27(2):161–86. [DOI] [PubMed] [Google Scholar]
- (131).Nelson RE, Stockmann C, Hersh AL, Pavia AT, Korgenksi K, Daly JA, et al. Economic analysis of rapid and sensitive polymerase chain reaction testing in the emergency department for influenza infections in children. Pediatr Infect Dis J. 2015;34(6):577–82. [DOI] [PubMed] [Google Scholar]
- (132).Stockdale D, McFerran D, Brazier P, Pritchard C, Kay T, Dowrick C, et al. An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv Res. 2017;17(1):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Doyle S, Lloyd A, Davis M. Public perception of atrial fibrillation and treatment-related adverse events in the UK. Br J Cardiol. 2011;18:88–93. [Google Scholar]
- (134).Ontario Public Service Employees Union. 2016–2019 OPSEU Hospital Profesionals Division central agreement [Internet]. North York (ON): The Union; 2016. [cited 2017 28 Dec]. Available from: https://opseu.org/sites/default/files/hpd_collective_agreement_20190331.pdf [Google Scholar]
- (135).Hansen MR, Gantz BJ, Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Meniere's disease. Otol Neurotol. 2013;34(9):1681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Ontario Ministry of Health and Long-Term Care Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto, ON: The Ministry; 2015. [cited 2017 Sept 1]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20160401.pdf. [Google Scholar]
- (137).Statistics Canada. Consumer Price Index: Table 326-0020 [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated 2017; cited 2017 Dec]. Available from: http://www5.statcan.gc.ca/cansim/a47 [Google Scholar]
- (138).SickKids. Cochlear implant program [Internet]. Toronto (ON): SickKids; 2017. [updated 2017; cited 2017 Dec]. Available from: http://sunnybrook.ca/content/?page=cochlear-implant-program-information [Google Scholar]
- (139).Merdad M, Wolter NE, Cushing SL, Gordon KA, Papsin BC. Surgical efficiency in bilateral cochlear implantation: a cost analysis. Cochlear Implants Int. 2014;15(1):43–7. [DOI] [PubMed] [Google Scholar]
- (140).Gaboury I, Coyle K, Coyle D, Le Saux N. Treatment cost effectiveness in acute otitis media: a watch-and-wait approach versus amoxicillin. Paediatr Child Health. 2010;15(7):e14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Ontario Drug Benefit Formulary [Internet]. Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2017. [updated 2017 Sept; cited 2017 Dec 28]. Available from: https://www.formulary.health.gov.on.ca/formulary/ [Google Scholar]
- (142).Ontario Ministry of Health and Long-Term Care Policies and procedures manual for the Assistive Devices Program [Internet]. Toronto (ON): The Ministry; 2016. [cited 2017 Dec 28]. Available from: http://www.health.gov.on.ca/en/pro/programs/adp/policies_procedures_manuals/docs/pp_adp_manual.pdf [Google Scholar]
- (143).Ontario Case Costing. OCC Costing Analysis Tool [Internet]. Toronto (ON): Ministry of Health and Long Term Care; 2016. Available from: https://hsim.health.gov.on.ca/hdbportal/ [Google Scholar]
- (144).Murthy JMK, Saxena AB. Bell's palsy: treatment guidelines. Ann Ind Acad Neurol. 2011;14 Suppl 1:S70–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Fitzpatrick E, Durieux-Smith A, Angus D, Olds J, Schramm D, Whittingham J. Economic evaluation of cochlear implants in children. J Speech Lang Pathol Audiol. 2006;30. [Google Scholar]
- (146).Statistics Canada. Labour Force Survey estimates: Can Sim Table 282-0151 [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated 2017 Aug; cited 2017 Dec 28]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=2820151 [Google Scholar]
- (147).Yong-xin L, Shuang L, De-min H. Research advances in post-operative rehabilitation following cochlear implant. J Otol 2007;2(2):92–6. [Google Scholar]
- (148).Thorrington D, Eames K. Measuring health utilities in children and adolescents: a systematic review of the literature. PloS One. 2015;10(8):e0135672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (150).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (151).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2017 Dec]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (152).Hearing loss of Canadians, 2012 and 2013 [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated 2015 Nov 27; cited 2017 Jan 12]. Available from: http://www.statcan.gc.ca/pub/82-625-x/2015001/article/14156-eng.htm [Google Scholar]
- (153).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (154).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2017 Dec]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (155).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (156).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]
- (157).Cochlear implants for children and adults with severe to profound deafness [Internet]. London, UK: National Institute for Health and Clinical Excellence; 2009. [cited 2017 Jun 30]. Available from: https://www.nice.org.uk/guidance/ta166 [Google Scholar]
- (158).IHP audiology appointment types/fee schedule/invoicing/documentation requirements [Internet]. Toronto (ON): Infant Hearing Program, City of Toronto; 2017. [cited 2017 Dec 28]. Available from: https://www.toronto.ca/wp-content/uploads/2017/11/9762-tph-hp-ihp-financial-audiology-appt-types-fee-sch-invoice-req-2017.pdf [Google Scholar]