Abstract
Prostaglandin E2 (PGE2), a major product of cyclooxygenase-2 (COX-2), plays an important role in the carcinogenesis of many solid tumors, including colorectal cancer. Because PGE2 functions by signaling through PGE2 receptors (EPs), which regulate tumor cell growth, invasion, and migration, there has been a growing amount of interest in the therapeutic potential of targeting EPs. In the present study, we investigated the role of EP4 on the effectiveness of cordycepin in inhibiting the migration and invasion of HCT116 human colorectal carcinoma cells. Our data indicate that cordycepin suppressed lipopolysaccharide (LPS)-enhanced cell migration and invasion through the inactivation of matrix metalloproteinase (MMP)-9 as well as the down-regulation of COX-2 expression and PGE2 production. These events were shown to be associated with the inactivation of EP4 and activation of AMP-activated protein kinase (AMPK). Moreover, the EP4 antagonist AH23848 prevented LPS-induced MMP-9 expression and cell invasion in HCT116 cells. However, the AMPK inhibitor, compound C, as well as AMPK knockdown via siRNA, attenuated the cordycepin-induced inhibition of EP4 expression. Cordycepin treatment also reduced the activation of CREB. These findings indicate that cordycepin suppresses the migration and invasion of HCT116 cells through modulating EP4 expression and the AMPK-CREB signaling pathway. Therefore, cordycepin has the potential to serve as a potent anti-cancer agent in therapeutic strategies against colorectal cancer metastasis.
Keywords: AMPK, Cell migration/invasion, Cordycepin, CREB, EP4
INTRODUCTION
Cordyceps species are rare herbs that are occasionally used in traditional medicine (1, 2). They have been used to treat various diseases, such as cancer, and are also known to have anti-tumor properties, the ability to scavenge free radicals, and immune-stimulating abilities (3–5). Cordycepin is the main bioactive component in Cordyceps species such as Cordyceps militaris and C. sinensis (2, 4). Cordycepin has many biological properties, including inflammation inhibition (6), platelet aggregation inhibition (7), anti-tumor effects (8), and chondrocyte hypertrophy inhibition (9). However, the molecular mechanism through which cordycepin inhibits cancer cell migration and invasion remains unclear.
The physiological functions and signaling of prostaglandin E2 (PGE2) are related to the activation of EP receptors (EP1-4), which are G-protein-coupled receptors (GPCRs) (10). PGE2, which is regulated by cyclooxygenase-2 (COX-2), promotes cell proliferation and the invasion of colorectal tumors (11). Signaling through the EP2 receptor activates the protein kinase A (PKA) pathway, which induces the phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) in the gastrointestinal tract (12). However, the molecular mechanism through which this intracellular mediator is related to cell invasion and migration in colorectal cancer remains unclear, along with the anti-inflammatory effects of PGE2 in colorectal cancer. Identifying the intracellular signaling mechanism that mediates cell invasion and movement, which in turn mediate the effects of PGE2, is critical to understanding the main properties of colorectal cancer and developing effective therapies.
AMP-activated protein kinase (AMPK) is a well-conserved serine/threonine protein kinase containing a catalytic subunit (α) and two regulatory subunits (β and γ) that is expressed in many tissues (13). Some studies have suggested that AMPK can function as a tumor suppressor by altering inflammation and causing cell-cycle arrest during tumorigenesis (14, 15). In addition, when activated by 5-aminoimidazole-4-carboxamideribonucleoside (AICAR) or phenformin, AMPK induces cell death through the mitogen-activated protein kinases pathway (16, 17). In addition, AMPK is mediated to Kahweol-induced glucose uptake in mouse embryo fibroblast cells (18). Taken together, these findings suggest that AMPK activation may be useful in controlling cell death in colorectal cancer cells.
Because cordycepin can greatly affect the pathogenesis of colorectal disease, we hypothesized that cordycepin down-regulates EP4 expression and its downstream signaling functions in human colorectal cancer cells. In order to examine the signaling pathway involved, we performed in vitro human cell-based assays. Our results suggest that cordycepin inhibits cell invasion and migration in lipopolysaccharide (LPS)-treated HCT-116 cells via the EP4-AMPK-CREB axis. These pathways provide new insights into the molecular mechanism of cell invasion and may reveal novel targets for therapeutic medications.
RESULTS
Cordycepin inhibits LPS-induced cell migration and invasion in HCT116 human colorectal carcinoma cells
In order to investigate the pharmacological potential of cordycepin on LPS-induced cell migration and invasion, we first determined the dose dependence of the cytotoxic effects of cordycepin in the absence or presence of LPS for 48 h in HCT116 cells using an MTT assay. Cordycepin at 25–50 μg/ml did not show any cytotoxic effect on HCT-116 cells with or without 2.5 μg/ml LPS (Fig. 1A). Therefore, a concentration of cordycepin within this range was employed in the remaining experiments. We next used gelatin zymography and Western blot analyses to investigate the inhibitory effects of cordycepin on the activation and expression of matrix metalloproteinase (MMP) proteins. Interestingly, compared to LPS alone, cotreatment with both cordycepin and LPS inhibited the activation and expression of MMP-9, but had no effect on MMP-2 activity or expression (Fig. 1B). In vitro invasion and migration assays were used to investigate the inhibitory effects of cordycepin on the invasive potency of LPS-treated HCT116 cells. As shown in Fig. 1C and D, LPS-stimulated cell migration and cell invasion were significantly inhibited by cordycepin. These results suggest that nontoxic concentrations of cordycepin have an inhibitory effect on the invasiveness of LPS-treated HCT-116 cells.
Fig. 1.
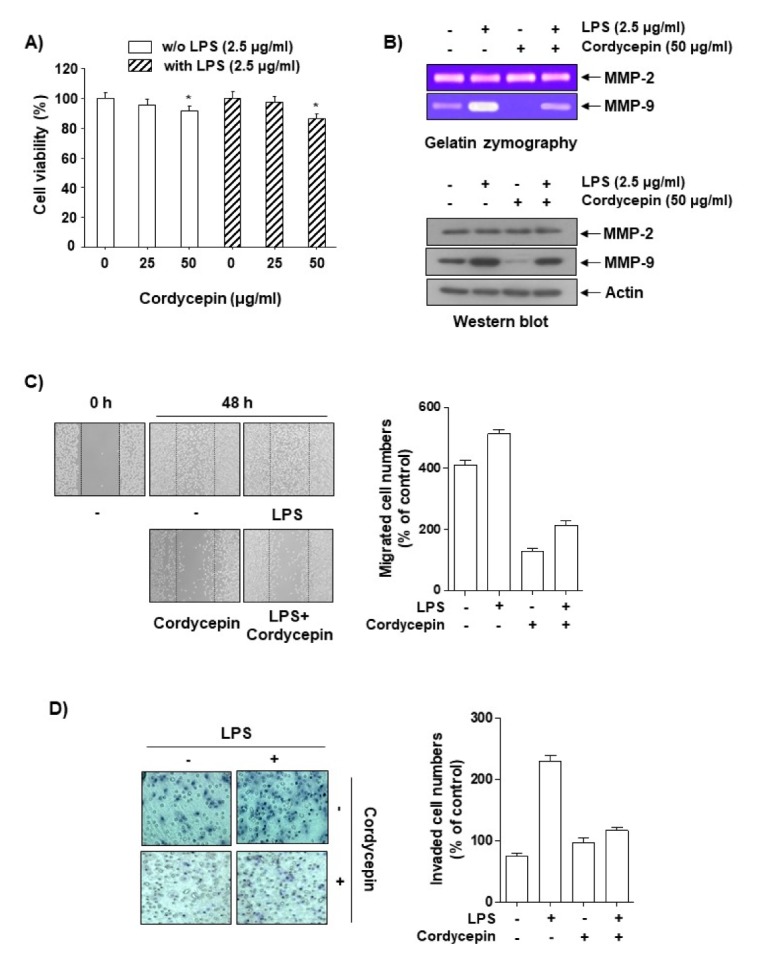
Inhibitory effects of cordycepin on the migration and invasion of human colorectal carcinoma HCT116 cells. (A) Cells were incubated with varying concentrations of cordycepin in the absence or presence of LPS for 48 h in serum-free medium, and proliferation was determined using an MTT assay. The data are expressed as the mean ± SD of triplicate experiments. *P < 0.05 compared to control. (B) Cells at 80% confluence were treated with various concentrations of cordycepin in serum-free medium. The conditioned medium was collected after 48 h, and gelatin zymography was performed in triplicate. Representative blots are shown. Equal amounts of cellular proteins were separated on SDS-polyacrylamide gels and transferred to PVDF membranes. The membranes were probed with specific antibodies and visualized using an ECL. Actin was used as an internal control. (C) Cells were scratched with a pipette tip and treated with cordycepin or LPS for 48 h. Migrated cells were imaged using phase-contrast microscopy. (D) The cells were plated onto the apical sides of Matrigel-coated filters in serum-free medium. Medium containing 20% FBS was placed in the basolateral chamber to act as a chemoattractant. After 48 h, cells on the apical side were removed using a Q-tip. The cells on the bottom of the filter were then stained using H&E and photographed.
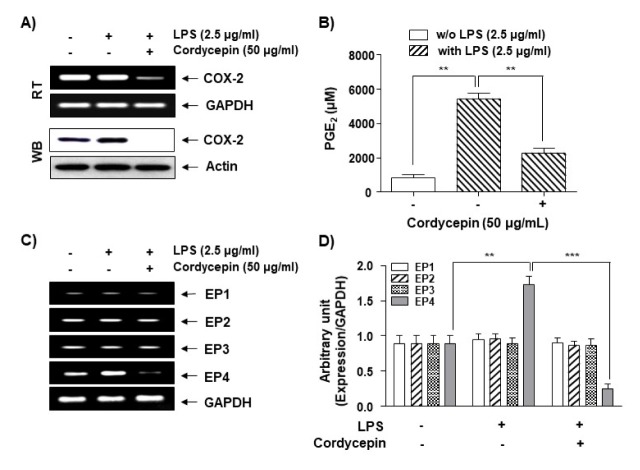
Cordycepin inhibits the expression of COX-2 and EP4 and the production of PGE2 in LPS-treated HCT116 cells
Previous reports have shown that cell migration and invasion increase the activation of the EPs as well as the expression of the COX-2 product PGE2 in several cancer cell lines (17–20). Thus, we investigated whether the EP4-COX-2-PGE2 cascade affects LPS-induced cell migration and invasion in HCT116 cells. While LPS did not alter the COX-2 mRNA level, it slightly increased the COX2 protein level (Fig. 2A). However, cordycepin dramatically inhibited LPS-induced COX-2 mRNA and protein expression. Cordycepin also showed an inhibitory effect on LPS-induced PGE2 production at a concentration of 50 μg/ml, as demonstrated by enzyme immunoassays (Fig. 2B), suggesting that the inhibitory effects of cordycepin on LPS-induced PGE2 production are controlled by COX-2 gene expression. In addition, we attempted to identify which of the PGE2 receptor subtypes is responsible for the LPS-mediated production of PGE2 in HCT-116 cells. Interestingly, only EP4 mRNA expression was affected by cordycepin, while EP2, EP3, and EP4 were not affected (Fig. 2C, D; Supplement Table 1). These results suggest that cordycepin inhibits EP4 and COX-2 expression to regulate PGE2 production in HCT116 cells.
Fig. 2.
Inhibitory effects of cordycepin on PGE2 production and expression of COX-2 and EPs in LPS-stimulated HCT-116 cells. Cells were pre-treated with 50 μg/ml of cordycepin for 1 h prior to incubation with LPS for 48 h. Total RNA (A; upper panel) and proteins (A; lower panel) were prepared and used for the RT-PCR analysis of COX-2 gene expression with GAPDH as an internal control. Actin was used as an internal control. (B) The culture supernatants were isolated, and the amount of PGE2 production was determined. The data are expressed as the mean ± SD of three independent experiments. **P < 0.01 compared to LPS alone. (C) Total RNA was prepared and used for RT-PCR analysis of EP1-4 gene expression. (D) The data represent the EP1-4 mRNA levels in the LPS-treated cultures compared with control cultures from three independent experiments (**P < 0.01, ***P < 0.001).
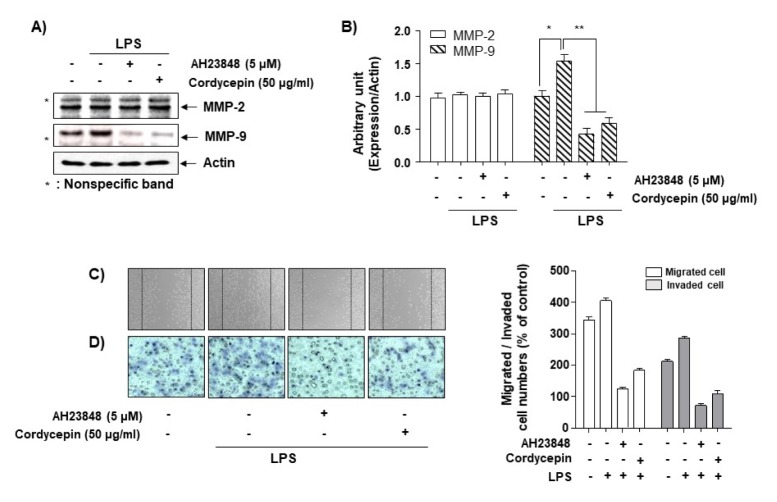
Cordycepin inhibits MMP9-mediated cell migration and invasion
In order to determine whether cordycepin could inhibit LPS-mediated MMP9 expression via EP4, we used AH23848, an EP4 antagonist. It dramatically inhibited LPS-induced MMP-9 expression, but not MMP-2 expression (Fig. 3A, B). In addition, cell migration and invasion assays were performed in order to examine whether cordycepin could inhibit cell invasion and migration in LPS-treated cells through inhibiting the EP4 receptor. While AH23848 inhibited LPS-mediated cell migration and invasion (Fig. 3C, D), overexpressed EP4 partially increased both of these (Supplement Fig. 1). These results indicate that cordycepin inhibited LPS-mediated cell migration and invasion via MMP and EP4.
Fig. 3.
Increase in cordycepin-induced anti-invasiveness by the inhibition of EP4 signaling in HCT-116 cells. (A, B) Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with AH23848 or cordycepin (50 μg/ml) for 48 h. The cells were lysed, and equal amounts of protein were subjected to Western blotting. (C, D) Cells were pretreated with LPS (2.5 μg/ml) for 1 h before being challenged with AH23848 or cordycepin (50 μg/ml) for 6 h. The cell migration (C) and Matrigel invasion (D) assays were performed. The experiment was repeated three times, and similar results were obtained.
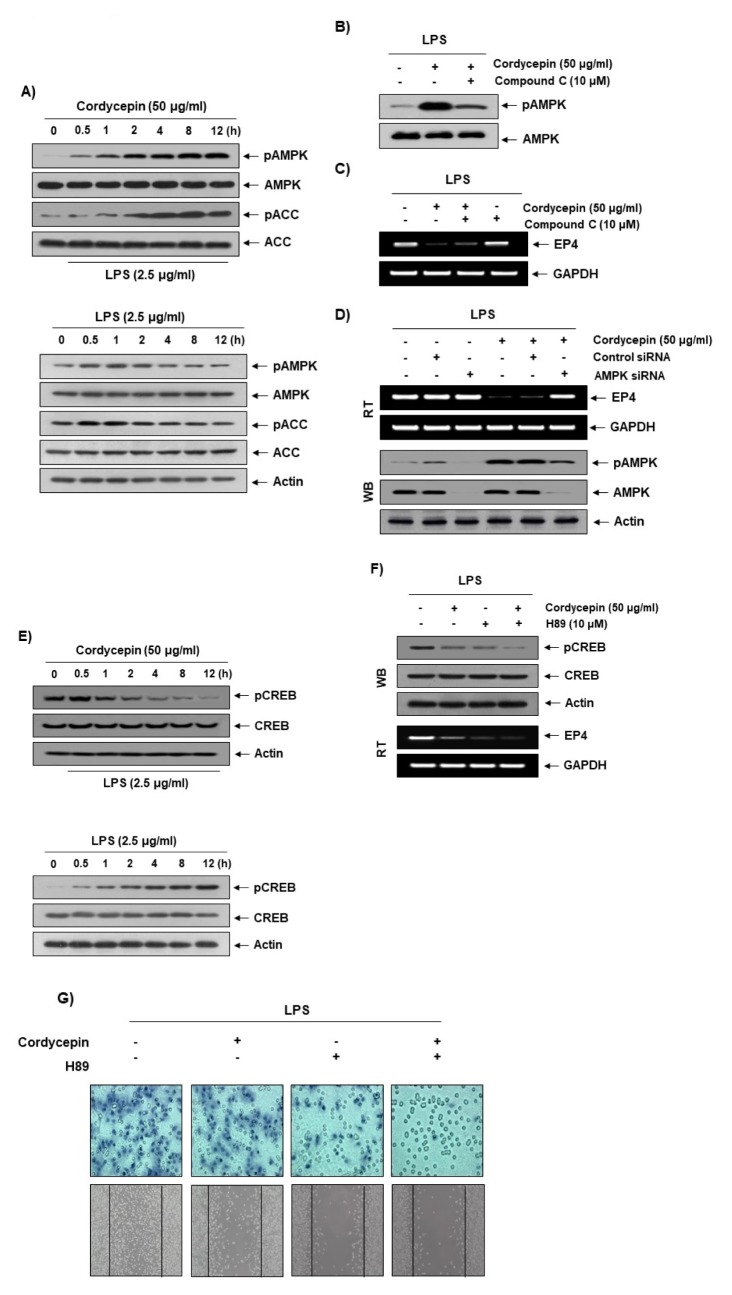
Cordycepin-activated AMPK inhibits EP4 gene expression in LPS-treated cells and Cordycepin decreases CREB activation by inhibiting EP4 gene expression
AMPK is related to many physiological phenomena, such as cell death (21, 22), autophagy (23), and metabolic control (24). In particular, AMPK can control cell polarity, migration, and cytoskeletal dynamics (25). As shown in Fig. 4A, the phosphorylation levels of AMPK and acetyl-CoA carboxylase (ACC), a downstream target kinase of AMPK, were increased by cordycepin treatment in a time-dependent manner, suggesting that AMPK signaling was activated. In order to determine whether cordycepin-activated AMPK can affect EP4 expression, we used compound C, an AMPK inhibitor. Cordycepin strongly inhibited EP4 gene expression (Fig. 4C), and compound C restored EP4 gene expression in LPS-treated cells. We employed COX-2 activity assay and PGE2 ELISA assay in order to determine whether compound C could partially restore COX-2 activity and PGE2 production. While cordycepin decreased LPS-mediated COX-2 activity and PGE2 production, compound C slightly increased both of these in LPS-treated cells (Supplement Fig. 2). The results from a genetic study using AMPK-specific siRNA (Fig. 4D) were the same as those from the pharmaceutical study (Fig. 4C), suggesting that cordycepin inhibits EP4 gene expression via the AMPK pathway in LPS-treated cells. In addition, many studies have reported that CREB may be a down-stream protein of the AMPK pathway (26, 27). Thus, we identified CREB activation in LPS-treated cells. Cordycepin dramatically inhibited CREB activation in LPS-treated cells in a time-dependent manner (Fig. 4E). H89, an inhibitor of PKA and MSK, was used to inhibit CREB activation (28). As expected, H89 strongly inhibited EP4 gene expression by inhibiting CREB activation in LPS-treated cells (Fig. 4F), indicating that CREB may be critical for EP4 gene expression. In order to determine whether cordycepin could inhibit LPS-mediated cell migration and invasion via CREB activation, we performed these assays. Cotreatment with cordycepin and H89 was shown to dramatically inhibit cell migration and invasion compared to cordycepin alone in LPS-treated cells (Fig. 4G). These results suggest that cordycepin strongly decreases CREB-mediated cell migration and invasion through the alteration of EP4 gene expression in LPS-treated cells.
Fig. 4.
Involvement of the AMPK pathway in the reduction of EP4 expression by cordycepin. (A; upper panel) Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with cordycepin (50 μg/ml) for the indicated times. (A; lower panel) Cells were treated with LPS (2.5 μg/ml) for the indicated times. Immunoblotting analyses were performed with anti-pAMPK, anti-AMPK, anti-pACC, and anti-ACC antibodies. (B) The effects of cordycepin and compound C on AMPK activation in LPS-stimulated HCT-116 cells. Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with either cordycepin (50 μg/ml) only or both cordycepin and compound C (10 μM) for 12 h. (C) The effects of cordycepin and compound C on EP4 expression in LPS-stimulated cells. Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with either cordycepin (50 μg/ml) only or both cordycepin and compound C (10 μM) for 12 h. (D) The cells were transfected with AMPK small interfering (siRNA) or control siRNA for 24 h. The transfected cells were treated with 50 μg/ml cordycepin for 48 h. (E; upper panel) Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with cordycepin (50 μg/ml) for the indicated times. (E; lower panel) Cells were treated with LPS (2.5 μg/ml) for the indicated times. Cells were pretreated with LPS (2.5 μg/ml) for 1 h prior to treatment with either cordycepin (50 μg/ml) only or both cordycepin and H89 (10 μM) for 12 h. Then, we performed Western blot and RT-PCR (F) as well as cell migration and invasion assay (G).
DISCUSSION
In this study, we examined the anti-cell migration and invasion effects of cordycepin in LPS-treated human colorectal carcinoma HCT-116 cells. Cordycepin had no cytotoxic effects on HCT-116 cells at the concentrations tested (Fig. 1), but it did show anti-inflammatory activity. Previous studies have shown that cordycepin has anti-cancer effects in human breast cancer cells (29), human lung cancer cells (30), oral squamous cell carcinoma (31), and human bladder cancer cells (32). Cordycepin has also been shown to have anti-cancer effects in human colorectal cancer cells, but there have been few reports on cordycepin’s role in altering early events (i.e., migration and invasion) to prevent the progression into cell transformation. Because most of the literature on cordycepin’s anti-tumor effects has focused thus far on cell apoptosis, the effects of cordycepin on early events, like cell migration and invasion during tumorigenesis, remain a matter of speculation. In particular, there have been no reports until now that cordycepin inhibits LPS-induced cell migration and invasion in human colorectal cancer cells, although cordycepin has been found to decrease tumor necrosis factor-α-induced cell migration and invasion in human bladder cancer cells (33). The major difference in these pathways is the function of the EP4 receptor versus the mediator of intracellular signaling nuclear factor-κB. This is a critical finding, because it is the first stage of signal transduction at the receptor as an early event.
Understanding the physiological characteristics of the EP4 receptor could provide further insight into the intracellular signaling mechanism that leads to protein complex formation of G-protein coupled receptors (34). Cordycepin decreased gene expression of the EP4 receptor, but not that of EP1, −2, or −3, and also reduced PGE2 production (Fig. 2C, D). This is unsurprising, because EP4 is preferentially expressed in the heart and intestines and expressed to a lesser degree in the lungs, kidneys, and brain (35). EP4 is a Gαs protein that stimulates cAMP production, and cordycepin dramatically increased AMPK activation (Fig. 4B) and significantly inhibited EP4 gene expression, suggesting that AMPK can specifically inhibit EP4 gene expression. Funahashi et al. (36) reported that EP-induced PGE2 expression abolished AMPK activity through protein kinase A signaling transmission. Reciprocally, a cordycepin-induced increase in AMPK activity could inhibit EP4 activity, thus preventing its physiological signaling. Taken together, these data suggest that LPS increases EP4 activation to produce PGE2, which induces cell migration and invasion by inhibiting AMPK, and that cordycepin inhibits this signaling mechanism, giving it anti-cancer properties that affect the early tumorigenesis stages.
Cordycepin inhibits CREB signaling in order to regulate its physiological functions. CREB-related gene expression is important for microenvironmental homeostasis under pathological conditions, and CREB has been considered as a major target of novel drugs. As seen in Fig. 4E, cordycepin inhibited LPS-induced CREB activation, and both H89 and forskolin induced CRE activity, suggesting that cordycepin decreased CREB activation via the EP4-AMPK pathway in order to prevent cell migration and invasion in HCT-116 cells.
In conclusion, our results demonstrate that the levels of CREB activation were dramatically decreased by the negative regulation of AMPK expression through the deactivation of EP4 in cordycepin-treated HCT116 cells. This study provides novel insights into the molecular mechanisms of cell migration in HCT-116 cells. Controlling the expression of the EP4 receptor may be a new strategy for preventing cell migration and invasion of HCT-116 cells. This study also highlights the potential for the therapeutic use of cordycepin in the treatment of colorectal cancer.
MATERIALS AND METHODS
Reagents
Cordycepin (MW, 251.2; Product No. C3394) from C. militaris, LPS (Escherichia coli 026:B6), Griess reagent, Tween-20, bovine serum albumin (BSA), H89, forskolin, compound C, and 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Antibodies against COX-2, MMP-2, MMP-9, AMPK, pAMPK, ACC, pACC, CREB, pCREB, and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The peroxidase-labeled donkey anti-rabbit immunoglobulin, peroxidase-labeled sheep anti-mouse immunoglobulin, and enhanced chemiluminescence (ECL) detection kit were purchased from Amersham Corp. (Arlington Heights, IL, USA). The enzyme-linked immunosorbent assay (ELISA) kit for PGE2 was obtained from R&D Systems (Minneapolis, MN, USA). COX-2, EP1-4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) oligonucleotide primers were purchased from Bioneer (Seoul, Korea). All other chemicals were purchased from Sigma-Aldrich Chemical Co.
Cell culture and MTT assay
HCT-116 human colon cancer cells were obtained from the American Type Culture Collection (Manassas, VA, USA). These cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco BRL, Gaithersburg, MD, USA) and 1% penicillin-streptomycin at 37°C in a humid environment containing 5% CO2. For the cell viability assay, HCT-116 cells were seeded in 6-well plates at a density of 2 × 105 cells per well. After 24 h of stabilization, the cells were treated with various concentrations of cordycepin for 1 h, then stimulated with LPS (2.5 μg/ml) for an additional 48 h. Cell viability was determined using the MTT assay, which is based on the conversion of MTT to MTT-formazan by mitochondrial enzymes.
In vitro wound-healing assay
HCT-116 cells were seeded in 6-well plates and grown overnight to confluence. The monolayers were then scratched with a 200-μl pipette tip in order to create a wound and washed twice with serum-free RPMI-1640 so as to remove floating cells; the medium was then replaced with serum-free medium supplemented with cordycepin baicalein in the presence or absence of LPS. The rate of wound closure was assessed and imaged 48 h later. Each value is derived from three randomly selected fields.
Matrigel invasion assay
HCT-116 cells were incubated in RPMI-1640 with 10% FBS and collected via trypsinization. Cells (2 × 105 cells/well) in serum-free medium were added to the inner cup of a 24-well transwell chamber (Corning Life Sciences, Oneonta, NY, USA) that had been coated with 50 ml of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA; 1:10 dilution in serum-free medium). Medium supplemented with 10% serum or the indicated agent was then added to the outer cup. After 48 h, cells that had migrated through the Matrigel and the 8-mm pore size membrane were fixed, stained with hematoxylin and eosin (H&E, Sigma-Aldrich Chemical Co.), then photographed under an inverted microscope. Each experiment was performed in triplicate.
Supplementary Information
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korea government (NRF-2016R1C1B1014724 for JWJ, 2016R1D1A 1B03932521 for KSS, and 2018R1A2B2005705 for YHC).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Yue K, Ye M, Zhou Z, et al. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65:474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Masuda M, Sakurai A, et al. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69:1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buenz EJ, Bauer BA, Osmundson TW, et al. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J Ethnopharmacol. 2005;96:19–29. doi: 10.1016/j.jep.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha B, Tanaka E, Han JG, et al. A brief chronicle of the genus cordyceps fr., the oldest valid genus in cordycipitaceae (hypocreales, ascomycota) Mycobiology. 2014;42:93–99. doi: 10.5941/MYCO.2014.42.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Devel Ther. 2014;8:1941–1953. doi: 10.2147/DDDT.S71957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HJ, Cho JY, Rhee MH, et al. Inhibitory effects of cordycepin (3′-deoxyadenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J Microbiol Biotechnol. 2007;17:1134–1138. [PubMed] [Google Scholar]
- 8.Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127:53–56. doi: 10.1016/j.jphs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Dou C, Li J, et al. Cordycepin inhibits chondrocyte hypertrophy of mesenchymal stem cells through PI3K/Bapx1 and Notch signaling pathway. BMB Rep. 2016;49:548–553. doi: 10.5483/BMBRep.2016.49.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev. 2011;63:471–538. doi: 10.1124/pr.110.003517. [DOI] [PubMed] [Google Scholar]
- 11.Greenhough A, Smartt HJ, Moore AE. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 12.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Saud SM, Young MR, et al. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6:7365–7378. doi: 10.18632/oncotarget.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petti C, Vegetti C, Molla A, et al. AMPK activators inhibit the proliferation of human melanomas bearing the activated MAPK pathway. Melanoma Res. 2012;22:341–350. doi: 10.1097/CMR.0b013e3283544929. [DOI] [PubMed] [Google Scholar]
- 17.Kim JI, Lakshmikanthan V, Frilot N, et al. Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 2010;8:569–577. doi: 10.1158/1541-7786.MCR-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek JH, Kim NJ, Song JK, Chun KH. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK) BMB Rep. 2017;50:566–571. doi: 10.5483/BMBRep.2017.50.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, Kim HS, Lee SH, et al. NDRG2 controls COX-2/PGE2-mediated breast cancer cell migration and invasion. Mol Cells. 2014;37:759–765. doi: 10.14348/molcells.2014.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter DG, Dubois RN. Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol. 2012;2012;723419 doi: 10.1155/2012/723419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011;585:981–985. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Al-Rashed F, Calay D, Lang M, et al. Celecoxib exerts protective effects in the vascular endothelium via COX-2-independent activation of AMPK-CREB-Nrf2 signalling. Sci Rep. 2018;8:6271. doi: 10.1038/s41598-018-24548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otero-Rodiño C, Velasco C, Álvarez-Otero R, et al. Changes in the levels and phosphorylation status of Akt, AMPK, CREB and FoxO1 in hypothalamus of rainbow trout under conditions of enhanced glucosensing activity. J Exp Biol. 2017;220:4410–4417. doi: 10.1242/jeb.165159. [DOI] [PubMed] [Google Scholar]
- 28.Cho IJ, Woo NR, Shin IC, et al. H89, an inhibitor of PKA and MSK, inhibits cyclic-AMP response element binding protein-mediated MAPK phosphatase-1 induction by lipopolysaccharide. Inflamm Res. 2009;58:863–872. doi: 10.1007/s00011-009-0057-z. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Zhang Y, Lu J, et al. Cordycepin, a natural antineoplastic agent, induces apoptosis of breast cancer cells via caspase-dependent pathways. Nat Prod Commun. 2016;11:63–68. [PubMed] [Google Scholar]
- 30.Hwang JH, Park SJ, Ko WG, et al. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am J Cancer Res. 2017;7:417–432. [PMC free article] [PubMed] [Google Scholar]
- 31.Su NW, Wu SH, Chi CW, et al. Metronomic cordycepin therapy prolongs survival of oral cancer-bearing mice and inhibits epithelial-mesenchymal transition. Molecules. 2017;22:E629. doi: 10.3390/molecules22040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao HL, Liu ZJ, Chang Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumour Biol. 2017;39 doi: 10.1177/1010428317706915. 1010428317706915. [DOI] [PubMed] [Google Scholar]
- 33.Lee EJ, Kim WJ, Moon SK. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother Res. 2010;24:1755–1761. doi: 10.1002/ptr.3132. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama U, Iwatsubo K, Umemura M, et al. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev. 2013;65:1010–1052. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 35.Bastien L, Sawyer N, Grygorczyk R, et al. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994;269:11873–11877. [PubMed] [Google Scholar]
- 36.Funahashi K, Cao X, Yamauchi M, et al. Prostaglandin E2 negatively regulates AMP-activated protein kinase via protein kinase A signaling pathway. Prostaglandins Other Lipid Mediat. 2009;88:31–35. doi: 10.1016/j.prostaglandins.2008.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.