Abstract
Sectioning of the paraffin-embedded tissue is widely used in histology and pathology. However, it is tedious. To improve this method, several commercial companies have devised complex section transfer systems using fluid water. To simplify this technology, we created a simple method using homemade equipment that combines cutting and floating within a simple thermostatic chamber; therefore, the sections automatically enter the water bath on the water surface. The hippocampus from adult mouse brains, adult mouse kidneys, embryonic mouse brains, and adult zebrafish eyes were cut using both conventional paraffin sectioning and the presented method for comparison. Statistical analysis shows that our improved method saved time and produced higher quality sections. In addition, paraffin sectioning of a whole specimen in a short time is easy for junior operators.
Keywords: Neuroscience, Issue 139, Morphology, Paraffin Sectioning, Static electricity, Section curling, Home-Made Equipment, Efficiency
Introduction
Morphological study is important in the biological research. Although new technology has allowed researchers to observe their targets directly from the whole tissue or organisms1,2,3, cutting the specimen into thin sections, followed by staining, remains the primary method fornot only tissue morphology but also protein targeting directly in the tissue. Light microscopy uses three section types: paraffin, frozen, and semithin. Although cryosectioning is common for protecting tissue antigenicity, and the specimen preparation is simple, the retained tissue morphology is poor and unsuitable for thin sectioning4,5. Paraffin sectioning is the most frequently used method for exhibiting well preserved morphology. As the specimens are dehydrated completely and embedded in wax, the paraffin blocks can be stored indefinitely. In addition, paraffin sectioning produces thin sections that improve biological probe access in further experiments and reduce cell layer overlay in the Z direction.
However, conventional paraffin sectioning is tedious and demands operator skill. Paraffin sections undergo fixation, dehydration, embedding, cutting, and floating. Importantly, transferring section ribbons from the knife holder to the water bath is necessary but difficult for junior operators. Especially in dry air, the section ribbons will twist due to static electricity and are difficult to unfold on the warm water surface. To improve section quality, moistening the exposed tissue surface between microtome blade passes, cooling the wax blocks by immersing them in ice water, or raising the humidity with a humidifier near the microtome are recommended6,7. Newer methods for improving paraffin sectioning include hybrid paraffin embedding, cryosectioning8, and commercial section transfer system assistance9. Although these methods partially improve paraffin sectioning speed and quality, they make sectioning much more cumbersome, and commercial section transfer systems are expensive.
In this protocol, we demonstrate how to create simple, cheap and flexible equipment step by step, which can be connected to the blade holder of a rotary microtome. This equipment is comprised of a section channel, a water bath, and a heater with a temperature detection switch. After cutting, dozens of sections flow into the section channel and enter the water bath directly, thus unfolding automatically. This improves the efficiency of paraffin sectioning and makes this technology more convenient. Using this method, more adult mouse hippocampal sections, adult mouse kidney sections, embryonic 15.5 day-old (E15.5) mouse brain sections, and adult zebrafish eye sections were harvested in less time and remained more intact morphologically. This method can also be used for other tissue samples that require accelerated paraffin sectioning while avoiding loss of section distinction.
Protocol
All methods described here have been approved by the Animal Care and Use Committee of Nanchang University.
1. Assemble the Equipment and Connect the Microtome
Design the parameter per the requirements (Supplementary Figure 1).
Submit the parameter to a local factory to manufacture the acrylic boards.
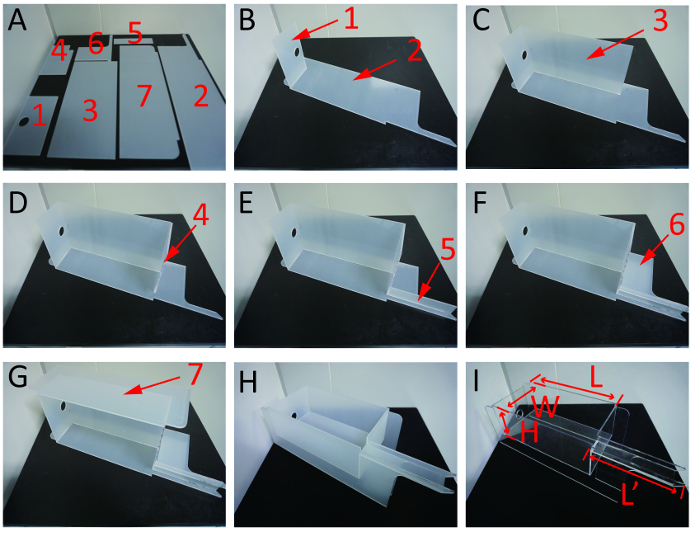
Assemble all parts in sequence: Use chloroform to combine 7 commercial acrylic boards into a tank with a section channel and water bath (Figure 1). CAUTION: Chloroform produces toxic substances when it meets light and oxygen. Assemble the apparatus in a fume hood.
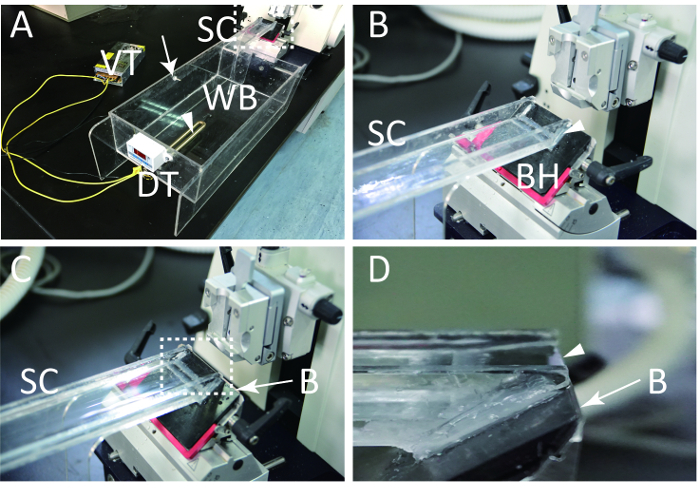
Install the tubular electric heating element through the hole in acrylic board #1 (Figure 2A, arrowhead).
To install the temperature controller, create a small hole on the #2 acrylic board using an electric drill, and install the temperature detector (Figure 2A, arrow). Install a digital thermostat on the tank (Figure 2A). NOTE: The temperature detector should be within 1 cm below the water surface.
To provide power access, adjust the voltage below 24 V before turning on the equipment.
Remove the section waste tray from the microtome.
Link the sections channel with the microtome blade holder with neutral silicone sealant (Figure 2B). NOTE: Do not smear any neutral silicone sealant above the top of the blade so that the old blade is easily replaced.
Dry overnight to allow the chloroform and neutral silicone sealant time to solidify.
Install the blade to the blade holder. Pour water into the water bath (Figure 2C). The water surface should just touch the top of the blade (Figure 2D, arrow head).
2. Paraffin Sectioning
NOTE: Debris often accumulate on the upper or lower edges of the wax block and the water surface. This debris should be cleared regularly.
- Perform Conventional paraffin sectioning10.
- Clamp the paraffin-embedded specimen on a rotary microtome and place the blade in a blade holder.
- Turn the handwheel and section the specimen.
- Transfer the paraffin sectioned ribbon to the 38.5 °C warm water bath.
- Pick up the sections onto the microscope slide from the water surface after unfolding.
- Dry the slides in an oven at 42 °C overnight.
- Improved paraffin sectioning (Figure 3)
- Turn on the equipment.
- Open the heater with temperature detection switch and set the targeted temperature between 38.0 °C to 40.0 °C for 1 h in advance to warm the water.
- Modify the paraffin block acquired by paraffin embedding; then place the paraffin block on the microtome's cassette clamp and lock it. NOTE: The superior and inferior borders of the paraffin block should be modified horizontally to prevent the section ribbon from forming an arc and becoming unable to flow through the narrow sections channel.
- Quickly approach the sample by cutting thicker sections.
- Adjust to a suitable section thickness (10 µm for adult mouse brains, adult mouse kidneys, and embryo mouse brains, 4 µm for zebrafish eyes). Turn the handwheel and the sections will automatically enter the sections channel and bath.
- Section the specimen in a slow and uniform cutting stroke (Figure 3A). NOTE: Thethickness of the first section will be altered after the handwheel has been stopped for a few minutes. The disposable blades should be replaced when the sections have consecutive scratches.
- Guide the sections into the bath chamber to float in a line (Figure 3B, 3C). NOTE: If the head section adheres to the chamber wall, use a brush guide to keep it floating.
- Adhere sections onto microscope slides. After the sections fully unfold in the water bath, separate several sections with tweezers. Then pick them up from the water bath onto the microscope slides (Figure 3D). NOTE: Sections should be picked up from the posterior of the water bath, as those in the sections channel and the anterior of the water bath will not yet be fully unfolded.
- Dry the slides by placing them in a 42 °C oven overnight. The slides can then be stored at room temperature indefinitely.
Use Hematoxylin-Eosin (HE) staining for the sake of evaluation of the improved method8.

Representative Results
The improved method increased the number of intact paraffin sections. We tested this new method on adult mouse hippocampal tissue, adult mouse kidneys, embryonic mouse brains, and zebrafish eyes. Water was added to the tank, and the water temperature was maintained between 38.0 °C to 40.0 °C. After a serially preparing the tissue samples, they were sectioned and compared to conventional sectioning. The new method avoided section loss and increased the proportion of intact sections in the adult mouse hippocampus, adult mouse kidneys, E15.5 mouse brains, and adult zebrafish eyes (Figure 4A, 4C, 4E, 4G). In conclusion, our method improved the sectioning by preventing section loss and harvesting more intact sections per specimen.
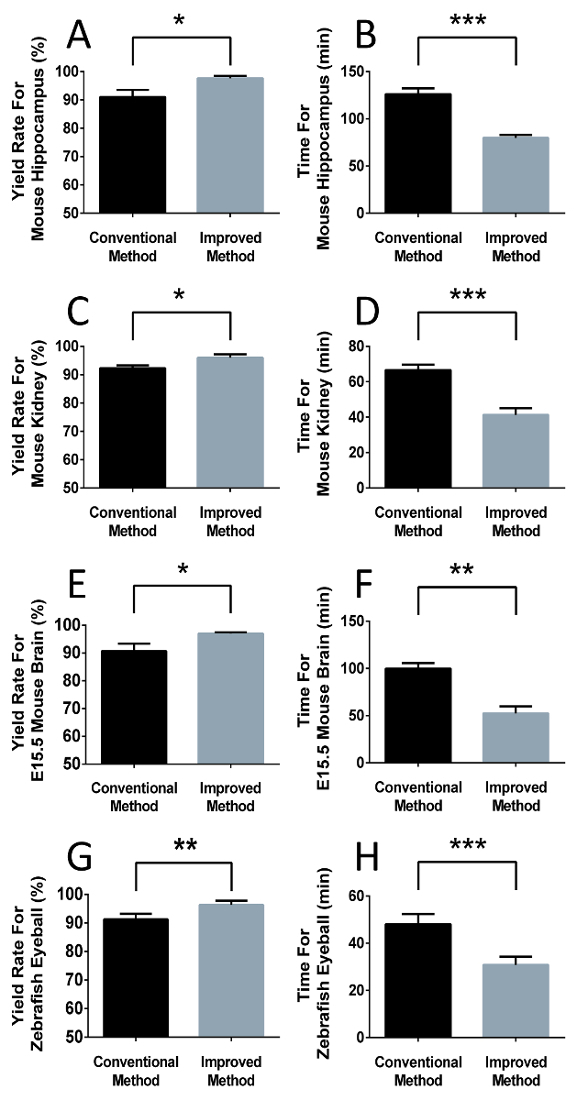
The improved method decreased the time spent per specimen. In comparing the time taken between the conventional method and our improved method, we performed whole-series sectioning (from the first section to the last section of an organ) on the adult mouse hippocampus, adult mouse kidneys, E15.5 mouse brains, and adult zebrafish eyes. The results suggest that the improved method accelerated the paraffin sectioning speed in all tested samples (Figure 4B, 4D, 4F, 4H). In general, our method allowed for faster high-quality paraffin sectioning.
Series sections of the zebrafish optic retina was observed by H and E staining. For series sectioning of the optic disc segment of the zebrafish retina,the sections harvested from the improved method were better has better integrity than those from the conventional paraffin sectioning method (Figure 5A, 5B). There was no significant difference between these two methods regarding the quality of most harvested sections (Figure 5C, 5D). Occasionally, the quality of sections harvested from the conventional method were relatively low (Figure 5E, 5F).
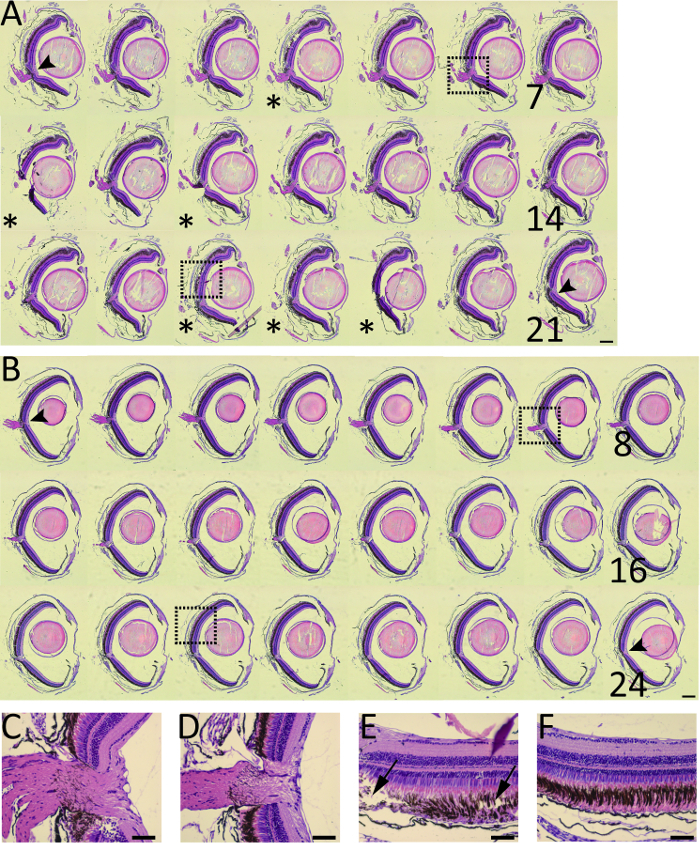
Figure 1: The process for assembling the 7 commercial acrylic boards into a tank. (A) Overall view of the 7 acrylic boards. (B-G) Stepwise assembly of the tank from board # 1 to board #7. (H) Primary tank after assembly. (I) General tank size; the protective membrane on the tank surface was removed for further installation. L = 400 mm, L' = 220 mm, H = 122 mm, W = 200 mm. Please click here to view a larger version of this figure.
Figure 2: The appropriately assembled equipment ready for use. (A) The intact equipment after being connected to the rotary microtome. Arrow indication, the temperature detector is installed through the small hole by electric drill. Arrow head, the heater (tubular electric heating element) is installed through the hole in board #1. (B) The amplified dashed area in A, the feature of the connection between the microtome blade holder and the sections channel. Arrow head, the blade holder and sections channel are connected by neutral silicone sealant. (C) Same as B, with the water poured and blade installed. (D) Side view of the dashed area in C. Arrow head, the water surface is close to the blade edge. VT: voltage transformer; WB: water bath; DT: digital thermostat; B: blade; BH: blade holder; SC: sections channel. Please click here to view a larger version of this figure.
Figure 3: The improved paraffin sectioning process. The figures located at the lower left corner are the amplified dashed areas in A-D respectively. Arrow indications, the first section. (A, A') The section enters the sections channel immediately and directly after cutting. (B, B') The sections ribbon crosses the border between the sections channel and water bath. (C, C') The sections float to the posterior of the water bath and are fully unfolded. (D, D') The sections are picked up by microscope slide when they reach the posterior of the water bath and are fully unfolded. SC: sections channel; WB, water bath. Please click here to view a larger version of this figure.
Figure 4: The improved sectioning method improved the efficiency and yield rate significantly for whole-series sections. The yield rate equaled the proportion of intact sections in all cut sections. Data are shown as the mean ± SEM and acquired by the same operator. (A, C, E, G) The improved method prevented section loss of the adult mouse hippocampus (91.04 ± 1.429% N = 3, vs 97.63 ± 0.4864% N = 3, p = 0.0120), adult mouse kidneys (92.30 ± 0.5689% N = 3, vs 95.99 ± 0.7055% N = 3, p = 0.0153), E15.5 mouse brain (90.67 ± 1.549%, N = 3 vs 97.01 ± 0.2816%, N = 3, p = 0.0158), and adult zebrafish eyes (91.27 ± 0.9734% N = 4 vs 96.32 ± 0.7217% N = 4, p = 0.0059). (B, D, F, H) The improved method saved time compared with the conventional method for the adult mouse hippocampus (126.0 ± 3.606 min vs 79.67 ± 1.856 min, N = 3, p = 0.0003), adult mouse kidneys (66.67 ± 1.764 min vs 41.33 ± 2.186 min, N = 3, p = 0.0008), E15.5 mouse brain (99.67 ± 3.480 min vs 52.33 ± 4.333 min, N = 3, p = 0.0010), and adult zebrafish eyes (48.00 ± 2.160 min vs 30.75 ± 1.797 min, N = 4, p = 0.0009). * P < 0.05, ** P < 0.01, *** P < 0.005. Please click here to view a larger version of this figure.
Figure 5: H and E staining of the whole-series sections of the optic disc segment of the zebrafish eyes acquired from the conventional methods and improved paraffin sectioning by the same operator are compared. (A) Conventional paraffin sectioning (21 sections). (B) Improved paraffin sectioning (24 sections). (C) Dashed area of A6. (D) Dashed area of B7. (E) Dashed area of A17. (F) Dashed area of B19. Asterisks, the sections with relatively poor quality. Arrow heads, optic disc; Arrows, severe tear of retinal tissues. Scale bar = 200 µm (A and B); Scale bar = 50 µm (C, D, E, F). Please click here to view a larger version of this figure.
Supplementary Figure 1: The dimension parameters of the 7 commercial acrylic boards. The number represents the length of corresponding board margin. Units: mm. Please click here to download this file.
Discussion
To improve paraffin section morphology and solve the problem of wasted time during conventional paraffin sectioning, we created an improved paraffin sectioning method that combines cutting and unfolding. This improved method relies on simple equipment that comprises a section channel, a water bath and a heater with a temperature detection switch. The section ribbon enters the water bath through the section channel and unfolds automatically while cutting. Therefore, this method improves paraffin sectioning quality and efficiency, which was verified by paraffin sectioning of the adult mouse hippocampus, adult mouse kidneys, E15.5 mouse brains, and adult zebrafish eyes.
To avoid section curling during conventional paraffin sectioning, many adjustments have been made, such as moistening the tissue surface, cooling the wax block, increasing the humidity, and combining cryosectioning6,7,8; however, these adjustments make paraffin sectioning more tedious. A commercial section transfer system that relies on fluid water has been more successful. In this system, the section contacts the water surface immediately after cutting, and the electric motor-driven water stream transfers the sections from the blade holder to the lower warm water bath9. Due to its mechanism, static electricity does not inhibit the sectioning, resulting in improved section quality. In addition, as this system avoids manually transferring sections from the blade holder to the water bath, easily damaging fragile sections, it also helps in acquiring high-quality thinner sections.
With our improved method, the simple equipment draws on the experience of the commercial section transfer system with some advantages. First, the equipment is cheaper, which could popularize this method in laboratories in developing regions and may complement the commercial section transfer system. Second, the water in the equipment is static and never disturbs the sections, making it possible to acquire thinner sections. We consecutively sectioned zebrafish eyes of 2 µm thickness using the improved method, and the acquired sections were of higher quality. Third, a larger water bath tank is used in the equipment, which permits at least 40 sections to unfold and float simultaneously, allowing two operators to cooperate. One person can cut the tissue specimen continuously and the other can directly fish up the sections. This reduces the time waiting for sections to unfold. Successive cutting with a uniform cutting stroke also improves section quality.
The structure and appearance of our simple equipment requires improvement. For example, the components, such as the power, a heater, and a temperature controller, can be integrated to make this equipment more integral and portable. Additionally, commercial rotary microtomes have different sizes. To connect to other microtomes, the angle and height of this equipment should be adjusted. Paraffin sectioning combined with other molecular techniques is widely used in neurobiology, tissue morphology and other research fields11,12,13. Potentially, this method can improve clinical inspection efficiency14,15 and other biological specimens beyond the three tissue types used here.
Disclosures
The authors have a patent on this device and declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31400936, 31460260) and the Natural Science Foundation of Jiangxi Province of China (20171BAB215020). We also thank the joint program between Nanchang University and Queen Mary University of London for supporting this work.
References
- Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nature Methods. 2013;10(6):508–513. doi: 10.1038/nmeth.2481. [DOI] [PubMed] [Google Scholar]
- Fujita S. Analysis of neuron differentiation in the central nervous system by tritiated thymidine autoradiography. Journal of Comparative Neurology. 1964;122(3):311–327. doi: 10.1002/cne.901220303. [DOI] [PubMed] [Google Scholar]
- Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends in Biotechnology. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Cryosectioning tissues. Cold Spring Harbor Protocols. 2008;3(8) doi: 10.1101/pdb.prot4991. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Luttenberg HP. A modified anti-roll plate as a remedy for the ill-effects of electrical charge during cryosectioning. Journal of Histochemistry and Cytochemistry. 1989;37(7):1157–1160. doi: 10.1177/37.7.2732459. [DOI] [PubMed] [Google Scholar]
- Onozato ML, Hammond S, Merren M, Yagi Y. Evaluation of a completely automated tissue-sectioning machine for paraffin blocks. Journal of Clinical Pathology. 2013;66(2):151–154. doi: 10.1136/jclinpath-2011-200205. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas NA, et al. High-throughput zebrafish histology. Methods. 2006;39(3):246–254. doi: 10.1016/j.ymeth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Chen TK, et al. Hybrid-Cut: An Improved Sectioning Method for Recalcitrant Plant Tissue Samples. Journal of Visualized Experiments. 2016. p. e54754. [DOI] [PMC free article] [PubMed]
- Kucherenko MM, et al. Paraffin-Embedded and Frozen Sections of Drosophila Adult Muscles. Journal of Visualized Experiments. 2010. p. e2438. [DOI] [PMC free article] [PubMed]
- Cornell WC, et al. Paraffin Embedding and Thin Sectioning of Microbial Colony Biofilms for Microscopic Analysis. Journal of Visualized Experiments. 2018. p. e57196. [DOI] [PMC free article] [PubMed]
- Whiteland JL, et al. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. Journal of Histochemistry and Cytochemistry. 1995;43(3):313–320. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- Tucker DK, Foley JF, Bouknight SA, Fenton SE. Sectioning Mammary Gland Whole Mounts for Lesion Identification. Journal of Visualized Experiments. 2017. p. e55796. [DOI] [PMC free article] [PubMed]
- Venegas-Pino DE, Banko N, Khan MI, Shi Y, Werstuck GH. Quantitative Analysis and Characterization of Atherosclerotic Lesions in the Murine Aortic Sinus. Journal of Visualized Experiments. 2013. p. e50933. [DOI] [PMC free article] [PubMed]
- Lau SK, Chu PG, Weiss LM. CD163: A specific marker of macrophages in paraffin-embedded tissue samples. American Journal of Clinical Pathology. 2004;122(5):794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining Protocols for Human Pancreatic Islets. Journal of Visualized Experiments. 2012. p. e4068. [DOI] [PMC free article] [PubMed]


