Abstract
This is a simple protocol for the quantitative determination of phycobiliprotein content in the model cyanobacterium Synechocystis. Phycobiliproteins are the most important components of phycobilisomes, the major light-harvesting antennae in cyanobacteria and several algae taxa. The phycobilisomes of Synechocystis contain two phycobiliproteins: phycocyanin and allophycocyanin. This protocol describes a simple, efficient, and reliable method for the quantitative determination of both phycocyanin and allophycocyanin in this model cyanobacterium. We compared several methods of phycobiliprotein extraction and spectrophotometric quantification. The extraction procedure as described in this protocol was also successfully applied to other cyanobacteria strains such as Cyanothece sp., Synechococcuselongatus, Spirulina sp., Arthrospira sp., and Nostoc sp., as well as to red algae Porphyridium cruentum. However, the extinction coefficients of specific phycobiliproteins from various taxa can differ and it is, therefore, recommended to validate the spectrophotometric quantification method for every single strain individually. The protocol requires little time and can be performed in any standard life science laboratory since it requires only standard equipment.
Keywords: Biochemistry, Issue 139, Phycobilisomes, phycobilins, phycocyanobilins, phycocyanin, allophycocyanin, pigments, light harvesting, protein, cyanobacteria, algae, spectrophotometry, extraction
Introduction
fPhycobiliproteins are water-soluble pigment-protein complexes that represent major components of the light-harvesting antennae in prokaryotic cyanobacteria (Cyanophyta) and several eukaryotic taxa (Glaucophyta, Rhodophyta, and Cryptophyta)1. They occur mainly as supramolecular complexes called phycobilisomes and they are typically attached to the surface of the photosynthetic membranes on the stromal side, with the exception of Cryptophyta, where the phycobiliproteins are localized in the thylakoid lumen2. Four types of phycobiliproteins have been identified up to date: the core allophycocyanin, and the peripheral phycocyanin, phycoerythrin, and phycoerythrocyanin1. As the main light-harvesting complexes, phycobilisomes represent one of the crucial factors of algae and cyanobacteria mass cultures productivity. It has been demonstrated that phycobilisomes truncation can enhance biomass accumulation under strong light3. On the other hand, under modest or low irradiance, the antenna truncation resulted in growth rates and biomass accumulation reduction3,4. Phycobiliproteins are commercially used as food colorants, pharmaceuticals, and food additives, in the cosmetic industry, and as fluorescence probes with applications in flow cytometry, fluorescent immunoassays, and fluorescence microscopy5.
This protocol focuses on the quantitative determination of phycobiliproteins in the model cyanobacterium Synechocystis. Cyanobacteria are the earliest oxygenic photosynthetic autotrophs; they have been forming the Earth's biosphere for more than 2.4 billion years6. They play a crucial role in global biogeochemical cycles of nitrogen, carbon, oxygen, and other elements. Among cyanobacteria, a unicellular strain Synechocystis gained a unique position since it was the first cyanobacterium with the entire genome sequenced7,8, it is naturally transformable by exogenous DNA9, and it performs stable and relatively fast growth10,11. In Synechocystis, the core antenna component, allophycocyanin, is associated with the integral membrane proteins, and the attached phycocyanin is located on the thylakoid membrane periphery.
Several methods for phycobiliprotein extraction and quantification are compared within this protocol. The final extraction procedure was successfully applied to Synechocystis, as well as to other cyanobacteria strains, including Cyanothece sp., Synechococcuselongatus, Spirulina sp., Arthrospira sp., and Nostoc sp., and it was also successfully applied to red algae Porphyridium cruentum. Therefore, the method developed in this protocol can be considered as a universal method for phycobiliprotein extraction. Even though some of the tested extraction methods resulted in higher total protein yields, the here described extraction procedure provided the highest phycobiliprotein yields together with the lowest content of chlorophyll a residue in the phycobiliprotein extract. Reducing the content of chlorophyll a was essential for the correct phycocyanin and allophycocyanin spectrophotometric quantification.
The phycobiliprotein absorption spectra can vary significantly among various algae and cyanobacteria species12,13,14,15,16,17 and even among several strains of a single cyanobacteria genus18. Therefore, the specific wavelengths and absorption coefficients as used for the determination of phycocyanin and allophycocyanin in Synechocystis are not generally applicable to other strains. Additionally, Synechocystis does not contain phycoerythrin and phycoerythrocyanin that can be found in some other algae and cyanobacteria. For the purpose of the determination of phycobiliproteins in strains other than Synechocystis, it is recommended to evaluate the spectrophotometric equations for each strain individually.
Although the protocol contains two longer steps (overnight freeze-drying of the cellular pellets and 1-hour protein extraction), the total labor time for the phycobiliproteins quantification is no longer than 2 hours.
Protocol
1. Cyanobacteria Cultivation
Cultivate Synechocystis cells in Erlenmeyer flasks or in photobioreactors10,19 in buffered BG11 medium20 to maintain a pH of < 10 (e.g., using 17 mM HEPES10). NOTE: Standard cultivation conditions require a controlled temperature (typically, 30 °C, the optimal temperature is 35 °C)21, illumination (typically, a white light of an intensity up to 800 µmol[photons]/[m2·s])21, and a CO2 supply (in the 400-mL flat-panel photobioreactor, the growth saturating CO2 concentration is 1,700 ppm)21.
To determine the culture density, measure the culture optical density spectrophotometrically at 730 nm (OD730) using a cuvette with a light path of 1 cm. Alternatively, count the cells under a microscope using a hemocytometer or an automated cell counter.
2. Samples Preparation
Work under low irradiance to prevent phycobiliprotein degradation.
Harvest 3 x 1 mL of culture suspension to safe-lock tubes. Use triplicates for the estimation of technical errors in the measurement. To perform the culture sampling under sterile conditions, harvest the cells in a laminar hood and follow the proper working and safety practices.
Centrifuge the cells at 15,000 x g at laboratory temperature for 5 min. Pay attention to the properly balanced centrifuge rotor. After centrifugation, discard the supernatants. Be sure not to disturb the pellet.
Put the samples in a freezer. For long-term storage, keep the samples at -80 °C. This is necessary to prevent phycobiliprotein degradation. For short-term storage, -20 °C is sufficient.
Freeze-dry the samples overnight. For a proper freeze-drying process, keep the temperature of the freeze-dryer condenser below -60 °C and the pressure in the freeze-dryer chamber around 1 hPa.
After finishing the freeze-drying cycle, close the tube as soon as possible to prevent the reabsorption of water from the air.
3. Cell Homogenization and Pigments Extraction
Add four pieces of glass beads (with a diameter of 2 mm) to each sample tube and close the tubes. NOTE: When the lid of the safe-lock tube used for homogenization is too thin, it can break during the homogenization; therefore, only safe-lock tubes with strong lids are recommended for this protocol.
Homogenize the samples with the glass beads for 15 s on a homogenizer at laboratory temperature. NOTE: A properly homogenized sample is spread over the whole inner surface of the safe-lock tubes.
Add 1 mL of PBS buffer (pH 7.4), precooled to 4 °C, to the samples in order to extract phycobiliproteins. NOTE: From this step on, keep the samples on ice to prevent the degradation of the extracted proteins. This is critical.
Mix the samples with PBS for 5 s on the homogenizer at laboratory temperature. NOTE: After mixing, the samples are greenish.
After mixing, keep the samples on ice for 60 min. Cover the ice bath with a lid to prevent pigments degradation.
After 60 min of phycobiliprotein extraction, centrifuge the samples at 15,000 x g at 4 °C for 5 min. Pay attention to properly balance the centrifuge rotor. NOTE: After the centrifugation, the supernatant has a cyan blue color.
4. Phycobiliprotein Quantification
Before the spectrophotometric measurement, calibrate the spectrophotometer to the baseline using the PBS buffer as a blank.
Once the spectrophotometer is calibrated, discard the PBS from one spectrophotometer cuvette and pipette the supernatant with extracted phycobiliproteins instead of with the discarded buffer.
- Quantify the phycobiliprotein concentration spectrophotometrically, using a slit width of 0.5 nm.
- Measure the absorbance of the phycocyanobilins in phycocyanin and allophycocyanin against the PBS buffer blank at 615 nm (A615) and 652 nm (A652), respectively, and measure the absorbance of the cellular debris at 720 nm (A720).
- Calculate the concentration of phycocyanin and allophycocyanin according to the equations (1) and (2) of Bennett and Bogorad12:

 NOTE: A615, A652, and A720 should fit to the linear absorbance range of the spectrophotometer. If necessary, dilute the sample with PBS buffer.
NOTE: A615, A652, and A720 should fit to the linear absorbance range of the spectrophotometer. If necessary, dilute the sample with PBS buffer.
Recalculate the pigment concentration in the original samples. The pigment concentration in the sample corresponds directly with the results of equations (1) and (2) when 1 mL of culture and 1 mL of the extraction buffer are used for the analysis. In case of using different volumes of cyanobacteria samples and/or PBS buffer, the final pigment concentration should be calculated according to equation (3):
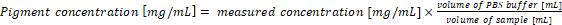 (3) NOTE: In case of a normalizing phycobiliprotein content per cell dry weight, the final pigment concentration should be calculated according to equation (4)
(3) NOTE: In case of a normalizing phycobiliprotein content per cell dry weight, the final pigment concentration should be calculated according to equation (4)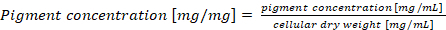 (4)
(4)
5. Determination of the Cell Dry Weight (Optional)
Weigh three empty safe-lock tubes on analytical balances. CAUTION: It is critical that the safe-lock tubes are dry. In case of storing the tubes in a wet environment, dry the tubes for several hours in a freeze dryer before weighing them. Manipulate the tubes gently and only with powder-free gloved hands to avoid any contact between the material and the researcher's fingers. Keep all holders and centrifuge clean to avoid the transfer of any material to the tubes.
Sample 1 x 15 mL of culture suspension to 15-mL conical tube.
Centrifuge the culture suspension in the 15-mL conical tubes at 4,000 x g at laboratory temperature for 10 min. Balance the centrifuge rotor with three additional 15-mL conical tubes, each containing 15 mL of water. After centrifugation, discard 12 mL of the supernatant.
Resuspend the pellet in the remaining supernatant and transfer 3 x 1 mL of the mixture to three 1.5-mL safe-lock tubes with a pipette. In case some leftovers of the pellet remain in the 15-mL conical tube, add 1.5 mL of deionized water to the conical tube, vortex or shake the tube to resuspend the remaining pellet, and transfer 3 x 0.5 mL of the mixture to the three 1.5-mL safe-lock tubes (already containing 3 x 1 mL of the pellet) with a pipette. NOTE: Dividing the pellet over three 1.5-mL safe-lock tubes will allow the estimation of any technical error of the dry weight measurement.
Centrifuge the cells in 1.5-mL safe-lock tubes at 15,000 x g at laboratory temperature for 5 min. Balance the centrifuge rotor with an additional 1.5-mL safe-lock tube that contains 1 mL of water. After the centrifugation, discard the supernatants.
Put the samples in the freezer. For long-term storage, keep the samples at -80 °C; for short-term storing, -20 °C is sufficient.
Freeze-dry the samples overnight. For a proper freeze-drying process, keep the temperature of the freeze dryer condenser below -90 °C and the pressure in the freeze-dryer chamber around 1 hPa.
After freeze-drying, close the tubes and weigh the samples on analytical balances. NOTE: The dry weight value can be used for the normalization of the phycobiliprotein content per cell dry weight.
Representative Results
For the initial method tests, Synechocystis was cultivated as batch cultures in Erlenmeyer flasks on a shaker in BG11 cultivation medium20 (supplemented with 17 mM HEPES) at 25 °C, under a warm white light of an intensity of 50 µmol(photons)/(m2·s) and with 1% CO2 in the culturing atmosphere. During the cultivation, the cultures were sampled to safe-lock tubes and centrifuged (15,000 x g at laboratory temperature for 5 min), the supernatant was discarded, and the samples were either stored at -80 °C and freeze-dried as preparation for the individual steps of this protocol or stored at -20 °C or -80 °C for comparative extraction and quantifications analysis.
The tested extraction procedures included cell disruption by sonication, homogenization with zirconia beads within the PBS buffer, and freeze-thawing. The results of different extraction methods are summarized in Figure 1. The method as described within this protocol provided the highest phycobiliproteins yields together with the lowest chlorophyll a contamination in the extraction buffer. Chlorophyll a was detected in the extraction buffer when sonication was used for the cell disruption. Repeating cycles of freezing and thawing the samples in an ultrasound bath on ice resulted in higher protein yields in the extraction buffer. However, at the same time, the concentration of chlorophyll a in the extraction buffer increased (Figure 1 inset), which excluded a proper phycobiliprotein content quantification.

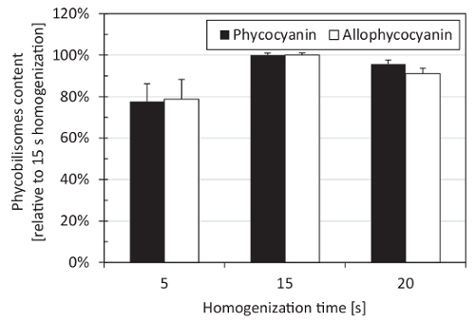
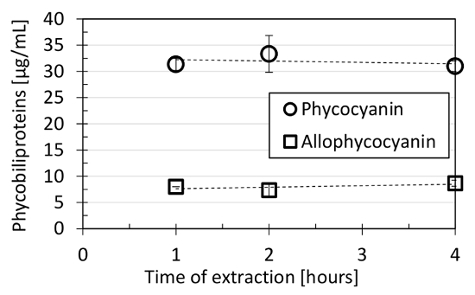
The optimal homogenization time was 15 s. Homogenization for both shorter (5 s) and longer (20 s) periods provided slightly lower phycobiliprotein yields, as shown in Figure 2. Homogenizing the cells with glass beads of a diameter of 2 mm resulted in the highest yields when compared with glass beads of a diameter of 0.5 mm, 1 mm, or 4 mm, with zirconia beads of a 0.5-mm diameter, or with sea sand grains. The PBS extraction buffer was tested as the optimal buffer for phycobiliproteins extraction (Figure 3). The addition of 100 mM NaCl or 150 mM KCl either to the PBS buffer or to water did not increase phycobiliprotein extraction efficiency, and the same went for using 20 mM sodium acetate buffer for extraction (Figure 3). The optimal phycobiliprotein extraction time was 60 minutes because, after more time (up to 240 minutes), the phycobiliprotein concentration in the extraction buffer did not change significantly (Figure 4).

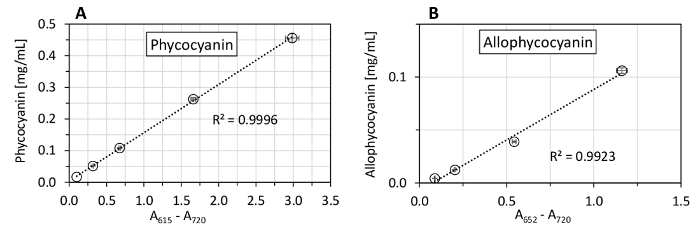
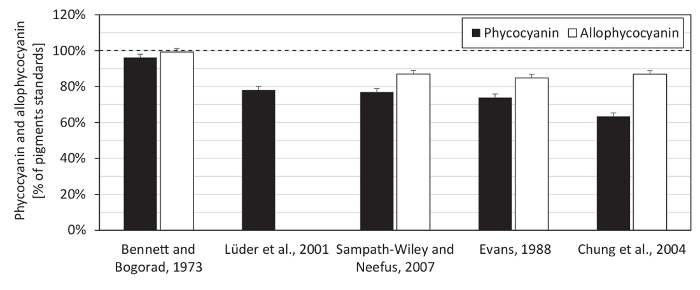
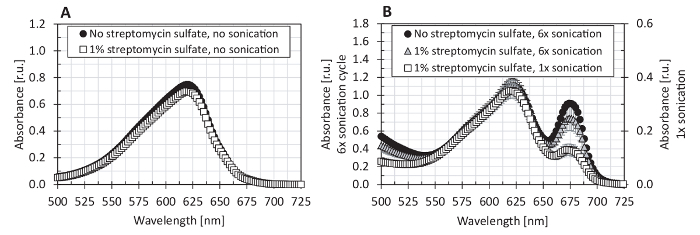
We also tested the spectrophotometer linearity range for the phycobiliprotein measurement (Figure 5) and compared several equations of phycobiliprotein quantification, using both phycocyanin and allophycocyanin standards (Figure 6). The spectrophotometer used in this protocol showed a linear absorbance range between 0.1 - 3.0 (Figure 5). Since the spectra of the protein extract had its maximal absorption around 620 nm (A620), and at 652 nm, the absorption (A652) was only 33% of A620 (Figure 7), the spectrophotometer linearity for A652 (maximum allophycocyanin absorbance) was tested only up to A652 of 1.16. The equations (1) and (2) of Bennett and Bogorad12 provided the best reconstruction of both phycocyanin and allophycocyanin standards among all tested equations (Figure 6). Streptomycin sulfate, used in several previous studies for the elimination of membrane fragments containing chlorophyll a, was shown as not necessary for the extraction (Figure 7).
For the representative experiment, Synechocystis was cultivated in a photobioreactor25 in a turbidostat regime, where the cells were maintained in exponential growth phase within a defined range of optical density (measured at 680 nm, OD680) by a controlled dilution by a fresh cultivation medium (BG11 medium buffered with 17 mM HEPES)11,26. The cultures were cultivated at 32 °C and the input air contained 0.5% CO2. The cultures were illuminated with a red light (λmax: 633 nm, λ1/2: 20 nm) of an intensity of 25 - 1100 µmol(photons)/(m2·s), together with a blue light (λmax: 445 nm, λ1/2: 20 nm) of an intensity of 25 µmol(photons)/(m2·s). The optical density of the culture suspension was measured by the photobioreactor base, and the range of OD680 was set to 0.52 - 0.58 (2 x 107 to 4 x 107 cells/mL). The cultures were cultivated under each specific light intensity for at least 24 hours to provide enough time for acclimation. After reaching growth stability, the cultures were sampled for dry weight (according to steps 2.1 - 2.8 of this protocol), cell count, and phycocyanin and allophycocyanin content. The results of the phycobiliprotein evaluation under increasing light intensities are shown in Figure 8. Synechocystis decreased the phycobiliprotein content from 12% of cellular dry weight (505 fg/cell) under the lowest irradiance to 3% of cellular dry weight (280 fg/cell) under the highest irradiance.
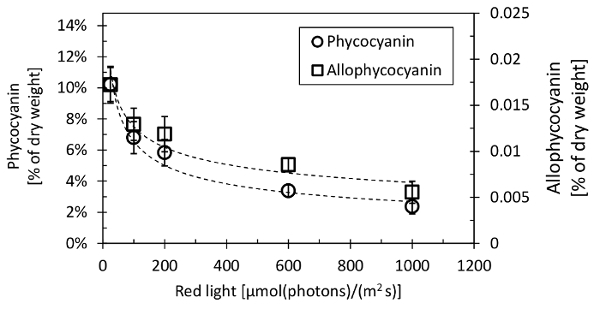
Figure 1: Comparison of the extraction efficiency of the method described in this protocol with several reference methods. The concentration of phycocyanin (black bars) and allophycocyanin (white bars) in the crude protein extract was measured after extraction in the PBS buffer for 60 min. Method A is described within this protocol. Method B was modified as follows: after harvesting, the cells were centrifuged (15,000 x g at laboratory temperature for 5 min) and stored at -20 °C overnight. The frozen samples were sonicated for 120 s at 4 °C, and the phycobiliproteins were extracted in PBS buffer for 60 min. Method C was modified from method B by storing the pellets of harvested cells at -80 °C for 30 min, freeze-drying the cellular pellets, and adding PBS buffer to the dry pellets. Method D was modified from Chung et al.16: after freeze-drying (same as in method C), 0.25 mL of Na-acetate with 1% streptomycin sulfate buffer (pH 5.5) was added to the dry cellular pellet, together with 0.25 mL of zirconium beads (with a diameter of 0.5 mm), and the samples were homogenized twice for 60 s on the homogenizer. After homogenization, another 0.5 mL of Na-acetate with 1% streptomycin sulfate buffer (pH 5.5) was added to the sample, the tubes were vortexed, and the proteins were extracted on ice for 30 min. Method E consisted of a single freezing-thawing cycle in PBS buffer and protein extraction for 24 h at 4 °C. Method F (inset figure) consisted of one and six cycles of freezing the cells (initially, freeze-dried and resuspended in PBS buffer) in liquid nitrogen, followed by sonication on ice. Due to significant chlorophyll a presence in the crude protein extract, the phycobiliproteins were not quantified within method F. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Pigment concentrations were calculated according to equations (1) and (2) of this protocol. Please click here to view a larger version of this figure.
Figure 2: Effect of the homogenization time on the phycobiliprotein extraction efficiency. The lyophilized pellets of Synechocystis cells (according to steps 3.1 - 3.5 of this protocol) were disrupted with glass beads on homogenizer for 5 s, 15 s, and 20 s. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Please click here to view a larger version of this figure.
Figure 3: Phycobiliprotein extraction efficiency in various extraction buffers. The phycobiliproteins were extracted in the PBS buffer, in the PBS buffer with an addition of 100 mM NaCl or 150 mM KCl, in deionized water with 100 mM NaCl or 150 mM KCl, according to Hemlata and Fareha22, and in 20 mM sodium acetate according to Chung et al.16. The extraction was performed at 4 °C according to steps 4.1 - 4.3 of this protocol. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Please click here to view a larger version of this figure.
Figure 4: Phycobiliprotein stability in the extraction buffer in time. After the extraction procedure, performed as described in steps 4.1 - 4.3 of this protocol, the phycobiliprotein extract in PBS buffer (pH 7.4) was stored at 4 °C for up to 4 hours, with no significant phycocyanin (circles) and allophycocyanin (squares) degradation. The dashed line represents the linear fit of the time points calculated by the least squares method. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Please click here to view a larger version of this figure.
Figure 5: Representative measurements of phycocyanin and allophycocyanin concentrations in Synechocystis. A dense culture suspension (phycocyanin: 456 µg/mL, allophycocyanin: 106 µg/mL) was gradually diluted up to a concentration of 19 µg/mL for both phycocyanin and allophycocyanin. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. The dotted line represents the linear fit of the concentration points calculated by the least squares method. Please click here to view a larger version of this figure.
Figure 6: Estimation of phycocyanin and allophycocyanin concentrations in pigments standards. The content of phycocyanin and allophycocyanin in pigment standards was measured spectrophotometrically according to the equations of Bennett and Bogorad12, Lüder et al.13, Sampath-Wiley and Neefus15, Evans14, and Chung et al.16. Protein content in the standards (as necessary for phycobiliproteins determination) was quantified using a solution of bicinchoninic acid, sodium carbonate, sodium tartrate, and sodium bicarbonate in 0.1 N NaOH (with a final pH of 11.25), in reaction with 4% (w/v) copper(II) sulfate pentahydrate27, using bovine serum albumin as a protein standard. The equations of Bennett and Bogorad provided the highest reconstruction of both pigments: 96% of phycocyanin standard and 99% of allophycocyanin standard. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. The dashed line highlights the 100% point. Please click here to view a larger version of this figure.
Figure 7: The effect of streptomycin sulfate on the absorption spectrum of the crude protein extract in the PBS buffer. (A) The extraction procedure as described in steps 4.1 - 4.3 of this protocol or (B) using sonication as described in the legend of Figure 1 for Method F was performed in PBS buffer (circles) and in PBS buffer supplemented with 1% streptomycin sulfate (squares). The phycobiliprotein quantification was performed according to steps 5.1 - 5.4 of this protocol. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Please click here to view a larger version of this figure.
Figure 8: Concentration of phycocyanin (circles) and allophycocyanin (squares) inSynechocystis cultivated under red-orange light of an intensity of 25 - 1100 µmol(photons)/(m2·s). Synechocystis cultures were cultivated in a photobioreactor at 32 °C, with 0.5% CO2 in the input air, in BG11 cultivation medium supplemented with 17 mM HEPES in a turbidostat regime according to Zavrel et al.11. The phycobiliprotein concentrations were measured according to this protocol and the final phycobiliprotein content was normalized per cellular dry weight, as described in section 2 of this protocol. The dashed lines represent the power fit of the measured points, calculated by the least squares method. The depicted values represent averages from three technical replicates, with the error bars representing standard deviations. Please click here to view a larger version of this figure.
Discussion
This protocol describes a simple, fast, and reproducible method for the quantification of phycobiliprotein content in the model cyanobacterium Synechocystis. Several methods of cell homogenization, protein extraction, and phycocyanin and allophycocyanin quantification are compared, and the final protocol represents a combination of the optimal steps of every single procedure. As representative data, the content of phycobiliproteins was quantified in Synechocystis cells under increasing light intensity. Even though the analysis requires similar time and laboratory equipment as some of the previously published methods12,13,14,15,16, the advantage of this protocol is that no chlorophyll a is released into the extraction buffer and, thus, the phycobiliprotein measurement can be estimated with high precision.
The amount of cell suspension required for phycobiliprotein analysis can vary with the culture density. The sample volume of 1 mL is optimized for cultures with an OD730 of 1.5, a cell density of approximately 2 x 107 cells/mL, or for cultures with a phycobiliprotein content of approximately 20 mg/L. In the case of diluted cultures, harvest a larger culture volume. On the other hand, in the case of dense cultures, harvest a lower culture volume-in high-density cultures, there is a risk that phycobiliproteins partially remain in the cells after the extraction. Similarly, the amount of cell suspension required for the dry weight determination can vary with the culture density. The sampling volume of 15 mL is optimized for the cultures with an OD730 of 1.5 or with a cell density of approximately 2 x 107 cells/mL. With lower density cultures or with lower volumes, the measurement error can increase. On the contrary, bigger culture volumes or denser cultures provide better method resolutions.
Critical steps of the protocol are the cell homogenization and phycobiliprotein extraction that should provide both high yields and high specificity. The highest yields and purity of the extracts with simultaneous maximum protein preservation was achieved by freeze-drying the samples, homogenizing the dry cellular pellets by glass beads, and performing the phycobiliprotein extraction in PBS buffer (Figure 1). Since even a small contamination of the crude protein extract by chlorophyll a can lead to a phycobiliprotein content overestimation, it is necessary to avoid any chlorophyll a extraction by adding the PBS buffer. Chlorophyll a was detected in the crude extract when sonication was used for cell disruption. As shown in the inset of Figure 1, with repeating sonication cycles, the amount of chlorophyll a in the extract increased. On the other hand, the amount of extracted proteins (as detected by the absorbance at 280 nm) also increased after repeating sonication cycles. Therefore, the use of sonication (in combination with freezing-thawing cycles in liquid nitrogen) appears as a suitable method for total protein extraction (both free and membrane-bound proteins are released from the cells); however, it is not recommended for the purpose of phycobiliprotein spectrophotometric quantification. Chung et al. recommended the use of 1% streptomycin sulfate in order to eliminate membrane fragments with chlorophyll a16. Here, the use of streptomycin sulfate appeared not to be necessary since it did not lead to a reduction of chlorophyll a in the protein extract (Figure 7). Even though a single sonication cycle for 120 s did not disrupt the cells efficiently (methods B and C, Figure 1), other methods (e.g., method F as presented in Figure 1 or the methods presented in Figure 7B) confirmed previous findings of sonication being an efficient cell disruption method22,28. On the other hand, simple freezing-thawing cycles, also previously reported as an efficient method for phycobiliproteins extraction22,28, provided the lowest yields in the tests described here (method E, Figure 1). Cell homogenization (within the extraction buffer, 2 x 60 s) by zirconium beads was tested as less efficient than the 15-s homogenization of the freeze-dried cellular pellet (method D, Figure 1). The homogenization procedure within the extraction buffer was described previously with a longer homogenization time (10 min)29. We did not test the homogenization for longer than 2 min since the samples heated up significantly during each homogenization cycle. In the literature, other cell disruption methods were also described, including the utilization of a French press16 or griding13,15,30; however, such methods were not tested in this study.
The protein extraction was performed for 15 s (Figure 2) in PBS buffer. The buffer pH was 7.4, which was reported previously as optimal for phycobiliprotein extraction22. The addition of NaCl or KCl did not improve phycobiliprotein extraction efficiency (Figure 3), which is contradicting previous observations22. However, as indicated in Figure 3, the phosphate-buffered saline solution as used in this protocol contained 154 mM NaCl (in addition to 5.6 mM Na2HPO4 and 1 mM KH2PO4), which did not allow us to establish the NaCl-free PBS extraction buffer as a control. We also compared PBS buffer and sodium acetate buffer16 extraction efficiencies. The combination of sodium phosphate and potassium phosphate within the PBS buffer provided higher phycobiliproteins yields (Figure 3). This finding corresponded with the previous findings of Hemlata et al.22.
The phycobiliprotein extraction time was optimized for 1 h. Longer extraction did not lead to significant phycobiliprotein degradation or extraction improvement (Figure 4), which corresponded with the previous work of Lawrenz et al.28. An extraction time shorter than 1 h was not tested. Similarly, extraction for more than 4 h was not tested since it was found previously that the extracted phycobiliproteins in the phosphate buffer are stable for at least 48 h28.
We compared several equations of spectrophotometric phycocyanin and allophycocyanin quantification as described previously in the literature, by recalculating the content of both phycobiliproteins in the commercial standards with known protein concentrations (Figure 6). The best reconstruction of both pigments was achieved with the equations of Bennett and Bogorad12. This result was not specific to the phosphate extraction buffer since such a buffer has been used previously12,13,15. It was neither specific to the pigment standards since the difference between A615, A618, and A620 (wavelengths used for phycocyanin estimation in previous works) in the phycocyanin standard was 1% and the difference between A650 and A652 (wavelengths used previously for allophycocyanin estimation12,13,14,16) in the allophycocyanin standard was 3%. Similarly, the difference between A615 and A620 in the protein extract of Synechocystis was only 2%. Such small differences in the absorption spectra cannot result in a final pigment variation of up to 36% (Figure 6). Therefore, the differences between the individual equations were rather connected to variations in phycobiliprotein extinction coefficients of the specific organisms12,13,14,15,16,17. Interestingly, the equations of Chung et al. provided the lowest phycocyanin reconstruction (Figure 6) although the authors also used Synechocystis for their experiments16.
Instead of the determination of single wavelengths, it is recommended to measure a continuous spectrum of the phycobiliprotein extract between 280 - 720 nm, to estimate the presence of chlorophyll a in the extract, which could interfere with both the allophycocyanin and the phycocyanin absorption. Furthermore, by measuring the absorbance at 280 nm (A280), a purity of both phycocyanin (A615/A280 or A620/A280)21,22 and allophycocyanin (A652/A280)24 within the extract can be estimated (0.7: food grade, 3.9: reactive grade, > 4.0: analytical grade)21.
As representative data, the concentration of both phycocyanin and allophycocyanin in Synechocystis was determined under increasing light intensity. Maintaining the culture density at a constant level within a turbidostat cultivation regime11,21 was essential for the direct comparative experiment. The phycocyanin and allophycocyanin concentrations of 2.4% - 10.2% and 0.6% - 1.7% of cellular dry weight, respectively, were similar as the previously reported values11,31,32. Also, the phycobiliprotein content per cell basis was similar as reported previously11,33. With the increasing light, the phycobiliproteins content decreased. Interestingly, even under the highest light intensity of 1100 µmol(photons)/(m2·s), the phycobiliproteins were not completely bleached.
Since the protocol requires only standard equipment and is relatively fast, it can be easily adopted in any laboratory in which phycobiliproteins are routinely analyzed.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The protocol was adopted from a previous publication11. T. Z., D. Ch., and J. Č. were supported by the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program I (NPU I), grant number LO1415. J. Č. was also supported by GA CR, Grant number 18-24397S. Access to instruments and other facilities was supported by the Czech research infrastructure for systems biology C4SYS (project no LM2015055). M. A. S. was supported by a grant from the Russian Science Foundation [no. 14-14-00904].
References
- Mimuro M, Kikuchi H. Antenna Systems and Energy Transfer in Cyanophyta and Rhodophyta. In: Green BR, Parson WW, editors. Light-Harvesting Antennas in Photosynthesis. Dordrecht, The Netherlands: Springer; 2003. pp. 281–306. [Google Scholar]
- Spear-bernstein L, Miller KR. Unique location of the phycobiliprotein light-harvesting pigment in the Cryptophyceae. Journal of Phycology. 1989;25(3):412–419. [Google Scholar]
- Kirst H, Formighieri C, Melis A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochimica et Biophysica Acta - Bioenergetics. 2014;1837(10):1653–1664. doi: 10.1016/j.bbabio.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Page LE, Liberton M, Pakrasi HB. Reduction of photoautotrophic productivity in the cyanobacterium Synechocystis sp. strain PCC 6803 by phycobilisome antenna truncation. Applied and Environmental Microbiology. 2012;78(17):6349–6351. doi: 10.1128/AEM.00499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonani RR. Recent advances in production, purification and applications of phycobiliproteins. World Journal of Biological Chemistry. 2016;7(1):100. doi: 10.4331/wjbc.v7.i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DA. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Springer Netherlands; 1994. [Google Scholar]
- Kaneko T, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Research. 1995;2:191–198. doi: 10.1093/dnares/2.4.153. [DOI] [PubMed] [Google Scholar]
- Kaneko T, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Research. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Grigorieva G, Shestakov S. Transformation in the cyanobacterium Synechocystis sp 6803. FEMS Microbiology Letters. 1982;13(4):367–370. [Google Scholar]
- Zavřel T, Sinetova MA, Búzová D, Literáková P, Červený J. Characterization of a model cyanobacterium Synechocystis sp: PCC 6803 autotrophic growth in a flat-panel photobioreactor. Engineering in Life Sciences. 2015;15(1) [Google Scholar]
- Zavřel T, Očenášová P, Červený J. Phenotypic characterization of Synechocystis sp. PCC 6803 substrains reveals differences in sensitivity to abiotic stress. PLoS One. 2017;12(12):e0189130. doi: 10.1371/journal.pone.0189130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A, Bogorad L. Complementary chromatic adaption in a filamentous blue-green alga. The Journal of Cell Biology. 1973;58:419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüder UH, Knoetzel J, Wiencke C. Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic antarctic red macroalga Palmaria decipiens. Polar Biology. 2001;24(8):598–603. [Google Scholar]
- Evans LV. The effects of spectral composition and irradiance level on pigment levels in seaweeds. In: Lobban CS, Chapman DJ, Kremer BP, editors. Experimental Phycology: A Laboratory Manual. Cambridge, New York, New Rochelle, Melbourne, Sydney: Cambridge University Press; 1988. pp. 123–133. [Google Scholar]
- Sampath-Wiley P, Neefus CD. An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta) Journal of Applied Phycology. 2007;19(2):123–129. doi: 10.1007/s10811-006-9118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Park YM, Moon YJ, Lee EM, Choi JS. Photokinesis of Cyanobacterium Synechocystis sp. PCC 6803. Journal of Photoscience. 2004;11(3):89–94. [Google Scholar]
- Sun L, et al. Phycobilisomes from Cyanobacteria. In: Gault PM, Marler HJ, editors. Handbook on Cyanobacteria: Biochemistry, Biotechnology and Applications. New York, NY: Nova Science Publishers, Inc; 2009. pp. 105–160. [Google Scholar]
- Six C, et al. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: A comparative genomics study. Genome Biology. 2007;8(12) doi: 10.1186/gb-2007-8-12-r259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinetova MA, Červený J, Zavřel T, Nedbal L. On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142. Journal of Biotechnology. 2012;162(1) doi: 10.1016/j.jbiotec.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriological Reviews. 1971;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavřel T, Sinetova MA, Búzová D, Literáková P, Červený J. Characterization of a model cyanobacterium Synechocystis sp. PCC 6803 autotrophic growth in a flat-panel photobioreactor. Engineering in Life Sciences. 2015;15(1):122–132. [Google Scholar]
- Hemlata G, Fareha B. Studies on Anabaena sp. nccu-9 with special reference to phycocyanin. Journal of Algal Biomass Utilization. 2011;2(1):30–51. [Google Scholar]
- Rito-Palomares M, Nuez L, Amador D. Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. Journal of Chemical Technology & Biotechnology. 2001;76(12):1273–1280. [Google Scholar]
- Zhang H, et al. Selenium-Containing Allophycocyanin Purified from Selenium-Enriched Spirulina platensis Attenuates AAPH-Induced Oxidative Stress in Human Erythrocytes through Inhibition of ROS Generation. Journal of Agricultural and Food Chemistry. 2011;59(16):8683–8690. doi: 10.1021/jf2019769. [DOI] [PubMed] [Google Scholar]
- Nedbal L, Trtílek M, Cervený J, Komárek O, Pakrasi HB. A photobioreactor system for precision cultivation of photoautotrophic microorganisms and for high-content analysis of suspension dynamics. Biotechnology and Bioengineering. 2008;100(5):902–910. doi: 10.1002/bit.21833. [DOI] [PubMed] [Google Scholar]
- Zavřel T, Knoop H, Steuer R, Jones PR, Červený J, Trtílek M. A quantitative evaluation of ethylene production in the recombinant cyanobacterium Synechocystis sp. PCC 6803 harboring the ethylene-forming enzyme by membrane inlet mass spectrometry. Bioresource Technology. 2016;202:142–151. doi: 10.1016/j.biortech.2015.11.062. [DOI] [PubMed] [Google Scholar]
- Smith PK, et al. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Lawrenz E, Fedewa EJ, Richardson TL. Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. Journal of Applied Phycology. 2011;23(5):865–871. [Google Scholar]
- Lea-Smith DJ, et al. Phycobilisome-Deficient Strains of Synechocystis sp. PCC 6803 Have Reduced Size and Require Carbon-Limiting Conditions to Exhibit Enhanced Productivity. Plant Physiology. 2014;165(2):705–714. doi: 10.1104/pp.114.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YC, et al. Stable isolation of phycocyanin from Spirulina platensis associated with high-pressure extraction process. International Journal of Molecular Sciences. 2013;14(1):1778–1787. doi: 10.3390/ijms14011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloupakis E, Cicchi B, Torzillo G. A bioenergetic assessment of photosynthetic growth of Synechocystis sp. PCC 6803 in continuous cultures. Biotechnology for Biofuels. 2015;8(1):133. doi: 10.1186/s13068-015-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloupakis E, Cicchi B, Benavides AMS, Torzillo G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.) Applied Microbiology and Biotechnology. 2016;100(3):1333–1341. doi: 10.1007/s00253-015-7024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Hihara Y. An AbrB-Like Transcriptional Regulator, Sll0822, Is Essential for the Activation of Nitrogen-Regulated Genes in Synechocystis sp. PCC 6803. Plant Physiology. 2008;148(1):660–670. doi: 10.1104/pp.108.123505. [DOI] [PMC free article] [PubMed] [Google Scholar]


