Abstract
Vascular leak, or plasma extravasation, has a number of causes, and may be a serious consequence or symptom of an inflammatory response. This study may ultimately lead to new knowledge concerning the causes of or new ways to inhibit or treat plasma extravasation. It is important that researchers have the proper tools, including the best methods available, for studying plasma extravasation. In this article, we describe a protocol, using the Evans blue dye method, for assessing plasma extravasation in the organs of FVBN mice. This protocol is intentionally simple, to as great a degree as possible, but provides high quality data. Evans blue dye has been chosen primarily because it is easy for the average laboratory to use. We have used this protocol to provide evidence and support for the hypothesis that the enzyme neprilysin may protect the vasculature against plasma extravasation. However, this protocol may be experimentally used and easily adapted for use in other strains of mice or in other species, in many different organs or tissues, for studies which may involve other factors that are important in understanding, preventing, or treating plasma extravasation. This protocol has been extensively optimized and modified from existing protocols, and combines reliability, ease of use, economy, and general availability of materials and equipment, making this protocol superior for the average laboratory to use in quantifying plasma extravasation from organs.
Keywords: Medicine, Issue 139, Vascular leak, plasma extravasation, Evans blue, substance P, urinary bladder, duodenum, neprilysin
Introduction
Vascular leak in the organs refers to extravasation, or leakage of blood plasma through gaps produced in the endothelium of post capillary venules in the organs. This plasma extravasation or increased vascular permeability, which may arise from some type of an inflammatory response, may have grave consequences. Thus, it is important that this phenomenon, its causes, modulators, and consequences, are studied and understood, and likewise, that investigators have good tools and protocols with which to study them. The endothelial gaps may be produced via a number of stimuli, but usually are produced by the action of peptide neurotransmitters and/or tachykinins on the endothelia. One of the major naturally occurring mediators of this process, which results in increased plasma extravasation, is the undecapeptide tachykinin neuropeptide, substance P1.
Methods to investigate and measure vascular permeability or plasma extravasation, which use the albumin-binding property of Evans blue dye, have been developed, and are usually known for their accuracy, simplicity, economy, safety, and ability to allow the determination of plasma extravasation from several tissues at once, if so desired2,3,4,5,6,7,8,9. This Evans blue protocol for assessing plasma extravasation in the organs of FVBN mice uses all these, but adds some important modifications that make it generally useful and adaptable for future studies, involving the average laboratory that conducts or will conduct important studies of factors associated with plasma extravasation or vascular permeability. In this protocol, substance P is introduced to the mice at 1 nmol/kg, which augments the extravasation of plasma by 1.5-fold. This increases the sensitivity of the protocol, resulting in more easily observable and obtainable results. Other factors that impact permeability, such as various other peptides, chemicals, or some forms of toxic injury, may be used or studied by other laboratories, as desired. Jugular vein injections are used in this protocol to introduce Evans blue and substance P systemically, which requires terminal surgery. However, jugular vein injections5,7,10, even after consideration of the necessary terminal surgical techniques, are easier to master and lead to the production of more consistent results than other venous injections, including tail vein injections4,9. Although it may be possible for Evans blue to be delivered by retro-orbital venous sinus injections, no references in the literature have been found that use this method of delivery of Evans blue. However, as for tail vein injections, the high degree of expertise and practice to reproducibly master this technique greatly limits its use for successful Evans blue injections. In contrast, the alternative jugular vein injection method as described in our protocol, offers a technically obtainable solution. A crucial procedure for perfusion of the mouse's veins, performed just after the sacrifice of the Evans blue-perfused mouse, removes excess Evans blue dye, and has been standardized in this protocol. Previously described methods of perfusion have been carefully examined and modified to obtain the present procedure.Other modifications described here are all optimized, straightforward, and inexpensive.
There are some important limitations of the Evans blue dye method. For example, low sensitivity sometimes associated with this method may prevent some additional gross pathological and histological examination of tissues from Evans blue-injected animals. However, these and other limitations have led to the development of alternative methods and models that, nonetheless, still use Evans blue. The measurement of Evans blue by fluorescence (rather than by visual-range) spectroscopy may increase the sensitivity of the method. Additionally, fluorescence microscopy of Evans blue-stained tissues was developed to allow for observation of vascular leak in more distinct locations11. Also, whole-body imaging and scanning of a live animal previously injected with Evans blue12 allows for investigation of Evans blue concentrations in a continuous manner, rather than at one specific chosen time point of the experiment. However, this method requires the availability of appropriate imaging facilities, and may be very expensive. Modifications involving Evans blue and performed in an in vitro type of model, such as in a cell culture or chick chorioallantoic model13 (CAM) have also been described. These models are monitored by fluorescence and intravital14 microscopy, and allow the quantification of vascular permeability changes over time, but may raise questions regarding accurate modeling of in vivo conditions and may also be expensive.
There have been other methods developed to determine and quantify vascular leak or permeability, which do not involve the administration of Evans blue. These methods may employ an appropriate fluorescent molecule (such as albumin or fluorescein), or an isotopically labeled or otherwise tagged molecule, to live animals (or to cell culture or chorioallantoic (CAM) models13, followed by non-invasive imaging (PET scanning, MRI, intravital microscopy, whole body scanning) or by invasive imaging (fluorescent microscopy)3,12,15. Although these techniques may offer a number of advantages over other Evans blue methods, they also have disadvantages, which may include their considerable complexities, requisite expertise, resources, and high monetary costs.
Neprilysin16 (the peptidase enzyme NEP, also known as CD10, MME, or Enkephalinase) has been suggested to be involved in inhibiting plasma extravasation, at least in part, through the enzymatic metabolism and inactivation of endogenous substance P. Thus, in tissues in which the cell surface peptidase NEP occurs, there may be an attenuation of the effect of substance P, presumably by the peptidase activity of NEP.
Initially, we tested for substance P-induced plasma extravasation utilizing this modified Evans blue protocol, with FVBN wild type (WT) and NEP knockout (KO) mice. NEP involvement in substance P-augmented plasma extravasation was suspected from these initial studies, and we describe these and further experiments involving NEP's role in plasma extravasation. However, the focus of this manuscript is not NEP or its role in plasma extravasation, but rather the plasma extravasation experiments themselves. The NEP results are representative of the kind of results that may be obtained through use of this modified protocol. The Evans blue method to measure plasma extravasation has been optimized and modified, as described in detail below for FVBN mice.
Protocol
All applicable international, national, and/or institutional guidelines for the care and use of animals (mice) were followed in the experiments described in this manuscript.
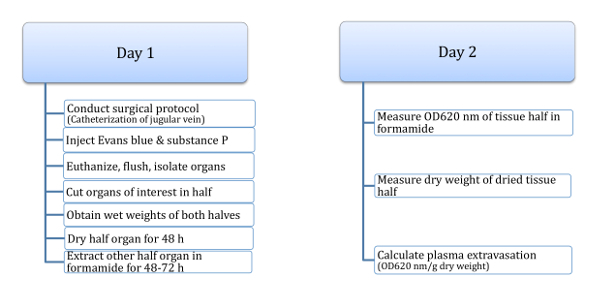
This method uses FVBN adult mice, aged 16 - 20 weeks, found to be optimal for the purposes of this study. Day 1 includes steps 1 - 5 and Day 2 includes steps 6 - 7 (Figure 1).
1. Equipment Preparation
Secure an adequate supply of sterile, disposable syringes and needles, if ketamine/xylazine is used as the anesthetic (as recommended). If isoflurane is used as the anesthetic, check the oxygen tank and the fluid level of isoflurane to make sure there are adequate supplies for the experiment before starting. Also, assemble the nosecone breathing circuits and attach them to the induction box; attach new charcoal canisters to the breathing circuits. Prepare the induction box by turning on the oxygen and ascertaining that the second stage reads approximately 50 psi.
Turn on the heating pad to 37 °C.
Prepare the rectal temperature probe for the surgery.
2. Mouse Preparation
This step includes anesthesia, hair removal, and positioning (adult FVBN mice-age 16 - 20 weeks).
Weigh the mice and record the weights.
- Anesthetize the mice.
- Administer ketamine and xylazine IP (80 - 100 mg/kg and 7.5 - 16 mg/kg, respectively). However, it is recommended to begin with lower doses of ketamine and xylazine (about 30 mg/kg and 6 mg/kg, respectively).
- Maintain the anesthesia with about 0.1 - 0.25 times initial doses of ketamine/xylazine throughout the surgery. Ketamine and xylazine were the anesthetic agent(s) of choice in this particular study, as more reproducibility and survivability were observed. The anesthetic agent(s) of choice in other studies may be found to be different. If isoflurane is used, put the mouse in an induction chamber and turn on isoflurane to 5% until the mouse loses full consciousness. Then use a nasal nosecone set at 1.5 - 2.5% isoflurane throughout the remainder of the procedure.
Monitor the mice every 2 - 3 min by toe pinch to check for appropriate depth of anesthesia.
Shave the ventral neck area of the mouse.
Place the mouse in a supine position on the preheated pad. Secure the paws and feet of the mouse to the surgical surface with tape.
Place artificial tear ointment onto the eyes to prevent drying out during surgery.
3. Surgical Details
Make a 1 cm incision in the right ventral neck over the jugular vein.
Apply one or two drops of lidocaine (1 - 2%) into the incision area for pain management and to promote vasodilation. Wait 2 min. for the lidocaine to take effect.
Expose and isolate the right internal jugular vein via blunt dissection. Tie the vein off with 4-0 suture and gently retract the rostral end of the vessel with a hemostat. Cut a hole, using fine scissors, about 3 mm below or caudal to the tie, approximately halfway through the diameter of the vein.
Mark a PV-1 polyvinyl catheter 1.5 cm from the end and insert it into the jugular vein through the hole using a vessel dilator, and thread the catheter towards the caudal end of the vessel, approximately 1.5 cm.
Tie the catheter securely within the caudal part of the vessel (below the cut), with 4-0 silk. Tie the rostral part of the vessel to the outside of the catheter with the loose ends of the suture which was used to tie off the rostral jugular vein, in step 3.3.
Tack the skin loosely back together around the catheter with 4-0 silk to help prevent loss of body heat and desiccation of tissues.
Connect the catheter to a syringe containing heparinized saline (10 U/mL) and flush the catheter.
4. Injections
Inject the Evans blue solution (50 μL of a 30 mg/mL solution in 0.9% normal, unheparinized saline, or approximately 50 mg/kg) into the jugular vein catheter, followed by a small volume of heparinized saline to flush the line.
2 min later, inject substance P (100 μL of a 0.3 μM solution in 0.9% normal, unheparinized saline, or 1 nmol/kg), followed by a small volume of heparinized saline to flush the line. Substance P augments the extravasation of plasma proteins through the endothelial layer; in this protocol, it routinely induces an augmentation of plasma extravasation values of approximately 1.5-fold, making plasma extravasation values easier to measure.
Wait 18 min after the substance P is injected. During this time, the Evans blue dye will equilibrate and circulate.
Terminate the experiment 18 min after the injection of substance P (20 min after Evans blue injection) by sacrificing the mouse with cervical dislocation. It is likely that the mouse can be directly cervically dislocated, without first giving an overdose of anesthetic, as the mouse is probably still well-anesthetized from the surgery. However, if it is necessary, an overdose of the anesthetics ketamine/xylazine, isoflurane, or pentobarbital may be given, followed by cervical dislocation.
5. Isolation of Organs
Cut open the chest cavity of the mouse and gravity-perfuse (from a height of about 51 cm or 20") the heart and blood vessels with 50 mL of 50 mM sodium citrate, pH 3.5. pH 3.5 presumably preserves Evans blue binding to albumin.
Excise 1 - 5 relevant organs (tissues) with a dissecting scalpel (e.g. urinary bladder, kidney, stomach, liver, pancreas, proximal or distal colon, ileum, duodenum, flank skin, ears, tail, heart, and/or lungs) and remove any residual contents, if present.
Rinse the organs in room temperature (RT) phosphate-buffered saline (PBS; 1.44 g of Na2HPO4, 0.24 g of KH2PO4, 8.0 g of NaCl, and 0.20 g of KCl in 1 L, pH 7.4).
Blot the organs with tissue, cut each organ in half, and weigh each half (wet weights, in g).
Dry one half of the tissue in a drying oven at 150 °C, on foil, for 48 h.
Place the other tissue half in a consistent volume (up to 200 µL) of formamide in a microfuge tube for 48 h (and up to 72 h) to extract the Evans blue.
6. Measurement of Tissue OD
Remove 50 µL of Evans blue-infused formamide (after 48 - 72 h RT incubation) from the microfuge tube and place into one well of a 96-well polystyrene plate. Be careful not to transfer tissue pieces along with the formamide.
Place 50 µL of new, pure formamide into each of two empty wells of the 96-well plate for the blanks.
Measure and record the OD620 of each well of the 96-well plate on an absorbance plate reader. 620 nm is the absorbance max of Evans blue.
7. Calculation of Plasma Extravasation
Weigh the dry tissue half which has been in the oven for 48 h.
Calculate the wet weight/dry weight ratio for the specific organ of interest from each individual mouse, starting with the wet weight of this tissue half (obtained in step 5.4), divided by the dry weight of this same tissue half (obtained in step 7.1).
Calculate the dry weight (in g) of the tissue half in formamide by dividing the wet weight of the tissue half before it was placed in formamide (obtained in step 5.4) by the wet: dry weight ratio for the specific organ of interest (calculated in step 7.2).
Calculate the corrected OD620 values. Starting with the OD620 value from each experimental well of the 96-well plate (containing Evans blue-infused formamide from each tissue, obtained in step 6.3), subtract the blank well OD620 value (the mean OD620 value of the two wells containing pure formamide, prepared in step 6.2, OD620 values obtained in step 6.3) from each experimental value.
Calculate the plasma extravasation value by dividing the corrected OD620 (calculated in step 7.4) by the dry weight of the tissue half in formamide (calculated in step 7.3). The units of plasma extravasation will be OD620/g dry weight.
Analyze data and express as the mean ± SEM. Statistically compare groups by t test or one-way analysis of variance and Scheffé's multiple-comparison test (lycofs01.lycoming.edu), as appropriate.
Representative Results
In Figure 1, a schematic of the procedure is shown, which has been found to result in the most reliable and consistent substance P-induced plasma extravasation values from organs of FVBN mice. This procedure usually takes two days of work, separated by at least 48 h of waiting time. It is possible to spread it out even more, if this is done consistently for all experiments to be compared. For example, after the organs are isolated on Day 1, the organs can be flash frozen in liquid nitrogen and stored at -80 °C; upon carefully thawing the organs (within a couple days to 1 week), they can be washed in PBS and blotted before the rest of the protocol. Also, although the drying of tissues should be done as stated (at 150 °C for 48 h), the extraction of Evans blue of the tissue half in formamide may be extended from 48 - 72 h, as long as the time in formamide is consistent for all experiments.
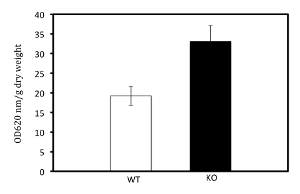
Shown in Figure 2 and Table 1 is data demonstrating substance P-induced plasma extravasation from the urinary bladders of FVBN wild type (WT) and NEP knockout (KO) mice17, which were available primarily due to this laboratory's long-standing interests in NEP and the regulation and actions of selected neuropeptides18,19. NEP KO mice do not express NEP in any tissue. However, NEP is expressed within the urinary bladder of WT mice, albeit at low levels20,21,22. Figure 2 and Table 1 suggest there is a difference in substance P-induced plasma extravasation from the urinary bladders from these two groups of mice. This implies a role for NEP in substance P-induced plasma extravasation from the urinary bladder, since the expression of NEP is potentially the main difference in the urinary bladders of WT and NEP KO mice.
Because the original data suggested a possible role for NEP in substance P-induced plasma extravasation in the urinary bladder (Figure 2 and Table 1), we decided to more fully investigate the involvement of NEP in plasma extravasation in another organ. First, we created a doubly transgenic mouse to overexpress NEP nearly universally23. This engineered mouse allowed a comparison of data from three types of FVBN mice: WT mice16, which naturally express a limited amount of NEP in many tissues; NEP KO mice17, which express no NEP in any tissue; and our doubly transgenic NEP overexpressor mice23, which overexpress NEP in nearly all tissues upon administration of doxycycline. Mice are allowed free access to doxycycline-containing chow for 1 week prior to surgery (Figure 1); doxycycline dose is 1 mg/kg of chow, undyed. The construction of these doubly transgenic mice that overexpress NEP has been detailed, with description of NEP assays, westerns, and NEP mRNA qPCR23.
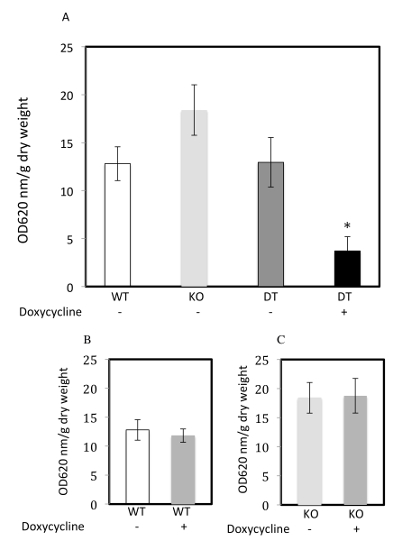
With these 3 types of mice, this protocol shows a role for NEP in plasma extravasation in tissues including the duodenum23 (Figure 3 and Table 2). In these experiments, the NEP overexpression in the doubly transgenic NEP overexpressor mice was turned on by feeding, for 1 week prior to day 1, chow containing doxycycline (undyed; 1 mg doxycycline/mg chow, Figure 3a). As a control, some WT and NEP KO mice were also fed the undyed doxycycline chow during the week preceding Day 1 (Figure 3b and 3c).
To confirm the role of NEP in plasma extravasation to in the duodenum of FVBN mice, the expression of NEP in the duodenum of FVBN WT and NEP (doubly transgenic) overexpressor mice fed doxycycline chow was confirmed by Western analysis with a polyclonal antibody against human NEP (which cross-reacts with mouse NEP)23. This analysis confirmed that WT duodenums express moderate amounts of the NEP protein, but the NEP overexpressor duodenums express 2 - 3x the amounts of NEP expressed by the WT duodenums. The NEP KO mice, by definition, do not express any NEP in the duodenum.
Figure 1: General schema of protocol for measuring substance P-induced plasma extravasation. Schematic of the protocol for measuring substance P-induced plasma extravasation from the organs of FVBN mice (age 16 - 20 weeks). This protocol involves the measurement of tissue plasma extravasation by the Evans blue dye method through injection into the jugular vein, and incorporates exogenously administered substance P, to obtain augmented and easily measured plasma extravasation values, in units of OD620/g dry weight. The described protocol has been found to result in consistent substance P-induced plasma extravasation values from various organs of FVBN mice, and takes a minimum of two days of work. Day 1 and day 2 are separated by at least 48 h of time waiting for tissues to dry and extract. Please click here to view a larger version of this figure.
Figure 2: Substance P-induced plasma extravasation from the urinary bladder of WT and NEP KO mice. Urinary bladder plasma extravasation from FVBN mice (without prior doxycycline) augmented by substance P administration and expressed as OD620 nm/g dry weight. Urinary bladders were isolated from FVBN wild type (WT; white bar, n = 12) or FVBN NEP knockout (KO; black bar, n = 7) mice and subjected to the protocol for plasma extravasation as detailed above. Individual values are given in Table 1. Lines above the bars represent the standard error of the mean (SEM). The WT and NEP KO values trended toward a significant difference p = 0.051 by t test. Please click here to view a larger version of this figure.
Figure 3: Substance P-induced plasma extravasation from the duodenum of WT, NEP KO, and NEP overexpressor mice. (A-C) Duodenal plasma extravasation augmented by substance P administration and expressed as OD620/g dry weight. (A) Duodenums were isolated from FVBN WT mice without prior doxycycline (-; white bar, n = 5), NEP KO mice without prior doxycycline (-; light gray bar, n = 5), NEP ovexpressor doubly transgenic mice without prior doxycycline (DT -; dark gray bar, n = 5) or NEP ovexpressor doubly transgenic mice with prior doxycycline (DT +; black bar, n = 4) and subjected to the protocol for plasma extravasation as detailed above. Lines above the bars represent the SEM. Although the NEP KO (-) values trended higher than the WT (-) or the DT (-) values, this difference was not statistically significant. Duodenal plasma extravasation values were decreased significantly only with the group of doxycycline fed, NEP ovexpressor doubly transgenic mice (DT+), compared to all others (*p <0.05, analysis of variance). (B,C) Duodenal plasma extravasation values from WT and NEP KO mice were not significantly affected by doxycycline administration (p <0.05). (B) WT mice without doxycycline (WT -; white bar, n = 5) and WT mice with doxycycline (WT +; medium gray bar, n = 7). (C) NEP KO mice without doxycycline (KO -; light gray bar, n = 5) and NEP KO with doxycycline (KO +; medium gray bar, n = 2). Individual values shown as a composite in A, B, and C, are given in Table 2. Reproduced with minor modification from Springer journal article23 with permission from Springer Science + Business Media. Please click here to view a larger version of this figure.
| Genotype | sex | wet/dry weight ratio of urinary bladder | wet weight of urinary bladder half (g; in 85 μL formamide) | calculated dry weight of urinary bladder half (g) | OD 620 nm - blank | OD 620 nm/g dry weight (urinary bladder) |
| WT | F | 4.737 | 0.0038 | 0.0008 | 0.0150 | 18.750 |
| WT | M | 4.632 | 0.0045 | 0.0010 | 0.0100 | 10.000 |
| WT | F | 4.700 | 0.0048 | 0.0010 | 0.0280 | 28.000 |
| WT | F | 4.625 | 0.0068 | 0.0015 | 0.0230 | 15.333 |
| WT | F | 4.546 | 0.0077 | 0.0017 | 0.0400 | 23.529 |
| WT | M | 4.800 | 0.0094 | 0.0020 | 0.0570 | 28.500 |
| WT | M | 5.500 | 0.0095 | 0.0017 | 0.0240 | 14.118 |
| WT | F | 4.316 | 0.0098 | 0.0023 | 0.0660 | 28.696 |
| WT | M | 5.061 | 0.0121 | 0.0024 | 0.0400 | 16.667 |
| WT | M | 5.061 | 0.0192 | 0.0038 | 0.0330 | 8.684 |
| Mean +/- SEM (of entries in column above) | 4.798 +/- 0.105 | 0.0088 +/- 0.0014 | 0.0018 +/- 0.0003 | 0.0336 +/- 0.0056 | 19.228 +/- 2.393 | |
| KO | M | 4.316 | 0.0196 | 0.0045 | 0.1610 | 35.778 |
| KO | M | 4.600 | 0.0134 | 0.0029 | 0.1020 | 35.172 |
| KO | M | 4.377 | 0.0104 | 0.0024 | 0.0410 | 17.083 |
| KO | M | 4.727 | 0.0258 | 0.0055 | 0.1410 | 25.636 |
| KO | F | 4.539 | 0.0025 | 0.0006 | 0.0250 | 41.667 |
| KO | M | 4.457 | 0.0149 | 0.0033 | 0.1420 | 43.030 |
| Mean +/- SEM (of entries in column above) | 4.503 +/- 0.062 | 0.0144 +/- 0.0032 | 0.0032 +/- 0.0007 | 0.1020 +/- 0.0233 | 33.061 +/- 4.065 |
Table 1: Substance P-induced plasma extravasation from the urinary bladder of individual WT and NEP KO mice. Plasma extravasation values from FVBN mice (without prior doxycycline) were individually expressed as OD620 nm/g dry weight, from FVBN WT (n = 12) or NEP KO (n = 7) mice and subjected to the protocol for plasma extravasation as detailed above. No gender differences were evident in the plasma extravasation values. Urinary bladders were isolated; individual values are given for the calculated wet/dry weight ratio of urinary bladder, the wet weight of the urinary bladder half in formamide, the calculated dry weight of the urinary bladder half in formamide (given in g), the corrected OD620 nm (sample OD620 nm minus the meanOD620 nmof the blanks (pure formamide), and the calculated plasma extravasation value in OD620 nm/g dry weight (the corrected OD620 nm, divided by the calculated dry weight). The resultant plasma extravasation values were used to construct Figure 2.
| Genotype and doxycycline chow, without (-) or with (+) | sex | wet/dry weight ratio of duodenum | wet weight of duodenum half (g; in 85 μL formamide) | calculated dry weight of duodenum half (g) | OD 620 nm - blank | OD620 nm/g dry weight (duodenum) |
| WT - | M | 4.927 | 0.0307 | 0.0062 | 0.0640 | 10.272 |
| WT - | F | 4.912 | 0.0172 | 0.0035 | 0.0460 | 13.138 |
| WT - | M | 5.333 | 0.0281 | 0.0053 | 0.0960 | 18.221 |
| WT - | F | 5.433 | 0.0375 | 0.0069 | 0.1010 | 14.634 |
| WT - | M | 5.179 | 0.0287 | 0.0055 | 0.0430 | 7.759 |
| Mean +/- SEM (of entries in column above) | 5.157 +/- 0.105 | 0.0284 +/- 0.0033 | 0.0055 +/- 0.0006 | 0.0700 +/- 0.0122 | 12.805 +/- 1.798 | |
| WT + | M | 5.342 | 0.0542 | 0.0101 | 0.1520 | 14.981 |
| WT + | M | 5.653 | 0.0477 | 0.0084 | 0.1120 | 13.272 |
| WT + | M | 5.700 | 0.0564 | 0.0099 | 0.1110 | 11.218 |
| WT + | F | 5.542 | 0.0498 | 0.0090 | 0.1430 | 15.914 |
| WT + | F | 5.723 | 0.0266 | 0.0046 | 0.0470 | 10.112 |
| WT + | F | 5.186 | 0.0262 | 0.0051 | 0.0470 | 9.304 |
| WT + | M | 5.543 | 0.0342 | 0.0062 | 0.0480 | 7.779 |
| Mean +/- SEM (of entries in column above) | 5.527 +/- 0.075 | 0.0422 +/- 0.0049 | 0.0076 +/- 0.0009 | 0.0943 +/- 0.0175 | 11.797+/- 1.142 | |
| KO - | F | 5.763 | 0.0209 | 0.0036 | 0.0690 | 19.025 |
| KO - | M | 4.232 | 0.0164 | 0.0039 | 0.1010 | 26.061 |
| KO - | M | 4.810 | 0.0227 | 0.0047 | 0.0880 | 18.647 |
| KO - | F | 5.650 | 0.0363 | 0.0064 | 0.0600 | 9.339 |
| KO - | M | 5.212 | 0.0178 | 0.0034 | 0.0640 | 18.739 |
| Mean +/- SEM (of entries in column above) | 5.133 +/- 0.282 | 0.0228 +/- 0.0035 | 0.0044 +/- 0.0005 | 0.0764 +/- 0.0078 | 18.362 +/- 2.659 | |
| KO + | M | 5.128 | 0.0193 | 0.0038 | 0.0600 | 15.941 |
| KO + | M | 5.030 | 0.0459 | 0.0091 | 0.1980 | 21.697 |
| Mean +/- range (of entries in column above) | 5.079 +/- 0.049 | 0.0326 +/- 0.0133 | 0.0065 +/- 0.0027 | 0.1290 +/- 0.0690 | 18.819 +/- 2.878 | |
| DT - | M | 5.426 | 0.0322 | 0.0059 | 0.0775 | 13.058 |
| DT - | M | 4.255 | 0.0360 | 0.0085 | 0.0895 | 10.579 |
| DT - | M | 4.818 | 0.0306 | 0.0064 | 0.1460 | 22.989 |
| DT - | M | 5.189 | 0.0197 | 0.0038 | 0.0380 | 10.010 |
| DT - | F | 5.500 | 0.0256 | 0.0047 | 0.0390 | 8.379 |
| Mean +/- SEM (of entries in column above) | 5.038 +/- 0.229 | 0.0288 +/- 0.0028 | 0.0059 +/- 0.0008 | 0.0780 +/- 0.0198 | 13.003 +/- 2.3607 | |
| DT + | M | 5.008 | 0.0694 | 0.0139 | 0.0270 | 1.948 |
| DT + | M | 4.750 | 0.0660 | 0.0139 | 0.0070 | 0.504 |
| DT + | M | 5.495 | 0.0587 | 0.0107 | 0.0692 | 6.478 |
| DT + | M | 5.233 | 0.0542 | 0.0104 | 0.0620 | 5.986 |
| Mean +/- SEM (of entries in column above) | 5.121 +/- 0.159 | 0.0621+/- 0.0034 | 0.0122+/- 0.0010 | 0.0413 +/- 0.0147 | 3.729 +/- 1.478 |
Table 2: Substance P-induced plasma extravasation from the duodenums of individual WT, NEP KO, and NEP overexpressor mice. Plasma extravasation values from FVBN mice (with or without prior doxycycline) were individually expressed as OD620 nm/g dry weight, from FVBN WT mice (n = 5 without, and n = 7 with, doxycycline), NEP KO mice (n = 5 without, and n = 2 with, doxycycline), and NEP overexpressor doubly transgenic mice (n = 5 without, and n = 4 with, doxycycline). Mice were subjected to the protocol for plasma extravasation as detailed above. No gender differences were evident in the plasma extravasation values. Duodenums were isolated; individual values used in calculating plasma extravasation were as described for Table 1. The resultant plasma extravasation values were used to construct Figure 3A-C.
Discussion
As discussed above, the study of plasma extravasation may ultimately lead to new knowledge concerning the causes of or new ways to inhibit or treat plasma extravasation. The successful use of the plasma extravasation protocol (above), using Evans blue dye, has been demonstrated in the current manuscript. Although the data shown back the hypothesis that NEP may protect the vasculature against plasma extravasation, this is a secondary goal presently, with the primary goal being to present an optimized protocol which may be readily used in the average laboratory for the future study of plasma extravasation. The protocol reported herein is focused on adaptability to future studies with different goals, animals, organs, and other conditions, and is especially helpful because this protocol is simple to use, reliable, economical, and takes into account the general availability of common materials, reagents, and competent personnel. It is noted, however, that this protocol will require a fair amount of repetitions per group of animals (8 - 12 repetitions suggested) to obtain statistically valid results.
There have been numerous studies and reports detailing attempts to measure vascular permeability and plasma extravasation. By far, most of these reports detail permutations of methods used along with the Evans blue dye method to investigate plasma extravasation and vascular permeability2,3,6,11,12,13. There have been fewer reports of vascular permeability methods which don't incorporate Evans blue at all3,13,15,24; these other methods are in general less accessible for the average laboratory, requiring complicated methods, specialized equipment and personnel, and are generally expensive.
The development of the Evans blue protocol above involved the testing of many different permutations and modifications. Early experiments were focused on attempts to inject Evans blue through the mouse tail vein. However, tail vein injections proved technically challenging and resulted in inconsistent results, necessitating the inclusion of the jugular vein injections and terminal surgical techniques described above. Many preliminary experiments were also done trying to find appropriate concentrations of Evans blue and substance P. The exact amount of Evans blue was not found to be imperative, as long as it was below about 200 mg/kg (compared to 50 mg/kg used presently), but the amount of substance P used in these experiments was important. After much experimentation, 1 nmole/kg was found to be the ideal concentration of exogenously added substance P, resulting in a 1.5-fold augmentation of (and thus more easily measurable) plasma extravasation values. Also, it was found that adequate time must be allowed for the Evans blue to equilibrate (>10 min). Perfusion of the blood vessels to remove excess Evans blue, after sacrifice of the mouse but prior to organ isolation, was found to be an essential component of the present protocol, as opposed to other protocols that do not include this perfusion step4. The perfusate (delivered by a gentle gravity gradient) is 50 mL of a pH 3.5 solution (which encourages binding of Evans blue and albumin); paraformaldehyde, prevalent in many other Evans blue protocols6,10,25, is omitted in the present protocol. Another factor that has been resolved in this protocol is that the dye used in doxycycline chow interferes with the OD620 nm measurement of Evans blue in tissues from the alimentary track. Thus, it was necessary to use undyed doxycycline chow in the above experiments. Also, an important and useful finding is that nearly any organ of the mouse is satisfactorily isolated and examined via the present protocol.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors wish to thank Andy Poczobutt and Dr. Jori Leszczynski for their valuable help and edits to this manuscript. Supported by Grants received from the National Heart, Lung and Blood Institute (NHLBI RO1 HL078929, PPG HL014985 and RO3 HL095439) and the Department of Veterans' Affairs (Merit Review).
References
- Snijdelaar DG, Dirksen R, Slappendel R, Crul BJ. Substance P. Eur J Pain. 2000;4(2):121–135. doi: 10.1053/eujp.2000.0171. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Iwamoto I, Ueki IF, Borson DB, Nadel JA. Recombinant neutral endopeptidase attenuates substance P-induced plasma extravasation in the guinea pig skin. Int Arch Allergy Appl Immunol. 1990;91(3):232–238. doi: 10.1159/000235122. [DOI] [PubMed] [Google Scholar]
- Szabo A, Menger MD, Boros M. Microvascular and epithelial permeability measurements in laboratory animals. Microsurgery. 2006;26(1):50–53. doi: 10.1002/micr.20210. [DOI] [PubMed] [Google Scholar]
- Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp. 2013. p. e50062. [DOI] [PMC free article] [PubMed]
- Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res. 2007;150(4):253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Figini M, et al. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol. 1997;272(4):785–793. doi: 10.1152/ajpgi.1997.272.4.G785. Pt 1. [DOI] [PubMed] [Google Scholar]
- Awad AS, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290(6):1516–1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- Lu B, et al. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med. 1997;3(8):904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- Gendron G, Simard B, Gobeil F, Sirois P, D'Orleans-Juste P, Regoli D. Human urotensin-II enhances plasma extravasation in specific vascular districts in Wistar rats. Can J Physiol Pharmacol. 2004;82(1):16–21. doi: 10.1139/y03-122. [DOI] [PubMed] [Google Scholar]
- Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42(3):789–794. [PubMed] [Google Scholar]
- Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983;8(1):41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Fricke IB, et al. In vivo bioluminescence imaging of neurogenesis - the role of the blood brain barrier in an experimental model of Parkinson's disease. Eur J Neurosci. 2017;45(7):975–986. doi: 10.1111/ejn.13540. [DOI] [PubMed] [Google Scholar]
- Pink DB, Schulte W, Parseghian MH, Zijlstra A, Lewis JD. Real-time visualization and quantitation of vascular permeability in vivo: implications for drug delivery. PLoS One. 2012;7(3):33760. doi: 10.1371/journal.pone.0033760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2(4):266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- Vandoorne K, Addadi Y, Neeman M. Visualizing vascular permeability and lymphatic drainage using labeled serum albumin. Angiogenesis. 2010;13(2):75–85. doi: 10.1007/s10456-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Nalivaeva NN. Proteinase dysbalance in pathology: the neprilysin (NEP) and angiotensin-converting enzyme (ACE) families. Cell Mol Biol (Noisy-le-grand) 2006;52(4):40–48. [PubMed] [Google Scholar]
- Lu B, Gerard NP, Kolakowski LF, Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulates septic shock. Ann N Y Acad Sci. 1996;780:156–163. doi: 10.1111/j.1749-6632.1996.tb15119.x. Mar 22. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, et al. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol. 2009;174(3):782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoor V, Oka M, Walchak SJ, Hersh LB, Miller YE, Dempsey EC. Neprilysin regulates pulmonary artery smooth muscle cell phenotype through a platelet-derived growth factor receptor-dependent mechanism. Hypertension. 2013;61(4):921–930. doi: 10.1161/HYPERTENSIONAHA.111.199588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P, Arber DA. Paraffin-section detection of CD10 in 505 nonhematopoietic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol. 2000;113(3):374–382. doi: 10.1309/8VAV-J2FU-8CU9-EK18. [DOI] [PubMed] [Google Scholar]
- Bircan S, Candir O, Kapucuoglu N, Serel TA, Ciris M, Karahan N. CD10 expression in urothelial bladder carcinomas: a pilot study. Urol Int. 2006;77(2):107–113. doi: 10.1159/000093901. [DOI] [PubMed] [Google Scholar]
- Koiso K, Akaza H, Ohtani M, Miyanaga N, Aoyagi K. A new tumor marker for bladder cancer. Int J Urol. 1994;1(1):33–36. doi: 10.1111/j.1442-2042.1994.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Wick MJ, et al. Protection against vascular leak in neprilysin transgenic mice with complex overexpression pattern. Transgenic Res. 2016;25(6):773–784. doi: 10.1007/s11248-016-9969-x. [DOI] [PubMed] [Google Scholar]
- Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF. Effects of acute hypoxia and hyperthermia on the permeability of the blood-brain barrier in adult rats. J Appl Physiol. 2009;107(4):1348–1356. doi: 10.1152/japplphysiol.91484.2008. (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen TE, et al. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol. 2001;166(2):1285–1291. doi: 10.4049/jimmunol.166.2.1285. [DOI] [PubMed] [Google Scholar]


