Abstract
IFNα is a cytokine essential to a vast array of immunologic processes. Its induction early in the innate immune response provides a priming mechanism that orchestrates numerous subsequent pathways in innate and adaptive immunity. Despite its beneficial effects in viral infections IFNα has been reported to be associated with several autoimmune diseases including autoimmune thyroid disease, systemic lupus erythematosus, rheumatoid arthritis, primary biliary cholangitis, and recently emerged as a major cytokine that triggers Type 1 Diabetes. In this review, we dissect the role of IFNα in T1D, focusing on the potential pathophysiological mechanisms involved.
Evidence from human and mouse studies indicates that IFNα plays a key role in enhancing islet expression of HLA-I in patients with T1D, thereby increasing autoantigen presentation and beta cell activation of autoreactive cytotoxic CD8 T-lymphocytes. The binding of IFNα to its receptor induces the secretion of chemokines, attracting monocytes, T lymphocytes, and NK cells to the infected tissue triggering autoimmunity in susceptible individuals. Furthermore, IFNα impairs insulin production through the induction of endoplasmic reticulum stress as well as by impairing mitochondrial function.
Due to its central role in the early phases of beta cell death, targeting IFNα and its pathways in genetically predisposed individuals may represent a potential novel therapeutic strategy in the very early stages of T1D.
Keywords: Interferon alpha, Type 1 Diabetes
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by immune-mediated pancreatic beta cell destruction and its incidence has been rapidly increasing worldwide in the past decades [1]. The progressive rise of T1D and the lower threshold for developing the disease have led to the conclusion that environmental factors are greatly influencing its etiology [2, 3]. Even though genetic risk factors play a major role in T1D development, the rapid increase in the incidence of T1D in the last 70 years demonstrates that environmental factors must play a crucial role, too [4, 5]. Indeed, the differences in the concordance rate between monozygotic twins, and seasonal and geographical variations in the incidence of T1D point to the involvement of nongenetic factors in T1D pathogenesis [6]
IFNα, crucial in both innate and adaptive immunity, has received increasing attention for its functional contribution to the development of autoimmune responses, representing a fundamental link between genetics, immune system, and environmental factors. Human and murine studies pinpoint a key role of IFNα in the pathophysiology of T1D; IFNα is responsible for generating an inflammatory milieu facilitating the diabetogenic adaptive response especially in mice or patients carrying T1D susceptibility genes. Of note, therapies that neutralize IFNα have been shown to suppress the beta cell dysfunction that precedes the onset of T1D [7–9].
Interferons were discovered in 1957, named for their ability to interfere with viral replication [10]. In addition to being antiviral agents, interferons have immune-modulating, antiproliferative, and antineoplastic effects and act as regulators of growth and differentiation. IFNα, in particular, is a key cytokine of the innate response produced by the immune system to combat unrecognized organisms and cells including viruses and tumor cells. Because of its strong anti-viral effect, until recently IFNα was the mainstay of treatment of chronic hepatitis C and is still used to treat certain cancers. Interestingly, IFNα has been associated with several autoimmune disorders besides T1D, including AITD (autoimmune thyroid disease), SLE (systemic lupus erythematosus), RA (rheumatoid arthritis), and PBC (primary biliary cholangitis) [11–17]. In this review, we dissect the literature linking IFNα to autoimmunity focusing on T1D and the potential molecular mechanisms underlying such association.
2. The functional role of IFNα in Type 1 Diabetes
2.1. Evidence from human studies
Islet autoimmunity has been reported in individuals following IFNα therapy for chronic hepatitis, as well as hematologic malignancies such as hairy cell leukemia, Kaposi’s sarcoma, and non-Hodgkin’s limphoma [18–20], suggesting a link between IFNα and islet autoimmunity. The first case of T1D related to IFNα therapy was reported by Fabris et al. in 1992 [21]; after that, in most reported cases, the onset of T1D occurred during or shortly after treatment with IFNα [22].
Supporting a role for IFNα in the etiology of T1D, early studies by Foulis and colleagues reported that the majority of beta cells collected from 37 patients with T1D were positive for IFNα, and that 93% of islets displayed over-expression of HLA-I [23]. Hyperexpression of HLAI is an early event that appears to be attributable to IFNα secretion, a feature observed in T1D patients that may enhance beta cell immunogenicity. More recently, pancreatic samples from the network of pancreatic organ donors (nPOD), living donors, and archival collection from the UK also exhibited hyperexpression of HLA-I in islets from T1D patients [16]. These new nPOD data are consistent with previous studies showing elevated expression of IFNα in the pancreatic tissue of recently diagnosed T1D patients, and beta cells from type 1 diabetic individuals contained immunoreactive IFNα [24, 25]. Similarly, increased expression of IFNα-stimulated genes were reported in pancreatic biopsies of patients with recent-onset T1D compared with islets from control organ donors [26]. Furthermore, Meyer at al. recently reported that selfreactive antibodies against IFNα were associated with protection against T1D in patients with autoimmune polyglandular syndrome type 1 that harbor mutations in the autoimmune regulator gene (AIRE gene). AIRE-deficient patients that generated self-neutralizing antibodies specific for IFNα were protected from T1D, whereas, patients that did not generate anti-IFNα antibodies developed T1D [27]. Interestingly, IFNα but not IFNγ production was higher in PBMCs isolated from patients with T1D compared with controls; and a specific subset of IFNα-producing dendritic cells (DCs) called plasmacytoid dendritic cells (pDCs) [28] have been detected in the blood of patients with T1D [29–31].
Few studies to date have focused on elucidating how IFNα signaling transforms the islet milieu to a diabetogenic environment. Most likely, a genetic predisposition (e.g. patients positive for DR3-DQ2 and/or DR4-DQ8 creates an islet environment permissive to IFNα-induced beta cell cytotoxicity by cytotoxic CD8+ T-cells [32]. Corroborating this hypothesis, recent GWAS studies in PBMCs from at-risk T1D children from Finnish (DIPP study) [33] and German (BABYDIET study) cohorts [34] revealed that IFNα signature is temporally increased in susceptible children prior to the development of autoantibodies, but not in patients with established T1D. Supporting this notion, a recent genetic analysis suggested a diabetogenic role for IFNα-induced genes in prediabetic children by identifying several IFNα signaling and antiviral immune response genes that were linked to T1D [35, 36]. A study comparing gene expression profiles of circulating blood cells from children at the onset of T1D, first-degree relatives of T1D children with positive antibodies, and healthy controls identified 107 distinct genes that were differentially expressed; one of the major gene clusters was regulated by IFNα [37].
2.2. Evidence from animal models
Various rodent models have been used to study the relationship between IFNα and T1D [38, 39]. In mice, IFNα has been shown to directly affect pancreatic beta cells by inducing cytokine and chemokine secretion and MHC-I expression, enhancing their susceptibility to be attacked by diabetogenic T-cells [40]. In 1993, Stewart and his group developed a transgenic mouse model over-expressing IFNα in beta cells: these mice exhibited inflammatory infiltrates within the islets alongside with beta cell necrosis, and fewer insulin-containing cells; the disease severity correlated with the genetic background, in genetically susceptible hosts [41–43].
More recent investigations by Qing Li et al. demonstrated that IFNα is an essential initiator of T1D in NOD mice [44, 45]. Interestingly, transcriptional profiling of NOD islets and pancreatic lymph nodes (isolated before T-cell infiltration) identified an IFNα-induced gene signature, showing that islet exposure to IFNα is a key precipitating event in T1D pathogenesis [46]. Elevated levels of pDCs, which are key producers of IFNα, were also reported in pancreatic draining lymph nodes of young (2–3 weeks old) NOD mice, supporting the notion that local IFNα produced by pDCs plays a critical role in T1D etiology [47]. The relationship between IFNα and T1D in NOD mice was also confirmed in mice lacking the interferon regulatory factor-1 (IRF-1−/− NOD mice) in which the development of insulitis and diabetes was completely suppressed [48].
Additional support for the role of IFNα in triggering T1D came from studies in BB rats (that spontaneously develop T1D) [49] and in low dose streptozotocin-treated mice that also develop a T1D-like disease [42]. Taken together, these murine T1D models strongly suggest that islet expression of IFNα is an early and critical step in the development of T1D and is likely involved in the breakdown of beta-cell self-tolerance.
Most of the studies described so far showed an association between IFNα expression and T1D, but not an actual causation. A more direct proof of causation came from studies demonstrating that blocking IFNα or its receptor using antibodies can prevent the development of diabetes in mice [41, 44]. Interestingly, IFNα but not IFNβ was identified as a required signal for autoreactive T-cells to enter the islets; similarly, blockade of IFNα but not IFNβ signaling prevented T1D by acting at the prediabetic stage in mice [9].
In summary, both human and rodent studies established a key role for local islet IFNα production in initiating the autoimmune process in T1D.
3. IFNα in other autoimmune disorders
3.1. Thyroid disease
Many studies have reported that up to 40% of patients receiving IFNα for HCV infection develop subclinical autoimmune thyroid disease (AITD) and up to 15% develop clinical disease; we have designated this association as IFNα-induced thyroiditis (IIT) [50]. IIT varies in presentation, and can be classified as autoimmune and non-autoimmune type. Autoimmune IIT can manifest by the development of thyroid antibodies without clinical disease, or by clinical disease including both Hashimoto’s thyroiditis and Graves’ disease [50–52]. In IIT, the production of antibodies against thyroid peroxidase (TPO) and thyroglobulin (Tg) can be induced de novo or increased by IFNα therapy, thus causing thyroid autoimmunity [53]. As in the islets IFNα can increase HLA class I expression on thyroid cells with cytotoxic T-cells activation, triggering proinflammatory cytokines associated with AITD (such as IL-6) [54]. In addition to its immune effects, we and others have shown that IFNα has direct toxic effects on the thyroid gland and that these effects significantly contribute to the development of nonautoimmune IIT (existing in two forms, destructive thyroiditis and hypothyroidism) in genetic predisposed patients [55–57]. We showed that IFNα can directly cause thyrocyte death as well as activate pathways of antigen presentation, pattern recognition receptors, and cytokines/chemokines in thyroid cells [56].
3.2. Systemic Lupus Erythematosus
A frequently reported rheumatologic complication of IFNα therapy is SLE. Patients receiving IFNα can experience typical manifestations of SLE such as malar rash and lupus nephritis while exhibiting lupus specific antibodies; of note, some patients present antibodies without clinical manifestations of lupus, developing a “lupus like” syndrome. Intriguingly, most of these cases resolve or improve after discontinuation of IFNα therapy strongly suggesting that IFNα directly triggers the induction or exacerbation of SLE [13]. Moreover, microarray studies evaluating PBMCs of SLE patients, have revealed gene expression signatures of IFNα inducible transcripts, showing that IFNα can be exploited as a marker of disease severity [58]. Additionally, SLE patients themselves were found to overproduce IFNα, and this phenomenon might have a pivotal role in the disease pathogenesis and in the premature development of atherosclerosis observed in SLE [59]. Indeed, sera from patients with SLE have been shown to form immune complexes with necrotic material and induce IFNα release from pDCs [60]. Murine models of lupus have also provided direct evidence of the pathogenic role of pDCs and targeting pDCs is being pursued as a potential treatment strategy [61, 62]. The strongest evidence for a direct pathogenic role of IFNα in SLE comes from recent clinical trials blocking IFNα or its receptor in SLE. For istance, anifrolumab, a monoclonal antibody against IFNα receptor has been successfully used in moderate to severe SLE, reducing proinflammatory cytokines (such as IL-6, IL-8 and and B cell differentiation in vitro [63].
3.3. Primary biliary cholangitis
PBC is a chronic cholestatic disease predominantly affecting females that is characterized by serum autoantibodies against mitochondrial antigens, elevated serum immunoglobulin M, progressive destruction of intrahepatic bile ducts, and, ultimately, liver cirrhosis and failure [64, 65]. The precise mechanisms leading to selective destruction of biliary epithelial cells are still unknown, although numerous immunomediated pathways have been proposed. Of note, the only currently established treatment for PBC is ursodeoxycholic acid (UDCA) and its active ingredient tauroursodeoxycholic acid (TUDCA), both chemical chaperons that reverse ER stress, suggesting an involvement of ER stress-induced apoptosis in the biliary epithelial lesion of PBC [66]. Epidemiological studies in PBC have suggested that the cause for this disease is likely to be a combination of both environmental factors and a genetic predisposition [67]. The disease has incomplete concordance in monozygotic twins and its incidence has increased over recent decades suggesting a strong role for environmental factors in its etiology [69]. Molecular mimicry by infectious agents has been proposed to be capable of breaking tolerance in genetically predisposed individuals, thus leading to onset of PBC [68]. This is a plausible mechanism because of the propensity of several viruses to specifically target the liver. Recent evidence indicates a role for infections in the induction of PBC; and numerous specific organisms, including viruses, have been investigated as possible agents involved in PBC [70–72]. Interestingly, polyinosinic polycytidylic acid (poly I:C), an inducer of IFNα, generated a PBClike cholangitis when administered to genetically susceptible mice [73]. Of note, IFNα signaling itself has been implicated in a murine model of PBC, suggesting that drugs targeting the IFNα pathway could be potentially beneficial in the earlier stages of PBC [74]. In PBC patients, the IFNα levels were significantly higher in portal tract and liver parenchyma as compared to levels in autoimmune hepatitis and chronic viral hepatitis patients indicating that IFNα pathways are involved in the pathophysiology of PBC [17]. Furthermore, PBC was exacerbated or induced by IFNα therapy [75–77].
3.4. Rheumatoid Arthritis and other diseases
RA is another disease that has been reported to ensue following IFNα therapy, but this is relatively less common compared with other autoimmune disorders [14, 78–80]. In 1979, Notkins and colleagues described for the first time the presence of IFNα in the serum of patients with RA [81, 82]. However, the exact role of IFNα in the pathophysiology of RA is controversial and remains under investigation. Genetic analyses document an association of IFNα-pathway genes with RA, similar to what was observed in SLE patients [83]. Of interest, several IFNα-related genes have been identified as risk loci for RA; moreover, recent data demonstrate co-expression of Toll-like receptors (TLRs, especially isoforms 3 and 7) and IFNα in synovial biopsies, suggesting that IFNα stimulated pathways could represent a link between innate and adaptive immune response in RA [84, 85]. While the relevance of the IFNα signature to RA disease activity and progression remains unclear, recent studies suggest that the presence of IFNα in circulation may predict the response to immunotherapy in RA [86, 87]. Patients with low levels of IFNα signature had better clinical response to therapy [86, 88], suggesting that IFNα can serve as a biomarker for predicting or monitoring the response to biologics in RA.
While much less common, other syndromes have also been observed in patients treated with IFNα, including (but not limited to) hypoparathyroidism [89, 90], celiac disease [91–93], myasthenia gravis [94, 95], Guillain-Barré syndrome [94], polymyositis [94], acute and chronic demyelinating polyneuropathy [94], psoriasis [96], vitiligo [97], both psoriasis and vitiligo [98], porphyria cutanea tarda [99], autoimmune hemolysis [100, 101], myositis and polymyositis [102], sarcoidosis [103] and retinal hemorrhage [104].
4. The diabetic islet: sources of IFNα
4.1. Viral sources
Several mechanisms have been proposed to explain the induction of islet-IFNα in T1D and how IFNα promotes early disease phases. Bacteria, vitamin D, wheat proteins, and cow’s milk have all been investigated with regard to T1D, but the strongest associations have been found with viral infections, corroborated by extensive epidemiological studies, as well [105, 106]. Viruses, specifically enteroviruses, have been shown to be one of the major environmental triggers of T1D [107, 108]. Early childhood infections are linked to islet autoimmunity and progression to T1D in children with genetic susceptibility [109]. Furthermore, epidemiological studies support a role for enteroviral infections in T1D development; in particular, increased incidence of T1D followed enterovirus epidemics [110]. Interestingly, staining of postmortem pancreatic tissues from T1D patients revealed enteroviral proteins [105, 111]. Similarly, Honkanen et al. showed that the presence of enteroviral RNA in stool (detected by PCR) preceded islet autoimmunity in genetically susceptible T1D children [112]. Moreover, antibodies against enterovirus immunoglobulin M and enteroviral RNA were detected in the blood of recent-onset T1D patients [113, 114]. Recently Krogvold et al performed islet biopsies in T1D patients 3–9 weeks after the diagnosis of T1D; interestingly, enteroviral capsid protein 1 was detected in 6/6 T1D islets and in 2/9 controls, and enteroviral RNA sequences were detected in 4/6 patients and none of the controls [115]. The same group showed that pancreatic islets from T1D donors had an increased frequency of viral receptors compared with controls [116], resulting in production of IFNα, induction of adhesion molecules, and increased interaction with immune cells [117]. Finally, our group recently showed that human pancreatic islets can be infected in vitro by the hepatitis C virus, providing the first direct evidence that viruses can infect islet cells and offering a new mechanism for the association between diabetes and HCV infection [118]. The mechanistic contributions of other viruses such as herpesvirus, rotaviruses, retroviruses, measles virus and influenza virus have also been explored in the pathophysiology of T1D [108, 119].
The presence of IFNα gene signature in the early phases of T1D and its absence in protected individuals strongly implicates viruses in the disease. A critical unanswered question is why only the beta cells become infected and then targeted by the immune system, whereas pancreatic alpha cells remain mostly unaffected; indeed different groups could detect major capsid VP1 protein only in insulin-containing islets [105, 111]. Recent studies suggest that the viral cycle is initiated in both alpha and beta cells but alpha cells are able to control viral amplification, leading to an abortive infection. The ability to clear infections could explain why VP1 labelling is not detected in the alpha cells of infected human islets after long infection times or among patients with T1D [120, 121].
Taken together these data suggest that viral infection of beta cells, even if short-lived, will lead to local production of IFNα that, in genetically predisposed individuals, will create a diabetogenic environment by triggering local cytokine and chemokine production and influx of antigen presenting cells and T-cells
4.1. Other sources
Whereas viruses are among the most potent inducers of IFNα, other agents are capable of triggering the production of this cytokine including nucleic acids, fixed viral particles, and recombinant viral proteins [122]. In T1D the apoptotic cellular debris, especially double-strand DNA and single-strand RNA, activate pDCs via TLR-7 and TLR-9, leading to the local production of large amounts of IFNα, which can perpetuate the diabetogenic islet milieu. According to this model, dying beta cells will release DNA that will bind to TLRs and activate IFNα production by pDCs cells promoting autoreactive T-cell priming [29]. Moreover, Lande et al. indentified in the sera of SLE patients immunogenic complexes composed of neutrophilderived antimicrobial peptides and self-DNA. These complexes were produced by activated neutrophils in the form of web-like structures known as neutrophil extracellular traps that have been previously shown to bind TLR-9 on pDCs inducing secretion of high levels of IFNα [123]. Hence, induction of IFNα by uptake of mammalian nucleic acids derived from apoptotic debris may amplify immunologic responses not only against foreign pathogens, but also against selfantigens in genetically predisposed individuals. IFNα can also be induced in human islets by hypoxia that develops as glucose levels rise in T1D patients [124].
5. Mechanisms underlying IFNα induction of T1D
5.1. Immunologic mechanisms
The critical role of IFNα in linking innate and adaptive immunity has been recently recognized both in mouse and human models leading to new insights into the possible immune-mediated mechanisms by which IFNα can induce autoimmunity under different physiological and pathological conditions [125]. Interestingly, autoimmune diseases triggered by IFNα include both T-cell-mediated and antibody-mediated disease, indicating that IFNα may exert a general stimulatory effect on the immune system in individuals genetically predisposed to a specific autoimmune condition. By binding to its receptor, IFNα activates several signaling pathways including JAK-STAT; upon phosphorylation, STAT proteins are translocated to the nucleus where they activate the expression of proinflammatory genes (including adhesion molecules and cytokine genes) that have been suggested to trigger T1D in genetically susceptible individuals [126].
One of the major effects of IFNα on beta cells is the upregulation of HLA class I and costimulatory molecules, leading to more efficient self-antigen presentation to previously quiescent low-affinity autoreactive T-cells. Indeed, overexpression of HLA class I can activate cytotoxic T-cells and direct the inflammatory response to the islets [127].
Furthermore, IFNα can stimulate IL-15 production from DCs leading to DCs maturation [128] or promote the differentiation of DCs enhancing the expression of HLA class I and II along with costimulatory molecules such as CD40, CD80, and CD86 [129]. Interestingly, DCs isolated from spleens of mice genetically deficient in IFNα exhibit a significant impairment in T-cell activity showing that IFNα is an essential cytokine for T-cell activation, by promoting their proliferation, survival, and increasing their effector functions [130]. The ability of IFNα to stimulate T-cell helper functions (increasing antibody production) and keeping memory cells alive in both mice and humans has been known for many years [131].
In T1D, IFNα can also be induced by products of tissue damage within the islets, thereby generating a self-perpetuating immune response. A study by Diana et al. demonstrated that tissue damage and pDCs have a critical role in the initiation of T1D; they showed that the accumulation of beta cell debris (such as self DNA) activates IFNα–secreting pDCs in the pancreas of NOD mice [46]. These data indicate the importance of innate immune cells crosstalk in early stages of T1D, as shown in other diseases [132]. However, recent studies support the notion that pDCs could harbor both pathogenic and protective functions during T1D development, depending on the course of the disease, the infectious content, and the localization of the cells [133, 134].
B cells have been proposed to be involved in T1D pathogenesis as professional self-antigen presenting cells, but there is little evidence for a diabetogenic role for islet autoantibodies. IFNα is known to have direct effects on B cells, such as on proliferation, survival, activation, autoantibody production, and Ig isotype switching [135]. Interestingly, an altered B cell receptor signaling threshold has also been observed in patients with T1D compared to healthy controls [136]. In addition, the alteration in immunoglobulin production and the decreased Treg function caused by IFNα can contribute to the development of T1D in genetically susceptible individuals [137]. Similarly, macrophages, NK cells, and neutrophils are also activated by IFNα [138, 139].
5.2. Non immunologic mechanisms
Prior to the symptomatic phase of T1D, pancreatic beta cells undergo critical pathological changes; several studies point to direct toxic effects of IFNα on the islets as playing a role in the pathogenesis of T1D. IFNα was shown to decrease insulin synthesis and secretion in vitro causing beta cell dysfunction [140, 141]. Of interest, our group has previously shown that IFNα can induce autoimmune thyroiditis by a direct toxic effect on thyroid cells [56], and we hypothesize that a similar mechanism could occur in the islets during T1D; in fact, we recently demonstrated that IFNα participates in the early stages of T1D by causing an endoplasmic reticulum (ER) stress-mediated impairment of insulin production [15]. In our study, insulin content was significantly reduced, with a significantly increased proinsulin:insulin ratio (a recognized marker of beta cell dysfunction in T1D) and a decreased expression of both proinsulin convertases PC1 and PC2 [15]. Consistent with our findings, ER stress has been recently proposed to play a role in inflammation-associated tissue destruction [142], beta cell inflammation, and upregulation of HLA class I molecules [143]. In particular, the ER stressinduced overexpression of HLA class I molecules is associated with activation of cytotoxic Tcells and is a prominent early feature in the development of T1D [144]. These findings may have translational implications since blocking IFNα is now being tested in other autoimmune diseases [145].
Another potential mechanism by which IFNα induces beta-cell damage is by inducing mitochondrial impairment [146, 147]; indeed, recent data suggest that effects of IFNα on mitochondrial gene expression and mitochondrial function may also be involved in the physiopathology of T1D. Specifically, IFNα can suppress several mitochondrial genes and can reduce electron transport and ATP levels, all of which can alter insulin production and secretion in beta cells [148–150]. Similarly, IFNα can induce apoptosis via mitochondrial permeability changes and, as mentioned before, apoptotic beta cells can themselves induce IFNα expression, thereby creating a vicious cycle of inflammation and beta cell death [151].
IFNα can also suppress cell division, decreasing the ability of beta cells to replicate and regenerate themselves, which can further worsen the progression of T1D [152]. Finally, IFNα has been reported to impair the ability of insulin to stimulate glucose clearance [153] a phenomenon that has been suggested to contribute to T1D [154].
Epigenetic modulation has been shown by us and others to be a critical factor in the etiology of T1D [155, 156]. Of interest, our group has recently demonstrated that IFNα confers susceptibility to AITD through epigenetic interactions with Tg variants [157], and it is likely that a similar effect of IFNα contributes to the development of T1D. Interestingly, Bonifacio and his group have demonstrated that children delivered by cesarean section have a higher risk of developing T1D compared to children born by vaginal delivery: this phenomenon is attributable to an interaction with immune-related genes, in particular the IFN-induced helicase 1 gene [158]. Finally, Miao and colleagues explored a novel epigenetic mechanism by which high glucose levels can induce IFNα-stimulated genes in T1D revealing that IFNα is also a major player in diabetic complications related to hyperglycemia [159]. Table 1 shows the different effects of IFNα in T1D; understanding these mechanistic pathways might offer potential therapeutic opportunities.
Table 1 –
IFNα effects in T1D
| Target | Effect | References | |
|---|---|---|---|
| Immunologic | Dendridic Cells | Increase IL-15 production; maturation; activation; survival; antigen presentation; expression of costimulatory molecules (CD80, CD86 and CD40) | [128–130] |
| T cells | Activation; proliferation; survival; Th1 deviation; increase effector functions with production of perforin, granzime and IFN | [131–134] | |
| B cells | Proliferation; survival; activation; Ig switching; antigen presentation | [135, 136] | |
| T reg cells | Decrease suppressive activity | [137 | |
| Macrophages | Production of pro-inflammatory cytokines (IL6, TNFa) | [138, 139] | |
| NK cells | Proliferation; cytotoxicity; ffNy production | [138, 139] | |
| Non-immunologic | Human beta cells | Impairment of insulin production; impairment of proinsulin convertases; impairment of replication; HLA-I upregulation |
[15, 140, 141 143, 144] |
| Mitochondria | Gene suppression; reduction of electron transport and ATP levels | [146–151] | |
| IFNa-induced genes | Epigenetic modifications | [158, 159] | |
6. Blocking IFNα in autoimmune diseases: therapeutic implications
The link between IFNα, ER stress and the pathogenesis of T1D has potential translational implications. Indeed, compounds targeting molecular processes altered in ER-stressed beta cells could represent an interesting new approach to prevent IFNα-induced beta cell death in the early onset of T1D. The specific inhibition of JAK could also provide a potential therapeutic strategy in T1D [160]. Given its important contribution to T1D development, targeting IFNα itself, its receptor, or the cells secreting it (pDCs) can potentially reverse T1D in the early stages of the disease. Recent data showed that blocking IFNα (but not IFNβ) signaling prior to clinical T1D disease using a specific antibody prevented beta cell death in mice, demonstrating that IFNα is essential for autoreactive T-cell entry into the islets [9]. Confirming the key role of IFNα in the etiology of autoimmune conditions, monoclonal antibodies targeting IFNα or its receptor were effectively used in recent clinical trials in SLE patients [63, 161]. Moreover, a therapeutic vaccine that induces anti-IFNα antibodies is currently in preclinical development for SLE [162]. Taken together, these data point to a new therapeutic approach in T1D using monoclonal antibodies or vaccines targeting IFNα; if used in the early stages of T1D, such an approach can help preserving beta cell function, thereby preventing the progression of the disease. However, blocking IFNα may suppress the normal antiviral responses; indeed, the identification of vaccines against key diabetogenic viruses could represent an alternative valid strategy to prevent beta cell death [163]. Also, some compounds have shown efficacy in reducing viral amplification, eradicating the virus and protecting human pancreatic islets [164–166]; antiviral treatment at the prediabetic stage could eliminate persistent infection reducing the risk of T1D.
7. Conclusion
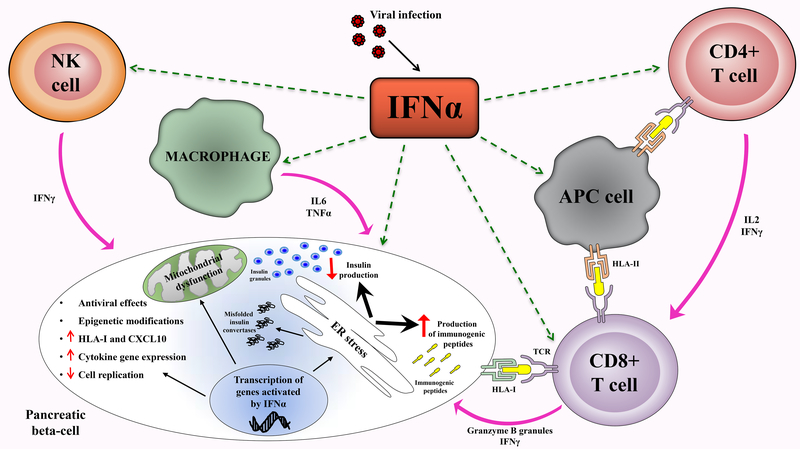
IFNα is the key cytokine of the innate immune response; it is induced by infections and tissue stress and damage, both of which are believed to trigger autoimmune diseases. Local IFNα production can trigger lymphocytic infiltration and activation. The binding of IFNα to its receptor induces overexpression of HLA class I proteins and secretion of chemokines, attracting monocytes, T lymphocytes, and NK cells to the infected tissue. This cascade of immune responses can trigger autoimmunity in susceptible individuals. Moreover, IFNα induces ER stress in human beta cells and the production of ER stress-modified autoantigens is another important pancreatic islet hallmark in early T1D (Figure 1). Its dual effects of activating innate and adaptive immune responses and causing direct tissue toxicity both play a decisive role in IFNα-mediated progressive destruction of pancreatic beta cells, especially in the early stages of the disease. In conclusion, these effects pinpoint IFNα as a fundamental modulator of inflammatory and ER stress responses in the early stage of T1D, contributing to the progressive destruction of pancreatic beta cells.
Figure 1. Mechanisms underlying IFNα-induced T1D.
IFNα is produced by PDCs in response to infection. Binding to its receptor, IFNα induces transcription of IFN-inducible genes leading to (1) antiviral effects, (2) epigenetic modifications, (3) production of chemokines and proinflammatory cytokines, (4) increased expression of HLAI, (5) decreased beta cell replication, (6) mitochondrial dysfunction, (7) ER stress and (8) stimulation of cytotoxic T cells.
Highlights.
Upregulation of IFNα is associated with several autoimmune disorders.
IFNα recently emerged as a key cytokine triggering T1D both in rodents and in humans.
IFNα triggers T1D through immune activation and direct beta-cell toxicity mechanisms.
IFNα upregulates HLA-I, cytokines, chemokines, and causes ER stress in pancreatic islets.
Targeting IFNα represents a potential therapeutic strategy in the early stages of T1D.
Acknowledgments
This work was supported in part by grant DK067555 from NIDDK (to YT).
Footnotes
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Redondo MJ, Steck AK, Pugliese A. Genetics of type 1 diabetes. Pediatric diabetes, 2018;19:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet, 2014;383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hasham A, Tomer Y. The recent rise in the frequency of type 1 diabetes: who pulled the trigger? Journal of autoimmunity, 2011;37:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes, 2002;51:3353–61. [DOI] [PubMed] [Google Scholar]
- [5].Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet, 2009;41:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Principi N, Berioli MG, Bianchini S, Esposito S. Type 1 diabetes and viral infections: What is the relationship? Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 2017;96:26–31. [DOI] [PubMed] [Google Scholar]
- [7].Devendra D, Eisenbarth GS. Interferon alpha--a potential link in the pathogenesis of viralinduced type 1 diabetes and autoimmunity. Clinical immunology, 2004;111:225–33. [DOI] [PubMed] [Google Scholar]
- [8].Stewart TA. Neutralizing interferon alpha as a therapeutic approach to autoimmune diseases. Cytokine Growth Factor Rev, 2003;14:139–54. [DOI] [PubMed] [Google Scholar]
- [9].Marro BS, Ware BC, Zak J, de la Torre JC, Rosen H, Oldstone MB. Progression of type 1 diabetes from the prediabetic stage is controlled by interferon-alpha signaling. Proceedings of the National Academy of Sciences of the United States of America, 2017;114:3708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Virus interference: I. The interferon. By Alick Isaacs and Jean Lindenmann, 1957. CA Cancer J Clin, 1988;38:280–90. [PubMed] [Google Scholar]
- [11].Stefan M, Wei C, Lombardi A, Li CW, Concepcion ES, Inabnet WB 3rd, et al. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proceedings of the National Academy of Sciences of the United States of America, 2014;111:12562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hasham A, Zhang W, Lotay V, Haggerty S, Stefan M, Concepcion E et al. Genetic analysis of interferon induced thyroiditis (IIT): evidence for a key role for MHC and apoptosis related genes and pathways. Journal of autoimmunity, 2013;44:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Niewold TB, Swedler WI. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol, 2005;24:178–81. [DOI] [PubMed] [Google Scholar]
- [14].Cacopardo B, Benanti F, Pinzone MR, Nunnari G. Rheumatoid arthritis following PEG-interferonalfa-2a plus ribavirin treatment for chronic hepatitis C: a case report and review of the literature. BMC Res Notes, 2013;6:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lombardi A, Tomer Y. Interferon alpha impairs insulin production in human beta cells via endoplasmic reticulum stress. Journal of autoimmunity, 2017;80:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia, 2016;59:2448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest, 2005;85:908–20. [DOI] [PubMed] [Google Scholar]
- [18].Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int, 2008;28:39–46. [DOI] [PubMed] [Google Scholar]
- [19].Guerci AP, Guerci B, Levy-Marchal C, Ongagna J, Ziegler O, Candiloros H et al. Onset of insulindependent diabetes mellitus after interferon-alfa therapy for hairy cell leukaemia. Lancet, 1994;343:1167–8. [DOI] [PubMed] [Google Scholar]
- [20].Nakamura K, Kawasaki E, Imagawa A, Awata T, Ikegami H, Uchigata Y et al. Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes care, 2011;34:2084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fabris P, Betterle C, Floreani A, Greggio NA, de Lazzari F, Naccarato R et al. Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet, 1992;340:548. [DOI] [PubMed] [Google Scholar]
- [22].Oka R, Hiroi N, Shigemitsu R, Sue M, Oshima Y, Yoshida-Hiroi M. Type 1 Diabetes Mellitus Associated with Pegylated Interferon-alpha Plus Ribavirin Treatment for Chronic Hepatitis C: Case Report and Literature Review. Clin Med Insights Endocrinol Diabetes, 2011;4:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morgan NG, Leete P, Foulis AK, Richardson SJ. Islet inflammation in human type 1 diabetes mellitus. IUBMB Life, 2014;66:723–34. [DOI] [PubMed] [Google Scholar]
- [24].Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes, 1995;44:658–64. [DOI] [PubMed] [Google Scholar]
- [25].Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet, 1987;2:1423–7. [DOI] [PubMed] [Google Scholar]
- [26].Lundberg M, Krogvold L, Kuric E, Dahl-Jorgensen K, Skog O. Expression of Interferon-Stimulated Genes in Insulitic Pancreatic Islets of Patients Recently Diagnosed With Type 1 Diabetes. Diabetes, 2016;65:3104–10. [DOI] [PubMed] [Google Scholar]
- [27].Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Karner J et al. AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell, 2016;166:582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S et al. The nature of the principal type 1 interferon-producing cells in human blood. Science, 1999;284:1835–7. [DOI] [PubMed] [Google Scholar]
- [29].Allen JS, Pang K, Skowera A, Ellis R, Rackham C, Lozanoska-Ochser B et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes, 2009;58:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chehadeh W, Weill J, Vantyghem MC, Alm G, Lefebvre J, Wattre P et al. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis, 2000;181:1929–39. [DOI] [PubMed] [Google Scholar]
- [31].Xia CQ, Peng R, Chernatynskaya AV, Yuan L, Carter C, Valentine J et al. Increased IFN-alphaproducing plasmacytoid dendritic cells (pDCs) in human Th1-mediated type 1 diabetes: pDCs augment Th1 responses through IFN-alpha production. Journal of immunology, 2014;193:1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes, 2008;57:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kallionpaa H, Elo LL, Laajala E, Mykkanen J, Ricano-Ponce I, Vaarma M et al. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes, 2014;63:2402–14. [DOI] [PubMed] [Google Scholar]
- [34].Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes, 2014;63:2538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Winkler C, Lauber C, Adler K, Grallert H, Illig T, Ziegler AG et al. An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes, 2011;60:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cabrera SM, Chen YG, Hagopian WA, Hessner MJ. Blood-based signatures in type 1 diabetes. Diabetologia, 2016;59:414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reynier F, Pachot A, Paye M, Xu Q, Turrel-Davin F, Petit F et al. Specific gene expression signature associated with development of autoimmune type-I diabetes using whole-blood microarray analysis. Genes Immun, 2010;11:269–78. [DOI] [PubMed] [Google Scholar]
- [38].Thomas VA, Woda BA, Handler ES, Greiner DL, Mordes JP, Rossini AA. Altered expression of diabetes in BB/Wor rats by exposure to viral pathogens. Diabetes, 1991;40:255–8. [DOI] [PubMed] [Google Scholar]
- [39].Sobel DO, Newsome J, Ewel CH, Bellanti JA, Abbassi V, Creswell K et al. Poly I:C induces development of diabetes mellitus in BB rat. Diabetes, 1992;41:515–20. [DOI] [PubMed] [Google Scholar]
- [40].Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nature medicine, 2005;11:138–45. [DOI] [PubMed] [Google Scholar]
- [41].Stewart TA, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan NJ. Induction of type I diabetes by interferon-alpha in transgenic mice. Science, 1993;260:1942–6. [DOI] [PubMed] [Google Scholar]
- [42].Huang X, Hultgren B, Dybdal N, Stewart TA. Islet expression of interferon-alpha precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity, 1994;1:469–78. [DOI] [PubMed] [Google Scholar]
- [43].Chakrabarti D, Hultgren B, Stewart TA. IFN-alpha induces autoimmune T cells through the induction of intracellular adhesion molecule-1 and B7.2. Journal of immunology, 1996;157:522–8. [PubMed] [Google Scholar]
- [44].Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America, 2008;105:12439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li Q, McDevitt HO. The role of interferon alpha in initiation of type I diabetes in the NOD mouse. Clinical immunology, 2011;140:3–7. [DOI] [PubMed] [Google Scholar]
- [46].Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nature medicine, 2013;19:65–73. [DOI] [PubMed] [Google Scholar]
- [47].Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nature reviews Immunology, 2015;15:471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nakazawa T, Satoh J, Takahashi K, Sakata Y, Ikehata F, Takizawa Y et al. Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. Journal of autoimmunity, 2001;17:119–25. [DOI] [PubMed] [Google Scholar]
- [49].Bortell R, Yang C. The BB rat as a model of human type 1 diabetes. Methods in molecular biology, 2012;933:31–44. [DOI] [PubMed] [Google Scholar]
- [50].Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology, 2006;43:661–72. [DOI] [PubMed] [Google Scholar]
- [51].Lee HJ, Lombardi A, Stefan M, Li CW, Inabnet WB 3rd, Owen RP et al. CD40 Signaling in Graves Disease Is Mediated Through Canonical and Noncanonical Thyroidal Nuclear Factor kappaB Activation. Endocrinology, 2017;158:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A et al. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology, 2007;148:5724–33. [DOI] [PubMed] [Google Scholar]
- [53].Lombardi A, Menconi F, Greenberg D, Concepcion E, Leo M, Rocchi R et al. Dissecting the Genetic Susceptibility to Graves’ Disease in a Cohort of Patients of Italian Origin. Front Endocrinol (Lausanne), 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Menconi F, Hasham A, Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferonalpha. Journal of endocrinological investigation, 2011;34:78–84. [DOI] [PubMed] [Google Scholar]
- [55].Caraccio N, Giannini R, Cuccato S, Faviana P, Berti P, Galleri D et al. Type I interferons modulate the expression of thyroid peroxidase, sodium/iodide symporter, and thyroglobulin genes in primary human thyrocyte cultures. The Journal of clinical endocrinology and metabolism, 2005;90:1156–62. [DOI] [PubMed] [Google Scholar]
- [56].Akeno N, Smith EP, Stefan M, Huber AK, Zhang W, Keddache M et al. IFN-alpha mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. Journal of immunology, 2011;186:4693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Caraccio N, Cuccato S, Pratesi F, Dardano A, Ursino S, Chimenti D et al. Effect of type I interferon(s) on cell viability and apoptosis in primary human thyrocyte cultures. Thyroid : official journal of the American Thyroid Association, 2009;19:149–55. [DOI] [PubMed] [Google Scholar]
- [58].Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ et al. Interferoninducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America, 2003;100:2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. The Journal of experimental medicine, 2017;214:1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis and rheumatism, 2004;50:1861–72. [DOI] [PubMed] [Google Scholar]
- [61].Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. The Journal of experimental medicine, 2014;211:1969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. The Journal of experimental medicine, 2014;211:1977–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P et al. Anifrolumab, an AntiInterferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis & rheumatology, 2017;69:376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Leung PS, Van de Water J, Coppel RL, Nakanuma Y, Munoz S, Gershwin ME. Molecular aspects and the pathological basis of primary biliary cirrhosis. Journal of autoimmunity, 1996;9:119–28. [DOI] [PubMed] [Google Scholar]
- [65].Kaplan MM, Gershwin ME. Primary biliary cirrhosis. The New England journal of medicine, 2005;353:1261–73. [DOI] [PubMed] [Google Scholar]
- [66].Sasaki M, Yoshimura-Miyakoshi M, Sato Y, Nakanuma Y. A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis. Journal of gastroenterology, 2015;50:984–95. [DOI] [PubMed] [Google Scholar]
- [67].Tanaka A, Borchers AT, Ishibashi H, Ansari AA, Keen CL, Gershwin ME. Genetic and familial considerations of primary biliary cirrhosis. The American journal of gastroenterology, 2001;96:8–15. [DOI] [PubMed] [Google Scholar]
- [68].Van de Water J, Ishibashi H, Coppel RL, Gershwin ME. Molecular mimicry and primary biliary cirrhosis: premises not promises. Hepatology, 2001;33:771–5. [DOI] [PubMed] [Google Scholar]
- [69].Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet, 2011;377:1600–9. [DOI] [PubMed] [Google Scholar]
- [70].Morshed SA, Nishioka M, Saito I, Komiyama K, Moro I. Increased expression of Epstein-Barr virus in primary biliary cirrhosis patients. Gastroenterologia Japonica, 1992;27:751–8. [DOI] [PubMed] [Google Scholar]
- [71].Xu L, Shen Z, Guo L, Fodera B, Keogh A, Joplin R et al. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proceedings of the National Academy of Sciences of the United States of America, 2003;100:8454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mason AL. The evidence supports a viral aetiology for primary biliary cirrhosis. Journal of hepatology, 2011;54:1312–4. [DOI] [PubMed] [Google Scholar]
- [73].Okada C, Akbar SM, Horiike N, Onji M. Early development of primary biliary cirrhosis in female C57BL/6 mice because of poly I:C administration. Liver Int, 2005;25:595–603. [DOI] [PubMed] [Google Scholar]
- [74].Bae HR, Hodge DL, Yang GX, Leung PSC, Chodisetti SB, Valencia JC et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology, 2018;67:1408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yoshikawa M, Mimura M, Shiroi A, Kojima H, Fukui H, Sugimoto Y et al. Primary biliary cirrhosis exacerbated by a course of acute hepatitis C and subsequent interferon therapy. Am J Gastroenterol, 2000;95:2396–7. [DOI] [PubMed] [Google Scholar]
- [76].Maeda T, Onishi S, Miura T, Iwamura S, Tomita A, Saibara T et al. Exacerbation of primary biliary cirrhosis during interferon-alpha 2b therapy for chronic active hepatitis C. Dig Dis Sci, 1995;40:1226–30. [DOI] [PubMed] [Google Scholar]
- [77].D’Amico E, Paroli M, Fratelli V, Palazzi C, Barnaba V, Callea F et al. Primary biliary cirrhosis induced by interferon-alpha therapy for hepatitis C virus infection. Dig Dis Sci, 1995;40:2113–6. [DOI] [PubMed] [Google Scholar]
- [78].Passos de Souza E, Evangelista Segundo PT, Jose FF, Lemaire D, Santiago M. Rheumatoid arthritis induced by alpha-interferon therapy. Clin Rheumatol, 2001;20:297–9. [DOI] [PubMed] [Google Scholar]
- [79].Sood A, Midha V, Sood N. Rheumatoid arthritis probably induced by pegylated interferon in a patient with chronic hepatitis C. Indian J Gastroenterol, 2004;23:28–9. [PubMed] [Google Scholar]
- [80].Ionescu C, Micu L, Constantinescu I, Hortopan M, Ursaciuc C, Voiculescu M. Prolonged treatment with interferon alpha and peginterferon induces rheumatoid arthritis syndrome and erythema nodosum. J Gastrointestin Liver Dis, 2008;17:211–2. [DOI] [PubMed] [Google Scholar]
- [81].Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. The New England journal of medicine, 1979;301:5–8. [DOI] [PubMed] [Google Scholar]
- [82].Gattorno M, Chicha L, Gregorio A, Ferlito F, Rossi F, Jarrossay D et al. Distinct expression pattern of IFN-alpha and TNF-alpha in juvenile idiopathic arthritis synovial tissue. Rheumatology, 2007;46:657–65. [DOI] [PubMed] [Google Scholar]
- [83].Sigurdsson S, Padyukov L, Kurreeman FA, Liljedahl U, Wiman AC, Alfredsson L et al. Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis and rheumatism, 2007;56:2202–10. [DOI] [PubMed] [Google Scholar]
- [84].Roelofs MF, Wenink MH, Brentano F, Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Annals of the rheumatic diseases, 2009;68:1486–93. [DOI] [PubMed] [Google Scholar]
- [85].Conigliaro P, Perricone C, Benson RA, Garside P, Brewer JM, Perricone R et al. The type I IFN system in rheumatoid arthritis. Autoimmunity, 2010;43:220–5. [DOI] [PubMed] [Google Scholar]
- [86].Mavragani CP, La DT, Stohl W, Crow MK. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: a post hoc analysis of a predominantly Hispanic cohort. Arthritis and rheumatism, 2010;62:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Thurlings RM, Boumans M, Tekstra J, van Roon JA, Vos K, van Westing DM et al. Relationship between the type I interferon signature and the response to rituximab in rheumatoid arthritis patients. Arthritis and rheumatism, 2010;62:3607–14. [DOI] [PubMed] [Google Scholar]
- [88].Wright HL, Thomas HB, Moots RJ, Edwards SW. Interferon gene expression signature in rheumatoid arthritis neutrophils correlates with a good response to TNFi therapy. Rheumatology, 2015;54:188–93. [DOI] [PubMed] [Google Scholar]
- [89].Muray S, Marco MP, Carrera I, Cao G, Craver L, Fernandez E. Relative hypoparathyroidism induced by interferon treatment in a hemodialysis patient. Clinical nephrology, 2005;64:163–6. [DOI] [PubMed] [Google Scholar]
- [90].Ueki K, Tsuchida A, Murakami H, Yano S, Kobayashi N, Omine M et al. Hypoparathyroidism during alpha-INF therapy in a patient with multiple myeloma. Journal of medicine, 1989;20:391–7. [PubMed] [Google Scholar]
- [91].Di Sabatino A, Pickard KM, Gordon JN, Salvati V, Mazzarella G, Beattie RM et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology, 2007;133:1175–87. [DOI] [PubMed] [Google Scholar]
- [92].Cammarota G, Cuoco L, Cianci R, Pandolfi F, Gasbarrini G. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. Lancet, 2000;356:1494–5. [DOI] [PubMed] [Google Scholar]
- [93].Monteleone G, Pender SL, Alstead E, Hauer AC, Lionetti P, McKenzie C et al. Role of interferon alpha in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut, 2001;48:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Congeni JP, Kirkpatrick RB. Pegylated interferon induced myasthenia crisis--a case report. Journal of clinical neuromuscular disease, 2013;14:123–5. [DOI] [PubMed] [Google Scholar]
- [95].Borgia G, Reynaud L, Gentile I, Cerini R, Ciampi R, Dello Russo M et al. Myasthenia gravis during low-dose IFN-alpha therapy for chronic hepatitis C. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 2001;21:469–70. [DOI] [PubMed] [Google Scholar]
- [96].Mendieta KL, Irfan M, Fernandez Faith E. Interferon-alpha induced psoriasis in a teenager. Pediatric dermatology, 2018;35:e136–e7. [DOI] [PubMed] [Google Scholar]
- [97].Oiso N, Sato M, Kawada A. Vitiligo after combination therapy of pegylated interferon-alpha-2a, ribavirin and vitamin D in a patient with chronic hepatitis C. The Journal of dermatology, 2013;40:772–3. [DOI] [PubMed] [Google Scholar]
- [98].Seckin D, Durusoy C, Sahin S. Concomitant vitiligo and psoriasis in a patient treated with interferon alfa-2a for chronic hepatitis B infection. Pediatric dermatology, 2004;21:577–9. [DOI] [PubMed] [Google Scholar]
- [99].Thevenot T, Bachmeyer C, Hammi R, Dumouchel P, Ducamp-Posak I, Cadranel JF. Occurrence of porphyria cutanea tarda during peginterferon/ribavirin therapy for chronic viral hepatitis C. Journal of hepatology, 2005;42:607–8. [DOI] [PubMed] [Google Scholar]
- [100].Said A, Elbahrawy A, Alfiomy M, Abdellah M, Shahat K, Salah M et al. Pegylated interferon de novo-induce autoimmune haemolytic anaemia in chronic hepatitis C patient. BMJ case reports, 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cauli C, Serra G, Chessa L, Balestrieri C, Scioscia R, Lai ME et al. Severe autoimmune hemolytic anemia in a patient with chronic hepatitis C during treatment with peginterferon alfa-2a and ribavirin. Haematologica, 2006;91:ECR26. [PubMed] [Google Scholar]
- [102].Higgs BW, Zhu W, Morehouse C, White WI, Brohawn P, Guo X et al. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-alpha monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Annals of the rheumatic diseases, 2014;73:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Buss G, Cattin V, Spring P, Malinverni R, Gilliet M. Two cases of interferon-alpha-induced sarcoidosis Koebnerized along venous drainage lines: new pathogenic insights and review of the literature of interferon-induced sarcoidosis. Dermatology, 2013;226:289–97. [DOI] [PubMed] [Google Scholar]
- [104].Andreoli MT, Lim JI. Cotton-wool spots and retinal hemorrhages. Interferon-associated retinopathy. JAMA ophthalmology, 2014;132:503–4. [DOI] [PubMed] [Google Scholar]
- [105].Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America, 2007;104:5115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes, 2008;57:2863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jaeckel E, Manns M, Von Herrath M. Viruses and diabetes. Ann N Y Acad Sci, 2002;958:7–25. [DOI] [PubMed] [Google Scholar]
- [108].Tomer Y, Davies TF. Infections and autoimmune endocrine disease. Bailliere’s clinical endocrinology and metabolism, 1995;9:47–70. [DOI] [PubMed] [Google Scholar]
- [109].Mustonen N, Siljander H, Peet A, Tillmann V, Harkonen T, Ilonen J et al. Early childhood infections precede development of beta-cell autoimmunity and type 1 diabetes in children with HLA-conferred disease risk. Pediatric diabetes, 2018;19:293–9. [DOI] [PubMed] [Google Scholar]
- [110].Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. Bmj, 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia, 2013;56:185–93. [DOI] [PubMed] [Google Scholar]
- [112].Honkanen H, Oikarinen S, Nurminen N, Laitinen OH, Huhtala H, Lehtonen J et al. Detection of enteroviruses in stools precedes islet autoimmunity by several months: possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia, 2017;60:424–31. [DOI] [PubMed] [Google Scholar]
- [113].Elfaitouri A, Berg AK, Frisk G, Yin H, Tuvemo T, Blomberg J. Recent enterovirus infection in type 1 diabetes: evidence with a novel IgM method. Journal of medical virology, 2007;79:1861–7. [DOI] [PubMed] [Google Scholar]
- [114].Schulte BM, Bakkers J, Lanke KH, Melchers WJ, Westerlaken C, Allebes W et al. Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral immunology, 2010;23:99–104. [DOI] [PubMed] [Google Scholar]
- [115].Krogvold L, Edwin B, Buanes T, Frisk G, Skog O, Anagandula M et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes, 2015;64:1682–7. [DOI] [PubMed] [Google Scholar]
- [116].Hodik M, Anagandula M, Fuxe J, Krogvold L, Dahl-Jorgensen K, Hyoty H et al. Coxsackieadenovirus receptor expression is enhanced in pancreas from patients with type 1 diabetes. BMJ open diabetes research & care, 2016;4:e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zanone MM, Favaro E, Ferioli E, Huang GC, Klein NJ, Perin PC et al. Human pancreatic islet endothelial cells express coxsackievirus and adenovirus receptor and are activated by coxsackie B virus infection. FASEB J, 2007;21:3308–17. [DOI] [PubMed] [Google Scholar]
- [118].Blackard JT, Kong L, Lombardi A, Homann D, Hammerstad SS, Tomer Y. A preliminary analysis of hepatitis C virus in pancreatic islet cells. Virology journal, 2017;14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG. The viral paradigm in type 1 diabetes: Who are the main suspects? Autoimmun Rev, 2016;15:964–9. [DOI] [PubMed] [Google Scholar]
- [120].Anagandula M, Richardson SJ, Oberste MS, Sioofy-Khojine AB, Hyoty H, Morgan NG et al. Infection of human islets of Langerhans with two strains of Coxsackie B virus serotype 1: assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. Journal of medical virology, 2014;86:1402–11. [DOI] [PubMed] [Google Scholar]
- [121].Gallagher GR, Brehm MA, Finberg RW, Barton BA, Shultz LD, Greiner DL et al. Viral infection of engrafted human islets leads to diabetes. Diabetes, 2015;64:1358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature, 2001;413:732–8. [DOI] [PubMed] [Google Scholar]
- [123].Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med, 2011;3:73ra–19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chakrabarti D, Huang X, Beck J, Henrich J, McFarland N, James RF et al. Control of islet intercellular adhesion molecule-1 expression by interferon-alpha and hypoxia. Diabetes, 1996;45:1336–43. [DOI] [PubMed] [Google Scholar]
- [125].Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends in immunology, 2002;23:201–8. [DOI] [PubMed] [Google Scholar]
- [126].Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science, 2002;297:2063–6. [DOI] [PubMed] [Google Scholar]
- [127].Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature, 2005;436:967–72. [DOI] [PubMed] [Google Scholar]
- [128].Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. Journal of immunology, 2001;167:1179–87. [DOI] [PubMed] [Google Scholar]
- [129].Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. The Journal of experimental medicine, 2000;191:1777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood, 2002;99:3263–71. [DOI] [PubMed] [Google Scholar]
- [131].Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science, 1996;272:1947–50. [DOI] [PubMed] [Google Scholar]
- [132].Kono H, Rock KL. How dying cells alert the immune system to danger. Nature reviews Immunology, 2008;8:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Diana J, Brezar V, Beaudoin L, Dalod M, Mellor A, Tafuri A et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. The Journal of experimental medicine, 2011;208:729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity, 2009;30:289–99. [DOI] [PubMed] [Google Scholar]
- [135].Mallone R, Brezar V. To B or not to B: (anti)bodies of evidence on the crime scene of type 1 diabetes? Diabetes, 2011;60:2020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. Journal of immunology, 2012;188:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Krause I, Valesini G, Scrivo R, Shoenfeld Y. Autoimmune aspects of cytokine and anticytokine therapies. The American journal of medicine, 2003;115:390–7. [DOI] [PubMed] [Google Scholar]
- [138].Corssmit EP, de Metz J, Sauerwein HP, Romijn JA. Biologic responses to IFN-alpha administration in humans. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 2000;20:1039–47. [DOI] [PubMed] [Google Scholar]
- [139].Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nature reviews Immunology, 2012;12:479–91. [DOI] [PubMed] [Google Scholar]
- [140].Shimizu F, Shimizu M, Kamiyama K. Inhibitory effect of interferon on the production of insulin. Endocrinology, 1985;117:2081–4. [DOI] [PubMed] [Google Scholar]
- [141].Rhodes CJ, Taylor KW. Effect of human lymphoblastoid interferon on insulin synthesis and secretion in isolated human pancreatic islets. Diabetologia, 1984;27:601–3. [DOI] [PubMed] [Google Scholar]
- [142].Lombardi A, Inabnet WB, Owen R 3rd, Farenholtz KE, Tomer Y. Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis. The Journal of clinical endocrinology and metabolism, 2015;100:E1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Marroqui L, Dos Santos RS, Op de Beeck A, Coomans de Brachene A, Marselli L, Marchetti P et al. Interferon-alpha mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia, 2017;60:656–67. [DOI] [PubMed] [Google Scholar]
- [144].Harrison LC, Campbell IL, Allison J, Miller JF. MHC molecules and beta-cell destruction. Immune and nonimmune mechanisms. Diabetes, 1989;38:815–8. [DOI] [PubMed] [Google Scholar]
- [145].Oon S, Wilson NJ, Wicks I. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clinical & translational immunology, 2016;5:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lombardi A, Trimarco B, Iaccarino G, Santulli G. Impaired mitochondrial calcium uptake caused by tacrolimus underlies beta-cell failure. Cell Commun Signal, 2017;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lombardi A, Gambardella J, Du XL, Sorriento D, Mauro M, Iaccarino G et al. Sirolimus induces depletion of intracellular calcium stores and mitochondrial dysfunction in pancreatic beta cells. Scientific reports, 2017;7:15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Shan B, Vazquez E, Lewis JA. Interferon selectively inhibits the expression of mitochondrial genes: a novel pathway for interferon-mediated responses. EMBO J, 1990;9:4307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lewis JA, Huq A, Najarro P. Inhibition of mitochondrial function by interferon. The Journal of biological chemistry, 1996;271:13184–90. [DOI] [PubMed] [Google Scholar]
- [150].Maechler P, Wollheim CB. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol, 2000;529 Pt 1:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yanase N, Ohshima K, Ikegami H, Mizuguchi J. Cytochrome c release, mitochondrial membrane depolarization, caspase-3 activation, and Bax-alpha cleavage during IFN-alpha-induced apoptosis in Daudi B lymphoma cells. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 2000;20:1121–9. [DOI] [PubMed] [Google Scholar]
- [152].Matsuoka M, Tani K, Asano S. Interferon-alpha-induced G1 phase arrest through up-regulated expression of CDK inhibitors, p19Ink4D and p21Cip1 in mouse macrophages. Oncogene, 1998;16:2075–86. [DOI] [PubMed] [Google Scholar]
- [153].Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes, 1989;38:641–7. [DOI] [PubMed] [Google Scholar]
- [154].Leslie RD, Taylor R, Pozzilli P. The role of insulin resistance in the natural history of type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association, 1997;14:327–31. [DOI] [PubMed] [Google Scholar]
- [155].Stefan M, Zhang W, Concepcion E, Yi Z, Tomer Y. DNA methylation profiles in type 1 diabetes twins point to strong epigenetic effects on etiology. Journal of autoimmunity, 2014;50:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature, 2008;455:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Stefan M, Jacobson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L et al. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alphamodulated mechanism. The Journal of biological chemistry, 2011;286:31168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bonifacio E, Warncke K, Winkler C, Wallner M, Ziegler AG. Cesarean section and interferoninduced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes, 2011;60:3300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Miao F, Chen Z, Zhang L, Wang J, Gao H, Wu X et al. RNA-sequencing analysis of high glucosetreated monocytes reveals novel transcriptome signatures and associated epigenetic profiles. Physiological genomics, 2013;45:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ronnblom L The importance of the type I interferon system in autoimmunity. Clinical and experimental rheumatology, 2016;34:21–4. [PubMed] [Google Scholar]
- [161].Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG et al. Sifalimumab, an antiinterferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Annals of the rheumatic diseases, 2016;75:1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lauwerys BR, Hachulla E, Spertini F, Lazaro E, Jorgensen C, Mariette X et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon alpha-kinoid. Arthritis and rheumatism, 2013;65:447–56. [DOI] [PubMed] [Google Scholar]
- [163].Larsson PG, Lakshmikanth T, Laitinen OH, Utorova R, Jacobson S, Oikarinen M et al. A preclinical study on the efficacy and safety of a new vaccine against Coxsackievirus B1 reveals no risk for accelerated diabetes development in mouse models. Diabetologia, 2015;58:346–54. [DOI] [PubMed] [Google Scholar]
- [164].Moell A, Skog O, Ahlin E, Korsgren O, Frisk G. Antiviral effect of nicotinamide on enterovirusinfected human islets in vitro: effect on virus replication and chemokine secretion. Journal of medical virology, 2009;81:1082–7. [DOI] [PubMed] [Google Scholar]
- [165].Berg AK, Olsson A, Korsgren O, Frisk G. Antiviral treatment of Coxsackie B virus infection in human pancreatic islets. Antiviral research, 2007;74:65–71. [DOI] [PubMed] [Google Scholar]
- [166].Alidjinou EK, Sane F, Bertin A, Caloone D, Hober D. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antiviral research, 2015;116:51–4. [DOI] [PubMed] [Google Scholar]