Abstract
Neural stem cells (NSCs) in the adult mammalian spinal cord are a relatively mitotically quiescent population of periventricular cells that can be studied in vitro using the neurosphere assay. This colony-forming assay is a powerful tool to study the response of NSCs to exogenous factors in a dish; however, this can also be used to study the effect of in vivo manipulations with the proper understanding of the strengths and limitations of the assay. One manipulation of the clinical interest is the effect of injury on endogenous NSC activation. Current models of spinal cord injury provide a challenge to study this as the severity of common contusion, compression, and transection models cause the destruction of the NSC niche at the site of the injury where the stem cells reside. Here, we describe a minimal injury model that causes localized damage at the superficial dorsolateral surface of the lower thoracic level (T7/8) of the adult mouse spinal cord. This injury model spares the central canal at the level of injury and permits analysis of the NSCs that reside at the level of the lesion at various time points following injury. Here, we show how the neurosphere assay can be utilized to study the activation of the two distinct, lineally-related, populations of NSCs that reside in the spinal cord periventricular region - primitive and definitive NSCs (pNSCs and dNSCs, respectively). We demonstrate how to isolate and culture these NSCs from the periventricular region at the level of injury and the white matter injury site. Our post-surgical spinal cord dissections show increased numbers of pNSC and dNSC-derived neurospheres from the periventricular region of injured cords compared to controls, speaking to their activation via injury. Furthermore, following injury, dNSC-derived neurospheres can be isolated from the injury site — demonstrating the ability of NSCs to migrate from their periventricular niche to sites of injury.
Keywords: Neuroscience, Issue 139, Minimal spinal cord injury, adult mouse, neural stem cells, periventricular dissection, neurosphere assay, stem cell kinetics, primitive neural stem cells, definitive neural stem cell, stem cell activation, stem cell migration, neuroscience
Introduction
The central nervous system contains a subpopulation of self-renewing, multipotent stem cells that have the capacity to give rise to all the different mature neural cell types1,2,3,4. These neural stem cells (NSCs) reside in specialized niches in the brain and spinal cord and can be activated following injury to proliferate, migrate, and differentiate into mature neural cells. NSCs and their progeny have been shown to migrate to the injury site in cortical injury models5,6. In the brain, NSCs have been shown to migrate from the lateral ventricles to the site of injury where they differentiate into astrocytes that contribute to glial scar formation7. In the spinal cord, however, few studies have been done to ask if these same endogenous NSCs can be harnessed to promote recovery following spinal cord injury. Indeed, there is currently a debate as to whether activation of the stem cell pool in the spinal cord requires a direct physical damage of the periventricular niche lining the central canal8 or if the damage to the spinal cord parenchyma (leaving the stem cell niche intact) is sufficient to activate endogenous NSCs9.
A number of spinal cord injury (SCI) models have been used to study the pathophysiology of acute and chronic injury. These models have also been used to test potential therapies to treat SCI through neuroprotection, immunomodulation, and developing cell transplantation/replacement strategies10,11,13. Current models include compression and/or contusion injuries, which cause large-scale functional deficits as well as extensive lesions and cavitations in the cord14,15. Resultant glial scars can span several spinal segments along with the majority of the width/circumference of the spinal cord16. Thus, while these models are clinically relevant, they afford significant challenges to studying the response of endogenous NSCs following injury. There are chemical models of injury that can be adapted to cause milder forms of injury that can spare the central canal17. However, these types of injury focus on the demyelination associated with SCI and are not clinically relevant models for the physical and/or mechanical damage associated with traumatic SCI.
To address the limitations of current injury models, we have adapted a needle track minimal SCI model, originally developed in the rat9, for the application in an adult mouse model. Our adapted injury model can create a consistent lesion of the dorsolateral region of the mouse spinal cord and spare the central canal at the level(s) of injury. The advantage of this model is that it permits the study of NSC kinetics following injury and their potential radial migration to the site of injury. The use of a mouse model also permits the use of transgenic mice that allow lineage tracking of endogenous NSCs and their progeny following injury. The properties of NSCs can further be assessed using a modified form of the in vitro neurosphere assay that is introduced in this protocol.
The neurosphere assay is an in vitro colony-forming assay that permits the isolation of NSCs in the presence of mitogens. At clonal plating densities, individual NSCs proliferate to give rise to free-floating spherical colonies of cells that are comprised of a small subpopulation of NSCs and a vast majority of progenitors18,19. In our protocol, we demonstrate the isolation of two distinct, lineally-related NSCs from the periventricular region of the spinal cord — under baseline conditions and following our minimal SCI model. Definitive neural stem cells (dNSCs) express nestin and the glial fibrillary acidic protein (GFAP) and are grown in the presence of epidermal growth factor (EGF), fibroblast growth factor (FGF), and heparin (together termed EFH)20. These dNSCs are rare in the naive spinal cord, giving rise to very few neurospheres in vitro. However, we show that dNSCs are activated following minimal SCI, expanding the numbers of neurospheres isolated from the periventricular region21. Primitive neural stem cells (pNSCs) are upstream of dNSCs in the neural stem cell lineage. pNSCs are exceedingly rare, express low levels of the pluripotency marker Oct4, and are leukemia inhibitory factor (LiF) responsive22. pNSCs do not form neurospheres when isolated from the adult mouse spinal cord due to the presence of myelin basic protein (MBP) in primary cultures; however, pNSC neurospheres can be isolated from MBP deficient mice and their numbers are expanded following injury — similar to dNSCs21. Finally, we show that dNSC-derived neurospheres can be isolated from the site of injury at early times following minimal SCI. These findings demonstrate that our injury model and assays can assess the activation characteristics of periventricular NSCs such as their ability to proliferate and migrate in response to injury.
Protocol
This protocol was approved by the Animal Care Committee at the University of Toronto and is in accordance with the "Guide to the Care and Use of Experimental Animals" (2nd Edition, Canadian Council on Animal Care, 2017).
1. Minimal Spinal Cord Injury Surgery
NOTE: Prior to surgery make sure that all surgical instruments and materials are sterilized by appropriate methods (Figure 1A).
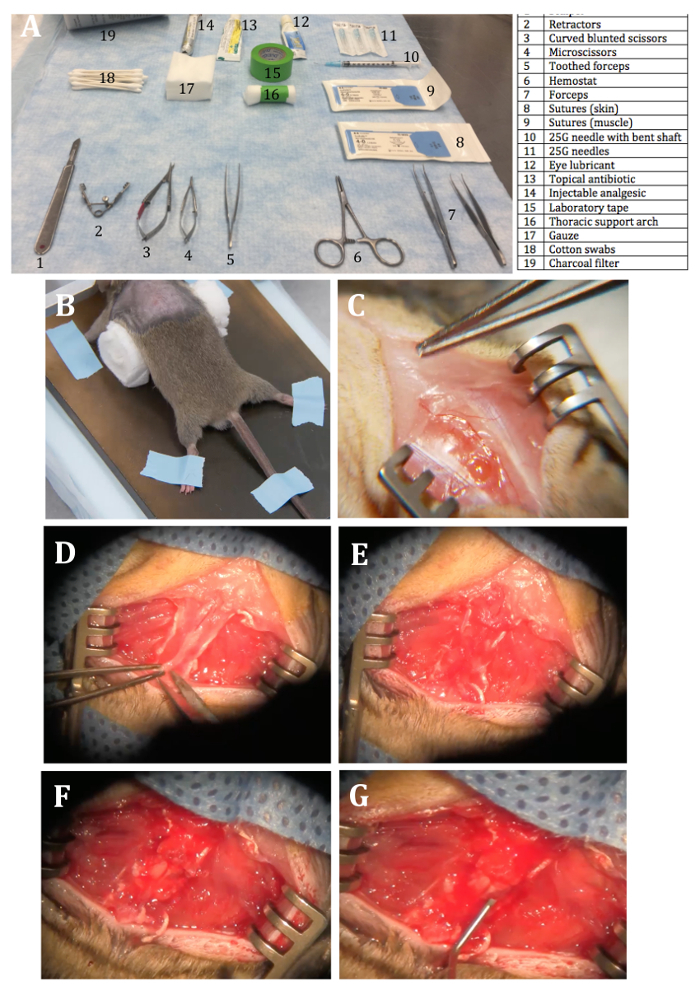
Build a thoracic support arch by rolling up 4–5 squares of gauze and taping them together in the middle to obtain a fixed roll of gauze.
Place the mouse in the induction chamber and initiate anesthesia with 5% isoflurane. Confirm the absence of a toe pinch reflex before proceeding, as well as throughout the procedure. As the surgery can take upwards of 30 min to complete, optimize for the minimal isoflurane exposure (5 min at 5% and the remaining time at 2–3%).
Transfer the mouse to a nose cone attached to the anesthetic machine at 2–3% isoflurane maintenance dose. Use hair clippers to remove fur from the dorsum of the animal from mid-back up to the neck/ears to expose a wide rectangular area of skin for surgery.
Transfer the mouse to a stereotaxic instrument by placing its nose in the attached nose cone and stabilizing the skull with ear bars. Maintain isoflurane at 5% during placement of the ear bars and lower back to 2–3% once the mouse is secured in the stereotaxic device.
Administer preoperative analgesic medications intraperitoneally — Meloxicam (2.0 mg/kg). Generously apply eye lubrication onto the open mouse eyes to prevent corneal desiccation when under anesthesia.
Place the thoracic support arch underneath the mouse abdomen while straightening out the mouse body and spine by lightly pulling on the base of the tail. Use laboratory labeling tape to secure the tail and all extended limbs in a star-like position. Once secure, push the thoracic support arch rostrally, from the mouse abdomen towards the upper thorax — in order to prop up the thoracic spine (Figure 1B).
Prepare a sterile surgical field by disinfecting the fur-clipped skin area prepared in step 1.3 with 70% ethanol, followed by povidone-iodine. Repeat twice. Apply a sterile surgical drape to keep a wide sterile field.
Using a #10 scalpel blade, make a vertical incision parallel to the longitudinal axis of the animal from the mid-point of both shoulder blades to the curvature of the thoracic spine. Retract the skin to expose soft tissue and the spinal column contour (Figure 1C).
Identify the lower border of the suprascapular fat pad (this demarcates the T4/5 vertebral level). With the same #10 blade, carefully but with force, cut along both sides of the vertebral bone T5–T8/9 to detach the back-muscle tendons from the column.
Insert the teeth of retractors into the incision sites on either side of the spine. Adjust the exposure by expanding retractors to sufficiently elevate the spine without putting too much strain on the retracted muscle layers (Figure 1D).
Under the surgical microscope, carefully clean the residual muscle and other soft tissue overlying the spine to expose the vertebral bone (Figure 1E). Identify the vertebrae that will be removed by clasping the spinous process of the vertebrae with toothed forceps and moving it slightly up and down. NOTE: This should allow identification of intervertebral joints and expose the small openings (intervertebral foramina) underneath the vertebrae of interest.
Insert one head of the curved blunted scissors into either side of the exposed intervertebral foramen, caudal to the vertebral lamina to be excised out, and cut the connecting intervertebral joints bilaterally.
Lift the lamina upwards and cut off the upper attachment of the lamina to isolate and remove the bone. NOTE: This will expose the intact dural sac containing the spinal cord (Figure 1F). There might be excessive bleeding which can be controlled by placing precut 1 cm x 1 cm gauze onto the affected areas and/or wash with sterile PBS.
With the dorsal midline vein serving as a landmark, insert a 45° bent shaft of a 30 G needle tip (with needle bevel facing upwards) into the dorsolateral surface of the spinal cord (approximately 1 mm deep) at approximately 0.5 mm lateral to either side of the midline.
Move the needle ~2 mm from caudal to rostral (parallel to the midline) so that the entire length of the bevel of the needle is inserted into the cord. Remove the needle by retracing via the path of entry (Figure 1G). NOTE: There should be no bleeding but swelling of the spinal tissue may be seen at this step.
To close the wound, remove the retractor and suture the back muscles on either side of the injury together in the midline using a 6-0 absorbable suture. Use a 4-0 sterile silk suture to subsequently close the overlying skin.
Apply antibiotic ointment to the top of the superficial sutured skin to prevent postoperative infection using the tip of a cotton swab. Administer post-operative analgesic of 0.1 mg/kg of Buprenorphine subcutaneously along with fluids (1 mL of lactated Ringer's solution).
Turn off the isoflurane vaporizer and oxygen. Remove the mouse from the stereotaxic device and place in a clean cage with no bedding.
Place the mouse in the cage about 30 cm away from a heat lamp for recovery from anesthesia and monitor behavior following waking. The mouse should wake up within 5–10 min and have the hind-limb function with possible paresis and/or tail weakness upon waking.
Monitor mouse behavior over the next few days and provide proper post-operative care and analgesics (i.e., mash, lactated Ringer's solution fluid subcutaneous, 0.1 mg/kg buprenorphine 2x daily subcutaneously for 2–3 days, and proper weight monitoring) in accordance to local animal facility regulations and on an individual basis as recovery may vary.
2. Neurosphere Assay Dissection
NOTE: Follow proper sterile tissue culture procedures throughout.
- Enzymatic Solution Preparation
- Add 32 mL of Earle's Balanced Salt Solution (EBSS) to the albumin ovomucoid inhibitor containing glass bottle and gently vortex until dissolved.
- Add 5 mL of EBSS to a pre-portioned papain glass vial and place in a 37 °C water bath to dissolve. The solution will become clear (Solution 1).
- Take 500 µL of EBSS and add to the pre-portioned DNase I glass vial to obtain a concentration of 1 mg/mL (Solution 2). Mix very gently by tilting the vial back and forth and add 250 µL of this mixture to Solution 1. NOTE: All solutions made (Solution 1 and 2) are enough for processing of two pieces of the tissue. If processing more pieces of tissue, accommodate with more solution prep.
- Tissue Preparation and Dissection
- Place a laboratory wipe soaked in isoflurane into the mouse cage and allow 2 - 3 min for vapors to completely anesthetize mouse. Following knockout, remove the mouse from the cage and confirm the absence of a toe-pinch reflex.
- Perform cervical dislocation with a blunt metal object (i.e., metal cage card) to euthanize the animal. Spray the euthanized animal from its neck to mid back generously with 70% ethanol.
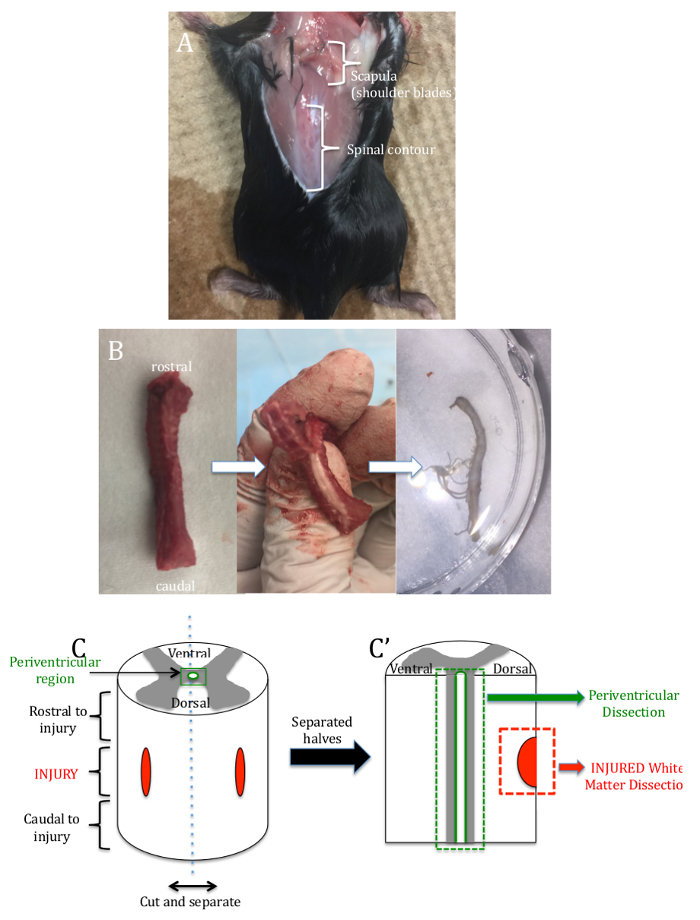
- At the base of the skull, use scissors to cut off the head and discard into a bio bag. Make a midline incision in the skin along the entire length of the back to expose the muscle and vertebral bone contour (Figure 2A).
- Lift the medial aspect of each scapula (i.e., shoulder blade — identified by overlying fat pad) and use scissors to cut away soft tissue (between scapulae and vertebrae). Separate fully to expose the underlying vertebral column.
- Following the curvature of the spinal column, insert the same pair of scissors into the upper thoracic aperture and isolate the vertebral column by cutting away attached ribs, muscle, and internal organs (Figure 2B).
- Insert scissors into the caudal vertebral foramen/opening and cut the intervertebral joints on either side of the laminae (dorsal surface). Take particular care to not damage the intact cord. Continue this process until the desired level and/or length of the cord is exposed.
- Carefully cut the spinal nerve(s) extending laterally from the cord prior to transferring the spinal cord tissue into a Petri dish with regular artificial cerebrospinal fluid. Keep the sample on ice (Figure 2C).
- Under a dissection microscope, cut the spinal cord tissue to include ~2 mm rostral and caudal to the injury site. Use two pairs of forceps to gently separate the cord into 2 halves longitudinally along the dorsal and ventral fissures (Figure 2C').
- With microscissors, cut out the injured dorsolateral white matter. Place the piece into a 15 mL conical tube.
- Holding one half of the spinal cord down with forceps, tease and remove white matter using another pair of fine forceps along the rostrocaudal extent of the spinal cord to isolate the desired periventricular region. Repeat for the other half of the spinal cord.
- Place pieces of periventricular tissue into a separate 15 mL conical tubes. Use microscissors and gently mince the tissue against the walls of the conical tubes. NOTE: Tissue Dissociation Modifications are from Papain Dissociation System Protocol23 and previously described tissue preparation procedures24.
- Add 2.5 mL of new Solution 1 (step 2.1.3) to the tissue sample and place on a rocker at 37 °C for 30 min.
- Gently triturate the tissue in solution with a 1,000 µL pipette tip. Centrifuge the cloudy cell suspension at 300 x g for 5 min at RT.
- Prepare Solution 3 with 2.7 mL of Earle's Balanced Salt Solution, 300 µL of ovomucoid inhibitor solution (step 2.1.1) and 150 µL of the DNase I solution (step 2.1.3).
- Discard the supernatant from step (step 2.2.13) and resuspend in 1.5 mL of Solution 3 from the previous step (step 2.2.14).
- Layer this new cell suspension (step 2.2.15) on top of 5 mL of ovoimucoid inhibitor solution (step 2.1.1) in a new 15 mL conical tube to create a discontinuous density gradient. Centrifuge at 300 x g for 5 min at RT.
- Discard supernatant and resuspend in 2 mL of neurobasal media. Centrifuge at 300 x g for 3 min at RT.
- Discard supernatant and resuspend in 1 mL of neurobasal media. Filter the suspension using a 20 µm cell strainer followed by 4 mL of media for a total volume of 5 mL.
- Plating
- Prepare culture media for neurosphere growth by supplementing neurobasal media with epidermal growth factor (20 ng/mL) / fibroblast growth factor (20 ng/mL)/ heparin (2 µg/mL) [for definitive NSCs] or leukemina inhibitory factor (10 ng/mL) [for primitive NSCs]. Add 10 mL of either media into a 25 mL tissue culture flask.
- Use a hemocytometer and Trypan blue dye to calculate the number of cells in suspension from (step 2.2.18)25. Once calculated, add cells to tissue culture flasks prepared in (step 2.3.1) to a clonal density of 10 cells/µL and place tissue culture flask with added cells into the incubator for 24 h (37 °C, 95% humidity, 5% CO2). NOTE: An example of the counting technique is as follows: Take 10 µL of the cell suspension and mix with 10 µL of Trypan blue dye. Add 10 µL of this mixture into a hemocytometer. Under a 10X light microscope, count the numbers of live cells and sum all cells counted within 4 of the 4 x 4 quadrants. Use the formula: 100,000/[(X cells/4) x 2 x 10 x 1,000] to give the volume of cell suspension to be plated in each 25 mL tissue culture flask to ensure clonal density (10 cells/µL).
- After 24 h, gently swirl the flask and pour contents into a 15 mL conical tube. Centrifuge at 300 x g for 5 min at RT.
- Discard the supernatant and re-suspend the cells in 1–2 mL of fresh neurobasal media.
- Repeat step 2.3.2 to count the number of cells in suspension and plate cells at clonal density in a 24-well tissue culture plate containing 500 µL each of their respective mitogen supplemented media (EFH for definitive NSC growth and LIF for primitive NSC growth). NOTE: Use the adapted formula (5,000/[X cells/4) x 2 x 10 x 1,000) to calculate the volume of the cell suspension to be plated in each well containing 500 µL of media.
- Place plates containing cells in the incubator (37 °C, 95% humidity, 5% CO2) to grow for 7 days.
- Counting
- Following 7 days in culture, remove plates and view under a light microscope. Quantify neurospheres by counting spherical colonies that are >80 µm in diameter for dNSCs cultures and >50 µm diameter for pNSCs cultures. NOTE: If desired, neurospheres can be further passaged26 or differentiated. If cells are no longer needed, dispose by adding accelerated hydrogen peroxide to the wells (approx. 1 mL) for 20 min so that the color of the medium turns yellow. Then discard.
Representative Results
Following surgery, the mice should experience minimal motor deficits which may include tail and possible hind-limb paresis for up to 24 h. After this time, the mice should experience no hind-limb paralysis and/or paresis and minimal changes in gait.
Figure 3 shows representative results from the neurosphere assay 5 days following the minimal spinal cord injury. The absolute numbers of dNSC-derived neurospheres (grown in EFH) is greater than the numbers of pNSC-derived neurospheres (grown in LIF) after injury (Figure 3A and 3B, respectively). Higher power images of a definitive neurosphere (Figure 3C) and a primitive neurosphere (Figure 3D) reveal differences in the appearance of these distinct colonies with definitive neurospheres being larger in diameter (≥80 µm) and usually having a large dark center. Primitive neurospheres are smaller in size (≥50 µm) and the cells are more tightly packed. Representative numbers of neurospheres arising from different parts of the injured cord are seen in Figure 3E (definitives) and 3F (primitives). Figure 3E shows that minimal SCI causes a significant increase in neurospheres grown from the central canal at the level of injury compared to a relatively modest increase at the rostral non-injured cord.
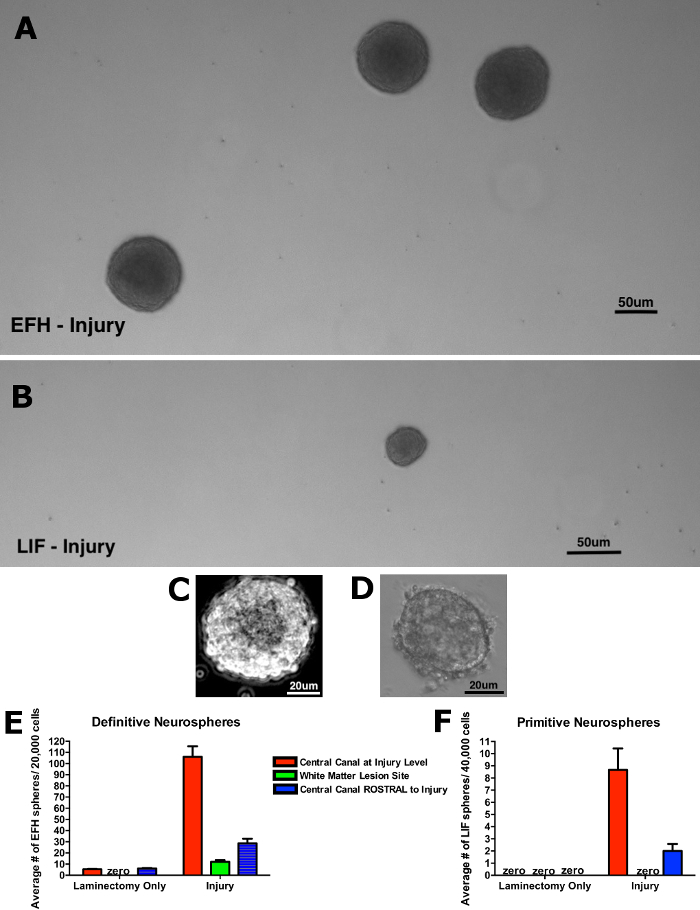
Interestingly, definitive neurospheres can be generated from the site of lesion, a region which does not contain neurosphere forming cells in laminectomy alone mice. Figure 3F demonstrates a similar increase in pNSCs post-injury with the exception of pNSC neurospheres at the lesion site. Hence, only dNSCs are able to migrate to the site of injury over this time course post-injury.
Figure 1: Surgical Procedure. (A) The layout of everything required, numbered for reference. (B) A picture of the mouse in a stereotaxic device with a straightened body, spine and limbs spread out and taped along with thoracic support arch placed underneath. (C) A picture of the mouse with a vertical skin incision exposing the muscular layer and the spinal column contour. (D) A picture of the mouse body after muscular incisions and exposure and isolation of the spine with retractors. Note taut muscle layers with a lack of tearing. (E) A picture of the isolated mouse spine with muscle layers removed, showing the bony surface of the vertebra (3 segments). This also shows the spinous process and possibly the intervertebral joint and lamina of middle vertebra to be removed. (F) A picture of the visible spinal cord post-laminectomy. Labeled is the dorsal midline vein. (G) A picture of the mouse with bilateral needle track injuries on either side of dorsal midline vein. There is a close-up insert of the 45° bent shaft of 30 G needle. Please click here to view a larger version of this figure.
Figure 2: Spinal Cord Dissection. (A) A picture of the decapitated mouse with the midline skin incision to expose the back muscle and the vertebral bone contour (B) A picture of the isolated vertebral column, showing rostral and caudal orientation. The second picture shows the cord isolation and the third shows the isolated spinal cord in a Petri dish. (C) A graphical representation of the fine dissection of the spinal cord, where different segments (periventricular, injury area) are removed and separated for culturing. Please click here to view a larger version of this figure.
Figure 3: Representative results from neurosphere assay following minimal spinal cord injury. (A) The numbers of dNSC-derived neurospheres (grown in EFH) cultured from the injured spinal cord is greater than (B) the number of pNSC-derived neurospheres (grown in LIF) when plated at equal density. (C, D) Higher power images of "typical" neurospheres in (C) dNSC cultures and (D) pNSC cultures. (E) Minimal needle tract injury results in significant increases in the numbers of dNSC-derived neurospheres from the central canal at the level of injury as well as the central canal rostral to the injury, and from the injury site. (F) pNSC-derived neurospheres cannot be isolated from the uninjured cord but can be isolated following injury from the central canal (at the level of the injury and from the rostral central canal). Error bars represent standard error of the mean. Please click here to view a larger version of this figure.
Discussion
During the surgical procedure, there are a few critical steps where the researcher should pay particular attention to in order to obtain optimal outcomes and minimize variability between animals. Care must be taken with the inhaled anesthesia (isoflurane) during surgery as the anesthetic has been shown to have neuroprotective effects with prolonged exposure27. Accordingly, when studying the regenerative capacity of the spinal cord following injury, make an effort to perform the surgery as quickly and efficiently as possible to prevent confounding variables. Maintaining the same isoflurane exposure time per mouse will reduce variability. The breathing rate of the mouse should be monitored throughout the surgery and should not be too slow (less than 1 breath every 2–3 s) or heavily labored (i.e., gasping). The maintenance dose of anesthesia can be lowered from 2–3% to prevent death due to prolonged anesthesia with the note of mice that received the lower dosing.
During the surgery, take extra care when performing muscular incisions on either side of the vertebral column. Ensure that the cuts are deep, and the blade is angled medially so that the edge of the blade rests against the bony vertebrae during muscular incision. If the blade is angled outwards, there is the possibility of excessive bleeding from vascular cuts. The researcher should also pay extra attention when performing the laminectomy to avoid deep angling of the scissors which will damage the spinal cord, causing unwanted tissue damage and functional deficits. The appropriate control for these experiments is a "laminectomy only" group (no injury), which will enable a comparison of the activation of NSCs attributed to the needle track injury as opposed to that which can result from the laminectomy only. We have shown that laminectomy alone can result in a small, albeit insignificant increase in NSC activation as revealed by an increase in neurosphere numbers from the periventricular region at the level of the lesion21. Laminectomy alone does not cause increases in neurosphere numbers from the periventricular region rostral or caudal to the laminectomy and no neurospheres are found in cultures of white matter isolated at the level of the laminectomy. Additionally, when performing the laminectomy, take care to remove the dorsal lamina in one piece to permit wide exposure of the cord. This will (1) allow sufficient access to perform the minimal needle track injury of the dorsolateral spinal cord, and (2) prevent segments of bone from being left behind and causing secondary damage following closure and post-recovery movement of the mouse. If the entire lamina is not taken as one piece, or segments of sharp bone protruding on either side of the laminectomy are observed, use instruments (such as toothed forceps and curved scissors) to remove these fragments prior to suturing.
The researcher should be extremely careful when applying sutures to the muscular layer during the surgical closure. One suture (double-knotted) should be placed caudal to the level of laminectomy/injury so that the suture lies on top of the intact vertebral bone. This is to prevent any secondary damage that may result from the muscular suture being in contact with the exposed cord when the mouse moves after recovery. Furthermore, the muscular suture caudal to the laminectomy/injury acts as a landmark for where the SCI was performed when isolating the spinal cord for analysis. Care should be taken when dissecting out the injured spinal cord area to avoid compromising the structure of the injured region so that it can remain recognizable during fine dissection under the dissecting microscope. In regard to the neurosphere assay, it is important to perform the mechanical trituration of cell pellets gently to avoid the production of air bubbles that can increase cell death. pNSC-derived neurospheres are even more sensitive to debris heavy, growth conditions — excessive trituration and prolonged exposure in enzymatic solutions — relative to dNSC cultures. pNSC derived spheres are more compact and smaller than dNSCs. Given the rarity of pNSCs, we recommend isolating at least 240,000 cells per sample.
The minimal SCI model is ideal for studying the cellular events following injury (such as activation of endogenous NSCs) but it does not permit the study of functional impairments. As noted previously, mice that recover from the needle track injury experience no notable behavioral deficits that persist and as such, the mice cannot be evaluated for the effectiveness of therapeutic interventions designed to improve functional outcomes. One important aspect of regenerative medicine is that a treatment should not only promote tissue repair (which can be evaluated using this model, in combination with the neurosphere assay, lineage tracing and immunohistochemistry/immunofluorescence) but should also demonstrate relevant functional improvement using behavioral paradigms where applicable. A number of behavioral tasks used to test functional outcomes in thoracic models of injury such as the foot fault test and the Basso, Beattie, Breshnan (BBB) Open Field Locomotor Scale28, are not sensitive enough to detect measurable deficits in our minimal SCI model. To overcome this shortcoming, one can make use of more sensitive digital scoring systems (e.g., CatWalk) which measures multiple parameters of gross and fine hind-limb locomotion parameters including gait analyses, which may detect the minimal deficits resulting from the minimal injury model29.
This method could also be adapted to study endogenous NSC activation as a potential therapy in models of cervical spinal cord injury. Cervical SCI is the most clinically relevant model and we propose that adapting this injury model to higher vertebral segments would provide insight into whether there are regional variations in the response of NSCs and their progeny (kinetics, migration, differentiation) and whether the lesion would result in more profound and measurable functional deficits. The currently used murine cervical injury models (such as transection, clip compression and/or contusion) require intensive post-operative care including the need to manually express bladders and constantly monitor the breathing of the mouse. Adapting the minimal injury model to the cervical spinal cord may reduce the mortality, morbidity and post-operative care associated with other cervical SCI models as well as permit one to examine the effectiveness of drugs/small molecules and/or rehabilitation outcomes on neural recovery. Our injury model can also be adapted to create a minimal injury in different parts of the cord (i.e., dorsal or lateral columns) and/or larger injuries with deeper penetration and/or the use of larger sized needles (smaller gauge). This allows the researcher to control/manipulate the type and size of the lesion and thus evaluate cellular and functional/behavioral recovery accordingly.
The minimal injury model in mice permits the use of transgenic animal models. The mouse models enable labeling of endogenous stem cells and/or progenitors prior to the injury. This can allow the researcher to track the fate of these pre-labeled cells following injury and evaluate neural precursor cell proliferation, migration, and differentiation following injury — potentially contributing to neural repair.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is funded by the Krembil Foundation (operating grant CMM). WX was the recipient of the Carlton Marguerite Smith student award. NL received an Ontario Graduate Scholarship.
References
- Johansson CB, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276(5309):66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Current Opinion in Neurology. 1999;9(1):135–141. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. Journal Of Cerebral Blood Flow And Metabolism. 2004;24(4):441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36(6):1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Faiz M, et al. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17(5):624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Ren Y, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Science Reports - UK. 2017;7 doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nature Reviews Neuroscience. 2006;7(8):628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Bethea JR, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. Journal of Neurotrauma. 1999;16(10):851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Experimental Neurology. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences USA. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Progress in Brain Research. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Metz GA, et al. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. Journal of Neurotrauma. 2000;17(1):1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. Journal of Neuroscience. 2004;24(9):2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan T, et al. Spinal cord injury models: a review. Spinal Cord. 2014;52(8):588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- Deleyrolle LP, Reynolds BA. Isolation, expansion, and differentiation of adult Mammalian neural stem and progenitor cells using the neurosphere assay. Neural Cell Transplantation: Methods and Protocols. 2009. pp. 91–101. [DOI] [PubMed]
- Singec I, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nature Methods. 2006;3(10) doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. The Journal of Comparative Neurology. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. Myelin basic protein regulates primitive and definitive neural stem cell proliferation from the adult spinal cord. Stem Cells. 2017;35(2):485–496. doi: 10.1002/stem.2488. [DOI] [PubMed] [Google Scholar]
- Sachewsky N, et al. Primitive neural stem cells in the adult mammalian brain give rise to GFAP-expressing neural stem cells. Stem Cell Reports. 2014;2(6):810–824. doi: 10.1016/j.stemcr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington Biochemical Corporation. Papain Dissociation System. 2018. Available from: http://www.worthington-biochem.com/PDS/default.html.
- Mothe A, Tator CH. Isolation of neural stem/progenitor cells from the periventricular region of the adult rat and human spinal cord. Journal of Visualized Experiments. 2015. [DOI] [PMC free article] [PubMed]
- Absher M. Hemocytometer counting. Tissue Culture. 1973. pp. 395–397.
- Azari H, Rahman M, Sharififar S, Reynolds BA. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. Journal of Visualized Experiments. 2010. [DOI] [PMC free article] [PubMed]
- Xiong L, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesthesia and Analgesia. 2003;96(1):233–237. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Research. 2000;883(2):165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma. 2006;23(3-4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]


