Abstract
Mouse body temperature measurement is of paramount importance for investigating allergies and anaphylactic symptoms. Rectal probes for temperature readings is common, and they have been proven to be accurate and invaluable in this regard. However, this method of temperature measurement requires the mice to be anesthetized in order to insert the probe without injury to the animal. This limits the ability to observe other phenotypes of the mouse simultaneously. In order to investigate other phenotypes while measuring temperatures, rectal probes are not ideal, and another method is desired. Here, we introduce a noninvasive method of temperature measurement that foregoes the requirement for mouse anesthesia while maintaining equal reliability to rectal probes in measuring body temperature. We use an infrared thermometer that detects body surface temperatures at ranges between 2 and 150 mm. This method of body temperature measurement is successful in reliably replicating temperature change trends during passive system anaphylaxis experiments in mice. We show that body surface temperatures are about 2.0 °C lower than rectal probe measurements, but the degree of temperature drop follows the same trend. Furthermore, we use the same technique to observe mice in a food allergy model to evaluate temperature and activity levels simultaneously.
Keywords: Immunology and Infection, Issue 139, Infrared thermometer, body temperature, noninvasive, passive systemic anaphylaxis, food allergy, mouse
Introduction
Measurement of body temperature has been an essential part of monitoring the effects of anaphylactic symptoms in animal models1,2. Temperature differences have been traditionally measured by rectal probe thermometers in mice3,4. With these measurements, investigators have reliably portrayed differences in temperature among variables; however, this method is a time-consuming procedure and causes distress to mice, which can increase the body core temperature. Rectal probing can also cause mucosal tearing and infection3. Moreover, the mice should be anesthetized in order to humanely insert the rectal probe to measure the temperature3. This is a slow process, and it prohibits the measurement of successive temperatures within a short period of time. Furthermore, mice activity phenotypes cannot be observed during this time until the anesthetic is completely worn off, which is another time-consuming process. More recently, other reliable methods to measure body temperature have used subcutaneously implanted passive infrared transponder tags or radio transmitters that include a temperature sensor3,5,6. Although they are accepted as the ideal practice by some researchers, these methods are not widely used because of high initial costs and distress to mice, due to the surgical implantation of a temperature sensor under the skin or another part of the body.
In order to demonstrate that a temperature difference is an accurate reflection of symptoms in a disease model1,2, mice must be awake during the temperature measurement and be able to return to their normal phenotypic activity immediately before and after the measurement. To this end, we sought a method by which this could be achieved.
Our goal was to accurately and inexpensively measure mouse body temperature, without the need for anesthesia and without restrictions on activity, to enable observation of behavioral phenotypes during and after the time of temperature measurement. To achieve this goal, it was apparent that a technique less invasive than the standard rectal temperature probes was required. Infrared thermometers have been used for decades in clinical medicine, especially in pediatrics, to obtain accurate temperature readings. It has been an alternative method that has allowed clinicians to quickly and accurately obtain temperature measurements in infants and fussy children that are actively mobile. We implemented this same technique in mice and have developed a successful method to obtain temperatures without anesthesia. Importantly, we show that this method is capable of replicating the well-established passive systemic anaphylaxis results regarding temperature changes, while also being able to observe the activity of the mouse throughout the measurement. Furthermore, we use the same method to evaluate body temperatures of food-allergic mice, while simultaneously investigating other symptoms, to demonstrate that body temperature is indeed an accurate reflection of the activity level and overall phenotype of the mouse.
Protocol
All animal experiments were approved by the Animal Care and Use Committee of the La Jolla Institute for Allergy and Immunology.
1. Mouse Body Temperature Measurement During Anesthetization
Place the mouse in an anesthesia induction box. Anesthetize by using 1 L/min flow of oxygen with 5% isoflurane. NOTE: Anesthetization is confirmed when the mouse ceases voluntary movement and has been immobile for over 30 s. Alternatively, monitor respiratory rate, and once mice are breathing at 1 breath per every 2 s or longer, anesthetization is confirmed.
Hold the mouse by the nape of the neck with an index finger and thumb and hold the tail with a pinky finger in order to expose the lower abdomen.
Place the infrared thermometer sensor below the lower abdomen while holding the mouse with its body parallel to the ground. NOTE: The outer flat surface of the thermometer (not the surface of the sensor) should be approximately 2–5 mm away from the surface of the abdomen. This replicate results of temperatures measured while mice are not anesthetized (described in section 2). It is important to determine the target site of the abdomen. Aiming between two upper nipples allows for consistent results.
Hold the trigger to measure the temperature. Ensure the stable holding of the mouse and thermometer.
2. Mouse Body Temperature Measurement Without Anesthetic
Pick up the mouse by the middle of the tail.
- Expose the abdomen of the mouse.
- Allow the mouse to hold on to a straight-edge surface, such as the lip of an open cage or cage-top with its forepaws. NOTE: This allows the mouse to stretch its upper body and expose the abdomen.
- Alternatively, allow the mouse to hold on to the upper straight-edge of the thermometer, and make the mouse sit on the outer flat surface of the thermometer with its abdomen just over the infrared sensor. NOTE: Any time the hind paws are resting on the surface of the thermometer, ensure that the feet are not obstructing the sensor from the abdominal surface. Thermometers with foot obstruction will measure a temperature lower than that of the abdomen.
Hold the trigger to measure the temperature. NOTE: Mice tend to move; take care in measuring the same location of the body consistently by taking temperature measurements when mice are relatively less mobile.
3. Passive Systemic Anaphylaxis7
- Day 0: Sensitize with immunoglobulin E (IgE).
- Prepare 200 µL (per mouse) of anti-dinitrophenyl (DNP) IgE at a concentration of 100 µg/mL in PBS.
- Intraperitoneally inject 200 µL of anti-dinitrophenyl (DNP) IgE prepared in step 3.1.1 or PBS only (PBS-only is the negative control). Use a 26 G needle for injection. Perform the injections just lateral to the midline, approximately between the two most inferior nipples.
- Day 1: Induce anaphylaxis with DNP-HSA.
- Prepare 100 µL (per mouse) of DNP-HSA at a concentration of 10 mg/mL in 0.9% NaCl.
- Anesthetize the mice as described in step 1.1.
- Measure the body temperature using the technique described in step 1.
- Intravenously inject 100 µL of DNP-HSA prepared in step 3.2.1. Use a 30 G needle for injection. Perform the injections on the retroorbital venous sinus. Insert the needle on the medial side of the eye at a shallow angle, aiming behind the eye.
- After injection, place the mice in individual cages. Ensure that the mice recover from anesthesia. Observe that they awaken and become voluntarily mobile.
- Measure body temperatures using the infrared thermometer and observe their activity every 10 min for 70 min.
4. Mouse Model of Food Allergy8,9
NOTE: The schematic is shown in Figure 2.
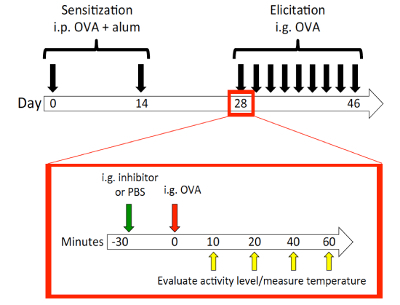
- Day 0: Sensitize the mice with OVA/alum.
- Prepare 100 µL (per mouse) of OVA (0.5 mg/mL) and alum (10 mg/mL) together in PBS. Vortex on the low setting for 30 min.
- Anesthetize the mice as described in step 1.1.
- Using a 26 G needle, intraperitoneally inject each mouse with 100 µL of the OVA/alum mixture prepared in step 4.1.1. NOTE: Vortex the OVA/alum mixture again, briefly prior to each injection, to best ensure a homogenized mixture. See step 3.1.3 for details on the injection site.
- After injection, place mice back in their original cages. Ensure that the mice recover from anesthesia. Observe that they awaken and become voluntarily mobile.
- Day 14: Perform a second sensitization with OVA/alum.
- Repeat steps 4.1.1, 4.1.2, and 4.1.3.
- Days 28-46: Challenge the mice with OVA every other day.
- Prepare 100 µL (per mouse) of OVA at a concentration of 250 mg/mL in PBS. NOTE: The mixture should be made by gently rocking back and forth by hand rather than with a vortex to minimize bubble formation.
- If using food allergy inhibitors, prepare the inhibitors9. Prepare 100 µL (per mouse) of inhibitor at a concentration of 1 mg/mL in PBS. This allows a delivery of 100 µg of inhibitor for each mouse. NOTE: Do not exceed 100 µL of inhibitor per mouse, as the mice are only able to handle 200 µL of total gavage volume during one challenge day. As 100 µL are necessary for the OVA challenge as well, 100 µL is the maximum recommended volume for inhibitor usage.
- Anesthetize the mice as described in step 1.1.
- Orally gavage each mouse with 100 µL of the OVA solution (25 mg of OVA in 100 µL of PBS gavaged per mouse) that was prepared in step 4.3.1.
- If using inhibitors, utilize the same techniques described in the following steps for the gavage of inhibitor (or PBS control) prepared in step 4.3.1.1 30 minutes prior to the OVA challenge. After 30 min, move on to the next step.
- Use a gavage needle with a 1 mL syringe. Take up 100 µL of OVA into the syringe.
- Insert the needle into the mouth; then, pointing the needle toward the left or right side of the throat, gently slide the needle through the esophagus. Inject 100 µL of OVA. NOTE: To ensure that the needle is not in the trachea, observe active breathing before injecting the OVA. The insertion of the needle will also be resisted early if it has entered the trachea rather than the esophagus.
- Place the mice into their individual cages. For easy observation of stool quality, use cages without bedding.
- Ensure recovery from anesthesia. Observe that the mice awaken and become voluntarily mobile.
- Measure body temperatures of the mice at timepoints 10, 20, 40, and 60 min using the technique described in section 2.
Representative Results
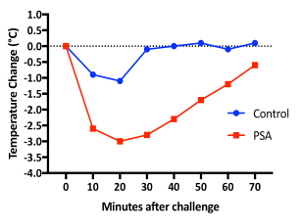
Passive systemic anaphylaxis: For iv injection, 10 week old female BALB/c mice were anesthetized. Prior to the injection, we measured their body temperatures (Video 1) as described in step 1. Figure 1 shows the temperature trend of both populations after iv injection. The IgE-sensitized mouse showed a maximum temperature drop of 3.0 °C at 20 min, while the PBS control mouse had a maximum drop of 1.1 °C at 20 min7. Furthermore, throughout this process, activity scores were evaluated based on the mobility of each mouse. Mice were first observed without any agitation. Then, they were observed with agitation by introducing the evaluator's hand into the cage and making soft contact with the mouse. Based on their reactions, the mice are evaluated and scored on a scale of 1 to 3. A score of 1 indicates a mouse that is immobile or minimally mobile with and without agitation; a score of 2 indicates a mouse that is immobile without agitation and is mobile with agitation without making quick evasive movements; and a score of 3 indicates a mouse that is either mobile or immobile without agitation, and mobile with agitation and makes quick evasive movements. Videos 2 and 3 provide examples of the two groups that exemplify the temperature measurement methods as well as the corresponding activity level measurements. Usage of the infrared thermometer allowed for temperature measurements without anesthesia, which in turn allowed for behavioral phenotype evaluation before, during, and after the temperature measurement; in contrast, a traditional rectal probe would require anesthesia and therefore preclude any behavioral evaluation within the several minutes surrounding the temperature measurements.
Mouse model of food allergy8: The schematic of the mouse model is shown in Figure 2. Mice are female BALB/c mice of ages 8 to 10 weeks old at the time of sensitization. Mice were observed at 10, 20, 40, and 60 min after each challenge for body temperature and activity. A representative result is shown in Figure 3 of the temperature change during challenge 7. The mice showed a maximal temperature drop at 10 min; therefore, the 10 min temperatures were used to correlate with body temperatures at the 10 min activity levels (with scoring based on the criteria depicted above). Activity score and temperature drop showed a statistically significant correlation (Figure 4). As mentioned in the passive systemic anaphylaxis section, behavioral observations were performed due to the lack of anesthesia, and the infrared thermometer was utilized. In contrast, rectal probes would forestall any behavioral observations or evaluations during the time of temperature measurement. Any time that temperature and other activity phenotypes must be recorded, it is essential to utilize a temperature-measuring system that does not require anesthesia.
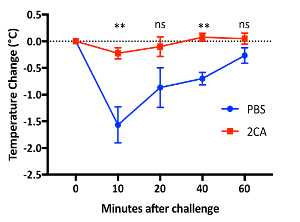
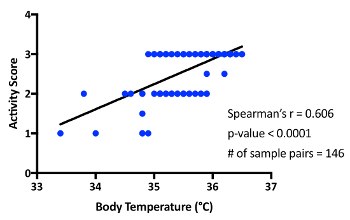
Figure 1: Passive systemic anaphylaxis in BALB/c mice. Two mice were ip injected with anti-DNP IgE on day 0, then iv injected with either normal saline or DNP-HSA on day 1. Body temperature was measured using an infrared thermometer before injection and every 10 min after injection for 70 min. Please click here to view a larger version of this figure.
Figure 2: Food allergy mouse model schematic. On day 0, BALB/c mice were sensitized ip with OVA and alum, and again on day 14. On day 28, 30 min prior to each challenge, mice were orally gavaged with either PBS control or a food allergy inhibitor (HRF-2CArecombinant histamine-releasing factor with its two cysteines exchanged with alanines)9. Mice were then challenged ig with 25 mg of OVA. Challenges were performed every other day for 9 challenges. Mice were observed for body temperature measurement and activity scoring at 10, 20, 40, and 60 min after the challenge. Please click here to view a larger version of this figure.
Figure 3: Food allergy model in BALB/c mice. Temperature change after the 7th OVA challenge was monitored by infrared thermometer. Typical results are shown from at least four independent experiments (**, p <0.01 between PBS and 2CA treatments by Student's t-test; ns: not significant). Error bars = SEM. Please click here to view a larger version of this figure.
Figure 4: Correlation between activity and temperature at 10 min in food allergy experiments. The results reflect measurements taken from 4 separate sets of experiments. Please click here to view a larger version of this figure.
 Video 1: Body temperature measurement while mice are anesthetized. Mice body temperature measurements can be taken with or without anesthesia. For consistent measurements, it is essential to always aim the thermometer at the same body location. Please click here to view this video. (Right-click to download.)
Video 1: Body temperature measurement while mice are anesthetized. Mice body temperature measurements can be taken with or without anesthesia. For consistent measurements, it is essential to always aim the thermometer at the same body location. Please click here to view this video. (Right-click to download.)
 Video 2: Activity level at 40 min after DNP-HSA iv injection. Mice are observed without agitation initially, then with agitation. Based on their reactions, the mice were evaluated and scored on an activity scale of 1 to 3. The mouse on the left (anti-DNP sensitized mouse) is given a score of 1, while the mouse on the right (PBS control mouse) is given a score of 3. Please click here to view this video. (Right-click to download.)
Video 2: Activity level at 40 min after DNP-HSA iv injection. Mice are observed without agitation initially, then with agitation. Based on their reactions, the mice were evaluated and scored on an activity scale of 1 to 3. The mouse on the left (anti-DNP sensitized mouse) is given a score of 1, while the mouse on the right (PBS control mouse) is given a score of 3. Please click here to view this video. (Right-click to download.)
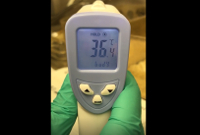 Video 3: Body temperature measurement at 40 min after DNP-HSA iv injection. This figure demonstrates the ability of the infrared thermometer to accurately and noninvasively measure body temperature without anesthetization. Moreover, the activity scores demonstrated in Video 2 reflect the degree of temperature drop measured in this video. Please click here to view this video. (Right-click to download.)
Video 3: Body temperature measurement at 40 min after DNP-HSA iv injection. This figure demonstrates the ability of the infrared thermometer to accurately and noninvasively measure body temperature without anesthetization. Moreover, the activity scores demonstrated in Video 2 reflect the degree of temperature drop measured in this video. Please click here to view this video. (Right-click to download.)
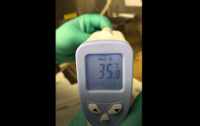 Video 4: Errors in temperature reading by hind paw obstruction of the sensor. This video demonstrates the difference in temperatures with and without the foot within sensor circumference. Please click here to view this video. (Right-click to download.)
Video 4: Errors in temperature reading by hind paw obstruction of the sensor. This video demonstrates the difference in temperatures with and without the foot within sensor circumference. Please click here to view this video. (Right-click to download.)
Discussion
The protocol described was established with the goal of measuring body temperature without the use of anesthesia. Despite its relative ease with which temperature readings can be obtained, there are several caveats that accommodate this technique, in addition to the more obvious effects such as handling stress and different ambient temperatures.
First, in order to maintain consistent temperature readings throughout the experiment, the location where the temperature is being measured must be predetermined using anatomic landmarks, and investigators must be able to replicate the measurement of said body location. By securing the mouse's forepaws on a straight-edge surface, such as the lip of a cage or the edge of the thermometer, the abdomen is readily exposed to the infrared sensor of the thermometer. Generally, the abdomen is a large surface area that allows some flexibility for consistent results. Furthermore, the abdomen has sparser hair than the dorsal surface of the mouse, and therefore allows for a more accurate body temperature reading.
Secondly, hind paws must always be monitored when taking a temperature measurement. When the thermometer is close to the abdomen, the mice tend to place their feet on the thermometer itself. When the feet or toes are within the circumference of the sensor, the temperature reading may be lower than that of the abdomen, as demonstrated in Video 4. It is important to allow the mouse to straddle with its feet on either side of the sensor, while keeping the circumference free of any body parts or objects besides the abdominal surface.
Thirdly, the abdominal surfaces of mice cannot be wet when taking the measurements. A wet surface will alter the thermometer's ability to measure temperature accurately; usually, the measured temperature will be lower than the actual value. This issue can be avoided by keeping the mice in cages with fresh bedding during the course of the observational period. This also ensures that the dryness of the abdominal surface is standardized each time the temperatures are measured.
Lastly, infrared thermometers have a larger margin of error than rectal probes, albeit only slightly. The margin of error for measurements with rectal probes is ± 0.1 °C, whereas for infrared thermometers, it is ± 0.2 °C. This margin of error was minimal compared to the overall differences in temperature drop between populations in the PSA experiment. More importantly, a project requiring a difference as subtle as ± 0.1 °C may also foresee other margin of error issues, regardless of whether an infrared thermometer or rectal probe is used. During the preparation of this protocol, it had also been shown that there is a very good correlation between body core temperatures measured by implantable temperature transponders and body surface temperatures measured by infrared thermometers during lipopolysaccharide-induced hypothermia6.
Temperature drop in the food allergy model used had not been observed previously8,10. The standard for evaluation had previously been to determine the phenotype severity by diarrhea scoring; however, this method lacked objectivity. By using the infrared thermometer, we showed that subtle temperature drops could be detected. We were then able to correlate these with activity levels, as the mice were not anesthetized during the procedure. This method allows for a fast, accurate, easy-to-use, noninvasive, and inexpensive way to measure body temperature in mice while being able to observe other phenotypes that would otherwise be missed if mice are anesthetized for rectal probe measurements.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Research in the Kawakami lab was supported by NIH grants: R01 AR064418-01A1, R01 HL124283-01, R21 AI 115534-01, and R41AI124734-01.
References
- Finkelman FD. Anaphylaxis: lessons from mouse models. The Journal of Allergy and Clinical Immunology. 2007;120(3):506–515. doi: 10.1016/j.jaci.2007.07.033. Quiz 516-507. [DOI] [PubMed] [Google Scholar]
- Lee JK, Vadas P. Anaphylaxis: mechanisms and management. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. 2011;41(7):923–938. doi: 10.1111/j.1365-2222.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- Newsom DM, Bolgos GL, Colby L, Nemzek JA. Comparison of body surface temperature measurement and conventional methods for measuring temperature in the mouse. Contemporary Topics in Laboratory Animal Science. 2004;43(5):13–18. [PubMed] [Google Scholar]
- Wong JP, Saravolac EG, Clement JG, Nagata LP. Development of a murine hypothermia model for study of respiratory tract influenza virus infection. Laboratory Animal Science. 1997;47(2):143–147. [PubMed] [Google Scholar]
- Quimby JM, Olea-Popelka F, Lappin MR. Comparison of digital rectal and microchip transponder thermometry in cats. Journal of American Association for Laboratory Animal Science. 2009;48(4):402–404. [PMC free article] [PubMed] [Google Scholar]
- Mei J, Riedel N, Grittner U, Endres M, Banneke S, Emmrich JV. Body temperature measurement in mice during acute illness: implantable temperature transponder versus surface infrared thermometry. Scientific Reports. 2018;8(1):3526. doi: 10.1038/s41598-018-22020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle E, Trosien J, Metz M. Protocols for the induction and evaluation of systemic anaphylaxis in mice. Methods in Molecular Biology. 2013;1032:133–138. doi: 10.1007/978-1-62703-496-8_10. [DOI] [PubMed] [Google Scholar]
- Brandt EB, et al. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of Clinical Investigations. 2003;112(11):1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, et al. Histamine-releasing factor enhances food allergy. The Journal of Clinical Investigations. 2017;127(12):4541–4553. doi: 10.1172/JCI96525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, et al. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. The Journal of Allergy and Clinical Immunology. 2009;123(1):53–58. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


