Abstract
Anogenital distance (AGD) is a sexually dimorphic attribute, twice longer in males than in females, and a marker of intrauterine hormonal environment. Interest in AGD measurements is increasing due to mounting evidence on their potential clinical implications. A parallel set of perineal measurements, the Pelvic Organ Prolapse Quantification System (POP-Q), include similar, but not exactly the same, landmarks: the perineal body (PB) and the genital hiatus (GH) lengths. However, clinical reproducibility of both perineal measurements and their usefulness to describe perineal anthropometry needs to be elucidated. To our knowledge, there is no publication in video format showing the methodology of these measurements. The main objective of this work is to show how to properly perform perineal anthropometry, including measurements of the AGD in its two variants [anoclitoral (AGDAC) and anofourchette (AGDAF)], genital hiatus (GH) and perineal body (PB). Moreover, we explored if there were differences in these measurements in women with and without Pelvic Organ Prolapse (POP). We research whether the anthropometric characteristics of the perineum, such as AGD (which is determined prenatally), may be altered in these women and be an independent etiological factor for pelvic floor dysfunction. We show two different ways of measuring perineal lengths, as they might be quite comparable. Our suggestion is that unifying perineal measurements could be useful for clinical and biomedical investigation. More studies are needed in order to compare GH and PB measurements and its AGD counterparts to analyze which procedures are more reproducible with less intra and interobserver variability.
Keywords: Medicine, Issue 139, Anogenital distance, anthropometry, perineum, genital hiatus, perineal body, pelvic organ prolapse (POP), Pelvic Organ Prolapse Quantification System (POP-Q)
Introduction
Anogenital distance (AGD) is an easily accessible and noninvasive anthropometric measurement (Figure 1). AGD is a sexual dimorphism in placental mammals, being almost twice longer in males than in females. It is considered a marker of intra uterus hormonal environment1,2. AGD has been related to prenatal exposure to endocrine disruptors3,4,5 and androgens during critical periods of genital development6,7. In prenatal consultations, AGD may be useful to determine fetus gender with great accuracy in the first trimester (scan at 11-13 ± 6 weeks of gestation)8,9. In adult women, AGD length is associated with the female reproductive function10,11,12,13. Longer AGD distances in adult women have been associated in cross-sectional studies to a higher number of ovarian follicles10 and to higher testosterone levels11. Young women with longer AGD were several times more likely to have had mothers with irregular menstrual cycles, suggesting that the potentially hyperandrogenic intrauterine environment of the mother was sufficient to modify the female offspring's reproductive tract12. Recently, Wu et al.14 have shown that the presence of polycystic ovarian syndrome (PCOS) is associated with longer AGD measurements in Chinese women, and Barrett et al.15 have also reported longer AGD in newborn daughters of women with PCOS15. Our research group have also confirmed this finding in adult Mediterranean women16. On the other hand, AGD is negatively and strongly associated with the presence and severity of endometriosis17, suggesting the potential of AGD as a biomarker of developmental antiandrogen/estrogen exposure. There is still controversy about the definitions and measurements of the female perineal and genital area. Previous human studies have applied a variety of measurements when referring to AGD. In women, some modification has been observed in the anatomical references for AGD measures. When AGD was firstly described and measured in women, the lower reference for the anatomical landmark was the "middle" anus. Nowadays, the lower reference is established as the top margin of the anus as it is a more accurate reference, making AGD a more reproducible measure18. AGD measurements must be corrected by weight or BMI as they are anthropometric measurements associated with body size19.
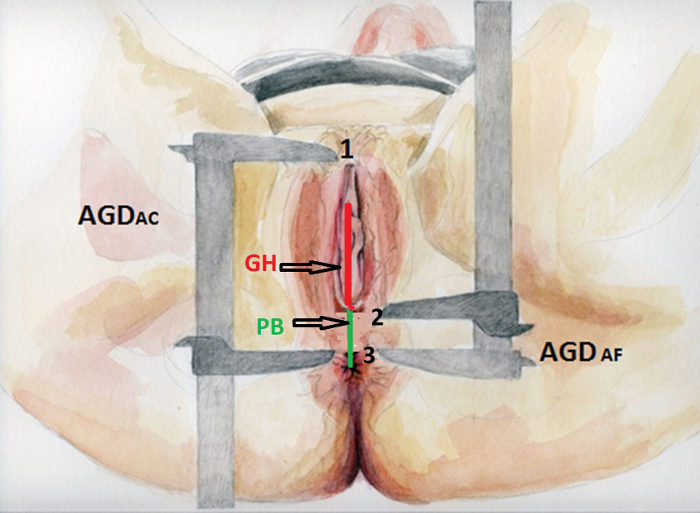
A similar set of measurements are the Pelvic Organ Prolapse Quantification System (POP-Q)20 that includes two perineal measurements: the perineal body (PB) length and genital hiatus (GH) length (Figure 1). These measurements have been standardized according to the International Continence Society and are commonly used in clinical practice and biomedical studies, especially in women with pelvic organ prolapse (POP)21. PB is the measurement taken from the posterior margin of the genital hiatus to the midanal opening and is equivalent to AGDAF (ano-fourchette). GH does not correlate exactly with DAGAC (anoclitoral), although it would be its analog measurement. Women with POP have a strong familial association22, and parity is also considered a risk factor for POP23. However, there is no data to presume that they have been subjected to abnormal hormonal environments and may associated to other reproductive problems. Because of the similarities of the measurements, however, we hypothesize that women with shorter PB would have shorter AGDAF as well.
In this paper, we present both procedures and assess their similarities to ensure uniformity of these measurements in research and clinical practice. To our knowledge, there is no video publication showing how to perform these measurements. The main objective of this study was to show how to perform both AGD measurements: ano-clitoral (AGDAC) and ano-fourchette (AGD AF), and how to measure GH and PB lengths. A secondary objective was to compare both sets of anthropometric measures in women with POP (cases) and asymptomatic women without pelvic floor dysfunction (control women).
A case-control study was conducted from August 2014 to June 2015 at the Department of Obstetrics and Gynecology of the University Clinical Hospital 'Virgen de la Arrixaca' in the Murcia Region (southeastern Spain). Cases were women over 40 years of age seeking care for genital lumps. If POP was confirmed in the gynecological examination and classified as stage II or more by the POP-Q classification20, women were invited to participate, regardless of the affected compartment (anterior, middle or posterior). Women with stress urinary incontinence that requires surgical treatment were excluded. Controls were women of similar age seeking routine gynecological exams with neither pelvic floor disease nor other gynecological conditions such as adnexal disease or uterine fibroids. The exclusion criteria for both cases and controls were the following: previous corrective surgery for pelvic floor disease or urinary incontinence; an active tumor that alters the mechanics and biometry of the pelvic floor; an active infection of external anogenital tract; and external hemorrhoids. Women having locomotor impairment preventing the physicians from taking the measurements were also excluded. A complete gynecological and obstetrical history was performed including menstrual and obstetric formulae (parity, vaginal and instrumented deliveries and birthweight) as well as medical and surgical history, and BMI. Women were questioned about symptoms of POP and/or urinary incontinence using the ICIQ-F questionnaires24 and Sandvik severity index25.
The pelvic status was assessed using the POP-Q classification system. A test of stress urinary incontinence was performed by emptying the bladder and introducing 300 mL of saline with a disposable urinary catheter. The patients performed Valsalva maneuvers with prolapse, and after reducing the prolapse, occult stress urinary incontinence was diagnosed. A 2D-transvaginal ultrasound was performed to rule out uterine or adnexal disease.
Protocol
This study was approved by the Ethics Research Committee of the University of Murcia. Written informed consent was obtained from all participants.
1. Perineum Measurements
NOTE: Before taking the measurements, two observers have to be trained to minimize inter-observer variability.
- Patient Preparation
- Place the patient in a lithotomy position, with thighs at an angle of 45° to the examination table.
- Use a digital caliper with a resolution length of 0.000500 inches (Figure 2A).
- To begin measuring, close the device and check that "0" appears on the display and press the calibration button.
- Select millimeters as the unit of measurement. NOTE: Other measurement units, such as inches, may be selected.
- AGD Measurements
- Perform the two AGD measurements in each woman using the digital caliper.
- Measure AGDAC from the anterior clitoral surface to the upper verge of the anus (Figure 1, point 1 to point 3).
- Measure AGDAF from the posterior fourchette or posterior margin of the hymen to the upper verge of the anus (Figure 1, point 2 to point 3).
- To improve accuracy, have two examiners perform each of these measurements three times, taking a total of six measures for AGDAF and AGDAC, respectively.
- Use the averaged values of the six measurements as the true estimate of the AGD in subsequent analyses.
- Blind each examiner to the other examiner´s results. NOTE: The caliper does not need to be sterilized after each use, but has to be cleaned up with soap and water and disinfectant, such as 2% alcoholic chlorhexidine solution.
- POP-Q Measurements
- Measure GH from the middle of the external urethral meatus to the posterior margin of the hymen (Figure 1).
- Measure PB from the posterior margin of the hymen to the mid-anal opening. NOTE: Bump et al.20 recommend expressing all measurements in centimeters. Measurements may be taken to the nearest 0.5 cm, and the authors consider that further precision is unlikely. All reports should clearly specify how measurements were taken and the accuracy of the instrument used as no specific instruments are recommended in the POP-Q system.
- Have two examiners collect each measurement 3 times. Take the average of the six measurements as the true value.
- Blind each examiner to the other examiner´s results.

Representative Results
Fifty-eight patients were included. Previous studies in women have reported that the minimum AGD distances was 24 mm12 with a standard deviation of about 10 mm12. The minimum sample size to detect a significant difference between the groups with respect to AGDAC (type I error α=0.05 and type II error β = 0.1) was estimated to be 25 individuals in each group (50 in total), based on a difference of at least 12 mm in AGDAC between the two groups. Eight more participants were added to mitigate potential losses. For a study power of 80%, the number of individuals per group would drop to 17. Patients that fulfilled the inclusion criteria were included in the study. Finally, 22 cases of prolapse and 36 controls were enrolled in the study.
Statistics were derived from the raw data. Continuous variables were summarized by arithmetic mean and standard deviation (SD), and categorical variables given as number of cases and percentages (%). Normal distribution of the data was confirmed with the Kolmogorov-Smirnov test. To study the differences between groups, unpaired Student's t-tests were used for quantitative variables and Chi-square for dichotomous variables. All tests were two-tailed at a 0.05 significance level.
The general characteristics of women participating in the study were evaluated, including the obstetrical history and other medical and surgical variables (Table 1). We found significant differences in age between the two groups of patients (mean value of 65.1 years in the patients with POP, compared to an average of 50 years in the controls (p = 0.0001)). Regarding the number of pregnancies, there was an average of 4.1 in POP patients compared to 2.3 in control women (p = 0.001). We also found differences in the number of vaginal deliveries (3.7 births in the group of POP patients, compared to the controls with 1.6 births (p = 0.0001)). Mean birthweight was also different in both groups (3,831 g in the population of patients with POP vs. 3,160 g in the control group (p = 0.0001)). However, there were no significant differences in the number of instrumented deliveries (18.1% in POP cases vs. 30.5% in the controls; p = 0.58) or in the number of cesarean sections (9% vs. 16.7% p = 0.5). Other personal, medical and surgical variables considered risk factors for POP were also evaluated, but there were no differences in any of them between the two groups. Figure 3 shows the box-plots of the four perineal measurements in both groups. There were significant differences between POP patients and the controls for AGDAC (88.1 ± 19.7 mm vs. 70.1 ± 11.7 mm, p=0.0001) (Figure 3A) and AGDAF (18 ± 5.4 mm in POP vs. 23 ± 5 mm in controls, p = 0.001) (Figure 3B). The length of the genital hiatus (GH) that was also longer in the POP cases than in the controls (53.7 ± 19.1 mm vs. 44.3 ± 10.3 mm) (p = 0.02) (Figure 3C). No significant differences were found in PB measurements between the two groups (Figure 3D).
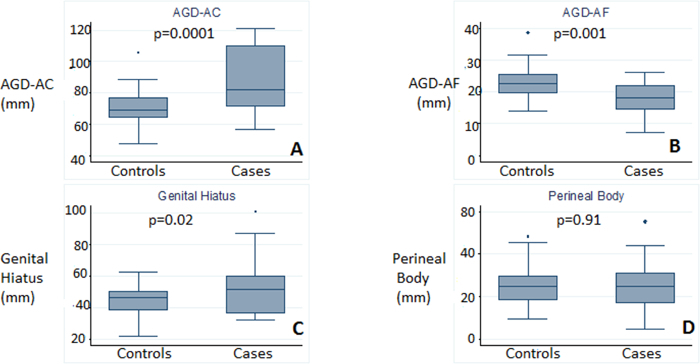
Figure 1. Benchmarks of perineal measurements. AGDAC, from the surface of the anterior clitoris to the upper edge of the anus (point 1 to point 3), and AGDAF, from the posterior fourchette to the upper edge of the anus (point 2 to point 3). Measurement of genital hiatus length (GH) is shown with a red line according to POP-Q: from the center of the urethral meatus to the posterior midline of the hymen or the leading edge of the nucleus of the perineum (identified by palpation of the levator ani and not by skin fold of the fourchette). Perineal body length (PB) according to POP-Q is shown with a green line: from the posterior margin of the urogenital hiatus or from the central node of the perineum to the center of the anus. Please click here to view a larger version of this figure.
Figure 2. Measuring instruments. A) Digital caliper. B) Sterile utility skin marker and ruler. Please click here to view a larger version of this figure.
Figure 3. Box plots that represent differences in anthropometric measures of the region of the perineum between patients and controls.A) AGDAC, B) AGDAF, C) genital hiatus, D) perineal body. Error bars correspond to standard deviation (SD). Please click here to view a larger version of this figure.
| CASES | CONTROLS | ||||||
| (n = 22) | (n = 36) | ||||||
| Characteristics | Mean | SD | Median | Mean | SD | Median | P |
| Age (years) | 65.1 | 9 | 67.5 | 50 | 7.7 | 49 | 0.0001 |
| Body mass index (BMI) (kg/m2) | 27.6 | 3.8 | 26.8 | 26.1 | 3.8 | 25.3 | 0.146 |
| Mean number of deliveries | 3.7 | 1.8 | 3 | 1.6 | 1.5 | 2 | 0.0001 |
| Birth weight (g) | 3831 | 404.5 | 4000 | 3160 | 414.5 | 3075 | 0.0001 |
| External genital injury (%) | 9.1 | 2.8 | 0.29 | ||||
| Perianal lesions (%) | 4.5 | 2.8 | 0.72 | ||||
| Chronic cough (%) | 4.5 | 2.9 | 0.73 | ||||
| COPD (%) | 4.5 | 2.9 | 0.76 | ||||
| Inguinal Repair % | 0 | 11.1 | 0.1 |
Table 1: General descriptive characteristics, obstetric and medical-surgical history of patients and controls.
Discussion
The article shows the procedure to carry out the perineal measurements according to both the AGD concept and the POP-Q system. Both procedures are described with different measurement systems: the AGD measurement with calipers in millimeters, and the POP-Q measurement with a ruler in centimeters. It would be desirable to unify the measuring instruments and the accuracy of the methods, and not just the landmarks. This is of great importance, since the more reproducible a measure is, the more reliable it will be. That will also guarantee the comparison of measures between different working groups and minimize the risk of bias among different observers. Nowadays, only PB and GH measures are standardized within the POP-Q prolapse classification system. However, AGD landmarks could replace the POP-Q landmarks if they were easier to measure and had less intra and interobserver variability (higher reproducibility).
This study shows that there are some significant differences in perineal anthropometric measurements among patients affected with POP and patients without this condition (controls). Regarding medical and surgical variables (Table 1), we found differences in the number of deliveries and fetal weight, which are both well known risk factors for POP. No other differences were found between controls and POP patients regarding risk factors for POP. We found differences between the AGDAF (which is shorter in cases of prolapse), AGDAC and GH length (which were longer in women with POP). This is an observational study that shows an association between pelvic floor disease and perineal measures but cannot determine whether differences in these distances are a cause or a consequence of POP. Therefore, it may be interesting to point out that there were statistically significant differences between AGDAF in both groups; meanwhile, there were no differences in the PB measurements, which would be its analogue measurement in the POP-Q system.
The landmarks of AGD measurement are more objective and concise than the ones of the POP-Q system. Therefore, as AGD´s landmarks may be easier to identify anatomically, they may be easier to measure and reproduce. Our group has previously reported low intra- and inter-examiner coefficients of variation for AGD (5% and 10% for AGDAF and AGDAC, respectively). Even more, intra-class correlation coefficients were above 0.95 for both AGD measurements16. Regarding the POP-Q system, in 1996, Hall et al. reported good Spearman's Correlation coefficients for GH and for PB in both inter and intra-observer studies, reporting good reproducibility of the system26. Later, Persu et al. confirmed good reliability of the POP-Q system27.
However, data and differences reported in this study are important. To our knowledge, PB and GH measurements have not been previously reported in women without pelvic floor disease, since these measurements are only used in the POP-Q system designed to evaluate women with POP. Moreover, we included AGD measurements in the perineal exploration of women with POP. AGD measurements have only been explored in women in relation to antral follicular count and serum testosterone levels, polycystic ovary syndrome and endometriosis, but POP-Q measurements were not included in these studies. When compared with controls, we have seen that AGD measurements are significantly different between patients with POP and patients without POP, which might suggest that AGD measurements may be useful to evaluate POP in women.
Future investigation is needed to evaluate comparability of AGD and POP-Q measurements, and to achieve a standardization of AGD measurements. It is important to point out that PB and GH measurements are not useful per se to quantify the grade of prolapse. GH measurements have been proposed as a prolapse risk factor28 and a risk factor for recurrence after repair29. Shorter PB measurement has also been described as a risk factor for medical treatment failure with pessary30. Apical prolapse has to be evaluated by other measures of the POP-Q system (Point C and Point D). Whether the equivalent measures of AGD could have similar clinical uses to GH and PB remains to be explored. Further studies are necessary to confirm our results and before suggesting modifications in the pelvic floor measurements procedure. The previously published results suggest the idea that the GH enlargement is a consequence of POP28. Our suggestion is that the anthropometric characteristics of the perineum, such as shorter AGDAF (which is determined prenatally) may be an independent etiological factor for pelvic floor dysfunction. In that case, AGD could be a cheaper and more accessible than others methods, like 3D ultrasound which is currently being used to calculate the volume of the genital area hiatus in women with POP31,32. Even more, a risk score prolapse could be established for women early in life that considers all risk factors and AGD. Patients might then be encouraged to perform activities to strengthen the pelvic floor during pregnancy and childbirth and to practice postpartum rehabilitation as soon as possible.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Ministry of Economy and Competitiveness, ISCIII (AES), grants no PI13/01237 and the Seneca Foundation, Murcia Regional Agency of Science and Technology, grant no 19443/PI/14. Funding to pay the Open Access publication charges for this article was provided by the Ministry of Economy and Competitiveness, ISCIII (AES), grant no PI13/01237.
References
- Greenham LW, Greenham V. Sexing mouse pups. Laboratory animals. 1977;11(3):181–184. doi: 10.1258/002367777780936620. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Jegatheesan P, Cunha GR, Baskin LS. Urethral development in the fetal rabbit and induction of hypospadias: a model for human development. The Journal of urology. 2000;164(5):1786–1792. [PubMed] [Google Scholar]
- Swan SH, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental health perspectives. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, et al. First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction. 2015;30(4):963–972. doi: 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag C-G, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environmental health perspectives. 2015;123(1):101–107. doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. The Journal of clinical endocrinology and metabolism. 2013;98(6):2230–2238. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Human Reproduction. 2013;28(9) doi: 10.1093/humrep/det286. [DOI] [PubMed] [Google Scholar]
- Arfi A, et al. First-trimester determination of fetal gender by ultrasound: measurement of the ano-genital distance. European journal of obstetrics, gynecology, and reproductive biology. 2016. pp. 177–181. [DOI] [PubMed]
- Sipahi M, Tokgöz VY, Alanya Tosun Ş. An appropriate way to predict fetal gender at first trimester: anogenital distance. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2018. pp. 1–5. [DOI] [PubMed]
- Mendiola J, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environmental health: a global access science source. 2012;11(1):90. doi: 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira-Escolano M, et al. Longer anogenital distance is associated with higher testosterone levels in women: a cross-sectional study. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121(11):1359–1364. doi: 10.1111/1471-0528.12627. [DOI] [PubMed] [Google Scholar]
- Mira-Escolano M-P, et al. Anogenital distance of women in relation to their mother's gynaecological characteristics before or during pregnancy. Reproductive BioMedicine Online. 2014;28(2):209–215. doi: 10.1016/j.rbmo.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Swan SH. Stability of proposed biomarkers of prenatal androgen exposure over the menstrual cycle. Journal of developmental origins of health and disease. 2015;6(2):149–157. doi: 10.1017/S2040174414000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhong G, Chen S, Zheng C, Liao D, Xie M. Human reproduction. 4. Vol. 32. Oxford, England: 2017. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure; pp. 937–943. [DOI] [PubMed] [Google Scholar]
- Barrett ES, et al. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. Journal of Developmental Origins of Health and Disease. 2018. pp. 1–8. [DOI] [PMC free article] [PubMed]
- Sánchez-Ferrer ML, et al. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Human Reproduction. 2017;32(11):2315–2323. doi: 10.1093/humrep/dex274. [DOI] [PubMed] [Google Scholar]
- Mendiola J, et al. Human reproduction. 10. Vol. 31. Oxford, England: 2016. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment; pp. 2377–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Ferrer ML, Moya-Jiménez LC, Mendiola J. Comparison of the anogenital distance and anthropometry of the perineum in patients with and without pelvic organ prolapse. Actas urologicas espanolas. 2016;40(10):628–634. doi: 10.1016/j.acuro.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Gallavan RH, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reproductive Toxicology. 1999;13(5):383–390. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Bump RC, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American journal of obstetrics and gynecology. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- Haylen BT, et al. An International Urogynecological Association (IUGA) / International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP) Neurourology and Urodynamics. 2016;35(2):137–168. doi: 10.1002/nau.22922. [DOI] [PubMed] [Google Scholar]
- Lince SL, van Kempen LC, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. International Urogynecology Journal. 2012;23(10):1327–1336. doi: 10.1007/s00192-012-1704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Śliwa J, et al. Analysis of prevalence of selected anamnestic factors among women with pelvic organ prolapse. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. 2018;27(2):179–184. doi: 10.17219/acem/68994. [DOI] [PubMed] [Google Scholar]
- Espuña Pons M, Rebollo Alvarez P, Puig Clota M. [Validation of the Spanish version of the International Consultation on Incontinence Questionnaire-Short Form. A questionnaire for assessing the urinary incontinence] Medicina clinica. 2004;122(8):288–292. doi: 10.1016/s0025-7753(04)74212-8. [DOI] [PubMed] [Google Scholar]
- Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourology and urodynamics. 2000;19(2):137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hall AF, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. American journal of obstetrics and gynecology. 1996;175(6) doi: 10.1016/s0002-9378(96)70091-1. [DOI] [PubMed] [Google Scholar]
- Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q) - a new era in pelvic prolapse staging. Journal of medicine and life. 2011;4(1):75–81. [PMC free article] [PubMed] [Google Scholar]
- Lowder JL, Oliphant SS, Shepherd JP, Ghetti C, Sutkin G. Genital hiatus size is associated with and predictive of apical vaginal support loss. American journal of obstetrics and gynecology. 2016;214(6) doi: 10.1016/j.ajog.2015.12.027. [DOI] [PubMed] [Google Scholar]
- Vakili B, Zheng YT, Loesch H, Echols KT, Franco N, Chesson RR. Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2005;192(5):1592–1598. doi: 10.1016/j.ajog.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Cheung RYK, Lee LLL, Chung TKH, Chan SSC. Predictors for dislodgment of vaginal pessary within one year in women with pelvic organ prolapse. Maturitas. 2018;108:53–57. doi: 10.1016/j.maturitas.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Khunda A, Shek KL, Dietz HP. Can ballooning of the levator hiatus be determined clinically? American journal of obstetrics and gynecology. 2012;206(3) doi: 10.1016/j.ajog.2011.10.876. [DOI] [PubMed] [Google Scholar]
- Dietz HP. Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2004;23(6):615–625. doi: 10.1002/uog.1072. [DOI] [PubMed] [Google Scholar]


