Abstract
Atherosclerosis is due to a chronic inflammatory response affecting vascular endothelium and is promoted by several factors such as hypertension, dyslipidemia, and diabetes. To date, there is evidence to support a role for circulating aldosterone as a risk factor for the development of cardiovascular disease. Transgenic mouse models have been generated to study cellular and molecular processes leading to atherosclerosis. In this manuscript, we describe a protocol that takes advantage of continuous infusion of aldosterone in ApoE-/- mice and generates atherosclerotic plaques in the aortic root after 4 weeks of treatment. We, therefore, illustrate a method for quantification and characterization of atherosclerotic lesions at the aortic root level. The added value of aldosterone infusion is represented by the generation of atherosclerotic lesions rich in lipid and inflammatory cells after 4 weeks of treatment. We describe in detail the staining procedures to quantify lipid and macrophage content within the plaque. Notably, in this protocol, we perform heart tissue-embedding in OCT in order to preserve the antigenicity of cardiac tissue and facilitate detectability of antigens of interest. Analysis of the plaque phenotype represents a valid approach to study the pathophysiology of atherosclerosis development and to identify novel pharmacological targets for the development of anti-atherogenic drugs.
Keywords: This Month in JoVE, Issue 139, Atherosclerosis, aldosterone, inflammation, ApoE-/-, aortic root, immunohistochemistry
Introduction
Atherosclerosis is one of the main causes of mortality and morbidity worldwide1. It is characterized by a chronic inflammatory state where blood vessels are infiltrated by lipids and leukocytes that determine the formation of atherosclerotic plaques. The majority of acute cardiac events are associated with thrombotic events due to plaque rupture. Plaques prone to rupture are defined "vulnerable" and are characterized by increased infiltration of pro-inflammatory leukocytes, a necrotic core, and a thin fibrous cap2. In the last decades, clinical and experimental studies have clarified the complex pathophysiology of the disease. Several animal models are used to investigate the molecular mechanisms involved in the induction of atherosclerosis3. Currently, the mouse is the most frequently used species to study atherosclerosis despite some species-specific differences existing in its pathophysiology in comparison with humans. In particular, circulating cholesterol is mainly composed by high-density lipoproteins (with antiatherogenic properties) in mouse models, while in humans circulating cholesterol is mainly transported as low-density lipoproteins (LDL), probably representing the main reason why wild-type mice do not develop spontaneous atherosclerosis4. Furthermore, wild type mice are generally resistant to absorbing cholesterol from the diet, whereas humans absorb around 50% of dietary cholesterol4. To overcome these limitations, several genetically modified mouse models have been generated. Apolipoprotein E–deficient mouse (ApoE−/−) and LDL receptor–deficient mouse (LDLr−/−) are widely used. On a high-fat high cholesterol diet, ApoE−/− mice develop plaque more rapidly compared to LDLr−/−mice, and for this reason, the use of ApoE−/− mice is more widespread than that of LDLr−/−mice5,6. The ApoE−/− mice develop lesions at any stage of atherosclerosis and are comparable to those observed in humans, although mouse plaques do not show an unstable phenotype7. Generally, ApoE−/− mice spontaneously develop atherosclerosis and this process is accelerated with a western diet3. The severity of atherosclerosis can be evaluated at the level of the carotid, pulmonary, femoral and brachiocephalic artery, and the aortic root6. In particular, the aortic root represents an anatomical site prone to develop atherosclerotic lesions in mice. For this reason, it is common practice to evaluate the formation of atherosclerotic plaques in this region.
Several studies have shown that aldosterone is implicated in the development of atherosclerosis8,9,10. Aldosterone infusion in ApoE−/− mice fed an atherogenic diet accelerates the development of aortic root atherosclerotic lesions inducing inflamed and lipid-rich plaque formation10.
In summary, we describe a protocol including aldosterone infusion, atherogenic diet feeding, embedding of heart tissues, quantification and characterization of atherosclerotic lesions in ApoE-/- mice. This procedure promotes an efficient formation of inflamed, lipid-rich atherosclerotic plaques and represents a valuable model for the study of atherogenesis.
Protocol
The study was approved by the Italian National Institutes of Health Care and Use Committees, authorization number 493/2016-PR. All procedures were conducted per the guidelines of the European Community for the use of experimental animals (European Directive, 2010/63/UE).
Note: Subcutaneous implantation of osmotic minipump containing vehicle (ethanol in saline solution) or aldosterone (240 µg · kg-1 · d-1) in 8-10 week-old male mice deficient for the ApoE gene. In general, 8-10 week-old male ApoE−/− mice weigh around 25-26 g.
1. Dissolving Aldosterone
Dissolve 5 mg of aldosterone powder in 1 mL of absolute ethanol.
Add 56.7 µL of aldosterone (dissolved in ethanol) to 68.3 µL of saline solution in order to obtain a concentration of 2.27 µg/µL. Fill the entire osmotic minipumps completely with 125 µL of this solution (maximum capacity of each minipump is 125 µL).
Use a minipump flow rate of 0.11 µL/h; therefore 2.64 µL of the solution is released every 24 h. Give each mouse around 6 µg · d-1 of aldosterone for 28 days.
2. Osmotic Pump Filling and Implantation
Note: Pumps are supplied in two separate parts: the main body of the pump and the flow regulator.
Fill and handle the minipumps using surgical gloves. Note: Skin oils may interfere with the performance of minipumps. The following steps should be performed in a sterile laminar flow hood.
Attach the filling tube (supplied with the minipumps) to a 1 mL syringe and draw up the aldosterone solution or vehicle. Note: It is important to avoid letting air inside the syringe while aspirating solutions from the tube to prevent the formation of air bubbles into the pump.
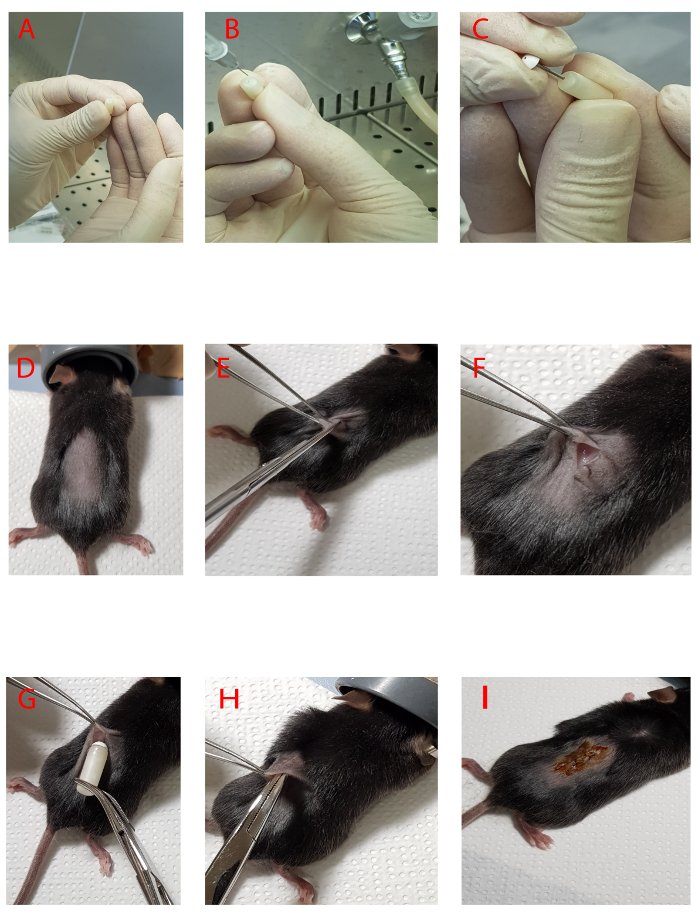
- Insert the filling tube through the hole at the top of the minipump until it can go no further (Figure 1A-1B). Slowly fill the pump with the aldosterone or vehicle. Notice the dark shadow inside the pump indicating the fluid level. Watch this level rise along with the filling pump.
- Stop filling the pump when a bead of fluid rises out of the pump body.
Carefully remove the filling tube and insert the flow regulator into the body of the minipump (Figure 1C). Make sure that the regulator is seated tightly against the pump body. Leave the filled osmotic pumps in a beaker containing sterile saline solution at 37 °C for up to 24 h before implantation in the mouse (priming procedure).
Conduct surgery in a disinfected area with 70% ethanol that promotes asepsis.
- Apply 1-3 drops at incision site of 0.25% bupivacaine and anesthetize the animal with 3% isoflurane. Turn on the supply gas and set the flowmeter to 500-1000 mL/min. Place the animal in the induction chamber and seal the top. Turn on the vaporizer to 5% isoflurane and monitor the mouse until recumbent.
- Switch the system to flow to the nosecone. Remove the animal from the chamber and position in the nosecone. In 2-3 minutes, the mouse starts to awaken. Restart gas flow with the flowmeter at 100-200 mL/min and the vaporizer at 3% isoflurane.
- Ensure adequate depth of anesthesia prior to performing procedures by testing pedal withdrawal and palpebral reflexes. Monitor respiration and response to stimulation during the procedure and adjust vaporizer as needed.
- Protect the corneas from drying out by applying an ophthalmic ointment.
Prepare the animal by removing hair from the surgical site using a razor (Figure 1D). The usual site for subcutaneous implantation of pumps is on the back.
Prepare the surgical site with nonwoven pad material saturated with 70% isopropyl alcohol.
Use a clean and dedicated space (disinfected with 70% ethanol) and covered with a sterile drape. Place the tip of the instruments in a glass bead sterilizer. Begin surgery with sterilized instruments and handle instruments aseptically.
Use surgical scissors to make a 0.8-1 cm incision (around 2 cm close to the tail), as shown in Figures 1E-1F. Maintain sterility by not touching anything outside the surgical dedicated space with sterile gloves or tips of instruments.
Take care to cut only the skin but not the underlying tissues. Use one hand to hold forceps to open the incision. Use the other hand to hold the trocar to spread the skin in order to create a pocket for the pump from the back upwards to the scapula (on the right or left side of the mouse).
Insert the pump into the incision. Gently push the pump completely into the pocket (Figure 1H). There should be enough skin free to close the wound with no tension or stretching of the skin needed. Once the pump has been inserted, suture the incision with surgery wire (polyglactin 910 with suture size 6-0) Note: The head of the regulator must be oriented toward the front of the mouse (Figure 1G).
Apply topical 10% povidone/iodine ointment with a clean swab (Figure 1I) and keep the animal on a warming stage. Do not leave mice in unattended until they recover sternal recumbency. Evaluate the hydration status and administer 0.5 mL of 0.9% saline solution subcutaneously if necessary. Return the animal to its routine housing.
- Follow the institutional guidelines for documentation (e.g., fill a surgical form with operative and post-operative information, update cage card with procedure and date) and provide analgesics (buprenorphine, 0.05 mg/kg) to reduce post-surgical pain and antibiotics (enrofloxacin 85 mg/kg) to avoid post-surgical infection.
- Continue daily monitoring and examine for signs of pain. Pay attention that wound is completely closed and minipump is maintained into the subcutaneous pocket. The wound may open and the mouse may lose the minipump.
3. Dissection of the Heart
At the time of sacrifice, anestethize mice with 80-100 mg/kg of Zoletil administered intramuscularly. Zoletil induces deep anesthesia. Note: check that mice are at an adequate depth of anesthesia prior to performing procedures by testing the pedal and palpebral reflexes; mice will be perfused while deeply anesthetized. Open the thoracic cavity, using scissors and forceps, by cutting the ribs laterally to the sternum, with the sternum being retracted toward the head.
Perfuse mouse via the left ventricle by gravity perfusion system with 10-15 mL of ice-cold Dulbecco’s phosphate buffered saline solution (PBS) and then with 40-50 mL of 10% of neutral buffered formalin to fix the vasculature. Fixation is indicated when the mice exhibit vigorous muscle contractions and become rigid. Note: The fixing process takes place in 10-20 min. It is possible to use the fixed organs and tissues to perform immunofluorescence or immunohistochemistry analyses. Importantly, such fixed tissues cannot be used for gene expression and western blotting analyses.
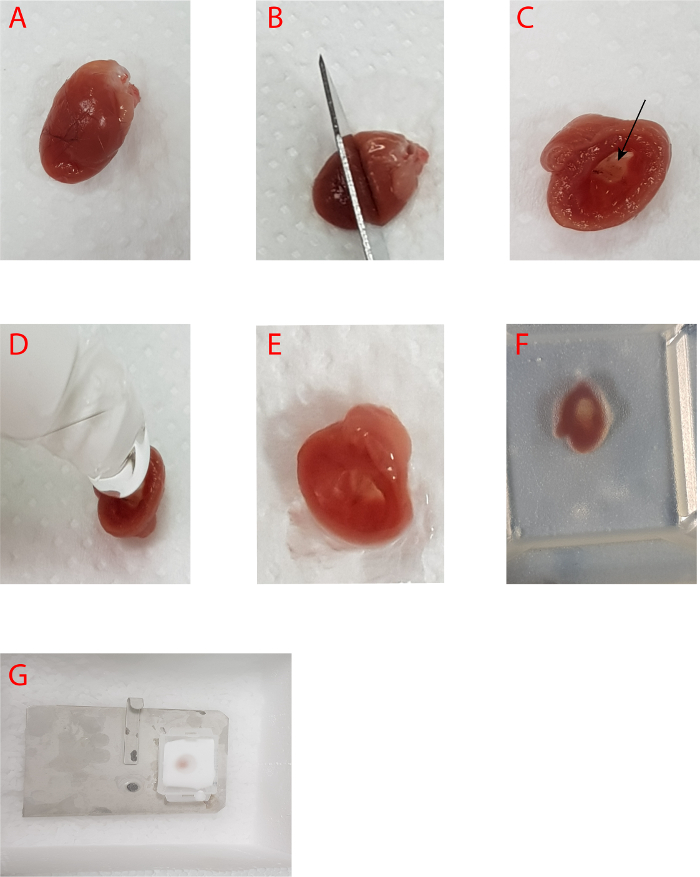
Separate the heart from the aorta by holding the heart with the forceps and cutting with either scissors or a scalpel blade, perpendicularly to the axis of the aorta, as close as possible to the heart without causing damage (Figure 2A). Use a scalpel blade to cut transversely along a straight line that joins the lower points of the right and left atrium (Figure 2B).
Fill the upper portion of the sectioned heart (through the cavity of the heart) with optimal cutting compound (OCT) (Figure 2D-2E). Put the tissue within a cryosection plastic mold with the axis of the aorta placed perpendicularly to the base of the rectangular mold and cover with OCT (Figure 2F). There should be no air bubble trapped. If any bubble is present, try to displace it to the edge.
Place a metal plate on dry ice and then carefully put the mold on (Figure 2G). When the OCT becomes white, wrap the mold with silver foil and then store at –80 °C.
4. Cutting sections of the Aortic Root
Set the cryostat machine to –20 °C. Before cutting, place the embedded tissue and leave it there for about 15 min to adjust to this temperature. Set the specimen holder temperature to -17 °C (Figure 3A).
Place the frozen heart block inside the cryostat machine to equilibrate the temperature of the block to that of the cryostat (Figure 3B) and take out the frozen heart block from the mold (Figure 3C). Cut the excess OCT from the frozen block to better adapt the block dimensions to the specimen holder (Figure 3D).
Mount the frozen heart block on the specimen holder oriented with the ventricular facing outward (Figure 3E-3I) and insert the specimen holder into the specimen orienting head (Figure 3J). Adjust the orientation of the specimen holder to allow cutting of the block in order to make the aortic root perpendicular to the blade (Figure 3K).
Cut the block and discard slices until the aortic root is reached (Figure 3L-3N). Collect serial sections and place them on the glass slides (Figure 3O). Monitor the aortic root anatomical profile under the microscope until all 3 aortic valves appear (Figure 3P-3Q).
Collect sections at 10 µm/section for Oil Red O and Picro Sirius Red staining, and at 6 µm/section for MAC3 staining (Figure 3R). Collect around 6-8 slides (for each heart) until one of the three valves disappears or is not intact anymore.

5. Immunohistochemistry
Note: All staining steps reported in the following protocols are performed in glass Coplin staining jars.
- Oil Red O Stain for Neutral Fats
- Fix sections in 70 mL of 37% formaldehyde for 5 min. Wash well in tap water and blot off excess water.
- Dilute 48 mL of Oil Red O (0.5% in isopropanol) in 32 mL of distilled water to obtain Oil Red O 0.3%.
- Stain sections in 70 mL of Oil Red O 0.3% for 10 min. Wash sections in tap water for 10 min.
- Stain for 1 min in 70 mL of Mayer’s Hematoxylin. Wash sections in tap water for 1 min.
- Immerse sections in 70 mL of 0.5% lithium carbonate. Wash in tap water for 1 min.
- Mount sections with an aqueous mounting medium and capture images using a digital camera mounted on a light microscope.
- Picro Sirius Red Stain for Collagen
- Cut 10 µm frozen sections and mount in the bottom of the slide.
- Fix sections in 70 mL of acetone for 5 min, air dry and rinse briefly in distilled water.
- Place sections in 70 mL of 0.2% phosphomolybdic acid for 5 min. Rinse briefly in distilled water.
- Place sections in 70 mL of 0.1% Picro Sirius solution for 90 min. Rinse one time with 70 mL of 0.01 N HCl. Discard solution.
- Place in 70 mL of 0.01 N HCl for 2 min. Rinse briefly in distilled water. Air dry.
- Place briefly in 70 mL of 70% ethanol.
- When completely dry, place in 70 mL of clearing agent of terpene origin and mount sections using poly(butyl methacrylate-co-methyl methacrylate) based mounting medium and capture images using a digital camera mounted on a light microscope. Note: One of the advantages of the Picro Sirius Red staining is that it is possible to distinguish the Type I (thick fibers, Yellow-Orange birefringence) and Type III (thin fibers, Green birefringence) collagen fiber networks by polarized light microscopy11.
- Mac3 Stain for Macrophage Staining
- Cut 6 µm frozen sections and mount in the bottom of the slide.
- Fix sections in 70 mL of cold acetone for 5 min. Immerse in 70 mL of PBS for 2 min.
- Using a pipette, cover sections with 1% of sodium dodecyl sulfate (SDS) solution and incubate for 5 min at room temperature (RT) for antigen retrieval. Immerse in 70 mL of PBS for 5 min.
- Using a pipette, cover the slices with 0.3% hydrogen peroxide for 20 min. Wash in 70 mL of PBS 3 times for 5 min.
- Use Avidin-Biotin Complexes (ABC)-HRP Kit for the following steps.
- Using a pipette, block sections in Normal Rabbit serum at room temperature for 20 min. Do not rinse off.
- Stain with MAC3 (1:300) for 90 min. at RT. Wash in 70 mL of PBS 3 times for 5 min.
- Stain with Secondary-biotinylated Anti-Rat IgG (1:200) for 45 min at RT. Rinse with 70 mL of PBS 3 times for 5 min.
- Cover the sections with Rabbit IgG ABC for 30 min at RT. Rinse with 30 mL of PBS 3 times for 5 min.
- Cover the sections with 3-amino-9-ethylcarbazole (AEC) for 10 min. Rinse with 70 mL of distilled water for 10 times.
- Stain for 1 min in 70 mL of Mayer’s Hematoxylin. Wash sections in tap water for 1 min.
- Immerse sections in 70 mL of 0.5% Lithium Carbonate. Wash in tap water for 1 min.
- Mount sections with an aqueous mounting medium and capture images using a digital camera mounted on a light microscope.
6. Image Analysis of Atherosclerotic Lesions in Aortic Root Sections
Note: Aortic root sections stained with Oil Red O or MAC3 or an antibody of interest can be analyzed using appropriate software (indicated in Table of Materials). Total pixels staining positive for the component of interest is normalized to the overall plaque area. Images were collected and analyzed by a treatment-blinded investigator.
- Calibration
- Open software and then select the image (in jpg) to be analyzed.
- Select the Home | Create | Quick Calibration.
- Set up the calibration of image by drawing a line along the scale bar of the image.
- Save the setup of calibration.
- Image Analysis
- Select the saved setup calibration from Options menu | Spatial Calibration Option.
- Select Calibration | Apply.
- Select the polygon tool on the Select menu and draw the perimeter along all atherosclerotic lesions.
- Select Count/Size | Types. From the Menu, add Area and Percent Area.
- From Count/Size menu, select Manual and a new window will open.
- From this Window click on the Pick color on image tool and select HSI mode.
- Point the mouse cursor on the positive pixels of the marker of interest to create a range of colors.
- Click on Count and the value Sum, observed in the Measurement Table, indicates the percentage of the area of the marker (also expressed in µm2) with respect to the total area of atherosclerotic lesions.
Representative Results
In 8-10 weeks Apo-/- mice, minipumps were implanted to infuse with vehicle or aldosterone (Figure 1E-1I) and fed an atherogenic diet (adjusted calories diet 42% from fat) for 4 weeks. At the end of treatment, mice were euthanized and perfused with PBS and 10% formalin as described above. The aortic root was separated from the apical portion of the heart and was embedded in OCT (Figure 2). Cross sections of the aortic roots were prepared for different staining by using a cryostat machine (Figure 3).
Atherosclerotic plaque composition can be characterized through the staining for specific components, which define the stage of plaque development. Oil Red O staining is used to determine lipid content of atherosclerotic lesions as described in step 5.1, Picro Sirius Red staining is used to determine collagen content as described in step 5.2, and Mac3 staining is employed to measure macrophage content described in step 5.3. After 4 weeks of treatment with aldosterone, mice displayed a significant increase in lipid (Figure 4A-4C) and macrophage content (Figure 4D-4F) in atherosclerotic plaques at the aortic root level, but no differences were observed in collagen content (Figure 4G-4I) compared to mice treated with the vehicle. Indeed, aldosterone accelerated atherogenesis in this model, inducing vascular inflammation but not fibrosis (Figure 4).
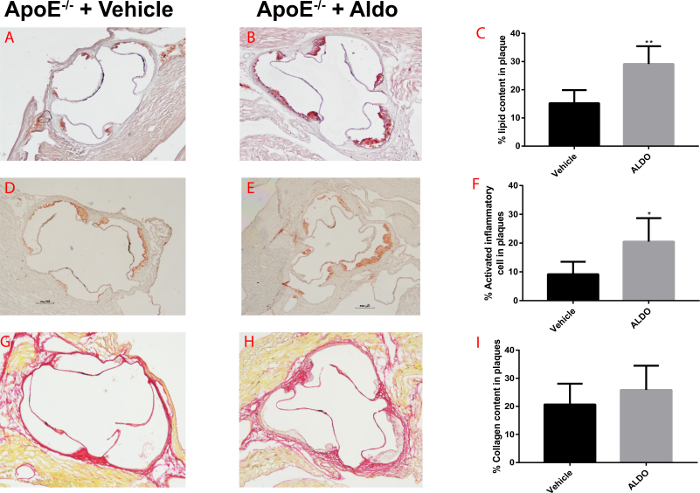
Figure 1. Preparation and implantation of osmotic minipump with vehicle or aldosterone. A-B) Steps to fill osmotic minipumps with vehicle or aldosterone. C) Steps to close the body of pump with the flow regulator. D-F) Steps to create an incision for the implantation of pump on the back of the mouse. G-I) Implantation of osmotic minipump and suturing of incision. Please click here to view a larger version of this figure.
Figure 2. Preparing the heart and the aortic root for embedding and sectioning. A-B) The heart is cut transversely along a straight line that joins the lower points of the right and left atrium. The apical part of the heart is processed to quantify atherosclerotic lesions composition in the aortic root. C) The aortic root from inside the heart. D-E) Filling of the heart cavity with OCT. F) Orientation of the dissected heart in a mold pre-filled with OCT (with the heart cavity in direction of the bottom of mold) G) Freezing of the dissected heart with OCT in the mold. Please click here to view a larger version of this figure.
Figure 3. Sectioning of aortic root using a cryostat microtome. A) Pre-cool the cryostat and set the right temperature for the machine and chuck. B-D) The solid OCT block is separated from the mold and the excess OCT around the heart is eliminated using a blade. E-I)The block of OCT is fixed, using liquid OCT, on the support. J-K) The support is inserted in the chuck and oriented with the ventricular facing outward. L-M) Section the block until the dissected heart is completely exposed. N-O) Set the thickness to 10 and/or 6 µm and collect the slices on the glass. P-Q) Check the presence of all three valves under the microscope. R) Subsequent slices are collected, as shown, until one or more valves are not observed anymore. Please click here to view a larger version of this figure.
Figure 4. Quantification of atherosclerotic plaque composition in aldosterone- or vehicle-treated ApoE-/-. A-B) Representative Red Oil O stained cross sections of aortic root in 8-10 week-old ApoE-/- mice treated with vehicle (A) or aldosterone (B) and fed an atherogenic diet for 4 weeks. C) Quantification of lipid content as percentage of positive staining/total plaque area. D-E) Representative Mac3 stained cross sections of aortic root. F) Quantification of macrophage content as percentage of positive staining/total plaque area. G-H) Representative Picro Sirius Red stained cross sections of aortic root. I) Quantification of collagen content as a percentage of positive staining/total plaque area. Values are expressed as mean ± Standard Error Mean (SEM; n=6 for each group). Please click here to view a larger version of this figure.
Discussion
Atherosclerosis is a chronic inflammatory disorder associated with large and medium vessels involving interactions between multiple cell types, such as macrophages, T-lymphocytes, endothelial cells and smooth muscle cells1. Despite the limitations of murine atherogenic models, a large body of evidence on the atherosclerotic process is available. These models have the advantage of rapidly generating experimental cohorts of a specific age and gender. Mice also show a defined and homogeneous genetic background.
The development of atherosclerotic plaques, observed in mouse models prone to atherosclerosis, partially resembles that observed in human subjects, except for unstable plaques formation4. Plaque rupture and subsequent thrombosis, the main cause of myocardial infarction in humans, is not observed in murine atherogenic models. In our recent papers, we showed that infusion of aldosterone is able to induce, after 4 weeks, an unstable plaque phenotype in ApoE-/-mice fed an HFD10. In fact, this treatment is able to increase plaque area, lipid content, and inflammatory area at the aortic root level, with no significant difference in collagen content compared to untreated mice10. Of note, these mice show increased development of hypertension-independent atherosclerosis, given that blood pressure was not significantly altered by aldosterone treatment10.
In the present manuscript, we illustrate in detail the technical procedure to embed the heart tissue in OCT as well as the sequential steps, i.e., aortic root sectioning and plaque analysis. Compared to paraffin, OCT allows section preparation with a high range of thicknesses (1-100 µm) with a cryostat microtome and minimal tissue processing. Limitations include the quality of frozen tissue, which is potentially affected by the formation of ice crystals and can alter subcellular details and generate artifacts detectable by immunohistological staining. Indeed, cryopreservation is thought to better preserve antigen and antigenicity. In fact, frozen tissues preserve the structure of antigens. Furthermore, frozen tissue processing for immunohistochemistry allows to avoid the use of formalin, which is a toxic and carcinogenic compound.
The aortic root is a site relatively straightforward to identify for histological processing. This anatomical site is a gold standard to study atherosclerotic lesion development in mice prone to atherosclerosis. Data obtained by the analysis of this specific region allow comparisons among mice belonging to the same group or different experimental groups. In fact, during sectioning of the aortic root, it is easy to recognize a specific region characterized by the presence of all three valves. In order to obtain such a specific region, it is strictly recommended to pay particular attention to several critical steps during embedding and sectioning. It is important to fill the cavity of the upper part of the sectioned heart with OCT to avoid the formation of air bubbles in the tissue that can compromise the integrity of the sections. Furthermore, to ensure that the cutting angle is exactly perpendicular to the ascending aorta during sectioning, it is necessary to properly orient the upper sectioned heart perpendicular to the bottom surface of the tissue mold. In order to obtain intact slices, the temperature of the cryostat should be exactly at -20 °C. In fact, a lower temperature could compromise the quality of sections (i.e., the blade could crumble the sections making them unusable).
Of note, when the three valves appear, it is recommended to cut the tissue with different thicknesses, depending on the type of the analysis that will be performed. In fact, some slides will be used for the determination of plaque progression, whereas the remaining slides could be used for different stainings (i.e., Picro Sirius, Mac3, etc.).
Atherosclerotic lesions in mice treated with aldosterone appear rich in lipid and macrophages characterized by the use of Oil Red O (for lipid staining), Mac3 (for macrophage staining), Sirius red (for the staining of collagen), or other specific antibodies for proteins of interest. It is important to highlight that OCT embedding, unlike paraffin embedding, avoids the use of organic solvents that remove the lipid content of the lesions. Therefore, lipid staining with Oil Red O is feasible in OCT-embedded sections and lipid content of atherosclerotic plaques can be precisely quantified.
In the present manuscript we showed that aldosterone infusion is a valuable method to promote atherosclerosis in ApoE-/- mice. In fact, aldosterone infusion in ApoE-/- mice is able to: 1) increase the activation of endothelial cells, as shown by the increased expression of molecules involved in the adhesion and migration of monocytes at the early stages of atherosclerotic plaque formation12,13; and 2) increase lipid and pro-inflammatory macrophages content at aortic root level10,13. These effects are not due to hemodynamic effects of aldosterone10. In conclusion, the proposed protocol represents a valid method to generate and characterize atherosclerosis in mice; this could help monitor early stages of disease and outcomes of therapeutic intervention and rehabilitation in atherosclerosis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the Italian Ministry of Health (Ricerca Corrente, GR-2009-1594563 and PE-2011-02347070 to M.C.)
References
- Ross R. Atherosclerosis--an inflammatory disease. New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Lafont A. Basic aspects of plaque vulnerability. Heart. 2003;89(10):1262–1267. doi: 10.1136/heart.89.10.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(5):1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Current Opinion in Lipidology. 2010;21(5):434–440. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis? Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(9):1734–1741. doi: 10.1161/ATVBAHA.116.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini Veseli B, et al. Animal models of atherosclerosis. European Journal of Pharmacology. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27(4):705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- de Rita O, Hackam DG, Spence JD. Effects of aldosterone on human atherosclerosis: plasma aldosterone and progression of carotid plaque. Canadian Journal of Cardiology. 2012;28(6):706–711. doi: 10.1016/j.cjca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Ivanes F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. European Heart Journal. 2012;33(2):191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- McGraw AP, et al. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. Journal of the American Heart Association. 2013;2(1):e000018. doi: 10.1161/JAHA.112.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods in Molecular Biology. 2017;1627:395–407. doi: 10.1007/978-1-4939-7113-8_26. [DOI] [PubMed] [Google Scholar]
- Caprio M, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circulation Research. 2008;102(11):1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolla V, et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. International Journal of Cardiology. 2017;232:233–242. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


