Abstract
Objective
Despite the high frequency of HFE gene mutations in Western Europe, widespread screening for HFE hemochromatosis is not recommended due to its variable phenotype. Joint pain and a premature osteoarthritis-like disease including the hip joints are the most frequent manifestation in patients with HFE hemochromatosis and iron overload. Therefore, screening of patients with severe osteoarthritis of the hip could identify patients with HFE hemochromatosis.
Methods
In this prospective cross-sectional study, 940 patients aged <70 years with end-stage osteoarthritis of the hip undergoing elective joint replacement surgery were screened for HFE hemochromatosis and compared to age- and sex-matched controls.
Results
No greater prevalence of C282Y homozygosity mutation or elevated serum ferritin or transferrin saturation levels was found in the study cohort with severe osteoarthritis of the hip than in controls from the general population.
Conclusion
Our screening approach could not identify an increased prevalence of HFE gene mutations and iron overload in younger patients with severe osteoarthritis of the hip.
Introduction
HFE hemochromatosis (HH) is a systemic disorder leading to iron overload in parenchymatous organs, eventually causing organ failure. Clinically manifest HH is associated with high morbidity and increased mortality. The classical triad was characterized by liver cirrhosis, diabetes and skin hyperpigmentation. The majority of symptomatic patients harbor a homozygous mutation leading to a cysteine-to-tyrosine substitution at amino acid position 282 (C282Y) in the High Iron Fe (HFE) gene on chromosome 6, which leads to inappropiate increased iron absorption from the gut [1]. The homozygous prevalence of this autosomal-recessive mutation is ~0.5% in Western Europe [2]. Other mutations such as the H63D mutation (histidine to aspartic acid) of the HFE gene can also be found frequently, but are usually clinically not relevant [3].
Phenotypic expression of C282Y homozygosity is variable and depends on environmental and host factors [4] and polymorphisms of TMPRSS6, GNPAT and PCSK7 genes relevant to iron absorption and cirrhosis risk [5, 6]. The rate of iron-overload-related disease in C282Y homozygotes was 28.4% for men and 1.2% for women in a large epidemiological study [7]. Joint pain often presents first and is the most common manifestation of HH. While symptoms do not differ from primary osteoarthritis (OA), the typical distribution of affected joints found in HH is remarkable. Metacarpophalangeal and ankle joints are frequently affected in HH, while hip joints are regularly involved in both disorders [8]. Site-specific arthropathy may be influenced by iron overload in HH patients. Arthropathy in HH was associated with iron overload in some studies [9], but not in others [10]. However, HH individuals seem to develop hip joint arthropathy earlier in life than patients with primary OA [11]. Therefore, we hypothesized that screening younger patients with severe OA of the hip could identify HH patients.
Patients and methods
Study population
Patients with severe OA of the hip were recruited sequentially in three orthopedic departments in Vienna over a period of three years for this cross-sectional study to identify clinically symptomatic HH patients. Individuals aged between 18 and 70 years old diagnosed with severe OA of the hip and scheduled for elective joint replacement surgery of the hip were eligible. Patients undergoing joint replacement surgery due to traumatic fractures and revision procedures were not included. Between 2012 and 2014 a total of 1001 patients gave written informed consent to participate in the study. After exclusion of patients with missing data, 940 patients could be included in the study. As control group, 940 age- and sex-matched individuals from a population-based prospective study without history of joint replacement surgery were randomly selected [12]. The study was approved by the Ethics Committee of the City of Vienna (EK 11-103-0811).
Patient assessment
Epidemiological data including age, sex and body mass index as well as history of former joint replacement surgeries were assessed. Iron status with serum ferritin (μg/l) and transferrin saturation (%) levels was obtained after overnight fasting and measured using standard laboratory methods. Blood samples for HFE genotyping were stored until further processing.
HFE gene mutation analysis
DNA was isolated from 300 μl of EDTA blood using Maxwell® 16 LEV Blood DNA Purification Kits (Promega) and the Maxwell® 16 IVD System (Promega) according to the manufacturer's protocol. DNA samples were analysed for C282Y and H63D mutations of the HFE gene by Sanger sequencing. Primer sets for PCR amplification of human HFE coding exons 2 and 4 were designed using Primer3Plus software. The resulting PCR products were subjected to fluorescence-based cycle sequencing using the BigDye® Terminator Cycle Sequencing Ready Reaction Kit, version 3.1 (Applied Biosystems). Samples were run and analysed on an Applied Biosystems® 3500 Genetic Analyzer. Sequencing electropherograms were assessed by visual inspection to identify C282Y and H63D mutations.
Statistical analysis
A three-fold increase in C282Y homozygous mutations in our patient cohort compared to the general population was estimated [11]. Statistical power for Fisher's exact test assuming alpha of 0.05 (two-tailed) was calculated using G*Power software (University of Duesseldorf, Germany). A case number of 2000 had 80% power to detect the assumed differences between groups. After a pre-planned halftime analysis the study was terminated early because of unexpectedly low prevalence of C282Y homozygosity in the patient group.
Data are presented as mean ± standard deviation (SD). Ferritin and transferrin saturation levels of patients with severe OA of the hip and controls were compared using Mann-Whitney U test. Distribution of genotypes between groups was analyzed by Pearson's chi-square test. Multivariate logistic regression analysis was used to determine the association of HFE genotypes with severe hip OA, adjusting for covariates age, sex, body mass index, history of diabetes and serum ferritin level. P-values <0.05 were considered statistically significant. SPSS statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
Mean age of 940 patients undergoing hip replacement surgery was 59.4 ± 9.5 years and ranged from 19 to 70 years. Male to female ratio of patients enrolled in the study was 1:1.4 and women were 1.7 years older in average. With a mean BMI of 27.9 ± 5.1, in overall patients could be classified pre-obese according to the World Health Organization. Mean BMI in the control group was 27.0 ± 4.8 (Table 1).
Table 1. Iron status and HFE genotype in patients with severe ostearthritis of the hip (OA) and controls.
| OA, n = 940 | Control, n = 940 | p | |||
|---|---|---|---|---|---|
| Men : women | 385 : 555 | 385 : 555 | |||
| Age, years | 59.4 ± 9.5 | 60.0 ± 10.1 | 0.27 | ||
| BMI | 27.9 ± 5.1 | 27.0 ± 4.8 | <0.05 | ||
| Ferritin, μg/l | 151.4 ± 157.6 | 200.4 ± 329.2 | <0.05 | ||
| Transferrin saturation, % | 26.1 ± 12.0 | 27.8 ± 12.0 | <0.05 | ||
| Elevated ferritin and transferrin saturation* | 20 | 29 | |||
| N/A | 57 | 108 | |||
| HFE genotype, n (%) | <0.05 | ||||
| Wild type | 650 (69.1) | 624 (67.2) | 0.36 | ||
| H63D/- | 203 (21.6) | 182 (19.6) | 0.28 | ||
| H63D/H63D | 13 (1.4) | 13 (1.4) | 0.98 | ||
| C282Y/- | 63 (6.7) | 82 (8.8) | 0.09 | ||
| C282Y/C282Y | 1 (0.1) | 6 (0.6) | 0.06 | <0.05 | |
| C282Y/H63D | 10 (1.1) | 22 (2.4) | <0.05 | ||
| N/A | 0 | 11 | |||
*Hyperferritinemia (≥150 μg/l in women, ≥300 μg/l in men) and increased transferrin saturation level (≥45%) according to National Institute of Health reference values.
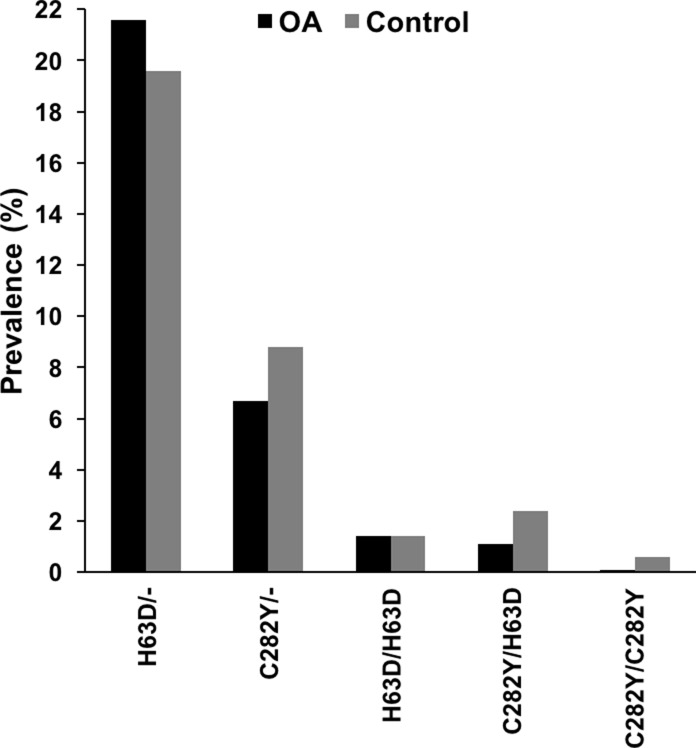
Serum ferritin and transferrin saturation levels were significantly higher in the control than in the patient group. C282Y homozygosity was found in only one (0.1%) out of 940 patients with end-stage OA of the hip. In the control group, six (0.6%) cases of C282Y homozygosity were identified. Compound heterozygosity (C282Y/H63D) was found in 10 (1.1%) versus 22 (2.4) of 940 individuals in the patient versus control cohort, respectively. Thus, C282Y homozygous and compound heterozygous mutations were significantly less prevalent in the hip OA group (p<0.05). According to this, multivariate regression analyses revealed a significantly lower prevalence of compound heterozygosity (C282Y/H63D) in OA patients than in controls (OR 0.44, 95%-CI 0.20–0.97, p<0.05). Prevalence of heterozygous C282Y and H63D mutations was 63 (6.7%) versus 82 (8.8%) and 203 (21.6%) versus 182 (19.6%) in the patient and control population, respectively. An equal number of 13 (1.4%) homozygous H63D mutations were found in both groups (Table 1 and Fig 1). HFE genotype was not available for 11 of 940 controls.
Fig 1. Prevalence of HFE genotype in patients with severe osteoarthritis of the hip (OA) and controls.
Discussion
Tissue iron accumulation in HH can lead to major complications such as liver cirrhosis and hepatocellular carcinoma. Iron depletion by phlebotomy is the treatment of choice with potential reversal of hepatic fibrosis [13]. Thus, early diagnosis of the disease to prevent complications is important and screening of at-risk populations warranted. In this regard, a case finding approach by targeted screening of vulnerable individuals with signs suggestive of iron overload, as well as offering testing to Caucasian men of Northern European ancestry, has been discussed [14].
Joint pain is the most frequent presentation in patients with HH and iron overload and often precedes other symptoms [8]. In contrast to primary OA, HH arthropathy frequently affects men and shows symmetric joint involvement. Symptoms of the typically affected second and third metacarpophalangeal joints may be subtle and do not necessarily lead patients to consult rheumatologists. Bilateral ankle joint involvement is also typical in HH, but its prevalence in patients is quite low [15]. Other affected joint regions include the wrists, as well as knee and hip joints. Importantly, HH was established as a risk factor for hip replacement at younger age [11]. An increased risk of musculoskeletal complications was confirmed in two independent population-based studies in Sweden and Australia [16, 17].
As an approach for screening of at-risk populations, we analyzed serum iron status and HFE genotypes in patients with end-stage hip OA under the age of 70. Given the previous findings from epidemiological studies, we estimated a three-fold increase in C282Y homozygous mutations in our patient cohort compared to the general population. Nevertheless, our data did not reveal a significant increase in HFE mutations nor laboratory signs of iron overload in our study population compared to age- and sex-matched individuals. In fact, C282Y homozygosity was even lower in prevalence than in the control population, the latter being in the expected range. Thus, our study does not suggest a benefit from HFE screening in younger patients with severe OA of the hip. A possible reason for not detecting an increase in C282Y homozygotes in the OA group is that subjects in our study were not primarily selected by iron overload and few suffered from elevated serum ferritin and transferrin saturation levels. Further, it cannot be excluded that hemochromatosis patients with joint pain are being diagnosed and treated at earlier stages in Austria. In fact, many rheumatologists include full iron status in their diagnostic work-up of newly presenting patients. Also, a referral bias of certain patient groups cannot be fully excluded, although all recruiting centers are part of the social insurance system.
Hence, other case identification approaches have to be developed and tested in HH. Regarding hemochromatosis arthropathy, although rare, patients with severe ankle OA without mechanical trigger factors may serve as potential candidates. Although we could not prove a benefit in testing patients with severe hip OA for HH, studies for screening of at-risk populations need to be continued in order to prevent the course of this potentially life-limiting disease by its early detection and therapy.
Acknowledgments
The authors would like to thank Franz Varga, Monika Rumpler and Silvia Spitzer for support, statistical and technical assistance.
Data Availability
All relevant data are within the manuscript.
Funding Statement
JZ received funding for this project by the Austrian Science Fund FWF (https://www.fwf.ac.at): KLI123-B00. Financial support from SPAR Austria to CD is gratefully acknowledged (https://www.spar.at). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Datz C, Lalloz MR, Vogel W, Graziadei I, Hackl F, Vautier G, et al. Predominance of the HLA-H Cys282Tyr mutation in Austrian patients with genetic haemochromatosis. J Hepatol. 1997;27(5):773–9. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen P, Milman N. Genetic screening for HFE hemochromatosis in 6,020 Danish men: penetrance of C282Y, H63D, and S65C variants. Ann Hematol. 2009;88(8):775–84. 10.1007/s00277-008-0679-1 [DOI] [PubMed] [Google Scholar]
- 3.Castiella A, Zapata E, de Juan MD, Otazua P, Fernandez J, Zubiaurre L, et al. Significance of H63D homozygosity in a Basque population with hemochromatosis. J Gastroenterol Hepatol. 2010;25(7):1295–8. 10.1111/j.1440-1746.2010.06247.x [DOI] [PubMed] [Google Scholar]
- 4.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393–408, e1-2. 10.1053/j.gastro.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 5.Stickel F, Buch S, Zoller H, Hultcrantz R, Gallati S, Osterreicher C, et al. Evaluation of genome-wide loci of iron metabolism in hereditary hemochromatosis identifies PCSK7 as a host risk factor of liver cirrhosis. Hum Mol Genet. 2014;23(14):3883–90. 10.1093/hmg/ddu076 [DOI] [PubMed] [Google Scholar]
- 6.McLaren CE, Emond MJ, Subramaniam VN, Phatak PD, Barton JC, Adams PC, et al. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology. 2015;62(2):429–39. 10.1002/hep.27711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221–30. 10.1056/NEJMoa073286 [DOI] [PubMed] [Google Scholar]
- 8.Sahinbegovic E, Dallos T, Aigner E, Axmann R, Manger B, Englbrecht M, et al. Musculoskeletal disease burden of hereditary hemochromatosis. Arthritis Rheum. 2010;62(12):3792–8. 10.1002/art.27712 [DOI] [PubMed] [Google Scholar]
- 9.Valenti L, Fracanzani AL, Rossi V, Rampini C, Pulixi E, Varenna M, et al. The hand arthropathy of hereditary hemochromatosis is strongly associated with iron overload. J Rheumatol. 2008;35(1):153–8. [PubMed] [Google Scholar]
- 10.Sinigaglia L, Fargion S, Fracanzani AL, Binelli L, Battafarano N, Varenna M, et al. Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol. 1997;24(9):1809–13. [PubMed] [Google Scholar]
- 11.Sahinbegovic E, Dallos T, Aigner E, Axmann R, Engelbrecht M, Schoniger-Hekele M, et al. Hereditary hemochromatosis as a risk factor for joint replacement surgery. Am J Med. 2010;123(7):659–62. 10.1016/j.amjmed.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 12.Stadlmayr A, Aigner E, Steger B, Scharinger L, Lederer D, Mayr A, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med. 2011;270(1):41–9. 10.1111/j.1365-2796.2011.02377.x [DOI] [PubMed] [Google Scholar]
- 13.Falize L, Guillygomarc'h A, Perrin M, Laine F, Guyader D, Brissot P, et al. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44(2):472–7. 10.1002/hep.21260 [DOI] [PubMed] [Google Scholar]
- 14.Phatak PD, Bonkovsky HL, Kowdley KV. Hereditary hemochromatosis: time for targeted screening. Ann Intern Med. 2008;149(4):270–2. [DOI] [PubMed] [Google Scholar]
- 15.Elstob A, Ejindu V, Heron CW, Kiely PDW. MRI ankle and subtalar characteristics in haemochromatosis arthropathy: a case-control study. Clin Radiol. 2018;73(3):323 e1– e8. [DOI] [PubMed] [Google Scholar]
- 16.Elmberg M, Hultcrantz R, Simard JF, Carlsson A, Askling J. Increased risk of arthropathies and joint replacement surgery in patients with genetic hemochromatosis: a study of 3,531 patients and their 11,794 first-degree relatives. Arthritis Care Res (Hoboken). 2013;65(5):678–85. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Gurrin LC, Wluka AE, Bertalli NA, Osborne NJ, Delatycki MB, et al. HFE C282Y homozygosity is associated with an increased risk of total hip replacement for osteoarthritis. Semin Arthritis Rheum. 2012;41(6):872–8. 10.1016/j.semarthrit.2011.11.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.