Abstract
Biofilm growth has been observed in Soviet/Russian (Salyuts and Mir), American (Skylab), and International (ISS) Space Stations, sometimes jeopardizing key equipment like spacesuits, water recycling units, radiators, and navigation windows. Biofilm formation also increases the risk of human illnesses and therefore needs to be well understood to enable safe, long-duration, human space missions. Here, the design of a NASA-supported biofilm in space project is reported. This new project aims to characterize biofilm inside the International Space Station in a controlled fashion, assessing changes in mass, thickness, and morphology. The space-based experiment also aims at elucidating the biomechanical and transcriptomic mechanisms involved in the formation of a “column-and-canopy” biofilm architecture that has previously been observed in space. To search for potential solutions, different materials and surface topologies will be used as the substrata for microbial growth. The adhesion of bacteria to surfaces and therefore the initial biofilm formation is strongly governed by topographical surface features of about the bacterial scale. Thus, using Direct Laser-Interference Patterning, some material coupons will have surface patterns with periodicities equal, above or below the size of bacteria. Additionally, a novel lubricant-impregnated surface will be assessed for potential Earth and spaceflight anti-biofilm applications. This paper describes the current experiment design including microbial strains and substrata materials and nanotopographies being considered, constraints and limitations that arise from performing experiments in space, and the next steps needed to mature the design to be spaceflight-ready.
Keywords: Bacteria, Fungi, Pseudomonas aeruginosa, Penicillium rubens, Direct laser-interference patterning (DLIP), Lubricant-impregnated surface (LIS)
1. Introduction
1.1. Biofilms
Biofilms are formed by groups of organisms that are adhered to each other by self-synthesized extracellular polymeric substances, and are ubiquitous in industrial and natural environments [1]. The formation of biofilms increases the risk of pathogen transmission in food handling facilities, drinking water systems, and medical devices. Furthermore, biofilms can decrease the efficiency and lifetime of equipment such as heat exchangers, air and water recycling systems, etc. [1,2]. Biofilm bacteria and fungi tend to have an increased resistance to disinfectants, antibiotics, and environmental stresses – such as salt, oxidizers, and low pH – making it difficult to address the problems that arise from their formation [3–5]. In addition to the challenges that emerge from their formation on surfaces, biofilms play an important role in several human diseases and infections, including endocarditis (bacterial infection of cardiac tissue), cystitis (a urinary tract infection), and otitis media (an inflammatory disease of the middle ear) [3], to name a few.
1.2. Biofilms in space
Microbial contamination was observed on piping and equipment behind panels on board Salyut 6; water recycling system, rubber of hatch locks, electrical connectors, and the thermal control system’s radiator on board Salyut 7; and air conditioning, oxygen electrolysis block, EVA suit’s headphone, water recycling unit, and thermal control system on board Mir [6]. Bacillus polymira, Penicillium chrysogenum (now called P. rubens), and Aspergfilus sp. were determined to be responsible for progressive destruction of a navigation window on board Mir (reviewed in Ref. [6]). An assessment of the microbes living in the Russian Mir space station revealed 234 different species of bacteria and fungi. Most of the isolated fungi were potential biodegradors of polymers, and thus presented a potential hazard to structural materials and components of several systems [7]. Novikova et al. [8] performed a six-year study aimed at characterizing the microbiome present on board ISS, and found that bacterial concentrations of up to 1 × 102CFU/mL were found in potable water with Sphingomonas sp. and Methylo-bacterium sp. being the dominant genera (same as the 100 CFU/mL limit described on the ISS’ Medical Operations Requirements Documents (MORD) [9]). Airborne bacteria were quantified to be as high as 7 × 102 CFU/m3 (the ISS MORD’s limit is 1 × 103CFU/m3) Staphylococcus sp. being the dominant genus. Samples collected from surfaces showed bacterial concentrations as high as 4 × 104 CFU/100 cm2 (Staphylococcus sp.) (MORD limit: 1 × 104 CFU/100 cm2) and fungal concentrations of up to 3 × 105 CFU/100 cm2 (Aspergillus sp. and Cladosporium sp.) (MORD limit: 1 × 102 CFU/100 cm2), where the dominant genera are indicated in parenthesis. The organic acids synthesized by microorganisms can degrade metallic surfaces, which can lead to hardware malfunctioning and short circuits. Checinska et al. [10] have reported more recent results about the ISS microbiome and concluded that Actinobacteria was the most common phylum on the space station. Recently, more profound investigations showed that the microbe biodiversity of the ISS was distinct to the one from the Spacecraft Assembly Facility (SAF) cleanrooms at JPL, suggesting the human skin-associated microbes play an important role on spacecraft microbial diversity [10–12]. This has also been corroborated via a metagenome profile study [13]. Figs. 1 and 2 show biofilm formation on different components used for water processing on board ISS, taken after their return to Earth. Fig. 1 shows how only one of 12 channels was not blocked by biofilm.
Fig. 1.

Biofilm formation inside the solenoid valve at the inlet to the International Space Station’s Water Processor Mostly Liquid Separator (immediately downstream of the Water Processor Waste Tank). The image was taken by United Technologies Aerospace Systems (UTAS) during disassembly of the valve at the supplier facility (ValveTech, Inc) after the valve’s return to Earth. Image: NASA.
Fig. 2.

Biofilm formation inside the condensate plumbing at the inlet to the Russian condensate processor. Image: NASA.
1.3. Biofilm investigations conducted in space
At least three investigations have been conducted in space to assess biofilm formation (Table 1). The first of these experiments, reported by Pyle et al. [2], took place in the European Space Agency’s PHORBOL cassettes hardware on board the Space Shuttle STS-81 mission. Burkholderia cepacia isolated from a Space Shuttle water system was used as the model organism. In this experiment, samples were grown in sterile reagent grade water (to simulate untreated water), tryptic soy broth (TSB) (to simulate wastewater), or an iodine solution (to simulate disinfected potable water), and exposed for six days to a stainless-steel coupon to allow for biofilm formation. The results showed that the spaceflight water-grown bacteria had a biofilm plate count (CFU/cm2) five times that of the Earth controls. On the other hand, the spaceflight TSB-grown culture biofilm population was one quarter of the ground controls. Analyses of the water- and iodine-grown planktonic bacteria showed a 3.5- and 2-fold increase in CFU/mL with respect to matched ground controls, respectively. Pyle et al. [2] concluded that spaceflight enhanced bacterial growth and diminished disinfectant (iodine) sensitivity in some conditions. Additionally, elongated cells and chains of cells were observed on the spaceflight samples with respect to their matched ground controls, especially on the sets grown in the iodine solution.
Table 1.
Previous biofilm investigations conducted in space.
| Year | Mission | Bacterial Species | Strain | Growth Medium | Biofilm growth substratum | Experiment duration | Ref. |
|---|---|---|---|---|---|---|---|
| 1997 | STS-81 | Burkholderia cepacia | Isolated from Shuttle water system | a) water b) tryptic soy broth c) iodine solution |
Stainless steel | 6 days | [2] |
| 1998 | STS-95 | Pseudomonas aeruginosa | PAO-1 | R2A broth | polycarbonate membrane (0.2-μm pore size) | 1 day, 8 days | [4] |
| 2010 | STS-132 | Pseudomonas aeruginosa | PA14 (WT, ΔmotABCD, ΔpilB) | Modified artificial urine media (mAUM) | 13 mm Millipore mixed cellulose ester membrane disc | 3 days | [14] |
| 2011 | STS-135 |
The next experiment used Instrumentation Technology Associates, Inc.’s Type III Osmotic Dewatering hardware to assess if Pseudomonas aeruginosa could form biofilms in microgravity. Bacterial cultures were exposed to 0.2 μm polycarbonate membranes, allowing them to form biofilms for either 1 or 8 days. Post-flight confocal laser scanning microscopy revealed that biofilms indeed formed during spaceflight but no differences were observed between space and ground samples in terms of morphology. Likely due to the limitations of spaceflight experimentation, this experiment consisted of four spaceflight cultures, of which two were used for microscopic analysis [4].
The most complete and systematic spaceflight biofilm investigation conducted thus far was reported by Kim et al. [14]. Two experiments were conducted using BioServe Space Technologies’ Fluid Processing Apparatus (FPA) during the STS-132 and -135 missions, with three strains of P. aeruginosa used as model organisms. The experiments assessed the role that phosphate, carbon source, bacterial motility, and oxygen availability play in biofilm formation in space. It was concluded that the number of viable cells, biomass and mean biofilm thickness was increased in space, regardless of phosphate concentration or carbon source. Interestingly, biofilms formed in space exhibited a “column-and-canopy” structure as opposed to the flat structures observed on the ground controls. However, this was only true for the biofilms formed by motile bacteria, as non-motile strains produced flat structures similar to those seen on the Earth samples. Additionally, it was concluded that oxygen availability diminished the differences observed between microgravity and 1g samples.
A low-shear modelled microgravity (LSMMG) experiment showed that, under the simulated microgravity environment, Escherichia coli formed a thicker biofilm and was more resistant to stressors – salt, ethanol and two antibiotics [3]. Separately, we observed an enhancement of planktonic cell aggregation during the Antibiotic Effectiveness in Space (AES-1) experiment conducted on board ISS [15,16] (similar to the observations reported in Wilson et al. [17]), which occurred in parallel to differential gene expression indicating cells in space were under higher levels of stress than matched Earth controls [18]. More recently, we also reported an activation of drug-resistance mechanisms in space as a result of these microgravity-derived stresses [19].
2. Scientific aims
In order to help determine the physical mechanisms of material/microorganism interaction in biofilms in space, this NASA-funded experiment aims to characterize biofilm mass, thickness, morphology, and the associated gene expression using various spaceflight-relevant microbial species and substrata materials. Additionally, this experiment has the aim of elucidating the biomechanical and transcriptomic mechanisms involved in the formation of the “column-and-canopy” biofilm architecture observed in space. Additional samples will allow for characterization of potential changes of the parameters being studied and on the genes that confer microorganisms with resistance to oxidative stress, acidity, and antimicrobials. This project also aims to investigate the role of material surface topology on biofilm formation, as well as to test a novel lubricant-impregnated surface, which may be used in the future to replace the current materials on biofilm-prone spacecraft components.
3. Preliminary spaceflight experiment design
The independent variables that drive the spaceflight experiment design are (i) gravity, (ii) microbial strains, (iii) substrata material/to-pographies, and (iv) time. Controlled parameters include temperature, growth medium, and hardware. The dependent variables are (i) biofilm mass, (ii) biofilm thickness, (iii) biofilm morphology (structure), and (iv) gene expression. Earth controls will replicate flight samples and are planned to be performed asynchronously to replicate crew operations and temperature profiles as closely as possible.
3.1. Approach to independent variables
3.1.1. Gravity
Gravity will have two values, microgravity on ISS (with the caveat of vibrations that occur on the Station) and 1g on Earth as the control. Two microbes (one bacterial and one fungal) are being considered for this experiment – although the final selection for spaceflight requires finishing Ground Testing: P. aeruginosa PA01 and Penicillium rubens ATCC* 28089™.
3.1.2. Microbial strains
P. aeruginosa is a gram-negative bacterium commonly found in man-made environments. It is an opportunistic and nosocomial pathogen that infects the airway, urinary tract, burns, wounds; and may also cause blood infections [20,21]. P. aeruginosa has been the model organism of at least 13 different space missions [4,14,22–25] and was collected post-flight from the Apollo 13 through 17 crews [26]. The use of this bacterial species represents a continuation of the biofilm investigations conducted in STS-95, -132, and –135 [4,14]. More specifically, PA01 (ATCC® BAA-47™, HER-1018 [PA01]) was the P. aeruginosa strain chosen for consideration for this experiment because it has been fully sequenced [27] and because it has been used in a previous spaceflight study from which proteomic and transcriptomic data (space vs. Earth) has been published [22].
Infections from P. chrysogenum (now called P. rubens as explained below) are primarily encountered among immunosuppressed people [28], which is a common case for astronauts [29]. On Earth, this fungus has been responsible for cases of endophthalmitis (inflammation of the internal coats of the eye) [30], fatal necrotizing esophagitis [28], invasive pulmonary mycosis (infections in the lungs) [31], among other types of infections [32]. P. chrysogenum was determined to be partly responsible for progressive destruction of a navigation window on board Mir [6]. The genome of P. chrysogenum Wisconsin 54–1255 has been fully sequenced [33] and later, based on a molecular phylogenetic study, P. chrysogenum was reclassified as Penicillium rubens [34]. Hence, P. rubens (ATCC® 28089™, a. k.a WIS 54–1255 [Wisconsin 54-1255; Wis. 51–20; Wis. 48–70; NRRL, 1951; ATCC 9480]) was selected for consideration to be the fungal model organisms of the spaceflight experiment. However, the final selection is dependent on finishing Ground Testing to ensure compatibility and compliance with the constraints and requirements derived from space-based research.
3.1.3. Substrata materials
Eight different materials, and three of these in three different topographies, each, are being tested for potential inclusion into the finalized experiment design. These materials were pre-selected mostly based on their relevance to (i) internal and external spacecraft structures, but also to (ii) space biology research, and (iii) nosocomial infections. Coupons of 1 cm × 1 cm and 1 mm thickness were prepared from each candidate material. The eight materials being assessed are:
3.1.3.1. Cellulose membrane.
Following on the research line started by Dr. Collins et al. (reported in Ref. [14]), biofilm growth on cellulose membrane will be assessed. The same membrane that was utilized on the STS-132 and -135 experiments (hydrophilic mixed cellulose 0.22 μm pore size membrane (Cat No. GSWP01300, Millipore, Billerica, MA)) will be used. In case the “column-and-canopy” structure is a feature unique to this type of substratum topography (it hasn’t yet been proven otherwise) this material would be used as control and to help confirm if the previous spaceflight observations can be replicated.
3.1.3.2. Aluminum 6061.
This is a material used in spacecraft structures, thermal control and cryogenic fuel systems, structures for electronic devices, panels, etc., making it a ubiquitous material in spacecraft [35–39]. According to NASA’s MAPTIS material database [10024], A16061 is a non-flammable, non-toxic material.
3.1.3.3. Titanium Ti-6Al-4V.
This titanium alloy features light specific gravity, high strength, and is ideal for high-temperature applications, making it a common selection for spacecraft structures, antennae, pressure vessels, brackets, fittings, propulsion tubing lines, and support tubes [40–42]. This titanium alloy is also used for implants, including cardiovascular, orthopedic, dental, craniofacial, and otorhinological [43–45].
3.1.3.4. Polycarbonate.
This material’s properties in terms of durability, safety, versatility and shatter resistance, make it a common go-to material for spaceflight instrumentation. This material is also commonly used for space biology research. Its use in the medical field include in renal dialysis, cardiac surgery products, and surgical instruments [46]. NASA MAPTIS categorizes it as toxicity “A”, permitting to have up to 501b in a single article [68558].
3.1.3.5. Quartz.
In addition to its optical properties, quartz is used for spacecraft windows due to its capability to withstand temperature extremes (including re-entry to Earth) while working as part of the pressure shell [47,48]. However, bacterial and fungal contamination (including P. chrysogenum) of quartz windows have been reported, as in the case of microbial growth-driven progressive destruction of a navigational window on board the Mir space station [6]. Quartz is also used in solar panels, semiconductors, lighting, coatings, adhesives and for other applications [49].
3.1.3.6. Silicone.
Due to its biocompatibility and biodurability, silicone is a material commonly used in the healthcare industry, e.g. on urological catheters, surgical incision drains, and respiratory devices [50]. Silicone is also commonly used for life sciences research devices, in electronics, and for a myriad of mechanical components (e.g. O-rings). NASA’s MAPTIS material database [06256] categorizes silicone as an “I” rating in terms of flammability and “A” in toxicity, indicating it is a benign material to work with.
3.1.3.7. Stainless steel 316.
Stainless steel 316 (SS316) is used on rocket engine components, environmental control and life support system tanks and tubing (including for potable water), and Extravehicular Mobility Unit (EMU) elements [51–53]. It is also used as a material for surgical equipment, and implants [54,55]. According to NASA’s MAPTIS material database [55747], SS316 is a non-flammable, nontoxic material.
3.1.3.8. Carbon fiber.
Carbon fiber’s mechanical (e.g. high tensile strength and low weight) and thermal (e.g. high temperature tolerance and low thermal expansion) properties make it a material commonly used for aeroshells and other applications in spacecraft [56–59]. The applied carbon fiber sheets are woven with epichlorohydrin and epoxy resin.
3.1.3.9. Lubricant-impregnated surf aces (LIS).
Given that adhesion is the first process preceding formation of a mature biofilm, adhesion prevention is a logical strategy in designing anti-fouling materials. Some strategies to create anti-adhesion surfaces include use of very smooth materials [60], modification of chemical properties, and modulation of surface hydrophobicity [61]. Hydrophobicity in particular has been explored for anti-biofouling properties; however, the influence is non-linear in that hydrophobic surfaces may cause greater initial attachment while also allowing for larger detachment rates [60]. Design of anti-adhesive surfaces is further complicated by microbial adaptations, as microbes modify gene expression and biofilm geometry to adapt to less habitable surfaces [61].
Lubricant-impregnated surfaces (LIS) are textured surfaces impregnated with a lubricating fluid that can impart remarkable mobility to both Newtonian and non-Newtonian fluids [62] (Fig. 3). The lubricating fluid is immiscible with the product it is in contact with and can be designed to be held stably within the solid textures via capillary and intermolecular forces [63,64]. A thermodynamic framework incorporating the properties of the product, lubricant, solid, and surrounding environment allows one to design stable LIS that can maximize product mobility [63,64]. For example, the lubricant-solid contact angle should be below a critical angle ΘC = cos−1(1 – ϕ)/(r – ϕ) for the lubricant to be stably impregnated within the textures and not be displaced by the contacting medium (φ is the solid fraction and r is roughness). It is possible to select lubricants that are biocompatible or bio-toxic, depending on the application.
Fig. 3.
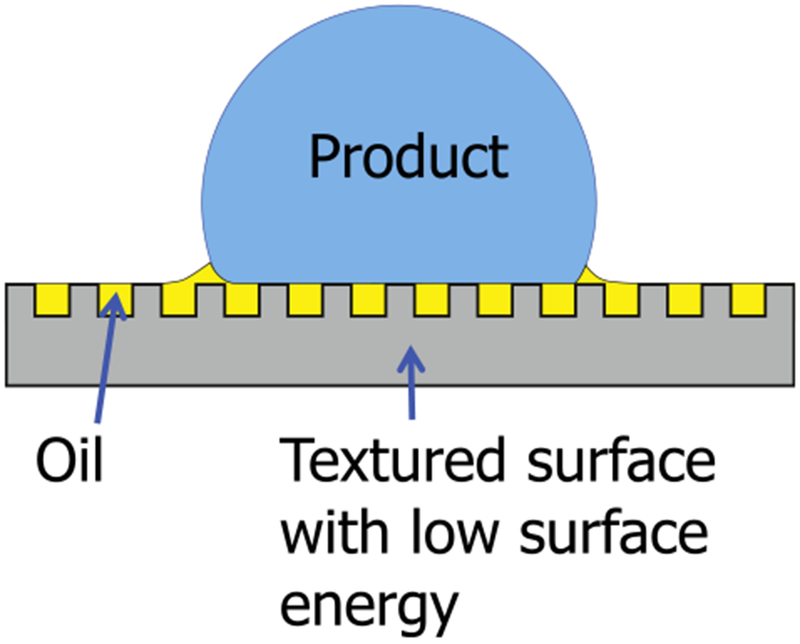
Cartoon of water drop on LIS. The lubricating layer is held stable by the nanotexture and creates a boundary between the water and the solid surface.
LIS eliminates adhesion without relying on altered chemical/physical properties, and have been previously demonstrated for consumer products such as food and cosmetics [65], anti-icing [66] and anti-fouling applications [66,67]. Therefore, LIS is a promising candidate for anti-biofouling surfaces that microbial colonies are incapable of adapting to.
LIS are fabricated by (1) creating nano/micro scale texture, (2) imparting appropriate surface chemistry and (3) impregnation of the lubricant. Here, a random nanotexture with very low solid fraction (termed nanograss) was fabricated using reactive ion etching (Surface Technology Systems) on a typical silicone wafer. This nanotextured wafer was then diced into 10 × 10 mm coupons using a laser cutter. Each coupon was cleaned using detergent, DI water, acetone, and IPA. Any remaining adsorbed organic species were then removed via plasma cleaning, and the surfaces were rendered hydrophobic using chemical vapor deposition of a flourosilane coating. Finally, silicone oil (viscosity of 100 cSt) was impregnated into the coupons using a dipcoater (KSV Nima) to form stable films and avoid excess lubricant [63,68]. Both silicone oil lubricant and silicone substrate are non-toxic and non-flammable.
3.1.4. Substrata topographies
The adhesion of bacteria to surfaces and therefore the initial biofilm formation is strongly governed by topographical surface features of about the bacterial scale – bacteria respond to surface topography and mechanics during the attachment phase by altering their signaling pathways between and within cells [69–71]. Thus, surface patterns at different scales (equal, above or below the size of bacteria) will be produced via Direct Laser-Interference Patterning (DLIP). The DLIP technique allows for surface patterning with lateral periodicities of about 500 nm to 50 μm [72]. The range of bacterial size (~ 1–2 μm) is thus covered by this technique, enabling the induction of unique interactions between surface topography and bacteria or fungi.
For the DLIP, a pulsed high energy laser beam is split up in several sub-beams, which are then recombined on the materials surface, thereby creating precise intensity patterns by interference effects. Once a material is irradiated, photo-thermal interactions translate these intensity patterns into topographical surface structures. The lateral periodicity of a pattern is governed by the laser wavelength and the angle between incident laser beams, the pattern type (e.g. line- or dot-like) is determined by the number of beams. Any kind of periodic surface pattern can be generated by using an Inverse Fourier Transformation to translate the 2D-pattern into a specific 3D geometrical setup of laser beams [72]. Nanotopographies on polycarbonate, woven carbon fiber sheet and silicone coupons will be tested during Ground Tests for potential inclusion into the spaceflight experiment.
3.1.5. Time
The concept of operations includes terminating the experiment by fixing the samples, either in paraformaldehyde (ACROS, Cat. No. 41678, New Jersey, USA) for morphological assessments, or in RNAProtect Bacteria (Qiagen, Cat. No. 76506, MD, USA) for gene expression analyses. The time at which samples will be fixed, as well as the appropriate fixative concentrations (fixative/sample ratios) to enable appropriate post-flight analyses, will be determined via Ground Testing.
3.2. Controlled parameters
Temperature and growth media will be controlled parameters. Ground Testing will determine culturing temperature but the preliminary experiment design is based on 37 °C and 25 °C for bacterial and fungal growth, respectively.
The growth media being considered for culturing P. aeruginosa include (i) sterile reagent grade water to simulate untreated water, (ii) 3% Tryptic soy broth (TSB) (as recommended by ATCC), (iii) 10% Tryptic soy broth (TSB) to simulate wastewater, (iv) 8.8 mgL−1 (weak) iodine solution to simulate disinfected potable water, (v) modified artificial urine media (mAUM) to simulate human urine as described in Ref. [14], (vi) LB broth, and (vii) LB broth supplemented with 5 g/L glucose.
The growth media being considered for culturing P. rubens include the same first four options described for P. aeruginosa, plus (v) potato dextrose broth (PDB), and (vi) PDB supplemented with 5 g/L glucose.
The experiment is planned to be performed in BioServe Space Technologies’ 12-Well BioCell, which is a culture system designed for space-based research. It is well-plate-sized and has customizable membranes (to change gas permeation rates), enabling for either aerobic or anaerobic growth.
3.3. Approach to dependent variables
Spaceflight and matched ground control samples will be fixed at experiment end. Half of the samples are planned to be fixed in paraformaldehyde (used for post-flight biofilm mass, thickness, and morphology analyses) and the other identical half will be fixed in RNAProtect Bacteria (for gene expression analyses).
3.3.1. Biofilm mass, thickness, and morphology (structure)
These three parameters are planned to be assessed post-flight via confocal laser scanning microscopy, calculating biomass, biofilm thickness, and structure from the three-dimensional images generated via software (e.g. Zeiss’ Zen, Vaa 3D, BitPlane Imaris, NIH Image J, ImageAnalysis Comstat, or 3i Slidebook Reader). Samples fixed in space with PFA are planned to be stained with 1 μg/mL propidium iodide and 1% Triton X-100 in PBS as described in Ref. [14], although other protocols will be assessed (e.g. DAPI stain). Scanning electron microscopy imaging will also be considered for detailed cellular surface morphological analysis.
3.3.2. Gene expression
Gene expression data acquisition is planned to be done via RNA Sequencing and differential gene expression will be performed per [18].
4. Preliminary microscopy analyses
Fluorescent microscopy images are currently being used to provide preliminary insight into the material-media combinations that best enable biofilm formation, and potential differences on the biofilms’ structures. Fig. 4 shows P. aeruginosa attached growth on quartz, after culturing in 3% TSB, at 37°C for 96 h. Samples were fixed in 4% final PFA and stained with FilmTracer™ SYPRO™ Ruby Biofilm Matrix Stain (ThermoFisher, Cat. No. F10318) for 30 miuntes. The stain was then washed off with dH2O and stained with 10 μg/mL DAPI Stain (ThermoFisher, Cat. No. 62248) for 10 min (seen in blue in Fig. 4). The presence of cells in focus and out of focus indicate the three-dimensionality of the biofilms structure.
Fig. 4.
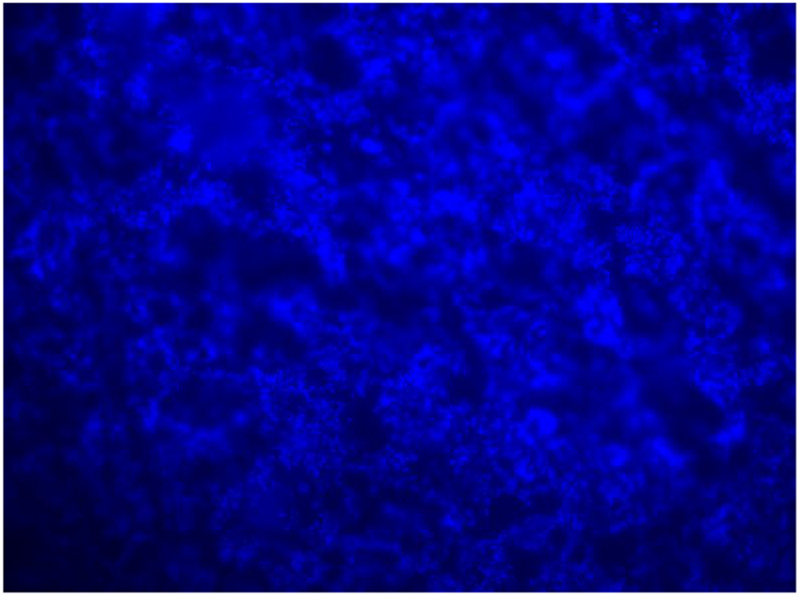
P. aeruginosa biofilm formed on quartz.
P. rubens was cultured in PDB for 96 h at room temperature with different material coupons. In this case, the coupons were fixed in 4% final PFA concentration and stained with Calcofluor white (CFW) and Acridine Orange (AO). CFW stains chitin (a cell wall component) blue, revealing the hyphae structure of the biofilm. AO stains double-stranded DNA green, exposing part of the overall extracellular matrix of the biofilm, which contains extracellular DNA as well as the DNA within the cells. Each coupon was stained with 10 μL of 0.1% CFW plus 10 μL of 1 mg 1−1 AO, waiting 15 min in the dark [73]. Visualization of all samples (P. aeruginosa and P. rubens) was done using the Nikon E600 Upright Wide field Microscope. Samples of P. rubens were cultured in BioServe’s Clinostat rotated at 6 RPM; Fig. 5 shows biofilm formed on aluminum in clinorotated samples. This separate investigation will be reported in detail separately.
Fig. 5.
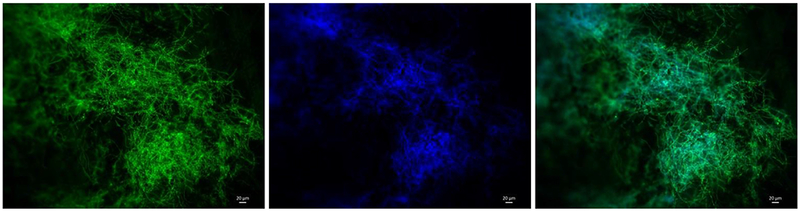
Penicillium rubens biofilm attached to an aluminum coupon after 96h incubation in a BioCell well, at room temperature under simulated microgravity. Left: AO reveals (in green) double-stranded DNA either within the cells or as part of the biofilm matrix as extracellular DNA. Center: CFW reveals (in blue) chitin, present in hyphae and spores cell wall, as well as in hyphal tip growth. Right: two-channel image revealing the biofilm structures. Overlapping regions appear “turquoise-blue”. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
5. Considerations for experiment design maturation
Space-based research generates constraints that normally do not exist in labs on Earth and that need to be considered in order to have a successful spaceflight experiment; a non-comprehensive list of these considerations is listed here. The amount of samples and kits that compose an experiment are limited by multiple factors, one being how much mass and volume can be sent to and returned from space. This usually translates into minimizing the number of samples that constitute the experimental matrix. Another consideration is temperature control for up- and down-mass, as well as in-space stowage. While manifesting items to be sent to space is difficult, it can be even more so to request them to be launched at a temperature that is not spacecraft-ambient. This tends to translate into performing Ground Testing, during the experiment design phase, to determine which items must be temperature controlled and which can afford not to be. A third consideration is crew time, as it is a finite resource. To address this, the experiment protocol can be designed in a fashion that minimizes crew interactions and required durations. Something else that needs to be taken into account is the time between experiment hand-over for integration into the launch vehicle – prior to lift-off – and experiment initiation, on orbit. Multiple events can occur between these two milestones. For example, for the AES-1 experiment, launch was delayed due to (i) cold temperatures at the spaceport, (ii) high space radiation on the spacecraft’s path to ISS due to a solar flare, and (iii) an ammonia pump needing unplanned maintenance on ISS [15]. Hence, the experiment must be designed in a way that allows flexibility and tolerance within the experiment performance times. This is also true for the post-experiment phase of the mission, as sample return may also be delayed for myriad reasons. Other considerations that are not discussed here are compliance with requirements driven by the launch/return vehicle as well as the spacecraft hosting the experiment (in this case, ISS), may they be related to safety, interfaces, compatibility, or any other type. While spaceflight-based research is now more accessible than ever, it is still far from being a routine operation.
6. Next steps towards a spaceflight-ready experiment design
The planned biofilm experiment described here will undergo a series of Ground Tests to inform the decisions that will enable its maturation into being spaceflight-ready. These tests include biocompatibility with the spaceflight hardware, determining the appropriate film (gas exchange rate) for microbial growth, cultivation temperature, method for adhering the material coupons onto the cell culture system without introducing a confounding factor, and characterizing the maximum durations under which experiment items (e.g. growth media, inocula, fixatives) can be stored and still be viable. Scientific and technical questions that need to be addressed include: Can the samples be launched already inoculated or do the inoculum and growth medium need to be launched separately? How long should the experiment run before fixing to enable the adequate formation of biofilms on the different material substrata being studied? What materials and nanotopographies should be included in the spaceflight experiment? For how long can the samples remain fixed and still allow for appropriate post-flight analyses?
Acknowledgments
This material is based upon work supported by the National Aeronautics and Space Administration under Grant No. 80NSSC17K0036. The work performed in the Moeller laboratory is supported by a grant from the German Aerospace Center (DLR grant DLR-FuW-Projekt ISS LIFE, Programm RF-FuW, Teilprogramm 475). SAM gratefully acknowledges that this work was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No.1122374. The authors would like to acknowledge Chris Brown (ISS Water Operations Lead for the Flight Operations Directorate) and Layne Carter (ISS Water Subsystem Manager) for providing the images of biofilm formation in ISS components (Figs. 1 and 2) and their expert advice. LZ, ZN, PR, and MC thank Dr. James Orth for his assistance with fluorescence microscopy.
Footnotes
This works was originally presented at the 68th International Astronautical Congress, Adelaide, Australia in 2017 IAC-17.A1.6.8×36309.
Declarations of interest
None.
References
- [1].Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I, Biofilm material properties as related to shear-induced deformation and detachment phenomena, J. Ind. Microbiol. Biotechnol 29 (6) (2002) 361–367. [DOI] [PubMed] [Google Scholar]
- [2].Pyle BH, McFeters GA, Broadaway SC, Johnsrud CK, Storga RT, Borkowski J, Bacterial Growth on surfaces and in suspensions, Biorack on Spacehab. Biological Experiments on Shuttle to Mir Missions 03, 05, and 06, European Space Agency, 1999SP–1222. [Google Scholar]
- [3].Lynch SV, Mukundakrishnan K, Benoit MR, Ayyaswamy PS, Matin A, Escherichia coli biofilms formed under low-shear modeled microgravity in a ground-based system, Appl. Environ. Microbiol 72 (2006) 7701–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McLean RJC, Cassanto JM, Barnes MB, Koo JH, Bacterial biofilm formation under microgravity conditions, FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett 195 (2) (2001) 115–119. [DOI] [PubMed] [Google Scholar]
- [5].Hall-Stoodley L, Costerton JW, Stoodley P, Bacterial biofilms: from the natural environment to infectious diseases, Nat. Rev. Microbiol 2 (2004) 95–108. [DOI] [PubMed] [Google Scholar]
- [6].Klintworth R, Reher HJ, Viktorov AN, Bohle D, Biological induced corrosion of materials II: new test methods and experiences from MIR station, Acta Astronautica 44 (7) (1999) 569–578. [DOI] [PubMed] [Google Scholar]
- [7].Novikova ND, Review of the knowledge of microbial contamination of the Russian manned spacecraft, Microb. Ecol 47 (2) (2004) 127–132. [DOI] [PubMed] [Google Scholar]
- [8].Novikova N, De Boever P, Poddubko S, Deshevaya E, Polikarpov N, Rakova N, … Mergeay M, Survey of environmental biocontamination on board the international space station, Res. Microbiol 157 (1) (2006) 5–12. [DOI] [PubMed] [Google Scholar]
- [9].NASA, International Space Station Medical Operations Requirements Document (ISS MORD), (2003) SSP 50260 Rev B May 2003. [Google Scholar]
- [10].Checinska A, Probst A, Vaishampayan P, White J, … Venkateswaran K, Microbiomes of the dust particles collected from the international space station and spacecraft assembly facilities, Microbiome 3 (2015) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mora M, Mahnert A, Koskinen K, Pausan MR, Oberauner-Wappis L, Krause R, … Moissl-Eichinger C, Microorganisms in confined habitats: microbial monitoring and control of intensive care units, operating rooms, cleanrooms and the International Space Station, Front. Microbiol 7 (2016) 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Venkateswaran K, Vaishampayan P, Cisneros J, Pierson DL, Rogers SO, Perry J, International Space Station environmental microbiome—microbial inventories of ISS filter debris, Appl. Microbiol. Biotechnol 98 (14) (2014) 6453–6466. [DOI] [PubMed] [Google Scholar]
- [13].Be NA, Avila-Herrera A, Allen JE, Singh N, Sielaff AC, Jaing C, Venkateswaran K, Whole metagenome profiles of particulates collected from the International Space Station, Microbiome 5 (1) (2017) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim W, Tengra FK, Young Z, Shong J, Marchand N, et al. , Spaceflight promotes biofilm formation by Pseudomonas aeruginosa, PLoS ONE 8 (4) (2013), 10.1371/journal.pone.0062437 e62437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zea L, Phenotypic and Gene Expression Responses of E. coli to Antibiotics during Spaceflight, Ph.D. Thesis University of Colorado, Boulder, 2015. [Google Scholar]
- [16].Zea L, Larsen M, Estante F, Qvortrup K, Moeller R, Dias de Oliveira S, … Klaus D, Phenotypic changes exhibited by E. coli cultured in space, Front. Microbiol 8 (2017) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilson JW, Ott CM, Honer zu Bentrup K, Ramamurthy R, Quick L, et al. , Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 16299–16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zea L, Prasad N, Levy SE, Stodieck L, Jones A, Shrestha S, Stodieck L, Klaus D, A molecular genetic basis explaining altered bacterial behavior in space, PLoS One 11 (11) (2016) e0164359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aunins TR, Erickson KE, Prasad N, Levy SE, Jones A, Shrestha S, … Zea L, Spaceflight modifies Escherichia coli gene expression in response to antibiotic exposure and reveals role of oxidative stress response, Front. Microbiol 9 (2018) 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lyczak JB, Cannon CL, Pier GB, Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist, Microb. Infect 2 (9) (2000) 1051–1060. [DOI] [PubMed] [Google Scholar]
- [21].Lodise TP, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, … McGregor JC, Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection, AAC (Antimicrob. Agents Chemother.) 51 (10) (2007) 3510–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crabbé A, Schurr MJ, Monsieurs P, Morici L, Schurr J, Wilson JW, … Nickerson CA, Transcriptional and proteomic responses of Pseudomonas aeruginosa PAO1 to spaceflight conditions involve Hfq regulation and reveal a role for oxygen, Appl. Environ. Microbiol 77 (4) (2011) 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Juergensmeyer MA, Juergensmeyer EA, Guikema JA, Long-term exposure to spaceflight conditions affects bacterial response to antibiotics, Microgravity Science and Technology 12 (1) (1999) 41–47. [PubMed] [Google Scholar]
- [24].Zaloguyev SN, et al. , Structural-functional changes in bacterial cells under spaceflight conditions, Dokl. Akad. Nauk SSSR 278 (5) (1984) 1236–1237 (English translation: USSR Report: Space, May 6, 1985, p.36 (JPRS-USP-85–004)). [PubMed] [Google Scholar]
- [25].Zea L, Stodieck L and Klaus D, The first fifty years of bacterial growth and antibiotic effectiveness research in space, ASGSR Conference, Pasadena, CA, October 22–26, 2014. [Google Scholar]
- [26].Taylor GR, Recovery of medically important microorganisms from Apollo astro-nauts, Aero. Med 45 (1974) 824–828. [PubMed] [Google Scholar]
- [27].Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen, Nature 406 (6799) (2000) 959. [DOI] [PubMed] [Google Scholar]
- [28].Hoffman M, Bash E, Berger SA, Burke M, Yust I, Fatal necrotizing esophagitis due to Penicillium chrysogenum in a patient with acquired immunodeficiency syndrome, Eur. J. Clin. Microbiol. Infect. Dis 11 (12) (1992) 1158–1160. [DOI] [PubMed] [Google Scholar]
- [29].Mermel LA, Infection prevention and control during prolonged human space travel, Clin. Infect. Dis 56 (1) (2013) 123–130, 10.1093/cid/cis861. [DOI] [PubMed] [Google Scholar]
- [30].Eschete ML, King JW, West BC, Oberle A, Penicillium chrysogenum endophthalmitis, Mycopathologia 74 (2) (1981) 125–127. [DOI] [PubMed] [Google Scholar]
- [31].Geltner C, Lass-Flörl C, Bonatti H, Müller L, Stelzmüller I, Invasive pulmonary mycosis due to penicillium chrysogenum: a new invasive pathogen, Transplantation 95 (4) (2013) e21–e23. [DOI] [PubMed] [Google Scholar]
- [32].Lyratzopoulos G, Ellis M, Nerringer R, Denning DW, Invasive infection due to Penicillium species other than P. marneffei, J. Infect 45 (3) (2002) 184–195. [DOI] [PubMed] [Google Scholar]
- [33].van den Berg MA, Albang R, Albermann K, Badger JH, Daran J-M, Driessen AJ, Garcia-Estrada C, Fedorova ND, Harris DM, Heijne WHM, Joarder V, Kiel JAKW, Kovalchuk A, Martín JF, Nierman WC, Nijland JG, Pronk JT, Roubos JA, van der Klei IJ, van Peij NNME, Veenhuis M, von Döhren H, Wagner C, Wortman J, Bovenberg RAL, Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum, Nat. Biotechnol 26 (2008) 1161–1168. [DOI] [PubMed] [Google Scholar]
- [34].Houbraken J, Frisvad JC, Samson RA, Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens, IMA fungus 2 (1) (2011) 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leidinger BJG, Kleinert-Alvarado G, Mueller R, Design and Analysis of a Spacecraft Water Evaporator (No. 901307). SAE Technical Paper, (1990). [Google Scholar]
- [36].Schuerger AC, Trigwell S, Calle CI, Use of non-thermal atmospheric plasmas to reduce the viability of Bacillus subtilis on spacecraft surfaces, Int. J. Astrobiol 7 (01) (2008) 47–57. [Google Scholar]
- [37].Destefanis R, Schäfer F, Lambert M, Faraud M, Selecting enhanced space debris shields for manned spacecraft, Int. J. Impact Eng 33 (1) (2006) 219–230. [Google Scholar]
- [38].Kumbar PB, Pandit JK, Jagadish T, & Thyagaraj MR Enhancing the fatigue life of an electronic package in spacecraft. [Google Scholar]
- [39].Gretchen S, Fluid loop radiators for orion, Spacecraft Thermal Control Workshop. February 27-March 1, 2007, 2007. [Google Scholar]
- [40].Özdemir N, Bilgin B, Interfacial properties of diffusion bonded Ti-6Al-4V to AISI 304 stainless steel by inserting a Cu interlayer, Int. J. Adv. Manuf. Technol 41 (5–6) (2009) 519–526. [Google Scholar]
- [41].Miyoshi K, Sanders JH, Hager CH, Zabinski JS, Vander Wal RL, Andrews R, … Abel PB, Wear behavior of low-cost, lightweight TiC/Ti–6Al–4V composite under fretting: effectiveness of solid-film lubricant counterparts, Tribol. Int 41 (1) (2008) 24–33. [Google Scholar]
- [42].Rawal S, Brantley J, Karabudak N, Additive manufacturing of Ti-6Al-4V alloy components for spacecraft applications, Recent Advances in Space Technologies (RAST), 2013 6th International Conference on (pp. 5–11). IEEE, 2013, June. [Google Scholar]
- [43].Cook SD, Thomas KA, Kay JF, Jarcho M, Hydroxyapatite-coated titanium for orthopedic implant applications, Clin. Orthop. Relat. Res 232 (1988) 225–243. [PubMed] [Google Scholar]
- [44].Hermawan H, Ramdan D, Djuansjah JR, Metals for Biomedical Applications, INTECH Open Access Publisher, 2011, pp. 411–430. [Google Scholar]
- [45].Niinomi M, Recent metallic materials for biomedical applications, Metall. Mater. Trans 33 (3) (2002) 477–486. [Google Scholar]
- [46].Powell DG, Medical applications of polycarbonate, MEDICAL PLASTIC AND BIOMATERIALS 5 (1998) 38–45. [Google Scholar]
- [47].Dino J, NASA Ames – Shuttle Glass FAQ, (2008) Retrieved from: http://www.nasa.gov/centers/ames/research/2007/faq-shuttleglass.html. [Google Scholar]
- [48].Wei Q, Liu H, He S, Yang D, Kinetics of radiation damage of quartz glass by lowenergy protons, J. Spacecraft Rockets 43 (3) (2006) 514–517. [Google Scholar]
- [49].The Quartz Corporation, Quartz Applications, (2014) Retrieved from: http://www.thequartzcorp.com/en/applications.html.
- [50].DOW, Medical & Healthcare Silicone Solutions, (2015) Retrieved from: http://www.dowcorning.com/content/discover/discovershowcase/healthcare.aspx.
- [51].Sutton GP, Biblarz O, Rocket Propulsion Elements, John Wiley & Sons, 2010. [Google Scholar]
- [52].Peterson L, Environmental Control and Life Support System (ECLSS) System Engineering Workshop. ISU SSP2009, Ames Research Center, (2009) Retrieved from: http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20090029327.pdf. [Google Scholar]
- [53].Campbell C, Advanced EMU portable life support system (PLSS) and shuttle/ISS EMU schematics, a comparison, 42nd International Conference on Environmental Systems, 2011, July, p. 3411. [Google Scholar]
- [54].Oh J, Hur B, U.S. Patent No. 6,498,421, U.S. Patent and Trademark Office, Washington, DC, 2002.
- [55].Bhatjiwale MG, Goel A, Muzumdar DP, A multiposition brain holder: a versatile appliance for microneurosurgical laboratory, J. Postgrad. Med 47 (1) (2001) 82. [PubMed] [Google Scholar]
- [56].Bacon R, Moses CT, Carbon fibers, from light bulbs to outer space, High Performance Polymers: Their Origin and Development, Springer; Netherlands, 1986, pp. 341–353. [Google Scholar]
- [57].Kirsch MT, Composite Crew Module: Primary Structure. NASA Technical Report NASA/TM-2011–217185 , (2011). [Google Scholar]
- [58].Chung D, Carbon Fiber Composites. Butterworth-heinemann, Butterworth-Heinemann, MA, USA, 1994. [Google Scholar]
- [59].Hashin Z, Thermoelastic properties and conductivity of carbon/carbon fiber composites, Mech. Mater 8 (4) (1990) 293–308. [Google Scholar]
- [60].Puckett SD, Taylor E, Raimondo T, Webster TJ, The relationship between the nanostructure of titanium surfaces and bacterial attachment, Biomaterials 31 (4) (2010) 706–713. [DOI] [PubMed] [Google Scholar]
- [61].Lorite Gabriela S., Janissen Richard, Clerici H, Joao, Rodrigues Carolina M., Tomaz Juarez P., Mizaikoff Boris, Kranz Christine, de Souza Alessandra A, Cotta Monica A., Surface physicochemical properties at the micro and nano length scales: role on bacterial adhesion and Xylella fastidiosa biofilm development, PLoS One 8 (9) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Solomon BR, Subramanyam SB, Farnham TA, Khalil KS, Anand S, Varanasi KK, Non-wettable Surfaces: Theory, Preparation, and Applications. Chapter 10: Lubricant-impregnated Surfaces, Royal Society of Chemistry, 2017. [Google Scholar]
- [63].Smith JD, Dhiman R, Varanasi KK, Cabello ER, 2011, Liquid-impregnated Surfaces, Methods of Making, and Devices Incorporating the Same. US Patent 8574704. [Google Scholar]
- [64].J David Smith Rajeev Dhiman, Anand Sushant, Ernesto Reza-Garduno Robert E. Cohen, Gareth H McKinley, Kripa K Varanasi, Droplet mobility on lubricant-impregnated surfaces, Soft Matter 9 (6) (2013) 1772–1780. [Google Scholar]
- [65].Smith JD, Dhiman R, Paxson AT, Love Christopher., Solomon BR., Varanasi KK, 2011, Self-lubricating Surfaces for Food Packaging and Food Processing Equipment. US Patent 8535779. [Google Scholar]
- [66].Subramanyam SB, Azimi G, Varanasi KK, Designing lubricant-impregnated textured surfaces to resist scale formation, Advanced Materials Interfaces 1 (2014) 1300068–1300074. [Google Scholar]
- [67].Epstein AK, Wong T-S, Belisle RA, Boggs EM, Aizenberg J, Liquid-infused structured surfaces with exceptional anti-biofouling performance, National Academy of Sciences U.S.A 109 (33) (2012) 13182–13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Seiwert J, Clanet C, Quere D, Coating of a textured solid, J. Fluid Mech 669 (2011) 55–63. [Google Scholar]
- [69].Edwards K, Rutenberg A, Microbial response to surface microtopography: the role of metabolism in localized mineral dissolution, Chem. Geol 180 (2001) 19–32. [Google Scholar]
- [70].Epstein AK, Hochbaum AI, Kim P, Aizenberg J, Control of bacterial biofilm growth on surfaces by nanostructural mechanics and geometry, Nanotechnology 22 (49) (2011) 494007. [DOI] [PubMed] [Google Scholar]
- [71].Epstein AK, Hong D, Kim P, Aizenberg J, Biofilm attachment reduction on bioinspired, dynamic, micro-wrinkling surfaces, N. J. Phys 15 (9) (2013) 095018. [Google Scholar]
- [72].Mücklich F, Lasagni A, Daniel C, Laser interference metallurgy: using interference as a tool for micro/nano structuring, Int. J. Mater. Res 97 (2006) 1–8 (awarded with Werner-Köster-Prize 2007). [Google Scholar]
- [73].Simoes LC, Simoes M, Lima N, Kinetics of biofilm formation by drinking water isolated Penicillium expansum, Biofouling 31 (2015) 349–362. [DOI] [PubMed] [Google Scholar]


