Abstract
The retinae of many bird species contain a depression with high photoreceptor density known as the fovea. Many species of raptors have two foveae, a deep central fovea and a shallower temporal fovea. Birds have six types of photoreceptors: rods, active in dim light, double cones that are thought to mediate achromatic discrimination, and four types of single cones mediating color vision. To maximize visual acuity, the fovea should only contain photoreceptors contributing to high-resolution vision. Interestingly, it has been suggested that raptors might lack double cones in the fovea. We used transmission electron microscopy and immunohistochemistry to evaluate this claim in five raptor species: the common buzzard (Buteo buteo), the honey buzzard (Pernis apivorus), the Eurasian sparrowhawk (Accipiter nisus), the red kite (Milvus milvus) and the peregrine falcon (Falco peregrinus). We found that all species, except the Eurasian sparrowhawk, lack double cones in the center of the central fovea. The size of the double cone-free zone differed between species. Only the common buzzard had a double cone-free zone in the temporal fovea. In three species, we examined opsin expression in the central fovea and found evidence that rod opsin positive cells were absent and violet-sensitive cone and green-sensitive cone opsin positive cells were present. We conclude that not only double cones, but also single cones may contribute to high-resolution vision in birds, and that raptors may in fact possess high-resolution tetrachromatic vision in the central fovea.
Keywords: birds of prey, retina, double cones, rods, visual ecology, RRID: AB_2156055, RRID: AB_2315274, RRID: AB_2158332, RRID: AB_2534069, RRID: AB_2534102
Introduction
Visual spatial resolution (or visual acuity) defines the detail that can be resolved in a visual scene. As in a camera, the spatial resolution of an eye is determined by the anterior focal length (hereafter, focal length) of the eye’s optical system and the density of the light sampling units in the neural retina (Land and Nilsson, 2012). The larger the eye, the longer the focal length and the larger the image projected onto the retina. The denser the receptor sampling array, the more spatial detail can be extracted from the retinal image (Miller, 1979). Thus, in order to achieve high spatial resolution, an animal has to have a large eye with a dense photoreceptor array in the retina (Meyer, 1977).
Among all animals, Accipitriform and Falconiform raptors have the most acute vision that has ever been measured (Fischer, 1969; Reymond, 1985). Behavioral studies show that large raptors such as Old World vultures (Fischer, 1969) and wedge-tailed eagles (Aquila audax) (Reymond, 1985) have twice the spatial resolution of humans (Land and Nilsson, 2012). These findings raise intriguing questions about the basis of enhanced spatial resolution in raptorial birds.
Despite having body sizes much smaller than that of humans, some birds of prey have eyes of equal or larger size (Martin, 1983; Reymond, 1985, 1987). These large eyes allow for a long focal length that results in a correspondingly large retinal image (Land and Nilsson, 2012). In addition, many birds have central or temporal regions in the retina with increased photoreceptor density (Meyer, 1977). These regions are referred to as “areae” and may or may not contain a fovea. The fovea is a region of the retina where photoreceptor densities are highest and other retinal layers are fully or partially displaced, resulting in a depression, which constitutes the ‘fovea’ proper. Unlike human eyes, which have only one shallow central fovea, the eyes of many raptor species (as well as swallows, martins, terns, kingfishers and some other birds; Rochon-Duvigneaud, 1943; Moroney and Pettigrew, 1987) have two foveae: a deep central fovea that views the lateral visual field, and a shallower temporal fovea that views the frontal visual field (e.g. Oehme, 1964; Reymond, 1985, 1987).
To maximize visual acuity, the fovea should only contain photoreceptors contributing to highresolution vision. Rods cannot operate in the bright-light conditions required for optimal foveal function (Snyder and Miller, 1977) and they are accordingly absent from the foveae of some species. For example, the primate fovea, including that of humans, is rod-free (e.g. Packer et al., 1989; Finlay et al., 2008) and the central areae or foveae of several bird species have been found to be rod-free (Bruhn and Cepko, 1996; Querubin et al., 2009; Coimbra et al., 2015). Furthermore, to achieve highest image quality the eye needs to avoid chromatic aberration, which is most severe for the short wavelength light (for more detail please see Discussion). Probably for this reason blue-sensitive cones are absent from the central-most part of some primate foveae (e.g. Wikler and Rakic, 1990; Martin and Grünert, 1999) leaving only green and red-sensitive cones for the tasks of highest acuity. These observations suggest that the demands of high-acuity vision select for a specific photoreceptor complement in the fovea.
Birds are thought to utilize different subsets of their photoreceptor complement for specific visual tasks (Hart, 2001b). As in many other vertebrate taxa, rod photoreceptors mediate dim light vision. Color vision is mediated by four types of single cone photoreceptors sensitive to different portions of the light spectrum: ultraviolet/violet-sensitive (UV/VS cones; with maximum sensitivity in the common buzzard [Buteo buteo] at 405 nm), blue-sensitive (S cones; 449 nm), green-sensitive (M cones; 504 nm) and red-sensitive (L cones; 567 nm) cones (Ödeen and Håstad, 2003; Lind et al., 2013). The double cones have broad spectral sensitivity, and their function is a matter of ongoing debate. Behavioral data suggest that double cones contribute to high-resolution achromatic vision (Osorio et al., 1999; Jones and Osorio, 2004; Martin and Osorio, 2008; Lind and Kelber, 2011). For example, in budgerigars (Melopsittacus undulatus) visual acuity for achromatic gratings was determined as 10.5 cycles/degree, while acuity for the red-green and blue-green gratings, which were isoluminant and had no detectable contrast for double cones, was 4.5 and 4.3 cycles/degree respectively (Lind and Kelber, 2011). Similar results have been found for domestic chickens (Gallus gallus domesticus; P. Olsson et al. unpublished data). It has also been suggested that double cones may contribute to color (Lind and Kelber, 2011) and motion vision (Campenhausen and Kirschfeld, 1998). While double cones typically constitute 40–55% of all photoreceptors in the mid-periphery of the bird retina (Hart, 2001a; Martin and Osorio, 2008), studies of the photoreceptor complement in the central retina are rare. Braekevelt (1993) reports that outside the foveal regions the red-tailed hawk (Buteo jamaicensis) has a rod : single cone : double cone ratio of 2:1:5 (i.e., 62.5% double cones), suggesting that functional specializations of double cones may be important for raptor vision renown for high spatial resolution. One might therefore be inclined to hypothesize that the raptor fovea should also contain a high density of this cell type.
Relatively few researchers have investigated the photoreceptors of raptor foveae in any detail and only using light microscopy. Oehme (1964) did not find any rods in foveal cross-sections of the common buzzard or the common kestrel (Falco tinnunculus). Surprisingly, he did not differentiate between single and double cones although he described both cone types in an earlier study on swifts and passerines (Oehme, 1962). Reymond (1985, 1987) carefully investigated tangential preparations of wedge-tailed eagle and brown falcon (Falco berigora) foveae and did not find any double cones. Although Reymond’s results seem to contradict the studies suggesting that double cones mediate high-resolution achromatic vision in birds (Osorio et al., 1999; Jones and Osorio, 2004; Lind and Kelber, 2011), they were rarely mentioned in later reviews of avian vision (Güntürkün, 2000; Hart, 2001b; Jones et al., 2007; Martin and Osorio, 2008), and have never been followed up.
In the present study, we used transmission electron microscopy (TEM) to investigate the claim that raptor foveae lack double cones. Furthermore, we asked whether raptor foveae, similarly to humans, lack rods and cone types sensitive to short-wavelength light. We studied four species of the order Accipitriformes, the common buzzard, the honey buzzard (Pernis apivorus), the Eurasian sparrowhawk (Accipiter nisus) and the red kite (Milvus milvus), and one species of the order Falconiformes, the peregrine falcon (Falco peregrinus).
Materials and methods
Biological samples
We used eyes from common buzzards (n=2 specimens, one adult and one juvenile), honey buzzard (n=1, juvenile), Eurasian sparrowhawks (n=2, one adult female and one juvenile), red kites (n=3, two adults and one juvenile), and peregrine falcon (n=1, juvenile) directly after the birds had been euthanized by cervical dislocation. All birds were severely injured wild specimens of unknown sex (except the adult female Eurasian sparrowhawk) cared for by a bird rescue station in southern Sweden. The birds were euthanized for reasons unrelated to this study. Juvenile birds were fully grown flying individuals still in their first set of plumage. The collection of the eyes from these specimens was approved by the Swedish Environmental Protection Agency (permit no. NV-00160–12). Some of the eyes were also used in an earlier study on ocular media transmittance (Lind et al., 2013) or in other studies currently in preparation.
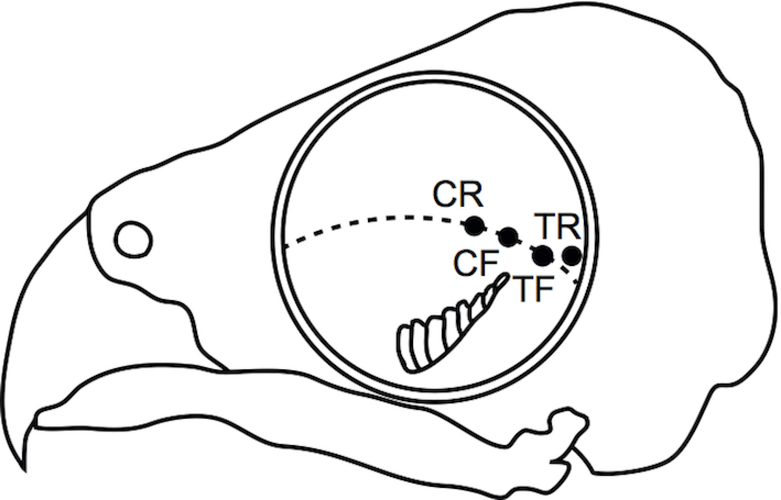
We used light microscopy (LM), transmission electron microscopy (TEM) and immunohistochemistry to study retinal samples from four defined locations: the central fovea (CF), the central retina (CR), the temporal fovea (TF) and the temporal retina (TF). Approximate locations, with respect to each other and to the pecten of the eye, are indicated in Figure 1. The central and temporal retinal pieces were taken approximately 3–6 mm from the central and temporal retinae respectively.
Fig. 1.
Schematic drawing of the eyecup in the skull with approximate positions, where the retina samples were taken: CF - central fovea, CR - central retina, TF - temporal fovea, TR - temporal retina. (Redrawn and modified from Oehme, 1964)
Light and transmission electron microscopy
One eye of common buzzard (juvenile), one eye of honey buzzard, one eye of Eurasian sparrowhawk (juvenile), three eyes of three red kites (adult and juvenile), and one eye of the peregrine falcon were used for LM and TEM studies. Only retinal samples from the central fovea were analyzed for the honey buzzard. The eyes were removed immediately after euthanasia, hemisected and placed in fixative (2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer) for 24 hours. The eyes were then transferred into fresh 0.1 M sodium cacodylate buffer and stored at 4°C until further processing. Small retinal samples (ca. 5 × 5 mm) were cut and post-fixed in 1% osmium tetroxide, dehydrated in a graded series of ethanol and acetone, and embedded in Epon. Thin retinal cross-sections (2 μm) were made with a microtome (11800 Pyramitome, LKB AB, Bromma, Sweden), stained with Azur II– Methylene Blue, coverslipped with Entellan New (Merck, Darmstadt, Germany) and visualized using a light microscope (Zeiss Axiophot equipped with a Nikon DC-Fi1c digital camera). The orientation of the samples was lost during the embedding procedure, and thus we cannot determine whether samples were sectioned along the nasotemporal or dorsoventral retinal axis. Ultra-thin retinal cross-sections (50 nm) were made with an ultra-microtome (Ultracut UCT, Leica), stained with 2% uranyl acetate and lead citrate in a LKB ultrastainer (LKB AB, Bromma, Sweden), and visualized by TEM (120 kV Jeol 1230 equipped with a Gatan Multiscan CCD camera, JEOL USA, MA).
To obtain tangential sections of the foveal region we used two different technical approaches. The first sample obtained in the study (the central fovea of the red kite) was embedded in such a way that it could be sectioned at a tangent to the photoreceptor axis and used directly for TEM studies. All other foveal samples were first sectioned parallel to the photoreceptor axis for inspection by LM until we found the deepest part of the fovea, then the samples were rotated 90 degrees and sectioned at a tangent to the receptor axis for inspection of cell crosss-ections by TEM. Because of this procedure the sections for TEM (except the central fovea of the red kite prepared with the first method) contained only half of the foveal region. We measured the extent of the double cone-free zones by scanning the samples directly in the TEM using high magnification, which allowed us to identify the cell profiles. Due to the difficulty in aligning the samples exactly parallel to the external limiting membrane and because the photoreceptor layer is not perfectly flat in the fovea (see Fig. 2), photoreceptors could not always be visualized at exactly the same level even in the same picture. The LM and TEM images were imported into Adobe Photoshop CS6, where brightness, contrast and image dimensions were adjusted.
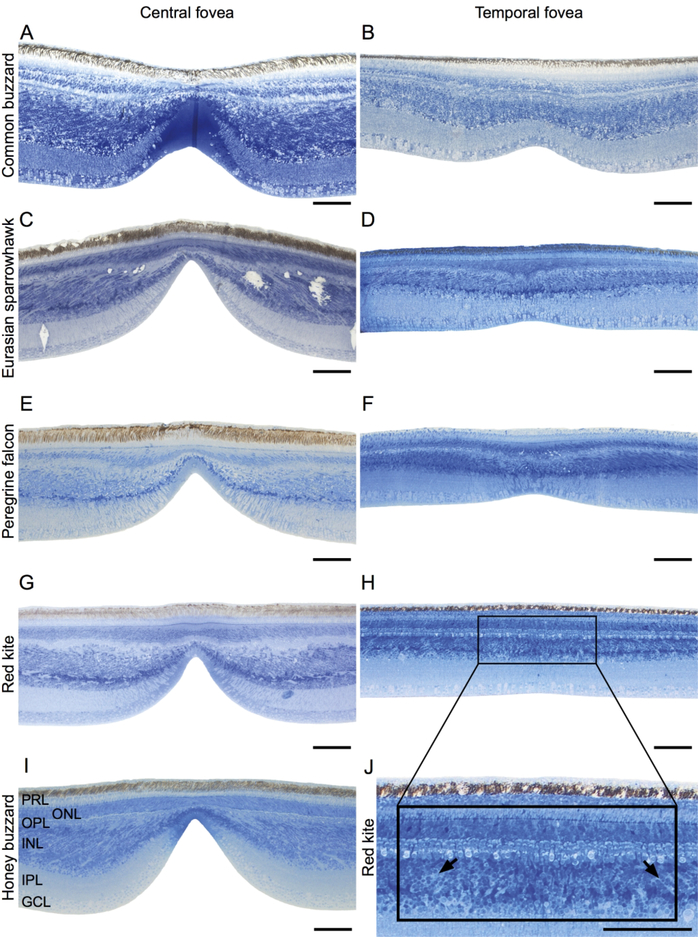
Fig. 2.
Raptor foveae. LM images of retinal cross-sections through the central (left column) and temporal (right column) foveae of the common buzzard (A, B), Eurasian sparrowhawk (C, D), peregrine falcon (E, F), red kite (G, H, J) and honey buzzard (I). Magnified image of the red kite temporal fovea (J) showing tilted retinal columns in the inner nuclear layer on the sides of the center of the fovea (indicated by arrows) that can also be found on all central and temporal foveae. There was no temporal fovea in the honey buzzard retina. The dark blue band in the center of the common buzzard central fovea (A) is a sectioning/staining artifact. PRL – photoreceptor layer; ONL – outer nuclear layer; OPL – outer plexiform layer; INL – inner nuclear layer; IPL – inner plexiform layer; GCL – ganglion cell layer. Scale bars: 100 μm.
To evaluate the relative proportion of the double cones we counted photoreceptor cross-sectional profiles in the 25 × 25 μm counting frames in the TEM or LM micrographs of the CF, CR and TF retinal regions, and in 50 × 50 μm counting frames in the LM micrographs of the TR regions. All cells inside the counting frame and those intersecting the acceptance lines, but not intersecting the rejection lines, were included in the counts (Gundersen, 1977). Because tissue shrinkage was not evaluated in this study, we did not measure photoreceptor dimensions or estimate the density.
Morphological identification of the double cones
We identified double cones in transmission electron micrographs by their typical paired inner segment cross-sectional profile, in which the principal and accessory members are directly apposed to each other, without intervening Müller cell processes (Nishimura et al., 1981; Braekevelt, 1993). In retinal regions outside the foveae, these profiles could also reliably be detected in light micrographs. We did not use other methods to discriminate double cones from the other receptor types of the bird retina, such as the size and coloration of the oil droplet (Kram et al., 2010), the larger diameter of inner segments visible in the wholemounted retina (Coimbra et al., 2015) or the position of the nuclei visible in cross-sectioned retina (Braekevelt, 1993, 1998), because all of these alternative methods turned out to be less reliable in the foveal region of the retina where photoreceptors are very narrow.
Immunohistochemical identification of photoreceptor subtypes in the fovea
Because of the limited number of samples we obtained, only one eye of the common buzzard (adult), one eye of the Eurasian sparrowhawk (adult), and one eye of the peregrine falcon (juvenile) were used for immunohistochemical studies. Only CF retinal samples were available for the Eurasian sparrowhawk and the peregrine falcon. The TF samples of these species were accidentally damaged during the following preparation procedure. The eyes were removed immediately after the euthanasia, hemisected, and placed in fixative (4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) with 3% sucrose) for one hour at room temperature. After rinsing in PBS (twice for 5 minutes), small retinal samples (ca. 4 × 4 mm) were cut, placed in 30% sucrose solution in PBS and incubated at 4°C. Once the retinal samples had equilibrated, an equal volume of cryostat freezing section medium (Neg-50™, Richard-Allan Scientific™) was added. After 2–3 hours the sucrose/freezing medium mixture was replaced with 100% freezing medium and incubated overnight at 4°C. Semi-thin retinal cross-sections (10 μm) were obtained using a cryostat (Microm HM 560, Thermo Scientific), collected on gelatin-chrome alum coated glass slides and stored at −80°C until further processing. The orientation of the samples was lost during the procedures and thus we cannot determine whether the samples were sectioned along the nasotemporal or dorsoventral retinal axis.
For immunohistochemical staining, retina sections were thawed at room temperature and washed three times in PBS. To facilitate the visualization of the photoreceptor outer segments that were hidden by the intercalated processes of the retinal pigment epithelium we bleached the sections with 10% hydrogen peroxide solution in PBS for 16 hours at 4°C following the methods of Manicam et al (2014). After rinsing and bleaching, we blocked the retinal sections in PBS with 0.5% Triton X-100, and 2% normal goat (A-11001, Life Technologies, RRID: AB_2534069; for rod opsin and green opsin primary antibodies) or 2% normal donkey serum (A-11055, Life Technologies, RRID: AB_2534102; for UV/Violet opsin antibody) for one hour at room temperature. We then applied the primary antibodies diluted in blocking buffer described above and incubated the sections for 16 hours at 4°C. We again rinsed the sections three times in PBS, applied the secondary antibodies diluted in blocking buffer, and incubated them in the dark for one hour at room temperature. Details of the antibodies and working dilutions are given below and in Table 1. Finally, the sections were rinsed three times in PBS, counterstained with 4’,6-diamidino-2-phenylindole (DAPI; D9542, Sigma Aldrich, St. Louis, MO) to label nuclei, and coverslipped with Vectashield mounting media (H-1200, Vector Laboratories, Burlingame, CA).
Table 1.
The primary and secondary antibodies used in this study
| Antibody | Immunogen | Source, host and clonality, cat #, RRID | Dilution |
|---|---|---|---|
| Primary antibody | Primary antibody dilution | ||
| Rho4D2 | Bovine (Bos taurus) rhodopsin: NGTEGPNFYVPFS NKTGVVRSPFEAP QYYLAEPWQFSM |
Abcam, mouse monoclonal, cat # ab98887, RRID:AB_2315274 | 1:500 |
| Ret-P1 | Rat (Rattus norvegicus) retina membrane fraction, epitope - rhodopsin: TEGPNFY |
Millipore, mouse monoclonal, cat # MAB5316, RRID:AB_2156055 |
1:300 |
| OPN1SW | Human (Homo sapiens) blue-sensitive opsin: EFYLFKNISSVGP WDGPQYH |
Santa Cruz, goat polyclonal, cat # SC14363, RRID:AB_2158332 | 1:300 |
| Secondary antibody | Secondary antibody dilution | ||
| Goat-antimouse IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugated | Gamma Immunoglobins Heavy and Light chains | Life Technologies, goat polyclonal, cat # A-11001, RRID:AB_2534069 | 1:1000 |
| Donkey-anti-goat IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugated | Gamma Immunoglobins Heavy and Light chains | Life Technologies, donkey polyclonal, cat # A-11055, RRID:AB_2534102 | 1:1000 |
The sections were imaged with a spinning disk confocal microscope (BX61WI; Olympus, Tokyo, Japan). The raw images from the confocal microscope were imported into Adobe Photoshop CS6, where the intensity profiles were adjusted and images from opsin staining were merged with DAPI images. For a given species the exposure times and image intensity adjustments were done the same way for all images.
Antibody characterization
To identify rod photoreceptors we used a monoclonal anti-rhodopsin antibody (Ret-P1, MAB5316, Millipore, RRID: AB_2156055), which was raised against amino acids 4–10 (TEGPNFY) at the N-terminus of rhodopsin of the membrane fraction from adult rat (Rattus norvegicus) retina (Barnstable, 1980; Silver et al., 1988; Coimbra et al., 2015). This antibody has been shown to specifically label rod photoreceptors in other vertebrates including fish, salamander, pigeon and mice (Barnstable, 1980; Querubin et al., 2009; Taylor et al., 2011). In several passerine species it has been shown to strongly label rods, but it also appears to have some cross-reactivity with Rh2 opsin and therefore weakly label green-sensitive cones (Coimbra et al., 2015).
To further parse the distribution of rods and green-sensitive cones we also stained retinae with a second monoclonal anti-rhodopsin antibody (Rho4D2, ab98887, Abcam, RRID: AB_2315274), which was raised against amino acids 2–39 at the N-terminus of bovine rhodopsin (NGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPWQFSM, Hicks and Molday, 1986). This antibody has been shown to reliably label rods in fish, amphibians and mammals (New et al., 2012), and strongly label both rods and green-sensitive cones in chickens (Fisher et al., 2007). The labelling of green-sensitive cones with anti-rhodopsin antibodies reflects the fact that green-sensitive cone opsin and rhodopsin share a high degree of amino acid homology (Okano et al., 1992). We interpreted the presence of Rho4D2 positive cells in regions where Ret-P1 staining was weak or absent as indicating the presence of green-sensitive cones and the absence of rods.
Finally, we labelled violet-sensitive cones using a goat polyclonal antibody (OPN1SW, SC14363, Santa Cruz, RRID: AB_2158332), which was raised against amino acids 8–27 at the N-terminus of human blue cone opsin (EFYLFKNISSVGPWDGPQYH, Schiviz et al., 2008) This antibody has been shown to be ultraviolet/violet cone-specific in birds (Nießner et al., 2011), and reflects the fact that human blue cone opsin (SWS1) is orthologous to the avian ultraviolet/violet-sensitive cone opsin (Hunt and Peichl, 2014).
Results
General observations about raptor foveae
The retinae of the common buzzard, the Eurasian sparrowhawk, the red kite and the peregrine falcon all had a deep central fovea and a shallower temporal fovea (Fig. 2). The temporal fovea of the red kite appeared as a very shallow indentation (Fig. 2H), but it was still possible to visually locate it in the unfixed and later in the fixed unstained retina. In the stained cross-sections the presence of the fovea was distinguished from artifactual indentation by the thickening of the retinal layers and tilting of the cell columns in the inner nuclear layer, on both sides of the fovea (Fig. 2J). We could not identify a temporal fovea of the honey buzzard either in fresh or fixed retina, suggesting that this species may instead have a temporal area without a foveal depression.
The raptor central fovea lacks rods
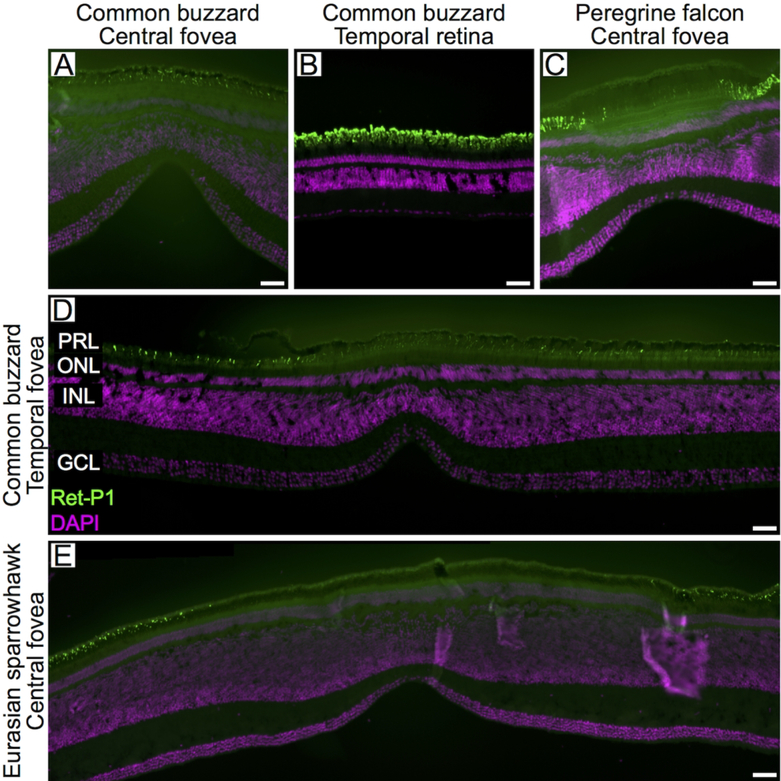
Anti-rhodopsin immunolabeling showed little or no reactivity in the central region of the Eurasian sparrowhawk and peregrine falcon central fovea (Figs. 3C, E). We detected Ret-P1 positive cells in the common buzzard central fovea (Fig. 3A); however, the intensity of labeling was much weaker than in non-foveal regions (Fig. 3B) suggesting that this was most likely green-sensitive cone opsin cross-reacting with the Ret-P1 antibody (Coimbra et al., 2015). Therefore, we conclude that rods are absent from the central most portion of the central fovea of these three species. We also did not detect Ret-P1-positive cells in the temporal fovea of the common buzzard (Fig. 3D). It was not possible to precisely measure the area of these rod-free zones in the retinal preparations. However, evaluation of the available images suggests that the size of the rod-free zone differs between species (Fig. 3).
Fig. 3.
Rhodopsin expression in the rods of the raptor retina. Confocal images of retinal cross-sections labeled with antibodies directed to rhodopsin (Ret-P1; green) of the central fovea (A), the temporal retina (B) and the temporal fovea (D) of the common buzzard, the central fovea of the peregrine falcon (C) and the central fovea of the Eurasian sparrowhawk (E). DAPI used to counter-stain nuclei is shown in purple. For labeling of retinal layers see legend of figure 2. Scale bars: 50 μm.
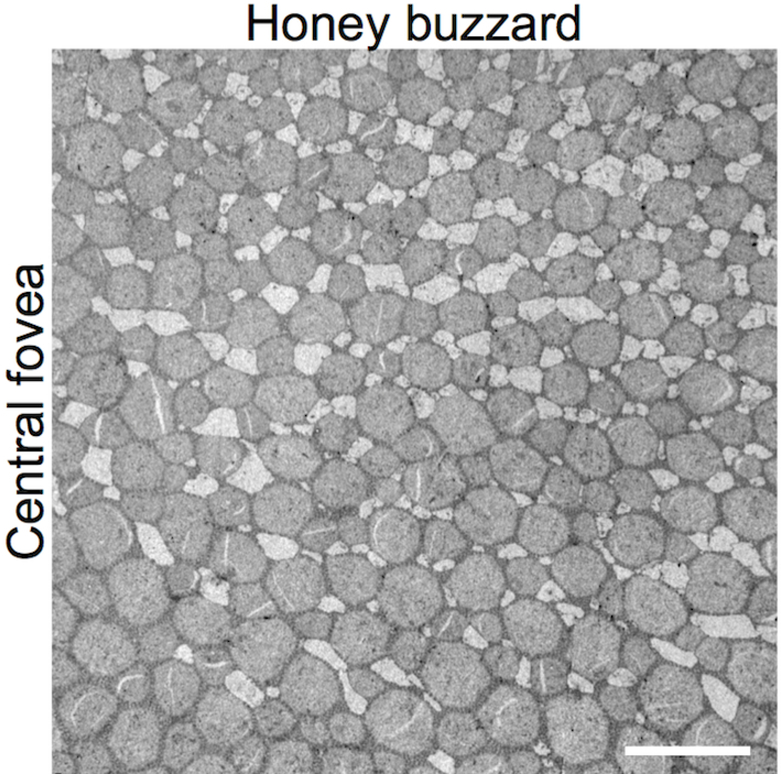
The raptor fovea lacks double cones
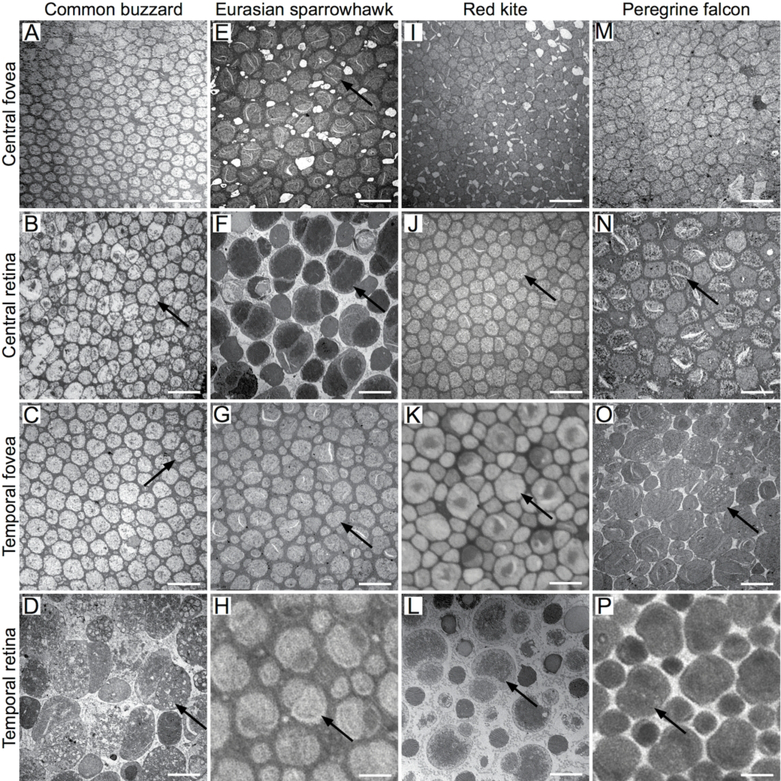
We did not observe double cones in the central fovea of the common buzzard, honey buzzard, red kite or peregrine falcon by TEM (Figs. 4A, 4I, 4M, 5). However, double cones were present in the central fovea of the Eurasian sparrowhawk (Fig. 4E). In the red kite central fovea, the double cone-free zone had a diameter of approximately 200 μm. The fovea was smaller in the common buzzard and the honey buzzard (approximately 100 μm), and smallest in the peregrine falcon (approximately 30 μm). Outside the double cone-free zone of the falcon (Fig. 4M), double cones were present, but rare. In the temporal fovea of the common buzzard we found a small zone (only approximately 25 μm in diameter) without double cones (Fig. 4C), but we did not detect a double cone-free zone in the temporal fovea of any of the other species (Figs. 4G, 4K, 4O). The proportion of double cones among all photoreceptors varied widely across the species (Table 2). As we only had one sample per species and per retinal area, we cannot determine the extent to which these differences are species-specific or due to inter-individual variation.
Fig. 4.
Tangential retinal sections through the photoreceptor inner segments of the common buzzard (A, B, C, D), Eurasian sparrowhawk (E, F, G, H), red kite (I, J, K, L) and peregrine falcon (M, N, O, P) retina. TEM (A-G, I, J, L-O) and LM (H, K, P) images of the central fovea (A, E, I, M), the central retina close to the fovea (B, F, J, N), the temporal fovea (C, G, K, O) and the temporal retina (D, H, L, P). Arrows point to the double cones. At the edge of the double cone-free zone of the common buzzard temporal fovea some double cones are also visible (arrow in C). Scale bars: 5 μm.
Fig. 5.
TEM micrograph of tangential retinal section through the photoreceptor inner segments of the honey buzzard central fovea. Scale bar: 5 μm.
Table 2.
Summary of the double cone and rod presence/absence in the retina of five raptor species investigated in this study.
| Retinal region | Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common buzzard | Honey buzzard | Eurasian sparrowhawk | Red kite | Peregrine falcon | ||||||
| Double cones | Rods | Double cones | Rods | Double cones | Rods | Double cones | Rods | Double cones | Rods | |
| Central fovea | No | No | No | n.a. | Yes (50.0%) | No | No | n.a. | No | No |
| Central retina | Yes (48.4%) | n.a. | n.a. | n.a. | Yes (37.0%) | n.a. | Yes (20.6%) | n.a. | Yes (12.3%) | n.a. |
| Temporal fovea | No | No | n.a. | n.a. | Yes (58.0%) | n.a. | Yes (24.1%) | n.a. | Yes (35.6%) | n.a. |
| Temporal retina | Yes (39.6%) | Yes | n.a. | n.a. | Yes (51.4%) | n.a. | Yes (21.3%) | n.a. | Yes (33.7%) | n.a. |
n.a. – not applicable (the sample was not available or not analysed with a certain method
Violet- and green-sensitive cones are present in raptor central foveae
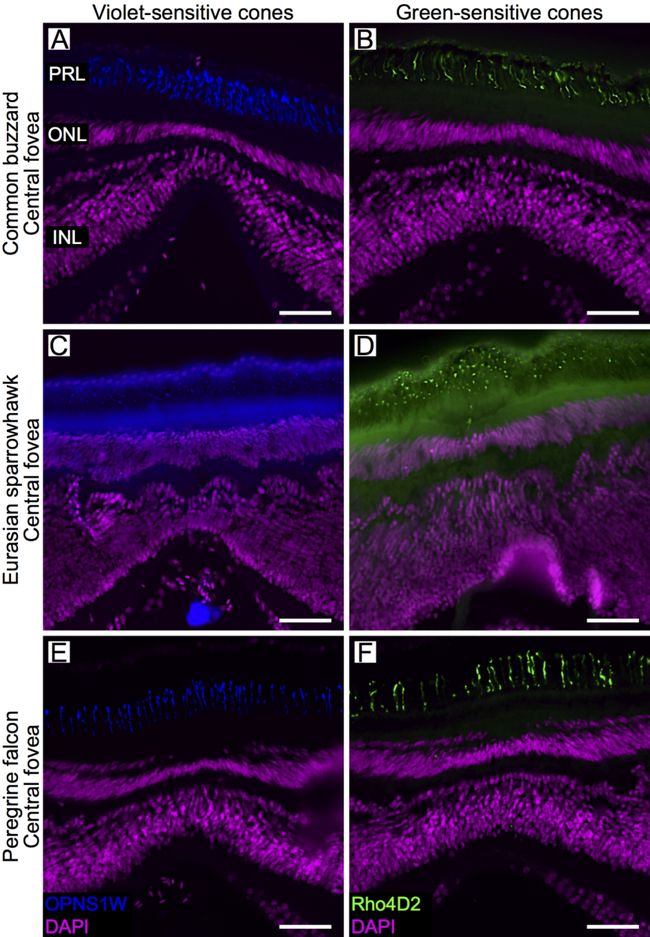
We were not able to discriminate between single cone types morphologically, but we did observe OPN1SW-positive cones in the common buzzard, the Eurasian sparrowhawk and the peregrine falcon retinae (Eurasian sparrowhawk and peregrine falcon temporal foveae were not available), including the central fovea of each species (Fig. 6). This indicates that violet-sensitive single cones are present in the central foveae of these species. We also observed Rho4D2 positive cells in the retinae of these three species (Eurasian sparrowhawk and peregrine falcon temporal foveae were not available). In the central fovea of the Eurasian sparrowhawk, the staining of outer segments is less obvious and only visible as dots (Fig. 6C, 6D), most likely because the outer segments were sectioned at an oblique angle in this sample. In the central foveae of these species we observed clear staining with Rho4D2, a marker of both rods and green-sensitive cones (Fig. 6). However, in these regions, staining with the more specific rod marker Ret-P1 was weak or absent (Fig. 3). We interpreted this pattern to indicate that the central fovea of these species lacks rods, but does contain green-sensitive cones.
Fig. 6.
Opsin expression in the single cones of the raptor central fovea. Confocal images of retinal cross-sections labeled with antibodies directed to two opsins: SWS1 (violet-sensitive cones; OPN1SW, blue, left column) and Rh2 (green-sensitive cones; Rho4D2, green, right column) of the common buzzard (A, B), Eurasian sparrowhawk (C, D) and peregrine falcon (E, F). DAPI used to counter-stain nuclei is shown in purple. For labeling of retinal layers see legend of figure 2. Scale bars: 50 μm.
Discussion
The goal of our study was to examine the photoreceptor complement of the raptor fovea to better understand the adaptations underlying the exceptional visual acuity of these birds. We had hypothesized that the fovea would be specifically populated with double cones, a class of photoreceptors that has been ascribed a range of functions including the mediation of highresolution achromatic vision in birds (Osorio et al., 1999; Jones and Osorio, 2004; Lind and Kelber, 2011). We also hypothesized that dim light-sensitive rods and violet-sensitive cones would be excluded from the fovea. We found that rods and double cones were absent from the foveae of several raptor species while violet-sensitive cones were present, suggesting that not only double cones, but also single cones may contribute to high-resolution vision in birds, and that raptors may in fact possess high-resolution tetrachromatic vision in the central fovea.
Rod-free zones in the foveae
Foveae of primates (Finlay et al., 2008), at least some fish (Collin and Collin, 1999) and some birds (Bruhn and Cepko, 1996; Querubin et al., 2009; Coimbra et al. 2015) have been observed to be rod-free. We found rod-free zones in and around the central fovea of the common buzzard, the Eurasian sparrowhawk and the peregrine falcon as well as the temporal fovea of the common buzzard (Fig. 3; red kite and honey buzzard were not investigated in this respect). While the absence of rods in the central fovea of the Eurasian sparrowhawk and the peregrine falcon is clear, there was some staining in the common buzzard foveae. The Ret-P1 antibody is known to have weak cross-reactivity with the Rh2 opsin expressed in greensensitive cones (Coimbra et al., 2015). The stained outer segments in the common buzzard foveae appear to be much narrower and have a fainter signal than in the temporal retina (Fig. 3B) or in the regions farther away from the temporal fovea (Fig. 3D). As Oehme (1964) also reported the absence of rods from the central and temporal foveae of the common buzzard and common kestrel, we consider it most likely that Ret-P1 cross-reacts with the Rh2 opsin in these regions.
Even though we could not precisely quantify the area of the rod-free zones in our histological preparations, the apparent cross-sectional diameter of these zones suggests that there might be large variation in the extent of the rod-free zone among raptor species, similar to what has recently been reported in some passerine birds (Coimbra et al., 2015). Rod-free zones have also been reported in the area centralis of chickens (Bruhn and Cepko, 1996) and in the fovea of pigeons (Columba livia; Querubin et al., 2009). The absence of rods in and around the fovea allows for a higher density of cones, which is needed for optimal high-acuity vision in bright light.
Double-cone free zones in foveae
In this study we confirm earlier suggestions that double cones are absent from the fovea of some raptor species (Reymond 1985, 1987). We found large differences in the extent of the double cone-free zone in the central foveae among the species we investigated. Unlike the foveae of the other raptors examined, the central fovea of the Eurasian sparrowhawk contained double cones. With the exception of the temporal foveae of the common buzzard, which had a small double cone-free zone, the temporal foveae of the red kite, the Eurasian sparrowhawk and the peregrine falcon all contained double cones.
In previous studies Reymond (1985, 1987) did not find double cones in the central and temporal foveae of the brown falcon. She also did not find double cones in the central fovea of the wedge-tailed eagle, but did not note the presence or absence of the double cones in the temporal fovea of this species. In neither of the studies does she mention the extent of the double cone-free zones. Reymond’s and our findings indicate variation in the double cone distribution among raptor foveae. Whether this is the case for other avian clades is currently unknown. However, variation in cone type proportions between different non-foveal retinal regions is well documented in many bird species (Hart, 2001a).
One of the possible explanations for the absence of double cones in the fovea may be adaptation to maximal receptor density. Two members of a double cone together have greater inner segment diameter than a single cone, and contribute two outer segments to this layer. However there are indications that both members function as a single receptive unit, because they are optically and electrically coupled. Two outer segments are optically isolated from other photoreceptors by pigmented apical processes of the retinal pigment epithelium cells, but not from each other (Young and Martin, 1984; Hart, 2001b). In addition, calculations indicate that due to the properties of the inner segments ‘optical cross-talk’ occurs between two members of the double cone (Wilby, 2014). Furthermore, the presence of gap junctions between the inner segments of the principle and accessory members suggests that they are sharing, at least partly, their electrical signals (Smith et al., 1985). Therefore, double cone inclusion in the fovea would limit the maximum possible receptive unit density essential for detailed spatial and chromatic tasks in bright light. The exclusion of double cones from the region of highest acuity in species renowned for their high-resolution vision suggests that not only double cones, but also single cones contribute to achromatic high-resolution vision in birds.
Single cones and the problem of chromatic aberration
To achieve highest image quality, the optical system needs to focus light of all wavelengths onto the same focal plane. However, light of different wavelengths is refracted to varying extents when passing through a lens making it impossible to focus all wavelengths on a single focal plane without additional optical adaptations. This phenomenon, known as chromatic aberration, is particularly pronounced for short wavelengths of light and large lenses, and can compromise visual acuity. To reduce this problem, the primate visual system filters out shorter wavelengths in the lens, thereby limiting the spectral range of light reaching the retina and (Douglas and Jeffery, 2014). In addition, blue-sensitive cones (that are homologous to the violet-sensitive cones of raptors) are absent from the central-most part of some primate foveae (e.g. Wikler and Rakic, 1990; Martin and Grünert, 1999). Thus, in these species, highr-esolution vision is restricted to longer wavelengths. The size of the blue-sensitive cone-free zone is smaller in smaller eyes and absent in the common marmoset (Callithrix jacchus), which has the smallest eye of the primate species so far investigated (Martin and Grünert, 1999). These examples indicate that smaller primate eyes with lower spatial resolution can cope with the degree of chromatic aberration they experience, however larger eyes may not. Spatial resolution in the foveae of raptors is similar to or even higher than in primates (Fischer, 1969; Reymond, 1985, 1987). As the ocular media of raptors transmit more short-wavelength light than those of primate eyes (Lind et al., 2013), chromatic aberration should be a more serious problem for these species than for primates. Therefore we were surprised to find violet-sensitive cones throughout the fovea. We consider it likely that the other two cone types, blue-sensitive (S) cones and red-sensitive (L) cones, are present in the raptor fovea as well, possibly allowing for tetrachromatic color vision in the central fovea.
How do raptors avoid the problem of chromatic aberration that is predicted to occur in a fovea containing cones with sensitivities ranging from below 400 nm (violet) to almost 700 nm (red)? Chromatic aberration is a serious problem only if the optical system has a wide pupil aperture as compared to the focal length of the eye. Thus, in order to avoid chromatic aberration raptors could close the pupil when they need a well-focused color image. Although Miller (1979) reports that raptors with shorter focal lengths than humans have larger pupil diameters even in the bright daylight, falconers report that their birds narrow the pupil when fixating on an object before taking flight (Simon Potier, personal communication). It has also been suggested that the multifocal lenses found in various vertebrates solve this problem by providing the retina with a well-focused image for all wavelengths (Kröger et al., 1999). Two accipitriform species have been found to have bifocal lenses (Lind et al., 2008), but so far, no functional evidence supporting this hypothesis has been published. Therefore, it remains unclear, which mechanisms allow raptors to use the highly resolved tetrachromatic image created in the central fovea.
Acknowledgements
We are very grateful to Kenneth Bengtsson for help with collecting samples, and to Eva Landgren, Carina Rasmussen and Ola Gustafsson for expert help with tissue processing. We thank João Paulo Coimbra for useful comments and suggestions on an earlier version, which improved the manuscript.
Acknowledgements for support
MM, PO and AK were funded by the Swedish Research Council (VR2012-2212), the Human Frontier Science Program grant #RGP0017/2011 and the K & A Wallenberg Foundation (Ultimate Vision). MT and JC were funded in part by Human Frontier Science Program grant #RGP0017/2011 and National Institutes of Health grants RO1EY026672 and RO1EY024958. MT was supported by fellowships from the National Science Foundation (Award #1202776), National Institutes of Health (5T32-EY013360-12), and the McDonnell Center for Cellular and Molecular Neurobiology at Washington University, St. Louis.
Footnotes
Conflict of interest statement
The authors have no known conflicts of interest that could inappropriately influence this work.
Role of authors
MM conceived the project and planned it in collaboration with PO, MT, JC and AK. MM and PO did the retinal sampling and TEM analysis. MT did the immunohistochemistry analysis. MM, PO, MT, JC and AK analyzed the data and wrote the paper.
Literature cited
- Barnstable CJ. 1980. Monoclonal antibodies which recognize different cell types in the rat retina. Nature 286:231–235. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR. 1993. Retinal photoreceptor fine structure in the red-tailed hawk (Buteo jamaicensis). Anat Histol Emryol 22:222–232. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR. 1998. Fine structure of the retinal photoreceptors of the emu (Dromaius novaehollandiae). Tissue Cell 30:137–148. [DOI] [PubMed] [Google Scholar]
- Bruhn SL, Cepko CL. 1996. Development of the pattern of photoreceptors in the chick retina. J Neurosci 16:1430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenhausen MV, Kirschfeld K. 1998. Spectral sensitivity of the accessory optic system of the pigeon. J Comp Physiol A 183:1–6. [Google Scholar]
- Coimbra JP, Collin SP, Hart NS. 2015. Variations in retinal photoreceptor topography and the organization of the rod-free zone reflect behavioral diversity in Australian passerines. J Comp Neurol 523:1073–1094. [DOI] [PubMed] [Google Scholar]
- Collin SP, Collin HB. 1999. The foveal photoreceptor mosaic in the pipefish, Corythoichthyes paxtoni (Syngnathidae, Teleostei). Histol Histopathol 14:369–382. [DOI] [PubMed] [Google Scholar]
- Douglas RH, Jeffry G. 2014. The spectral transmission of ocular media suggests ultraviolet sensitivity is widespread among mammals. Proc R Soc B 281:20132995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Franco ECS, Yamada ES, Crowley JC, Parsons M, Muniz JAP, Silveira LCL. 2008. Number and topography of cones, rods and optic nerve axons in New and Old World primates. Vis Neurosci 25:289–299. [DOI] [PubMed] [Google Scholar]
- Fischer AB. 1969. Laboruntersuchungen und Freilandbeobachtungen zum Sehvermögen und Verhalten von Altweltgeiern. Zool Jb Syst Bd 96:S81–132. [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. 2007. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol 500:1154–1171. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. 1977. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 111:219–223. [Google Scholar]
- Güntürkün O 2000. Sensory physiology: Vision In: Whittow GC, editor. Sturkie’s Avian Physiology, 5th ed. London: Academic Press; p 1–19. [Google Scholar]
- Hart NS. 2001a. Variations in cone photoreceptor abundance and the visual ecology of birds. J Comp Physiol A 187:685–697. [DOI] [PubMed] [Google Scholar]
- Hart NS. 2001b. The visual ecology of avian photoreceptors. Prog Retin Eye Res 20:675–703. [DOI] [PubMed] [Google Scholar]
- Hicks D, Molday RS. 1986. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res 42:55–71. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Peichl L. 2014. S cones: evolution, retinal distribution, development, and spectral sensitivity. Vis Neurosci 31:115–138. [DOI] [PubMed] [Google Scholar]
- Jones C, Osorio D. 2004. Discrimination of oriented visual textures by poultry chicks. Vision Res 44:83–89. [DOI] [PubMed] [Google Scholar]
- Jones MP, Pierce KE, Ward D. 2007. Avian vision: a review of form and function with special consideration to birds of prey. J Exot Pet Med 16:69–87. [Google Scholar]
- Kram YA, Mantey S, Corbo JC. 2010. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 5(2):e8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger RHH, Campbell MCW, Fernald RD, Wagner H-J. 1999. Multifocal lenses compensate for chromatic defocus in vertebrate eyes. J Comp Physiol A 184:361–369. [DOI] [PubMed] [Google Scholar]
- Land MF, Nilsson D-E. 2012. Animal eyes. 2nd ed. Oxford, UK: Oxford University Press. [Google Scholar]
- Lind O, Kelber A. 2011. The spatial tuning of achromatic and chromatic vision in budgerigars. J Vis 11:1–9. [DOI] [PubMed] [Google Scholar]
- Lind O, Kelber A, Kröger RHH. 2008. Multifocal optics and pupil dynamics of birds. J Exp Biol 211:2752–2758. [DOI] [PubMed] [Google Scholar]
- Lind O, Mitkus M, Olsson P, Kelber A. 2013. Ultraviolet sensitivity and colour vision in raptor foraging. J Exp Biol 216:1819–1826. [DOI] [PubMed] [Google Scholar]
- Manicam C, Pitz S, Brochhausen C, Grus FH, Pfeiffer N, Gericke A. 2014. Effective melanin depigmentation of human and murine ocular tissues: An improved method for paraffin and frozen sections. PLoS One 9: e102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. 1983. Schematic eye models in vertebrates In Autrum H, Ottoson D, Perl ER, Schmidt RF, Shimazu H, Willis WD, editors. Progress in Sensory Physiology, Vol 4 Berlin: Springer-Verlag; p. 43–81. [Google Scholar]
- Martin GR, Osorio D. 2008. Vision in birds In: Masland RH, Albright T, editors. The Senses. A Comprehensive Reference, Vol 1 London: Academic Press; p 25–52. [Google Scholar]
- Martin PR, Grünert U. 1999. Analysis of the short wavelength-sensitive (“blue”) cone mosaic in the primate retina: Comparison of New World and Old World monkeys. J Comp Neurol 406:1–14. [DOI] [PubMed] [Google Scholar]
- Meyer DB. 1977. The avian eye and its adaptations In: Crescitelli F, editor. Handbook of Sensory Physiology, Vol VII/5 Berlin: Springer-Verlag; p 549–611. [Google Scholar]
- Miller WH. 1979. Ocular optical filtering In: Autrum H, editor. Handbook of Sensory Physiology, Vol VII/6A Berlin: Springer-Verlag; p 69–143. [Google Scholar]
- Moroney MK, Pettigrew JD. 1987. Some observations on the visual optics of kingfishers (Aves, Coraciformes, Alcedinidae). J Comp Physiol A 160:137–149. [Google Scholar]
- New STD, Hemmi JM, Kerr GD, Bull CM. 2012. Ocular anatomy and retinal photoreceptors in a skink, the sleepy lizard (Tiliqua rugosa). Anat Rec 295:1727–1735. [DOI] [PubMed] [Google Scholar]
- Nießner C, Denzau S, Gross JC, Peichl L, Bischof H-J, Fleissner G, Wiltschko W, Wiltschko R. 2011. Avian Ultraviolet/Violet cones identified as probable magnetoreceptors. PLoS ONE 6(5):e20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Smith RL, Shimai K. 1981. Junction-like structure appearing at apposing membranes in the double cone of chick retina. Cell Tissue Res 218:113–116. [DOI] [PubMed] [Google Scholar]
- Oehme H 1962. Das Auge von Mauersegler, Star und Amsel. J Ornithol 103:187–212. [Google Scholar]
- Oehme H 1964. Vergleichende Untersuchungen an Greifvogelaugen. Z Morph Ökol Tiere 53:618–635. [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. 1992. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci 89:5932–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Jones CD, Vorobyev M. 1999. Accurate memory for colour but not pattern contrast in chicks. Curr Biol 9:199–202. [DOI] [PubMed] [Google Scholar]
- Ödeen A, Håstad O. 2003. Complex distribution of avian colour vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. 1989. Photoreceptor topography of the retina in the adult pigtail macaque (Macaca nemestrina). J Comp Neurol 288:165–183. [DOI] [PubMed] [Google Scholar]
- Querubin A, Lee HR, Provis JM, O’Brien KMB. 2009. Photoreceptor and ganglion cell topographies correlate with information convergence and high acuity regions in the adult pigeon (Columba livia) retina. J Comp Neurol 517:711–722. [DOI] [PubMed] [Google Scholar]
- Reymond L 1985. Spatial visual acuity of the eagle Aquila audax: a behavioural, optical and anatomical investigation. Vision Res 25:1477–1491. [DOI] [PubMed] [Google Scholar]
- Reymond L 1987. Spatial acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vision Res 27:1859–1874. [DOI] [PubMed] [Google Scholar]
- Rochon-Duvigneaud A 1943. Les Yeux et la Vision des Vertébrés. Paris: Masson. [PubMed] [Google Scholar]
- Schiviz AN, Ruf T, Kuebber-Heiss A, Schubert C, Ahnelt PK. 2008. Retinal cone topography of artiodactyl mammals: influence of body height and habitat. J Comp Neurol 507:1336–1350. [DOI] [PubMed] [Google Scholar]
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. 1988. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res 253:189–198. [DOI] [PubMed] [Google Scholar]
- Smith RL, Nishimura Y, Raviola G. 1985. Inter-receptor junction in the double cone of the chicken retina. J Submicrosc Cytol Pathol 17:183–186. [PubMed] [Google Scholar]
- Snyder AW, Miller HM. 1977. Photoreceptor diameter and spacing for highest resolving power. J Opt Soc Am 67:696–698. [DOI] [PubMed] [Google Scholar]
- Taylor SM, Loew ER, Grace MS. 2011. Developmental shifts in functional morphology of the retina in Atlantic tarpon, Megalops atlanticus (Elopomorpha: Teleostei) between four ecologically distinct life-history stages. Vis Neurosci 28:309–323. [DOI] [PubMed] [Google Scholar]
- Wilby D 2014. Optics and photoreception in the avian retina (Doctoral dissertation). University of Bristol, Bristol, UK. [Google Scholar]
- Wikler KC, Rakic P. 1990. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci 10:3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Martin GR. 1984. Optics of retinal oil droplets: a model of light collection and polarization detection in the avian retina. Vision Res 24:129–137. [DOI] [PubMed] [Google Scholar]