Abstract
Assessment of reproductive competence is critical for understanding the impact of a treatment or genetic manipulation on the reproductive axis, also termed the hypothalamic-pituitary-gonadal axis. The reproductive axis is a key integrator of environmental and internal input adapting fertility to favorable conditions for reproduction. Prior to embarking upon a fertility study in mice and rats, sexual maturity is evaluated to exclude the possibility that the observed reproductive phenotypes are caused by delayed or absent pubertal onset. This protocol describes a non-invasive approach to assess pubertal onset in males through the determination of preputial separation, and in females through vaginal opening and first estrus. After the confirmation of the completion of puberty and the achievement of sexual maturity, a fertility study can be initiated. The procedure describes the optimal breeding conditions for mice and rats, how to set up a fertility study, and what parameters to evaluate and determine if the treatment or gene deletion has an impact on fertility.
Keywords: Biology, Issue 140, Pubertal onset, fertility assay, reproductive competence, preputial separation, vaginal opening, first estrus, mouse, rodent, rat, male, female, body weight
Introduction
The transition through puberty is required to attain sexual maturity and reproductive competence. The pubertal transition and the maintenance of fertility in adulthood is regulated by the reproductive axis, also termed the hypothalamic-pituitary-gonadal axis (Figure 1). The timing of pubertal onset and maintenance of fertility is tightly regulated by internal as well as environmental factors to increase the chances of survival of offspring and parents1,2. This protocol provides a non-invasive approach to determine pubertal onset in mice and rats to confirm sexual maturity prior to setting up a fertility study to assess reproductive competence.
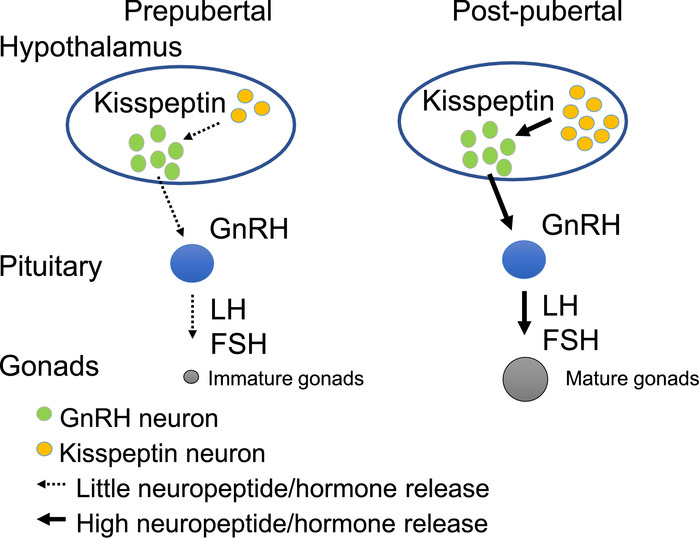
A fertility study is performed in sexually mature animals and can be initiated after the animals have gone through puberty. Prior to pubertal onset, the reproductive axis is quiescent, and the key driver of sexual maturation, gonadotropin-releasing hormone (GnRH), is released onto the pituitary in insufficient amounts to initiate puberty (Figure 1). Pubertal onset is a complex process that results in increased GnRH release at the median eminence. GnRH promotes luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion from the pituitary, two hormones essential for gonadal maturation and reproductive function (Figure 1)3,4,5.
Insults to the reproductive axis result in reduced fertility and can also advance or delay pubertal onset. Conditions known to influence the timing of pubertal onset and reproductive competence include the exposure to endocrine disrupting chemicals6,7, increased/decreased body weight1,8, changes in day length2,9 and genetic mutations10,11,12,13,14,15.
The onset of sexual maturity is a critical step that needs to be completed prior to setting up a fertility assay. The advantages of determining pubertal onset through preputial separation, vaginal opening and first estrus, are the non-invasive characteristics of these procedures, as they do not require blood collection or sacrifice of the animal16,17.
After pubertal onset is determined, correctly setting up a fertility study will provide important information about the integrity of the reproductive axis, and usually has the second advantage of generating experimental animals for further studies (refinement)18. The fertility study setup described in this protocol can detect both minor and major deficits in reproductive competence in males and females. Key parameters evaluated include 1) time to the first litter, 2) number of litters generated in a given time frame and 3) litter size. Finally, recommendations for the type of follow up studies which can be conducted to identify the cause of fertility impairment are included.
The described protocol refers to mice and the representative data reflect work done in transgenic mice. However, all the included protocols are equally valid in rats.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee of Michigan State University and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
1. Determine Pubertal Onset
Follow institutional guidelines for clothing, at a minimum, it is necessary to wear a clean lab coat and clean gloves. Always handle mice wearing clean gloves.
- Prepare the work area by placing a pad on the table.
- Place a clean mouse cage top with the grid facing upwards on the pad.
- Set up a scale in the work area. Place a clean 500 – 1000 mL beaker on the scale and tare.
- Place a sheet next to the work area to record body weight and preputial separation, vaginal opening or first estrus.
Identify the mice for the study. Perform daily inspections for pubertal onset until puberty is reached. Perform the inspection at the same time of day throughout the study. Pubertal onset can occur as early as postnatal day 11-1219, however, in most experimental setups, pubertal onset monitoring starts at ~22 days.
Place the mouse cage in the work area (Step 1.2). Open the cage and place the lid, water bottle and food holder on the table.
- Determining preputial separation in male mice.
- While holding the mouse by the tail, place it on the clean mouse cage top from Step 1.2.1. While gently holding onto the tail, let the mouse explore the grid of the cage top for ~5 s.
- Gently pull the tail backward; this usually results in the animal moving forward while holding on to the grid with its forelegs. Approach the other hand to the skin close by the neck and ears.
- Grasp the loose skin between the shoulders and neck with the thumb and forefinger. It is important to get a good hold of the skin, as it will prevent the mouse from turning its head around to bite.
- Grab the skin on the back with the remaining fingers and hold it by pressing against the hand. Hold firmly without obstructing breathing, blood flow, or damaging bones, muscles or skin. Turn the hand to expose the belly of the mouse, while supporting the back of the mouse within the palm of the hand.
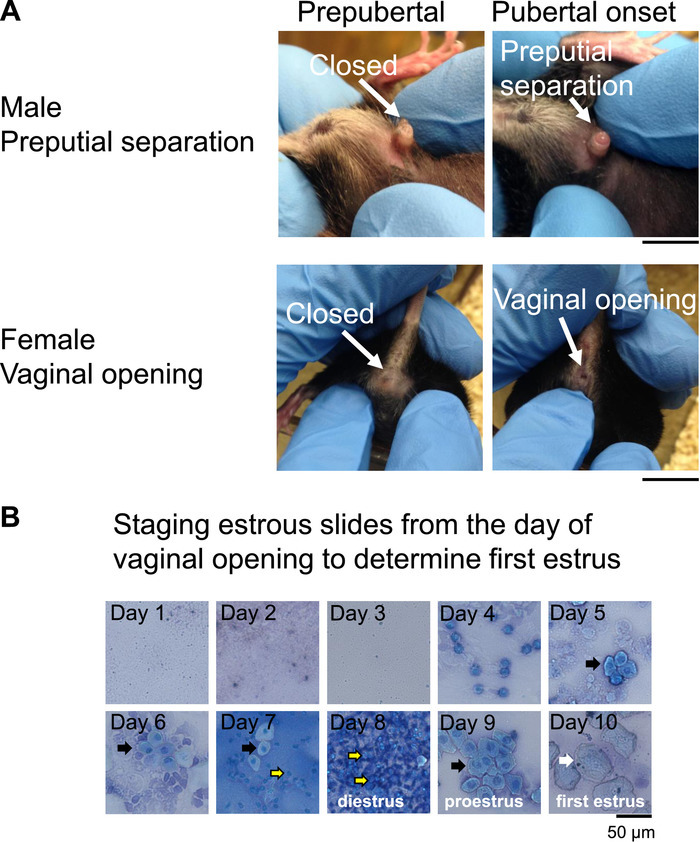
- With the free hand, gently push back the skin around the penis without forcing. At preputial separation, the preputial skin slides backward exposing the glans penis (Figure 2A, arrow showing preputial separation). Record the presence or absence of preputial separation.
- Weigh the mouse by gently placing it in the 500-1000 mL beaker from Step 1.2.2 and record the weight.
- Reintroduce the mouse in the housing cage by holding the beaker 0-2 cm over the cage floor and slowly tip over the beaker allowing the mouse to exit on its own. Clean the beaker with water and mild detergent and dry it off before returning it to the scale and taring the scale.
- Determining vaginal opening in female mice.
- Place a cotton ball, beaker and a bottle of sterile water in the work area (Step 1.2). Pour the water into the beaker. Humidify the cotton ball with the sterile water and place it next to the cage top from Step 1.2.1.
- Place the mouse cage in the work area (Step 1.2) and transfer the mouse from its home cage to the cage top (Step 1.2.1) by holding it at the base of the tail. Gently pull back the tail to encourage a forward movement of the mouse.
- Lift the tail while supporting the hips with the free fingers. Allow the hindlimbs to maintain contact with the top of the cage. If the mouse moves too much, hold it in the hand as described in Steps 1.5.1-1.5.4.
- Gently clean the vulva with the water humidified cotton ball from Step 1.6.1. Use a clean humidified cotton ball for each mouse. To determine vaginal opening, examine the vulva and determine whether the vagina is completely open (Figure 2A, female vaginal opening).
- Record if vaginal opening has occurred. Weigh the mouse and return it to the home cage as described in Steps 1.5.6-1.5.7.
- First estrus an indicator of first ovulation.
- Place a beaker with sterile water, a labeled glass slide and a 200 µL pipette with a clean tip in the work area (Step 1.2).
- Hold the female as described in Step 1.6.2-1.6.3. Place the tip of the pipette containing ~50 µL of sterile water at the vaginal opening.
- Gently flush the cells from the vaginal wall by introducing and reabsorbing ~50 µL of water 2-4 times. Smear the content from the pipette tip onto a labeled glass microscope slide.
- Carefully observe the cellular morphology on a brightfield microscope using a 10X or 20X objective or let the smear airdry for ~1 h and then counterstain with a 0.1% methylene blue solution (dissolved in ddH2O) (Figure 2B). To counterstain, dip the dried slide into the 0.1% methylene blue solution for ~ 30 s. Let the slide air dry for 1 h.
- Observe the slides from Step 1.7.4 on a bright field microscope at 10X or 20X. Establish the stage of the estrous cycle (Figure 2B)20 by observing the cell morphology which allows to distinguish the four distinct stages of the estrous cycle (Figure 2B). Note: Metestrus is characterized by a mixture of cornified squamous epithelial cells and leukocytes, diestrus by leukocytes, proestrus by a mixture of leukocytes and nucleated epithelial cells, and estrus by cornified epithelial cells (Figure 2B)20.
- Record the body weight and return the mouse to its home cage as described in Steps 1.5.6-1.5.7. Vaginal smears are terminated on the day when the mouse has its first estrus (Figure 2B).
2. Desirable Breeding Room Conditions
Ensure that the breeding room has a temperature of ~20-24 °C, with 55 ± 10% humidity, and homogenous room light of an intensity of ~300-400 lux approximately 1 m above the floor21. This light intensity allows the desirable mid-cage light intensity of 25-80 lux. To test light intensity, use a light meter.
Use an automated system to control the lighting in the breeding room to ensure appropriate timing of lighting throughout the experiment. Optimal day-length conditions to assess fertility in rodents range from a 12 h day (12 h light and 12 h darkness, LD12:12), to “summer-like” conditions with 14 h of light and 10 h darkness (LD14:10)21.
- Prepare breeding cages.
- Prepare a clean mouse cage with lid, water, food, bedding and nesting per breeding pair.
- Fill out the cage cards with all the required information, including sex, date of birth, mouse strain, and the date when the mating is set up.
3. Fertility Study
Identify the animals to be used for the fertility study. Ensure that the animals are sexually mature (Step 1). A typically used age range to set up a fertility study in mice is 10-16 weeks of age, an age where sexual maturation has been completed in most experimental conditions.
On a clean table, place the breeding cage prepared in Step 2.3.
- Catch and hold the mouse.
- Slowly introduce a gloved hand into the mouse cage. Leave the hand in the cage for a short period of time (~5-30 s), allowing the mice to adapt to the smell of the glove and hand. Avoid hectic and jerky movements.
- Lower the cupped hand close to the bottom of the cage and slowly approach it to the mice. They will run away. When they run over the hand, trap the tail of one mouse between the thumb and index finger.
- Lift the mouse by the tail for 2-3 s. If the mouse needs to be held longer, hold onto the tail and place the mouse in the cupped hand maintaining the hold on the tail.
Transfer 1 male and thereafter 1 female into the breeding cage by holding them by the tail (Step 3.3). Ensure that the females are in the same estrous stage when setting up the fertility assay. Determine the estrous stage of the females by performing a vaginal smear as described in Step 1.7.
Close the cage, and ensure that the mice have food, water and nesting material. Attach the cage card holder with the cage card to the cage.
Place the breeding cage on the housing rack. Leave the breeding cage as undisturbed as possible for the full duration of the fertility study. Conduct the fertility study for 30 days. For each breeding pair, keep detailed records and note 1) the date the mating is set up, 2) the date litters are born and 3) the number of pups born, including dead and live pups. Perform litter checks daily throughout the study.
Prolong the fertility study for an additional 30-60 days if no or a weak impact on fertility is established in Step 3.6.
Representative Results
The presented results are from two different transgenic mouse models where the transcription factor Ventral anterior homeobox 1 (Vax1) has been deleted in the whole body on one allele, here referred to as heterozygote mice (HET)13, or Vax1 has been conditionally deleted within GnRH neurons22, here termed conditional KO (cKO). Prior to setting up the fertility study, it is important to confirm pubertal onset in all the mice.
First, the day and weight at vaginal opening and preputial separation were established. In the HET mice, vaginal opening and preputial separation occurred at the same age and weight as in the control mice (Figure 3A and F, respectively). In contrast, vaginal opening and preputial separation were significantly delayed in cKO females and males and associated with increased bodyweight (Figure 3B and G, respectively). Pubertal onset is to some degree associated with body weight, and this is particularly true in females23. To determine if the delay in vaginal opening and preputial separation was associated with a delay in weight gain, the body weight at the average age of vaginal opening and preputial separation in controls (4.5 weeks of age) was compared to the body weight in cKO in males and females at 4.5 weeks of age. At age 4.5 weeks, female controls and cKO had comparable body weight (Figure 3C), whereas cKO males were slightly heavier than controls (Figure 3H). This indicates that the delay in vaginal opening and preputial separation in cKO is not associated with a delay in weight gain.
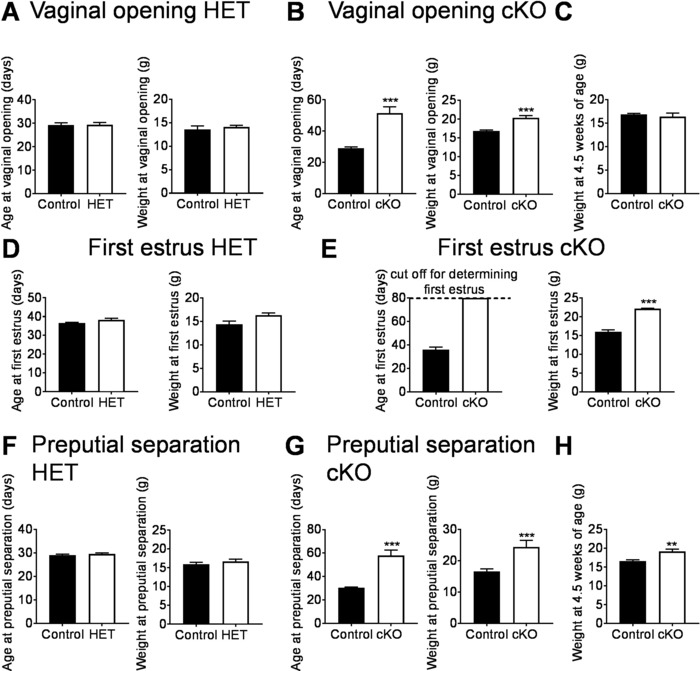
Vaginal opening is an early marker of sexual maturation and first estrus an indicator that the first ovulation has occurred, a marker of completion of puberty17. To determine if HET and cKO females reached first estrus at the same age and weight as controls, vaginal smears were performed daily from the day of vaginal opening until first estrus (Figure 3E and 3D). In the HET mice, first estrus and the weight at first estrus was comparable between controls and HETs (Figure 3D). In contrast, cKO did not progress through the estrous cycle and did not have first estrus at the age of 80 days (Figure 3E). This delay was not due to reduced body weight (Figure 3E).
The HET mice with confirmed pubertal onset were used to set up the fertility study. The fertility assay was set up in 12 h light and 12 h dark conditions (LD12:12) in transgenic mice on a C57BL/6J genetic background. The mice were aged 10-16 weeks at the start of the experiment. Four groups of mice were studied: control matings (WTxWT), control males with HET females (WTxHET), HET males with WT females (HETxWT), and HET males with HET females (HETxHET). First, the number of litters generated in 3 months was determined. Control matings (WTxWT) generated an average of 2.8 litters in 3 months, which was comparable to HET males paired with control females (HETxWT mating, Figure 4A). HET females, whether paired with a WT male (control), or a HET male, generated significantly fewer litters in the 3 month fertility study, revealing that HET females are subfertile13. To illustrate the importance of confirming pubertal onset prior to setting up a fertility assay, reproductive competence was evaluated in cKO mice, where the males had delayed preputial separation (Figure 3G) and the females did not have their first estrus by 80-days of age (Figure 3E). The fertility study was set up >1 week after males had preputial separation, and in females at >80 days of age. During the 70-day fertility study, control matings generated an average of 2.2 litters, whereas no litters were generated from either the cKO female paired with a WT male, or the cKO male paired with a control (WT) female (Figure 4B), showing that these mice are infertile5,22.
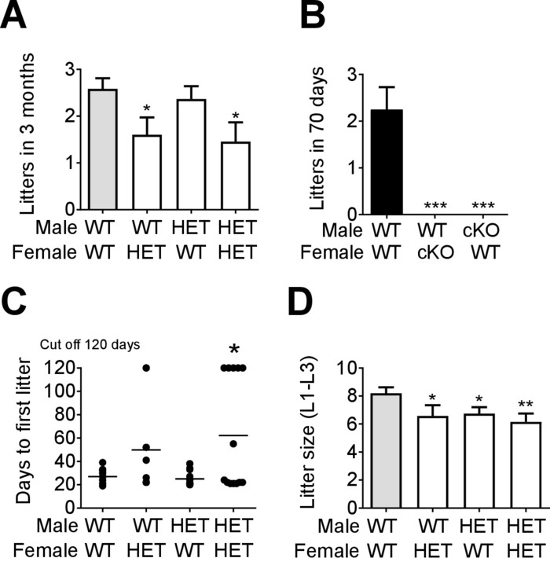
To better characterize the fertility phenotype of the HET females, we recorded the number of days it took to generate the first litter. WTxWT matings generated their first litter in an average of 22 days (Figure 4C), which was comparable to HET males paired with WT females. HET females paired with WT males took an average of 58 days to produce their first litter, which was similar to HETxHET matings (Figure 4C). However, the delay in generating the first litter of the HET females was very heterogenous, and delayed more in HETxHET matings, suggesting a semi-penetrance of the phenotype, and a contribution of the HET male to the subfertility observed in HETxHET matings13.
Evaluating the litter size can give important information about ovulation/implantation and sperm production. The average size of the first 3 litters was determined. Interestingly, all mating combinations (WTxHET, HETxWT, HETxHET) produced significantly smaller litters as compared to controls (WTxWT, Figure 4D), showing that both male and female HET mice are subfertile.
Figure 1: The hypothalamic-pituitary-gonadal axis controls sexual maturation and reproduction. At the apex of the reproductive axis are the hypothalamic kisspeptin neurons (yellow circles) and gonadotropin-releasing hormone (GnRH) neurons (green circles). In the juvenile period (prepubertal), kisspeptin neurons release little kisspeptin onto GnRH neurons. After pubertal onset, kisspeptin release on GnRH neurons is augmented (post-pubertal). Increased GnRH release in the late juvenile period dramatically increases luteinizing hormone (LH) and follicle stimulating hormone (FSH) release from the pituitary, leading to gonadal maturation and reproductive function in adulthood. Please click here to view a larger version of this figure.
Figure 2: Determination of pubertal onset in mice. An external marker of pubertal onset in mice is (A) preputial separation in the males, and vaginal opening in the females. Scale bar = 1 cm. (B) Example images from vaginal lavage used to determine first estrus. Slides were counterstained for 30 s with 0.1% methylene blue, airdried and observed at 20X magnification. Black arrows indicate nucleated epithelial cells, yellow arrows with black outline indicate leukocytes and white arrows indicate cornified epithelial cells. First estrus occurred on day 10 after vaginal opening. Please click here to view a larger version of this figure.
Figure 3: Representative data which can be generated from a pubertal onset study. (A, B) Vaginal opening and weight at vaginal opening in the transgenic mouse models. (C) Weight at 4.5 weeks in control and cKO females. (D, E) Age and weight at first estrus. (F, G) Age and weight at preputial separation. (H) Weight at 4.5 weeks in control and cKO males. Data represent means ± SEM. N = 5-10 per group, statistical analysis Student's-t test. **p<0.01; ***p<0.001. These figures have been modified from previous publication13,22. Please click here to view a larger version of this figure.
Figure 4: Representative analysis from a fertility study of transgenic mice. Fertility assessment of (A) Vax1 HET mice and (B) cKO was performed in virgin 10- to 16-week-old mice and the number of litters recorded in the indicated time frames. Data represent means ± SEM. Statistical analysis was performed by one-way ANOVA followed by Dunnett's multiple comparison test. *p<0.05; ***p<0.001. (C) The number of days to first litter (n = 5-12), and (D) average litter size of the first three litters (n = 10-13) was determined. Data represent means ± SEM. Statistical analysis was done by Student's-t test as compared with WT x WT. *p<0.05; **p<0.01. These figures have been modified from previous publication13,22. Please click here to view a larger version of this figure.
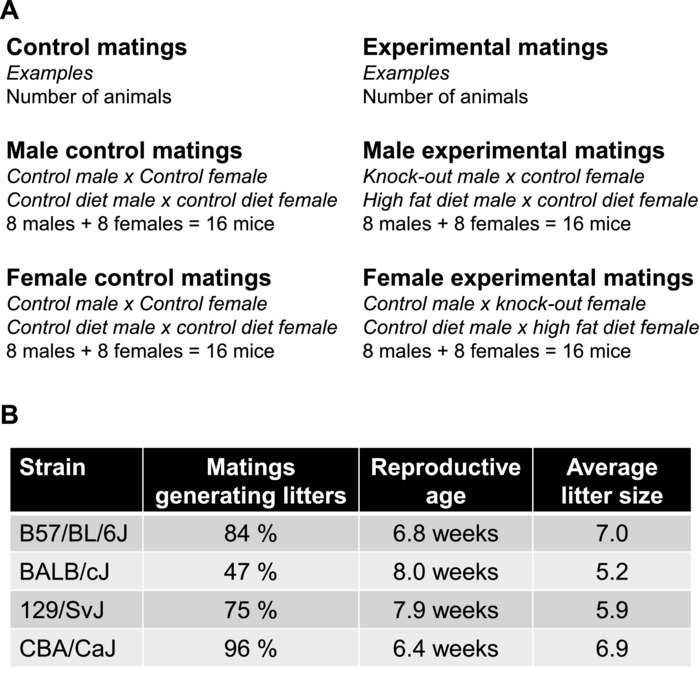
Figure 5: Setting up a fertility assay. (A) Example of breeding scheme and suggested number of animals required for a fertility assay. The total number of animals required for a study with 2 conditions such as a control versus KO or control diet versus high fat diet where fertility is assessed in both males and females is: 2 mice per mating (1 male x 1 female) x 8 animals per group x 2 conditions x 2 genders (to evaluate both male and female fertility) = total of 64 animals per study. If the male and female fertility study is done in parallel, one set of control x control matings can usually serve to compare both the impact on male and female reproduction. (B) When setting up a fertility assay, the mice used need to have reached sexual maturity. Different strains of mice have different reproductive characteristics and reach sexual maturity at different ages24. For information on rats, see previous publication25. Please click here to view a larger version of this figure.
Discussion
The overall wellbeing of the mice is critical for a successful fertility assay21. When performing a fertility assay, it is important to not physically check on the mice every day as this can cause stress. Further avoid frequent cage changes, as these are also stressful. Ideally cage changes will be done no more than 1-2 times per week. Light exposure during the dark phase negatively impacts breeding in nocturnal rodents. Do not turn on lights in the breeding room during the dark hours. If entry to the room is required during the dark hours, use dim red light illumination. Another stressor is the presence of excessive odors, vibrations or noise in the vivarium for all or parts of the study. To help alleviate stress and improve mouse wellbeing and breeding, give the mice nesting material or other types of enrichment. Nest building is an important behavior in mice and will increase breeding success. The nests also allow the mice a place to hide and play. Healthy and well-nourished animals breed well, therefore it is important to provide ad libitum access to high quality food and water. If breeding pairs start to exhibit symptoms of disease, such as dermatitis (frequent in C57BL/6J), dental malocclusion or other, exclude these mice from the study. In addition, if the study is being done in immunodeficient mice, it is key that the mouse room is kept clean to preserve fertility.
To appropriately set up a fertility study, it is important to decide if the study will be performed in virgin mice or proven breeders as well as carefully select the mice used for control matings. Control matings should be run in parallel with the experimental breeders. This is imperative as unknown or unanticipated stressors and changes in the environment can cause changes in breeding rate. Control matings are preferentially composed of litter mates to the experimental mice (Figure 5A). This is particularly important when the fertility study is done in mice with mixed genetic background due to the great variations in breeding characteristics between mouse strains (Figure 5B).
Identifying a subfertility phenotype can be challenging due to the prolonged length of the study, and the need to analyze additional parameters to reveal its cause. The HET mouse in the representative data is a good example of a modest subfertility phenotype (Figure 4), and the extended analysis required to identify the male subfertility. As shown in the Representative Results (Figure 3 and 4), normal onset of puberty (Figure 3A, D, and F) can be associated with both male and female subfertility (Figure 4A, C, D). In this case, the mice were only sub-fertile, and thus the length of the study was extended to 120 days. This is important, as a shorter fertility study (e.g., 60 days) would not have been able to reveal the phenotype. The male subfertility was modest, and first revealed when HET males were paired with HET females, which also were subfertile (Figure 4A). Indeed, the subfertility of HET males was confirmed when evaluating the average size of litters sired (Figure 4D). Follow up studies confirmed the male subfertility and revealed a poor sperm quality in the HET males, with an ~80 % reduction in motile sperm13.
When performing a fertility and pubertal onset study in mice with a major delay in pubertal onset (>5 days), it is important to consider the different factors which regulate pubertal onset26. Although vaginal opening and first estrus most often happen concomitantly in rats, first estrus in mice usually occurs ~10 days after vaginal opening25,27. Therefore, it is recommended to ensure that both vaginal opening and first estrus are established when working in both rats and mice18,28. Pubertal onset is linked to metabolic status and body weight in females and to a minor degree in males1,7,9,29, and as such, a treatment or genetic mutation slowing animal growth will in some cases result in a delayed pubertal onset. To determine if the delay/advancement in pubertal onset may be associated with rapid/slow growth, it is important to weigh the mice daily during the assessment of pubertal onset. As seen in Figure 3B and C, as well as G and H, the delay in pubertal onset was not associated with reduced body weight, but was a true delay in pubertal onset, as confirmed by gonadal histology and circulating hormone levels22. One of the major limitations of using preputial separation and vaginal opening as markers of pubertal onset is that both will often happen eventually over time due to mechanisms other than increased activity of the reproductive axis18,30,31 (Figure 1). An example of this is the delayed vaginal opening in the cKO females (Figure 3B). The cKO females never become fertile due to an absence of ovulation22 (Figure 3E). Indeed, further studies evaluating hormonal levels and gonadal histology of the cKO mice demonstrated that these mice never completed puberty and were infertile22 (Figure 4B). This shows the limit of using external markers to determine pubertal onset. Completion of puberty and reaching sexual maturity requires increased GnRH release and activation of the reproductive axis (Figure 1), and to fully confirm sexual maturity both gonadal morphology and circulating hormone levels need to be evaluated22,31. In males, measuring testosterone, and evaluating seminal vesicle size and testis morphology and size in addition to sperm quality will confirm puberty was achieved16,22,32. In females, measuring progesterone and estrogen levels in combination with uterine and ovarian weight and morphology will confirm the completion of puberty17,19,22,33.
Litter size can impact both gestation length and age at pubertal onset. Most mouse strains have a gestation period of 18-22 days. However, if the mother is expecting a large litter, the gestation period tends to be reduced34,35. On the other hand, if the mother is nursing a previous litter, this can result in an extension of the gestational period5. Pups in large litters also tend to have delayed pubertal onset. This is caused by their growth being slightly delayed1,9,34,35. If a fertility study is performed and one of the mouse groups (control or experimental) produces very large litters, it is recommended to homogenize litter sizes, also termed culling, allowing the studied litters to have comparable sizes.
Reproduction can only be assessed in vivo due to the multiple organs involved in reproduction (Figure 1) and the extensive feedback mechanisms regulating the reproductive axis3. Certain aspects of reproductive competence can be rapidly evaluated through a vaginal plugging assay, where the capacity of the male to mount the female and leave a physical plug in the vagina is observed13,36. Although this is a useful approach to assess male sexual behavior, this type of study does not allow the detection of minor problems in reproduction, nor does it assess maintained fertility for a long period of time or resulting litter size and parental behavior. The protocol described here presents numerous advantages over a plugging assay, the major being the capacity to identify both minor and major impacts on reproductive competence in males and females (Figure 4).
Fertility decreases with age, and in addition the female estrous cycle becomes longer and irregular during aging5,28. It is therefore recommended to start a fertility study in young and sexually mature animals. A typically used age range to set up a fertility study is 10-16 weeks of age. However, it is important to be familiar with the specific reproductive characteristics of the mouse strain used in the study, as major differences in reproduction and sexual maturity exist between different mouse or rat strains (Figure 5B). Additional information about reproductive characteristics can be found in the JAX Mouse Phenome Database https://phenome.jax.org/. Performing a longer (>4 month) fertility study has the advantage of being able to detect early reproductive decline, where the mice might breed normally for the first 1-2 months of the fertility assay, but then have an early decline in reproduction starting before 6 months of age.
Disclosures
The author has nothing to disclose.
Acknowledgments
I thank the authors contributing to the initial work which is the basis of this publication. Thanks to Aitor Aguirre, Genevieve E. Ryan and Erica L. Schoeller for help preparing the manuscript. Thanks to Jessica Sora Lee and Austin Chin for technical assistance with the manuscript. H.M.H. was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R00HD084759.
References
- Schneider JE. Energy balance and reproduction. Physiology and Behavior. 2004;81(2):289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Frontiers Neuroendocrinology. 2012;32(3):303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Mellon PL. A small population of hypothalamic neurons govern fertility: the critical role of VAX1 in GnRH neuron development and fertility maintenance. Neuroscience communications. 2016;2 [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides. 2009. [DOI] [PMC free article] [PubMed]
- Bronson FH, Dagg CP, Snell GD. Reproduction. New York: Dover Publications, Inc; 1966. [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997. [DOI] [PubMed]
- Yoshimura S, Yamaguchi H, Konno K, Ohsawa N, Noguchi S, Chisaka A. Observation of Preputial Separation is a Useful Tool for Evaluating Endocrine Active Chemicals. J Toxicologic Pathology. 2005;18:141–157. [Google Scholar]
- Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. Journal of Clinical Investigation. 1997;99(3):391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen TM, et al. A short-day photoperiod delays the timing of puberty in female mice via changes in the kisspeptin system. Frontiers in Endocrinology. 2018;9(FEB):1–9. doi: 10.3389/fendo.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences. 2005. [DOI] [PMC free article] [PubMed]
- Hoffmann HM, Mellon PL. A small population of hypothalamic neurons govern fertility: the critical role of VAX1 in GnRH neuron development and fertility maintenance. Neuroscience communications. 2016;2:5–9. [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, et al. Role of Neurokinin B in the Control of Female Puberty and Its Modulation by Metabolic Status. Journal of Neuroscience. 2012;32(7):2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Tamrazian A, Xie H, Pérez-Millán MI, Kauffman AS, Mellon PL. Heterozygous deletion of ventral anterior homeobox (Vax1) causes subfertility in mice. Endocrinology. 2014;155(10):4043–4053. doi: 10.1210/en.2014-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, et al. The Kisspeptin Receptor GPR54 Is Required for Sexual Differentiation of the Brain and Behavior. Journal of Neuroscience. 2007;27(33):8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MG, et al. Brief report: A GPR54-activating mutation in a patient with central precocious puberty. New England Journal of Medicine. 2008. [DOI] [PMC free article] [PubMed]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biology of reproduction. 1977. [DOI] [PubMed]
- Gaytan F, et al. Development and validation of a method for precise dating of female puberty in laboratory rodents: The puberty ovarian maturation score (Pub-Score) Scientific Reports. 2017;7(March):1–11. doi: 10.1038/srep46381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. Assessing reproductive status/stages in mice. Current Protocols in Neuroscience. 2010. pp. 1–11. [DOI] [PMC free article] [PubMed]
- Mayer C, et al. Timing and completion of puberty in female mice depend on estrogen receptor -signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences. 2010;107(52):22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. Journal of Visualized Experiments. 2012. pp. 4–9. [DOI] [PMC free article] [PubMed]
- Hedrich H. The Laboratory Mouse. Academic Press; 2012. [Google Scholar]
- Hoffmann HM, Trang C, Gong P, Kimura I, Pandolfi EC, Mellon PL. Deletion of Vax1 from Gonadotropin-Releasing Hormone (GnRH) Neurons Abolishes GnRH Expression and Leads to Hypogonadism and Infertility. Journal of Neuroscience. 2016;36(12):3506–3518. doi: 10.1523/JNEUROSCI.2723-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS ONE. 2009. [DOI] [PMC free article] [PubMed]
- Manual R. Breeding Strategies for Maintaining Colonies of Laboratory Mice. Management. 2007.
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. The Journal of Physiology. 1963. [DOI] [PMC free article] [PubMed]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biology of reproduction. 1990. [DOI] [PubMed]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biology of reproduction. 1982. [DOI] [PubMed]
- Falconer DS. Weight and age at puberty in female and male mice of strains selected for large and small body size. Genetical Research. 1984. [DOI] [PubMed]
- Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Developmental Biology. 1997. [DOI] [PubMed]
- Lomniczi A, Wright H, Ojeda SR. Epigenetic regulation of female puberty. Frontiers in Neuroendocrinology. 2015;36:90–107. doi: 10.1016/j.yfrne.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmanoff MK, Goldman BD, Ginsburg BE. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology. 1977;100(1):122–127. doi: 10.1210/endo-100-1-122. [DOI] [PubMed] [Google Scholar]
- Larder R, Clark DD, Miller NLG, Mellon PL. Hypothalamic Dysregulation and Infertility in Mice Lacking the Homeodomain Protein Six6. Journal of Neuroscience. 2011;31(2):426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CH, Maltz E, Docherty AH. Milk yield and composition in mice: Effects of litter size and lactation number. Comparative Biochemistry and Physiology -- Part A: Physiology. 1986;84(1):127–133. doi: 10.1016/0300-9629(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Chahoud I, Paumgartten FJR. Influence of litter size on the postnatal growth of rat pups: is there a rationale for litter-size standardization in toxicity studies. Environmental research. 2009;109(8):1021–1027. doi: 10.1016/j.envres.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Pandolfi EC, Hoffmann HM, Schoeller EL, Gorman MR, Mellon PL. Haploinsufficiency of SIX3 Abolishes Male Reproductive Behavior Through Disrupted Olfactory Development, and Impairs Female Fertility Through Disrupted GnRH Neuron Migration. Molecular Neurobiology. 2018. [DOI] [PMC free article] [PubMed]


