Abstract
The gut-blood barrier (GBB) controls the passage of nutrients, bacterial metabolites and drugs from intestinal lumen to the bloodstream. The GBB integrity is disturbed in gastrointestinal, cardiovascular and metabolic diseases, which may result in easier access of biologically active compounds, such as gut bacterial metabolites, to the bloodstream. Thus, the permeability of the GBB may be a marker of both intestinal and extraintestinal diseases. Furthermore, the increased penetration of bacterial metabolites may affect the functioning of the entire organism.
Commonly used methods for studying the GBB permeability are performed ex vivo. The accuracy of those methods is limited, because the functioning of the GBB depends on intestinal blood flow. On the other hand, commonly used in vivo methods may be biased by liver and kidney performance, as those methods are based on evaluation of urine or/and peripheral blood concentrations of exogenous markers. Here, we present a direct measurement of GBB permeability in rats using an in vivo method based on portal blood sampling, which preserves intestinal blood flow and is virtually not affected by the liver and kidney function.
Polyurethane catheters are inserted into the portal vein and inferior vena cava just above the hepatic veins confluence. Blood is sampled at baseline and after administration of a selected marker into a desired part of the gastrointestinal tract. Here, we present several applications of the method including (1) evaluation of the colon permeability to TMA, a gut bacterial metabolite, (2) evaluation of liver clearance of TMA, and (3) evaluation of a gut-portal blood-liver-peripheral blood pathway of gut bacteria-derived short-chain fatty acids. Furthermore, the protocol may also be used for tracking intestinal absorption and liver metabolism of drugs or for measurements of portal blood pressure.
Keywords: Retraction, Issue 140, Gut-blood barrier, intestinal permeability, liver clearance, portal blood sampling, gut bacteria, TMA, short chain fatty acids
Introduction
The gut-blood barrier (GBB), also known as the intestinal barrier, is a complex multilayer system that separates the gut lumen from the bloodstream in order to limit the passage of harmful compounds while allowing the absorption of nutrients1. It consists of the three main layers: the mucus layer, epithelium and lamina propria.
Numerous factors may affect the GBB integrity and function2. It has been shown that GBB is disturbed in both gastrointestinal and extraintestinal diseases, including cardiovascular and metabolic diseases3, which may lead to an increased passage of gut bacterial metabolites to the bloodstream4. An increased penetration of gut bacterial metabolites may affect the functioning of the entire organism. For example, recent studies show a significant impact of bacterial metabolites, such as indoles, H2S, short-chain fatty acids (SCFA), and trimethylamine N-oxide, on the circulatory system functions5,6,7,8,9. Finally, it has been proposed that an increased GBB permeability may serve as a marker of cardiovascular and metabolic diseases which are associated with morphological and functional alterations in the intestines10. Therefore, tracking the gut-portal blood-liver-systemic blood pathway of bacterial metabolites may be of interest for both basic and clinical sciences.
Commonly utilized experimental methods for the evaluation of GBB permeability are performed in vitro using resected intestinal segments, fragments of mucosa, or artificial membranes11,12. The accuracy of those methods is compromised by the fact that proper functioning of the GBB requires constant intestinal blood flow. On the other hand, the available in vivo methods are based on the evaluation of urine or peripheral blood concentrations of exogenous markers13. However, peripheral blood and urine concentration of exogenous compounds is influenced by kidney function, i.e., glomerular filtration rate and tubular excretion, as well as by liver metabolism, i.e., first pass metabolism. Both parameters may differ significantly between study subjects independently of the GBB function.
This paper describes a direct measurement of the GBB permeability in rats using portal blood sampling. This in vivo method preserves the intestinal blood flow and is virtually not influenced by liver and kidney function. The described approach is not commonly used, possibly because of some methodological difficulties. We describe in detail the catheterization of the portal vein and inferior vena cava just above the hepatic vein confluence. Blood sampling from the portal vein and inferior vena cava allows evaluation of the GBB permeability and liver clearance as well as tracking of gut-portal blood-liver-systemic blood pathway of molecules of interest, such as gut bacterial metabolites or medicines. We also present several applications of the method that were tested in our laboratory. These include the evaluation of the colon permeability to TMA, a gut bacterial metabolite, evaluation of liver clearance of TMA, and evaluation of a gut-portal blood-liver-systemic blood pathway of SCFA.
To evaluate gut-blood barrier permeability, the following protocol steps should be followed, in order: 1 (insertion of the line for intraintestinal administrations), 3 (portal vein catheterization), 4 (portal vein blood sampling), 6 (administration of a gut permeability marker), 4.
To evaluate liver clearance and a gut-portal blood-liver-systemic blood pathway, the following protocol steps should be followed, in order: 1 (insertion of the line for intraintestinal administrations), 2 (inferior vena cava catheterization), 3 (portal vein catheterization), 4 (portal vein blood sampling), 5 (inferior vena cava blood sampling), 6 (administration of a gut permeability marker), 4, 5, 7 (calculation of liver clearance).
Protocol
The experiments were performed on male Wistar Kyoto rats according to Directive 2010/63 EU on the protection of animals used for scientific purposes and were approved by the I Local Bioethical Committee in Warsaw.
1. Insertion of the Line for Intraintestinal Administration
NOTE: Here we propose intracolonic administration of a marker using a catheter. It may be modified by oral administration or gavage at various levels of the digestive tract e.g. stomach or duodenum. Remember to use disposable surgical clothing, including surgical gown, hood and gloves, and ensure to follow the safety precautions related to the sharp tools used in surgery (needles, etc.) during procedures 1-6.
Fast animals overnight before the procedure. Perform all procedures during general anesthesia, i.e., obtained by injection of urethane 1.5 g/kg bw i.p. Assess proper anesthetization by the lack of palpebral and corneal reflexes, and by toe-pinch and tail-pinch method.
Use a pediatric Foley catheter (10F or 8F) as a colonic catheter. Mark the catheter to indicate the part that will be inserted into the colon (approximately 8 cm).
Check the anal region and the stool content in the rectum before inserting the catheter into the colon. If stool is present, empty the rectum by massaging the rectal area.
Put a lubricant (e.g. glycerin or petrolatum) along the catheter. Moisten the anus and its surroundings with the lubricant.
Insert the catheter with a guide wire approximately 8 cm through the external anal sphincter. Make slow forward-backward and circular movements. NOTE: Keep on checking the location of the catheter by abdominal palpation while inserting the catheter.
2. Inferior Vena Cava Catheterization
Shave fur in the groin. Alternately disinfect the skin with alcohol and povidone iodine 3 times and cover the groin area with surgical drapes.
Try to feel the pulse on the femoral artery and cut the skin longitudinally for the length of about 2.0 cm in the place where the pulse is palpable.
Dissect the fascia and muscles to visualize the neurovascular bundle.
Dissect the femoral vein from the neurovascular bundle: first nerves, then the femoral artery, and then the vein. NOTE: Be careful during the dissection of the neurovascular bundle, since tiny branches of the femoral vein may easily be damaged, producing bleeding.
Put two ligatures on the femoral vein. Do not tie the knots yet. Catch the ends of the proximal ligature with a needle holder.
Carefully pull the ligature ends with the holder upwards to close the proximal part of the vein. Wait until the vein is filled with blood and tie the distal knot.
Make a small incision (ca. 1 mm) on the vein between the knot and proximal ligature, using microsurgical scissors. Insert the catheter using tweezers or the needle with the curved end. NOTE: Puncture the vein and use the bended tip of the needle as a guide for the catheter. Loosen the proximal ligature while inserting the catheter. Insert the catheter for 6-7.0 cm.
Secure the catheter in the femoral vein with two single surgical knots. Tie the proximal ligature as well.
Check the patency of the catheter by attempting to draw blood with a syringe. Rinse the catheter with 0.3 mL of the heparinized saline (100 units/mL).
Close the surgical wound with two layers of single stitches.
3. Portal Vein Catheterization
Figure 1: Portal catheter. The portal catheter consists of a needle OD: 0.9 mm with a length of about 25.0 mm [A], a flexible polyurethane catheter OD: 0.025", length about 100.0 mm [B], a flexible polyethylene tip of the catheter OD: 0.040", approximately 15.0 mm long [C], a plug [D], and a ligature 3/0 with a length of 100.0 mm [E]. Please click here to view a larger version of this figure.
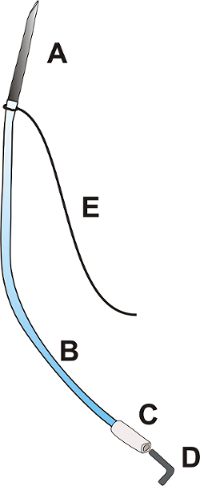
- Prepare the portal catheter according to Figure 1.
- Insert the cut end of the needle (OD: 9 mm) into the polyurethane catheter OD: 0.025".
- Tie the ligature 3/0 at the junction of the needle and catheter. NOTE: Ensure that the longer part of the ligature is at least 6 cm long.
- Insert the end of the catheter OD: 0.025" into the polyethylene catheter OD: 0.040".
- Close the catheter with a metal or plastic plug.
- Midline laparotomy
- Shave fur in the abdomen, alternately disinfect the skin with alcohol and povidone iodine 3 times, and cover the area with surgical drapes.
- Cut the skin longitudinally from the xiphoid of the sternum to the navel.
- Cut the muscles of the abdominal wall along the white line.
- Expand the cut rostrally in the Y shape so that the xiphoid cartilage is between two cuts.
- Portal vein dissection
- Moisten the surgical swabs with saline.
- Exteriorize the cecum, ascending and transverse colon, and small intestine loop. Put the intestines on the left side to expose the root of the mesentery. NOTE: Cover the intestines with gauze moistened with a physiological saline to protect the intestines from drying.
- To expose the portal vein, carefully move the hepatic lobes to the sides or upwards towards the diaphragm with the moistened swabs.
- Localize the part of the portal vein that is not covered with the mesentery (in the hepatic hilum, about 5 mm long) and pass the ligature 3/0 (15 cm long) under the portal vein. NOTE: To protect tissues from damage while placing the ligature, moisten the ligature with a physiological saline solution.
- Clamp the ends of ligature with forceps and tighten it gently to stabilize the vessel.
- Insertion and stabilization of the catheter
- Pass the longer part of the portal catheter's ligature under the free part of the portal vein and pull it so that the catheter is located just next to the portal vein.
- Insert the needle into the upper mesenteric vein 3 mm below the junction of upper mesenteric vein and the portal vein. Hold the needle at a 30° angle and, after entering into the vein, reduce the angle and advance the needle almost horizontally, in parallel to the portal vein. NOTE: Insert the needle for a length of approximately 6-7 mm. The stabilizing ligature should gently tighten the portal vein while inserting the catheter.
- Apply 1-2 drops of tissue glue at the place where the needle is inserted. Remove the swabs that cover the liver.
- Put the intestines back into the abdominal cavity.
- Moisten the intestines with a warmed up saline solution and cover it with moistened sterile gauze.
- Check the patency of the catheter and rinse the catheter with 0.3 mL of the heparinized saline (100 units/mL). NOTE: Venous blood spontaneously backflows in the catheter.
- Ending of the surgery
- After 5 minutes, check the color of the intestines and peristaltic movements, make sure that the proper mesenteric blood flow is maintained.
- Close the abdominal cavity with 3 stitches: wall peritoneum with the inner layer of the abdominal wall muscles - a continuous, absorbable suture; remaining muscles of the abdominal wall - a continuous, absorbable suture; skin and subcutaneous tissue - single, non-absorbable sutures. NOTE: Exteriorize the distal part of the catheter around the navel.
4. Portal Vein Blood Sampling
Sample portal vein blood at times according to the specific testing protocol used; see Table 1.
| Short protocol | Long protocol |
| t0 – baseline (before intracolonic administration) | t0 – baseline (before intracolonic administration) |
| t1 – 5 min after intracolonic administration | t1 – 30 min after intracolonic administration |
| t2 – 30 min after intracolonic administration | t2 – 60 min after intracolonic administration |
Table 1: Portal blood sampling protocols for gut permeability assessment.
NOTE: The time between consecutive blood sampling depends mainly on the bioavailability of the tested substances and the site of administration (colon, stomach, etc.).
Open the portal catheter plug and let the blood flow freely.
Use syringe (vol. 2 mL) and blunt needle OD: 0.9 mm. Collect no more than 0.7 mL of blood.
Rinse the catheter with 0.3 mL of heparinized saline (100 units/mL) and close the catheter plug.
5. Inferior Vena Cava Blood Sampling
Sample inferior vena cava blood at times according to the specific testing protocol used; see Table 2.
| Portal vein | Inferior vena cava |
| t0 – baseline (before intracolonic administration) | t0 – baseline (before intracolonic administration) |
| t1 – 30 min after intracolonic administration | t1 – 30 min after intracolonic administration |
Table 2: Protocol of blood sampling for liver clearance measurement and tracking the gut-portal blood-liver-systemic blood pathway.
Open the inferior vena cava catheter plug and let the blood flow freely.
Collect no more than 0.7 mL of blood using syringe (vol. 2 mL) and broken needle OD: 0.9mm.
Rinse the catheter with 0.2-0.3 mL of heparinized saline (100 units/mL) and close the catheter plug.
6. Administration of a Gut Permeability Marker
Remove the guide wire and inflate the colonic catheter balloon, using adequate volume of sterile water (usually 1 mL but check actual balloon size before insertion). NOTE: The balloon diameter should not exceed 1 cm.
Place the rat head down (inclination about 15%) to minimize the risk of the outflow of the administered solution from the colon.
Slowly administer the tested substance (e.g. trimethylamine, 100 mg/kg bw) using a drainage port in colonic catheter. NOTE: Do not exceed the volume of 0.75 mL of the administered solution and the feeding speed of 0.5 mL/min to prevent the outflow of the administered solution from the anus.
After 10 min deflate the catheter balloon.
Sample blood from the inferior vena cava and the portal vein according to the specific testing protocol used; see Table 1 and Table 2.
Euthanize animal via approved method.
7. Calculation of Liver Clearance
Express liver clearance, understood as hepatic extraction, by the difference between portal blood concentration and inferior vena cava blood concentration or by the ratio of inferior vena cava to portal blood concentration, (1 - (inferior vena cava concentration/portal vein concentration)).
8. Evaluation of the Test Substance Concentration n Blood Samples
Depending on the test substance and test methodology, subject the sample to appropriate laboratory procedures (centrifugation, etc.). In the proposed protocols, we evaluate TMA/TMAO and SCFA concentration using liquid chromatography coupled with triple-quadrupole mass spectrometry. Please find a detailed description of the method in Supplemental Material.
Representative Results
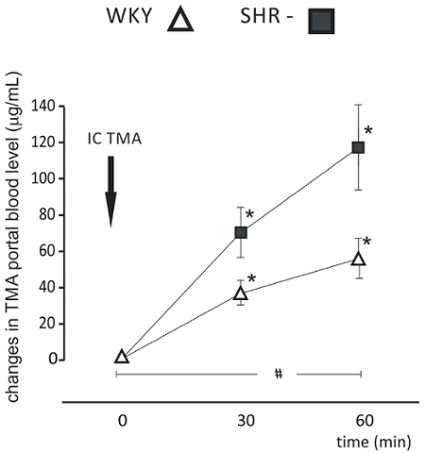
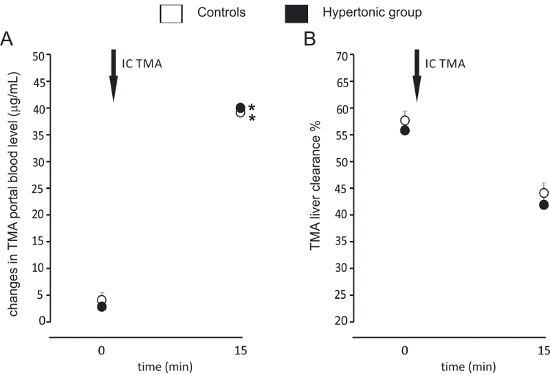
We have successfully measured the GBB permeability and liver clearance of TMA in rats. We have demonstrated that hypertensive rats have an increased colon permeability to TMA in comparison to normotensive rats (Figure 2)4. In another study we found that high salt intake does not affect the GBB permeability and liver clearance of TMA (Figure 3)14.
Measuring the concentration of SCFA in stools, portal blood, and peripheral blood, we traced the path of the molecules from the intestine to the peripheral blood. The exemplary results for those experiments are presented in Table 3.
Figure 2: Hypertension-associated changes in gut-blood barrier permeability. Intracolonic administration of TMA produced a significant increase in portal blood TMA in each group (n=12 for each group). The increase in portal blood TMA in the hypertensive (SHR) group was significantly higher than in normotensive (WKY) group. We used the long protocol consisting of blood sampling 30 min and 60 min after TMA administration (IC TMA). Values are means, + SE, *p < 0.05 vs baseline, #p < 0.05 WKY vs SHR. This figure has been modified from Jaworska et al.4 Please click here to view a larger version of this figure.
Figure 3:Gut-blood barrier permeability and liver clearance after high salt intake. (A) Intracolonic administration of TMA produced a significant increase in portal blood TMA. The size of the increase was similar between the groups (n=7 for each group). We used a simplified protocol, taking blood samples at baseline (0) and 15 min after administration of TMA (IC TMA). (B) TMA liver clearance was similar between the groups at baseline, and 15 min after the intracolonic administration of TMA. Values are means, + SE. *p < 0.05 vs baseline. This figure has been modified from Bielinska et al.14 Please click here to view a larger version of this figure.
| SCFA | Stool concentration (µM) | Portal blood concentration (µM) | Peripheral blood concentration (µM) |
| AA- acetic acid (C2) | 15998.40 ± 4317.58 | 564.22 ± 155.34 | 149.89 ± 31.74 |
| IPA- propionic acid (C3) | 5390.70 ± 1016.19 | 138.25 ± 55.50 | 5.36 ± 3.25 |
| IBA- isobutyric acid (C4) | 191.20 ± 123.87 | 4.51 ± 1.60 | 1.14 ± 1.16 |
| BA- butyric acid (C4) | 4159.80 ± 3141.68 | 143.14 ± 68.42 | 6.43 ± 4.18 |
| 2MeB- 2 methylbutyric acid (C5) | 80.90 ± 59.86 | 2.02 ± 0.88 | 1.14 ± 1.42 |
| IVA- isovaleric acid (C5) | 109.10 ± 56.05 | 2.59 ± 1.07 | 0.90 ± 1.22 |
| VA- valeric acid (C5) | 281.9 ± 158.20 | 8.55 ± 3.56 | 0.72 ± 1.02 |
| ICA- isocaproic acid/ 4-methylvaleric acid (C6) | 5.9 ± 2.95 | 0.61 ± 0.15 | 1.76 ± 0.87 |
| CA- caproic acid (C6) | 287.00 ± 309.68 | 11.19 ± 4.94 | 1.12 ± 0.93 |
Table 3: SCFA concentration in stool, portal blood, and peripheral blood (n=7).
| Test substance | Possible application |
| Bacterial metabolites: trimethylamine (TMA), short chain fatty acids (SCFA), hydrogen sulfide, etc. | GBB permeability studies Tracking a gut-portal blood-liver-systemic blood pathway Hepatic clearance studies |
| Classic permeability markers: FITC-dextran, polysaccharides, PEG, etc. | GBB permeability studies |
| Drugs | absorption and hepatic clearance studies |
Table 4: Exemplary test substances with possible applications.
Discussion
The described direct, in vivo, method of measuring the GBB permeability maintains closetophysiological conditions in the gastrointestinal system (preserves the intestinal blood flow), and is virtually not influenced by liver and kidney function.
The critical step of this technique is the insertion of the portal catheter. This must be done gently and decisively at the same time. A mild, short bleeding may occur from the correctly performed puncture of the portal vein; however, it stops when the needle is inserted into the vessel. Persistent bleeding indicates that the portal vein is perforated. To facilitate the catheter insertion, the portal vein should be well exposed. After exteriorizing the intestines, when the mesenteric root is well exposed, the upper mesenteric vein should also be visible (mesenteric vein enters cranially into the portal vein). The portal vein is usually covered by the hepatic lobes, which have to be moved to the sides. Also, the proper stabilization of the portal catheter is crucial for a successful procedure, since the catheter's movement may produce portal vein rupture and bleeding, especially in longer experiments. Additional stabilization of the catheter may be achieved by attaching the catheter to mesentery by sticking it to a mesentery with tissue glue or by applying two single stitches (thread 6/0). After closing the abdominal cavity to secure the placement of the catheter, a purse-string suture may be applied on the catheter.
There are several minor difficulties that may occur during the experiment. After catheterization of the femoral vein, if the venous blood does not backflow in the catheter, try the following solutions: flush the catheter with heparinized saline, gently pull the catheter 1-2 mm from the vein, remove the surgical knots, and tie a new one, pull the catheter out and reinsert, or replace with a new catheter. Remember to confirm the proper placement of the catheter after the experiment. The catheter should be inserted for 6-7 cm, depending on the size of the animal, to place the proximal tip of the catheter in the inferior vena cava just above the hepatic vein confluence. When it comes to colon catheterization, if you have problems with advancing the catheter you may inject 0.3-0.5 mL of saline or leave the catheter in the colon for 5-10 minutes, and try again. Do not use force while inserting a catheter to avoid perforation of the intestine.
In our studies, we used a gut bacteria-derived molecule, trimethylamine (TMA), as a marker of the colon GBB permeability, as TMA is produced mostly by colonic bacteria. However, many other substances, including classic permeability markers like FITC-dextran or sugars, may be used as well (see Table 4). When preparing a solution of the test substance, take into account its irritating effect on the intestinal mucosa and appropriately choose the concentration of the substance. Further laboratory procedures of the blood samples must be adjusted to the selected marker.
In our protocol, we propose intracolonic administration of a marker; however, it may be modified by oral administration or gavage at various levels of the digestive tract. The variable speed of peristalsis and possible interactions with enzymes and gastric acid should be taken into account while administering a marker into upper parts of the gastrointestinal tract e.g. stomach or duodenum. Accordingly, time of blood sampling after administration of a marker needs to be adjusted.
There are several limitations of the presented method, including adverse effects of anesthesia and fasting overnight, that may both influence GBB function. It should be taken into account as the procedure is terminal and involves blood sampling during not fully physiological conditions. However, as mentioned before, it has still many advantages over other experimental methods assessing GBB permeability, especially performed in vitro11. For example, an Ussing chamber measures the conductance and particle flux through the intestinal epithelial cells. The main weakness of this technique lies in its excessive simplification. It is difficult to describe the complex physiological system of the intestinal mucosa using a small number of measurements on epithelial cell layer alone. Some researchers use whole-thickness intestine for Ussing chamber studies, but this procedure is accompanied by several methodological complications15. Furthermore, the accuracy of the method is compromised by a limited viability of tissues isolated from the organism. Some in vitro methods used in pharmacokinetic studies use artificial membranes as a model of the intestinal barrier12. However, those methods, similarly to the Ussing chamber, do not reflect the complexity of the GBB structure and functions.
There are also in vivo permeability assays available in experimental and clinical studies. They are mostly based on urine or peripheral blood sampling after oral or colonic administration of various markers13. The widely used sugar test involves oral intake of mono- and oligosaccharides, which are not metabolized in mammalian organism, e.g. mannitol and lactulose. The method is non-invasive and may be employed in both experimental and clinical use16,17; however, the results are affected by first-pass liver metabolism and kidney function, which may differ significantly between the study subjects. In contrast to the above mentioned indirect methods, collecting blood from the portal vein allows direct evaluation of the GBB permeability12. This method is not dependent on liver and kidney function and virtually preserves physiological conditions in the intestines which is an important advantage over ex vivo or in vitro methods.
The techniques described in this paper also allow for a relatively accurate liver clearance evaluation, as the blood is collected from the portal vein and inferior vena cava just above the hepatic vein confluence. The representative results for the hepatic extraction are presented in Figure 3 (for TMA) and Table 3 (for SCFA). Our data suggest that three main SCFA, acetate, propionate, and butyrate, are characterized by different hepatic clearance, which is supported by previous studies, where Bloemen et al. shown that intestinal release of butyrate and propionate, but not acetate, is almost equaled by hepatic uptake18. Therefore, the presented protocol is suitable for tracking intestinal absorption and liver metabolism of drugs, which can be used in pharmacokinetic studies.
The techniques may also be adjusted to other experimental purposes. Catheterization of the portal vein may be used to measure portal blood pressure or for administration of drugs directly to the portal vein, in order to study hepatic circulation. For instance, in our previous work, we administered hydrogen sulfide donors to the portal vein to assess its influence on hepatic circulation and portal pressure19.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The work is supported by the Ministry of Science and Higher Education Republic of Poland, Diamond grant no: DI2017 009247.
References
- Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2012;24(6):503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2010;22(7):718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. Journal of Gastroenterology and Hepatology. 2003;18(5):479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- Jaworska K, et al. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PloS one. 2017;12(12):e0189310. doi: 10.1371/journal.pone.0189310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Therapeutic Apheresis and Dialysis: Official Peer-Reviewed Journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2011;15(2):125–128. doi: 10.1111/j.1744-9987.2010.00883.x. [DOI] [PubMed] [Google Scholar]
- Tomasova L, et al. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide: Biology and Chemistry. 2016;60:50–58. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2013;14(12):950–959. doi: 10.1111/obr.12068. [DOI] [PubMed] [Google Scholar]
- Ufnal M, et al. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. The Canadian Journal of Cardiology. 2014;30(12):1700–1705. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Huc T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacological Research. 2018;130:172–179. doi: 10.1016/j.phrs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Ufnal M, Pham K. The gut-blood barrier permeability - A new marker in cardiovascular and metabolic diseases? Medical Hypotheses. 2017;98:35–37. doi: 10.1016/j.mehy.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Le Ferrec E, et al. In vitro models of the intestinal barrier. The report and recommendations of ECVAM Workshop 46. European Centre for the Validation of Alternative methods. Alternatives to Laboratory Animals: ATLA. 2001;29(6):649–668. doi: 10.1177/026119290102900604. [DOI] [PubMed] [Google Scholar]
- Bohets H, et al. Strategies for absorption screening in drug discovery and development. Current Topics in Medicinal Chemistry. 2001;1(5):367–383. doi: 10.2174/1568026013394886. [DOI] [PubMed] [Google Scholar]
- Grootjans J, et al. Non-invasive assessment of barrier integrity and function of the human gut. World Journal of Gastrointestinal Surgery. 2010;2(3):61–69. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska K, et al. High salt intake increases plasma trimethylamine N-oxide (TMAO) concentration and produces gut dysbiosis in rats. Nutrition. 2018;54:33–39. doi: 10.1016/j.nut.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Clarke LL. A guide to Ussing chamber studies of mouse intestine. American journal of physiology. Gastrointestinal and Liver Physiology. 2009;296(6):G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denno DM, et al. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2014;59 Suppl 4:S213–S219. doi: 10.1093/cid/ciu541. [DOI] [PubMed] [Google Scholar]
- Lugea A, Salas A, Casalot J, Guarner F, Malagelada JR. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut. 2000;46(4):515–521. doi: 10.1136/gut.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen JG, et al. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clinical Nutrition. 2009;28(6):657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Huc T, et al. Colonic hydrogen sulfide produces portal hypertension and systemic hypotension in rats. Experimental Biology and Medicine. 2018;243(1):96–106. doi: 10.1177/1535370217741869. [DOI] [PMC free article] [PubMed] [Google Scholar]


