Abstract
The acute respiratory distress syndrome is a relevant intensive care disease with an incidence ranging between 2.2% and 19% of intensive care unit patients. Despite treatment advances over the last decades, ARDS patients still suffer mortality rates between 35 and 40%. There is still a need for further research to improve the outcome of patients suffering from ARDS. One problem is that no single animal model can mimic the complex pathomechanism of the acute respiratory distress syndrome, but several models exist to study different parts of it. Oleic acid injection (OAI)-induced lung injury is a well-established model for studying ventilation strategies, lung mechanics and ventilation/perfusion distribution in animals. OAI leads to severely impaired gas exchange, deterioration of lung mechanics and disruption of the alveolo-capillary barrier. The disadvantage of this model is the controversial mechanistic relevance of this model and the necessity for central venous access, which is challenging especially in smaller animal models. In summary, OAI-induced lung injury leads to reproducible results in small and large animals and hence represents a well-suited model for studying ARDS. Nevertheless, further research is necessary to find a model that mimics all parts of ARDS and lacks the problems associated with the different models existing today.
Keywords: Medicine, Issue 140, ARDS, lung injury, oleic acid, pig, animal model, method
Introduction
The acute respiratory distress syndrome (ARDS) is an intensive care syndrome that has been extensively studied since its first description about 50 years ago1. This body of research led to a better understanding of the pathophysiology and causes the development of ARDS resulting in improved patient care and outcome2,3. Nevertheless, the mortality in patients suffering from ARDS remains very high with about 35-40%4,5,6. The fact that about 10% of ICU admissions and 23% of ICU patients who require mechanical ventilation is due to ARDS underscores the relevance for further research in this field.
Animal models are widely used in research to examine pathophysiologic changes and potential treatment modalities for different kinds of diseases. Due to the complexity of ARDS, there is no single animal model to mimic this disease, but different models representing different aspects7. One well-established model is oleic acid injection (OAI)-induced lung injury. This model has been used in a wide array of animals, including mice8, rats9, pigs10, dogs11, and sheep12. Oleic acid is an unsaturated fatty acid and the most common fatty acid in the body of healthy humans13. It is present in human plasma, cell membranes, and adipose tissue13. Physiologically, it is bound to albumin while it is carried through the bloodstream13. Increased levels of fatty acids in the blood stream are associated with different pathologies and the severity of some diseases correlates with serum fatty acid levels13. The oleic acid ARDS-model was developed in an attempt to reproduce ARDS caused by lipid embolism as seen in trauma patients14. Oleic acid has direct effects on innate immune receptors in the lungs13 and triggers neutrophil accumulation15, inflammatory mediator production16, and cell death13. Physiologically, oleic acid induces rapidly progressing hypoxemia, increase in pulmonary arterial pressure and accumulation of extravascular lung water. Furthermore, it induces arterial hypotension and myocardial depression7. The disadvantages of this model are the necessity for central venous access, the questionable mechanistic relevance and the potential lethal progress caused by rapid hypoxemia and cardiac depression. The advantage of this model compared to other models is the usability in small and large animals, the valid reproducibility of the pathophysiological mechanisms in ARDS, the acute onset of ARDS after injection of oleic acid, and the possibility to study isolated ARDS without systemic inflammation like in many other sepsis models7. In the following article, we give a detailed description of the oleic acid-induced lung injury in pigs and provide representative data to characterize the stability of the compromises in lung function. There are different protocols for OAI-induced lung injury. The protocol provided here is able to reliably induce acute lung injury.
Protocol
All animal experiments described here have been approved by the institutional and state animal care committee (Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany; approval number G14-1-077) and were conducted in accordance with the guidelines of the European and German Society of Laboratory Animal Sciences. The experiments were conducted in anesthetized male pigs (sus scrofa domestica) of 2-3 months age, weighing 27-29 kg.
1. Anesthesia, Intubation and Mechanical Ventilation
Withhold food for 6 h before anesthesia to reduce the risk of aspiration but allow free access to water to reduce stress.
For sedation, inject a combination of Ketamine (4 mg kg-1) and Azaperone (8 mg kg-1) in the neck or the gluteal muscle of the pig with a needle for intramuscular injection (20 G) while the animal is in the animal box. Caution: Use gloves when working with the animal.
Insert peripheral vein catheter (20 G) in an ear vein after local disinfection with alcohol.
Inject fentanyl (4 µg kg-1), propofol (3 mg kg-1) and atracurium (0.5 mg kg-1) intravenously for the induction of anesthesia.
When the pig stops breathing, place it in supine position on the stretcher and immobilize it with bandages.
Start monitoring the peripheral oxygen saturation (SpO2) by clipping the sensor on to one of the ears or the tail of the animal.
Ventilate the pig with a mask for ventilating dogs, size 2, with a peak inspiratory pressure below 20 cm H2O, a positive end expiratory pressure (PEEP) of 5 cm H2O, a respiratory rate of 14-16 /min and an inspiratory oxygen fraction (FiO2) of 1.0.
Start a continuous infusion with balanced electrolyte solution (5 mLkg-1 h-1), propofol (8-12 mg kg-1 h-1) and fentanyl (0.1-0.2 mg kg-1 h-1) to maintain anesthesia.
For the intubation, prepare a common endotracheal tube suitable for the animal (e.g., weight of 25-30 kg, endotracheal tube internal diameter (ID) 6-7mm) armed with endotracheal tube introducer and a common laryngoscope with a Macintosh Blade 4. NOTE: Two people are necessary for the intubation.
Person 1: Pull out the tongue with one hand and press the snout dorsally with the other.
Person 2: Insert laryngoscope and advance it as usual until the epiglottis comes into view.
Pull the laryngoscope ventrally to visualize the vocal cords. NOTE: Sometimes the epiglottis "sticks" to the soft palatine. In this case, mobilize it with the tip of the tube.
Insert the tube through the vocal cords and pull out the introducer.
Block the cuff of the tube with a syringe with 10 mL of air.
Connect the tube to the ventilator.
Check for the correct positioning of the tube by regular exhalation of carbon dioxide (CO2) with capnography and equal ventilation of both lungs with auscultation.
Start mechanical ventilation (tidal volume 6-8 mL/kg, positive PEEP 5 cm H20, FiO2 to keep peripheral oxygen saturation (SpO2) between 94 – 98%17, respiratory rate to keep end tidal pressure of carbon dioxide (etCO2) between 35 – 45 mmHG).
2. Instrumentation
Retract the hindlegs with bandages to stretch the skin above the femoral area for catheterizing necessary vessels.
Prepare a 5 mL syringe, a 10 mL syringe, a Seldinger's needle, 3 introducer sheaths (5 Fr, 6 Fr, 8 Fr) with guidewires, a central venous catheter with 3 ports (7 Fr, 30 cm) with guidewire and a pulmonary artery catheter (7,5 Fr, 110 cm).
Generously disinfect the femoral area with skin disinfectant applying a wipe down technique.
Completely fill the catheters with saline.
Place the ultrasound-probe on the right inguinal ligament and scan for femoral vessels.
Turn the probe 90° to fully visualize the femoral artery in the long axis.
Cannulate right femoral artery under in-line ultrasound visualization with the Seldinger's needle. NOTE: There are different ways to gain vascular access with or without ultrasound. Ultrasound-guided vascular cannulation is not necessary for this model.
When pulsating bright blood flows out, introduce the guidance wire and retract the needle.
Visualize the femoral vein and cannulate the vein under in-line ultrasound visualization and continuous aspiration with the needle.
When venous blood is aspirable, disconnect the syringe and insert the guidance wire.
Retract the needle.
Check the position of the wires with ultrasound.
Insert the arterial introducer sheath (5 Fr) and central venous catheter using Seldinger's technique (for details on Seldinger's technique, refer to published method18).
Repeat the arterial and venous puncture on other side and insert the introducer sheaths using Seldinger´s technique as described above (artery 6 Fr, vein 8 Fr).
Connect the arterial introducer sheath and central venous catheter to a transducer system suitable to the monitoring equipment.
Calibrate the invasive monitoring against atmosphere (zero) by opening the three-way-stopcocks to the atmosphere and press Zero all on the monitor.
Turn the three-way-stopcocks back to measure hemodynamics.
Start monitoring hemodynamics.
Place all pressure transducers at the height of the right atrium.
Switch the infusion of propofol (8-12 mg kg-1 h-1) and fentanyl (0.1-0.2 mg kg-1 h-1) to one of the ports of the central venous line to maintain anesthesia.
3. Ultrafast Measurement of Partial Oxygen Pressure (pO2)
NOTE: The measurement of pO2 with the probe for ultrafast pO2-measurement is not obligatory but helps visualizing the real-time changes in pO2.
Open software NeoFox viewer and click Options.
Choose the Calibration tab and click the Open Calibration button.
Choose calibration file and click Open and Download.
Confirm the pop-up window by clicking Yes.
Open the Options dialogue.
Choose the Calibration tab and click Single point calibration.
Enter 21% in the field Oxygen and the temperature in the field Temperature.
Click Use current Tau and Download. Afterwards, confirm the pop-up window by clicking Yes.
Insert the probe for ultrafast measurements of pO2 through the left arterial introducer sheath.
4. INSERTING PULMONARY ARTERY CATHETER
Check the balloon of pulmonary artery catheter for damage.
Connect to the transducer system suitable to the monitoring equipment.
Calibrate the pulmonary arterial pressure monitoring against the atmosphere (zero) by opening the three-way-stopcock to the atmosphere and press Zero on the monitor.
Turn the three-way-stopcock back to measure pulmonary arterial pressure.
Start monitoring the pulmonary arterial pressure.
Insert the pulmonary artery catheter through the left venous introducer sheath (balloon deflated).
When the pulmonary artery catheter has passed through the introducer sheath, inflate the balloon with 1 mL of air.
Advance the pulmonary artery catheter and monitor the typical waveforms (venous vessels, right atrium, right ventricle, pulmonary arteria, and pulmonary capillary wedge pressure). Deflate the balloon and check, if it is possible to aspirate blood through all ports of the pulmonary artery catheter. NOTE: For detailed instruction on how to insert pulmonary artery catheter, refer to previous publication19.
5. Induction of Lung Injury
Prepare oleic-acid solution: 0.1 mL kg-1 of oleic acid in a 20 mL syringe and connect it to a 3-way-stopcock.
Take 2 mL of blood in another 20 mL syringe and add saline to a total volume of 20 mL in both syringes.
Connect the second syringe also to the 3-way-stopcock. CAUTION: Use gloves and eye protection when working with oleic acid.
Prepare norepinephrine (0.1 mg/mL) for continuous infusion and for bolus injection (10 µg/mL).
Connect the norepinephrine syringe pump to one of the ports of the central venous catheter without starting it.
Start the ultrafast pO2-measurement.
Before the induction of lung injury, record the values (baseline) from all relevant parameters.
Set the FiO2 to 1.0 and conduct a lung recruitment maneuver (plateau pressure 40 cm H2O for 10 s).
Connect the 3-way-stopcock to the proximal port of the pulmonary artery catheter.
Mix the oleic acid and the blood/saline mixture thoroughly by injecting it repetitively from one syringe into the other via the 3-way-stopcock and keep mixing all the time.
When it is a homogenous emulsion, inject 2 mL of the emulsion and continue mixing. NOTE: If mixing is stopped, the emulsion may separate into a lipophilic and a hydrophilic part.
Closely monitor the hemodynamics after the injection of oleic acid and keep the norepinephrine at hand. If necessary, give norepinephrine as bolus injection (10 – 100 µg) or continuous infusion to keep mean arterial pressure above 60 mmHg.
Repeat the injection of 2 mL of the solution every 3 min until the arterial partial pressure of oxygen (PaO2)/FiO2-ratio is below 200 mmHg.
If the syringe is empty before the PaO2/FiO2-ratio is between 100 and 200 mmHg, prepare 2 more syringes as described in step 5.1.
Wait 30 min and re-evaluate the PaO2/FiO2-ratio. If it is still over 200 mmHg, repeat steps 5.5-5.8 until PaO2/FiO2-ratio falls between 100 and 200 mmHg.
If PaO2/FiO2-ratio is between 100 and 200 mmHg, wait for 30 min and check again.
If it is persistent below 200 mmHg start experiment/treatment, otherwise prepare 2 more syringes as described in step 5.1 and repeat steps 5.5-5.9.
Set the ventilation according to the suggestions from the ARDS network20.
6. End of Experiment and Euthanasia
Inject 0.5 mg of fentanyl additionally to the continuous anesthesia and wait for 5 min. Inject 200 mg of propofol and 40 mmol of potassium chloride to euthanize the animal in deep anesthesia.
Representative Results
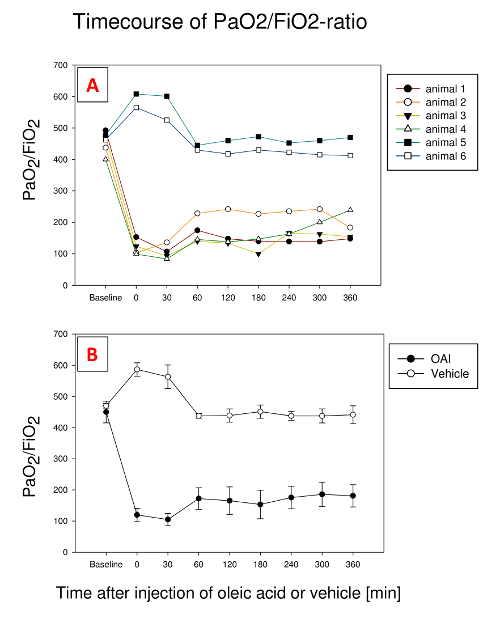
PaO2/FiO2-ratio decreases after fractionated application of oleic acid (Figure 1). In the presented study, 0.185 ± 0.01 ml kg-1 oleic acid was necessary for the induction of lung injury. All animals showed an impaired oxygenation after the induction of lung injury, with varieties in the further time course. In animal 1 and 3, it remained at one level with little fluctuations; in animal 2, we observe an initial increase, followed by a decrease at the end, while animal 4 shows a constant rise. Nevertheless, we find a marked impairment in oxygenation in all 4 animals after 6 h. Therefore, it is necessary to closely monitor PaO2/FiO2-ratio while inducing the lung injury. We use an ultrafast pO2-measurement probe to monitor the decrease in PaO2 in real time21. A different option is to take regular arterial blood gas samples from the time the SpO2 starts dropping. In vehicle-treated animals (5 and 6), there is no decrease in PaO2/FiO2-ratio.
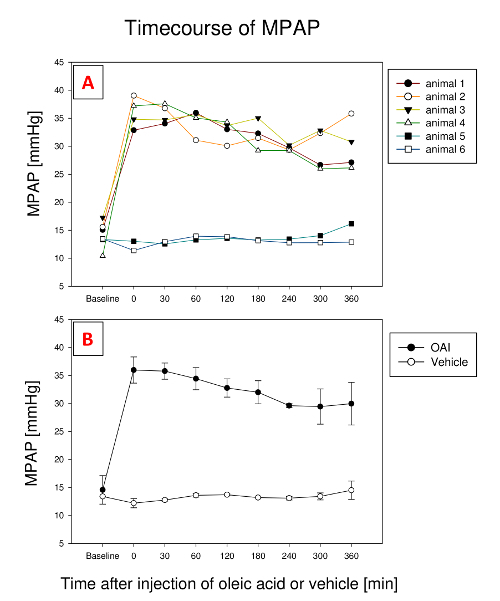
The decrease in PaO2/FiO2-ratio is paralleled by an increase in pulmonary arterial pressure (PAP), which usually remains elevated for the rest of the experiment (Figure 2). Similar to PaO2/FiO2-ratio, it sometimes fluctuates a bit. In one animal (animal 3), MPAP stayed at this level afterwards; in two animals (animal 1 and 4), it fell a little; in one animal (animal 2), it initially fell to rise afterwards. In vehicle-treated animals (5 and 6), MPAP didn't change during the experiment.
Lung injury is also visually detectable in lungs taken out after the death of the animal. Figure 3 shows representative lungs of a pig with OAI-induced lung injury after the euthanasia. In histologic slices, processed according to earlier publications22, alveolar edema and hemorrhage are visible (Figure 4).
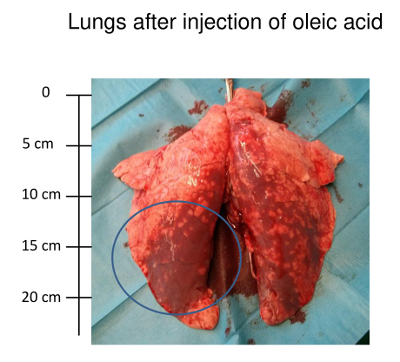
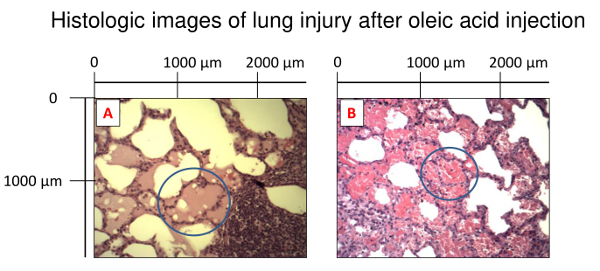
Figure 1: Development of PaO2/FiO2-Ratio during 6 h after the injection of oleic acid in 4 exemplary pigs and 2 pigs treated with vehicle. (A). Representative plots showing stable values with little fluctuations (animals 1 and 3), initial rise followed by a decrease (animal 2) or continuous rise (animal 4). Vehicle treated pigs (animals 5 and 6) show little variation over time. (B). Mean and standard deviation for all animals. Please click here to view a larger version of this figure.
Figure 2: Development of mean pulmonary artery pressure (MPAP) during 6 h after injection of oleic acid in 4 exemplary pigs and 2 pigs treated with vehicle. (A). Representative plots showing an initial rise in all 4 animals. In one animal (animal 3), MPAP stayed at this level afterwards; in two animals (animal 1 and 4), it fell a little; in one animal (animal 2), it initially fell to rise afterwards. Vehicle treated pigs (animals 5 and 6) show little variation over time. (B). Mean and standard deviation for all animals. Please click here to view a larger version of this figure.
Figure 3: Lungs after the injection of oleic acid. Photo of lungs 6 h after the injection of oleic acid. Hemorrhagic areas are visible. Please click here to view a larger version of this figure.
Figure 4: Histologic images of lung injury after the oleic acid injection. The lungs were fixed in 10% formalin for paraffin sectioning and haematoxylin/eosin staining. Image magnification: 10X. (A). Alveolar edema. (B). Hemorrhage. Please click here to view a larger version of this figure.
| Animal 1 | Animal 2 | Animal 3 | Animal 4 | Animal 5 | Animal 6 | |
| Body weight [kg] | 27 | 28 | 27 | 27 | 27 | 29 |
| Right upper lobe wet [g] | 96 | 83 | 116 | 116 | 60 | 44 |
| Right upper lobe dry [g] | 14 | 13 | 13 | 11 | 11 | 9 |
| Wet-to-dry | 6,9 | 6,4 | 8,9 | 10,5 | 5,5 | 4,9 |
Table 1: This table shows the weight of the animals, wet weight, dry weight and wet-to-dry-ratio of the right upper lobe of the animals' lungs.
Discussion
This article describes one method of oleic acid-induced lung injury as a model for studying various aspects of severe ARDS. There are also other protocols with different emulsions, different injection sites, and different temperatures of the emulsion23,24,25,26,27,28,29. Our method offers a reproducible and stable deterioration in lung function. As the effect of oleic acid is dose dependent, it is necessary to define the individual threshold for the PaO2/FiO2-ratio, depending on the desired study, and find the necessary dose of oleic acid to achieve this ratio.
When using this method, there are some pitfalls. The first is the lipophilicity of the oleic acid. To keep it emulsified in the blood/saline mixture, it is necessary to continuously mix it. Another problem is the sudden change in hemodynamics after the injection of oleic acid. Directly after the injection of oleic acid, PAP values can increase abruptly to more than 60 mmHg, which can result in the sudden hemodynamic decompensation and the death of the animal. Therefore, it is necessary to keep sufficient rescue medication, e.g., norepinephrine, prepared and at hand. Nevertheless, the hemodynamic decompensation sometimes results in sudden death of the animal which cannot be prevented. The last pitfall is the after-effect of oleic acid. Similar to human ARDS, the time to symptom onset may vary and it is neither possible to predict exactly how much oleic acid is necessary in a given pig for the induction of lung injury, nor to predict the impact of a given dose on PaO2/FiO2-ratio. PaO2/FiO2-ratios can be nearly stagnating; but they might also improve or further decline. This is displayed in Figure 1. Once the PaO2/FiO2-ratio is between 100 and 200 mmHg at a PEEP ≥ 5 cm H2O, we require oxygenation to remain impaired and below this threshold for more than 30 min. Usually, PaO2/FiO2 remains relatively constant during this time course, though it may drop further. Seldom, even an improvement is possible, reaching values above 200 mmHg. Under these circumstances, more oleic acid is needed.
The induction of lung injury by oleic acid does have certain limitations. The main disadvantage is the need for central venous access, which can be challenging particularly in small animals. Another is the question about the mechanistic relevance of this model. The oleic acid ARDS-model was developed in an attempt to reproduce ARDS due to lipid embolism as seen in trauma patients14. But trauma is only causative for about 10% of ARDS cases30 and whether or not other causes like sepsis or pneumonia share the same mechanism is still under discussion. The final disadvantage of this pig model for ARDS is the complex instrumentation and clinical experience needed to maintain anesthesia in hypoxic large animals with sudden hemodynamic changes. Therefore, only investigators with experience in large animal research and intensive care medicine should work with this model or at least closely supervise unexperienced researchers.
There are, however, distinct advantages to this model. It produces the basic pathologic changes of human ARDS – inflammatory lung injury with permeability changes, impairment in gas exchange and lung mechanics – very well and with good reproducibility7,31. This makes it superior to other models that usually lack one or more of the pathologic effects. Surfactant depletion by lavage induces only little alveolar epithelial changes7,19 and lipopolysaccharide administration, a sepsis model, usually induces only minimal changes of the alveolo-capillary barrier7. Oleic acid injection is feasible in large and small animals, so it can be used in various laboratories that use animal models8,9,10,12. Third, it not only mimics the early phase of ARDS, but also the later phases with deposition of fibrin on the alveolar surface16. Furthermore, when using large animals, it is possible to use extended clinical monitoring and instrumentation that is not fully available in small animals. This resembles the situation of a bedside setting which intensive care physicians are used to, thus allowing easier access for clinicians to this method and facilitating faster implementation in treatment algorithms.
Disclosures
All authors disclose no financial or any other conflict of interest.
Acknowledgments
The authors want to thank Dagmar Dirvonskis for excellent technical support.
References
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. The Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- Brower RG, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England Journal of Medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Briel M, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- Bellani G, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Chiumello D, et al. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Critical Care. 2017;21(1):240. doi: 10.1186/s13054-017-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T, Zochios V, Parhar K. Re-examining Permissive Hypercapnia in ARDS: A Narrative Review. Chest. 2017. [DOI] [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295(3):379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, et al. Thromboxane A2 exacerbates acute lung injury via promoting edema formation. Scientific Reports. 2016;6:32109. doi: 10.1038/srep32109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Liu Z, Yu T, Yang H, Feng L. Ghrelin ameliorates acute lung injury induced by oleic acid via inhibition of endoplasmic reticulum stress. Life Sciences. 2017. [DOI] [PubMed]
- Kamuf J, et al. Endexpiratory lung volume measurement correlates with the ventilation/perfusion mismatch in lung injured pigs. Respiratory Research. 2017;18(1):101. doi: 10.1186/s12931-017-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Wang S, Li Z, Liu J. Sevoflurane Posttreatment Attenuates Lung Injury Induced by Oleic Acid in Dogs. Anesthesia & Analgesia. 2017;124(5):1555–1563. doi: 10.1213/ANE.0000000000002034. [DOI] [PubMed] [Google Scholar]
- Prat NJ, et al. Low-Dose Heparin Anticoagulation During Extracorporeal Life Support for Acute Respiratory Distress Syndrome in Conscious Sheep. Shock. 2015;44(6):560–568. doi: 10.1097/SHK.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves-de-Albuquerque CF, Silva AR, Burth P, Castro-Faria MV, Castro-Faria-Neto HC. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediators of Inflammation. 2015;2015 doi: 10.1155/2015/260465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DP. ARDS: clinical lessons from the oleic acid model of acute lung injury. American Journal of Respiratory and Critical Care Medicine. 1994;149(1):245–260. doi: 10.1164/ajrccm.149.1.8111590. [DOI] [PubMed] [Google Scholar]
- Goncalves-de-Albuquerque CF, et al. Oleic acid induces lung injury in mice through activation of the ERK pathway. Mediators of Inflammation. 2012;2012:956509. doi: 10.1155/2012/956509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard-Croft C, Wang D, Sumpter LR, Zhou X, Zwischenberger JB. Large-animal models of acute respiratory distress syndrome. The Annals of Thoracic Surgery. 2012;93(4):1331–1339. doi: 10.1016/j.athoracsur.2011.06.107. [DOI] [PubMed] [Google Scholar]
- O'Driscoll BR, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:90. doi: 10.1136/thoraxjnl-2016-209729. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Ettrup KS, et al. Basic surgical techniques in the Gottingen minipig: intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. Journal of Visualized Experiments. 2011. p. 2652. [DOI] [PMC free article] [PubMed]
- Russ M, et al. Lavage-induced Surfactant Depletion in Pigs As a Model of the Acute Respiratory Distress Syndrome (ARDS) Journal of Visualized Experiments. 2016. p. 53610. [DOI] [PMC free article] [PubMed]
- Brower RG, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England Journal of Medicine. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- Hartmann EK, et al. Influence of respiratory rate and end-expiratory pressure variation on cyclic alveolar recruitment in an experimental lung injury model. Critical Care. 2012;16(1) doi: 10.1186/cc11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann EK, et al. Inhalation therapy with the synthetic TIP-like peptide AP318 attenuates pulmonary inflammation in a porcine sepsis model. BMC Pulmonary Medicine. 2015;15:7. doi: 10.1186/s12890-015-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien M, Hoeffel JM, Flick MR. Oleic acid lung injury in sheep. Journal of Applied Physiology. 1986;60(2):433–440. doi: 10.1152/jappl.1986.60.2.433. [DOI] [PubMed] [Google Scholar]
- Wiener-Kronish JP, et al. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. Journal of Clinical Investigation. 1988;82(4):1422–1429. doi: 10.1172/JCI113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahagi N, et al. Low molecular weight dextran attenuates increase in extravascular lung water caused by ARDS. American Journal of Emergency Medicine. 2000;18(2):180–183. doi: 10.1016/s0735-6757(00)90014-7. [DOI] [PubMed] [Google Scholar]
- Eiermann GJ, Dickey BF, Thrall RS. Polymorphonuclear leukocyte participation in acute oleic-acid-induced lung injury. The American Review of Respiratory Disease. 1983;128(5):845–850. doi: 10.1164/arrd.1983.128.5.845. [DOI] [PubMed] [Google Scholar]
- Townsley MI, Lim EH, Sahawneh TM, Song W. Interaction of chemical and high vascular pressure injury in isolated canine lung. Journal of Applied Physiology. 1990;69(5):1657–1664. doi: 10.1152/jappl.1990.69.5.1657. [DOI] [PubMed] [Google Scholar]
- Young JS, et al. Sodium nitroprusside mitigates oleic acid-induced acute lung injury. The Annals of Thoracic Surgery. 2000;69(1):224–227. doi: 10.1016/s0003-4975(99)01130-3. [DOI] [PubMed] [Google Scholar]
- Katz SA, et al. Catalase pretreatment attenuates oleic acid-induced edema in isolated rabbit lung. Journal of Applied Physiology. 1988;65(3):1301–1306. doi: 10.1152/jappl.1988.65.3.1301. [DOI] [PubMed] [Google Scholar]
- El-Haddad H, Jang H, Chen W, Soubani AO. Effect of ARDS Severity and Etiology on Short-Term Outcomes. Respiratory Care. 2017;62(9):1178–1185. doi: 10.4187/respcare.05403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. European Surgical Research. 2008;40(4):305–316. doi: 10.1159/000121471. [DOI] [PubMed] [Google Scholar]


