Abstract
Purpose of review:
Avoidant/restrictive food intake disorder (ARFID) was added to the psychiatric nomenclature in 2013, but little is known about its optimal treatment. The purpose of this paper is to review the recent literature on ARFID treatment and highlight a novel cognitive-behavioral approach presently under study.
Recent findings:
The current evidence base for ARFID treatment relies primarily on case reports, case series, and retrospective chart reviews, with only a handful of randomized controlled trials in young children. Studies in adults are lacking. ARFID treatments recently described in the literature include family-based treatment and parent training; cognitive-behavioral approaches; hospital-based re-feeding including tube feeding; and adjunctive pharmacotherapy. A novel form of outpatient cognitive-behavioral therapy for ARFID (CBT-AR) is one treatment currently under study. CBT-AR is appropriate for children, adolescents, and adults ages 10 and up; proceeds through four stages across 20–30 sessions; and is available in both individual and family-supported versions.
Summary:
There is no evidence-based psychological treatment suitable for all forms of ARFID at this time. Several groups are currently evaluating the efficacy of new psychological treatments for ARFID—particularly family-based and cognitive-behavioral approaches—but results have not yet been published.
Keywords: Avoidant/restrictive food intake disorder, ARFID, family-based treatment, cognitive-behavioral therapy, tube feeding
Introduction
Avoidant/restrictive food intake disorder (ARFID) made its diagnostic debut in 2013 with the publication on DSM-5 [1]. ARFID is a reformulation and expansion of the former DSM-IV diagnosis of feeding disorder of infancy and early childhood, and can occur across the lifespan. The hallmark feature of ARIFD is food avoidance or restriction, motivated by sensitivity to the sensory characteristics of food, fear of aversive consequences of eating, or lack of interest in eating or food. To meet criteria for ARFID, the food restriction or avoidance must lead to one or more consequences such as weight loss or faltering growth, nutritional deficiency, dependence on oral nutritional supplements or tube feeding, or psychosocial impairment. DSM-5 describes three example presentations of ARFID. In the first, individuals eat a very limited range of foods due to an inability to tolerate certain tastes and textures. In the second, individuals avoid specific foods or categories of food, or may stop eating altogether, for fear of aversive consequences of eating, such as choking, vomiting, anaphylaxis, or gastrointestinal distress. In the third, individuals exhibit a lack of interest in food or eating. It is important to note that these three presentations are not mutually exclusive and can co-occur within the same individual [2].
In addition to the heterogeneity of clinical presentation, ARFID is also quite diverse in terms of age, demographics, and comorbidities, highlighting the difficulty in identifying a universally applicable treatment approach. For example, ARFID has been reported in very young children [3 **], adolescents [4 *], and adults [5], and several studies have highlighted that both males and females present with the disorder [6,7]. Other investigations have underscored numerous potential psychiatric and medical comorbidities, including autism spectrum disorder [8] and gastrointestinal disorders [6], which may further individualize treatment needs.
Available data on the treatment of ARFID
Because ARFID is so new, there is currently no evidence-based treatment suitable for all forms of the disorder. A robust literature that pre-dates DSM-5 supports the efficacy of behavioral interventions for young children with pediatric feeding disorders [9,10]. However, the generalizability of these approaches to individuals with ARFID—especially adolescents and adults—remains unclear. Below we summarize studies published since the 2013 advent of DSM-5 that describe the treatment of ARFID specifically. ARFID treatments recently described in the literature include family-based treatment and parent training; cognitive-behavioral approaches; hospital-based re-feeding including tube feeding; and adjunctive pharmacotherapy.
Family-based treatment and parent training
Several recently published case reports have described the use of family-based treatment (FBT) for children and adolescents with ARFID [11,12,13]. Such approaches are similar to FBT for anorexia nervosa (AN) in that parents are charged with the task of feeding, but differ from FBT for AN in that parents are asked to support their children in increasing not only dietary volume, but also dietary variety through repeated exposure to novel foods. At least two clinical trials of FBT for ARFID are currently underway [14,15]. Another case report described the use of a behavioral parent-training intervention comprising differential reinforcement, gradual exposure to novel foods, and contingency management, resulting in the acceptance of 30 novel foods in a six-year-old with limited dietary variety [16].
Cognitive-behavioral approaches
Multiple published case reports and case series have described the use of various forms of cognitive-behavioral therapy (CBT) for children [13,17,18] and adults [19,5] with ARFID. Common elements across CBT interventions for ARFID include regular eating [5,13], self-monitoring of food intake [5], exposure and response prevention [13,16], relaxation training [17,16, and behavioral experiments [5]. In one case study, a 16-year-old boy was able to significantly increase his consumption of proteins, fruits, and vegetables, and significantly decrease his eating-related distress after 11 sessions of CBT supplemented with in-home meal interventions in which his mother reinforced the consumption of novel foods [16].
Hospital-based re-feeding including tube feeding
Several hospital-based re-feeding programs have reported positive outcomes on eating and weight for children and adolescents with low-weight ARFID. One randomized controlled study prospectively evaluated the efficacy, among 20 boys and girls (ages 13–72 months) with ARFID, of a five-day manualized behavioral treatment comprising structured mealtimes, escape extinction, and reinforcement procedures in a day hospital setting. Patients randomized to the study treatment exhibited significantly greater bite acceptance, grams of food consumed at mealtime, and fewer mealtime disruptions post-treatment compared to those in the wait list control condition 3 **]. Another study described treatment response among 32 children and adolescents with ARFID treated in an eating disorders partial hospitalization program, reporting significant increases in weight and significant decreases in eating pathology and anxiety from pre- to post-treatment after an average of seven weeks [4 *]. Treatment gains were maintained for at least 12 months in the subset of 20 patients who completed a follow-up assessment [20].
Several case studies have described the use of tube feeding to support inpatient nutritional rehabilitation among low-weight children and adolescents (ages 5–17 years old) with ARFID [21,22,23]. Of note, at least two studies have reported that patients with ARFID were significantly more likely than those with other eating disorders to require tube feeding during inpatient hospitalization [24,25 *]. Although tube feeding can be a life-saving measure in some cases of acute food refusal, a recent review described potentially iatrogenic effects of tube feeding, including long-term tube dependence and decreased oral intake [26], highlighting the urgent need for future research on effective tube weaning protocols for individuals who require tube feeding.
Adjunctive pharmacotherapy
Three groups have recently published studies on pharmacotherapy as an adjunct to hospital-based treatment to facilitate meal consumption and/or weight gain in low-weight children and adolescents with ARFID. In one retrospective chart review, 14 children and adolescents demonstrated a significantly faster rate of weight gain after (versus before) being prescribed mirtazapine [27 *]. In another retrospective chart review, nine youth who took olanzapine showed significant increases in weight from pre- to post-treatment [28 *]. The only double-blind randomized placebo-controlled trial of medication for ARFID evaluated the efficacy of D-cycloserine (DCS) augmentation of a five-day behavioral intervention for chronic and severe food refusal in 15 children (ages 20–58 months). Those randomized to the DCS condition showed a significantly greater percentage of bites rapidly swallowed, and significantly fewer mealtime disruptions, compared to those receiving placebo [29 **].
Summary of available data
Available data on the treatment of ARFID are sparse, and limited to child and adolescent populations. Studies are limited to case reports, case series, and retrospective chart reviews, with a handful of randomized controlled trials in very young children treated in day hospital settings. Findings in adults are limited to case reports, with no larger-scale studies on patients over the age of 18. Several groups are currently evaluating the efficacy of new psychological treatments for ARFID [14,15,30], but results have not yet been published. Case reports and case series have highlighted the promise of family-based treatment, cognitive-behavioral therapy, and hospital-based re-feeding, with pharmacotherapy as an adjunctive rather than a stand-alone treatment. Prospective randomized controlled trials are needed, particularly for adolescents and adults.
The cognitive-behavioral formulation of ARFID
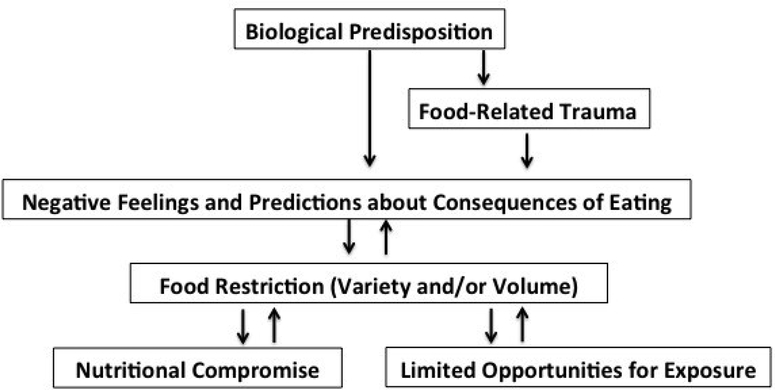
To fill the need for manualized treatments suitable for testing in randomized controlled trials, our team at Massachusetts General Hospital has developed a novel form of cognitive-behavioral therapy for ARFID that is currently being tested in an open trial in which 20 participants ages 10–22 are receiving either individual of family-based versions of the treatment [30,31 **]. The goal of CBT-AR is to help patients achieve a healthy weight, resolve nutrition deficiencies, increase variety to include multiple foods from each of the five basic food groups, eliminate dependence on nutritional supplements, and reduce psychosocial impairment. CBT-AR is based on our cognitive-behavioral conceptualization of the disorder (Figure 1), which posits that some individuals have a biological predisposition to sensory sensitivity, fear of aversive consequences, and/or lack of interest in food or eating [2]. Specifically, those with sensory sensitivity may have heightened response to unfamiliar tastes and smells, those with fear of aversive consequences may have high trait anxiety, and those with lack of interest in eating or food may have lower homeostatic or hedonic appetites.
Figure 1.
Cognitive-behavioral model of avoidant/restrictive food intake disorder
The CBT model posits that individuals with such predispositions will be vulnerable to developing negative feelings and predictions about eating. For example, the patient with sensory sensitivity might feel disgust about novel foods and predict, “Every time I have tasted a vegetable, I have gagged, so I will probably hate any other vegetable.” These negative feelings and predictions would logically lead the patient to begin restricting food intake. Unfortunately, this food avoidance has both physiological and psychological consequences that reinforce negative feelings and predictions. Physiologically, the patient may experience nutritional compromise, such as weight loss or nutrition deficiencies. Under these auspices the patient may experience the predictable consequences of starvation such as becoming satisfied on smaller portions of food, and experiencing altered taste perception from nutrition deficiencies, thus reinforcing the cycle of restricting volume. Psychologically, the more the patient relies on the same foods again and again, the greater the just noticeable difference will become between the patient’s preferred foods and novel foods, thus reinforcing the cycle of restricting variety.
Cognitive-behavioral therapy for ARFID (CBT-AR)
Based on our cognitive-behavioral model of ARFID, CBT-AR is designed reduce nutritional compromise and increase opportunities for exposure to novel foods to reduce negative feelings and predictions about eating. CBT-AR is appropriate for the outpatient treatment of children, adolescents, and adults with ARFID (ages 10 and up). CBT-AR is a flexible, modular treatment designed to last approximately 20 (for patients who are not underweight) to 30 (for patients who have significant weight to gain) sessions over six to 12 months. CBT-AR is appropriate for individuals with ARFID who are medically stable, currently accepting at least some food by mouth, and not receiving tube feeding. Patients who are under the age of 16 and/or older adolescents and young adult patients who have significant weight to gain can be offered a family-supported version of CBT-AR, whereas patients ages 16 years and up without significant weight to gain can be treated with an individual version.
CBT-AR proceeds through four broad stages (Table 1) [31 **]. In Stage 1, the therapist provides psychoeducation about ARFID and CBT-AR. In addition, the therapist encourages the patient to establish a pattern of regular eating and self-monitoring by relying primarily on preferred foods, but also encourages early change by asking the patient who is not underweight to begin introducing minor variations in the presentation of preferred foods and/or reintroducing previously dropped foods. In contrast, the therapist encourages early change for patients who are underweight by asking them (often with family support) to increase their intake by at least 500 calories per day to support a weight gain of approximately 1–2 lbs per week.
Table 1.
Four stages of cognitive-behavioral therapy for avoidant/restrictive food intake disorder (CBT-AR)
| Stage | Primary interventions |
|---|---|
| 1. Psychoeducation and early change (2–4 sessions) |
• Psychoeducation on ARFID and its treatment • Self- or parent-monitoring of food intake • Establishing a pattern of regular eating to normalize hunger cues • Increasing volume of preferred foods (for patients who are underweight) and variety (for all patients) • Individualized formulation of mechanisms that maintain avoidant/restrictive eating (i.e., sensory sensitivity, fear of aversive consequences, lack of interest in eating or food) |
| 2. Treatment planning (2 sessions) | • Continue increasing volume and/or variety • Reviewing intake from Primary Food Group Building Blocks and selecting foods to learn about in Stage 3 |
| 3. Maintaining mechanisms in order of priority (14–22 sessions) | • Sensory sensitivity: Systematic desensitization to novel foods by repeated in-session exploration of sight, smell, texture, taste, chew; specific, detailed plans for out-of-session practice with tasting and incorporation • Fear of aversive consequences: Psychoeducation about how avoidance maintains anxiety, development of fear/avoidance hierarchy, graded exposure to feared foods and situations in which choking, vomiting, or other feared consequence may occur • Apparent lack of interest in eating or food: Interoceptive exposure to bloating, fullness, and/or nausea; in-session exposure to highly-preferred foods |
| 4. Relapse prevention(2 sessions) | • Evaluating whether treatment goals have been met, identifying treatment strategies to continue at home, and developing a plan for maintaining weight gain (if needed) continuing to learn about novel foods |
In Stage 2, the therapist provides psychoeducation about nutrition deficiencies and supports the patient in selecting novel fruits, vegetables, proteins, dairy, and grains to learn about in Stage 3 that will support resolution of these deficiencies, encourage further weight gain, and/or ameliorate psychosocial impairment.
In Stage 3—the heart of the treatment—the therapist selects the module(s) most appropriate to the patient’s ARFID maintaining mechanisms(s) including sensory sensitivity, fear of aversive consequences, and/or lack of interest in food or eating. For patients with multiple maintaining mechanisms, the therapist starts with the module addressing the primary or most impairing mechanism. Although Stage 3 interventions differ based on the specific module, the common element across all modules is exposure. For patients with sensory sensitivity, the therapist invites the patient (or family) to bring five novel foods to each session and asks the patient to non-judgmentally describe each food’s appearance, feel, smell, taste, and texture. The patient then selects foods to practice tasting throughout the week to facilitate habituation, and later works to incorporate larger portions of these novel foods into his or her day-to-day diet. For patients with fear of aversive consequences, the therapist works with the patient (or family) to create a fear and avoidance hierarchy of foods and eating-related situations that the patient fears will lead to negative outcomes. The therapist then conducts in-session exposures to these foods and situations, and asks the patient to repeat these exposures for homework, to test the patient’s predictions that the feared outcome will actually occur. Lastly, for patients with lack of interest in eating, the therapist introduces a series of interoceptive exposures (e.g., pushing one’s belly out, gulping water, and spinning in a chair) to help the patient habituate to sensations associated with eating and fullness. The therapist also helps the patient remember what he or she enjoys about his or her preferred foods by describing their appearance, feel, smell, taste, and texture.
Lastly, in Stage 4, the therapist supports the patient in evaluating progress, co-creating a relapse prevention plan, and setting goals for the future.
Conclusion and future directions
The addition of ARFID to DSM-5 has drawn attention to the urgent need for research into its optimal treatment. Available data are limited to case reports, case series, and randomized controlled trials in specialized populations of children and adolescents; treatment studies in adults are lacking. New psychological therapies are currently being tested. One such approach is a novel form of cognitive-behavioral therapy for children, adolescents, and adults that can be offered over 20–30 sessions in an individual or family-supported format. Given the heterogeneity of ARFID, it is likely that different presentations will require different interventions, and that once clinical trials have been completed, patients can be matched to the treatment that is the best fit for their unique clinical needs.
Key points.
There are no evidence-based psychological treatments suitable for all forms of avoidant/restrictive food intake disorder at this time.
The current evidence base for ARFID treatment relies primarily on case reports, case series, retrospective chart reviews, and a handful of randomized controlled trials in very young children. Treatment studies in adults are lacking.
ARFID interventions recently described in the literature include family-based treatment and parent training; cognitive-behavioral approaches; hospital-based re-feeding including tube feeding; and adjunctive pharmacotherapy.
New psychological treatments are currently being tested, including a novel form of cognitive-behavioral therapy for children, adolescents, and adults that can be offered over 20–30 sessions in an individual or family-supported format.
Acknowledgments
Disclosure of funding. The authors would like to gratefully acknowledge funding for the work described in this paper from the National Institute of Mental Health (1R01MH108595), Hilda and Preston Davis Foundation, and American Psychological Foundation.
Footnotes
Conflicts of interest. Drs. Thomas and Eddy will receive royalties from Cambridge University Press for the sale of their book Cognitive-Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents, and Adults, scheduled to be published in late 2018.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric Pub; 2013. [Google Scholar]
- 2.Thomas JJ, Lawson EA, Micali N et al. Avoidant/restrictive food intake disorder: a three-dimensional model of neurobiology with implications for etiology and treatment. Current psychiatry reports. 2017; 19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. **.Sharp WG, Stubbs KH, Adams H et al. Intensive, manual-based intervention for pediatric feeding disorders: results from a randomized pilot trial. Journal of pediatric gastroenterology and nutrition. 2016; 62:658–63. This randomized wait list controlled trial describes an intensive five-day manualized behavioral intervention for young children with ARFID. [DOI] [PubMed] [Google Scholar]
- 4. *.Ornstein RM, Essayli JH, Nicely TA et al. Treatment of avoidant/restrictive food intake disorder in a cohort of young patients in a partial hospitalization program for eating disorders. International Journal of Eating Disorders. 2017; 50:1067–74. This retrospective chart review describes outcomes for children and adolescents with ARFID treated in a partial hospitalization program for eating disorders, which utilizes techniques from family-based treatment and cognitive-behavioral therapy. [DOI] [PubMed] [Google Scholar]
- 5.Steen E, Wade TD. Treatment of co‐occurring food avoidance and alcohol use disorder in an adult: possible avoidant restrictive food intake disorder?. International Journal of Eating Disorders. 2018; 51:373–377. [DOI] [PubMed] [Google Scholar]
- 6.Eddy KT, Thomas JJ, Hastings E et al. Prevalence of DSM‐5 avoidant/restrictive food intake disorder in a pediatric gastroenterology healthcare network. International Journal of Eating Disorders. 2015; 48:464–70. [DOI] [PubMed] [Google Scholar]
- 7.Forman SF, McKenzie N, Hehn R et al. Predictors of outcome at 1 year in adolescents with DSM-5 restrictive eating disorders: report of the national eating disorders quality improvement collaborative. Journal of Adolescent Health. 2014; 55:750–6. [DOI] [PubMed] [Google Scholar]
- 8.Lucarelli J, Pappas D, Welchons L, Augustyn M. Autism spectrum disorder and avoidant/restrictive food intake disorder. Journal of Developmental & Behavioral Pediatrics. 2017; 38:79–80. [DOI] [PubMed] [Google Scholar]
- 9.Lukens CT, Silverman AH. Systematic review of psychological interventions for pediatric feeding problems. Journal of pediatric psychology. 2014. June 13;39(8):903–17. [DOI] [PubMed] [Google Scholar]
- 10.Sharp WG, Volkert VM, Scahill L et al. A systematic review and meta-analysis of intensive multidisciplinary intervention for pediatric feeding disorders: how standard is the standard of care?. The Journal of pediatrics. 2017; 181:116–24. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick KK, Forsberg SE, Colborn. Family-based therapy for avoidant restrictive food intake disorder: Families Facing Food Neophobias In: Family Therapy for Adolescent Eating and Weight Disorders. 1 Loeb K. (Ed.), Le Grange D. (Ed.), Lock J. (Ed.). New York: Routledge; 2015. pp. 276–296 [Google Scholar]
- 12.Norris ML, Spettigue WJ, Katzman DK. Update on eating disorders: current perspectives on avoidant/restrictive food intake disorder in children and youth. Neuropsychiatric disease and treatment. 2016; 12:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JJ, Brigham KS, Sally ST et al. Case 18–2017—an 11-year-old girl with difficulty eating after a choking incident. New England journal of medicine. 2017; 376:2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesser J, Eckhardt S, Ehrenreich-May J, et al. Integrating family based treatment with the unified protocol for the transdiagnostic treatment of emotional 351 disorders: a novel treatment for avoidant restrictive food intake disorder. Clinical Teaching Day presentation at the International Conference on Eating Disorders; 2017; Prague, Czech Republic. [Google Scholar]
- 15.Sadeh-Sharvit S, Robinson A, Lock J. FBT-ARFID for younger patients: lessons from a randomized controlled trial. Workshop presented at the International Conference on Eating Disorders; 2018; Chicago, Illinois. [Google Scholar]
- 16.Murphy J, Zlomke KR. A behavioral parent-training intervention for a child with avoidant/restrictive food intake disorder. Clinical Practice in Pediatric Psychology. 2016; 4:23–34. [Google Scholar]
- 17.Fischer AJ, Luiselli JK, Dove MB. Effects of clinic and in-home treatment on consumption and feeding-associated anxiety in an adolescent with avoidant/restrictive food intake disorder. Clinical Practice in Pediatric Psychology. 2015; 3:154–166. [Google Scholar]
- 18.Bryant Waugh R Avoidant restrictive food intake disorder: an illustrative case example. International Journal of Eating Disorders. 2013; 46:420–3. [DOI] [PubMed] [Google Scholar]
- 19.King LA, Urbach JR, Stewart KE. Illness anxiety and avoidant/restrictive food intake disorder: cognitive-behavioral conceptualization and treatment. Eating behaviors. 2015; 19:106–9. [DOI] [PubMed] [Google Scholar]
- 20.Bryson AE, Scipioni AM, Essayli JH et al. Outcomes of low‐weight patients with avoidant/restrictive food intake disorder and anorexia nervosa at long‐term follow‐up after treatment in a partial hospitalization program for eating disorders. International Journal of Eating Disorders. 2018; 51:470–474. [DOI] [PubMed] [Google Scholar]
- 21.Guvenek-Cokol PE, Gallagher K, Samsel C. Medical traumatic stress: a multidisciplinary approach for iatrogenic acute food refusal in the inpatient setting. Hospital pediatrics. 2016; 6:693–8. [DOI] [PubMed] [Google Scholar]
- 22.Pitt PD, Middleman AB. A focus on behavior management of avoidant/restrictive food intake disorder (ARFID): a case series. Clinical pediatrics. 2018; 57:478–80. [DOI] [PubMed] [Google Scholar]
- 23.Schermbrucker J, Kimber M, Johnson N et al. Avoidant/restrictive food intake disorder in an 11-year old south American boy: medical and cultural challenges. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2017; 26:110–113. [PMC free article] [PubMed] [Google Scholar]
- 24.Strandjord SE, Sieke EH, Richmond M, Rome ES. Avoidant/restrictive food intake disorder: illness and hospital course in patients hospitalized for nutritional insufficiency. Journal of Adolescent Health. 2015; 57:673–8. [DOI] [PubMed] [Google Scholar]
- 25. *.Peebles R, Lesser A, Park CC et al. Outcomes of an inpatient medical nutritional rehabilitation protocol in children and adolescents with eating disorders. Journal of eating disorders. 2017; 5:1–14. This paper describes the Children’s Hospital of Philadelphia (CHOP) Malnutrition Protocol for the inpatient re-feeding of children and adolescents with restrictive eating disorders, including ARFID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dovey TM, Wilken M, Martin CI, Meyer C. Definitions and clinical guidance on the enteral dependence component of the avoidant/restrictive food intake disorder diagnostic criteria in children. Journal of Parenteral and Enteral Nutrition. 2018; 42:499–507. [DOI] [PubMed] [Google Scholar]
- 27. *.Gray E, Chen T, Menzel J et al. Mirtazapine and weight gain in avoidant and restrictive food intake disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2018; 57:288–9. This retrospective chart review describes adjuctive pharmacotherapy with mirtazipine for children and adolescents with ARFID. [DOI] [PubMed] [Google Scholar]
- 28. *.Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. Journal of child and adolescent psychopharmacology. 2017; 27:920–2. This retrospective chart review describes adjunctive pharmacotherapy with olanazapine for children and adolescents with ARFID. [DOI] [PubMed] [Google Scholar]
- 29. **.Sharp WG, Allen AG, Stubbs KH et al. Successful pharmacotherapy for the treatment of severe feeding aversion with mechanistic insights from cross-species neuronal remodeling. Translational psychiatry. 2017; 7:1–9. This double blind randomized placebo controlled trial describes adjunctive pharmacotherapy with D-cycloserine for young children with chronic and severe food refusal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas JJ, Becker KR, Wons O et al. Cognitive behavioral therapy for avoidant/restrictive food intake disorder (CBT-AR): A pilot study demonstrating feasibility, efficacy, and acceptability. Submitted to the XXIVth Annual Meeting of the Eating Disorders Research Society 2018. [Google Scholar]
- 31. **.Thomas JJ, Eddy KT. Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults. Cambridge, UK: Cambridge University Press; in press. This book describes a novel cognitive-behavioral model of the maintenance of ARFID and is the first treatment manual to describe the implementation of cognitive-behavioral therapy for the disorder. [Google Scholar]