Abstract
Growing evidence suggests chronic low-grade inflammation (LGI) as a possible mechanism underlying the aging process. Some biological and pharmaceutical compounds may reduce systemic inflammation and potentially avert functional decline occurring with aging. The aim of the present meta-analysis was to examine the association of pre-selected interventions on two established biomarkers of inflammation, interleukin-6 (IL-6), and C-reactive protein (CRP) in middle-age and older adults with chronic LGI.
We reviewed the literature on potential anti-inflammatory compounds, selecting them based on safety, tolerability, acceptability, innovation, affordability, and evidence from randomized controlled trials. Six compounds met all five inclusion criteria for our systematic review and meta-analysis: angiotensin II receptor blockers (ARBs), metformin, omega-3, probiotics, resveratrol and vitamin D. We searched in MEDLINE, PubMed and EMBASE database until January 2017. A total of 49 articles fulfilled the selection criteria. Effect size of each study and pooled effect size for each compound were measured by the standardized mean difference. I2 was computed to measure heterogeneity of effects across studies.
The following compounds showed a significant small to large effect in reducing IL-6 levels: probiotics (−0.68 pg/ml), ARBs (−0.37 pg/ml) and omega-3 (−0.19 pg/ml). For CRP, a significant small to medium effect was observed with probiotics (−0.43 mg/L), ARBs (−0.2 mg/L), omega-3 (−0.17 mg/L) and metformin (−0.16 mg/L). Resveratrol and vitamin D were not associated with any significant reductions in either biomarker.
These results suggest that nutritional and pharmaceutical compounds can significantly reduce established biomarkers of systemic inflammation in middle-age and older adults. The findings should be interpreted with caution, however, due to the evidence of heterogeneity across the studies.
Keywords: chronic inflammation, interleukin-6, C-reactive protein, nutrient, pharmaceutical, adults
1. Introduction
Older age is often associated with a higher burden of comorbidities that lead to declines in physical and cognitive function and ultimately, disability and death (Marengoni et al., 2009). In recent years inflammation has been shown to contribute to most if not all chronic diseases typical of old age (Stepanova et al., 2015). Moreover, aging itself could result in immune system dysregulation leading to chronic low-grade inflammation (LGI) (Ferrucci et al., 2005). Growing evidence suggests chronic low level elevation of proinflammatory cytokines and chemokines, a process also defined as “inflammaging” specifically contributes to age-related decline in function and increases risk of morbidity and mortality (Franceschi and Campisi, 2014).
The origin of inflammaging currently remains unclear (Baylis et al., 2013; Fougere et al., 2016; Franceschi et al., 2000; Fulop et al., 2014). Although there is likely a genetic predisposition (Capurso et al., 2007), many other factors can contribute to the inflammatory process. Some identified exogenous triggers include smoking (Behnia et al., 2016), air pollution (Fougere et al., 2015), persistent infections (Derhovanessian et al., 2011; Oppermann et al., 2012) and overweight or obesity (Giugliano et al., 2006). Several endogenous factors also play a relevant role, including: overproduction of reactive oxygen species (ROS) (Zhang et al., 2016) and advanced glycation end-products (AGEs) (Yamagishi and Matsui, 2016), mitochondrial dysfunction (Lopez-Lluch et al., 2015), renin-angiotensin system (RAS) deregulation (Duprez, 2006), hormonal changes (Epel and Lithgow, 2014; Gubbels Bupp, 2015), visceral adiposity (Palmer and Kirkland, 2016), changes in the gut microbiota (Biagi et al., 2010) and accumulation of cell debris due to a defective autophagy (Franceschi et al., 2016).
In humans, two of the most well accepted markers of systemic inflammation are interleukin 6 (IL-6) and C-reactive protein (CRP) (Michaud et al., 2013). The levels of both biomarkers typically increase with aging (Singh and Newman, 2011; Wyczalkowska-Tomasik et al., 2016), which leads to an increased risk of morbidity and mortality in older adults (Alley et al., 2007; Ferrucci et al., 1999; Harris et al., 1999). Higher IL-6 levels have been associated with indicators of physical frailty such as slower walking speed, impaired muscle strength, and lower extremity performance (Cesari et al., 2004; Taaffe et al., 2000), and sarcopenia (Haddad et al., 2005) which are predictive of future disability in nondisabled older adults (Ferrucci et al., 1999). Moreover, in the Longitudinal Aging Study of Amsterdam, moderately elevated CRP levels (3–10 mg/L) were associated with 3-year incident frailty (Puts et al., 2005). Elevated levels of both IL-6 and CRP have also been related to a decline in cognitive function (Schram et al., 2007) and Alzheimer’s disease (AD) (Akiyama et al., 2000).
The rise of IL-6 and CRP levels are mechanistically linked to the activation of pro-inflammatory transcription factors, including nuclear factor kappa B (NF-κB) (Maggio et al., 2006). A consistent body of evidence suggests NF-κB is an attractive target for anti-inflammatory therapies (Gupta et al., 2010). Thus, the inhibitors, at various levels, of NF-κB pathway could lead to a reduction of inflammatory biomarkers (such as IL-6 and CRP) and potentially avert or slow the functional decline that occurs with aging.
A recent review by Gupta et al. (2010), based on in vitro data, provided a comprehensive overview on potential compounds that can inhibit the NF-κB pathway, including natural and synthetic products, proteins, and peptides. Although there is a growing body of evidence to suggest many of these compounds can reduce established inflammatory biomarkers, to date, the findings have not been systematically examined in middle-age and older adults with chronic LGI. Thus, the goal of this review was to examine the state of evidence from relevant randomized controlled trials (RCTs) to identify the most promising biological and pharmacological compounds that reduce inflammation in middle-age and older adults with elevated circulating levels of IL-6 and/or CRP.
2. Methods
2.1. Search Strategy and Study Selection
This systematic literature review and meta-analysis followed the requirements of the PRISMA statement (Moher et al., 2009). The review was registered in PROSPERO, the international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO; registration number: CRD42017059820)
Three study authors (C.C., R.T.M. and S.A.L.) independently conducted a systematic search of the databases MEDLINE, PubMed, and EMBASE (all years to January 31st 2017).
To initially identify the potential compounds, we considered the molecules listed in a recent review by Gupta et al. (2010), since this review provided, on a molecular base, a comprehensive overview of targets of NF-κB. Furthermore, to maximize the public health impact of our meta-analysis, we selected compounds based on four criteria: safety, tolerability and acceptability, innovation, and affordability. First, we evaluated compounds in terms of their safety record in the general population, risk of adverse events, and potential interactions with other drugs. Next, we took into account tolerability and acceptability, considering side effects that may reduce quality of life and adherence to the treatment. For our innovation criterion, we prioritized compounds that did not already have an indication for anti-inflammatory therapy (e.g., non-steroidal anti-inflammatory drugs, corticosteroids and interleukin (IL)-1 beta inhibitor were excluded). Next, we considered costs of specific compounds and the potential for individuals to take specific compounds on a regular basis. To be included in the present meta-analysis, compounds had to meet all four criteria listed above.
Once we identified potential compounds, we applied the fifth criterion which was to select compounds that had sufficient evidence from four or more RCTs conducted in middle-age and older adults with chronic LGI (indicated by either elevated IL-6 or CRP levels). After a preliminary search in the above-mentioned databases, we excluded compounds that had less than four eligible studies. Several compounds that met our initial criteria (e.g. curcumin, vitamin-B6, -C, -E, β -carotene, melatonin) were subsequently excluded due to lack of sufficient evidence from clinical trials in middle-age and older adults with chronic LGI. Table 1 reports the results of the selection process.
Table 1.
Selection criteria of compounds to include in the systematic review.
| Compounds | Safety | Tolerability, acceptability |
Innovation | Affordability | Evidence from RCTs |
|---|---|---|---|---|---|
| ACEIs | + | − | + | + | + |
| ARBs | + | + | + | + | + |
| Beta-blockers | + | − | + | + | + |
| Spironolactone | + | + | + | + | − |
| Metformin | + | + | + | + | + |
| ω-3 | + | + | + | + | + |
| Probiotics | + | + | + | + | + |
| Resveratrol | + | + | + | + | + |
| Vitamin D | + | + | + | + | + |
| Calcitriol | − | − | + | + | − |
| Thiazolidinediones | − | − | + | + | + |
| Sulfonylureas | − | − | + | + | + |
| Statins, fibrates | − | − | + | + | + |
| Anti-TNF-α, -IL6,-IL1; methotrexate, leflunomide, thalidomide, rapamycin, cyclosporin A, tacrolimus |
− | − | − | − | + |
| Corticosteroids, aspirin, paracetamol, NSAIDs, cox-2 inhibitors, sulfasalazine, Mesalamine |
− | − | − | + | + |
| N-acetylcysteine | + | + | + | − | − |
| Imatinib, gemcitabine | − | − | + | − | − |
| Fluoroquinolones, Macrolides |
− | − | + | + | − |
| Ribavirin, ritonavir | − | − | + | − | − |
| Estrogen, Raloxifene | − | − | + | + | + |
| Melatonin | + | + | + | + | − |
| Ursodeoxycholic acid | ? | ? | + | + | − |
| Vitamin B6, C, E; β- Carotene |
+ | + | + | + | − |
| Foods, herbs and spices (apple juice, black raspberry extract, blueberry, cocoa, curcumin, garlic, Ginkgo biloba extract, ginseng, pomegranate extract, strawberry extracts) |
+ | + | + | + | − |
| Other micronutrients (anthocyanins, astaxanthin, bromelain, capsaicin, |
+ | + | + | + | − |
| epigallocatechin-3-gallate, genistein, glucosamine, glutamine, quercetin) |
“+” positive evidence, “−” negative evidence, “?” evidence lacking. In bold the compounds that fulfilled all selection criteria. ARBs= angiotensin II receptor blockers, ACEIs= angiotensin-converting enzyme inhibitors, NSAIDs= non-steroidal anti-inflammatory drugs, Cox-2= cyclooxygenase-2.
Six compounds met all five inclusion criteria and were included in our systematic review and meta-analysis: angiotensin II receptor blockers (ARBs), metformin, omega-3, probiotics, resveratrol, and vitamin D. Search terms included combinations of the following keywords: (“losartan” OR “candesartan” OR “valsartan” OR “irbesartan” OR “telmisartan” OR “olmesartan” OR “eprosartan” OR “azilsartan” OR “fimasartan” OR “metformin” OR “omega-3 fatty acids” OR “n-3 polyunsaturated fatty acid” OR “n-3 pufa” OR “probiotic” OR “resveratrol” OR “vitamin D” OR “cholecalciferol” OR “ergocalciferol”) AND (“inflammation” OR “interleukin-6” OR “c-reactive protein”).
To be included in this review, studies were limited to RCTs (including both parallel and cross-over study designs) to ensure the effects of interventions on outcomes were compared to placebo or control group receiving no treatment. Studies were required to meet the following inclusion criteria: (1) conducted in humans aged 45 years or older; (2) included at least one specific nutritional or pharmacological intervention arm; (3) assessed the effect of treatment on an outcome of interest (IL-6 or CRP); (4) conducted in adults with baseline levels of IL-6 between 2.5 and 30 pg/ml and/or baseline levels of CRP between 2 and 10 mg/L, according to the most well accepted cut-off levels indicating chronic LGI (Ferrucci et al., 1999; Ockene et al., 2001; Ridker et al., 2008; Ridker et al., 2001; Sabatine et al., 2007; Steinmetz et al., 1995); (5) carried out for four weeks or longer; (6) with a sample size of at least 15 per group; and (7) written in the English language. In addition, studies with the following characteristics were excluded: (1) involving patients with infections, acute inflammatory diseases, acute coronary syndrome, chronic kidney disease, chronic liver and lung diseases, inflammatory bowel diseases, autoimmune disorders, cancers, or participants undergoing surgical procedures; (2) with another intervention co-occurring; and (3) with intravenous administration of treatment.
Articles were initially screened based on title and abstract by three study authors (C.C., R.T.M. and S.A.L.), with the full text sought if the abstract did not provide sufficient information to draw a conclusion regarding eligibility for inclusion in the current review.
Reference lists of the articles were reviewed to identify additional relevant articles. Disagreement was resolved by discussion or in consultation with a senior author (S.D.A.). We contacted authors of primary studies to obtain any missing information.
2.2. Data extraction
The following details were extracted from each study: first author’s name, publication year, sample size, details of study population (age, body mass index -BMI-, health status), study duration, study design, outcomes of interest measured at baseline, and at follow-up after the intervention. For example, the findings from studies testing different dosages of compounds or including different sub-studies, were extracted separately. In the article of Bahr et al. (2011) we excluded results referring to treatment with telmisartan 80 mg because mean age in that group was less than 45, therefore, we considered only findings from groups under telmisartan 160 mg and placebo. All values were converted to the same units of measure: pg/ml for IL-6 and mg/L for CRP.
2.3. Risk of Bias assessment
All included studies were assessed for quality using the Cochrane Collaboration’s tool (Higgins et al., 2011) from a study author (C.C.). Each study was assigned a rating (low, unclear or high risk of bias) related to six domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, and selective outcome reporting. The reliability of assessment was ensured by revision and consultation with a senior author (S.D.A.).
2.4. Statistical analysis
Each study’s effect size, or standardized mean difference (SMD) was calculated by comparing mean and standard deviation of IL-6 or CRP at follow-up measurement, between treatment and control group (Borenstein, 2009). Hedges’g was used to adjust the effect size, and an overall weighted average effect size for each compound was calculated based on each study’s sample size (Borenstein, 2009). In accordance with convention, effect sizes of compounds were classified as small (−0.2), moderate (−0.5) and large (−0.8) (Cohen, 1992). Z statistics were calculated by comparing effect size difference over pooled standard deviation between every two compounds. The z-test was used to perform pairwise comparisons of effects between compounds and to test if each compound was significantly different from the overall mean of all compounds (Borenstein, 2009). Fixed-effects model was used as we applied strict inclusion criteria and assumed the population and effect would be similar across studies (Borenstein, 2009). Heterogeneity of effects across studies was estimated by I2 statistics. It measures percentage of variation that is caused by heterogeneity between studies, and is larger when heterogeneity increases (Borenstein, 2009). A meta-regression was conducted, for all the selected compounds, to estimate the influence of dosage and treatment duration on effect size. Funnel plots and Egger’s tests were utilized to detect bias in meta analyses (Borenstein, 2009). All statistical analyses were performed in R 3.3.2 (R Core Team, 2013). Each P-value is based on two-sided alternative hypothesis, and a level of 0.05 or below was considered statistically significant.
3. Results
3.1. Search Results and Study Selection
A total of 858 articles were identified and assessed for eligibility. Based on titles and abstracts, 569 papers were excluded. Following full text revision of remaining 289 articles, other 240 studies were excluded. Overall, 49 RCTs (Bahr et al., 2011; Bays et al., 2013; Bitzur et al., 2010; Bo et al., 2016; Breslavsky et al., 2013; Caballero et al., 2004; Chandler et al., 2014; Darghosian et al., 2015; Dawczynski et al., 2013; De Jager et al., 2005; de Jager et al., 2014; Duggan et al., 2015; Ebrahimi et al., 2009; Feher et al., 2014; Gagnon et al., 2014; Goldberg et al., 2014; Hass et al., 2014; Hutchins et al., 2013; Krysiak et al., 2011, 2013; Link et al., 2006; Magyar et al., 2012; Mazloom et al., 2013; Militaru et al., 2013; Mohamadshahi et al., 2014; Murphy et al., 2007; Nodari et al., 2011; Ogino et al., 2010; Paoli et al., 2015; Persson et al., 2006; Pooya et al., 2010; Pot et al., 2009; Pradhan et al., 2009; Rajkumar et al., 2014; Sadiya et al., 2015; Schiano et al., 2008; Shaseb et al., 2016; Sinha-Hikim et al., 2015; Sokol et al., 2012; Stricker et al., 2012; Tome-Carneiro et al., 2012; Tousoulis et al., 2008; Troseid et al., 2009; Valentini et al., 2015; van der Zijl et al., 2011; Witte et al., 2014; Xu et al., 2015; Zhao et al., 2009; Zittermann et al., 2009) were selected for the final analysis (one RCT (Rajkumar et al., 2014) was eligible among both omega −3 and probiotics). Fig. 1 presents the flow diagram of the study selection process, and the stated reason specific articles were excluded followed the order of inclusion/exclusion criteria listed in the Methods section.
Fig. 1.
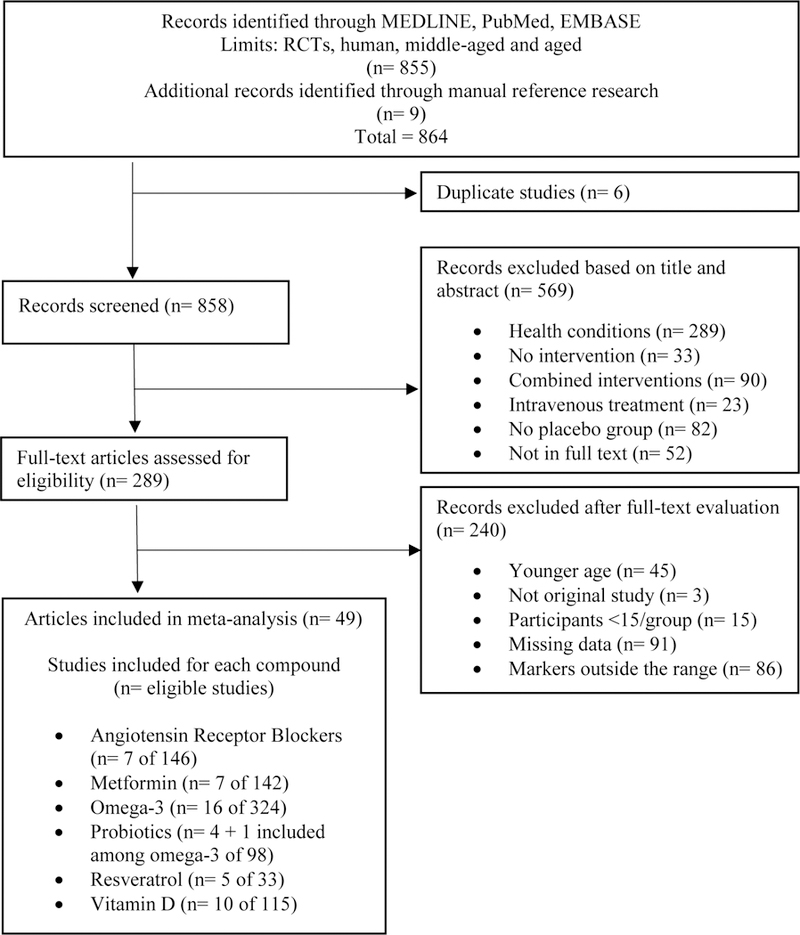
Flow diagram of study selection process.
3.2. Quality Assessment
Fig. 2 and Fig. A.1 (Supplementary materials) summarize the distribution of the risk of bias across all included studies. Incomplete outcome data, allocation concealment, random sequence generation, and blinding of participants and personnel were the major concerns as potential sources of bias. Thirty studies had unclear risk of bias, eight studies had high risk of bias in one category, and two had high risk of bias in two categories.
Fig. 2.
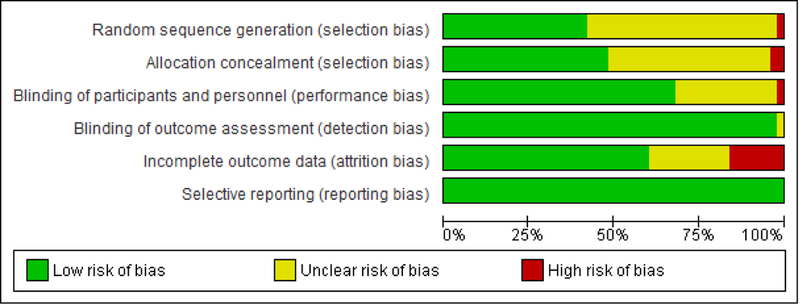
Risk of bias assessment. Percentage of studies having high, unclear or low risk of bias.
3.3. Study Findings for Specific Compounds
3.3.1. ARBs
Seven of 146 potential articles involving 528 participants met our inclusion criteria (Bahr et al., 2011; Hass et al., 2014; Link et al., 2006; Ogino et al., 2010; Persson et al., 2006; Tousoulis et al., 2008; van der Zijl et al., 2011). The included studies tested five different ARBs: losartan, valsartan, candesartan, irbesartan and telmisartan. The majority of the trials were double-blind and placebo-controlled. Only two studies did not have a placebo group, but the control group was represented by people who received usual antihypertensive treatment (other than RAS inhibitors) or no antihypertensive treatment (Hass et al., 2014; Tousoulis et al., 2008). Table 2 presents key characteristics of the included studies.
Table 2.
General characteristics of randomized controlled trials testing the effect of angiotensin receptor blockers (ARBs) supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/ Health status |
Duration | Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/ml) studies | ||||||||
| Bahr et al. (2011) | telmisartan 160 mg: 19 PLA: 18 |
telmisartan 160 mg: 50±10.4 PLA: 45.1±11.9 |
telmisartan 160 mg: 34.6±6.5 PLA: 32.7±7.1 |
Patients with metabolic syndrome |
14 weeks | telmisartan 160 mg/d, or placebo. |
telmisartan 160 mg: 2.5±1.9 PLA: 2.1±1.7 |
telmisartan 160 mg: +0.2 PLA: +0.1 |
| Ogino et al. (2010) | 16 | 63±4 | 25.8±1.4 | Patient with CHF | 16 weeks | cross-over study design with losartan 100 mg/d (3 patients 50 mg/d) vs placebo |
4.0±0.5 | losartan: − 1.3* PLA: −0.2 |
| Persson et al.(2006) | irbesartan: 143 PLA: 126 |
irbesartan: 57.3±8.0 PLA: 58.4±9.0 |
irbesartan: 29.8±4.4 PLA: 30.4±4.3 |
Patients with T2DM and microalbuminuria |
96 weeks | irbesartan 300 mg/d vs placebo |
birbesartan: 2.98 (2.65– 3.36) PLA: 3.12 (2.80–3.48) |
irbesartan: +0.15**(vs PLA) PLA: +0.37 |
| Link et al. (2006) | telmisartan: 21 PLA: 21 |
telmisartan: 56.4±7.1 PLA: 58.5±11.6 |
telmisartan: 27.3±3.6 PLA: 26.9±2.9 |
Patients with stable CAD and essential hypertension |
12 weeks | telmisartan 40 mg/d vs placebo |
telmisartan: 2.5±0.6 PLA: 3.8±1.4 |
telmisartan: −1.1 PLA: −1.2 |
| CRP (mg/L) studies | ||||||||
| Hass etal. (2014) | candesartan 32 mg: 22 candesartan 16 mg: 23 CNT: 22 |
candesartan 32 mg: 63.1±6.2 candesartan 16 mg: 61.6±8.2 CNT: 62.7±8.0 |
candesartan 32 mg: 32.4±3.4 candesartan 16 mg: 30.6±4.2 CNT: 31.5±4.6 |
Patients with hypertension |
24 weeks | candesartan 32 mg or candesartan 16 mg or patients that received antihypertensive treatment other than ARBs or ACEIs |
candesartan 32 mg: 7±5 candesartan 16 mg: 4±4 CNT: 3±5 |
candesartan 32 mg: +2 candesartan 16 mg: +1 CNT: +5 |
| van der Zijl et al.(2011) | valsartan: 21 PLA: 20 |
56.7±1 | valsartan: 29.0±0.7 PLA: 28.2±1.0 |
Individuals with impaired glucose metabolism |
26 weeks | valsartan 320 mg/d or placebo |
valsartan: 2.8±1.1 PLA: 2.8±0.6 |
valsartan: − 0.64 PLA: +0.18 |
| Tousoulis et al.(2008) | irbesartan: 20 CNT: 20 |
irbesartan: 58.4±1.9 CNT: 60.0±2.9 |
irbesartan: 24.5±0.27 CNT: 24.1±0.28 |
Normotensive patients with CAD |
4 weeks | irbesartan 75 mg/d or no antihypertensive agent |
irbesartan: 4.08±0.58 CNT: 3.5±0.79 |
irbesartan: −1.43* CNT: −1.2 |
| Persson et al.(2006) | irbesartan: 143 PLA: 126 |
irbesartan: 57.3±8.0 PLA: 58.4±9.0 |
irbesartan: 29.8±4.4 PLA: 30.4±4.3 |
Patients with T2DM and microalbuminuria |
96 weeks | irbesartan 300 mg/d vs placebo |
birbesartan: 3.13 (2.62– 3.74) PLA: 2.96 (2.43–3.61) |
irbesartan: −0.29*** (vs PLA) PLA: +0.49 |
| Link et al. (2006) | telmisartan: 21 PLA: 21 |
telmisartan: 56.4±7.1 PLA: 58.5±11.6 |
telmisartan: 27.3±3.6 PLA: 26.9±2.9 |
Patients with stable CAD and essential hypertension |
12 weeks | telmisartan 40 mg/d vs placebo |
telmisartan: 3.1±0.5 PLA: 3.0±0.5 |
telmisartan: −0.1 PLA: +2.1 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, PLA= placebo, CNT= control, CHF= chronic heart failure, T2DM= type 2 diabetes mellitus, CAD= coronary artery disease, ACEIs= angiotensin-converting enzyme inhibitors.
= All values are means (± standard deviation (SD)).
= mean (95% confidence interval (CI)).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001
In the intervention groups, mean levels of IL-6 and CRP at baseline were 2.8±1.4 pg/ml and 4.4±3.3 mg/L, respectively. Overall, after treatment, IL-6 levels (SMD: −0.37, 95% confidence interval (CI): −0.59 to −0.16, p < 0.001; Fig. 3) and CRP levels (SMD: −0.2, 95% CI: −0.39 to −0.02, p < 0.05; Fig. 4) significantly decreased compared to placebo/control groups. There was not significant dosage or treatment duration effects for either IL-6 or CRP. We found a high heterogeneity across the studies (I2= 93.8% for IL-6 studies, I2= 85.1% for CRP studies), but no significant risk of small-study effect (Fig. A.2, Supplementary materials).
Fig. 3.
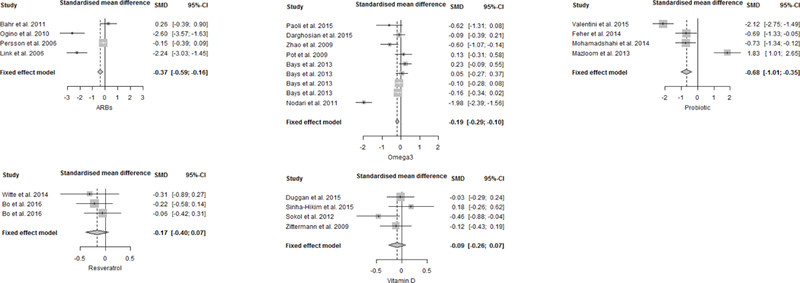
Forest plots for effect of interventions compared to placebo group on IL-6 levels. A standardized difference in means <0 favours intervention and >0 favours the placebo arm. Box size represents study weighing. Diamond represents overall effect size and 95% confidence intervals.
Fig. 4.
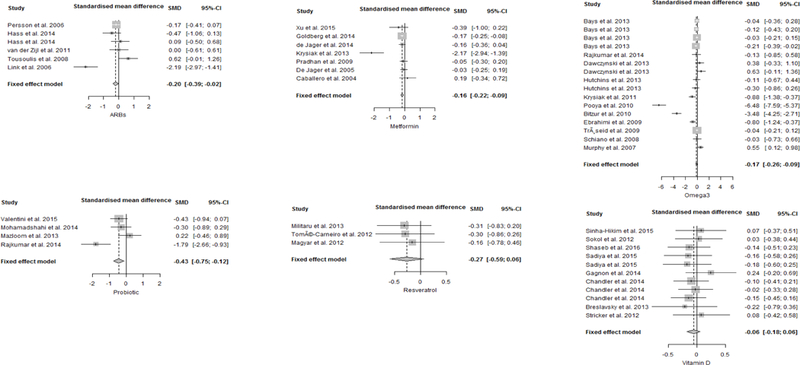
Forest plots for effect of interventions compared to placebo group on CRP levels. A standardized difference in means <0 favours intervention and >0 favours the placebo arm. Box size represents study weighing. Diamond represents overall effect size and 95% confidence intervals.
3.3.2. Metformin
Seven of 142 potential studies described the effects of metformin in 3,247 participants with mean CRP levels of 3.8 mg/L (Caballero et al., 2004; De Jager et al., 2005; de Jager et al., 2014; Goldberg et al., 2014; Krysiak et al., 2013; Pradhan et al., 2009; Xu et al., 2015). No study with the above mentioned inclusion criteria reported IL-6 levels. Thus, all the included studies investigated the effect of metformin on CRP levels. Table 3 shows the characteristics of each trial.
Table 3.
General characteristics of randomized controlled trials testing the effect of metformin supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/ Health status |
Duration | Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| CRP (mg/L) studies | ||||||||
| Xu et al.(2015) | INT: 21 PLA: 21 |
INT: 55.2±10.4 PLA: 55.1±10.9 |
- | Patients with carotid artery atherosclerosis |
12 weeks | metformin 1000 mg/d |
aINT: 3.5 (1.3–9.9) PLA: 3.1 (0.9–7.8) |
INT: −2.2** PLA: −0.2 |
| Goldberg et al.(2014) | MET: 1073 PLA: 1082 |
MET: 50.9±10.3 PLA: 50.3±10.4 |
MET: 33.9±6.6 PLA: 34.2±6.7 |
Participants at high risk for T2DM |
3.4 years | metformin 1700 mg/d or placebo or intensive lifestyle |
bMET: 3.34 (3.13–3.57) PLA: 3.52 (3.30–3.75) |
MET: − 0.36* (vs PLA) PLA: −0.08 |
| de Jager et al.(2014) | MET: 196 PLA: 194 |
MET: 64±10 PLA: 59±11 |
MET: 30±5 PLA: 30±5 |
Patients with T2DM treated with insulin |
4.3 years | metformin 850 mg or placebo (one to three times daily) added to insulin therapy |
bMET: 3.06 (2.63–3.56) PLA: 3.06 (2.61–3.58) |
MET: − 0.45* (vs PLA) PLA: +0.06 |
| Krysiak et al.(2013) | FEN+MET: 22 FEN+PLA: 20 |
FEN+MET: 50±4 FEN+PLA: 51±4 |
FEN+MET: 28.5±2.6 FEN+PLA: 28.3±2.2 |
Patients with isolated IGT |
12 weeks | fenofibrate 200 mg/d + metformin 3000 mg/d or fenofibrate 200 mg/d + placebo |
FEN+MET: 2±0.5 FEN+PLA: 2.2±0.5 |
FEN+MET: −0.8** FEN+PLA: −0.1 |
| Pradhan et al.(2009) | MET: 126 PLA: 124 |
MET: 53.8±11.5 PLA: 54.0±10.9 |
MET: 36.2±8.1 PLA: 37.2±8.2 |
Patients with T2DM |
14 weeks | 2×2 factorial trial of insulin glargine and placebo- controlled metformin (500 mg/d up-titrated to max 2000 mg/d) |
bMET: 4.3 (3.6–5.1) PLA: 4.7 (3.9–5.6) |
MET: −0.7 PLA: −0.9 |
| De Jager et al.(2005) | INT: 150 PLA: 163 |
INT: 63±9 PLA: 59±11 |
INT: 29.9±5.2 PLA: 29.5±4.6 |
Patients with T2DM treated with insulin |
16 weeks | metformin 850 mg or placebo (one to three times daily) added to insulin therapy |
bINT: 3.26 (2.80–3.78) PLA: 3.27 (2.80–3.81) |
INT: −0.02 PLA: +0.07 |
| Caballero et al.(2004) | INT: 29 PLA: 26 |
INT: 47.7±9.8 PLA: 49.3±9.6 |
INT: 30.0±6.2 PLA: 29.8±3.5 |
Participants with IGT |
16 weeks | metformin 2000 mg/d or placebo |
INT: 5.5±1.3 PLA: 5.3±0.7 |
INT: −0.1 PLA: −0.1 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, CNT= control, PLA= placebo, T2DM= type 2 diabetes, MET= metformin, IGT= impaired glucose tolerance, FEN= fenofibrate.
= All values are means (± standard deviation (SD)).
= median (interquartile range).
= mean (95% confidence interval (CI)).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001
Metformin treatment significantly reduced serum CRP concentrations compared to placebo (SMD: −0.16, 95% CI: −0.22 to −0.09, p < 0.0001, I2= 79.9%; Fig. 4). Analysis adjusted for the dosages showed that a greater reduction of CRP levels was associated with higher doses of metformin (−0.87, standard error (SE): 0.39; p < 0.05), but there were not significant treatment duration effects. Although visual inspection of the Funnel plot showed asymmetry (Fig. A.3, Supplementary materials) confirmed by Egger’s test (p= 0.05), excluding data from Krysiak et al. (2013), the effect size of metformin treatment did not change significantly.
3.3.3. Omega-3
Sixteen of 324 potential studies described the effects of omega-3 in 2,576 participants (Bays et al., 2013; Bitzur et al., 2010; Darghosian et al., 2015; Dawczynski et al., 2013; Ebrahimi et al., 2009; Hutchins et al., 2013; Krysiak et al., 2011; Murphy et al., 2007; Nodari et al., 2011; Paoli et al., 2015; Pooya et al., 2010; Pot et al., 2009; Rajkumar et al., 2014; Schiano et al., 2008; Troseid et al., 2009; Zhao et al., 2009). Characteristics of included studies are displayed in Table 4.
Table 4.
General characteristics of randomized controlled trials testing the effect of omega-3 supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/ Health status |
Duration | Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/ml) studies | ||||||||
| Paoli et al.(2015) | MD+ω 3: 16 MD: 18 |
MD+ω3: 58.1±6.0 MD: 56.3±5.1 |
MD+ω3: 29.2±2.3 MD: 29.3±2.4 |
Male overweight participants |
4 weeks | ketogenic MD with and without supplementatio n of omega-3 230 mg/d |
MD+ω3: 6.55±1.41 MD: 6.13±0.81 |
MD+ω3: − 2.9* MD: −1.75 |
| Darghosian et al.(2015) | INT: 126 PLA: 64 |
INT: 62±12 PLA: 61±11 |
INT: 30.1±7.2 PLA: 31.9±7.3 |
Patients with paroxysmal or persistent atrial fibrillation |
24 weeks | 4 g/d of omega-3 or placebo (corn oil) |
aINT: 2.8 (1.5–4.4) PLA: 2.5 (1.3–4.5) |
INT: −0.6 PLA: −0.1 |
| Zhao et al.(2009) | INT: 38 PLA: 37 |
INT: 74±6 PLA: 71±10 |
INT: 24.7±3.6 PLA: 24.0±2.9 |
Patients with CHF | 12 weeks | 2 g/d of omega 3 or placebo |
INT: 11.5±7.9 PLA: 11.1±10.2 |
INT: −3.6** PLA: +1.7 |
| Pot et al. (2009) | INT: 39 PLA: 38 |
INT: 58±4.3 PLA: 59.5±5.3 |
INT: 26.5±3.2 PLA: 26.6±3.6 |
Healthy participants |
12 weeks | 1.5 g/d of omega-3 or placebo (high oleic sunflower oil) |
aINT: 8.00 (1.66–31.3) PLA: 3.42 (2.64–50) |
INT: −1.16 PLA: 0 |
| Bays et al.(2013) | IPE 4g/d: 77 IPE 2g/d: 76 PLA: 76 |
IPE 4g/d: 51.9±10.2 IPE 2g/d: 53.4±9.3 PLA: 53.4±8.3 |
IPE 4g/d: 30.4±4.3 IPE 2g/d: 30.8±4.2 PLA: 31.0±4.2 |
Hypertriglyceridem ic patients from MARINE study |
12 weeks | IPE 4g/d vs IPE 2g/d vs placebo |
aIPE 4g/d: 2.3 (3.34) IPE 2g/d: 3.0 (2.78) PLA: 2.5 (4.12) |
IPE 4g/d: +0.1 IPE 2g/d: 0 PLA: −0.2 |
| Bays et al.(2013) | IPE 4g/d: 233 IPE 2 g/d: 236 PLA: 233 |
IPE 4g/d: 61.6±10 IPE 2g/d: 61.8±9.4 PLA: 61.2±10.5 |
IPE 4g/d: 32.7±4.9 IPE 2g/d 32.9±4.9 PLA: 33.0±5.0 |
Hypertriglyceridem ic patients from ANCHOR study |
12 weeks | IPE 4 g/d vs IPE 2g/d vs placebo |
aIPE 4g/d: 2.7 (2.61) IPE 2g/d: 2.4 (2.01) PLA: 3.2 (3.23) |
IPE 4g/d: − 0.1 IPE 2g/d: +0.3 PLA: −0.3 |
| Nodari et al.(2011) | INT: 67 PLA: 66 |
INT: 61±11 PLA: 64±9 |
INT: 25.9±2.3 PLA: 25.7±2.22 |
Patients with CHF | 48 weeks | 2 g/d of omega 3 or placebo (olive oil) |
INT: 11.0±6.0 PLA: 10.1±4.5 |
INT: − 7.47*** PLA: +1.1*** |
| CRP (mg/L) studies | ||||||||
| Rajkumar et al.(2014) | ω3: 15 PRO: 15 PRO+ω 3: 15 PLA: 15 |
49 | 28.79 | Overweight healthy adults |
6 weeks | VSL#3 1 cps/d, omega- 3: 180 mg EPA + 120 mg DHA/d, VSL#3+ omega-3 or placebo |
ω3: 5.60±0.74 PRO: 5.60±0.52 PRO+ω3: 6.20±0.41 PLA: 5.30±0.58 |
ω3: −0.34* PRO: − 1.24** PRO+ω3: − 1.90** PLA: +0.05 |
| Dawczynski et al.(2013) | HIGH: 16 LOW: 17 PLA: 14 |
HIGH: 61.8±7.1 LOW: 61.6±11.8 PLA: 58.2±7.4 |
HIGH: 25.9±3.6 LOW: 26.8±3.9 PLA: 26.1±3.8 |
hypertriacylglycero lemic participants |
10 weeks | Yogurt supplemented with omega-3 0.8 g/d (LOW), 3 g/d (HIGH) or placebo (commercial fruit yoghurt) |
HIGH: 2.93±2.76 LOW: 3.87±4.19 PLA: 1.50±1.64 |
HIGH: +1.11 LOW: −1.24 PLA: 0.25 |
| Hutchins et al.(2013) | 25 | 58.6±6.3 | 30.4±5.3 | Overweight or obese individuals with pre-diabetes |
12 weeks | Crossover trial with 0, 2.9 g/d (LOW), or 5.8 g/d (HIGH) of ALA |
HIGH: 3.2±2.8 LOW: 3.0±3.2 CNT: 2.9±3.0 |
HIGH: − 0.4±1.2 LOW: +0.4±2.4 CNT: +0.9±2.1 |
| Bays et al.(2013) | IPE 4g/d: 77 IPE 2g/d: 76 PLA: 76 |
IPE 4g/d: 51.9±10.2 IPE 2g/d: 53.4±9.3 PLA: 53.4±8.3 |
IPE 4g/d: 30.4±4.3 IPE 2g/d: 30.8±4.2 PLA: 31.0±4.2 |
Hypertriglyceridem ic patients from MARINE study |
12 weeks | IPE 4 g/d vs IPE 2 g/d vs placebo |
aIPE 4g/d: 2.2 (3.10) IPE 2g/d: 2.0 (2.70) PLA: 1.8 (3.05) |
IPE 4g/d: 0** (vs PLA) IPE 2g/d: +0.4 PLA: +0.7 |
| Bays et al.(2013) | IPE 4g/d: 233 IPE 2 g/d: 236 PLA: 233 |
IPE 4g/d: 61.6±10 IPE 2g/d: 61.8±9.4 PLA: 61.2±10.5 |
IPE 4g/d: 32.7±4.9 IPE 2g/d 32.9±4.9 PLA: 33.0±5.0 |
Hypertriglyceridem ic patients from ANCHOR study |
12 weeks | IPE 4 g/d vs IPE 2 g/d vs placebo |
aIPE 4g/d: 2.2 (2.70) IPE 2g/d: 1.9 (2.90) PLA: 2.2 (4.00) |
IPE 4g/d: −0.2*** (vs PLA) IPE 2g/d: +0.6 PLA: +0.4 |
| Krysiak et al.(2011) | ω3: 34 PLA: 32 |
ω3: 53.1±3.5 PLA: 52.5±3.1 |
ω3: 28.6±2.8 PLA: 28.3±2.4 |
hypertriglyceridae mic participants |
12 weeks | omega-3 2 g/d or placebo |
ω3: 3.3±0.5 PLA: 3.1±0.3 |
ω3: −0.8 PLA: −0.2 |
| Pooya et al.(2010) | INT: 40 PLA: 41 |
INT: 56.4±9.2 PLA: 52.7±10.6 |
INT: 27.7±3.4 PLA: 26.1±5.0 |
Patients with T2DM |
8 weeks | 2.7 g/d of omega-3 or placebo (sunflower oil) |
INT: 2.70±0.02 PLA: 3.15±0.36 |
INT: −0.22 PLA: +0.65 |
| Bitzur et al.(2010) | INT: 34 PLA: 33 |
INT: 51.0±1.9 PLA: 48.3±1.6 |
INT: 27.8±0.8 PLA: 28.0±0.6 |
Participants with mixed hyperlipidemia |
12 weeks | 1.6 g free plant sterols and 1.3 g EPA+DHA or 4 g corn oil (placebo) daily. |
INT: 3.1±0.6 PLA: 3.3±0.4 |
INT: −0.6 ** (vs PLA) PLA: +1.2 |
| Ebrahimi et al.(2009) | INT: 47 CNT: 42 |
INT: 53.5±12.7 CNT: 52.3±11.1 |
INT: 30.3±5.2 CNT: 30.4±6.1 |
Participants with metabolic syndrome |
24 weeks | single capsule containing 180 mg EPA and 120 mg DHA daily, or no supplementatio n (control) |
aINT: 9.37 (5.4–19.39) CNT: 7.73 (5.02–16.32) |
INT: − 6.25** CNT: −0.93 |
| Troseid et al.(2009) | ω3: 282 PLA: 281 |
a70 (67–73) |
a26.5 (24.1– 28.7) |
Elderly men with high risk of cardiovascular disease |
144 weeks | Randomized 2 x 2 factorial- designed trial comparing Mediterranean like diet, omega-3 2.4 g/d, or combination. |
aω3: 3.58 (4.08) PLA: 3.13 (4.23) |
ω3: −0.66* PLA: −0.09 |
| Schiano et al.(2008) | INT: 16 CNT: 16 |
aINT: 66 (58–71) CNT: 66 (59–73) |
aINT: 27 (25.8– 30) CNT: 26.8 (24.4– 29.7) |
Patients with peripheral arterial disease |
12 weeks | 2 g/d of omega-3 |
aINT: 2.6 (2.1–5.8) CNT: 3.1 (2.1–3.9) |
INT: +0.3 CNT: −0.1 |
| Murphy et al.(2007) | INT: 42 CNT: 44 |
INT: 50.4±14.5 CNT: 50.2±9.4 |
INT: 31.4±5.0 CNT: 32.4±5.1 |
Overweight volunteers with high levels of triacylglycerols |
24 weeks | omega-3- enriched foods to achieve an EPA + DHA intake of 1 g/d or control foods (not enriched) |
INT: 5.2±0.7 CNT: 3.9±0.4 |
INT: +0.4 CNT: +1.2 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, CNT= control, PLA= placebo, CHF= chronic heart failure, PAD= peripheral artery disease, T2DM= type 2 diabetes, EPA= eicosapentaenoic acid, DHA= docosahexaenoic acid, MD= Mediterranean diet, PRO= probiotics, ω3= omega-3, IPE= icosapent ethyl.
= All values are means (± standard deviation (SD)).
= median (interquartile range).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001.
On average, at baseline in omega-3 groups, IL-6 levels were 10.6±6.3 pg/ml and CRP levels were 3.5±2.0 mg/L. IL-6 levels were significantly lower after omega-3 supplementation compared to the respective controls (SMD: −0.19, 95% CI: −0.29 to −0.10, p < 0.0001; Fig. 3). Omega 3 interventions resulted in a significantly more pronounced decrease in CRP levels as compared to placebo (SMD: −0.17, 95% CI: −0.26 to −0.09, p < 0.0001; Fig. 4). Meta-regression identified a significant decrease of IL-6 levels as the duration of the treatment increased (−0.04, SE: 0.01, p < 0.0001), but no significant treatment duration effects for CRP. There were also not significant dosage effects for either IL-6 or CRP. Heterogeneity was high for both IL-6 (I2= 90.9%) and CRP studies (I2= 93.6%). There was no evidence of small-study effect (Egger’s test: p= 0.32) for IL-6. Since there was evidence of small-study effect for CRP (Egger’s test: p < 0.001, Fig. A.4, Supplementary materials), we performed a sensitivity analysis excluding data from Pooya et al. (2010) and Bitzur et al. (2010). After removal of these two studies, the CRP-lowering effect of omega-3 was still significant.
3.3.4. Probiotics
Five of 98 potential studies investigated the effect of probiotics in a total of 210 participants (Feher et al., 2014; Mazloom et al., 2013; Mohamadshahi et al., 2014; Rajkumar et al., 2014; Valentini et al., 2015). Table 5 shows the characteristics of included studies.
Table 5.
General characteristics of randomized controlled trials testing the effect of probiotics supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/Health status | Duratio n |
Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/ml) studies | ||||||||
| Valentini et al. (2015) | DIET: 31 DIET+VSL# 3: 31 |
70.1±3.9 | 26.8±3.6 | Healthy persons aged 65–85 years |
8 weeks | Web-based dietary advice (RISTOMED platform) alone or with supplementati on of VSL#3 (2 cps/d) 112.5×109 CFU/capsule |
DIET: 29.9±9.1 DIET+VSL# 3: 12.3±1.8 |
DIET: +4.8 DIET+VSL# 3: +5.4 |
| Feher et al.(2014) | INT: 20 CNT: 20 |
INT: 45.15±4.7 3 CNT: 45.95±6.6 5 |
- | Patients with irritable eye syndrome |
8 weeks | three softgels/d each containing lysate of L. acidophilus (1.25×109 CFU) and B. longum (1.35×109 CFU) vs control group (cod liver oil) |
INT: 17.42±10.52 CNT: 17.21±5.26 |
INT: − 4.55*** CNT: +0.1 |
| Mohamadshahi et al.(2014) | INT: 22 CNT: 22 |
INT: 53±5.9 CNT: 49±7.08 |
INT: 28.36±4. 14 CNT: 29.22±3. 2 |
Patients with T2DM |
8 weeks | 300 g/d probiotic and conventional yogurts with L. delbrueckii subsp. bulgaricus and S. thermophilus. The probiotic yogurt also enriched with B. animalis subsp. lactis Bb12 and L. acidophilus strain La5 (3.7 × 106 CFU/g of both) |
INT: 22.6±2.81 CNT: 23.64±3.31 |
INT: −0.42 CNT: +0.55 |
| Mazloom et al. (2013) | INT: 16 PLA: 18 |
INT: 55.4±8 PLA: 51.8±10.2 |
INT: 27.97±3. 81 PLA: 27.24±2. 73 |
Patients with T2DM |
6 weeks | probiotic capsules (L. acidophilus, L. bulgaricus, L. bifidum, and L. casei) 1500 mg/ bid vs placebo (1000 mg magnesium stearate/ bid) |
INT: 4.51±0.45 PLA: 4.38±0.80 |
INT: −0.68 PLA: −1.29* |
| CRP (mg/L) studies | ||||||||
| Valentini et al. (2015) | DIET: 31 DIET+VSL# 3: 31 |
70.1±3.9 | 26.8±3.6 | Healthy persons aged 65–85 years |
8 weeks | Web-based dietary advice (RISTOMED platform) alone or with supplementati on of VSL#3 (2 cps/d) |
DIET: 3.6±0.6 DIET+VSL# 3: 2.9±0.7 |
DIET: +0.2 DIET+VSL# 3: −0.5 |
| Rajkumar et al. (2014) | PRO: 15 ω3: 15 PRO+ω3: 15 PLA: 15 |
49 | 28.79 | Overweight healthy adults |
6 weeks | VSL#3 1 cps/d (112.5×109 CFU/capsule), omega-3: 180 mg EPA + 120 mg DHA/d, VSL#3+ omega-3 or placebo |
PRO: 5.60±0.52 ω3: 5.60±0.74 PRO+ω3: 6.20±0.41 PLA: 5.30±0.58 |
PRO: −1.24** ω3: −0.34* PRO+ω3: −1.90** PLA: +0.05 |
| Mohamadshahi et al.(2014) | INT: 22 CNT: 22 |
INT: 53±5.9 CNT: 49±7.08 |
INT: 28.36±4. 14 CNT: 29.22±3. 2 |
Patients with T2DM |
8 weeks | 300 g/d probiotic and conventional yogurts with L. delbrueckii subsp. bulgaricus and S. thermophilus. The probiotic yogurt also enriched with B. animalis subsp. lactis Bb12 and L. acidophilus strain La5 (3.7×106 CFU/g of both) |
INT: 3.26±1.36 CNT: 3.08±1.54 |
INT: −0.46 CNT: +0.21 |
| Mazloom et al. (2013) | INT: 16 PLA: 18 |
INT: 55.4±8 PLA: 51.8±10.2 |
INT: 27.97±3. 81 PLA: 27.24±2. 73 |
Patients with T2DM |
6 weeks probiotic | probiotic capsules (L. acidophilus, L. bulgaricus, L. bifidum, and L. casei) 1500 mg/ bid vs placebo (1000 mg magnesium stearate/ bid) |
INT: 3.17±0.7 PLA: 2.17±0.41 |
INT: +1.16 PLA: +1.89 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, CNT= control, PLA= placebo, PRO= probiotics, ω3= omega-3, T2DM= type 2 diabetes.
All values are means (± standard deviation (SD)).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001.
On average, concentration of IL-6 and CRP at baseline in intervention groups were 13.8±2.0 pg/ml and 3.1±1.0 mg/L, respectively. Probiotic supplementation significantly reduced both inflammatory biomarkers. The pooled effect sizes were −0.68 (95% CI: −1.01 to −0.35, p < 0.0001; Fig. 3) for IL-6 and −0.43 (95% CI: −0.75 to −0.12, p < 0.01; Fig. 4) for CRP. Moreover, meta-regression analysis showed a significant reduction of IL-6 levels as the duration of probiotic treatment increased (−1.13, SE: 0.39, p < 0.01), but there were not significant dosage effects for IL-6. There were not a sufficient number of studies to evaluate the dosage or treatment duration effects of probiotics on CRP. Heterogeneity was high for IL-6 studies (I2= 94.7%) as well as CRP studies (I2 = 77.3%), with evidence of asymmetry at Funnel plot for IL-6 studies (Fig. A.5, Supplementary materials).
3.3.5. Resveratrol
Five of 33 potential studies were identified upon resveratrol effect in a total of 372 participants (Bo et al., 2016; Magyar et al., 2012; Militaru et al., 2013; Tome-Carneiro et al., 2012; Witte et al., 2014). Table 6 presents main characteristics of the studies.
Table 6.
General characteristics of randomized controlled trials testing the effect of resveratrol supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/ Health status |
Duration | Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/ml) studies | ||||||||
| Bo et al.(2016) | HIGH: 62 LOW: 59 PLA: 58 |
HIGH: 65.0±7.6 LOW: 64.9±8.6 PLA: 65.4±8.8 |
HIGH: 28.8±3.9 LOW: 29.5±3.8 PLA: 28.2±3.9 |
Patients with T2DM |
24 weeks | resveratrol 500 mg/day (HIGH), resveratrol 40 mg/day (LOW) or placebo |
HIGH: 2.55 (2.15) LOW: 2.71 (2.37) PLA: 2.83 (1.91) |
HIGH: − 0.09 LOW: +0.04 PLA: +0.22 |
| Witte et al. (2014) | INT: 23 PLA: 23 |
INT: 64.8±6.8 PLA: 63.7±5.3 |
INT: 27.4±1.9 PLA: 27.7±1.6 |
Healthy older adults |
26 weeks | 200 mg of resveratrol and 320 mg of quercetin or placebo |
INT: 2.9±1.2 PLA: 7.9±19.8 |
INT: − 0.9** PLA: −3.9* |
| CRP (mg/L) studies | ||||||||
| Militaru et al.(2013) | INT: 29 CNT: 29 |
INT: 64.9±5.8 CNT: 64.2±7.1 |
c24–27 | Participants with stable angina pectoris |
8 weeks | resveratrol 20 mg/d | INT: 6.9±2.5 CNT: 6.6±2.4 |
INT: −1.7* CNT: − 0.7* |
| Tome-Carneiro et al.(2012) | GE- RES: 25 GE: 25 PLA: 24 |
GE-RES: 62±9 GE: 56±11 PLA: 63±9 |
GE-RES: 32±9 GE: 31±5 PLA: 29±3 |
Participants at high risk of CVD |
48 weeks | GE-RES: grape extract + 8.1±0.5 mg of resveratrol per capsule. One cps/d for the first 6 months and 2 cps/d for the following 6 months, GE: conventional grape extract lacking resveratrol or placebo |
GE-RES: 5±3.7 GE: 3.2±2.5 PLA: 4.4±4.3 |
GE-RES: − 1.3* GE: +0.1 PLA: +0.4 |
| Magyar et al. (2012) | INT: 20 PLA: 20 |
INT: 65.3±9.7 PLA: 67.4±7.7 |
INT: 29.3±2.1 PLA: 28.1±3.2 |
Participants with stable CAD |
12 weeks | resveratrol 10 mg/day | INT: 3.64±0.57 PLA: 3.27±0.35 |
INT: +2.87 PLA: +3.76 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, CNT= control, PLA= placebo, T2DM= type 2 diabetes, RES= resveratrol, GE= grape extract, CVD= cardiovascular disease, CAD= coronary artery disease.
All values are means (± standard deviation (SD)).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001.
Baseline serum concentrations of IL-6 and CRP in participants undergoing intervention were respectively 2.7±2.1 pg/ml and 4.4±2.8 mg/L. No significant effect was shown after resveratrol supplementation, with mean IL-6 decrease of −0.17 (95% CI: −0.40 to 0.07, p > 0.05; Fig. 3) and mean CRP decrease of −0.27 (95% CI: −0.59 to 0.06, p > 0.05; Fig. 4). There were not a sufficient number of studies to evaluate the dosage or treatment duration effects of resveratrol on either IL-6 or CRP. Heterogeneity was low for both IL-6 and CRP studies (I2= 0%) and there was no evidence of small-study effect (Fig. A.6, Supplementary materials).
3.3.6. Vitamin D
Ten of 115 potential studies investigated the relationship between vitamin D supplementation (cholecalciferol or ergocalciferol) and levels of inflammatory biomarkers in 1,314 adults (Breslavsky et al., 2013; Chandler et al., 2014; Duggan et al., 2015; Gagnon et al., 2014; Sadiya et al., 2015; Shaseb et al., 2016; Sinha-Hikim et al., 2015; Sokol et al., 2012; Stricker et al., 2012; Zittermann et al., 2009). Table 7 shows main characteristics of included trials.
Table 7.
General characteristics of randomized controlled trials testing the effect of vitamin D supplementation on IL-6 and CRP.
| Study | N | Age (years)^ |
BMI (kg/m2)^ |
Population/ Health status |
Duration | Intervention | Baseline levels^ |
Δ levels from baseline^ |
|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/ml) studies | ||||||||
| Duggan et al. (2015) | INT: 109 PLA: 109 |
INT: 60.3±5.3 PLA: 59.0±4.7 |
INT: 32.3±5.5 PLA: 32.5±6.1 |
postmenopausal, overweight or obese women with vitamin-D Insufficiency |
48 weeks | vitamin D3 2000 IU/d + a lifestyle- based weight-loss program or placebo + a lifestyle-based weight-loss program |
bINT: 3.89 (3.33–4.53) PLA: 3.89 (3.15–4.79) |
bINT: − 0.48 PLA: − 0.37 |
| Sinha-Hikim et al. (2015) | INT: 40 PLA: 40 |
INT: 51.6±7.7 PLA: 52.4±6.7 |
INT: 32.5±4.4 PLA: 32.9±4.5 |
Participants with pre-diabetes and hypovitaminosis D |
48 weeks | vitamin D3 supplementation average dose (±SD) 85300 IU ± 16000/week i.m. |
INT: 3.4±2 PLA: 3.2±2 |
INT: +0.3 PLA: +0.1 |
| Sokol et al.(2012) | INT: 45 PLA: 45 |
INT: 55±9.6 PLA: 56.9±11.6 |
INT: 29.6±6.2 PLA: 30.9±7.6 |
Participants with CAD and vitamin D deficiency |
12 weeks | ergocalciferol 50000 IU/week |
aINT: 4.1 (6.8) PLA: 4.8 (4.5) |
aINT: +0.59 PLA: +0.76 |
| Zittermann et al.(2009) | INT: 82 PLA: 83 |
INT: 47.4±10.3 PLA: 48.8±10.1 |
INT: 33.7±4.1 PLA: 33.0±4.3 |
Healthy Overweight participants and hypovitaminosis D |
48 weeks | vitamin D3 3332 IU/d or placebo while participating in a weight- reduction program |
INT: 8.9±15.2 PLA: 7.8±12.3 |
INT: − 3.5±14.0 PLA: − 1.9±10.9 |
| CRP (mg/L) studies | ||||||||
| Shaseb et al. (2016) | INT: 55 PLA: 57 |
INT: 54±6.13 PLA: 55.9±5.24 |
INT: 27.5± 2.8 PLA: 26.9±2.8 |
Patient with T2DM and CAD |
8 weeks | single-dose administration of either vitamin D 300000 IU i.m. or placebo |
INT: 3.4±3.2 PLA: 3.0±2.3 |
INT: 0 PLA: +0.8 |
| Sadiya et al. (2015) | INT: 45 PLA: 42 |
INT: 49±8 PLA: 48±8 |
INT:37.9±6.1 PLA: 37.6±7.7 |
vitamin D- deficient obese, T2DM participants |
24 weeks | Two phases of 3 months each. In phase 1: vitamin D3 6000 IU/d vs placebo capsules. In phase 2: vitamin D3 3000 IU/d vs placebo |
INT: 7.4±7.6 PLA: 8.0±9.2 |
12 weeks: INT: +0.4 PLA: +1.5 24 weeks: INT: +0.6 PLA: +2 |
| Sinha-Hikim et al. (2015) | INT: 40 PLA: 40 |
INT: 51.6±7.7 PLA: 52.4±6.7 |
INT: 32.5±4.4 PLA: 32.9±4.5 |
Participants with pre-diabetes and hypovitaminosis D |
48 weeks | vitamin D3 supplementation average dose (±SD) 85300 IU ± 16000/week i.m. |
INT: 5.6±2.4 PLA: 5.6±2.7 |
INT: +0.2 PLA: 0 |
| Gagnon et al. (2014) | INT: 35 PLA: 45 |
INT: 53.8±11.9 PLA: 55.3±11.1 |
INT: 31.1±5.7 PLA: 31.9±6.2 |
Vitamin D- Deficient participants at risk of T2DM |
24 weeks | CA 1200 mg/d + vitamin D3 2000– 6000 IU/d to target 25(OH)D >75 nmol/L |
INT: 5.52±10.82 PLA: 2.44±2.15 |
INT: −1.9 PLA: +0.13 |
| Chandler et al. (2014) | 328 | a51 | a31 | Healthy black Population |
12 weeks | vitamin D3 1000 IU/d, 2000 IU/d or 4000 IU/d or placebo |
a4000 IU: 2.23 (0.66– 5.35) 2000 IU: 2.18 (0.6– 6.74) 1000 IU: 1.95 (0.79– 5.02) PLA: 2.74 (1.14–4.56) |
a4000 IU: −0.25 2000 IU: +0.21 1000 IU: +0.26 PLA: − 0.28 |
| Breslavsky et al.(2013) | INT: 24 PLA: 23 |
INT: 66.8±9.2 PLA: 65.8±9.7 |
INT: 27.9±5.2 PLA: 30.6±5.1 |
Patients with T2DM |
48 weeks | vitamin D3 1000 IU/d |
INT: 6±5 PLA: 4±3 |
INT: −2 PLA: +1 |
| Stricker et al. (2012) | INT: 31 PLA: 31 |
INT: 72.9±8.7 PLA: 74.8±14.6 |
- | Patients with PAD | 4 weeks | Single oral supplementation of vitamin D3 100000 IU or placebo |
INT: 2.7±2.8 PLA: 3.7±5.2 |
INT: +0.3 PLA: −0.9 |
| Sokol et al.(2012) | INT: 45 PLA: 45 |
INT: 55±9.6 PLA: 56.9±11.6 |
INT: 29.6±6.2 PLA: 30.9±7.6 |
Participants with CAD and vitamin D deficiency |
12 weeks | ergocalciferol 50000 IU/week |
aINT: 2.6 (5.2) PLA: 1.8 (3.5) |
aINT: − 0.17 PLA: − 0.05 |
IL= interleukin, CRP= C-reactive protein, N= number of participants, Δ= change, INT= intervention, CNT= control, PLA= placebo, CA= calcium carbonate, 25(OH)D= 25-hydroxyvitamin D, T2DM= type 2 diabetes, PAD= peripheral artery disease, CAD= coronary artery disease.
= All values are means (± standard deviation (SD)).
= median (interquartile range)
= mean (95% confidence interval (CI)).
Change data are defined as the follow-up minus the baseline value.
: p< 0.05
: p< 0.01
: p< 0.001.
At baseline in intervention groups, mean IL-6 and CRP levels were respectively 5.6±9.4 pg/ml and 5.8±6.6 mg/L. Vitamin D supplementation did not produce any significant reduction in either inflammatory biomarker compared to placebo. The pooled effect sizes were −0.09 (95% CI: −0.26 to 0.07, p > 0.05; Fig. 3) for IL-6 and −0.06 (95% CI: −0.18 to 0.06; p > 0.05, Fig. 4) for CRP. There was not significant dosage or treatment duration effects for either IL-6 or CRP. Heterogeneity was low for both IL-6 (I2= 36.4%) and CRP studies (I2= 0%) and there was no evidence of small-study effect (Fig. A.7, Supplementary materials).
3.4. Pooled analysis
Compared to the average effect size of all compounds, probiotics significantly reduced IL-6 levels (−0.68 vs −0.3, z= −2.72 p < 0.01). Conversely, vitamin D had a smaller effect than the mean change for all compounds (−0.09 vs −0.3, z= 2.54, p < 0.05). The effect of probiotics on IL-6 levels was also significantly larger compared to those of vitamin D (−0.68 vs −0.09, z= −3.13, p 0.01), resveratrol (−0.68 vs −0.17, z= −2.47, p < 0.05) and omega-3 (−0.68 vs −0.19, z= −2.80, p 0.01), Table 8, Fig. 5. Furthermore, ARBs effect was significantly larger compared to that of vitamin D (−0.37 vs −0.09, z= −2.02, p < 0.05; Table 8, Fig. 5).
Table 8.
Z-scores in pairwise comparison of IL-6 decrease across compounds.
| ARBs | Omega-3 | Probiotic | Resveratrol | Vitamin D | |
|---|---|---|---|---|---|
|
ARBs (P-value) |
−1.501 (0.133) |
1.543 (0.123) |
−1.231 (0.218) |
−2.025 (0.043) |
|
|
Omega-3 (P-value) |
2.797 (0.005) |
−0.155 (0.877) |
-1.029 (0.303) |
||
|
Probiotic (P-value) |
−2.467 (0.014) |
−3.134 (0.002) |
|||
|
Resveratrol (P-value) |
−0.546 (0.585) |
||||
|
Vitamin D (P-value) |
Significant differences (p<0.05) are in bold. Negative z-scores indicate the compound on the left margin produced greater mean decreases compared with the compound on the top margin.
Fig. 5.
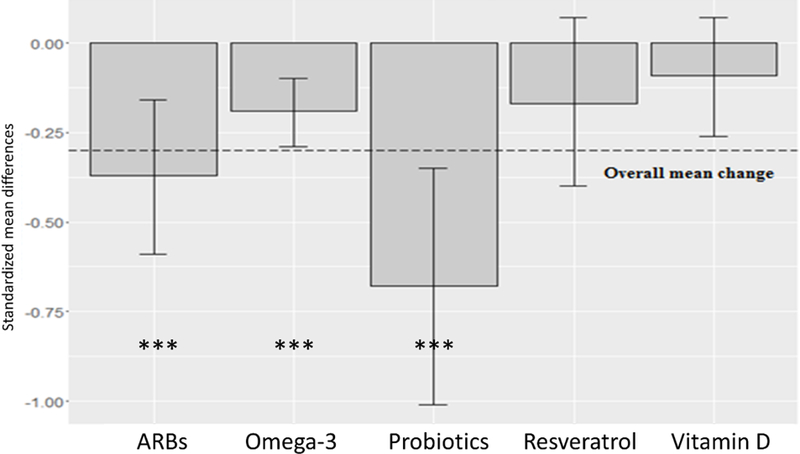
Mean decrease in IL-6 by compound. Values are given as standardized mean difference (SMD) and 95% confidence interval (CI). Dashed line shows mean change of all compounds. P-values compared to placebo/control groups = *: p <0.05; **: p <0.01; ***: p <0.001.
Average change of CRP levels considering all the included compounds was −0.21. None of the analyzed interventions yielded a significant difference in change of CRP concentrations compared to the mean effect of all compounds. However, in pairwise comparison, probiotics showed a significantly larger effect compared to vitamin D (−0.43 vs −0.06, z= −2.15, p < 0.05; Table 9, Fig. 6).
Table 9.
Z-score in pairwise comparison of CRP decrease across compounds.
| ARBs | Metformin | Omega-3 | Probiotics | Resveratrol | Vitamin D | |
|---|---|---|---|---|---|---|
|
ARBs (P-value) |
−0.400 (0.689) |
−0.289 (0.773) |
1.234 (0.217) |
0.367 (0.714) |
−1.244 (0.213) |
|
|
Metformin (P-value) |
0.183 (0.855) |
1.645 (0.100) |
0.651 (0.515) |
−1.436 (0.151) |
||
|
Omega-3 (P-value) |
1.562 (0.118) |
0.583 (0.560) |
−1.466 (0.143) |
|||
|
Probiotic (P-value) |
−0.693 (0.488) |
−2.151 (0.031) |
||||
|
Resveratrol (P-value) |
−1.188 (0.235) |
|||||
|
Vitamin D (P-value) |
Significant differences (p<0.05) are in bold. Negative z-scores indicate the compound on the left margin produced greater mean decreases compared with the compound on the top margin.
Fig. 6.
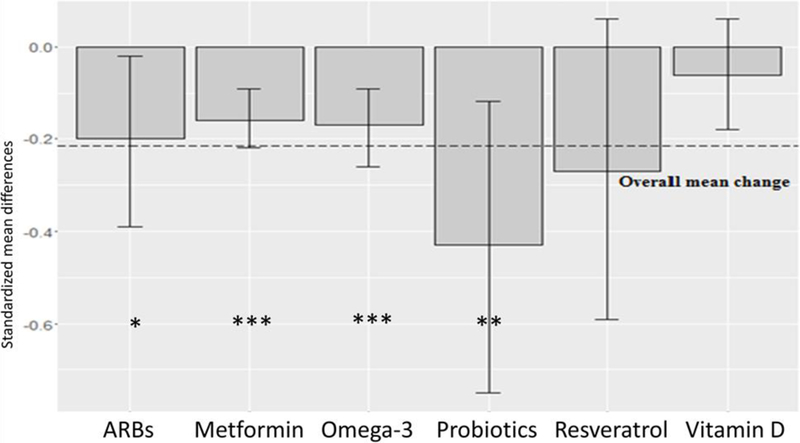
Mean decrease in CRP by compound. Values are given as standardized mean difference (SMD) and 95% confidence interval (CI). Dashed line shows mean change of all compounds. P-values compared to placebo/control groups = *: p <0.05; **: p <0.01; ***: p <0.001.
4. Discussion
The results of our meta-analysis suggest that many but not all compounds selected across a broad range of potential anti-inflammatory molecules have a significant effect on levels of IL-6 and CRP in middle-age and older adults with chronic LGI. Compared to placebo, ARBs, omega-3 and probiotics significantly reduced IL-6 levels, and ARBs, metformin, omega-3 and probiotics significantly reduced CRP levels. Effects ranged in size from small to large according to established definitions (Cohen, 1992). Two compounds, resveratrol and vitamin D, however, did not show any significant effect on the investigated inflammatory biomarkers.
In this review, among RAS inhibitors, we prioritized ARBs, also known as sartans, because of greater tolerability compared to angiotensin-converting enzyme inhibitors (ACEIs)(Caldeira et al., 2012). ARBs are widely used for the treatment of hypertension and cardiovascular diseases. They exhibit excellent safety profiles with minimal effects in normotensive patients. Our findings show that consumption of ARBs may provide a small to moderate reduction of IL-6 and CRP. Takagi et al. (2013) in a previous meta-analysis showed that telmisartan therapy is effective in reducing IL-6 (−0.38 pg/ml) and tumor necrosis factor (TNF)-α levels. The biological basis of ARBs effect on chronic LGI involves the blockade of angiotensin II type 1 receptor leading to inhibition of NF-κB signaling (Kleiber et al., 2010). In animal experiments, sartans have been shown to reduce IL-6 levels (Lin et al., 2014), improve memory (Ongali et al., 2014) and motor performance (Villapol et al., 2012) and protect against muscle loss (Burks et al., 2011). Moreover, a recent meta-analysis of RCTs and observational studies reported that the use of ARBs can reduce incidence risks by 35% for cognitive impairment and by 20% for AD (Zhuang et al., 2016). To date, no conclusion can be drawn on potential differences within drug class, due to dearth of available studies for each included sartan. Overall, these results suggest that people already treated with ARBs could take advantage from the anti-inflammatory effect of this therapy.
Metformin is the most commonly prescribed oral hypoglycemic drug and first line therapy in type 2 diabetes mellitus (T2DM). It has been used for over 60 years with an excellent safety record. A consistent body of data shows that metformin can increase health span and longevity through anti-inflammatory and anti-apoptotic pathways independent of its hypoglycemic effect (Barzilai et al., 2016; Saisho, 2015). Our results are consistent with these data, supporting a small but significant reduction of CRP levels following metformin administration. Evidence from the Diabetes Prevention Program study suggests the CRP lowering effect could be driven by weight loss achieved during metformin treatment (Haffner et al., 2005). The findings of our meta-analysis indicate this effect is significantly dependent upon metformin dosage; increased doses are associated with a greater reduction of CRP. This is in line with some findings from experimental models in which metformin reduced levels of several inflammatory mediators (IL-6, IL-1β, TNF-α, prostaglandin E2, nitric oxide) in a dose-dependent manner (Hyun et al., 2013). Also for metformin, one of the biological mechanisms underpinning this anti-inflammatory effect was the inhibition of transcription factor NF-κB (Hyun et al., 2013; Li et al., 2009). Taken together these findings indicate metformin as a potential alternative strategy to caloric restriction in reducing inflammaging.
A large body of evidence indicates that omega-3, targeting NF-κB pathway, could relieve inflammatory processes (Calder, 2015). Indeed, we found that omega −3 produce a small but significant reduction in both IL-6 and CRP. In line with our findings, three recent systematic reviews identified omega-3 supplementation as one of the most promising treatments targeting inflammation in older adults. The reviews by Molfino et al. (2014) and Ticinesi et al. (2016), however, did not focus on a population with chronic LGI and additionally, the results were from critically ill patients in which omega-3 was administered intravenously. Another meta-analysis by Li et al. (2014) confirmed the effectiveness of omega-3, highlighting that higher reduction of IL-6 and CRP levels can be achieved in chronic non-autoimmune diseases (IL-6: −0.22 pg/ml, CRP: −0.20 mg/L). Evidence of efficacy in healthy participants, however, are still inconsistent (Li et al., 2014; Rangel-Huerta et al., 2012). Interestingly, our findings indicate that the IL-6 lowering effect is directly proportional to the duration of omega-3 treatment. This finding, together with the safety and tolerability of this compound, should encourage a long-term use of n-3 polyunsaturated fatty acids (PUFAs) in primary prevention among middle-age and older adults with chronic LGI. Fortunately, such a trial is under way and results from the ENRGISE (Enabling Reduction of low-Grade Inflammation in SEniors) trial should be reported in approximately one year (Manini et al., 2017).
Probiotics are food supplements that contain live microorganisms such as Bifidobacteria and Lactobacilli. The rationale of probiotic intervention in inflammaging is built on the evidence that age–related changes in human gut microbiota composition (from fermentative to putrefactive bacterial flora) (Lakshminarayanan et al., 2014) could be related with elevated inflammatory markers and other geriatric conditions (e.g. frailty (Claesson et al., 2012), sarcopenia (Rampelli et al., 2013), cognitive impairment and AD (Porter et al., 2000; Widner et al., 1999)) by reduced production of short-chain fatty acids (SCFAs) which have a powerful immunoregulatory activity (Shapiro et al., 2014). Probiotics have the potential to rebalance gut microbiota and modulate gut immune response inhibiting the NF-κB pathway (Kim et al., 2012). Our findings suggest a significant moderate to large effect size of probiotics supplementation on reduction of inflammatory biomarkers, but it should be considered that this result could be overestimated by a significant small-study effect, at least for IL-6 studies. We also showed longer treatment durations were associated with greater reductions in IL-6. A recent meta-analysis by Mazidi et al. (2017) reported a significant reduction of serum CRP (−1.35 mg/L) following probiotics supplementation. However, the 20 RCTs that they included had mean age ranging from 6 months to 85 years, some of them had short follow-up (7 days), and also involved participants with acute inflammation. Other meta-analyses on patients with T2DM did not support a significant effect of probiotics on IL-6 and CRP levels (Samah et al., 2016; Yao et al., 2017). Perhaps the anti-inflammatory action of probiotics is stronger in specific conditions (i.e., acute disease or chronic LGI compared to others) or is dependent on the strain of microorganisms. Further research is needed to elucidate these aspects.
Another important finding of our meta-analysis was that resveratrol did not show a significant effect on IL-6 and CRP levels in chronic LGI. For resveratrol, our results are in line with those of a previous meta-analysis in which intervention did not lead to any significant reduction of CRP levels (Sahebkar et al., 2015). Interestingly, Timmers et al. (2011) demonstrated that 30 days of resveratrol supplementation induces metabolic changes in obese humans, mimicking the effects of caloric restriction, but they did not find any relation with IL-6 levels. Considering the relatively small sample size of studies included in the present review, larger clinical trials should be encouraged to draw definitive conclusions about the efficacy of resveratrol in chronic systemic inflammation.
Additionally, vitamin D did not show significant effects on either IL-6 or CRP levels. Our findings are in contrast with results of a previous meta-analysis by Chen et al. (2014) that reported a reduction of CRP levels by 1.08 mg/L after vitamin D supplementation, with a more pronounced effect in the subgroup with baseline CRP ≥5 mg/L (−2.21 mg/L). However, they included RCTs carried out in a younger population and in participants with acute inflammation as defined by CRP levels >10 mg/L. Two different systematic reviews are consistent with our findings, and they concluded that evidence of a relationship between vitamin D supplementation and inflammatory biomarkers are still weak in human studies (Chagas et al., 2012; Ticinesi et al., 2016).
4.1. Strengths and limitations
To our knowledge, this is the first meta-analysis summarizing the associations of promising nutritional and pharmacological compounds on well accepted inflammatory biomarkers (IL-6 and CRP). The six compounds examined in this meta-analysis were chosen among a broad range of compounds with anti-inflammatory properties because they met criteria representing promising, safe, tolerable, affordable and acceptable strategies for reducing chronic LGI in middle-age and older adults. This distinguishes our findings from those of other systematic reviews that included populations with acute inflammatory conditions (Chen et al., 2014; Mazidi et al., 2017; Molfino et al., 2014; Takagi et al., 2013; Ticinesi et al., 2016). Furthermore, we included only studies with a RCT design to ensure higher quality of the effects.
This review has also some limitations. First, none of the included studies were specifically designed to treat individuals with chronic LGI; this could explain the wide heterogeneity observed across the studies despite of the strict inclusion/exclusion criteria. Second, several studies had relatively small sample sizes that could potentially lead to overestimation of treatment effects. Nevertheless, we performed sensitivity analysis excluding the studies at higher risk of publication bias, and we did not detect any significant change in the results. Third, for most of the studies the assessment of chronic LGI was based only on a single baseline value which could lead to a false positive indication of an inflammatory state. Fourth, we did not investigate the “gray literature”, therefore there could be studies not published for negative outcomes that could change the magnitude of effects. Fifth, the majority of the included trials were carried out in strict population subgroups (e.g., hypertensive, diabetic, hyperlipidemic) and with different dosage of compounds, so we are unable to generalize the effectiveness in people with chronic LGI. Finally, the assays for biochemical measurements of serum IL-6 and CRP varied across studies.
4.2. Clinical perspective
In clinical practice, the presence of multiple comorbidities as infections, diabetes, hypertension, obesity, hyperlipidemia, smoking, among others are common in patients, all of which are potential causes or contributors to the chronic inflammatory state. Since the decline in functional status and development of sarcopenia in older adults frequently correlates with elevated levels of IL-6 and other inflammatory cytokines (Cesari et al., 2004; Ferrucci et al., 1999), the clinical assessment of functional status may be an indirect way of identifying the presence of an elevated inflammatory state.
Accumulating evidence also indicates that lifestyle changes involving caloric restriction and/or intermittent fasting may increase health span and longevity through a modulation of reactive oxygen species and inflammatory cytokines (Anton and Leeuwenburgh, 2013; Lee and Longo, 2011). Although diet represents a modifiable factor, these approaches typically require high levels of compliance that are difficult to achieve for most individuals. The traditional Mediterranean diet, which provides high amounts of n-3 PUFAs and polyphenols (typically lacking in modern Western diets) has been found to reduce inflammatory biomarkers (e.g. IL-6, TNF-α) independent of caloric restriction or weight loss (Hermsdorff et al., 2009).
The anti-inflammatory mechanisms of action of some of these compounds, as well as development of new medications designed to preserve muscle function are of interest. Some examples include: ARBs, which are often used as second line therapy for hypertension, renal protection activity and prevention of cardiac remodeling in congestive heart failure; metformin, a medication with proven value in diabetes especially in the setting of obesity, weight reduction without hypoglycemic risks; and omega 3, a natural compound with potential to reduce cardiovascular risk factors. Although this review did not find evidence of benefit from vitamin D supplementation for reducing levels of IL-6 or CRP, vitamin D deficiency has been associated with reduction in muscular strength and increased risk of falls, especially in elderly patients with concomitant polypharmacy.
The data generated by this review, through better understanding mechanisms of action of new and current medications, could be a foundation for their potential use as treatments reducing functional decline during aging. In clinical practice, it is also important to convey to patients that many of the readily available supplements have not been carefully evaluated in terms of their potential risks or even contraindications with some medications.
5. Conclusion
In conclusion, our findings provide support for the potential of ARBs, metformin, omega-3 fatty acids and probiotics to significantly reduce established biomarkers of systemic inflammation in middle-age and older adults with chronic LGI. Resveratrol and vitamin D, however, were not found to be effective in reducing markers of systemic inflammation in clinical trials conducted to date.
Ultimately, practical nutritional and pharmacological interventions targeting inflammaging that are safe, affordable, and acceptable could represent new therapeutic opportunities toward the promotion of successful aging in the near future.
6. Future directions
More studies are needed to better define the reference range of inflammatory biomarkers (e.g. IL-6, CRP) for chronic LGI. At the same time, larger RCTs targeting older adults with chronic LGI should be encouraged to clarify the relation between anti-inflammatory activity of these compounds and age-related pathologies. Specifically, future studies should test if compounds that reduce systemic inflammation can avert the decline in mobility and physical function that typically occurs among older adults.
Supplementary Material
Acknowledgements:
Drs. Anton, Mankowski, and Pahor are supported by the University of Florida’s Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740). Dr. Custodero is supported by the Dipartimento Interdisciplinare di Medicina, Clinica Medica Cesare Frugoni, University of Bari Aldo Moro.
Footnotes
Declarations of interest: none
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol Aging 21, 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L, 2007. Three-year change in inflammatory markers in elderly people and mortality: the Invecchiare in Chianti study. J Am Geriatr Soc 55, 1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton S, Leeuwenburgh C, 2013. Fasting or caloric restriction for healthy aging. Exp Gerontol 48, 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr IN, Tretter P, Kruger J, Stark RG, Schimkus J, Unger T, Kappert K, Scholze J, Parhofer KG, Kintscher U, 2011. High-dose treatment with telmisartan induces monocytic peroxisome proliferator-activated receptor-gamma target genes in patients with the metabolic syndrome. Hypertension 58, 725–732. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a Tool to Target Aging. Cell Metab 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis D, Bartlett DB, Patel HP, Roberts HC, 2013. Understanding how we age: insights into inflammaging. Longev Healthspan 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN, 2013. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 13, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia F, Sheller S, Menon R, 2016. Mechanistic Differences Leading to Infectious and Sterile Inflammation. Am J Reprod Immunol 75, 505–518. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W, 2010. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5, e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzur R, Cohen H, Cohen T, Dror TW, Herzog Y, Lifshitz Y, Lubish T, Harats D, Rubinstein A, 2010. The metabolic effects of omega-3 plant sterol esters in mixed hyperlipidemic subjects. Cardiovasc Drugs Ther 24, 429–437. [DOI] [PubMed] [Google Scholar]
- Bo S, Ponzo V, Ciccone G, Evangelista A, Saba F, Goitre I, Procopio M, Pagano GF, Cassader M, Gambino R, 2016. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol Res 111, 896–905. [DOI] [PubMed] [Google Scholar]
- Borenstein M, 2009. Introduction to meta-analysis John Wiley & Sons, Chichester, U.K. [Google Scholar]
- Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M, 2013. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr 32, 970–975. [DOI] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD, 2011. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med 3, 82ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero AE, Delgado A, Aguilar-Salinas CA, Herrera AN, Castillo JL, Cabrera T, Gomez-Perez FJ, Rull JA, 2004. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab 89, 3943–3948. [DOI] [PubMed] [Google Scholar]
- Caldeira D, David C, Sampaio C, 2012. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs 12, 263–277. [DOI] [PubMed] [Google Scholar]
- Calder PC, 2015. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851, 469–484. [DOI] [PubMed] [Google Scholar]
- Capurso C, Solfrizzi V, D’Introno A, Colacicco AM, Capurso SA, Semeraro C, Capurso A, Panza F, 2007. Interleukin 6 variable number of tandem repeats (VNTR) gene polymorphism in centenarians. Ann Hum Genet 71, 843–848. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L, 2004. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 59, 242–248. [DOI] [PubMed] [Google Scholar]
- Chagas CE, Borges MC, Martini LA, Rogero MM, 2012. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients 4, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, Chan AT, Bennett GG, Hollis BW, Giovannucci EL, Emmons KM, Fuchs CS, 2014. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila) 7, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ, 2014. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients 6, 2206–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW, 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychol Bull 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Darghosian L, Free M, Li J, Gebretsadik T, Bian A, Shintani A, McBride BF, Solus J, Milne G, Crossley GH, Thompson D, Vidaillet H, Okafor H, Darbar D, Murray KT, Stein CM, 2015. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol 115, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M, Grun M, Nicolaou A, Jahreis G, 2013. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clinical nutrition (Edinburgh, Scotland) 32, 686–696. [DOI] [PubMed] [Google Scholar]
- De Jager J, Kooy A, Lehert P, Bets D, Wulffele MG, Teerlink T, Scheffer PG, Schalkwijk CG, Donker AJ, Stehouwer CD, 2005. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. Journal of internal medicine 257, 100–109. [DOI] [PubMed] [Google Scholar]
- de Jager J, Kooy A, Schalkwijk C, van der Kolk J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD, 2014. Long-term effects of metformin on endothelial function in type 2 diabetes: a randomized controlled trial. J Intern Med 275, 59–70. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G, 2011. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol 92, 2746–2756. [DOI] [PubMed] [Google Scholar]
- Duggan C, de Dieu Tapsoba J, Mason C, Imayama I, Korde L, Wang CY, McTiernan A, 2015. Effect of Vitamin D3 Supplementation in Combination with Weight Loss on Inflammatory Biomarkers in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev Res (Phila) 8, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DA, 2006. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens 24, 983–991. [DOI] [PubMed] [Google Scholar]
- Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, Hosseininezhad SJ, Tavallaei S, Vejdani A, Azimi-Nezhad M, Shakeri MT, Rad MA, Mobarra N, Kazemi-Bajestani SM, Ferns GA, 2009. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol 64, 321–327. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lithgow GJ, 2014. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69 Suppl 1, S10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J, Pinter E, Kovacs I, Helyes Z, Kemeny A, Markovics A, Plateroti R, Librando A, Cruciani F, 2014. Irritable eye syndrome: neuroimmune mechanisms and benefits of selected nutrients. Ocul Surf 12, 134–145. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL, 2005. The origins of age-related proinflammatory state. Blood 105, 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ, 1999. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47, 639–646. [DOI] [PubMed] [Google Scholar]
- Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M, 2016. Chronic Inflammation: Accelerator of Biological Aging. J Gerontol A Biol Sci Med Sci [DOI] [PubMed]
- Fougere B, Vellas B, Billet S, Martin PJ, Gallucci M, Cesari M, 2015. Air Pollution modifies the association between successful and pathological aging throughout the frailty condition. Ageing Res Rev 24, 299–303. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J, 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69 Suppl 1, S4–9. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S, 2016. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab [DOI] [PubMed]
- Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G, 2000. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol 35, 879–896. [DOI] [PubMed] [Google Scholar]
- Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A, 2014. On the immunological theory of aging. Interdiscip Top Gerontol 39, 163–176. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, Jean S, Ebeling PR, 2014. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and beta-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One 9, e109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K, 2006. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol 48, 677–685. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Temprosa MG, Mather KJ, Orchard TJ, Kitabchi AE, Watson KE, 2014. Lifestyle and metformin interventions have a durable effect to lower CRP and tPA levels in the diabetes prevention program except in those who develop diabetes. Diabetes care 37, 2253–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp MR, 2015. Sex, the aging immune system, and chronic disease. Cell Immunol 294, 102–110. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB, 2010. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 1799, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR, 2005. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98, 911–917. [DOI] [PubMed] [Google Scholar]
- Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E, Diabetes Prevention Program Research, G., 2005. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 54, 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr., Heimovitz H, Cohen HJ, Wallace R, 1999. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106, 506–512. [DOI] [PubMed] [Google Scholar]
- Hass A, Oz H, Mashavi M, Shargorodsky M, 2014. Role of RAAS and adipokines in cardiovascular protection: effect of different doses of angiotensin II receptor blocker on adipokines level in hypertensive patients. J Am Soc Hypertens 8, 709–714. [DOI] [PubMed] [Google Scholar]
- Hermsdorff HH, Zulet MA, Abete I, Martinez JA, 2009. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine 36, 445–451. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G, 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE, 2013. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res 33, 367–375. [DOI] [PubMed] [Google Scholar]
- Hyun B, Shin S, Lee A, Lee S, Song Y, Ha NJ, Cho KH, Kim K, 2013. Metformin Down-regulates TNF-alpha Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw 13, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim HG, Kim JY, Kim NR, Jung BJ, Jeong JH, Chung DK, 2012. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol Lett 328, 13–19. [DOI] [PubMed] [Google Scholar]
- Kleiber AC, Zheng H, Sharma NM, Patel KP, 2010. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298, H1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysiak R, Gdula-Dymek A, Okopien B, 2011. The effect of bezafibrate and omega-3 fatty acids on lymphocyte cytokine release and systemic inflammation in patients with isolated hypertriglyceridemia. Eur J Clin Pharmacol 67, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysiak R, Gdula-Dymek A, Okopien B, 2013. Monocyte-suppressing effect of high-dose metformin in fenofibrate-treated patients with impaired glucose tolerance. Pharmacol Rep 65, 1311–1316. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan B, Stanton C, O’Toole PW, Ross RP, 2014. Compositional dynamics of the human intestinal microbiota with aging: implications for health. J Nutr Health Aging 18, 773–786. [DOI] [PubMed] [Google Scholar]
- Lee C, Longo VD, 2011. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene 30, 3305–3316. [DOI] [PubMed] [Google Scholar]
- Li K, Huang T, Zheng J, Wu K, Li D, 2014. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS One 9, e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, Wang TH, Deng HP, 2009. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels 24, 446–453. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yang H, Xue QL, Chuang YF, Roy CN, Abadir P, Walston JD, 2014Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol 58, 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Lenz M, Legner D, Bohm M, Nickenig G, 2006. Telmisartan inhibits beta2-integrin MAC-1 expression in human T-lymphocytes. J Hypertens 24, 1891–1898. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Santos-Ocana C, Sanchez-Alcazar JA, Fernandez-Ayala DJ, Asencio-Salcedo C, Rodriguez-Aguilera JC, Navas P, 2015. Mitochondrial responsibility in ageing process: innocent, suspect or guilty. Biogerontology 16, 599–620. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L, 2006. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 61, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, Szabados E, 2012. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc 50, 179–187. [DOI] [PubMed] [Google Scholar]
- Manini TM, Anton SD, Beavers DP, Cauley JA, Espeland MA, Fielding RA, Kritchevsky SB, Leeuwenburgh C, Lewis KH, Liu C, McDermott MM, Miller ME, Tracy RP, Walston JD, Radziszewska B, Lu J, Stowe C, Wu S, Newman AB, Ambrosius WT, Pahor M, investigators E.P.s., 2017. ENabling Reduction of Low-grade Inflammation in SEniors Pilot Study: Concept, Rationale, and Design. J Am Geriatr Soc 65, 1961–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L, 2009. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 265, 288–295. [DOI] [PubMed] [Google Scholar]
- Mazidi M, Rezaie P, Ferns GA, Vatanparast H, 2017. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloom Z, Yousefinejad A, Dabbaghmanesh MH, 2013. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci 38, 38–43. [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F, 2013. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14, 877–882. [DOI] [PubMed] [Google Scholar]
- Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI, 2013. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition 29, 178–183. [DOI] [PubMed] [Google Scholar]
- Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F, 2014. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–1012. [DOI] [PubMed] [Google Scholar]
- Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M, 2014. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients 6, 4058–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, Barden A, Puddey IB, Beilin LJ, Howe PR, 2007. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr 97, 749–757. [DOI] [PubMed] [Google Scholar]
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L, 2011. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 57, 870–879. [DOI] [PubMed] [Google Scholar]
- Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E, 2001. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem 47, 444–450. [PubMed] [Google Scholar]
- Ogino K, Kato M, Furuse Y, Kinugasa Y, Kaetsu Y, Mizuta E, Sugihara S, Ishida K, Yanagihara K, Hisatome I, Shigemasa C, 2010. Addition of losartan to angiotensin-converting enzyme inhibitors improves insulin resistance in patients with chronic heart failure treated without beta-blockers. Circ J 74, 2346–2352. [DOI] [PubMed] [Google Scholar]
- Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, Lecrux C, Imboden H, Hamel E, 2014. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis 68, 126–136. [DOI] [PubMed] [Google Scholar]
- Oppermann RV, Weidlich P, Musskopf ML, 2012. Periodontal disease and systemic complications. Braz Oral Res 26 Suppl 1, 39–47. [DOI] [PubMed] [Google Scholar]
- Palmer AK, Kirkland JL, 2016. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol 86, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli A, Moro T, Bosco G, Bianco A, Grimaldi KA, Camporesi E, Mangar D, 2015. Effects of n-3 polyunsaturated fatty acids (omega-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar Drugs 13, 996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk C, Tarnow L, Parving HH, 2006. Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes 55, 3550–3555. [DOI] [PubMed] [Google Scholar]
- Pooya S, Jalali MD, Jazayery AD, Saedisomeolia A, Eshraghian MR, Toorang F, 2010. The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr Metab Cardiovasc Dis 20, 326–331. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Lunn BS, Walker LL, Gray JM, Ballard CG, O’Brien JT, 2000. Cognitive deficit induced by acute tryptophan depletion in patients with Alzheimer’s disease. Am J Psychiatry 157, 638–640. [DOI] [PubMed] [Google Scholar]
- Pot GK, Brouwer IA, Enneman A, Rijkers GT, Kampman E, Geelen A, 2009. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eur J Clin Nutr 63, 1353–1359. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM, 2009. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA 302, 1186–1194. [DOI] [PubMed] [Google Scholar]
- Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P, 2005. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 63, 403–411. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statisical Computing, Vienna, Austria. [Google Scholar]
- Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP, 2014. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators of inflammation 2014, 348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O’Toole PW, Brigidi P, 2013. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 5, 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A, 2012. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr 107 Suppl 2, S159–170. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JS, 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359, 2195–2207. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr., Air Force/Texas Coronary Atherosclerosis Prevention Study, I., 2001. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 344, 1959–1965. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E, Investigators P, 2007. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 115, 1528–1536. [DOI] [PubMed] [Google Scholar]
- Sadiya A, Ahmed SM, Carlsson M, Tesfa Y, George M, Ali SH, Siddieg HH, Abusnana S, 2015. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: a randomized controlled double-blinded clinical trial. Eur J Clin Nutr 69, 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, Mikhailidis DP, Rizzo M, Rysz J, Sperling LS, Lip GY, Banach M, Lipid, Blood Pressure Meta-analysis Collaboration, G., 2015. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 189, 47–55. [DOI] [PubMed] [Google Scholar]
- Saisho Y, 2015. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr Metab Immune Disord Drug Targets 15, 196–205. [DOI] [PubMed] [Google Scholar]
- Samah S, Ramasamy K, Lim SM, Neoh CF, 2016. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 118, 172–182. [DOI] [PubMed] [Google Scholar]
- Schiano V, Laurenzano E, Brevetti G, De Maio JI, Lanero S, Scopacasa F, Chiariello M, 2008. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr 27, 241–247. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frolich M, Hofman A, Jolles J, Breteler MM, Westendorp RG, 2007. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc 55, 708–716. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Thaiss CA, Levy M, Elinav E, 2014. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol 30, 54–62. [DOI] [PubMed] [Google Scholar]
- Shaseb E, Tohidi M, Abbasinazari M, Khalili D, Talasaz AH, Omrani H, Hadaegh F, 2016. The effect of a single dose of vitamin D on glycemic status and C-reactive protein levels in type 2 diabetic patients with ischemic heart disease: a randomized clinical trial. Acta Diabetol 53, 575–582. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB, 2011. Inflammatory markers in population studies of aging. Ageing Res Rev 10, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Duran P, Shen R, Lee M, Friedman TC, Davidson MB, 2015. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African-American subjects with pre-diabetes and hypovitaminosis D. Horm Metab Res 47, 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SI, Srinivas V, Crandall JP, Kim M, Tellides G, Lebastchi AH, Yu Y, Gupta AK, Alderman MH, 2012. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med 17, 394–404. [DOI] [PubMed] [Google Scholar]
- Steinmetz HT, Herbertz A, Bertram M, Diehl V, 1995. Increase in interleukin-6 serum level preceding fever in granulocytopenia and correlation with death from sepsis. J Infect Dis 171, 225–228. [DOI] [PubMed] [Google Scholar]
- Stepanova M, Rodriguez E, Birerdinc A, Baranova A, 2015. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget 6, 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G, 2012. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg 44, 307–312. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE, 2000. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 55, M709–715. [DOI] [PubMed] [Google Scholar]
- Takagi H, Mizuno Y, Yamamoto H, Goto SN, Umemoto T, All-Literature Investigation of Cardiovascular Evidence, G., 2013. Effects of telmisartan therapy on interleukin-6 and tumor necrosis factor-alpha levels: a meta-analysis of randomized controlled trials. Hypertens Res 36, 368–373. [DOI] [PubMed] [Google Scholar]
- Ticinesi A, Meschi T, Lauretani F, Felis G, Franchi F, Pedrolli C, Barichella M, Benati G, Di Nuzzo S, Ceda GP, Maggio M, 2016. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients 8, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P, 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC, 2012. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol 110, 356–363. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Kourtellaris P, Antoniades C, Vasiliadou C, Papageorgiou N, Tentolouris C, Siasos G, Stefanadi E, Stefanadis C, 2008. Effects of irbesartan and perindopril on forearm reactive hyperemia and inflammatory process, in normotensive patients with coronary artery disease. Int J Cardiol 124, 127–129. [DOI] [PubMed] [Google Scholar]
- Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I, 2009. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism 58, 1543–1549. [DOI] [PubMed] [Google Scholar]
- Valentini L, Pinto A, Bourdel-Marchasson I, Ostan R, Brigidi P, Turroni S, Hrelia S, Hrelia P, Bereswill S, Fischer A, Leoncini E, Malaguti M, Blanc-Bisson C, Durrieu J, Spazzafumo L, Buccolini F, Pryen F, Donini LM, Franceschi C, Lochs H, 2015. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin Nutr 34, 593–602. [DOI] [PubMed] [Google Scholar]
- van der Zijl NJ, Serne EH, Goossens GH, Moors CC, Ijzerman RG, Blaak EE, Diamant M, 2011. Valsartan-induced improvement in insulin sensitivity is not paralleled by changes in microvascular function in individuals with impaired glucose metabolism. J Hypertens 29, 1955–1962. [DOI] [PubMed] [Google Scholar]
- Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ, 2012. Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology 37, 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D, 1999. Degradation of tryptophan in neurodegenerative disorders. Adv Exp Med Biol 467, 133–138. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Margulies DS, Floel A, 2014. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci 34, 7862–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, Paczek L, 2016. Inflammatory Markers Change with Age, but do not Fall Beyond Reported Normal Ranges. Arch Immunol Ther Exp (Warsz) 64, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Deng YY, Yang L, Zhao S, Liu J, Zhao Z, Wang L, Maharjan P, Gao S, Tian Y, Zhuo X, Zhao Y, Zhou J, Yuan Z, Wu Y, 2015. Metformin ameliorates the proinflammatory state in patients with carotid artery atherosclerosis through sirtuin 1 induction. Transl Res 166, 451–458. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Matsui T, 2016. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 32, 157–165. [DOI] [PubMed] [Google Scholar]
- Yao K, Zeng L, He Q, Wang W, Lei J, Zou X, 2017. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Medical science monitor : international medical journal of experimental and clinical research 23, 3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W, 2016. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev 2016, 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YT, Shao L, Teng LL, Hu B, Luo Y, Yu X, Zhang DF, Zhang H, 2009. Effects of n-3 polyunsaturated fatty acid therapy on plasma inflammatory markers and N-terminal pro-brain natriuretic peptide in elderly patients with chronic heart failure. J Int Med Res 37, 1831–1841. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Wang HF, Wang X, Li J, Xing CM, 2016. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: A meta-analysis. J Clin Neurosci 33, 32–38. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R, 2009. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 89, 1321–1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


