Abstract
Background
The relative risk of lung cancer decreases with years since quitting (YSQ) smoking, but risk beyond 25 YSQ remains unclear. Current lung cancer screening guidelines, which exclude smokers with more than 15 YSQ, may not detect lung cancers in this population.
Methods
We analyzed data from Framingham Heart Study Original (n = 3905) and Offspring cohort (n = 5002) participants for lifetime smoking and lung cancer incidence from 1954 to 1958 (Exam 4) and 1971 to 1975 (Exam 1), respectively, through 2013. We used multivariable-adjusted Cox proportional hazards regression models to compare current, former, and never smokers and lung cancer risk. Smoking status and covariates were time-updated every two years (Original) or four years (Offspring). Primary analyses were restricted to heavy ever smokers with more than 21.3 pack-years; additional analyses included all ever smokers.
Results
On follow-up (median = 28.7 years), 284 lung cancers were detected: incidence rates/1000 person-years in current, former, and never smokers were 1.97 (95% confidence interval [CI] = 1.66 to 2.33), 1.61 (95% CI = 1.34 to 1.93), and 0.26 (95% CI = 0.17 to 0.39), respectively. Heavy former (vs never) smokers had elevated lung cancer risk at all YSQ (<5: hazard ratio [HR] = 12.12, 95% CI = 6.94 to 21.17; 5–9: HR = 11.77, 95% CI = 6.78 to 20.45; 10–14: HR = 7.81, 95% CI = 3.98 to 15.33; 15–24: HR = 5.88, 95% CI = 3.19–10.83; ≥25: HR = 3.85, 95% CI = 1.80 to 8.26). Heavy former (vs current) smokers had 39.1% lower lung cancer risk within five YSQ. Among all former smokers, 40.8% of lung cancers occurred after more than 15 YSQ.
Conclusions
Among heavy former smokers, lung cancer risk drops within five YSQ relative to continuing smokers, yet it remains more than threefold higher than never smokers after 25 YSQ. Four of ten lung cancers occurred in former smokers with more 15 YSQ, beyond the screening window of the current guideline.
Worldwide, smoking causes 1.69 million deaths per year from lung cancer (1,2), the leading cause of cancer death for men and women. In the United States, 87% of lung cancer deaths are attributable to cigarette smoking (3). Lung cancer screening can reduce the relative risk of dying from lung cancer by 20% (4), but when combined with successful smoking cessation, this benefit has been estimated to be as high as 38% (5). Smoking cessation reduces the risk of lung cancer mortality (6–11) and lung cancer incidence (12–15) in former smokers relative to current smokers, yet lung cancer risk relative to never smokers is known to remain elevated for years. Limitations of published data leave important questions about the time course and magnitude of lung cancer risk reduction in former smokers.
Guidelines adopted by the Centers for Medicare and Medicaid Services (CMS) mandate insurance coverage of low-dose computed tomography scan (LDCT) screening for current or former smokers who meet age and pack-year criteria, but they exclude former smokers with more than 15 years since quitting (YSQ) (16). The YSQ threshold was carefully selected by experts using available data in order to optimize the risk-benefit ratio of screening (17). However, if the risk of lung cancer in fact remains elevated for more than 15 YSQ, then extending the screening window in former smokers could augment early detection without diminishing screening effectiveness.
The objective of this investigation was to relate comprehensive lifetime smoking history to the risk of lung cancer in a large and well-characterized prospective cohort study, the Framingham Heart Study (FHS), which began in 1948 with enrollment of the Original cohort. We further sought to replicate results in a second FHS cohort (the Offspring cohort), which was recruited beginning in 1971 and followed a similar methodology. To assess the degree to which bias may be minimized by frequent, prospective capture of smoking status and lung cancer status at regular intervals (18,19), we conducted a sensitivity analysis by varying the number of follow-up assessments in which smoking status and cigarettes per day were assessed.
Methods
Study Samples
The present investigation evaluated participants in the Framingham Heart Study (FHS) Original cohort who attended their fourth examination cycle (1954–1958) and Offspring cohort participants who attended their first examination cycle (1971–1975), when smoking data were first reliably collected. The Vanderbilt University Medical Center and the Boston Medical Center Institutional Review Board approved this study protocol.
From a total of 4541 participants at the fourth Original cohort examination cycle and 5122 participants at the first Offspring cohort examination cycle who had at least one vital status update, we excluded individuals with a history of lung cancer at baseline and those missing pack-years at baseline or with an unclear smoking history, yielding final sample sizes of 3905 and 5002 for the Original and Offspring cohorts, respectively (Supplementary Figure 1, available online). Participants were followed through the end of 2013 for development of lung cancer. Smoking status and other variables were ascertained every two years in the Original cohort, starting with the fourth examination (median = 16 assessments), and every four years in the Offspring cohort, starting with the first examination (median = 9 assessments).
Quantification of Smoking Duration and Intensity
At the baseline (ie, first available) examination, data on current and prior smoking habits were collected, allowing categorization of participants as current, former, or never smokers. For current and former smokers, we obtained information on age at which the participant started smoking, usual cigarettes smoked per day in the past, age at quitting (for former smokers), and current number of cigarettes smoked per day (for current smokers). We used these variables to calculate cumulative pack-years at baseline for both current and former smokers, as well as initial YSQ for former smokers. Pack-years and YSQ were time-updated at the intervals noted above into cumulative variables of smoking exposure in order to incorporate periods of smoking abstinence as well as variation in smoking rate, which can also influence lung cancer risk (20).
For a given participant, smoking status (current, former, never) could change over time, such that each participant contributed person-examinations and person-time to the category reflecting his or her status at each assessment. If an individual developed lung cancer, it was counted only in the smoking status category to which the individual belonged at the time of the diagnosis (Supplementary Figure 2, available online).
Outcome Events
All FHS participants are under continuous surveillance for the development of new cancer events (21). Lung cancer incidence was surveyed through 2013. Known lung cancer cases are formally adjudicated using standardized protocols, including medical record review and pathology and laboratory reports (21). In 281 cases, there was a pathology report available, and histological types were categorized as small cell lung carcinoma (SCLC), non–small cell lung carcinoma (NSCLC), and other. In three cases, a pathology report was unavailable, in which case clinical diagnoses or death certificates were used as confirming evidence.
To avoid carrying forward potentially inaccurate smoking information, participants were censored after a single missed exam plus an additional year without an update (ie, five years for the Original cohort and nine years for the Offspring cohort). Once censored, participants were ineligible to re-enter the sample. As follow up may end due to other outcomes related to smoking, that is, death, we note that in all cases we are estimating the cause-specific hazards ratios, as opposed to subdistribution hazards ratios.
Statistical Analysis
Sample missingness was relatively low. In the Original cohort, 53.0% of person-exams had no missing data. In the Original cohort, current alcohol consumption (46.4% missingness) was not collected at examination cycles 5, 6, 8–11, 16, 24, and 25. In the Offspring cohort, 95.2% of person-exams had no missing data. Smoking status was missing at 13.7% and 0.1% of person-exams in the Original and Offspring cohorts, respectively, and all other variables had less than 5% missingness. Five complete data sets in each cohort were imputed to account for the missingness. Continuous variables were imputed through the use of predictive mean matching in order to produce imputed values that were clinically plausible. We imputed categorical variables using the discriminant function with a noninformative Jeffrey’s prior.
Utilizing combined data from the Original and Offspring cohorts, we calculated incidence rates of lung cancer per 1000 person-years stratified by time-updated smoking status (current, former, never). Among current and former smokers, we further stratified by median cumulative pack-years. Using smoking status or the combination of smoking status and cumulative pack-years as time-varying covariates, we implemented Cox proportional hazards regression to estimate unadjusted cause-specific hazard ratios for lung cancer comparing former and current smokers with never smokers. Lung cancer risk is known to be elevated with greater years of smoking and higher smoking rate (12). Among ever smokers, 92.7% of cancers occurred among heavier smokers who were at or above the median of 21.3 pack-years. Therefore, adjusted analyses focused on these heavier smokers.
To determine the time course and magnitude of lung cancer risk reduction in former smokers relative to current smokers, we performed two analyses. First, we created a categorical variable of YSQ: less than 5, 5 to less than 10, 10 to less than 15, 15 to less than 25, and 25 or more, with current smokers as the reference group. We then used Cox proportional hazards models to estimate lung cancer incidence (outcome) in relation to YSQ (time since end of exposure). In our second analysis, we modeled YSQ as a continuous variable (up to 25), assigning current smokers a value of 0. The former smokers with more than 25 YSQ were assigned a value of 26 years of abstinence. In Cox proportional hazards regression models, this continuous YSQ variable was modeled using restricted cubic splines with three knots to allow a nonlinear association with the log-hazards of lung cancer. We then plotted the association between continuous YSQ and lung cancer risk.
We used analogous methods to determine the time course and magnitude of lung cancer risk reduction in former smokers relative to never smokers. In the models with categorical YSQ, never smokers served as the reference group, while in those with continuous YSQ, never smokers were assigned a value of 50 YSQ (ie, well above all former smokers, whose YSQ were truncated after 25).
After verifying that the proportional hazards assumption held through assessing interactions with time (Combined cohorts P = .82; Original cohort P = .37; Offspring cohort P = .99), Cox proportional hazards regression models were run in the Original and Offspring cohorts separately and combined. In combined analyses, we allowed baseline hazards to differ by cohort. We adjusted all models for age, sex, education level, decade of examination, and alcohol consumption. We placed fourth-order polynomials on age, with a quadratic term on decade of examination to allow a nonlinear association between the log hazards of lung cancer and these continuous predictors (22). In secondary analyses, we placed second-order polynomials on cumulative pack-years to account for a nonlinear association between this comprehensive variable of smoking exposure and risk of lung cancer. All dynamic predictors (age, decade of examination, alcohol consumption, YSQ, and cumulative pack-years) were updated at each exam and were modeled as time-varying covariates, while static predictors (sex, education level) remained constant across the follow-up period.
Sensitivity analyses were conducted with either six or two assessments of smoking status, approximating the design of prior studies (8,23). Additional sensitivity analyses included all current and former smokers, without restriction by cumulative pack-years. A final sensitivity analysis restricted ever smokers to at least 30 pack-years of exposure. Statistical significance was assessed using a P value of less than .05, and all statistical analyses were performed by MSD in conjunction with RAG using SAS software version 9.4. (Cary, NC). All statistical tests were two-sided.
Results
There were 3905 participants from the FHS Original cohort and 5002 participants from the FHS Offspring cohort who were free of baseline lung cancer (Table 1). The Original cohort tended to be older, less educated, and have more Cardiovascular Disease (CVD) risk factors, including a higher smoking prevalence. Restricting analyses to “heavy” current and former smokers with at least 21.3 pack-years, current and former smokers had similar distributions of cumulative pack-years (Supplementary Figure 3, available online).
Table 1.
Baseline characteristics of sample
| Characteristic* | Original cohort (total n = 3905) |
Offspring cohort (total n = 5002) |
||
|---|---|---|---|---|
| No. | Summary | No. | Summary | |
| Age, y | 3905 | 50.2 ± 8.5 | 5002 | 36.2 ± 10.5 |
| Male sex | 3905 | 1596 (40.9) | 5002 | 2411 (48.2) |
| Education | 3859 | − | 3781 | − |
| Less than high school Graduate | − | 1614 (41.8) | − | 306 (8.1) |
| High school graduate | − | 1169 (30.3) | − | 1297 (34.3) |
| More than high school | − | 1076 (27.9) | − | 2178 (57.6) |
| Systolic blood pressure, mmHg | 3905 | 133.2 ± 22.8 | 5000 | 121.9 ± 16.5 |
| Diastolic blood pressure, mmHg | 3905 | 83.3 ± 12.1 | 5000 | 78.6 ± 10.9 |
| Antihypertensive medication | 3895 | 138 (3.5) | 4991 | 164 (3.3) |
| Hypertension | 3902 | 1462 (37.5) | 4994 | 970 (19.4) |
| Body mass index, kg/m2 | 3897 | 25.7 ± 4.1 | 4998 | 25.1 ± 4.4 |
| Diabetes | 3869 | 81 (2.1) | 4909 | 94 (1.9) |
| Total cholesterol, mg/dL | 3863 | 239.0 ± 44.5 | 4971 | 199.3 ± 40.5 |
| Current drinkers | 3862 | 2627 (68.0) | 4955 | 4207 (84.9) |
| Smoking status | 3905 | − | 5002 | − |
| Current | − | 1955 (50.1) | − | 2227 (44.5) |
| Former | − | 283 (7.3) | − | 941 (18.8) |
| Never | − | 1667 (42.7) | − | 1834 (36.7) |
| Cigarettes per day† | 1955 | 18.8 ± 10.5 | 2227 | 21.6 ± 12.2 |
| Cumulative pack-years | − | − | − | − |
| Current smokers | 1955 | 24.3 ± 18.1 | 2227 | 20.7 ± 18.2 |
| Former smokers | 283 | 19.4 ± 21.6 | 941 | 18.7 ± 19.8 |
| Years since quitting‡ | 283 | 4.7 ± 5.3 | 941 | 7.7 ± 6.1 |
Summary statistics are displayed as mean ± SD for continuous variables and as No. (%) for categorical variables, unless otherwise noted.
Among current smokers only.
Among former smokers only.
Most people (89.5%) who were smoking at baseline quit during follow-up and never relapsed. Among baseline ever smokers, there were 651 current and former smokers who relapsed (ie, returned to smoking after reporting abstinence during at least one clinic visit) either one, two, three, or four times: 575, 66, 9, and 1, respectively. Abstinence periods ranged from 0.6 to 39.7 years, with a median of 2.9 (Q1, Q3 = 1.14, 5.1) years.
During a median follow-up time of 28.7 years (25.1 and 33.6 years for the Original and Offspring cohorts, respectively), there were 284 (158 and 126, respectively) incident lung cancer diagnoses (Table 2). Among ever smokers, the majority of lung cancers (92.7%) occurred among heavy smokers, with 21.3 or more cumulative pack-years of smoking. For both cohorts, former and current smokers had statistically significantly higher rates of incident lung cancer as compared with never smokers, but among current and former smokers with 21.3 or more pack-years, the unadjusted lung cancer risk was more than 10-fold higher than never smokers. Incidence rates/1000 person-years in current, former, and never smokers were 1.97 (95% confidence interval [CI] = 1.66 to 2.33), 1.61 (95% CI = 1.34 to 1.93), and 0.26 (95% CI = 0.17 to 0.39), respectively (Table 2). Among 281 cases with available histology, 215 (76.5%) were NSCLC, 40 (14.2%) SCLC, and 26 (9.3%) other types.
Table 2.
| Smoking status | Person-exams | Lung cancers | Person-years | Incidence rate per 1000 PY (95% CI) | Unadjusted hazards ratio‡ (95% CI) | P‡,§ |
|---|---|---|---|---|---|---|
| Combined cohorts | ||||||
| Never (referent) | 30 847 | 25 | 94 902 | 0.26 (0.17 to 0.39) | 1.00 | − |
| Former, pack-years | 22 873 | 120 | 74 313 | 1.61 (1.34 to 1.93) | 6.47 (4.20 to 9.96) | <.001 |
| <21.3‖ | 12 389 | 17 | 42 414 | 0.40 (0.23 to 0.64) | 1.61 (0.87 to 2.99) | .13 |
| ≥21.3 | 10 484 | 103 | 31 899 | 3.23 (2.64 to 3.92) | 12.35 (7.98 to 19.11) | <.001 |
| Current, pack-years | 22 085 | 139 | 70 489 | 1.97 (1.66 to 2.33) | 7.55 (4.94 to 11.55) | <.001 |
| <21.3 | 7967 | 2 | 28 153 | 0.07 (0.009 to 0.26)¶ | 0.28 (0.07 to 1.20)¶ | .09 |
| ≥21.3 | 14 118 | 137 | 42 336 | 3.24 (2.72 to 3.83) | 12.06 (7.98 to 18.42) | <.001 |
| Original cohort | ||||||
| Never (referent) | 20 384 | 13 | 43 107 | 0.30 (0.16 to 0.52) | 1.00 | − |
| Former, pack-years | 12 300 | 59 | 26 014 | 2.27 (1.73 to 2.93) | 7.50 (4.12 to 13.66) | <.001 |
| <21.3 | 6008 | 7 | 12 776 | 0.55 (0.22 to 1.13) | 1.81 (0.73 to 4.53) | .20 |
| ≥21.3 | 6292 | 52 | 13 238 | 3.93 (2.93 to 5.15) | 12.96 (7.07 to 23.77) | <.001 |
| Current, pack-years | 15 231 | 86 | 32 215 | 2.67 (2.14 to 3.30) | 8.86 (4.95 to 15.84) | <.001 |
| <21.3 | 5268 | 1 | 11 033 | 0.09 (0.002 to 0.50)¶ | 0.30 (0.04 to 2.31)¶ | .25 |
| ≥21.3 | 9963 | 85 | 21 181 | 4.01 (3.21 to 4.96) | 13.28 (7.43 to 23.74) | <.001 |
| Offspring cohort | ||||||
| Never (referent) | 10 463 | 12 | 51 795 | 0.23 (0.12 to 0.40) | 1.00 | − |
| Former, pack-years | 10 573 | 61 | 48 299 | 1.26 (0.97 to 1.62) | 5.36 (2.89 to 9.95) | <.001 |
| <21.3 | 6381 | 10 | 29 637 | 0.34 (0.16 to 0.62) | 1.44 (0.62 to 3.33) | .39 |
| ≥21.3 | 4192 | 51 | 18 662 | 2.73 (2.03 to 3.59) | 11.64 (6.21 to 21.82) | <.001 |
| Current, pack-years | 6854 | 53 | 38 274 | 1.38 (1.04 to 1.81) | 6.13 (3.28 to 11.47) | <.001 |
| <21.3 | 2699 | 1 | 17 120 | 0.06 (0.001 to 0.33)¶ | 0.26 (0.03 to 2.02)¶ | .20 |
| ≥21.3 | 4155 | 52 | 21 155 | 2.46 (1.84 to 3.22) | 10.66 (5.72 to 19.88) | <.001 |
Hazards ratios are from an unadjusted model with a five-level variable constructed on the basis of smoking status and cumulative pack-years. Never smokers serve as the reference group. CI = confidence interval; PY = pack-years.
All cells are time-updated; that is, as individuals begin and quit smoking, they contribute person-exams and person-time to various groups. An individual’s lung cancer diagnosis only contributes to the group he or she was in at the time of diagnosis.
Hazards ratio and P value columns include data from all five multiple imputations. Other columns are based on data in the first imputation only.
P values were derived from a two-sided χ2 test.
21.3 cumulative pack-years is the median cumulative pack-years among former smokers at the time of quitting.
Because fewer than five lung cancer events contributed to the estimation of the incidence rates and hazard ratios in these cells, these estimates should be interpreted with caution
In models adjusted for age, sex, education level, decade of examination, and alcohol consumption, as compared with current smokers, the risk of lung cancer was lower among heavy former smokers (Figure 1), and this lower risk was detectable within five YSQ (hazards ratio [HR] = 0.61, 95% CI = 0.40 to 0.93, P = .02) (Table 3). In secondary analyses, further adjustment for cumulative pack-years (ie, incorporating time-updated changes in smoking rate and periods of abstinence) confirmed the lower risk of lung cancer among heavy former vs current smokers in all YSQ categories, except for the former smokers with five to nine YSQ. This group had a lower risk of lung cancer that was not statistically significant (P = .13) (Supplementary Table 1, available online). However, as compared with never smokers, long-term former smokers demonstrated persistently elevated lung cancer risk even after 25 YSQ (Figure 1 displays combined cohort results; Table 4 shows combined and individual cohort analyses). Heavy former (vs never) smokers had elevated lung cancer risk at all YSQ (<5: HR = 12.12, 95% CI = 6.94 to 21.17; 5–9: HR = 11.77, 95% CI = 6.78 to 20.45; 10–14: HR = 7.81, 95% CI = 3.98 to 15.33; 15–24: HR = 5.88, 95% CI = 3.19 to 10.83; ≥25: HR = 3.85, 95% CI = 1.80 to 8.26) (Table 4).
Figure 1.
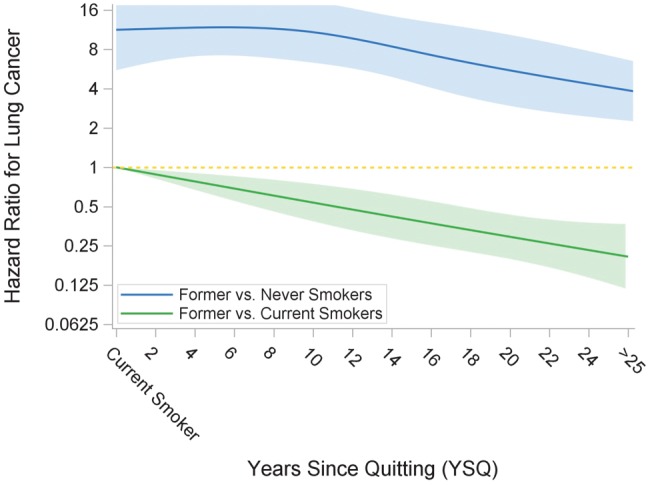
Restricted cubic splines: risk of lung cancer by YSQ in former smokers compared with current and never smokers (combined cohort). Restricted cubic splines analyses are adjusted for age, sex, education, examination decade, and alcohol consumption. All variables are time-updated. This analysis is limited to current smokers and former smokers with at least 21.3 cumulative pack-years. YSQ = years since quitting.
Table 3.
Adjusted risk of lung cancer in heavy former vs current smokers*
| YSQ category | Person-exams | Lung cancers | Adjusted hazards ratio† (95% CI) | P‡ |
|---|---|---|---|---|
| Combined cohorts | ||||
| Current smokers (referent) | 14 118 | 137 | 1.00 | − |
| <5 | 2828 | 27 | 0.61 (0.40 to 0.93) | .02 |
| 5 to <10 | 2301 | 27 | 0.59 (0.39 to 0.89) | .01 |
| 10 to <15 | 1564 | 15 | 0.39 (0.23 to 0.67) | <.001 |
| 15 to <25 | 2332 | 22 | 0.29 (0.18 to 0.48) | <.001 |
| 25+ | 1459 | 12 | 0.19 (0.10 to 0.37) | <.001 |
| Original cohort | ||||
| Current smokers (referent) | 9963 | 85 | 1.00 | − |
| <5 | 1882 | 13 | 0.48 (0.27 to 0.87) | .02 |
| 5 to <10 | 1409 | 11 | 0.42 (0.23 to 0.78) | .006 |
| 10 to <15 | 951 | 9 | 0.41 (0.20 to 0.83) | .01 |
| 15 to <25 | 1280 | 12 | 0.31 (0.16 to 0.59) | <.001 |
| 25+ | 770 | 7 | 0.23 (0.10 to 0.54) | <.001 |
| Offspring cohort | ||||
| Current smokers (referent) | 4155 | 52 | 1.00 | − |
| <5 | 946 | 14 | 0.81 (0.44 to 1.48) | .50 |
| 5 to <10 | 892 | 16 | 0.79 (0.45 to 1.40) | .42 |
| 10 to <15 | 613 | 6 | 0.36 (0.15 to 0.85) | .02 |
| 15 to <25 | 1052 | 10 | 0.28 (0.13 to 0.59) | <.001 |
| 25+ | 689 | 5 | 0.15 (0.05 to 0.42) | <.001 |
This analysis is limited to current and former smokers with at least 21.3 cumulative pack-years. CI = confidence interval; YSQ = years since quitting.
Hazards ratios are adjusted for age, sex, education, examination decade, and alcohol consumption. All variables are time-updated.
P values were derived from a two-sided χ2 test.
Table 4.
Adjusted risk of lung cancer in heavy former vs never smokers*
| YSQ category | Person-exams | Lung cancers | Adjustedhazard ratio† (95% CI) | P‡ |
|---|---|---|---|---|
| Combined cohorts | ||||
| <5 | 2828 | 27 | 12.12 (6.94 to 21.17) | <.001 |
| 5 to <10 | 2301 | 27 | 11.77 (6.78 to 20.45) | <.001 |
| 10 to <15 | 1564 | 15 | 7.81 (3.98 to 15.33) | <.001 |
| 15 to <25 | 2332 | 22 | 5.88 (3.19 to 10.83) | <.001 |
| 25+ | 1459 | 12 | 3.85 (1.80 to 8.26) | <.001 |
| Never smokers (referent) | 30 847 | 25 | 1.00 | − |
| Original cohort | ||||
| <5 | 1882 | 13 | 11.60 (5.47 to 24.61) | <.001 |
| 5 to <10 | 1409 | 11 | 10.46 (4.67 to 23.42) | <.001 |
| 10 to <15 | 951 | 9 | 10.30 (4.18 to 25.41) | <.001 |
| 15 to <25 | 1280 | 12 | 7.81 (3.44 to 17.74) | <.001 |
| 25+ | 770 | 7 | 5.96 (2.15 to 16.54) | <.001 |
| Never smokers (referent) | 20 384 | 13 | 1.00 | − |
| Offspring cohort | ||||
| <5 | 946 | 14 | 12.32 (5.38 to 28.20) | <.001 |
| 5 to <10 | 892 | 16 | 12.33 (5.64 to 26.96) | <.001 |
| 10 to <15 | 613 | 6 | 5.74 (2.07 to 15.89) | <.001 |
| 15 to <25 | 1052 | 10 | 4.46 (1.81 to 10.97) | .001 |
| 25+ | 689 | 5 | 2.69 (0.88 to 8.24) | .08 |
| Never smokers (referent) | 10 463 | 12 | 1.00 | − |
This analysis is limited to never smokers and former smokers with at least 21.3 cumulative pack-years. CI = confidence interval; YSQ = years since quitting.
Hazard ratios are adjusted for age, sex, education, examination decade, and alcohol consumption. All variables are time-updated.
P values were derived from a two-sided χ2 test.
Compared with the primary analyses that used all available follow-up time points, sensitivity analyses with fewer (ie, 6 or 2) assessments of smoking status tended to produce lower risk estimates in heavy former smokers relative to both heavy current and never smokers, especially as YSQ increased (Supplementary Tables 2 and 3, respectively, available online). In sensitivity analyses that included all ever smokers, results did not change appreciably from analyses that were restricted to heavier ever smokers (Supplementary Table 4, available online). In sensitivity analyses that restricted ever smokers to 30 or more pack-years, results for former vs current smokers (Supplementary Table 5, available online) and former vs never smokers (Supplementary Table 6, available online) were similar to the main analysis.
Notably, only 58.7% (152) of lung cancer cases among former and current smokers in our sample met the current screening eligibility criteria at the time of diagnosis: 49 lung cancer diagnoses occurred among former smokers with more than 15 YSQ (40.8% of diagnoses in former smokers); 29.7% occurred before age 55 years or after age 75 years, and 15.4% occurred among those with fewer than 30 pack-years.
Discussion
In this prospective community-based sample with a median follow-up time of almost 30 years, among ever smokers with at least median (21.3) pack-year exposure, individuals who quit smoking within the last five years had a 39.1% lower lung cancer risk compared with current smokers. This window of relative risk reduction for incident lung cancer is more rapid than what has been reported from prospectively collected data (3,13) and underscores the health benefits of quitting smoking. As YSQ increased, lung cancer risk in former smokers decreased relative to current smokers, yet lung cancer risk remained elevated more than threefold relative to never smokers, even after 25 YSQ. The persistence of heightened risk in former smokers has important implications for lung cancer screening given that 40.8% of the lung cancers in former smokers occurred after 15 YSQ, which is beyond the window of screening eligibility as currently defined. While some of these particular individuals would have been ineligible for lung cancer screening on the basis of other criteria (ie, age or pack-years), it has been estimated that nationally, removing the 15-year threshold would add about 3 million individuals to the lung cancer screening pool (24).
Potential concerns about relaxing current lung cancer screening criteria are valid. So-called downward eligibility creep could blunt the expected benefits of screening and augment harms by screening individuals who are at lower risk than that observed in the National Lung Screening Trial (NLST), on which the US Preventive Services Task Force (25) recommendations and final CMS decision were largely based. Furthermore, the NLST, which used the 15-year threshold, is the only published trial demonstrating a mortality benefit for lung cancer screening with LDCT (26,27). Nevertheless, the current data reflect the most complete capture of longitudinal cigarette smoking status and lung cancer published to date. The fact that these findings are present in two separate cohorts within the Framingham Heart Study strongly supports their validity. However, altering screening criteria based on these results alone would be premature. Rather, these findings should inform continuing research, including modeling studies using FHS cohort data to examine the effects of different criterion cut-points on the number needed to screen and the associated cost-effectiveness, in order to maximize benefit (28–30).
This study is consistent with earlier work reporting that cigarette smoking increases the risk of lung cancer and that quitting smoking reduces the risk of lung cancer relative to persistent smokers. This study further extends prior work by employing uniquely rigorous methodology, featuring frequent time-updated smoking status collected in person at regular visits, to minimize bias. Indeed, sensitivity analyses using fewer assessments of smoking tended to overestimate the risk reduction in former vs current smokers and underestimate the degree of elevated risk in former vs never smokers, especially as YSQ increased. Additionally, analyses were conducted in two separate cohorts with median baseline ages of 50 and 36 years and a median follow-up time of almost 30 years. Results are consistent with prior studies demonstrating the preponderance of SCLC in current and former smokers. Results were consistent with, but were not designed to confirm, existing knowledge that smoking cessation reduces risk of all types of lung cancer, with the greatest reduction seen for SCLC and lower but substantial benefits for other NSCLC histologic types such as adenocarcinoma and large cell carcinoma (31,32).
Despite numerous strengths, there are several limitations to these data. The sample size was smaller than that of some prior studies, although large enough to address the main objective. Information on some factors that influence lung cancer risk was not included in the analysis, such as other sources of combustible tobacco (33), radon exposure (34), dietary intake of isothiocyanates (contained in cruciferous vegetables) or genetic variation in neuronal nicotinic acetylcholine receptors or hepatic enzymes that metabolize isothiocyanates (35), and genetic variation in enzymes that metabolize nicotine and tobacco-specific nitrosamines (36). However, cigarette smoking is the major driving etiology of lung cancer risk, and this study offers the most completely captured longitudinal smoking data available. In addition, the FHS is primarily comprised of white individuals of European ancestry who live in the northeastern United States, and, therefore, results based on our samples may not be generalizable to the general population of ever smokers. Finally, while some of the mechanisms by which tobacco smoking initiates and promotes tumor development are understood (37–40), further biological insights are needed to probe why and how some former smokers remain at prolonged elevated risk of lung cancer.
In conclusion, relative to current smokers, former smokers have a 39.1% lower risk of incident lung cancer within five YSQ, which continues to fall with increasing YSQ. Thus the importance of smoking cessation cannot be overstated. Yet relative to never smokers, risk of incident lung cancer in former smokers remains elevated more than threefold, even after 25 YSQ. Persistently elevated lung cancer risk in former smokers is important given that four of every 10 lung cancers diagnosed in former smokers occur in people with more than 15 YSQ, who are beyond the current window of eligibility for lung cancer screening.
Funding
This work was supported by the Vanderbilt Center for Tobacco, Addiction, and Lifestyle (ViTAL; directed by Dr. Tindle) and the Vanderbilt Center for Clinical Cardiovascular Outcomes Research and Trials Evaluation (V-CREATE; directed by Dr. Freiberg). The Framingham Heart Study is supported by a contract (NO1-HC-38038) with the National Heart, Lung, and Blood Institute.
Notes
Affiliations of authors: Vanderbilt University Medical Center, Nashville, TN (HAT, MSD, RAG, SK, PPM, MSF); the Medical Service, Section of Pulmonary and Critical Care Medicine, Veterans Affairs Tennessee Valley Healthcare System, Nashville, TN (PPM); Geriatric Research Education and Clinical Centers, Veterans Affairs Tennessee Valley Healthcare System, Nashville, TN (HAT, RAG, MSF); Framingham Heart Study, Framingham and Boston University Schools of Medicine and Public Health, Boston, MA (MSD, VSR).
Funders of the FHS had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors acknowledge Stephen King for editorial assistance. The authors do not have any conflicts of interest to disclose.
Supplementary Material
References
- 1. World Health Organization. Tobacco fact sheet. http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed February 7, 2018.
- 2. World Health Organization. Cancer fact sheet. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed February 7, 2018.
- 3.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2014.
- 4. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanner NT, Kanodra NM, Gebregziabher M, et al. The association between smoking abstinence and mortality in the National Lung Screening Trial. Am J Respir Crit Care Med. 2016;193(5):534–541. [DOI] [PubMed] [Google Scholar]
- 6. Lim SH, Tai BC, Yuan JM, et al. Smoking cessation and mortality among middle-aged and elderly Chinese in Singapore: The Singapore Chinese Health Study. Tob Control. 2013;22(4):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carreras G, Pistelli F, Falcone F, et al. Reduction of risk of dying from tobacco-related diseases after quitting smoking in Italy. Tumori. 2015;101(6):657–663. [DOI] [PubMed] [Google Scholar]
- 8. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doll R, Peto R.. Mortality in relation to smoking: 20 years' observations on male British doctors. BMJ. 1976;2(6051):1525–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammond EC. Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr. 1966;19:127–204. [PubMed] [Google Scholar]
- 11. Rogot E, Murray J.. Cancer mortality among nonsmokers in an insured group of U.S. veterans. J Natl Cancer Inst. 1980;65(5):1163–1168. [PubMed] [Google Scholar]
- 12. Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebbert JO, Yang P, Vachon CM, et al. Lung cancer risk reduction after smoking cessation: Observations from a prospective cohort of women. J Clin Oncol. 2003;21(5):921–926. [DOI] [PubMed] [Google Scholar]
- 14. Lubin JH, Blot WJ, Berrino F, et al. Modifying risk of developing lung cancer by changing habits of cigarette smoking. BMJ. 1984;288(6435):1953–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong KY, Seow A, Koh WP, et al. Smoking cessation and lung cancer risk in an Asian population: Findings from the Singapore Chinese Health Study. Br J Cancer. 2010;103(7):1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed March 12, 2018.
- 17. Chin J, Syrek Jensen T, Ashby L, et al. Screening for lung cancer with low-dose CT—translating science into Medicare coverage policy. N Engl J Med. 2015;372(22):2083–2085. [DOI] [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services. The Health Benefits of Smoking Cessation. Washington, DC: Department of Health and Human Services; 1990. [Google Scholar]
- 19. Kuller LH, Ockene JK, Meilahn E, et al. Cigarette smoking and mortality. MRFIT Research Group. Prev Med. 1991;20(5):638–654. [DOI] [PubMed] [Google Scholar]
- 20. Godtfredsen NS, Prescott E, Osler M.. Effect of smoking reduction on lung cancer risk. JAMA. 2005;294(12):1505–1510. [DOI] [PubMed] [Google Scholar]
- 21. Kreger BE, Splansky GL, Schatzkin A.. The cancer experience in the Framingham Heart Study cohort. Cancer. 1991;67(1):1–6. [DOI] [PubMed] [Google Scholar]
- 22. Peto J. That the effects of smoking should be measured in pack-years: Misconceptions 4. Br J Cancer. 2012;107(3):406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinsky PF, Zhu CS, Kramer BS.. Lung cancer risk by years since quitting in 30+ pack year smokers. J Med Screen. 2015;22(3):151–157. [DOI] [PubMed] [Google Scholar]
- 24. Pinsky PF, Berg CD.. Applying the National Lung Screening Trial eligibility criteria to the US population: What percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19(3):154–156. [DOI] [PubMed] [Google Scholar]
- 25. Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 26. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: A systematic review. JAMA. 2012;307(22):2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: A systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159(6):411–420. [DOI] [PubMed] [Google Scholar]
- 28. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tammemagi MC, Church TR, Hocking WG. et al. Evaluation of the lung cancer risks at which to screen ever- and never smokers: Screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khuder SA, Mutgi AB.. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120(5):1577–1583. [DOI] [PubMed] [Google Scholar]
- 32. Kenfield SA, Wei EK, Stampfer MJ, et al. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17(3):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker F, Ainsworth SR, Dye JT, et al. Health risks associated with cigar smoking. JAMA. 2000;284(6):735–740. [DOI] [PubMed] [Google Scholar]
- 34. Pershagen G, Akerblom G, Axelson O, et al. Residential radon exposure and lung cancer in Sweden. N Engl J Med. 1994;330(3):159–164. [DOI] [PubMed] [Google Scholar]
- 35. Lam TK, Gallicchio L, Boyd K, et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(1):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wassenaar CA, Ye Y, Cai Q, et al. CYP2A6 reduced activity gene variants confer reduction in lung cancer risk in African American smokers—findings from two independent populations. Carcinogenesis. 2015;36(1):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spira A, Beane J, Shah V, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101(27):10143–10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beane J, Sebastiani P, Liu G, et al. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8(9):R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Varella-Garcia M, Chen L, Powell RL, et al. Spectral karyotyping detects chromosome damage in bronchial cells of smokers and patients with cancer. Am J Respir Crit Care Med. 2007;176(5):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rahman SM, Ji X, Zimmerman LJ, et al. The airway epithelium undergoes metabolic reprogramming in individuals at high risk for lung cancer. JCI Insight. 2016;1(19):e88814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


