Abstract
Background
A beneficial effect of supplementation with selenium, vitamin E, and beta-carotene was observed on total and cancer mortality in a Chinese population, and it endured for 10 years postintervention, but longer durability is unknown.
Methods
A randomized, double-blind, placebo-controlled trial was conducted in Linxian, China, from 1986 to 1991; 29 584 residents age 40 to 69 years received daily supplementations based on a factorial design: Factors A (retinol/zinc), B (riboflavin/niacin), C (vitamin C/molybdenum), and/or D (selenium/vitamin E/beta-carotene), or placebo for 5.25 years, and followed for up 25 years. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the intervention effects on mortalities were estimated using Cox proportional hazards models.
Results
Through 2016, the interventions showed no effect on total mortality. The previously reported protective effect of Factor D against total mortality was lost 10 years postintervention. The protective effect of Factor D for gastric cancer was attenuated (HR = 0.93, 95% CI = 0.85 to 1.01), but a newly apparent protective effect against esophageal cancer was found for Factor B (HR = 0.92, 95% CI = 0.85 to 1.00, two-sided P = .04). Other protective/adverse associations were observed for cause-specific mortalities. Protective effects were found in people younger than age 55 years at baseline against non–upper gastrointestinal cancer death for Factor A (HR = 0.80, 95% CI = 0.69 to 0.92) and against death from stroke for Factor C (HR = 0.89, 95% CI = 0.82 to 0.96). In contrast, increased risk of esophageal cancer was found when the intervention began after age 55 years for Factors C (HR = 1.16, 95% CI = 1.04 to 1.30) and D (HR = 1.20, 95% CI = 1.07 to 1.34).
Conclusions
Multiyear nutrition intervention is unlikely to have a meaningful effect on mortality more than a decade after supplementation ends, even in a nutritionally deprived population. Whether sustained or repeat intervention would provide longer effects needs further investigation.
Debate regarding the association between nutritional supplementation and cancer risk has continued for decades. More than 20 major randomized controlled trials (RCTs) were conducted to test the effects of nutritional interventions on cancer prevention, but few reported important effects for the nutrients tested. Studies including the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) (1), the Beta-Carotene and Retinol Efficacy Trial (CARET) (2), and the Selenium and Vitamin E Cancer Prevention Trial (SELECT) (3) found adverse results for the primary hypothesis tested in their respective interventions, which led to cautionary advice from the US Preventive Services Task Force against use of nutritional supplements in healthy adults without special nutritional needs (4). In contrast, other studies such as the Linxian General Population Nutrition Intervention Trial (NIT) (5,6), the Supplementation en Vitamines et Mineraux Antioxydants Study (SU.VI.MAX) (7), and the Physicians’ Health Study II (PHSII) (8) reported statistically significant benefits from nutritional intervention in specific populations. Among the nutritional interventions that found statistically significant effects, few subsequently reported the duration of effects after cessation of the intervention. Among those that did report duration, most effects regressed within six or fewer years post-trial.
The Linxian NIT study was a landmark study because it was the first randomized, double-blind, placebo-controlled nutritional intervention trial to report a reduction in total and cancer mortality following supplementation (5). The uniqueness of the NIT was further evident when a 10-year post-trial follow-up showed that the beneficial effects of selenium, vitamin E, and beta-carotene supplementation on total mortality and gastric cancer mortality lasted up to 10 years (6). However, still longer follow-up was needed to determine the durability of these post-trial effects.
Here we report a 25-year post-trial follow-up analysis of the effects of supplementation on the a priori end points. This large and long-term assessment of a nutritional intervention will inform the utility of multiyear interventions for future public health campaigns.
Methods
Study Design and Post-trial Follow-up of the NIT Study
The design of the Linxian General Population NIT and its extended follow-up have been described before (5,6,9); 29 584 residents age 40 to 69 years received daily supplementations based on a factorial design by four Factors (10): A (retinol/zinc), B (riboflavin/niacin), C (vitamin C/molybdenum), and/or D (selenium/vitamin E/beta-carotene), or placebo (Supplementary Table 1, available online). After a baseline survey, participants were randomly assigned to one of eight intervention groups, which received Factors ABCD, AB, AC, AD, BC, BD, CD, or placebo. With this design, half of the subjects received and half did not receive each of the four factors. The intervention lasted for 5.25 years, from March 1986 to May 1991. The cohort was followed postsupplementation for an additional 25 years through March 2016 (Supplementary Methods, available online).
In the post-trial follow-up, the village health workers contacted participants monthly. Cancer diagnoses were verified by the panel of American and Chinese experts (1991 to 1996) or senior Chinese diagnosticians from Beijing (1996 to 2016), and death end points were cross-checked with death registration quarterly. Through the 30 years of observation (March 1986 to 2016), case ascertainment was considered complete and loss to follow-up minimal (n = 381, 1.3%). Due to delayed ascertainment of outcomes, the number of deaths reported here is slightly higher than in previous reports (5,6).
The Linxian NIT and follow-up studies were approved by the institutional review boards of the Cancer Hospital/ Institute of Chinese Academy of Medical Sciences and the US National Cancer Institute, and written informed consent was obtained from all participants. The trial was registered as ClinicalTrials.gov number NCT00342654.
Statistical Analysis
The primary outcomes were total, total cancer, esophageal cancer, and gastric cancer mortality. Secondary outcomes were non–upper gastrointestinal (non-UGI) cancer, cerebrovascular disease, heart disease, and other disease mortality.
Participants were censored at their last known follow-up date, date of death, or the administrative closure of follow-up for the study (March 2016), whichever came first. The 5.25-year trial plus 25-year post-trial follow-up was analyzed as a single unit, and in two separate 15-year periods: the earlier 15-year period (March 1986 to May 2001) and the later 15-year period (June 2001 to March 2016). The 15-year cut-point was chosen to facilitate comparison with the earlier 15-year follow-up results (6). We used a time-dependent indicator of follow-up beyond 15 years to test for heterogeneity of effects over time.
We tabulated baseline frequencies and percentages by demographic factors for participants in the different intervention groups. As for our previous analyses (5,6), Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for each factor, adjusting for the other three factors, sex, age at entry (continuous), and commune (four communes). These analyses were conducted on 29 553 of 29 584 initial study participants (31 were excluded before the intervention began) (Supplementary Figure 1, available online). Models were stratified by baseline age (<55 and ≥55 years) and sex. To test for interactions, we included interaction terms in the Cox models. The 55-year age cut-point was chosen as the midpoint of the 40–69-year age range of the population at baseline (6). Kaplan-Meier estimates of survival rates were plotted to compare time to death for each intervention factor, for all subjects and by age group. To test the proportional hazards assumption, the heterogeneity of the treatment hazard ratios across the initial and later 15-year follow-up periods was tested for each of the analyses by testing for interaction with a time-dependent indicator of more than 15 years of follow-up. All P values are two-sided, and P values of less than .05 were considered statistically significant unless otherwise indicated. In addition, we used a Bonferroni correction for each of the subgroups and end points. The cut-points for statistically significant P values after Bonferroni correction are described with each table. Moreover, we performed an analysis on the loss per 100 person-years of observation (Supplementary Table 2, available online). Analyses were conducted using SAS version 9.3 (SAS Institute, Inc, Cary, NC), and figures were produced using the R survival package (version 3.3.1).
Results
Demographic Information
Through March 2016, a total of 588 401 person-years of follow-up were accumulated. Baseline demographic characteristics, smoking and alcohol use, and family history of UGI cancer for all subjects are shown in Table 1. As expected, because of the random assignment, there were no statistically significant differences between any of these baseline characteristics by treatment group assignment.
Table 1.
Baseline demographic characteristics of subjects
| Characteristic | All participants(% of total) | Range of 8 treatment arms |
|---|---|---|
| No. of participants | 29 553 | 3687–3706 |
| Age, y | ||
| <50 | 12 364 (41.8) | 41.6–42.2 |
| 50–59 | 10 255 (34.7) | 34.4–35.0 |
| ≥60 | 6934 (23.5) | 23.3–23.6 |
| Sex | ||
| Women | 16 378 (55.4) | 55.1–55.6 |
| Men | 13 175 (44.6) | 44.4–44.9 |
| Cigarette smoking* | ||
| Nonsmoker | 20 613 (70.0) | 69.8–70.8 |
| Smoker | 8836 (30.0) | 29.2–30.2 |
| Alcohol drinking† | ||
| Nondrinker | 22 535(76.5) | 75.6–76.8 |
| Drinker | 6913 (23.5) | 23.2–24.5 |
| Family history of UGI cancer‡ | ||
| Yes | 9443 (32.0) | 31.0–32.4 |
| No | 20 110 (68.1) | 67.6–69.0 |
| BMI, mean | 21.9 | 21.9–22.0 |
| Fruit, mean, times/y | 15.7 | 14.9–16.4 |
| Fresh vegetable, mean, times/y | 737.3 | 730.8–746.7 |
| Egg and meat, mean, times/y | 54.8 | 52.2–56.5 |
Ever smoking cigarettes for six or more months; data on smoking was not available for 104 subjects. There was a statistically significant sex difference with respect to smoking: 67% of the males but only 0.2% of the females reported smoking. BMI = body mass index; UGI = upper gastrointestinal.
Any alcoholic beverages in the last 12 months; data on drinking were not available for 105 subjects.
Family history of UGI cancer was defined as a diagnosis of any UGI cancer (esophageal, gastric cardia, or gastric noncardia cancer) in a first-degree relative (parents, siblings, children).
Overall Intervention Effect Through the Total 30-Year Follow-up
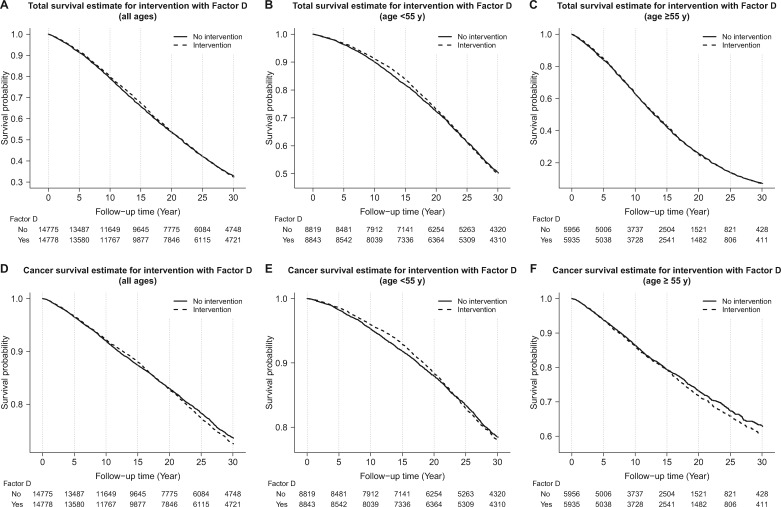
A total of 19 734 deaths (66.8% of participants) were ascertained through 30 years. Cerebrovascular diseases (32.1%), cancer (29.3%), and heart disease (24.4%) ranked as the top three causes of the death. The top two cancers were esophageal cancer (n = 2603, 45.0% of cancer deaths, 13.2% of all deaths) and gastric cardia cancer (n = 1410, 24.4% of cancer deaths, 7.1% of all deaths). Adjusted hazard ratios (95% CIs) for associations of each intervention factor with total and cause-specific deaths through 30 years are shown in Table 2. For the 30-year follow-up overall, no differences in total mortality were found between the intervention and nonintervention groups for Factors A, B, C, or D, nor did total mortality differ for any of the treatment in age or sex subgroups. Figure 1 shows that the 5.25-year nutritional intervention by Factor D had no effect on total or cancer mortality through the entire follow-up period, either in the whole population or in age subgroups (Figure 1).
Table 2.
Hazard ratios and 95% CIs over 30 years of total follow-up for death by cause and intervention factor*
| Group, cause of death | No. | Vitamin and mineral treatment factor† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor A |
Factor B |
Factor C |
Factor D |
||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Total‡ | |||||||||
| Total deaths | 19 734 | 1.02 (0.99 to 1.05) | .14 | 0.99 (0.97 to 1.02) | .58 | 0.99 (0.96 to 1.02) | .39 | 1.00 (0.97 to 1.03) | .93 |
| Cancer | 5783 | 0.99 (0.94 to 1.04) | .75 | 0.98 (0.93 to 1.03) | .37 | 1.06 (1.01 to 1.12)§ | .02 | 1.03 (0.98 to 1.08) | .27 |
| Esophageal | 2603 | 1.09 (1.01 to 1.18)§ | .03 | 0.92 (0.85 to 1.00)§ | .04 | 1.07 (0.99 to 1.15) | .11 | 1.11 (1.03 to 1.20)§ | .01 |
| Gastric | 1971 | 0.96 (0.88 to 1.05) | .34 | 1.01 (0.93 to 1.11) | .77 | 1.09 (1.00 to 1.19) | .06 | 0.93 (0.85 to 1.01) | .10 |
| Cardia | 1410 | 0.93 (0.83 to 1.03) | .14 | 1.02 (0.92 to 1.13) | .77 | 1.14 (1.02 to 1.26)§ | .02 | 0.92 (0.83 to 1.02) | .12 |
| Noncardia | 560 | 1.04 (0.88 to 1.23) | .61 | 1.01 (0.86 to 1.19) | .91 | 0.98 (0.83 to 1.15) | .79 | 0.95 (0.80 to 1.12) | .52 |
| Non-UGI cancer | 1210 | 0.86 (0.76 to 0.96)§ | .007 | 1.04 (0.93 to 1.16) | .52 | 1.01 (0.90 to 1.13) | .84 | 1.04 (0.93 to 1.17) | .45 |
| Cerebrovascular | 6343 | 1.06 (1.01 to 1.11)§ | .03 | 1.00 (0.95 to 1.05) | .89 | 0.93 (0.89 to 0.98)‖ | .005 | 1.02 (0.97 to 1.07) | .55 |
| Heart disease | 4821 | 1.01 (0.95 to 1.07) | .80 | 0.99 (0.93 to 1.05) | .70 | 0.98 (0.93 to 1.04) | .55 | 0.97 (0.92 to 1.03) | .35 |
| Other | 2787 | 1.03 (0.96 to 1.11) | .46 | 1.02 (0.95 to 1.10) | .56 | 0.98 (0.91 to 1.06) | .60 | 0.96 (0.89 to 1.04) | .29 |
| Age at baseline, y | |||||||||
| Age < 55 y¶, total deaths | 8719 | 1.02 (0.98 to 1.06) | .37 | 0.99 (0.95 to 1.03) | .69 | 0.98 (0.94 to 1.02) | .32 | 1.00 (0.96 to 1.05) | .84 |
| Cancer | 3180 | 0.97 (0.90 to 1.04) | .34 | 0.98 (0.91 to 1.05) | .54 | 1.04 (0.97 to 1.11) | .32 | 1.01 (0.94 to 1.08) | .82 |
| Esophageal | 1400 | 1.09 (0.99 to 1.22) | .09 | 0.90 (0.81 to 1.00) | .06 | 1.00 (0.90 to 1.10) | .92 | 1.03 (0.93 to 1.15) | .55 |
| Gastric | 1043 | 0.94 (0.83 to 1.06) | .32 | 1.04 (0.92 to 1.18) | .50 | 1.08 (0.96 to 1.22) | .21 | 0.90 (0.80 to 1.02) | .10 |
| Cardia | 762 | 0.92 (0.80 to 1.06) | .25 | 1.04 (0.90 to 1.20) | .57 | 1.09 (0.95 to 1.26) | .24 | 0.85 (0.74 to 0.98)§ | .03 |
| Noncardia | 281 | 1.00 (0.79 to 1.26) | .97 | 1.04 (0.83 to 1.32) | .73 | 1.06 (0.84 to 1.34) | .62 | 1.06 (0.84 to 1.34) | .63 |
| Non-UGI cancer | 737 | 0.80 (0.69 to 0.92)‖ | .002 | 1.04 (0.90 to 1.21) | .57 | 1.05 (0.91 to 1.22) | .47 | 1.12 (0.97 to 1.30) | .11 |
| Cerebrovascular | 2658 | 1.07 (0.99 to 1.15) | .09 | 0.99 (0.92 to 1.07) | .75 | 0.89 (0.82 to 0.96)‖ | .002 | 1.04 (0.96 to 1.12) | .32 |
| Heart disease | 1608 | 1.00 (0.91 to 1.10) | .97 | 1.03 (0.94 to 1.14) | .52 | 1.02 (0.92 to 1.12) | .75 | 0.97 (0.88 to 1.07) | .51 |
| Other | 1273 | 1.08 (0.97 to 1.21) | .15 | 0.98 (0.88 to 1.10) | .73 | 0.99 (0.89 to 1.11) | .88 | 0.97 (0.87 to 1.08) | .59 |
| Age ≥ 55 y¶, total deaths | 11 015 | 1.02 (0.98 to 1.06) | .34 | 1.00 (0.96 to 1.04) | .92 | 1.00 (0.97 to 1.04) | .88 | 1.00 (0.96 to 1.04) | .92 |
| Cancer | 2603 | 1.02 (0.95 to 1.10) | .60 | 0.98 (0.91 to 1.06) | .57 | 1.10 (1.02 to 1.19)§ | .02 | 1.05 (0.98 to 1.14) | .19 |
| Esophageal | 1203 | 1.09 (0.97 to 1.22) | .14 | 0.95 (0.85 to 1.06) | .37 | 1.16 (1.04 to 1.30)§ | .01 | 1.20 (1.07 to 1.34)‖ | .002 |
| Gastric | 928 | 0.98 (0.86 to 1.11) | .71 | 0.99 (0.87 to 1.12) | .83 | 1.10 (0.97 to 1.26) | .13 | 0.95 (0.84 to 1.08) | .44 |
| Cardia | 648 | 0.93 (0.80 to 1.08) | .34 | 0.99 (0.85 to 1.15) | .89 | 1.20 (1.03 to 1.40)§ | .02 | 1.00 (0.86 to 1.17) | .96 |
| Noncardia | 279 | 1.09 (0.86 to 1.38) | .47 | 0.98 (0.78 to 1.24) | .88 | 0.91 (0.72 to 1.15) | .43 | 0.84 (0.67 to 1.07) | .16 |
| Non-UGI cancer | 473 | 0.95 (0.80 to 1.14) | .59 | 1.03 (0.86 to 1.23) | .75 | 0.96 (0.80 to 1.15) | .62 | 0.93 (0.77 to 1.11) | .40 |
| Cerebrovascular | 3685 | 1.04 (0.98 to 1.11) | .19 | 1.01 (0.94 to 1.07) | .86 | 0.97 (0.91 to 1.04) | .41 | 1.00 (0.94 to 1.06) | .93 |
| Heart disease | 3213 | 1.00 (0.94 to 1.08) | .91 | 0.97 (0.91 to 1.04) | .46 | 0.98 (0.91 to 1.05) | .47 | 0.98 (0.91 to 1.05) | .54 |
| Other | 1514 | 0.98 (0.89 to 1.09) | .75 | 1.07 (0.96 to 1.18) | .22 | 0.98 (0.89 to 1.09) | .71 | 0.95 (0.86 to 1.05) | .35 |
| Sex | |||||||||
| Women¶, total deaths | 10 094 | 1.02 (0.98 to 1.06) | .46 | 1.00 (0.97 to 1.04) | .83 | 1.00 (0.96 to 1.04) | .85 | 1.01 (0.97 to 1.05) | .66 |
| Cancer | 2655 | 1.03 (0.96 to 1.11) | .41 | 0.98 (0.91 to 1.06) | .67 | 1.09 (1.01 to 1.17)§ | .03 | 1.00 (0.92 to 1.08) | .93 |
| Esophageal | 1317 | 1.17 (1.05 to 1.31)§ | .004 | 0.91 (0.82 to 1.01) | .08 | 1.07 (0.96 to 1.19) | .25 | 1.06 (0.95 to 1.18) | .28 |
| Gastric | 756 | 0.93 (0.80 to 1.07) | .30 | 1.02 (0.88 to 1.17) | .84 | 1.10 (0.96 to 1.27) | .18 | 0.91 (0.79 to 1.05) | .20 |
| Cardia | 552 | 0.94 (0.80 to 1.11) | .47 | 1.02 (0.86 to 1.21) | .82 | 1.15 (0.97 to 1.35) | .11 | 0.89 (0.76 to 1.06) | .19 |
| Noncardia | 204 | 0.89 (0.68 to 1.18) | .42 | 1.00 (0.76 to 1.32) | .98 | 1.00 (0.76 to 1.31) | .98 | 0.96 (0.73 to 1.26) | .77 |
| Non-UGI cancer | 582 | 0.89 (0.76 to 1.05) | .16 | 1.13 (0.96 to 1.33) | .14 | 1.12 (0.95 to 1.32) | .18 | 0.97 (0.83 to 1.14) | .73 |
| Cerebrovascular | 3537 | 1.01 (0.95 to 1.08) | .71 | 1.03 (0.97 to 1.10) | .34 | 0.93 (0.87 to 0.99)§ | .02 | 1.05 (0.98 to 1.12) | .17 |
| Heart disease | 2569 | 0.97 (0.90 to 1.05) | .42 | 0.99 (0.92 to 1.07) | .78 | 1.02 (0.94 to 1.10) | .65 | 0.98 (0.91 to 1.06) | .60 |
| Other | 1333 | 1.08 (0.97 to 1.20) | .18 | 1.00 (0.90 to 1.12) | .97 | 0.97 (0.88 to 1.09) | .63 | 0.99 (0.89 to 1.10) | .80 |
| Men¶, total deaths | 9640 | 1.03 (0.99 to 1.07) | .16 | 0.98 (0.94 to 1.02) | .27 | 0.98 (0.94 to 1.02) | .31 | 0.99 (0.95 to 1.03) | .74 |
| Cancer | 3128 | 0.96 (0.89 to 1.03) | .25 | 0.97 (0.90 to 1.04) | .39 | 1.04 (0.97 to 1.11) | .30 | 1.06 (0.99 to 1.13) | .12 |
| Esophageal | 1286 | 1.02 (0.91 to 1.13) | .78 | 0.94 (0.84 to 1.04) | .23 | 1.06 (0.95 to 1.19) | .27 | 1.15 (1.03 to 1.29)§ | .01 |
| Gastric | 1215 | 0.98 (0.87 to 1.10) | .70 | 1.01 (0.90 to 1.13) | .84 | 1.08 (0.96 to 1.21) | .19 | 0.94 (0.84 to 1.05) | .25 |
| Cardia | 858 | 0.92 (0.80 to 1.05) | .20 | 1.01 (0.89 to 1.16) | .84 | 1.13 (0.98 to 1.29) | .09 | 0.94 (0.82 to 1.07) | .35 |
| Noncardia | 356 | 1.14 (0.93 to 1.40) | .22 | 1.01 (0.82 to 1.25) | .91 | 0.96 (0.78 to 1.19) | .73 | 0.94 (0.76 to 1.15) | .54 |
| Non-UGI cancer | 628 | 0.83 (0.71 to 0.97)§ | .02 | 0.96 (0.82 to 1.12) | .58 | 0.92 (0.79 to 1.08) | .30 | 1.12 (0.95 to 1.31) | .17 |
| Cerebrovascular | 2806 | 1.12 (1.04 to 1.21)§ | .003 | 0.95 (0.88 to 1.02) | .18 | 0.94 (0.87 to 1.01) | .10 | 0.98 (0.91 to 1.05) | .51 |
| Heart disease | 2252 | 1.06 (0.97 to 1.15) | .19 | 0.99 (0.91 to 1.07) | .73 | 0.94 (0.87 to 1.03) | .17 | 0.97 (0.89 to 1.05) | .40 |
| Other | 1454 | 0.99 (0.89 to 1.09) | 0.80 | 1.04 (0.94 to 1.15) | 0.46 | 0.99 (0.89 to 1.10) | 0.82 | 0.94 (0.85 to 1.04) | 0.23 |
Factor A = vitamin A (5000 IU/d) + zinc (22.5 mg/d); Factor B = riboflavin (3.2 mg/d) + niacin (40 mg/d); Factor C = ascorbic acid (120 mg/d) + molybdenum (30 μ g/d); Factor D = selenium (50 μ g/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d). CI = confidence interval; HR = hazard ratio; UGI = upper gastrointestinal.
Hazard ratios (95% CIs) were mutually adjusted for each of the factors in the table, including age at entry (continuous), sex, and intervention Factor received, as well as commune (four communes).
The Bonferroni-corrected critical P = .05/10 = .005.
Uncorrected P value was less than .05.
Uncorrected P value was less than the Bonferroni-corrected critical P value.
The Bonferroni-corrected critical P = .05/20 = .0025.
Figure 1.
Effect of Factor D (a combination of 50 µg selenium, 30 mg vitamin E, and 1.5 mg β-carotene daily for 5.25 years) on total mortality and total cancer mortality. A–C) Effects on total mortality for all subjects, subjects younger than age 55 years at random assignment, and subjects age 55 years and older at random assignment, respectively. D–F) Effects on cancer mortality for the same three age groups. Dashed lines represent participants who received Factor D; solid lines represent participants who did not receive Factor D. The number of subjects at risk is shown underneath each figure for people who received Factor D and those who did not receive Factor D.
The effects of Factors A, B, and C did not vary by time across all analyses, and no heterogeneity was found between the earlier and later 15-year follow-up periods (all Pheterogeneity > .05, data not shown). The extended analysis found that the previously observed increased risk of stroke death for Factor A and the reduced risk of stroke death for Factor C remained (HR = 1.06, 95% CI = 1.01 to 1.11, P = .02; HR = 0.93, 95% CI = 0.89 to 0.98, P = .005, respectively). In addition, several suggestive effects identified at 15 years became evident after 30 years of follow-up. These included protective effects for non-UGI cancer with Factor A (HR = 0.86, 95% CI = 0.76 to 0.96, P = .007) and esophageal cancer with Factor B (HR = 0.92, 95% CI = 0.85 to 1.00, P = .04), and adverse effects for esophageal cancer with Factor A (HR = 1.09, 95% CI = 1.01 to 1.18, P = .03), gastric cardia cancer with Factor C (HR = 1.14, 95% CI = 1.02 to 1.26, P = .02), and total cancer with Factor C (HR = 1.06, 95% CI = 1.01 to 1.12, P = .02) (Table 2).
For Factor D, however, the effect of the intervention varied by follow-up period. The protective effect of Factor D identified during the intervention period and the initial 10 years of postintervention follow-up against gastric cancer death was diminished (from HR = 0.89, 95% CI = 0.79 to 1.00, P = .04 [6]; to HR = 0.93, 95% CI = 0.85 to 1.01, P = .10) and an adverse effect on esophageal cancer became evident after 30 years (HR = 1.11, 95% CI = 1.03 to 1.20, P = .01). In addition, statistically significant heterogeneity was found for the time-specific effects of Factor D. Risk of esophageal cancer death was higher in the second half of follow-up than in the first half (HR1–15y = 1.01, 95% CI = 0.92 to 1.12; HR16–30y = 1.25, 95% CI = 1.11 to 1.41, Pheterogeneity = .008), and this contributed to a higher risk of total cancer death (HR1–15y = 0.95, 95% CI = 0.89 to 1.02; HR16–30y = 1.14, 95% CI = 1.06 to 1.24, Pheterogeneity = .0004) and total death (HR1–15y = 0.95, 95% CI = 0.91 to 0.99; HR16–30y = 1.06, 95% CI = 1.02 to 1.10, Pheterogeneity = .0002) in the second half of follow-up as well (Table 3). These adverse results in the later 15 years neutralized the beneficial effects of Factor D in the earlier 15 years, and cumulatively resulted in no overall effect on total mortality through the full 30 years of observation.
Table 3.
Hazard ratios for causes of death by Factor D and different follow-up periods stratified by age and sex groups, estimated from adjusted Cox proportional hazards models*
| Cause of death by years of follow-up, y | No. of cases | Intervention vs nonintervention groups for Factor D |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Women < 55 y |
Men < 55 y |
Women ≥ 55 y |
Men ≥ 55 y |
|||||||
| HR† (95% CI) | Pheterogeneity‡,§ | HR‖ (95% CI) | Pheterogeneity‡,¶ | HR‖ (95% CI) | Pheterogeneity‡,¶ | HR‖ (95% CI) | Pheterogeneity‡,¶ | HR‖ (95% CI) | Pheterogeneity‡,¶ | ||
| Total deaths | |||||||||||
| ≤15 | 10037 | 0.95 (0.91 to 0.99)# | .0002** | 0.88 (0.80 to 0.97)# | .0008** | 0.90 (0.81 to 1.00)# | .01# | 0.98 (0.91 to 1.05) | .22 | 0.98 (0.92 to 1.04) | .62 |
| >15 | 9696 | 1.06 (1.02 to 1.10)# | 1.08 (1.01 to 1.16)# | 1.06 (0.98 to 1.14) | 1.05 (0.97 to 1.13) | 1.01 (0.92 to 1.11) | |||||
| All cancers | |||||||||||
| ≤15 | 3282 | 0.95 (0.89 to 1.02) | .0004** | 0.85 (0.73 to 0.99)# | .01# | 0.87 (0.75 to 1.02) | .009# | 0.97 (0.85 to 1.12) | .38 | 1.04 (0.92 to 1.17) | .08 |
| >15 | 2501 | 1.14 (1.06 to 1.24)**,†† | 1.10 (0.97 to 1.25) | 1.14 (1.01 to 1.29)# | 1.09 (0.88 to 1.37) | 1.29 (1.04 to 1.61)# | |||||
| Esophageal cancer | |||||||||||
| ≤15 | 1524 | 1.01 (0.92 to 1.12) | .008# | 0.82 (0.66 to 1.02) | .009# | 0.87 (0.68 to 1.10) | .04# | 1.06 (0.87 to 1.28) | .30 | 1.23 (1.03 to 1.47)# | .38 |
| >15 | 1079 | 1.25 (1.11 to 1.41)**,‡‡ | 1.21 (1.00 to 1.46) | 1.20 (0.98 to 1.47) | 1.29 (0.94 to 1.76) | 1.45 (1.04 to 2.04)# | |||||
| Gastric cancer | |||||||||||
| ≤15 | 1187 | 0.89 (0.80 to 1.00) | .30 | 0.87 (0.66 to 1.16) | .74 | 0.84 (0.66 to 1.07) | .44 | 0.92 (0.72 to 1.17) | .97 | 0.92 (0.77 to 1.11) | .21 |
| >15 | 784 | 0.98 (0.85 to 1.13) | 0.93 (0.71 to 1.22) | 0.95 (0.78 to 1.16) | 0.92 (0.60 to 1.43) | 1.19 (0.83 to 1.69) | |||||
| Gastric cardia cancer | |||||||||||
| ≤15 | 850 | 0.90 (0.79 to 1.03) | .61 | 0.88 (0.64 to 1.21) | .73 | 0.78 (0.58 to 1.05) | .43 | 0.90 (0.68 to 1.19) | .31 | 0.99 (0.79 to 1.24) | .43 |
| >15 | 560 | 0.95 (0.81 to 1.12) | 0.81 (0.59 to 1.12) | 0.91 (0.72 to 1.15) | 1.25 (0.71 to 2.19) | 1.20 (0.79 to 1.82) | |||||
| Gastric noncardia cancer | |||||||||||
| ≤15 | 336 | 0.88 (0.71 to 1.09) | .28 | 0.86 (0.47 to 1.56) | .28 | 0.98 (0.63 to 1.54) | .78 | 0.97 (0.60 to 1.58) | .25 | 0.80 (0.57 to 1.10) | .32 |
| >15 | 224 | 1.06 (0.82 to 1.37) | 1.32 (0.80 to 2.17) | 1.07 (0.72 to 1.59) | 0.59 (0.29 to 1.19) | 1.16 (0.60 to 2.25) | |||||
| Non-UGI cancer | |||||||||||
| ≤15 | 572 | 0.90 (0.76 to 1.06) | .02# | 0.89 (0.61 to 1.29) | .37 | 0.98 (0.68 to 1.41) | .12 | 0.84 (0.60 to 1.19) | .70 | 0.9 (0.68 to 1.18) | .32 |
| >15 | 638 | 1.19 (1.02 to 1.39)# | 1.09 (0.85 to 1.38) | 1.40 (1.08 to 1.80)# | 0.94 (0.60 to 1.48) | 1.19 (0.74 to 1.93) | |||||
| Cerebrovascular | |||||||||||
| ≤15 | 3109 | 0.98 (0.92 to 1.06) | .22 | 0.98 (0.82 to 1.18) | .31 | 1.06 (0.86 to 1.32) | .57 | 1.01 (0.89 to 1.13) | .45 | 0.95 (0.84 to 1.06) | .81 |
| >15 | 3233 | 1.05 (0.98 to 1.12) | 1.10 (0.98 to 1.23) | 0.99 (0.86 to 1.14) | 1.08 (0.94 to 1.23) | 0.97 (0.83 to 1.14) | |||||
| Heart disease | |||||||||||
| ≤15 | 2116 | 0.95 (0.87 to 1.03) | .39 | 0.91 (0.70 to 1.17) | .42 | 0.84 (0.64 to 1.09) | .31 | 0.96 (0.83 to 1.11) | .74 | 0.99 (0.86 to 1.12) | .87 |
| >15 | 2705 | 0.99 (0.92 to 1.07) | 1.03 (0.88 to 1.19) | 0.99 (0.82 to 1.18) | 0.99 (0.87 to 1.13) | 0.97 (0.83 to 1.13) | |||||
| Other | |||||||||||
| ≤15 | 1530 | 0.89 (0.80 to 0.98)# | .02# | 0.77 (0.60 to 0.99)# | .02# | 0.84 (0.66 to 1.06) | .08 | 0.95 (0.78 to 1.16) | .41 | 0.93 (0.79 to 1.09) | .71 |
| >15 | 1257 | 1.06 (0.95 to 1.18) | 1.12 (0.92 to 1.37) | 1.10 (0.89 to 1.36) | 1.08 (0.86 to 1.36) | 0.88 (0.68 to 1.13) | |||||
Hazard ratio, adjusted for the other three treatments factors, and commune (four communes). Factor D = selenium (50 μ g/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d). CI = confidence interval; HR = hazard ratio; UGI = upper gastrointestinal.
The Bonferroni-corrected critical P = .05/20 = .0025 for the period-specific HR test in the whole population.
P value was tested for the heterogeneity of the HRs for the earlier (1–15th years) and later (16–30th years) 15-year follow-up periods, using Cox model.
The Bonferroni-corrected critical P = .05/10 = .005 for the heterogeneity test in the whole population.
The Bonferroni-corrected critical P = .05/80 = .000625 for the period-specific HR test in the age- and sex- subgroups.
The Bonferroni-corrected critical P = .05/40 = .00125 for the heterogeneity test in the age- and sex-subgroups.
Uncorrected P value was less than .05
Uncorrected P value was less than the Bonferroni-corrected critical P value.
Uncorrected P = .0007.
Uncorrected P = .0002.
Effect of Intervention in Different Age and Sex Subgroups
Although there were no uniformly evident interactions between age and intervention factors through the entire follow-up period, the effects of intervention appeared to differ by age (Table 2). Results in the subgroup of persons younger than age 55 years at baseline showed three intervention effects (P < .05), and all three were protective: deaths decreased for non-UGI cancer death with Factor A (HR = 0.80, 95% CI = 0.69 to 0.92, P = .002), stroke with Factor C (HR = 0.89, 95% CI = 0.82 to 0.96, P = .002), and gastric cardia cancer with Factor D (HR = 0.85, 95% CI = 0.74 to 0.98, P = .03). In contrast, four intervention effects (P < .05) were found in persons age 55 years or older at entry, and all four were adverse: deaths increased for esophageal cancer with Factor C (HR = 1.16, 95% CI = 1.04 to 1.30, P = .01), gastric cardia cancer with Factor C (HR = 1.20, 95% CI = 1.03 to 1.40, P = .02), total cancer mortality with Factor C (HR = 1.10, 95% CI = 1.02 to 1.19, P = .02), and esophageal cancer with Factor D (HR = 1.20, 95% CI = 1.07 to 1.34, P = .002).
Overall, no statistically significant differences were found between sexes for the intervention effects on total mortality and cancer mortality (all Pinteraction > .05, data not shown). The subjects were further stratified into subgroups by age and sex (Table 4 and 5), and effects appeared to vary among subgroups for some specific end points, although these results should be considered exploratory. For both sexes, Factor A apparently lowered risk of non-UGI cancer, and younger males seemed to benefit the most (HR = 0.72, 95% CI = 0.58 to 0.88, P = .002). Similarly, hazard ratios were uniformly less than 1 for stroke in both sexes for Factor C, with the strongest protective effect seen in younger females (HR = 0.88, 95% CI = 0.79 to 0.97, P = .009). Finally, adverse effects on esophageal cancer death appeared most pronounced for Factor A in younger females (HR = 1.26, 95% CI = 1.09 to 1.46, P = .002), for Factor C in older females (HR = 1.19, 95% CI = 1.01 to 1.41, P = .04), and for Factor D in older males (HR = 1.27, 95% CI = 1.09 to 1.49, P = .003).
Table 4.
Hazard ratios for causes of death by treatment factors for women stratified by age, estimated from adjusted Cox proportional hazards models*
| Cause of death by age at baseline, y | No. of cases | Intervention vs nonintervention groups, HR† (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor A |
Factor B |
Factor C |
Factor D |
||||||
| HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | ||
| Total | |||||||||
| <55 | 4660 | 1.04 (0.98 to 1.10) | .18 | 1.01 (0.95 to 1.07) | .73 | 0.97 (0.91 to 1.02) | .26 | 1.01 (0.95 to 1.07) | .73 |
| ≥55 | 5434 | 1.00 (0.95 to 1.06) | .95 | 1.00 (0.95 to 1.05) | .98 | 1.02 (0.97 to 1.08) | .44 | 1.01 (0.96 to 1.06) | .78 |
| All cancers | |||||||||
| <55 | 1532 | 1.07 (0.97 to 1.19) | .17 | 1.01 (0.91 to 1.12) | .84 | 1.01 (0.91 to 1.12) | .85 | 0.99 (0.89 to 1.09) | .81 |
| ≥55 | 1123 | 0.99 (0.88 to 1.11) | .81 | 0.95 (0.84 to 1.07) | .37 | 1.20 (1.07 to 1.35)§ | .002 | 1.01 (0.89 to 1.13) | .93 |
| Esophageal cancer deaths | |||||||||
| <55 | 746 | 1.26 (1.09 to 1.46)§ | .002 | 0.87 (0.75 to 1.00) | .06 | 0.98 (0.85 to 1.13) | .75 | 1.02 (0.88 to 1.18) | .79 |
| ≥55 | 571 | 1.07 (0.91 to 1.26) | .43 | 0.96 (0.82 to 1.13) | .64 | 1.19 (1.01 to 1.41)§ | .04 | 1.12 (0.95 to 1.32) | .19 |
| Gastric cancer deaths | |||||||||
| <55 | 410 | 0.96 (0.79 to 1.16) | .65 | 1.16 (0.95 to 1.41) | .14 | 1.06 (0.88 to 1.29) | .54 | 0.91 (0.75 to 1.10) | .31 |
| ≥55 | 346 | 0.90 (0.73 to 1.11) | .34 | 0.87 (0.70 to 1.07) | .19 | 1.15 (0.93 to 1.42) | .19 | 0.92 (0.74 to 1.13) | .43 |
| Cardia gastric cancer deaths | |||||||||
| <55 | 304 | 0.98 (0.78 to 1.22) | .84 | 1.17 (0.93 to 1.46) | .18 | 1.05 (0.84 to 1.31) | .68 | 0.84 (0.67 to 1.06) | .14 |
| ≥55 | 248 | 0.90 (0.70 to 1.16) | .43 | 0.86 (0.67 to 1.11) | .25 | 1.27 (0.99 to 1.63) | .06 | 0.96 (0.75 to 1.23) | .74 |
| Noncardia gastric cancer deaths | |||||||||
| <55 | 106 | 0.90 (0.61 to 1.32) | .59 | 1.14 (0.78 to 1.66) | .51 | 1.10 (0.75 to 1.61) | .62 | 1.11 (0.76 to 1.62) | .61 |
| ≥55 | 98 | 0.90 (0.60 to 1.33) | .59 | 0.88 (0.59 to 1.31) | .53 | 0.90 (0.61 to 1.34) | .60 | 0.82 (0.55 to 1.23) | .34 |
| Non-UGI cancer deaths | |||||||||
| <55 | 376 | 0.88 (0.72 to 1.08) | .21 | 1.17 (0.96 to 1.44) | .12 | 1.02 (0.84 to 1.25) | .83 | 1.02 (0.84 to 1.25) | .83 |
| ≥55 | 206 | 0.92 (0.70 to 1.20) | .52 | 1.06 (0.80 to 1.39) | .69 | 1.31 (1.00 to 1.73) | .05 | 0.88 (0.67 to 1.16) | .35 |
| Cerebrovascular | |||||||||
| <55 | 1574 | 1.03 (0.94 to 1.14) | .53 | 1.00 (0.90 to 1.10) | .93 | 0.88 (0.79 to 0.97)§ | .009 | 1.06 (0.96 to 1.17) | .24 |
| ≥55 | 1963 | 1.01 (0.92 to 1.10) | .92 | 1.06 (0.97 to 1.16) | .19 | 0.97 (0.89 to 1.06) | .50 | 1.04 (0.95 to 1.13) | .44 |
| Heart disease | |||||||||
| <55 | 913 | 0.98 (0.86 to 1.11) | .75 | 1.06 (0.93 to 1.21) | .35 | 1.03 (0.91 to 1.18) | .64 | 0.99 (0.87 to 1.13) | .91 |
| ≥55 | 1656 | 0.97 (0.89 to 1.07) | .60 | 0.95 (0.86 to 1.05) | .31 | 1.01 (0.92 to 1.11) | .82 | 0.98 (0.89 to 1.08) | .66 |
| Other | |||||||||
| <55 | 641 | 1.08 (0.92 to 1.26) | .35 | 0.97 (0.83 to 1.13) | .72 | 1.02 (0.87 to 1.19) | .83 | 0.97 (0.83 to 1.13) | .68 |
| ≥55 | 692 | 1.09 (0.94 to 1.26) | .26 | 1.03 (0.89 to 1.20) | .67 | 0.94 (0.81 to 1.09) | .39 | 1.01 (0.87 to 1.17) | .95 |
Factor A = vitamin A (5000 IU/d) + zinc (22.5 mg/d); Factor B = riboflavin (3.2 mg/d) + niacin (40 mg/d); Factor C = ascorbic acid (120 mg/d) + molybdenum (30 μ g/d); Factor D = selenium (50 μ g/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d). CI = confidence interval; HR = hazard ratio; UGI = upper gastrointestinal.
Hazard ratios adjusted for the other three treatments factors and commune (four communes).
Uncorrected P values were two-sided and calculated using the Cox proportional hazard model; the Bonferroni-corrected critical P = .05/40 = .00125 for the age and sex subgroup analyses, but none of the results were statistically significant at the Bonferroni-corrected critical P values.
Uncorrected P value was less than .05.
Table 5.
Hazard ratios for causes of death by treatment Factors for Men stratified by age, estimated from adjusted Cox proportional hazards models*
| Cause of death by age at baseline, y | No. of cases | Intervention vs nonintervention groups, HR† (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor A |
Factor B |
Factor C |
Factor D |
||||||
| HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | HR (95% CI)† | P‡ | ||
| Total | |||||||||
| <55 | 4059 | 1.00 (0.94 to 1.06) | .90 | 0.97 (0.91 to 1.03) | .34 | 0.99 (0.93 to 1.06) | .81 | 1.00 (0.94 to 1.06) | .95 |
| ≥55 | 5581 | 1.04 (0.98 to 1.09) | .18 | 0.99 (0.94 to 1.05) | .82 | 0.99 (0.94 to 1.04) | .60 | 0.99 (0.94 to 1.04) | .67 |
| All cancers | |||||||||
| <55 | 1648 | 0.88 (0.80 to 0.97)§ | .008 | 0.95 (0.86 to 1.04) | .28 | 1.06 (0.97 to 1.17) | .22 | 1.03 (0.93 to 1.13) | .58 |
| ≥55 | 1480 | 1.05 (0.95 to 1.16) | .36 | 1.00 (0.90 to 1.11) | .99 | 1.03 (0.93 to 1.14) | .62 | 1.09 (0.98 to 1.21) | .10 |
| Esophageal cancer deaths | |||||||||
| <55 | 654 | 0.93 (0.80 to 1.08) | .33 | 0.94 (0.81 to 1.10) | .44 | 1.02 (0.87 to 1.19) | .83 | 1.05 (0.90 to 1.22) | .55 |
| ≥55 | 632 | 1.11 (0.95 to 1.30) | .19 | 0.94 (0.80 to 1.10) | .42 | 1.13 (0.97 to 1.32) | .13 | 1.27 (1.09 to 1.49)§ | .003 |
| Gastric cancer deaths | |||||||||
| <55 | 633 | 0.93 (0.79 to 1.09) | .35 | 0.97 (0.83 to 1.14) | .72 | 1.10 (0.94 to 1.28) | .25 | 0.90 (0.77 to 1.06) | .20 |
| ≥55 | 582 | 1.02 (0.87 to 1.20) | .80 | 1.06 (0.90 to 1.25) | .45 | 1.07 (0.91 to 1.26) | .39 | 0.97 (0.83 to 1.14) | .72 |
| Cardia gastric cancer deaths | |||||||||
| <55 | 458 | 0.88 (0.74 to 1.06) | .19 | 0.97 (0.80 to 1.16) | .71 | 1.12 (0.93 to 1.34) | .23 | 0.86 (0.72 to 1.03) | .11 |
| ≥55 | 400 | 0.94 (0.77 to 1.15) | .55 | 1.08 (0.89 to 1.31) | .45 | 1.15 (0.94 to 1.40) | .17 | 1.03 (0.85 to 1.26) | .74 |
| Noncardia gastric cancer deaths | |||||||||
| <55 | 175 | 1.06 (0.79 to 1.42) | .72 | 0.99 (0.73 to 1.33) | .93 | 1.04 (0.77 to 1.40) | .80 | 1.03 (0.77 to 1.39) | .85 |
| ≥55 | 181 | 1.21 (0.90 to 1.62) | .20 | 1.05 (0.78 to 1.40) | .77 | 0.91 (0.68 to 1.22) | .54 | 0.86 (0.64 to 1.15) | .30 |
| Non-UGI cancer deaths | |||||||||
| <55 | 361 | 0.72 (0.58 to 0.88)§ | .002 | 0.92 (0.75 to 1.13) | .44 | 1.09 (0.89 to 1.34) | .42 | 1.24 (1.01 to 1.53)§ | .04 |
| ≥55 | 267 | 0.98 (0.77 to 1.25) | .87 | 1.01 (0.79 to 1.28) | .95 | 0.75 (0.59 to 0.95)§ | .02 | 0.96 (0.76 to 1.22) | .76 |
| Cerebrovascular | |||||||||
| <55 | 1084 | 1.12 (1.00 to 1.27) | .05 | 0.98 (0.87 to 1.10) | .70 | 0.91 (0.81 to 1.02) | .11 | 1.01 (0.90 to 1.14) | .88 |
| ≥55 | 1722 | 1.09 (0.99 to 1.20) | .07 | 0.95 (0.86 to 1.04) | .25 | 0.98 (0.89 to 1.08) | .64 | 0.96 (0.87 to 1.05) | .34 |
| Heart disease | |||||||||
| <55 | 695 | 1.03 (0.88 to 1.19) | .74 | 1.00 (0.86 to 1.16) | .96 | 1.00 (0.86 to 1.16) | .95 | 0.94 (0.81 to 1.09) | .39 |
| ≥55 | 1557 | 1.04 (0.94 to 1.15) | .45 | 0.99 (0.90 to 1.10) | .89 | 0.94 (0.85 to 1.04) | .22 | 0.98 (0.89 to 1.08) | .66 |
| Other | |||||||||
| <55 | 632 | 1.09 (0.94 to 1.28) | .26 | 0.99 (0.85 to 1.16) | .91 | 0.97 (0.83 to 1.13) | .66 | 0.97 (0.83 to 1.14) | .74 |
| ≥55 | 822 | 0.91 (0.79 to 1.04) | .15 | 1.09 (0.95 to 1.25) | .22 | 1.02 (0.89 to 1.17) | .78 | 0.91 (0.80 to 1.05) | .19 |
Factor A = vitamin A (5000 IU/d) + zinc (22.5 mg/d); Factor B = riboflavin (3.2 mg/d) + niacin (40 mg/d); Factor C = ascorbic acid (120 mg/d) + molybdenum (30 μ g/d); Factor D = selenium (50 μ g/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d). CI = confidence interval; HR = hazard ratio; UGI = upper gastrointestinal.
Hazard ratios adjusted for the other three treatments factors and commune (four communes).
Uncorrected P values were two-sided and calculated using the Cox proportional hazard model; the Bonferroni-corrected critical P = .05/40 = .00125 for the age and sex subgroup analyses, but none of the results were statistically significant at the Bonferroni-corrected critical P values.
Uncorrected P value was less than .05.
Discussion
The four nutritional intervention factors showed no effect on total mortality overall or by age or sex during the full 30-year observation period. The previously observed beneficial effects on mortality (6) for Factor D—the combination of selenium, vitamin E, and beta-carotene—waned with further observation and were no longer apparent, thus establishing 10 years as the duration of efficacy in the post-trial period for this nutritional intervention.
This level of durability is consistent with other major nutritional interventions in cancer prevention. The ATBC study previously reported a beneficial effect of vitamin E on prostate cancer, but harmful effects of beta-carotene on lung cancer and total mortality; the postintervention follow-up showed that these effects dissipated over an interval of roughly three to 6.5 years postintervention (1,11). The CARET study originally found increased lung cancer incidence and mortality and total mortality in participants randomly assigned to beta-carotene and retinol (2,12). All three adverse outcomes showed marked reductions over the course of a six-year postintervention follow-up, although mortality from lung cancer remained elevated (2,12). The SU.VI.MAX trial found reduced cancer incidence and total mortality in males supplemented with multivitamins and minerals, but these effects were no longer evident five years postintervention (13). Although the durability of these postintervention effects has been variable, eventually most effects regressed. At 10 years’ postintervention, NIT stands out as the longest durable beneficial effect among the major nutritional intervention trials in cancer prevention conducted to date. Of note, long-term postintervention beneficial effects have also been observed with non-nutritional agents for cancer prevention. The most well-known agent is tamoxifen, which has shown durable beneficial effects on breast cancer incidence for up to 15 years postintervention in high-risk populations (14,15).
The NIT study identified a noteworthy age pattern on the intervention effects. Beneficial effects were identified only in persons younger than age 55 years at baseline, while harmful effects were found among persons who were older (55+ years) at study entry. A similar finding of greater benefit in younger women (<50 years) was also noted in a tamoxifen trial (14). We previously proposed a hypothesis to explain the heterogeneity of response to nutritional interventions at different ages that we termed the “point of no return” (6). This hypothesis suggests that timely supplementation of essential nutrients at a younger age may delay carcinogenesis while supplementation later in carcinogenesis may fuel the process. A similar result has been speculated to explain the results of folate supplementation in colorectal cancer. Folate supplementation prior to the existence of preneoplastic lesions may prevent or slow progression to colorectal cancer, whereas intervention after early lesions are established may increase tumorigenesis (16,17). However, the appropriate timing and duration of a nutritional intervention are difficult to determine due to the long latent period for cancer and the inability to stage whole cohorts of individuals with precision.
Because many previous nutritional intervention RCTs (eg, ATBC, SELECT, and PHSII) have investigated males only, we investigated sex-specific effects in NIT where 55% of participants were female. Evidence from the SU.VI.MAX study found benefit only in men but not in women. Similarly, RCTs conducted in women only, including the Women’s Health Study, the Women’s Antioxidant Cardiovascular Study (WACS), the Women’s Health Initiative, and the Women’s Antioxidant and Folic Acid Cardiovascular Study, found no effects of nutritional supplementation on cancer prevention (18–20), with the single exception of increased lung cancer among women who received vitamin C in the WACS study. In contrast to these results, we observed benefits for total and cancer mortality in women for Factor D in the first 10-year postintervention follow-up period of the NIT (6), although these effects subsequently waned. In contrast, there was some evidence for increased risks of esophageal cancer death after 30 years in women who received Factor A or Factor C, but decreased risk of stroke in women who received Factor C. However, none of these effects withstood Bonferroni correction, and they should be interpreted with caution.
Different baseline nutritional status of trial populations and different intervention doses may also contribute to the variable results found in the nutritional intervention trials. Populations that benefitted from nutritional supplementation (eg, males in SU.VI.MAX and all participants in NIT) were low in certain nutrients at baseline (7,21,22). A modification of the intervention effect by baseline nutritional status has been seen in several studies. The Nutritional Prevention of Cancer Trial found a benefit for selenium supplementation on cancer that was largely limited to persons with lower baseline selenium levels (23). Similarly, participants with poorer nutritional status obtained greater intervention benefits than others in SU.VI.MAX (23,24). It seems plausible, even likely, that a beneficial effect of supplementation on cancer may only be evident under the circumstance when suboptimal nutritional status is corrected to optimal nutrition levels (25). A “U”-shaped dose response curve may exist where either deficiency or supraphysiologic doses of micronutrients are harmful (26). This hypothesis is consistent with, and may help explain, the apparently contradictory results from many observational studies that showed the lowest cancer risk in people with the highest nutrient intake levels, while some RCTs that tested nutritional supplements with high, even supraphysiologic, doses that exceeded the Recommended Dietary Allowances have reported harmful results. The effects of interventions are also likely influenced by risk factors (eg, cigarette smoking status) and the predominant cancer types of trial populations. For example, the most common cancers in both sexes in the NIT were esophageal and gastric cancers, whereas breast cancer was the most common cancer in women in the WHS and SU.VI.MAX studies (19,24). We might expect that different tumors would show different responses to different nutritional and micronutrient supplementations.
This current analysis established 10 years as the duration of the protective effect for the multiyear use of selenium, vitamin E, and beta-carotene on total and cancer mortality in this trial. Cohort analyses of the same trial participants suggested that the main protective agent in this population was selenium (21,27,28). It is possible that a strong public health benefit might be evident with longer supplementation of selenium. An alternative to individual supplementation to increase selenium intake and selenium blood levels on a population-wide basis is selenium fortification of fertilizer in areas with selenium-deficient soil, which has been done successfully in Finland (29). However, further research is required before exploring this alternative in selenium-deficient populations such as that in Linxian, China.
Major strengths of this study include the randomized, double-blind, placebo-controlled design, the large study size, the excellent compliance, the accurate and complete ascertainment of end points, and the particularly long follow-up. The study has limitations as well. We tested nine different vitamins/mineral micronutrients, but as they were combined into four factors, we could not evaluate the effects of the individual vitamins and minerals, only the combinations. Our use of a fractional factorial design meant that we could not evaluate all two- and three-way interactions, although we had full power to evaluate all main effects. This study was conducted in a nutritionally deprived population with extremely high UGI cancer mortality. Improvements in diets likely occurred over the 30-year follow-up, but its effects should have been evenly distributed across different randomized groups and should not bias the results (6). We took advantage of this cohort to do a 25-year post-trial follow-up analysis for the intervention effects of the a priori end points and several secondary end points. For exploratory analyses, we applied Bonferroni correction to the end points analyzed in Tables 2–5 and found robust age-specific effects and time trend influences on the intervention results. However, we acknowledge that some findings in the subgroup analyses for specific causes of death lost naïve statistical significance after correction for multiple comparisons and therefore may be due to chance. Evidence from the NIT may inform similar high UGI cancer incidence populations such as those in Iran, Central Asia, or East Africa, but may have only limited generalizability to well-nourished populations with low UGI cancer mortality. It is possible, however, that the beneficial effects observed for non-UGI cancer from Factor A and on stroke from Factor C in this study may have relevance to populations with lower UGI cancer rates but high rates of these other diseases. Furthermore, if the effects of intervention are due to the replacement of essential nutrients in a nutritionally deprived population, these results can be useful in subgroups of Western populations who rely on diverse starchy food staples and may still be deficient in some micronutrients (30).
In summary, none of the nutritional interventions tested statistically significantly altered total mortality across the total 30 years of observation in this rural Chinese population. The previously observed beneficial effects of the combination of selenium, vitamin E, and beta-carotene on mortality waned and were no longer apparent, thus establishing 10 years as the duration of efficacy of this combination of nutrients in the post-trial period. According to our results, multiyear supplementation with vitamins and minerals may reduce mortality over the short term but is unlikely to have a meaningful effect on total mortality more than a decade after the supplementation ends, even in a nutritionally deprived population. Whether longer or sustained intervention or re-intervention at intervals would provide more durable effects is not known and needs further investigation.
Funding
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under contracts N01-RC-91030, N01-RC-47701, and N02CP21009-56 to the Cancer Hospital/Institute, Chinese Academy of Medical Sciences.
Notes
Affiliations of authors: National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China (SMW, JHF, HL, YLQ); Metabolic Epidemiology Branch (SMW, PRT, GAM, SMD, CCA) and Biostatistics Branch (RMP, MHG), Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD.
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. All authors declare no potential conflicts of interests.
The authors thank all the people who participated in the study and the many individuals not specifically mentioned in the paper who have supported the study.
SMW, CA, and PT wrote the manuscript with input from SD, GM, and YLQ; SMW and RF did the statistical analysis with input from MG; JHF, HL, and YLQ conducted the field work and collected the follow-up data. All authors have seen and approved the final version of the Abstract for publication.
Registration: ClinicalTrials.gov number NCT00342654.
Supplementary Material
References
- 1. Virtamo J, Pietinen P, Huttunen JK, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: A postintervention follow-up. JAMA. 2003;290(4):476–485. [DOI] [PubMed] [Google Scholar]
- 2. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. [DOI] [PubMed] [Google Scholar]
- 3. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–834. [DOI] [PubMed] [Google Scholar]
- 5. Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. [DOI] [PubMed] [Google Scholar]
- 6. Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: Follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101(7):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164(21):2335–2342. [DOI] [PubMed] [Google Scholar]
- 8. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: The Physicians' Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. [DOI] [PubMed] [Google Scholar]
- 10. Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3(6):577–585. [DOI] [PubMed] [Google Scholar]
- 11. Virtamo J, Taylor PR, Kontto J, et al. Effects of alpha-tocopherol and beta-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int J Cancer. 2014;135(1):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743–1750. [DOI] [PubMed] [Google Scholar]
- 13. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: A postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127(8):1875–1881. [DOI] [PubMed] [Google Scholar]
- 14. Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. [DOI] [PubMed] [Google Scholar]
- 16. Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. JAMA. 2007;297(21):2351–2359. [DOI] [PubMed] [Google Scholar]
- 17. Song J, Sohn KJ, Medline A, et al. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/-Msh2-/- mice. Cancer Res. 2000;60(12):3191–3199. [PubMed] [Google Scholar]
- 18. Lee IM, Cook NR, Manson JE, et al. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The Women's Health Study. J Natl Cancer Inst. 1999;91(24):2102–2106. [DOI] [PubMed] [Google Scholar]
- 19. Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women's Health Study: A randomized controlled trial. JAMA. 2005;294(1):56–65. [DOI] [PubMed] [Google Scholar]
- 20. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J Natl Cancer Inst. 2009;101(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92(21):1753–1763. [DOI] [PubMed] [Google Scholar]
- 22. Yang CS, Sun Y, Yang QU, et al. Vitamin A and other deficiencies in Linxian, a high esophageal cancer incidence area in northern China. J Natl Cancer Inst. 1984;73(6):1449–1453. [PubMed] [Google Scholar]
- 23. Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91(7):608–612. [DOI] [PubMed] [Google Scholar]
- 24. Galan P, Briancon S, Favier A, et al. Antioxidant status and risk of cancer in the SU.VI.MAX study: Is the effect of supplementation dependent on baseline levels? Br J Nutr. 2005;94(1):125–132. [DOI] [PubMed] [Google Scholar]
- 25. Hercberg S, Czernichow S, Galan P.. Antioxidant vitamins and minerals in prevention of cancers: Lessons from the SU.VI.MAX study. Br J Nutr. 2006;96(Suppl 1):S28–S30. [DOI] [PubMed] [Google Scholar]
- 26. Hashemian M, Poustchi H, Abnet CC, et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: Results from the Golestan Cohort Study. Am J Clin Nutr. 2015;102(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor PR, Qiao YL, Abnet CC, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1414–1416. [DOI] [PubMed] [Google Scholar]
- 28. Abnet CC, Qiao YL, Dawsey SM, et al. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control. 2003;14(7):645–655. [DOI] [PubMed] [Google Scholar]
- 29. Alfthan G, Eurola M, Ekholm P, et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J Trace Elem Med Biol. 2015;31:142–147. [DOI] [PubMed] [Google Scholar]
- 30. Angelo G, Drake VJ, Frei B.. Efficacy of multivitamin/mineral supplementation to reduce chronic disease risk: A critical review of the evidence from observational studies and randomized controlled trials. Crit Rev Food Sci Nutr. 2015;55(14):1968–1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.