Abstract
Background
This study investigated the effects of metformin and weight loss on biomarkers associated with breast cancer prognosis.
Methods
Overweight/obese postmenopausal breast cancer survivors (n = 333) were randomly assigned to metformin vs placebo and to a weight loss intervention vs control (ie, usual care). The 2 × 2 factorial design allows a single randomized trial to investigate the effect of two factors and interactions between them. Outcomes were changes in fasting insulin, glucose, C-reactive protein (CRP), estradiol, testosterone, and sex-hormone binding globulin (SHBG). The trial was powered for a main effects analysis of metformin vs placebo and weight loss vs control. All tests of statistical significance were two-sided.
Results
A total of 313 women (94.0%) completed the six-month trial. High prescription adherence (ie, ≥80% of pills taken) ranged from 65.9% of participants in the metformin group to 81.3% of those in the placebo group (P < .002). Mean percent weight loss was statistically significantly higher in the weight loss group (–5.5%, 95% confidence interval [CI] = –6.3% to –4.8%) compared with the control group (–2.7%, 95% CI = –3.5% to –1.9%). Statistically significant group differences (ie, percent change in metformin group minus placebo group) were –7.9% (95% CI = –15.0% to –0.8%) for insulin, –10.0% (95% CI = –18.5% to –1.5%) for estradiol, –9.5% (95% CI = –15.2% to –3.8%) for testosterone, and 7.5% (95% CI = 2.4% to 12.6%) for SHBG. Statistically significant group differences (ie, percent change in weight loss group minus placebo group) were –12.5% (95% CI = –19.6% to –5.3%) for insulin and 5.3% (95% CI = 0.2% to 10.4%) for SHBG.
Conclusions
As adjuvant therapy, weight loss and metformin were found to be a safe combination strategy that modestly lowered estrogen levels and advantageously affected other biomarkers thought to be on the pathway for reducing breast cancer recurrence and mortality.
There are more than 3.5 million (1) breast cancer survivors alive today with a keen interest in steps they can take to reduce their risk of cancer recurrence and death (2). Studies linking modifiable health behaviors with breast cancer prognosis offer insight for these women and their clinicians.
Obesity increases the risk of breast cancer (3) and may adversely affect aspects of cancer survivorship, including quality of life, lymphedema, cancer recurrence, and mortality (4–6). Although definitive data showing that weight loss improves breast cancer morbidity and mortality are needed, weight management is recommended for breast cancer survivors by numerous agencies such as the National Cancer Institute, the National Comprehensive Cancer Network, and the American Institute for Cancer Research (7).
Type 2 diabetes is hypothesized to contribute to cancer risk through similar mechanisms as obesity, including elevated insulin concentrations, sex hormones, and inflammation (8). Diabetes medications modify the diabetes-cancer connection: metformin is generally associated with reduced breast cancer recurrence and mortality, while insulin and insulin secretagogues are associated with increased recurrence and mortality (9). Considerable evidence supports the therapeutic effects of metformin on the primary prevention and treatment of breast and other cancers (9–14). Metformin reduces hepatic glucose production, increases hepatic fatty acid oxidation, reduces inflammation, and improves peripheral insulin sensitivity (15–17). These activities reduce circulating glucose and insulin levels, although the exact mechanisms by which metformin achieves these effects are not well understood (18). Activation of AMPK by metformin also suppresses aromatase (19), which could reduce the production of estrogen in postmenopausal women (20).
This paper presents the primary results of the Reach for Health Study, a randomized trial of overweight/obese, postmenopausal breast cancer survivors designed to investigate the impact of metformin and weight loss on biological systems (ie, biomarkers) associated with breast cancer outcomes. This trial used a 2×2 factorial design to efficiently test two interventions in a single study powered for a main effects analysis of metformin vs placebo and weight loss vs control.
Methods
Study Design
The Reach for Health trial was part of the University of California (UC) San Diego Transdisciplinary Research in Energetics and Cancer Center initiative to examine the role of insulin resistance and inflammation in breast cancer risk (1U54CA155435-01; PI: Patterson). Overweight/obese postmenopausal breast cancer survivors (n = 333) were randomly assigned in equal numbers to 1) metformin vs placebo and 2) a weight loss intervention vs control (ie, usual care). The National Institutes of Health ClinicalTrials.gov identifier is NCT01302379. Measurements and fasting blood specimens were collected at baseline and the final six-month visit. Details regarding the study design, recruitment strategies, and interventions have been published (21). The Human Research Protections Program at UC San Diego approved the study, and participants signed informed consent forms. An independent Data Safety and Monitoring Board met annually to review study activities and adverse events.
Participants and Recruitment
Breast cancer survivors were recruited between August 2011 and May 2015 from San Diego and surrounding communities using cancer registry mailings, physician referrals, community outreach, and mass media approaches. Eligible participants were postmenopausal breast cancer survivors with a body mass index (BMI) of 25.0 kg/m2 or greater with a diagnosis of primary operable stage IA–IIIC breast cancer within the past 10 years. Participants completed chemotherapy and/or radiation therapy prior to enrollment. Women taking adjuvant endocrine or biological therapy for breast cancer continued that therapy throughout the six-month intervention period to prevent changes in endogenous hormones or other biomarker concentrations associated with therapy cessation. Women were excluded if they had diabetes (unless well controlled with diet and lifestyle alone), were using hormone replacement therapy, or had serious medical conditions such as renal insufficiency or congestive heart failure.
Intervention Groups
Metformin vs Placebo
Participants were randomly assigned to receive metformin or placebo pills (we received a Food and Drug Administration waiver to provide metformin to these nondiabetic women). Participants and study staff were blinded to medication group assignment. To enhance drug tolerance, participants began taking the pills with dinner at a low dose (one 500 mg metformin pill or placebo). After one week, the dose was increased to two pills at dinner. After one month, the dose was increased to three pills (one pill in the morning and two at dinner, equal to 1500 mg). Adherence was supported by structured telephone interviews at two weeks, one month, and three months. Safety data were collected during these calls by querying participants regarding adverse events in a nonleading manner. Participants returned unused medication at the final clinic visit, and the remaining pills were counted. High adherence was defined as taking 80% or more of the prescribed medication (22).
Weight Loss Intervention vs Control
Participants were randomly assigned to a telephone-based weight loss intervention or control. The study goal was a mean of 7% weight loss. Trained lifestyle coaches delivered the intervention using strategies outlined by Social Cognitive Theory (23) focused on goal setting and building self-efficacy. The counseling protocol included 12 motivational interviewing calls over the six-month intervention period. Intervention weight loss strategies included encouraging portion control, healthy eating, and counting calories to reduce daily energy intake by 500 to 1000 calories. Participants were given pedometers and encouraged to increase their physical activity levels (primarily through walking) toward a goal of 300 minutes per week of moderate-intensity physical activity. We defined high adherence as 5% or greater weight loss, a weight loss considered clinically meaningful by the US Preventive Services Taskforce (24).
Women randomly assigned to the control group were provided with the US Dietary Guidelines for Americans, 2010. Study staff contacted women in the control groups at two weeks, one month, and three months to support pill adherence.
Primary Outcome Measures
We selected biomarkers based on biologic mechanisms known to link adiposity with cancer risk: 1) glucoregulation, 2) chronic systemic inflammation, and 3) endogenous sex hormones (25–33). The biomarkers most strongly supported by the literature are fasting insulin, estradiol, and C-reactive protein (CRP), each of which has been associated with an approximately twofold increased risk of incident or recurrent breast cancer (34–37). Other outcomes supported by the literature are glucose, testosterone, and sex hormone–binding globulin (SHBG). We focused on bioavailable hormone concentrations because the literature indicates that bioavailable hormone fractions are most strongly associated with breast cancer risk (38).
Glucose, Insulin, and CRP Assays
Fasting plasma glucose concentrations were measured using a glucose oxidase method (YSI Inc., Yellow Springs, OH, US). Plasma insulin and CRP concentrations were determined using high-sensitivity immunoassays (Meso Scale Discovery (Rockville, MD, US), catalog #K15164C and #K15198D, respectively). Intraplate and interplate coefficients of variance (CV), respectively, were insulin (3.5%, 6.5%), glucose (2.1%, 3.2%), and CRP (8.4%, 18.0%).
Serum Estradiol, Testosterone, and SHBG
Serum estradiol, testosterone, and SHBG were measured at the Reproductive Endocrine Research Laboratory at the University of Southern California (Director: Frank Z. Stancyzk) using radioimmunoassay after organic solvent extraction and celite column chromatography to increase assay sensitivity. Assay sensitivities for testosterone and estradiol were 1.5 ng/dL and 2 pg/mL, respectively; values for all participants were above the assay sensitivities. Intra- and interassay CV ranged from 3% to 6% and 9% to 12%, at low and high levels, respectively, in quality control samples. Bioavailable or non-SHBG-bound (free plus albumin-bound) testosterone and estradiol concentrations were calculated using law of mass action equations (39). Serum SHBG was measured using the Immulite 2000 (Siemens Healthineers, Munich, Germany) analyzer and a two-site chemiluminometric sandwich assay). SHBG assay sensitivity was 0.1 nmol/L, and the intraassay CV was 7.0%.
Other Measurements
Demographic data, smoking status and history, alcohol consumption, and medical comorbidities were obtained via self-report questionnaires at baseline. Height and weight were measured using standard procedures. Medical charts were abstracted to obtain information regarding the original cancer diagnosis and treatment. Details of measurement procedures have been published (21).
Statistical Considerations and Analysis
Participants were assigned to the study arms using a random permuted-block design that included strata for stage at diagnosis (stage I vs stages II and III) and BMI (<30.0 kg/m2 vs ≥30.0 kg/m2). Random assignments were performed using the study’s relational database. Study personnel were blinded to the medication group assignment (metformin vs placebo). Sample size estimates were based on main effects comparisons of metformin vs placebo and weight loss vs control. Assuming a two-sided test with an alpha of .05 and 80 participants per each of the four arms (ie, 320 total), there was 90% power to detect a main effect of 0.37 for a standardized group mean difference in change (ie, effect size) for a composite marker outcome.
Biomarker outcomes were log-transformed to better approximate Gaussian residual distributions and presented as geometric means and 95% confidence intervals (CIs). Repeated measures mixed models (40) compared six-month changes in biomarker concentrations between study groups using intention-to-treat methods. The mixed effects paradigm includes all available data in the analysis without directly imputing missing outcome values. Given the multiple correlated biomarker outcomes, we first created a composite outcome defined as the sum of z-scores of the six biomarkers: insulin, glucose, CRP, estradiol, testosterone, and SHBG. Specifically, we calculated a standardized biomarker score by subtracting the baseline sample mean and dividing by the standard deviation for that biomarker. We summed the standardized scores across the biomarkers to create a composite score for each participant. A mixed model was fit with this composite score (at baseline and six months) as the dependent variable, which served as an omnibus test to assess the overall intervention effect on the joint multiple biomarker outcomes. There were statistically significant group*time interactions for the interventions, indicating overall improvements in the set of markers (data not shown).
Given the positive findings from the omnibus test, we proceeded to fit single-marker, repeated-measures mixed effects models to quantify intervention effects on individual biomarkers. Models included subject-specific intercepts, fixed effects for time (baseline, six months), treatment, and treatment × time interactions. Treatment effects were calculated as the difference (compared with the appropriate control group) in absolute and percent changes in biomarkers from baseline to six months with 95% confidence intervals.
As a sensitivity analysis, we added age, BMI, and cancer stage to the mixed models and re-estimated treatment effects. We tested additive vs synergistic effects of the two treatments by including a three-way interaction term for time*metformin*weight-loss intervention. Secondary analyses examined the intervention effects across the four individual treatment arms. Exploratory post hoc analyses included stratifying analyses by intervention adherence (<or≥80% adherence to metformin/placebo; < or≥ 5% weight loss). Three-way interaction terms (ie, treatment*time*stratifying variables) tested whether intervention adherence had a statistically significant impact on biomarker changes.
Results
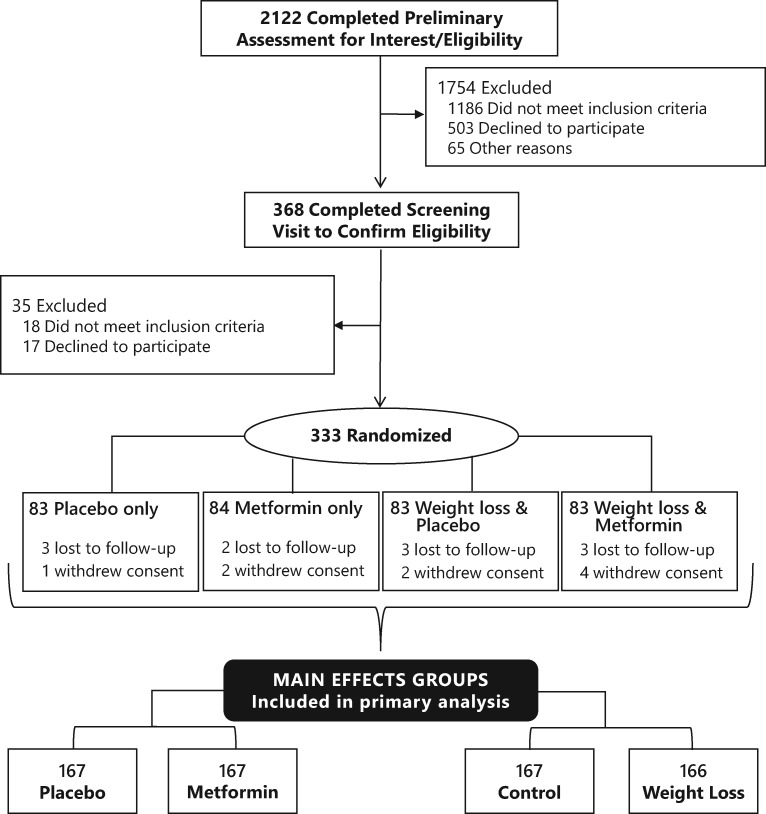
From August 2011 through May 2015, we enrolled 333 women from the San Diego region. As shown in the Consort diagram (Figure 1), 2122 women were screened by telephone, 368 attended a screening visit, 333 were randomly assigned, and 313 (94.0%) completed the trial (Figure 1).
Figure 1.
Consort diagram for 2 × 2 factorial randomized controlled trial of metformin and weight loss among breast cancer survivors.
Random assignment balanced participants’ demographic and breast cancer characteristics, and there were no statistically significant differences by group assignment (21). As shown in Table 1, study participants were a mean age (SD) of 62.6 (6.9) years and predominantly white (83.5%). The average time between breast cancer diagnosis and enrollment (SD) was 2.7 (2.0) years. Overall, 48.4% of participants were diagnosed with stage I cancer, with 72.1% estrogen receptor (ER)+ or progesterone receptor (PR)+ human epidermal growth factor receptor 2 (HER2)- tumors and 9.0% triple-negative breast cancer.
Table 1.
Baseline characteristics of breast cancer survivors in a 2 × 2 factorial randomized controlled trial of metformin and weight loss (n = 333)*
| Characteristic | Metformin and weight loss | Weight loss only | Metformin only | Control | Full sample |
|---|---|---|---|---|---|
| No. | 83 | 83 | 84 | 83 | 333 |
| Age, mean (SD), y | 62.1 (7.1) | 62.9 (6.8) | 62.1 (6.3) | 63.1 (7.4) | 62.6 (6.9) |
| Education, No. (%) | |||||
| Graduate school | 28 (33.7) | 26 (31.3) | 28 (33.3) | 22 (26.5) | 104 (31.2) |
| College graduate | 20 (24.1) | 10 (12.0) | 16 (19.0) | 21 (25.3) | 67 (20.1) |
| Some college | 29 (34.9) | 36 (43.4) | 37 (44.0) | 30 (36.1) | 132 (39.6) |
| High school or less | 6 (7.2) | 11 (13.3) | 3 (3.6) | 10 (12.0) | 30 (9.0) |
| Time since diagnosis (SD), y | 2.4 (1.9) | 3.1 (2.4) | 2.6 (1.9) | 2.5 (1.7) | 2.7 (2.0) |
| Body mass index (SD), kg/m2 | 31.4 (5.2) | 30.8 (4.6) | 30.8 (4.7) | 31.5 (5.4) | 31.1 (5.0) |
| Waist circumference (SD), cm | 99.2 (12.6) | 97.6 (11.4) | 98.6 (11.4) | 99.3 (12.6) | 98.7 (12.0) |
| Race, No. (%) | |||||
| White | 69 (83.1) | 68 (81.9) | 70 (83.3) | 70 (84.3) | 278 (83.5) |
| Black or African American | 3 (3.6) | 2 (2.4) | 5 (6.0) | 2 (2.4) | 12 (3.6) |
| Asian | 3 (3.6) | 0 (0.0) | 1 (1.2) | 2 (2.4) | 6 (1.8) |
| Mixed race or other race | 8 (9.6) | 13 (15.7) | 8 (9.5) | 9 (10.8) | 37 (11.1) |
| Ethnicity, No. (%) | |||||
| Non-Hispanic | 72 (87.8) | 71 (85.5) | 80 (95.2) | 71 (85.5) | 295 (88.6) |
| Hispanic | 10 (12.2) | 12 (14.5) | 4 (4.8) | 12 (14.5) | 38 (11.4) |
| Cancer stage, No. (%) | |||||
| Stage I | 41 (49.4) | 40 (48.2) | 40 (47.6) | 40 (48.2) | 161 (48.4) |
| Stage II | 30 (36.1) | 31 (37.3) | 28 (33.3) | 27 (32.5) | 116 (34.8) |
| Stage III | 12 (14.5) | 12 (14.5) | 16 (19.0) | 16 (19.3) | 56 (16.8) |
| Receptor status, No. (%) | |||||
| ER+ or PR+ HER2- | 52 (62.7) | 63 (75.9) | 62 (73.8) | 63 (75.9) | 240 (72.1) |
| HER2+ | 15 (18.1) | 11 (13.3) | 11 (13.1) | 14 (16.9) | 51 (15.3) |
| Triple-negative (ER-, PR-, HER2-) | 10 (12.0) | 5 (6.0) | 10 (11.9) | 5 (6.0) | 30 (9.0) |
| Missing data | 6 (7.2) | 4 (4.8) | 1 (1.2) | 1 (1.2) | 12 (3.6) |
| Any current alcohol intake, No. (%) | |||||
| Yes | 66 (79.5) | 68 (81.9) | 66 (78.6) | 66 (79.5) | 225 (67.6) |
There were no statistically significant differences across the study arms. ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
As shown in Table 2, percent initial weight loss was statistically significantly higher in the weight loss groups (–5.5%, 95% CI = –6.3% to –4.8%) compared with the control group (–2.7%, 95% CI = –3.5% to –1.9%). Participants prescribed metformin lost statistically significantly more weight (–5.3%, 95% CI = –6.1% to –4.6%) than those prescribed placebo (–2.9%, 95% CI = –3.7% to –2.1%). The proportion of participants with high adherence to pill prescriptions (defined as ≥80% adherence) was statistically significantly lower in the metformin (65.9%) vs the placebo group (81.3%, P = .002). Pill adherence was approximately 70% in the weight loss and the control groups (P = .10).
Table 2.
Adherence to metformin/placebo prescription and weight loss intervention among 333 breast cancer survivors in a 2 × 2 factorial randomized controlled trial, grouped by intervention
| Intervention adherence | Metformin intervention groups |
Weight loss intervention groups |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 166) | Metformin (n = 167) | Group differences | Control (n = 167) | Weight loss (n = 166) | Group differences | |
| Geometric mean weight (95% CI), kg | ||||||
| Baseline | 82.40 (80.36 to 84.50) | 81.82 (79.78 to 83.89) | 81.90 (79.87 to 84.98) | 82.32 (80.28 to 84.42) | ||
| 6 mo | 80.03 (78.03 to 82.07) | 77.44 (75.51 to 79.41) | 79.70 (77.72 to 81.73) | 77.75 (75.81 to 79.75) | ||
| Absolute change | –2.38 (–3.05 to –1.70) | –4.4 (–5.0 to –3.7) | –2.00 (–2.95 to –1.05) | –2.20 (–2.86 to –1.53) | –4.57 (–5.24 to –3.89) | –2.37 (–3.32 to –1.42) |
| Percent change | –2.88 (–3.69 to –2.08) | –5.34 (–6.14 to –4.56) | –2.46 (–3.59 to –1.34) | –2.68 (–3.48 to –1.88) | –5.54 (–6.34 to –4.75) | –2.87 (–3.99 to –1.74) |
| P* | <.001 | <.001 | ||||
| Pill adherence, % | ||||||
| 6 mo | ||||||
| Adherent (≥80%) | 81.3 | 65.9 | .002 | 71.9 | 75.3 | .10 |
| Taking some (1%–79%) | 10.8 | 25.7 | 22.2 | 14.5 | ||
| Not taking pills (0%) | 7.8 | 8.4 | 6.0 | 10.2 | ||
| P† | ||||||
Based on two-sided t statistic for the group*time interaction in a mixed model analysis comparing metformin with placebo arm and weight loss intervention with control arm. CI = confidence interval.
Based on chi-square tests comparing metformin with placebo arm and weight loss intervention with control arm.
Table 3 presents changes in breast cancer–related biomarkers from baseline to six months for the main intervention effects. Statistically significant group differences (ie, percent change in metformin group minus placebo group) were –7.9% (95% CI = –15.0% to –0.8%) for insulin, –10.0% (95% CI = –18.5% to –1.5%) for estradiol, –9.5% (95% CI = –15.2% to –3.8%) for testosterone, and 7.5% (95% CI = 2.4% to 12.6%) for SHGB. Statistically significant group differences (ie, % change in weight loss group minus placebo group) were –12.5% (95% CI = –19.6% to –5.3%) for insulin and 5.3% (95% CI = 0.2% to 10.4%) for SHBG, with no changes in estradiol or testosterone. Neither study treatment affected glucose or CRP concentrations. We conducted a sensitivity analyses by adding age, BMI, waist circumference, and cancer stage to the above mixed models and re-estimated treatment effects; biomarker results were essentially unchanged.
Table 3.
Main effects of metformin and weight loss on breast cancer–related biomarkers among 333 breast cancer survivors in a 2 × 2 factorial randomized controlled trial, grouped by intervention (geometric means and 95% CI)
| Biomarkers | Metformin intervention groups |
Weight loss intervention groups |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 166) | Metformin (n = 167) | Group differences | Control (n = 167) | Weight loss (n = 166) | Group differences | |
| Insulin, pg/mL | ||||||
| Baseline | 476.94 (440.72 to 516.13) | 484.55 (447.86 to 524.24) | 466.00 (431.72 to 504.17) | 495.9 (458.27 to 536.67) | ||
| 6 mo | 430.49 (397.40 to 466.34) | 399.16 (368.48 to 432.40) | 431.90 (398.85 to 467.70) | 397.86 (367.14 to 431.14) | ||
| Absolute change | –46.44 (–73.01 to –19.88) | –85.39 (–111.79 to –58.98) | –38.94 (–76.4 to –1.49) | –34.09 (–60.15 to –8.03) | –98.07 (–125.11 to –71.02) | –63.97 (–101.53 to –26.42) |
| Percent change | –9.74 (–14.99 to –4.48) | –17.62 (–22.43 to –12.81) | –7.88 (–15.01 to –0.76) | –7.32 (–12.68 to –1.95) | –19.77 (–24.48 to –15.07) | –12.46 (–19.6 to –5.32) |
| P* | .03 | <.001 | ||||
| Glucose, mg/dL | ||||||
| Baseline | 102.8 (100.99 to 104.65) | 101.84 (100.04 to 103.66) | 102.25 (100.45 to 104.08) | 102.39 (100.58 to 104.23) | ||
| 6 mo | 102.62 (100.78 to 104.49) | 100.36 (98.56 to 102.19) | 102.4 (100.58 to 104.26) | 100.57 (98.76 to 102.41) | ||
| Absolute change | –0.19 (–1.73 to 1.36) | –1.48 (–3.00 to 0.05) | –1.29 (–3.47 to 0.88) | 0.15 (–1.38 to 1.69) | –1.82 (–3.36 to –0.28) | –1.98 (–4.15 to 0.2) |
| Percent change | –0.18 (–1.69 to 1.32) | –1.45 (–2.94 to 0.04) | –1.27 (–3.39 to 0.85) | 0.15 (–1.35 to 1.65) | –1.78 (–3.27 to –0.29) | –1.93 (–4.05 to 0.19) |
| P* | .24 | .08 | ||||
| C-reactive protein, mg/L | ||||||
| Baseline | 3.29 (2.77 to 3.9) | 3.1 (2.62 to 3.68) | 3.35 (2.82 to 3.97) | 3.05 (2.57 to 3.61) | ||
| 6 mo | 3.27 (2.75 to 3.88) | 2.62 (2.21 to 3.12) | 3.28 (2.76 to 3.9) | 2.61 (2.19 to 3.1) | ||
| Absolute change | –0.02 (–0.47 to 0.43) | –0.48 (–0.88 to –0.08) | –0.46 (–1.06 to 0.14) | –0.07 (–0.52 to 0.39) | –0.44 (–0.84 to –0.04) | –0.37 (–0.98 to 0.23) |
| Percent change | –0.64 (–14.39 to 13.11) | –15.51 (–27.16 to –3.86) | –14.87 (–32.88 to 3.15) | –1.95 (–15.39 to 11.50) | –14.39 (–26.29 to –2.48) | –12.44 (–30.4 to 5.52) |
| P* | .10 | .17 | ||||
| Bioavailable estradiol, pg/mL | ||||||
| Baseline | 3.07 (2.76 to 3.42) | 3.01 (2.7 to 3.35) | 2.94 (2.64 to 3.27) | 3.15 (2.83 to 3.51) | ||
| 6 mo | 3.08 (2.76 to 3.43) | 2.71 (2.43 to 3.02) | 2.88 (2.58 to 3.2) | 2.9 (2.6 to 3.24) | ||
| Absolute change | 0.00 (–0.19 to 0.20) | –0.30 (–0.48 to –0.11) | –0.3 (–0.57 to –0.03) | –0.06 (–0.24 to 0.12) | –0.24 (–0.44 to –0.05) | –0.18 (–0.45 to 0.08) |
| Percent change | 0.14 (–6.14 to 6.43) | –9.84 (–15.51 to –4.16) | –9.98 (–18.45 to –1.51) | –2.12 (–8.23 to 3.99) | –7.75 (–13.59 to –1.91) | –5.63 (–14.08 to 2.83) |
| P* | .02 | .19 | ||||
| Bioavailable testosterone, ng/dL | ||||||
| Baseline | 11.84 (10.87 to12.88) | 11.24 (10.33 to 12.23) | 11.64 (10.7 to 12.67) | 11.42 (10.5 to 12.43) | ||
| 6 mo | 11.49 (10.55 to12.52) | 9.84 (9.04 to 10.72) | 10.91 (10.02 to 11.87) | 10.38 (9.52 to 11.3) | ||
| Absolute change | –0.34 (–0.85 to 0.17) | –1.39 (–1.87 to –0.92) | –1.05 (–1.74 to –0.35) | –0.74 (–1.23 to –0.25) | –1.05 (–1.53 to –0.56) | –0.31 (–1 to 0.38) |
| Percent change | –2.90 (–7.13 to 1.34) | –12.39 (–16.22 to –8.55) | –9.49 (–15.2 to –3.77) | –6.33 (–10.39 to –2.27) | –9.18 (–13.17 to –5.18) | –2.85 (–8.55 to 2.85) |
| P* | .001 | .33 | ||||
| Serum hormone binding globulin, nmol/L | ||||||
| Baseline | 44.29 (40.88 to 47.98) | 44.86 (41.42 to 48.59) | 42.63 (39.36 to 46.18) | 46.6 (43.01 to 50.49) | ||
| 6 mo | 45.93 (42.37 to 49.78) | 49.89 (46.04 to 54.06) | 44.67 (41.22 to 48.4) | 51.29 (47.32 to 55.6) | ||
| Absolute change | 1.64 (0.13 to 3.15) | 5.03 (3.39 to 6.66) | 3.39 (1.16 to 5.61) | 2.03 (0.58 to 3.49) | 4.69 (3.00 to 6.38) | 2.66 (0.43 to 4.89) |
| Percent change | 3.70 (0.25 to 7.16) | 11.20 (7.49 to 14.92) | 7.5 (2.43 to 12.57) | 4.77 (1.30 to 8.24) | 10.07 (6.37 to 13.77) | 5.3 (0.23 to 10.37) |
| P* | .004 | .04 | ||||
Based on a two-sided t statistic for the group*time interaction in a mixed model analysis comparing metformin arm with placebo and weight loss intervention with control arm. CI = confidence interval.
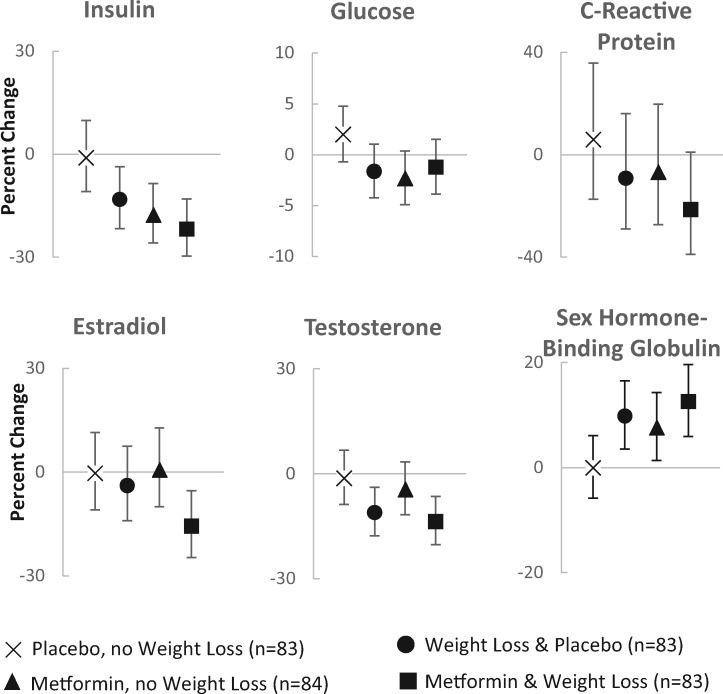
Figure 2 illustrates the baseline to six-month change in the cancer-related biomarkers by the four study arms. Combination therapy (metformin and weight loss) had statistically significant impacts on insulin, estradiol, testosterone, and SHBG. There was no statistical evidence that metformin and weight loss had synergistic impacts on biomarkers because the three-way interaction terms for time*metformin*weight loss intervention were not statistically significant in the mixed models.
Figure 2.
Effects of metformin and weight loss on mean percent change (95% confidence limits) in breast cancer–related biomarkers among breast cancer survivors in a 2 × 2 factorial randomized controlled trial.
At three months (mid-trial), self-reported symptoms of any kind were 3.0% in the placebo groups and 8.4% in the metformin groups (data not shown). Approximately 80% of these symptoms were gastrointestinal in nature. There were five serious adverse events in five participants: an injury due to a fall, abdominal pain, slurred speech, a transient neurological defect, and a transient ischemic attack. The study physician and the Data Safety and Monitoring Board judged these events as unrelated to the study. These patients completed the study.
Exploratory models stratified by adherence (<or≥80% adherence to metformin or placebo; < or ≥5% weight loss) showed no evidence that degree of adherence to metformin or weight loss statistically significantly modified the changes in biomarkers (three-way interaction P > .05) (Supplementary Table 1, available online). We had inadequate power to test whether the interventions were more (or less) effective for subgroups, such as receptor status or use of tamoxifen.
Discussion
To our knowledge, this is the first trial to evaluate the combined effects of metformin and weight loss on biomarkers associated with breast cancer recurrence and survival. In this sample of 333 overweight/obese breast cancer survivors, metformin had statistically significant, favorable effects on insulin, estradiol, testosterone, and SHBG concentrations. Weight loss had statistically significant favorable effects on insulin and SHBG concentrations. The combined impacts of the two interventions were additive.
Our finding that metformin statistically significantly lowered insulin in nondiabetic postmenopausal women is consistent with a six-month interim analysis of a randomized trial of metformin vs placebo in early-stage breast cancer (n = 492). This study found that metformin use was associated with decreased insulin (consistent with our findings) and deceased glucose and CRP concentrations (not consistent with our findings) (41). Our results are similar to recent publications showing that among nondiabetic women, metformin resulted in statistically significant reductions in estradiol and testosterone concentrations (20, 42). However, the Diabetes Prevention Program found that metformin did not alter estradiol or testosterone levels (43).
Numerous studies have reported that weight loss favorably impacts sex hormones (44), although few were conducted in breast cancer survivors. Consistent with our results, the Diabetes Prevention Program found that weight loss in prediabetic adults resulted in statistically significant decreases in fasting insulin concentrations and SHBG, but did not alter estradiol or testosterone levels (43,45). Our findings were not consistent with a trial of weight loss and physical activity among 439 postmenopausal women that found statistically significant reductions in circulating estradiol, testosterone, and CRP (46,47). However, the greater levels of weight loss achieved in this study may have resulted in larger effects on biomarkers.
The influence of metformin and weight loss on biomarkers has clinical relevance for breast cancer survival. Insulin appears to affect breast carcinogenesis in a variety of ways, including insulin receptor activation, which can accelerate cell growth and division (48). High circulating concentrations of insulin are associated with recurrence and early death among breast cancer survivors (35). Insulin also interacts with estrogens by inducing expression of adipose stromal cell aromatase and tumor cell sex steroid hormone receptor expression and suppressing expression of SHBG, which enhances estrogen synthesis and bioactivity with consequent promotion of estrogen-dependent breast cancer (49). Higher levels of estrogen, and estrogen fractions, are documented risk factors for poor breast cancer survival (50). Research also suggests that testosterone increases the risk of breast cancer directly (51,52) or via aromatization to estradiol, which increases cell proliferation and breast cancer risk (53). Although there is abundant evidence that testosterone influences breast cancer risk independent of estradiol (36,54,55), little is known about the association of testosterone with breast cancer recurrence and survival. In a prospective study of 194 postmenopausal breast cancer survivors, Micheli et al. found that testosterone levels of 0.40 ng/mL or higher were associated with a higher risk of breast cancer events (hazard ratio = 1.8, 95% CI = 1.1 to 3.0) (56); however, testosterone did not predict recurrence in a nested case–control cohort of 306 women from the WHEL trial (57).
In this trial, metformin and weight loss showed modest effects on biomarker concentrations. The literature on the association of biomarkers with breast cancer prognosis is sparse and difficult to interpret. In a study of breast cancer survivors (n = 306), Rock et al. reported that a relatively modest difference in bioavailable estradiol between cases (12.5 pg/mL) and controls (6.1 pg/mL) was associated with a statistically significantly lower risk of breast cancer recurrence (57). In a prospective study of breast cancer survivors (n = 512), Goodwin et al. found that quartile 1 to 2 differences in insulin as modest as 21.4 to 31.1 pmol/L were associated with statistically significant increases in recurrence and death (35). More research is needed to quantitatively extrapolate the impact of circulating biomarker concentrations on the risk of breast cancer recurrence and mortality over time.
The major limitations of this trial are that the weight loss achieved was modest and metformin adherence varied by study group. Nonetheless, our adherence analysis did not indicate that poor adherence to metformin or weight loss explains the null finding for glucose and CRP or the null findings for the impact of weight loss on sex hormones. In addition, the sample size was underpowered to support subgroup analyses by tumor receptor status or breast cancer treatment (eg, only a small percentage of women were taking tamoxifen). Finally, this was a relatively homogeneous sample consisting mostly of white, well-educated, postmenopausal women, which limits generalizability of these findings. Strengths of this study include the randomized design with double-blinded pill arms and high completion rate (94%).
The enthusiasm surrounding the potential role of metformin in breast cancer treatment is tempered with concerns about compliance. Approximately 25% of women prescribed anti-estrogen therapy to reduce the risk of recurrence after surgery don’t start taking the medicine or stop taking it early, largely due to side effects such as hot flashes (58). Similarly, 20% to 30% of Americans with diabetes discontinue metformin use, generally because of gastrointestinal side effects (59). However, the difficulty of successful weight loss is also well documented (60,61). Taken together, these data suggest that multiple strategies should be initiated and tailored to a woman’s preference, initial response, and ability to adhere. This multipronged approach to simultaneously initiate lifestyle and pharmacologic therapy has been endorsed for type 2 diabetes by the American Association of Clinical Endocrinologists and the American College of Endocrinology (62).
In summary, our findings suggest that as adjuvant therapy, weight loss and metformin are a safe combination strategy that modestly lower estrogen levels and advantageously affect other biomarkers thought to be on the pathway for reducing breast cancer recurrence and mortality. Results are eagerly awaited for phase III randomized trials in breast cancer survivors testing metformin vs placebo (41) and weight loss vs usual care on clinical outcomes (63).
Funding
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by funding from the National Cancer Institute (U54 CA155435-01). Dr. Marinac was supported by the National Cancer Institute of the National Institutes of Health under Award Number F31CA183125; Dr. Hartman was supported under Award Number K07CA181323; Dr. Cadmus-Bertram was supported under Award Number K07CA178870; and Dr. Adriana Villaseñor was supported by a Diversity Research Supplement from the Continuing Umbrella of Research Experiences training program, as part of the National Cancer Institute Center to Reduce Cancer Health Disparities (3U54 CA155435-04S1).
Notes
Affiliations of authors: Department of Family Medicine and Public Health (REP, DDS, JK, SJH, AV, GAL, LN), Department of Medicine (DDS, BAP), and Moores UC San Diego Cancer Center (REP, DDS, JK, SJH, AV, SWF, JOC, BAP, LN), UC San Diego (SG, HL), La Jolla, CA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA (CRM); Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA (CRM); Department of Kinesiology, University of Wisconsin, Madison, WI (LCB).
We want to thank the resilient and resourceful study team and the countless hours of help from student and staff volunteers that made recruitment for the Reach for Health trial successful. We also gratefully acknowledge the dedicated and selfless efforts of our study participants.
Supplementary Material
References
- 1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute. Cancer stat facts: Female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html. Accessed January 24, 2017.
- 3. Goodwin PJ. Obesity and breast cancer outcomes: How much evidence is needed to change practice? J Clin Oncol. 2016;34(7):646–648. [DOI] [PubMed] [Google Scholar]
- 4. Playdon MC, Bracken MB, Sanft TB, et al. Weight gain after breast cancer diagnosis and all-cause mortality: Systematic review and meta-analysis. J Natl Cancer Inst. 2015;107(12):djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodwin PJ, Ambrosone CB, Hong CC.. Modifiable lifestyle factors and breast cancer outcomes: Current controversies and research recommendations. Adv Exp Med Biol. 2015;862:177–192. [DOI] [PubMed] [Google Scholar]
- 7. World Cancer Research Fund, American Institute for Cancer Research. Breast Cancer 2010 Report: Food, Nutrition, Physical Activity and the Prevention of Breast Cancer. Continuous Update Project, Keeping the Science Current. London, UK: WCRF International; 2010. [Google Scholar]
- 8. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33(7):1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calip GS, Yu O, Hoskins KF, et al. Associations between diabetes medication use and risk of second breast cancer events and mortality. Cancer Causes Control. 2015;26(8):1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wojciechowska J, Krajewski W, Bolanowski M, et al. Diabetes and cancer: A review of current knowledge. Exp Clin Endocrinol Diabetes. 2016;124(5):263–275. [DOI] [PubMed] [Google Scholar]
- 11. Gong Z, Aragaki AK, Chlebowski RT, et al. Diabetes, metformin and incidence of and death from invasive cancer in postmenopausal women: Results from the Women's Health Initiative. Int J Cancer. 2016;138(8):1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Col NF, Ochs L, Springmann V, et al. Metformin and breast cancer risk: A meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135(3):639–646. [DOI] [PubMed] [Google Scholar]
- 13. Lega IC, Austin PC, Gruneir A, et al. Association between metformin therapy and mortality after breast cancer: A population-based study. Diabetes Care. 2013;36(10):3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vissers PA, Cardwell CR, van de Poll-Franse LV, et al. The association between glucose-lowering drug use and mortality among breast cancer patients with type 2 diabetes. Breast Cancer Res Treat. 2015;150(2):427–437. [DOI] [PubMed] [Google Scholar]
- 15. Pernicova I, Korbonits M.. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–156. [DOI] [PubMed] [Google Scholar]
- 16. Miller RA, Chu Q, Xie J, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng J, Woo SL, Hu X, et al. Metformin and metabolic diseases: A focus on hepatic aspects. Front Med. 2015;9(2):173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi YK, Park KG.. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013;36(4):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao H, Orhan YC, Zha X, et al. AMP-activated protein kinase and energy balance in breast cancer. Am J Transl Res. 2017;9(2):197–213. [PMC free article] [PubMed] [Google Scholar]
- 20. Campagnoli C, Berrino F, Venturelli E, et al. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin Breast Cancer. 2013;13(6):433–438. [DOI] [PubMed] [Google Scholar]
- 21. Patterson RE, Marinac CR, Natarajan L, et al. Recruitment strategies, design, and participant characteristics in a trial of weight-loss and metformin in breast cancer survivors. Contemp Clin Trials. 2016;47:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker EA, Molitch M, Kramer MK, et al. Adherence to preventive medications: Predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29(9):1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 24. Moyer VA, Force USPST. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373–378. [DOI] [PubMed] [Google Scholar]
- 25. Renehan AG, Frystyk J, Flyvbjerg A.. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17(8):328–336. [DOI] [PubMed] [Google Scholar]
- 26. Roberts DL, Dive C, Renehan AG.. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu Rev Med. 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 27. Becker S, Dossus L, Kaaks R.. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115(2):86–96. [DOI] [PubMed] [Google Scholar]
- 28. Cirillo D, Rachiglio AM, la Montagna R, et al. Leptin signaling in breast cancer: An overview. J Cell Biochem. 2008;105(4):956–964. [DOI] [PubMed] [Google Scholar]
- 29. Kelesidis I, Kelesidis T, Mantzoros CS.. Adiponectin and cancer: A systematic review. Br J Cancer. 2006;94(9):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 31. Nunez NP, Hursting SD, Yakar S, et al. Obesity provides a permissive milieu in inflammation-associated carcinogenesis: Analysis of insulin and IGF pathways. Methods Mol Biol. 2009;512:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Renehan AG, Roberts DL, Dive C.. Obesity and cancer: Pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. [DOI] [PubMed] [Google Scholar]
- 33. Trayhurn P, Wood IS.. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. [DOI] [PubMed] [Google Scholar]
- 34. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: Approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101(6):384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. [DOI] [PubMed] [Google Scholar]
- 36. Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. [DOI] [PubMed] [Google Scholar]
- 37. Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. [DOI] [PubMed] [Google Scholar]
- 39. Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 40. Diggle PJ, Heagerty P, Liang KY, et al. Analysis of Longitudinal Data. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- 41. Goodwin PJ, Parulekar WR, Gelmon KA, et al. Effect of metformin vs placebo on weight and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107(3):djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campagnoli C, Pasanisi P, Abba C, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: A randomized study. Clin Breast Cancer. 2012;12(3):175–182. [DOI] [PubMed] [Google Scholar]
- 43. Kim C, Kong S, Laughlin GA, et al. Endogenous sex hormone changes in postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab. 2012;97(8):2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Gemert WA, Schuit AJ, van der Palen J, et al. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: The SHAPE-2 trial. Breast Cancer Res. 2015;17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. J Clin Oncol. 2012;30(19):2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012;72(9):2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Escudero CA, Herlitz K, Troncoso F, et al. Pro-angiogenic role of insulin: From physiology to pathology. Front Physiol. 2017;8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rose DP, Vona-Davis L.. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19(6):R225–R241. [DOI] [PubMed] [Google Scholar]
- 50. National Cancer Institute. Hormone therapy for breast cancer. https://www.cancer.gov/types/breast/breast-hormone-therapy-fact-sheet.
- 51. Kuller LH. The etiology of breast cancer—from epidemiology to prevention. Public Health Rev. 1995;23(2):157–213. [PubMed] [Google Scholar]
- 52. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. [DOI] [PubMed] [Google Scholar]
- 53. Glaser R, Dimitrakakis C.. Testosterone and breast cancer prevention. Maturitas. 2015;82(3):291–295. [DOI] [PubMed] [Google Scholar]
- 54. Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: The European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. [DOI] [PubMed] [Google Scholar]
- 55. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer: Does the association vary by a woman's predicted breast cancer risk? J Clin Oncol. 2006;24(12):1823–1830. [DOI] [PubMed] [Google Scholar]
- 56. Micheli A, Meneghini E, Secreto G, et al. Plasma testosterone and prognosis of postmenopausal breast cancer patients. J Clin Oncol. 2007;25(19):2685–2690. [DOI] [PubMed] [Google Scholar]
- 57. Rock CL, Flatt SW, Laughlin GA, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(3):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boccuzzi SJ, Wogen J, Fox J, et al. Utilization of oral hypoglycemic agents in a drug-insured U.S. population. Diabetes Care. 2001;24(8):1411–1415. [DOI] [PubMed] [Google Scholar]
- 60. Anderson JW, Konz EC, Frederich RC, et al. Long-term weight-loss maintenance: A meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. [DOI] [PubMed] [Google Scholar]
- 61. Dulloo AG, Montani JP.. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: An overview. Obes Rev. 2015;16(suppl 1):1–6. [DOI] [PubMed] [Google Scholar]
- 62. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract. 2017;23(2):207–238. [DOI] [PubMed] [Google Scholar]
- 63. Yung RL, Ligibel JA.. Obesity and breast cancer: Risk, outcomes, and future considerations. Clin Adv Hematol Oncol. 2016;14(10):790–797. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.