Abstract
Background:
Disrupted rest-activity rhythms (RAR) have been associated with incident dementia and mild cognitive impairment (MCI) in older women; no longitudinal studies have examined RAR and cognitive decline in older men.
Methods:
We used data from the Osteoporotic Fractures in Men (MrOS) and ancillary Outcomes of Sleep Disorders in Men (MrOS Sleep) studies (n=2754; mean age 76.0 ± 5.3 years). The Modified Mini-Mental State examination (3MS) was used to assess cognition at baseline (2003–05) and follow-up exams (2005–06 and 2007–09). Wrist actigraphy was used to measure 24-hour activity counts at baseline. RAR variables included amplitude (strength of the activity rhythm), mesor (mean activity level), pseudo F-statistic (overall circadian rhythm robustness) and acrophase (time of daily peak activity).
Results:
After an average of 3.4 ± 0.5 years, men with lower amplitudes, mesor and pseudo F-statistic showed greater decline in 3MS performance (amplitude: −0.7 point Q1 vs. −0.5 point Q4, p<0.001; mesor: −0.5 point Q1 vs. −0.2 point Q4, p=0.01; pseudo F-statistic: −0.5 point Q1 vs. −0.3 point Q4, p<0.001). Lower amplitude and pseudo-F statistic were associated with increased odds of clinically significant cognitive decline (≥5 point decrease) [OR (95% CI): amplitude Q1 vs. Q4: 1.4 (1.0, 1.9); pseudo-F statistic Q1 vs. Q4: 1.4 (1.0, 1.9)]. Men with phase advanced acrophase had increased odds of clinically significant cognitive decline [OR (95% CI): 1.8 (1.2, 2.8)]. Results were adjusted for multiple confounders.
Discussion:
Several parameters of disrupted RAR (lower amplitude, pseudo F-statistic, mesor and phase advanced acrophase) were associated with greater cognitive decline in older, community-dwelling men. These findings contribute to a growing body of evidence suggesting that altered RAR are associated with cognitive decline in older adults.
Keywords: cognitive decline, rest-activity rhythm, men
INTRODUCTION
Circadian rhythms are critically involved in control of sleep-wake cycles and numerous physiological processes, including cognitive processing.1 Aging is associated with altered rest-activity circadian rhythm (RAR), including decreased amplitude (a measure of peak activity),2 fragmentation or loss of rhythms, 3–8 alterations in entrainment and decreased sensitivity to phase resetting signals, including light and sleep medications.9 Also, the timing of activity often shifts with age, resulting in changes to both the onset of sleepiness and waking time. 4 Despite these changes in circadian rhythm with aging, relatively few studies have examined the association between disrupted RAR and cognition among older adults.10–13 In a study of older women, disrupted RAR, including decreased RAR amplitude, robustness and delayed rhythm, were related to increased risks mild cognitive impairment (MCI) and dementia. 14 However, whether this same association exists in men has not been well studied.
The primary aim of the current study was examine whether disrupted RAR is associated with cognitive impairment and decline in men enrolled in the multicenter Osteoporotic Fractures in Men (MrOS) Study. We used validated wrist-worn actigraphs to longitudinally measure RAR measures that were related to cognitive decline in women (RAR amplitude, robustness and delayed rhythm)14. In secondary analyses, we further examined whether other sleep parameters found to be associated to cognitive decline in this cohort (sleep efficiency15, nocturnal hypoxemia16 or level of activity (physical activity, hours worked) would impact the association between circadian rhythm disruption and cognitive decline.
METHODS
Participants
During the MrOS baseline examination (3/2000–4/2002), 5,994 community-dwelling men 65 years or older were enrolled at six clinical centers in the United States. 17,18 In order to participate, men needed to be able to walk without assistance and could not have had a bilateral hip replacement.
The ancillary Outcomes of Sleep Disorders in Men (MrOS Sleep) Study (12/2003–3/2005) recruited 3,135 MrOS participants for a comprehensive sleep assessment. Men were screened for nightly use of mechanical devices, including pressure masks for sleep apnea (positive airway pressure or oral appliance devices), or nocturnal oxygen therapy, and were excluded if they could not forgo use of these devices during a polysomnography recording.
The present analysis used longitudinal cognitive data collected at the Sleep Visit (12/2003–3/2005), MrOS Visit 2 (3/2005–5/2006) and MrOS Visit 3 (3/2007–3/2009) (Figure 1). Men had to have technically adequate RAR data at the Sleep Visit (≥ 24 hours of data collection), 19 as well as data on cognitive change from the Sleep Visit to MrOS Visit 2 or MrOS Visit 3. Men were excluded from the longitudinal analysis if they had “probable dementia” at the Sleep Visit (3MS score <80 or on a medication for dementia). No exclusion criteria were used for participation in MrOS Visit 2 or MrOS Visit 3. There were 2,754 men in the final longitudinal analytic cohort (Figure 1).
Figure 1:
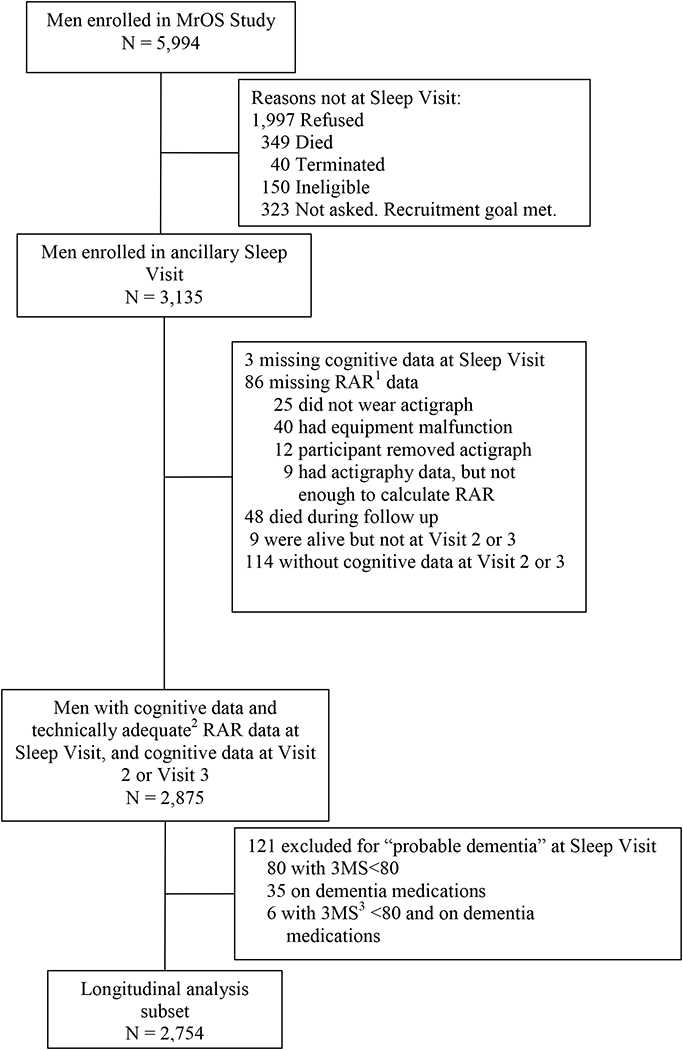
Progression of participants through the MrOS and MrOS Sleep studies
Reasons for not including participants are given on the right.
MrOS enrollment (March 2000-April 2002)
Sleep Visit (December 2003-March 2005)
MrOS Visit 2 (March 2005-May 2006)
MrOS Visit 3 (March 2007-March 2009)
1RAR, rest-activity rhythm
2Technically adequate, ≥72 hours of continuous data collection
33MS, Modified Mini-Mental State examination
In accordance with the Helsinki Declaration, all men provided written informed consent, and the study was approved by the Institutional Review Board at each participating study site.
Actigraphy
At the Sleep Visit, rest-activity rhythms were examined using a wrist actigraph (SleepWatch-O ®, Ambulatory Monitoring, Inc.). Movement was measured by a piezoelectric linear accelerometer, which generated a voltage each time the actigraph was moved. These voltages were gathered continuously and summarized over 1-minute epochs. Activity data were collected in the proportional integration mode (PIM). Men were asked to wear the actigraphs continuously for a minimum of three 24-hour periods in accordance with the Centers for Medicare Services (CMS) (Current Procedural Technology (CPT®) code for actigraphy monitoring (code 95803). 20
An extension to the traditional cosine model was used to map the circadian activity rhythm to the activity data. This approach was based on the assumption that the MrOS RAR data have a cosinor-like distribution, but the modified cosine algorithm allowed for greater flexibility with the data compared to traditional cosinor methods. 21 Four RAR parameters were calculated. Amplitude (counts/minute), a measure of the strength of activity rhythm, is the peak to nadir difference in activity at the point of greatest activity. Mesor (counts/minute) is the mean level of activity. Pseudo F-statistic (no unit) represents robustness of circadian activity rhythm, with higher pseudo-F values indicating stronger rhythms. Acrophase (portions of hours) is the time of day of peak activity. 22,23
Circadian amplitude, mesor and pseudo-F statistic were examined based on quartile distributions. To allow for a potential u-shaped association, acrophase was examined in terms of the deviation from the population mean (2:17 PM). “Phase advanced” participants were defined as having an acrophase of < −1.5 standard deviations (SD) from the mean (<12:28 PM), and “phase delayed” participants were defined as having an acrophase of > +1.5 SD from the mean (>4:06 PM). Participants in the reference category had an acrophase within 1.5 SD of the mean (12:28 PM-4:06 PM).
Participants completed sleep diaries for the time period they wore the actigraph. Each minute the actigraph was worn was determined to be “sleep” or “wake” using the sleep scoring algorithm provided by the manufacturer. Sleep efficiency, a measure of sleep fragmentation, was defined as the percentage of time in bed after “lights off” spent sleeping. 24 Values for sleep efficiency were averaged over all nights the participant wore the device.
Ascertainment of Cognitive Function
The Modified Mini-Mental State examination (3MS) is a global measurement of cognitive function; scores range from 0 to 100. Higher scores represent better cognitive functioning, and a decrease in 3MS score represents cognitive decline. 25 The 3MS test was administered at clinic visits by trained staff.
Development of clinically significant cognitive decline from the Sleep Visit to Visit 3 (mean of 3.4 ± 0.5 years later) was defined as a decline in 5 points on the 3MS. 26
The Trails B test was also used to assess cognitive function. 27 A shorter Trails B completion time represents better cognitive functioning; increased completion time represents cognitive decline.
Other Measurements
All participants completed questionnaires at the Sleep Visit, including questions about demographics, self-reported medical history and health status, physical activity (Physical Activity Scale for the Elderly, PASE)28, hours worked in the past week (paid or volunteer), smoking and alcohol use. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms. 29All medications used within the preceding 30 days were entered into an electronic database as described previously30 Functional status was assessed based on five instrumental activities of daily living (IADL). 31,32 Self-reported caffeine intake was calculated based on caffeinated coffee, tea and soda. 33 A comprehensive examination included measurements of body weight and height, which were used to calculate body mass index (BMI). Nocturnal hypoxemia was measured with polysomnography as described elsewhere25 and defined as the percentage of time during overnight sleep in which arterial oxygen saturation was below 90%, categorized as <1% vs. ≥1%.
Statistical Analysis
Characteristics of participants were compared by amplitude and acrophase using chi-square tests for categorical variables, ANOVA or Kruskal-Wallis tests for continuous variables.
The 3MS score was transformed (squared) to meet normality assumptions of the models used, due to skewness. All results were back transformed for presentation.
Random-effects models, which account for between-participant variation and within-participant correlation of repeated outcomes, 34 were used to study the association between RAR and changes in cognition over time (repeated measures at Sleep Visit, Visit 2 and Visit 3). The random effect terms included the intercept and the slope of the 3MS score over time. Variances and covariances were estimated using the restricted maximum likelihood method. Time was modeled as a continuous covariate. A quadratic term for time was considered to account for a nonlinear time trend; the interactions of the quadratic term and the RAR parameters were not significant, so time was modeled linearly. All continuous covariates were centered (value-mean) for use in the models. Change in cognition is presented as average annualized change, calculated using coefficients derived from the random-effects models.
Logistic regression was used to assess the associations of the RAR parameters with clinically significant cognitive decline. Results are presented as odds ratios and their 95% confidence intervals (CIs).
In models with the RAR predictors of amplitude, mesor or pseudo F-statistic, quartile 4 served as the reference. A test for linear trend across quartiles was also completed. All models were minimally adjusted for age and clinic site. Additional covariates were selected for inclusion in a multivariable model by examining both the univariate association of the covariate and the RAR parameters and the association with 3MS in age adjusted models. Those covariates associated to both a RAR parameter and an outcome at P <0.10 were kept in all multivariable models (e.g., race, education, BMI, depression, history of comorbid conditions, presence of an IADL impairment, benzodiazepine use, antidepressant use, self-reported health status, alcohol use, caffeine intake and smoking status). Models were further adjusted for physical activity, sleep efficiency (sleep fragmentation), hours worked and nocturnal hypoxemia 16 to determine if associations were independent of these parameters.
All significance levels reported were two-sided, and all analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the Study Population
This analytic cohort was comprised of 2754 primarily Caucasian (90.8%) men with an average age of 76.0 ± 5.3 years at the Sleep Visit.
We examined characteristics of participants by quartile of amplitude. On average, younger age, lower BMI and more hours spent working were associated with higher (e.g., more favorable) amplitude values. The proportions of men with an IADL impairment, a comorbid condition, benzodiazepine use, antidepressant use, depression and nocturnal hypoxemia decreased as amplitude increased. Greater alcohol and caffeine consumption, physical activity, self-reported health and sleep efficiency were associated with higher amplitude (Table1). Participants did not significantly differ across quartiles of amplitude in education, current smoking or use of sleep medications (data not shown). Participant characteristics by acrophase are presented in Supplementary Table S1.
Table 1.
Participant characteristics across quartiles of amplitude (difference between peak to nadir activity of the fitted curve), Mean ± SD or n (%)
| Characteristic | Overall (n=2754) |
Q1: <2962.5 (n=688) | Q2: 2962.5 to <3568.9 (n=689) | Q3: 3568.9 to <4218.8 (n=688) | Q4: ≥4218.8 (n=689) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 76.0 ± 5.3 | 77.6 ± 5.6 | 76.5 ± 5.4 | 75.5 ± 5.0 | 74.5 ± 4.7 | <.0001 |
| Body mass index (kg/m2) | 27.2 ± 3.8 | 28.0 ± 4.2 | 27.3 ± 3.7 | 27.0 ± 3.6 | 26.7 ± 3.6 | <.0001 |
| Caucasian | 2501 (90.8) | 632 (91.9) | 626 (90.9) | 629 (91.4) | 614 (89.1) | 0.31 |
| ≥ 1 IADL impairment | 533 (19.4) | 241 (35.0) | 125 (18.1) | 92 (13.4) | 75 (10.9) | <.0001 |
| Any medical condition* | 1108 (40.2) | 357 (51.9) | 266 (38.6) | 258 (37.5) | 227 (33.0) | <.0001 |
| Current use of benzodiazepines | 116 (4.2) | 42 (6.1) | 28 (4.1) | 26 (3.8) | 20 (2.9) | 0.02 |
| Current use of antidepressants | 189 (6.9) | 68 (9.9) | 34 (4.9) | 52 (7.6) | 35 (5.1) | 0.0005 |
| GDS score ≥6 | 151 (5.5) | 65 (9.5) | 31 (4.5) | 29 (4.2) | 26 (3.8) | <.0001 |
| Average drinks/day in past 30 days | 3.5 ± 4.3 | 2.9 ± 4.0 | 3.4 ± 4.2 | 3.4 ± 4.2 | 4.3 ± 4.6 | <.0001 |
| Average caffeine intake (mg/d) | 237.2 ± 244.9 | 209.3 ± 226.9 | 241.3 ± 261.3 | 229.8 ± 231.4 | 268.3 ± 254.9 | 0.0001 |
| PASE score | 148.6 ± 71.1 | 120.6 ± 66.7 | 142.1 ± 65.6 | 158.0 ± 69.6 | 173.5 ± 71.4 | <.0001 |
| Hours worked in past week | 6.3 ±11.5 | 5.0 ± 10.3 | 5.0 ± 9.8 | 7.5 ± 12.3 | 7.9 ± 13.1 | <.0001 |
| Self-reported health status | <.0001 | |||||
| Poor/very poor | 28 (1.0) | 10 (1.5) | 7 (1.0) | 4 (0.6) | 7 (1.0) | |
| Fair | 317 (11.5) | 132 (19.2) | 78 (11.3) | 52 (7.6) | 55 (8.0) | |
| Good/excellent | 2409 (87.5) | 546 (79.4) | 604 (87.7) | 632 (91.9) | 627 (91.0) | |
| Sleep efficiency (%) | 78.6 ± 11.7 | 75.1 ± 15.1 | 79.7 ± 10.2 | 80.1 ± 9.7 | 79.3 ± 10.2 | <.0001 |
| Nocturnal hypoxemia (n, %) | 1332 (51.4) | 379 (57.8) | 338 (51.7) | 316 (50.0) | 299 (46.1) | 0.0004 |
P-values for continuous variables are from ANOVA for normally distributed data; the Kruskal-Wallis test was used for skewed data. P-values for categorical data are from a chi-square test for homogeneity.
Medical conditions include stroke, diabetes, Parkinson’s disease, chronic obstructive pulmonary disease and cardiovascular disease (myocardial infarction, angina or congestive heart failure). GDS, geriatric depression scale. IADL, instrumental activities of daily living. PASE, physical activity scale for the elderly
Longitudinal Association of RAR Parameters and Cognitive Decline
By Visit 2 (1.18 ± 0.32 years after the Sleep Visit), men had begun to show signs of cognitive decline, with an average decrease of 0.5 ± 4.6 points in 3MS score. This trend continued at Visit 3 (3.4 ± 0.5 years after Sleep Visit). The unadjusted average 3MS score was lower by 1.3 ± 5.5 points compared to the Sleep Visit. At Visit 3, 18.7% of men were considered to have cognitive decline (5 point decline in 3MS score).
The adjusted annualized changes in 3MS score by RAR parameters are summarized in Figure 2. Men with lower amplitude (quartile 1) had a greater decline in 3MS score compared to those in quartile 4 (0.66 vs. 0.45 point decline; p<0.001). Similar associations were seen for the associations of mesor and decline in 3MS (0.52 vs. 0.22 point decline; p=0.01) and of rhythm robustness (pseudo F-statistic) and decline in 3MS (0.54 vs. 0.30 point decline; p<0.001). Acrophase was not associated with adjusted annualized change in 3MS score. All associations were not attenuated by further adjustment for physical activity, sleep efficiency, nocturnal hypoxemia or hours worked (data not shown).
Figure 2:
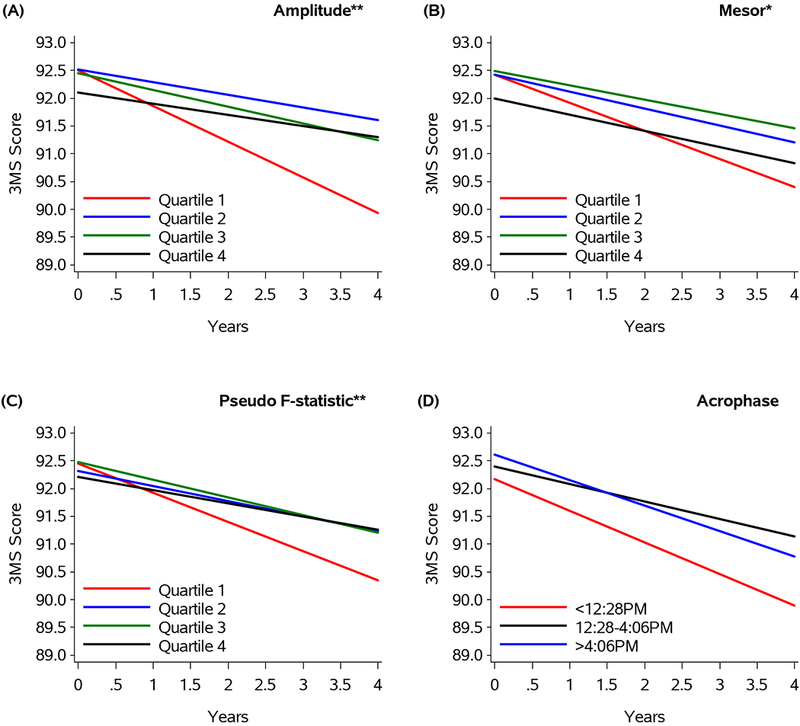
Changes in 3MS Score by amplitude (A), mesor (B), pseudo F-statistic (C) and acrophase (D)
**Quartile 1 vs. quartile 4 (p≤0.001)
*Quartile 1 vs. quartile 4 (p≤0.01)
Footnotes: Figure 2 represents estimated longitudinal decline based on the model. Year 0 = Sleep Visit
The associations of RAR measurements and clinically significant decline in 3MS are shown in Table 2 and Supplementary Table S2. After multivariable adjustment, lower levels of amplitude and robustness (pseudo F-statistic) were associated with an approximate 1.4-fold increase in odds of clinically significant cognitive decline. Results remained significant after adjustment for physical activity and hours worked. Significance was attenuated (p=0.05) after adjustment for sleep efficiency for both predictors, and after adjustment for nocturnal hypoxemia for robustness. Compared to those with acrophase from 12:28 to 4:06PM, those with acrophase <12:28PM (phase advanced) had a 1.8-fold increase in odds of clinically significant cognitive decline in 3MS. This association remained after adjustment for physical activity, sleep efficiency, hours worked and nocturnal hypoxemia. Mesor was not associated with clinically significant decline.
Table 2.
Adjusted longitudinal association of rest-activity rhythms and development of clinically significant cognitive decline (assessed by 3MS score1) in older men (n=2754)
| Outcome/ Predictor |
Category | N of events (%) | Age + Clinic Adjusted Odds Ratio (95% CI) |
Multivariable Adjusted* Odds Ratio (95% CI) |
|---|---|---|---|---|
| Amplitude (counts/min) | Q1: <2962.5 | 154 (26.9) | 1.5 (1.1, 2.0) | 1.4 (1.0, 1.9) |
| Q2: 2962.5 to <3568.9 | 93 (15.1) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | |
| Q3: 3568.9 to <4218.8 | 108 (17.1) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.4) | |
| Q4: ≥4218.8 | 103 (16.5) | Ref | Ref | |
| p-trend | 0.03 | 0.12 | ||
| Mesor (counts/min) | Q1: <1868.4 | 137 (23.1) | 1.13 (0.9, 1.5) | 1.1 (0.8, 1.4) |
| Q2: 1868.4 to <2143.8 | 105 (17.2) | 0.8 (0.6, 1.1) | 0.9 (0.6, 1.2) | |
| Q3: 2143.8 to <2431.7 | 102 (16.4) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | |
| Q4: ≥2431.7 | 114 (18.4) | Ref | Ref | |
| p-trend | 0.37 | 0.63 | ||
| Pseudo F-statistic | Q1: <705.9 | 151 (25.7) | 1.5 (1.2, 2.1) | 1.4 (1.0, 1.9) |
| Q2: 705.9 to <978.9 | 100 (16.6) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | |
| Q3: 978.9 to <1320.6 | 105 (16.8) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.3) | |
| Q4: ≥1320.6 | 102 (16.1) | Ref | Ref | |
| p-trend | <0.01 | 0.04 | ||
| Acrophase2 (time) | <12:28PM | 39 (30.7) | 1.9 (1.3, 2.8) | 1.8 (1.2, 2.8) |
| 12:28PM to 4:06PM | 392 (17.9) | Ref | Ref | |
| >4:06PM | 27 (20.8) | 1.2 (0.7, 1.8) | 1.03 (0.6, 1.6) |
Decline on 3MS of 5 points
<12:28 PM (phase advanced), >4:06 PM (phase delayed)
Multivariate models are adjusted by age, clinic site, race (white vs. nonwhite), body mass index, education, depression, history of comorbid conditions, benzodiazepine use, anti-depressant use, self-reported health status, IADL impairment, alcohol use, caffeine use and smoking. P-trends are from a test of linear trend across quartiles. All other p-values are pairwise comparisons of each quartile to the reference.
The adjusted annualized changes in Trails B test time by RAR parameters are summarized in Supplementary Figure S1. Lower amplitude and phase advanced acrophase were associated with significant worsening (i.e., prolongation) in Trails B time (amplitude quartile1: +3.97 seconds/year vs. quartile 4: +2.27 seconds/year, p=0.007; acrophase <12:28PM: +5.43 seconds/year vs. 12:28 to 4:06PM: +2.11 seconds/year, p=0.015). Neither mesor nor pseudo-F statistic were associated with adjusted annualized change in Trails B time. No associations between RAR parameters and clinically significant cognitive decline (as assessed by Trails B) were observed (data not shown).
DISCUSSION
In this cohort of community-dwelling men age 65 and older, we observed longitudinal associations between several RAR indices and cognitive decline after an average of 3.4 years of follow up. Lower amplitude, mesor and rhythm robustness (pseudo F-statistic) were associated with significant decline in 3MS score. Furthermore, men with lower amplitude and rhythm robustness, as well as men with phase advanced acrophase, had a higher likelihood of developing clinically significant cognitive decline, as measured by 3MS score. These associations were not attenuated after adjustment for numerous covariates related to health and lifestyle, although adjustment for sleep efficiency attenuated the associations of lower amplitude, as well as rhythm robustness, and likelihood of clinically significant cognitive decline. We also observed associations between lower amplitude and phase advanced acrophase and worsening of Trails B test time. Overall, these data support our hypothesis that disrupted circadian rhythms of activity are associated with cognitive decline.
Cognitive test results at the Sleep Visit were comparable to those of other studies of free living adults without dementia. 35,36 The participants in this cohort were considered cognitively healthy overall at the Sleep Visit, and associations between disrupted RAR and cognition became evident as cognition declined over time.
Relatively few studies of community-dwelling, older adults have examined longitudinal associations between measures of RARand cognition, and to our knowledge, none have used the same rhythm parameters and longitudinal cognitive outcomes as the present study. Prospective analyses of sleep disturbances and risk of incident cognitive impairment have been largely based on self-report of sleep and have reported conflicted findings. 6–9,11,12. It is interesting to compare our findings with similar analyses using objectively measured activity rhythms. Fragmented rest-activity rhythms were negatively correlated with mental speed, memory and cognitive function, independent of age, in men and women. 11 In a separate analysis of the MrOS cohort, lower daytime activity at baseline was associated with reduction in 3MS score and worsened performance on the Trails B test. 37 In older women, lower amplitude was associated with worsened Trails B test performance and categorical fluency; lower mesor was associated with worsened categorical fluency. 13 Women with lower amplitude and pseudo F-statistic had an increased likelihood of developing dementia or MCI. 14
In contrast to the present study, phase delayed acrophase has been previously associated with cognitive regression. Older adults with cognitive decline had later acrophase compared to those with intact cognition.12 Elderly women with phase delayed acrophase (+1 SD from the mean) performed worse in categorical fluency and were at greater risk for dementia and MCI after 5 years. 13,14 Gender, age and different definitions of phase-delayed acrophase may account for the differences between these12,14 and the present study. Additionally, factors such as sleep behavior, chronotype, and environmental exposures to light may have differed between studies and influenced findings related to acrophase. Further research is needed.
Disturbances of the sleep-wake cycle, which are reflected in less robust activity rhythms, are particularly pronounced in age-associated cognitive impairments, 38 delirium 39 and neurodegenerative diseases including Alzheimer’s disease (AD).40,41 Shifts in activity phase in adults with dementia have been shown to predict a shorter survival 41 and are hypothesized to be a primary cause of institutionalization. 42,43 Chronotype has been shown to impact several cerebral mechanisms and cognitive processing; 44 bidirectional interactions between cognitive performance and circadian processes suggest that time of day is an important consideration for a variety of cognitive tasks.45 At the molecular level, multiple circadian oscillators (clock-related genes) demonstrated altered expression patterns across several brain regions in AD patients compared with controls. 46 Furthermore, a pronounced age-related variation in the biosynthesis of vasopressin in the suprachiasmatic nucleus (SCN) may impact neurologic functioning. 47
This study has a number of strengths, including a large sample size, use of validated outcome measures and adjustment for multiple confounders. Participants in this prospective cohort were enrolled from the community and not selected based on sleep complaints or cognition. However, participants were primarily healthy Caucasian men, so generalizability to other populations is limited. Since men unable to forgo using mechanical devices during sleep or nocturnal oxygen therapy were excluded from the study, results of this analysis are not generalizable to these patients. Wrist actigraphy may be subject to masking by imposed schedules,48 and RAR do not directly assess SCN circadian output compared to melatonin or temperature rhythms. 48 Actigraphy recordings were limited to approximately five days of assessment at one time point. Decline outcomes required men to have data from Visits 2 and 3, so men who died before these visits or were unable to attend were not included in the analysis. Adjustment for numerous covariates was performed, but the possibility of residual confounding remains. Finally, it was difficult to observe the full spectrum of associations between RAR and cognitive function due to the fairly low prevalence of severely disrupted circadian rhythms in this cohort.
Results of the present analysis require confirmation in other longitudinal cohort analyses but could inform future intervention studies to slow cognitive decline. Markers of circadian rhythm may be useful as prognostic indicators in identifying older adults at risk for cognitive impairment, though more research is needed. Others49,50 have utilized a nonparametric approach to analyze circadian data in humans; these methods have not yet been applied to MrOS data but could be explored in future analyses.
In this cohort of older community-dwelling men, disrupted RAR (lower amplitude, mesor, pseudo F-statistic, and phase advanced acrophase) were associated with greater cognitive decline (as measured by 3MS score) over an average of 3.4 years. These findings contribute to a growing body of evidence suggesting that dysregulated rest-activity rhythms are associated with cognitive decline in older adults.
Supplementary Material
Supplementary Figure S1. Changes in Trails B Score by amplitude (A), mesor (B), pseudo F-statistic (C) and acrophase (D)
**Quartile 1 vs. quartile 4 (p≤0.01)
*Phase advanced (<12:28 PM) vs. 12:28 PM to 4:06 PM (p≤0.05)
ACKNOWLEDGEMENTS
Financial Disclosure: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, R01 HL070839 and R21 AG051380.
Sponsor’s Role: The National Institutes of Health had no role in the design of the study, collection or analysis of the data, or preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Impact Statement:
The authors certify that this work is novel. This analysis contributes to the geriatrics field as the first longitudinal study to report associations between rest-activity rhythms and cognitive decline in older men.
REFERENCES
- 1.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 2002;133(1):95–108. [DOI] [PubMed] [Google Scholar]
- 2.Kripke DF, Youngstedt SD, Elliott JA et al. Circadian phase in adults of contrasting ages. Chronobiol Int 2005;22(4):695–709. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Monk TH, Carrier J et al. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep 2005;28(11):1365–1376. [DOI] [PubMed] [Google Scholar]
- 4.Czeisler CA, Dumont M, Duffy JF et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 1992;340(8825):933–936. [DOI] [PubMed] [Google Scholar]
- 5.Duffy JF, Zeitzer JM, Rimmer DW et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab 2002;282(2):E297–303. [DOI] [PubMed] [Google Scholar]
- 6.Weitzman ED, Moline ML, Czeisler CA et al. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging 1982;3(4):299–309. [DOI] [PubMed] [Google Scholar]
- 7.Yoon IY, Kripke DF, Elliott JA et al. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc 2003;51(8):1085–1091. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai N, Sasaki M. An activity monitor study on the sleep-wake rhythm of healthy aged people residing in their homes. Psychiatry Clin Neurosci 1998;52(2):253–255. [DOI] [PubMed] [Google Scholar]
- 9.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 2006;5(1):33–51. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 11.Oosterman JM, van Someren EJ, Vogels RL et al. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res 2009;18(1):129–135. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study. J Neural Transm (Vienna) 2012;119(10):1233–1239. [DOI] [PubMed] [Google Scholar]
- 13.Walsh CM, Blackwell T, Tranah GJ et al. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep 2014;37(12):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tranah GJ, Blackwell T, Stone KL et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011;70(5):722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackwell T, Yaffe K, Laffan A et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 2014;37(4):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackwell T, Yaffe K, Laffan A et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2015;63(3):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 2005;26(5):557–568. [DOI] [PubMed] [Google Scholar]
- 19.Rogers TS, Blackwell TL, Lane NE et al. Rest-activity patterns and falls and fractures in older men. Osteoporosis International 2017;28(4):1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coding FAQ. 2014; http://www.aasmnet.org/codingfaq.Aspx. Accessed 3 October 2016.
- 21.Marler MR, Gehrman P, Martin JL et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med 2006;25(22):3893–3904. [DOI] [PubMed] [Google Scholar]
- 22.Paudel ML, Taylor BC, Ancoli-Israel S et al. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int 2010;27(2):363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paudel ML, Taylor BC, Ancoli-Israel S et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int 2011;28(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone KL, Blackwell TL, Ancoli-Israel S et al. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc 2014;62(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48(8):314–318. [PubMed] [Google Scholar]
- 26.Kuller LH, Lopez OL, Newman A et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22(1):13–22. [DOI] [PubMed] [Google Scholar]
- 27.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271–276. [Google Scholar]
- 28.Washburn RA, Smith KW, Jette AM et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46(2):153–162. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh JYJ. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol 1986;5:165–173. [Google Scholar]
- 30.Pahor M, Chrischilles EA, Guralnik JM et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 31.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1 1987(21):1–115. [PubMed] [Google Scholar]
- 32.Pincus T, Summey JA, Soraci SA, Jr. et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 1983;26(11):1346–1353. [DOI] [PubMed] [Google Scholar]
- 33.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol 1996;34(1):119–129. [DOI] [PubMed] [Google Scholar]
- 34.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–974. [PubMed] [Google Scholar]
- 35.Barnes DE, Tager IB, Satariano WA et al. The relationship between literacy and cognition in well-educated elders. J Gerontol A Biol Sci Med Sci 2004;59(4):390–395. [DOI] [PubMed] [Google Scholar]
- 36.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry 2001;46(6):506–510. [DOI] [PubMed] [Google Scholar]
- 37.Zeitzer JM, Blackwell T, Hoffman AR et al. Daily Patterns of Accelerometer Activity Predict Changes in Sleep, Cognition, and Mortality in Older Men. J Gerontol A Biol Sci Med Sci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackwell T, Yaffe K, Ancoli-Israel S et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2006;61(4):405–410. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald JM, Adamis D, Trzepacz PT et al. Delirium: a disturbance of circadian integrity? Med Hypotheses 2013;81(4):568–576. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S, Klauber MR, Jones DW et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 1997;20(1):18–23. [PubMed] [Google Scholar]
- 41.Gehrman P, Marler M, Martin JL et al. The timing of activity rhythms in patients with dementia is related to survival. J Gerontol A Biol Sci Med Sci 2004;59(10):1050–1055. [DOI] [PubMed] [Google Scholar]
- 42.Bliwise DL. Sleep in normal aging and dementia. Sleep 1993;16(1):40–81. [DOI] [PubMed] [Google Scholar]
- 43.Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol 2000;35(9–10):1229–1237. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt C, Peigneux P, Leclercq Y et al. Circadian preference modulates the neural substrate of conflict processing across the day. PLoS One 2012;7(1):e29658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gritton HJ, Kantorowski A, Sarter M et al. Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem 2012;19(3):126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cermakian N, Lamont EW, Boudreau P et al. Circadian clock gene expression in brain regions of Alzheimer ‘s disease patients and control subjects. J Biol Rhythms 2011;26(2):160–170. [DOI] [PubMed] [Google Scholar]
- 47.Hofman MA, Swaab DF. Influence of aging on the seasonal rhythm of the vasopressin-expressing neurons in the human suprachiasmatic nucleus. Neurobiol Aging 1995;16(6):965–971. [DOI] [PubMed] [Google Scholar]
- 48.Ancoli-Israel S, Cole R, Alessi C et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 49.Cespedes Feliciano EM, Quante M, Weng J et al. Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 2017;40(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goncalves BS, Adamowicz T, Louzada FM et al. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev 2015;20:84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Changes in Trails B Score by amplitude (A), mesor (B), pseudo F-statistic (C) and acrophase (D)
**Quartile 1 vs. quartile 4 (p≤0.01)
*Phase advanced (<12:28 PM) vs. 12:28 PM to 4:06 PM (p≤0.05)


