Abstract
Background/objectives:
Stroke is associated with acute and chronic functional decline, but it is unclear whether subclinical brain infarcts (SBI) are associated with functional decline independently of clinical vascular events. We aimed to test associations between SBI and functional decline independently of intervening clinical vascular events and other vascular risk factors.
Design:
Longitudinal follow-up for a mean 7.3 years. Generalized estimating equation models tested associations between SBI, number of perivascular spaces (PVS), and baseline Barthel index (BI) and change in BI, adjusting for sociodemographic, vascular, and cognitive risk factors, and stroke and myocardial infarction occurring during follow-up.
Setting:
Population-based prospective cohort study
Participants:
In the race/ethnically diverse Northern Manhattan Study, 1290 stroke-free individuals.
Exposure:
SBI and PVS on baseline brain MRI.
Measurements:
Annual functional assessments using the Barthel index (BI; range 0–100).
Results:
Mean age was 70.6 (standard deviation [SD] 9.0) years, 40% of participants were male, 66% Hispanic; 193 (16%) had SBI and 508 (42%) had large PVS. SBI were not associated with baseline BI. In a fully adjusted model, there was a decline of −0.85 BI points per year (95% CI −1.01 to −0.69); among those with SBI there were −0.88 additional points annually (−1.43 to −0.32). There were no associations between PVS and baseline BI or change in BI.
Conclusions:
In a large population-based study, we found a strong and independent association between “subclinical” markers of cerebrovascular injury and important clinical, patient-centered functional trajectories. Future research would clarify the evolution of such subclinical markers over time and test strategies to prevent their progression and minimize related disability.
Keywords: subclinical infarct, disability, epidemiology
Introduction
Subclinical brain infarcts (SBI) are at least 5 times as prevalent as clinical strokes.1 Vascular risk factors are associated with incident SBI.2 SBI have been associated with increased risk of stroke, mortality,3–5 cognitive impairment and reduced function.6–8 The pathophysiology of SBI is under some debate, and it is unclear whether their effect is similar to other brain pathological findings such as perivascular spaces (PVS).9 Also, there is little data regarding the time course of change in function in relation to SBI and the specific aspects of function that are compromised by SBI.
We hypothesized that SBI was independently associated with worse baseline functional status and steeper slope of change in those free of stroke at baseline, but measures of perivascular spaces (PVS) were not.
Methods
The Northern Manhattan Study (NOMAS) MRI study included 1290 individuals: 1) age ≥50 years, 2) without MRI contraindications, 3) without clinical stroke and 4) able to provide signed informed consent. Imaging was performed on a 1.5T MRI (Philips Medical Systems, Best, Netherlands), including axial T1, axial T2, axial proton density, dual-spin echo, diffusion weighted imaging, and FLAIR sequences. IRBs at Columbia University and University of Miami approved this study.
Baseline Evaluation
Bilingual research assistants interviewed participants and collected data using standardized questions regarding: hypertension, diabetes mellitus, hypercholesterolemia, cigarette smoking, alcohol use, and cardiac conditions.10 A thorough baseline examination including comprehensive medical history, physical examination, review of medical records, quality of life (QOL) assessed by the Spitzer QOL index, and fasting blood samples.
Follow-up
Participants were followed annually via phone screening to detect change in vital status, new neurological or cardiac symptoms and events, interval hospitalizations, cognitive function, and functional status via the BI. Only two subjects were lost to follow-up after their baseline examination, and the average annual contact rate was 99%.
A positive screen for any potential cardiac or neurological event was followed by an in-person assessment to determine whether a vascular outcome occurred, and a review of medical records from relevant hospitalizations. Also, all admissions and discharges of NOMAS study participants at Columbia University Medical Center -- where ~70% of hospitalized vascular events occur -- were screened for possible outcome events. Hospital records were reviewed to classify outcomes as previously reported.11 Stroke included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, and ≥2 stroke neurologists classified all stroke cases. MI was defined by criteria adapted from 2 trials,12, 13 and was adjudicated by cardiologists. As of the end of 2014, 53 first definite and probable MIs occurred during follow-up, and 64 first strokes (59 infarcts, 3 intracerebral hemorrhages, and 2 subarachnoid hemorrhages).
Study outcome
The Barthel index (BI)14, 15 measures performance in 10 activities of daily living (ADLs) and ranges from 0–100 in 5-point increments, with 100 indicating normal physical functioning. Phone assessments using the BI are reliable.16 Although it is an ordinal scale, recent research has advocated analyzing the scale as a continuous variable due to increased power to detect associations, ability to describe the course of change over time in linear form, and avoidance of potential misclassification due to crude categorization.17–19
Explanatory variables
We defined SBI in two ways: 1) Brain MRI lesions >3 mm, of similar intensity as cerebrospinal fluid. SBI location was classified as cortical or subcortical. This definition of SBI was used for consistency with earlier definitions in previous publications. 2) Pathology-informed SBI (pSBI) represented a re-reading of the original MRI scans and were informed by pathological understanding of the stroke mechanism as follows: lacunar infarcts were in penetrating artery territories; subcortical but likely embolic infarcts were in medullary arteries territories; cortical infarcts were likely embolic; and cerebellar infarcts were located in the cerebellum. In addition, the appearance of the rim of lesions on FLAIR was used to identify infarct. SBI location was coded as cortical or subcortical.
In 1228 participants, small perivascular spaces (SPVS) and large perivascular spaces (LPVS) were ascertained, according to modified criteria as previously described.20 In brief, SPVS were parenchymal T1 hypointensities <3 mm in effective axial diameter without associated FLAIR hyperintensity, and number of SPVS by brain region was categorized as 0=none, 1=1–3 voids, 2=4 or more voids in each of 12 anatomical brain regions. The total SPVS score was a sum of scores across all brain regions. Lesions >3 mm in effective diameter were characterized as LPVS or SBI according to previously described criteria.20 Briefly, we used the FLAIR rim as the strongest determinant of infarction. Compared to STRIVE criteria and previous studies, for lesions in the brain stem and in the upper basal ganglia, we tolerated a fainter FLAIR signal to identify infarct. The location of pSBI was determined by consensus by two vascular neurologists (JG and MSE) who rated each void separately with an agreement of >95%.20
An operator traced dura mater, and non-brain structures were manually removed from images. Modeling of pixel-intensity histograms for cerebral spinal fluid (CSF) and brain white and gray matter was performed. Semi-automated measurements of pixel distributions were made to identify the optimal pixel-intensity threshold to distinguish CSF from brain matter. Total cranial volume (TCV) constituted the sum of whole brain volume voxels from the T1 segmentation process. White matter hyperintensity volume (WMHV) was calculated as the sum of voxels ≥3.5 standard deviations above mean image intensity multiplied by pixel dimensions and section thickness, divided by TCV.21
Covariates
Analytic models were adjusted for: demographics (age, sex, race-ethnicity), medical risk factors (body mass index [body weight in kilograms divided by the square of height in meters], hypercholesterolemia [defined by self-report, lipid lowering therapy use, or fasting total cholesterol level >240 mg/dL], diabetes mellitus [defined by self-report, fasting blood glucose level ≥126 mg/dL, or insulin/oral hypoglycemic use], hypertension [defined as a systolic blood pressure (BP) recording ≥140 mmHg or diastolic BP recording ≥90 mmHg based on the average of two BP measurements or self-report of hypertension or antihypertensive use]), smoking (nonsmoker vs. smoker within the last year), alcohol use (with moderate alcohol use classified as 1 drink/month to 2 drinks/day), any physical activity (versus none), social variables (marital status, insurance status [classified as uninsured/Medicaid versus Medicare/private insurance], number of friends [individuals whom the participant knows well enough to visit in their homes], years living in the community), and cognitive/mood factors (depressed mood, performance on mini-mental state examination [analyzed as a continuous variable], and Spitzer QOL index score).
Statistical analysis
We analyzed associations of SBI with baseline BI and slope of decline over time. We first calculated the distributions of SBI, pSBI, pSBI subtype (penetrating artery, medullary artery, embolic cortical, and cerebellar), SPVS, LPVS, baseline covariates, and BI.
Due to correlations among repeated measures of BI in each individual, regression models using generalized estimating equations (GEE) with an identity link function were used to assess the association between primary predictors (SBI, SPVS, LPVS, pSBI, penetrating artery pSBI, and SBI location) and repeated measurements of BI, adjusting sequentially for: baseline demographic variables, medical risk factors, smoking and alcohol use and physical activity, social variables, and cognitive/mood factors, as defined above. Each primary predictor was tested in a separate model.
In order to assess whether MRI variables were associated with change in outcomes over time, we included interaction terms between time of follow-up assessment and the main predictor variable. For each model, there were 3 main estimates of interest: 1) annual change in BI, 2) additional annual change in the presence of the predictor of interest (e.g. SBI), 3) baseline difference in BI in the presence of the predictor of interest. Various model diagnostics including tests of linearity, residual plots, and goodness of fit measures were used to evaluate the final model. There was no evidence to suggest lack of linearity in the final models. As a working correlation structure for the GEE models we chose the exchangeable (intraclass) structure and compared the quasi-likelihood under the independence model criterion obtained with this model with one using the unstructured working correlation structure. In order to assess whether interval vascular events such as clinical stroke and MI were implicated in the trajectory of functional status, we ran a second set of models in which stroke and MI were included as time-varying covariates. We tested whether the relationship between SBI and functional status remained even after adjusting for these events. We also tested whether the relationship between SBI and functional trajectories remained after adjusting for WMHV.
A sensitivity analysis was performed among those with BI 95 or 100 at baseline (the closest BI measurement to the MRI date). The association between SBI location (cortical, subcortical, and both) and trajectories of functional status was examined using a multi-level categorical location variable.
Results
Table 1 shows distributions of baseline variables stratified by presence or absence of SBI; those with SBI were older, more often male, and more often had hypertension. 1136 (88.8%) had BI of 95 or 100 at baseline. The mean (SD) baseline BI was 96.5 (8.3). There were 193 (15.6%) with SBI, 246 (19.1%) with pSBI, 508 (42.1%) with ≥1 LPVS, and most individuals had a total PVS score of 4 (193, 16.0%), with a range of 0–22. Among those with no LPVS (n=695), mean WMHV was −0.028 (SD 0.985) and 62 (8.9%) had SBI, and among those with ≥1 LPVS, mean WMHV was −0.021 (SD 0.948) and 123 (24.2%) had SBI. Eighty-three (6.7%) had cortical SBI location, 88 (7.1%) had subcortical SBI, and 22 (1.8%) had both. There were 39 (3.0%) with cerebellar pSBI, 37 (2.9%) with cortical pSBI, 143 (11.1%) with pSBI in the territory of medullary arteries, and 85 (6.6%) with pSBI in the territory of penetrating arteries. The mean follow-up was 7.3 years (SD 2.1).
Table 1.
Baseline characteristics of the cohort:
| SBI | No SBI | p-value | |
|---|---|---|---|
| Variable | No. (%)* | No. (%)* | |
| Number of participants, No. (%) | 193 (100) | 1045 (100) | |
| Age, mean (SD), y | 74.7 (8.6) | 69.8 (8.8) | <0.0001 |
| Body mass index, mean (SD), kg/m2 | 27.5 (4.8) | 28.0 (4.8) | 0.23 |
| Male | 96 (49.7) | 404 (38.7) | 0.0039 |
| Other | 7 (3.6) | 20 (1.9) | |
| Received at least high school education | 99 (51.3) | 479 (45.8) | 0.16 |
| Marital status, married | 77 (39.9) | 448 (42.9) | 0.4 |
| Medicare or private insurance | 104 (53.9) | 551 (52.7) | |
| Hypertension | 151 (78.2) | 670 (64.1) | 0.0001 |
| Heavy drinker | 8 (4.2) | 20 (1.9) | |
| Any | 104 (54.2) | 580 (56.2) | |
| Diabetes mellitus | 38 (19.7) | 189 (18.1) | 0.60 |
| Current | 28 (14.5) | 145 (13.9) | |
| Hypercholesterolemia | 112 (58.0) | 648 (62.0) | 0.30 |
| History of coronary heart disease | 29 (15.0) | 138 (13.2) | 0.50 |
| Hamilton Depression Scale, mean (SD) | 2.7 (3.4) | 3.2 (3.9) | 0.10 |
| Mini Mental State, mean (SD) | 26.7 (3.4) | 26.8 (3.3) | 0.77 |
| Spitzer Quality of Life Index score | 9.3 (1.1) | 9.3 (1.0) | 0.73 |
| 5 or more | 125 (64.8) | 713 (68.2) | |
| Number of years living in community | 28.6 (17.1) | 24.6 (14.3) | 0.0007 |
unless otherwise indicated
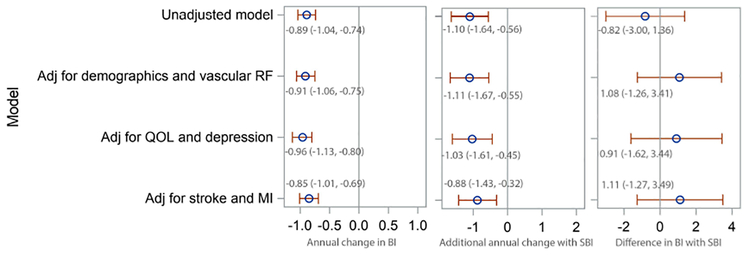
SBI was not associated with baseline functional status but was strongly and consistently associated with accelerated decline in function over time. The magnitude of the association between SBI and the rate of decline varied between the unadjusted model (-1.10 BI points per year, 95% CI −1.64, −0.56) and the full adjusted model (-0.88, 95% CI −1.43, −0.32) (Figure 1). The magnitude and significance of this association did not vary substantially when WMHV was added to the models (Supplementary Table 1). Also, SBI showed a similar association with mobility and non-mobility domains of the BI (Supplementary Table 2), proportional to the portion of the BI comprising each domain.
Figure 1. Unadjusted and adjusted models of the association between subclinical brain infarcts and change in Barthel index.
BI=Barthel index; CI=confidence interval; SBI=subclinical brain infarct; MI=myocardial infarction; RF=risk factor; QOL=quality of life; Adj=adjusted for Demographics and vascular risk factor model is adjusted for age at time of MRI, sex, race, diabetes, hypertension, coronary artery disease, hypercholesterolemia, physical activity, alcohol use, smoking, and body mass index at the time of MRI QOL and depression model is additionally adjusted for: marital status, insurance, number of friends, years lived in the community, mini-mental state score, and Spitzer quality of life index and depression Stroke and MI model is additionally adjusted for stroke and MI occurring during follow-up, as time-varying covariates
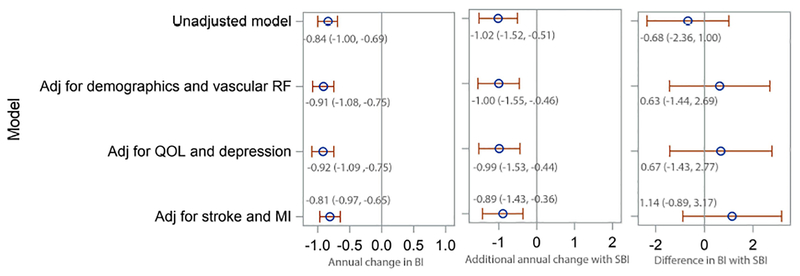
Examining pSBI, results were similar (Figure 2), with −1.02 additional BI points per year (95% CI −1.52, −0.51) with pSBI in an unadjusted model, and −0.89 additional points per year (95% CI −1.43, −0.36) in a fully adjusted model. Results were similar when the number of pSBI was tested, with an additional decline of −0.40 points per year (95% CI −0.72, −0.08) with each additional pSBI in a fully adjusted model. pSBI showed a similar association with mobility and non-mobility domains of the BI, proportional to the portion of the BI comprising each domain: −0.34 points per year (95% CI-0.56, −0.13) for mobility domains and −0.54 (-0.89, −0.19) for non-mobility domains.
Figure 2. Unadjusted and adjusted models of the association between pathology-informed subclinical brain infarcts and change in Barthel index.
BI=Barthel index; CI=confidence interval; SBI=subclinical brain infarct; MI=myocardial infarction; pSBI=pathology-informed subclinical brain infarct; RF=risk factor; QOL=quality of life; Adj=adjusted for Demographics and vascular risk factor model is adjusted for age at time of MRI, sex, race, diabetes, hypertension, coronary artery disease, hypercholesterolemia, physical activity, alcohol use, smoking, and body mass index at the time of MRI QOL and depression model is additionally adjusted for: marital status, insurance, number of friends, years lived in the community, mini-mental state score, and Spitzer quality of life index and depression Stroke and MI model is additionally adjusted for stroke and MI occurring during follow-up, as time-varying covariates
There was a significant and consistent association between presence of penetrating artery infarcts and accelerated decline in functional status over time, with a change of −1.39 additional BI points per year (95% CI −2.23, −0.55) with penetrating artery infarcts in an unadjusted model, and −1.24 points per year (95% CI −2.11, −0.37) in a fully adjusted model.
In contrast to findings for SBI and pSBI, there were no significant associations between LPVS and baseline BI or change in BI over time in unadjusted or adjusted models, either with a dichotomous definition of LPVS or one that incorporated the number of LPVS per individual. Similarly, when the SPVS score was tested, there were no significant associations with baseline BI score or change in BI over time, in unadjusted or adjusted models, and when mobility and non-mobility domains of the BI were tested separately (results not shown).
Sensitivity analysis was performed among those with BI score of 95 or 100 at baseline (n=1136). Although the magnitude of overall decline and the magnitude of additional decline with MRI variables were both slightly reduced in most models, there were still highly significant associations paralleling the findings in models among the entire cohort. For example, among those with BI of 95 or 100 at baseline SBI presence was associated with an additional decline of −0.79 points per year (95% CI −1.34, −0.24).
Table 2 shows models testing the association between SBI location and functional status. When tested in separate models, cortical (-0.79 points per year, 95% CI −1.63, 0.06) and subcortical location (-1.11, 95% CI −1.81, −0.41) were both associated with accelerated decline in functional status over time, but not with baseline BI score. When tested in the same model, subcortical location was associated with accelerated decline over time (-0.90 additional BI points per year, 95% CI −1.60, −0.20) but not cortical location (-0.49, 95% CI −1.36, 0.38), and neither was associated with baseline BI score. Individuals with both cortical and subcortical SBI had more than double the additional decline in functional status than those with subcortical SBI alone (-2.68 points per year, 95% CI −5.03, −0.32). With pSBI, cortical (-0.95 points per year, 95% CI −1.72, −0.17) and subcortical (-1.35, 95% CI −2.37, −0.33) pSBI were individually associated with accelerated decline in functional status over time, and there was a trend for an association of similar magnitude with both cortical and subcortical pSBI (-1.23, 95% CI −2.63, 0.17).
Table 2.
Association of subclinical brain infarcts by location with change in functional status *
| Variable | Estimate | 95% CI | p-value |
|---|---|---|---|
| Model 1: SBI, superficial/cortical location:* | |||
| Annual change in BI | −0.92 | −1.08, −0.76 | <.0001 |
| Difference in BI with cortical SBI location** | 0.27 | −3.29, 3.83 | 0.9 |
| Additional annual change with cortical SBI location** | −0.79 | −1.63, 0.06 | 0.068 |
| Model 2: SBI, subcortical location:* | |||
| Annual change in BI | −0.88 | −1.04, −0.72 | <.0001 |
| Difference in BI with subcortical SBI location** | 1.21 | −1.87, 4.29 | 0.4 |
| Additional annual change with subcortical SBI location** | −1.11 | −1.81, −0.41 | 0.002 |
| Model 3: SBI, testing location:* | |||
| Annual change in BI | −0.85 | −1.01, −0.69 | <.0001 |
| Difference in BI with cortical SBI location† | 0.70 | −2.93, 4.34 | 0.7 |
| Difference in BI with subcortical SBI location† | 1.81 | −1.14, 4.76 | 0.2 |
| Difference in BI with both cortical and subcortical SBI location† | 0.73 | −9.15, 10.62 | 0.9 |
| Additional annual change with cortical SBI location† | −0.49 | −1.36, 0.38 | 0.3 |
| Additional annual change with subcortical SBI location† | −0.90 | −1.60, −0.20 | 0.01 |
| Additional annual change with both cortical and subcortical SBI location† | −2.68 | −5.03, −0.32 | 0.03 |
| Model 4: pSBI, testing location:* | |||
| Annual change in BI | −0.82 | −0.98, −0.66 | <.0001 |
| Difference in BI with cortical pSBI location† | 2.33 | −0.31, 4.98 | 0.084 |
| Difference in BI with subcortical pSBI location† | 3.54 | −0.45, 7.53 | 0.082 |
| Difference in BI with both cortical and subcortical pSBI location† | −0.74 | −6.84, 5.36 | 0.8 |
| Additional annual change with cortical pSBI location† | −0.95 | −1.72, −0.17 | 0.017 |
| Additional annual change with subcortical pSBI location† | −1.35 | −2.37, −0.33 | 0.009 |
| Additional annual change with both cortical and subcortical pSBI location† | −1.23 | −2.63, 0.17 | 0.086 |
SBI=subclinical infarct; pSBI=pathology-informed SBI; BI=Barthel index; CI=confidence interval; MI=myocardial infarction
models are additionally adjusted for: age at the time of MRI, sex, race-ethnicity, diabetes, hypertension, coronary artery disease, hypercholesterolemia, physical activity, alcohol use, smoking, body mass index, marital status, insurance status, number of friends, mini-mental state score, and stroke and myocardial infarction occurring during follow-up
versus no SBI
versus no SBI in this location
Discussion
In this large population-based MRI study with a mean follow-up of seven years, we found a strong, consistent, and independent association of SBI with accelerated decline in function over time over and above the annual decline in function associated with age. Despite healthy risk factor profiles and good functional status, 19.1% of the cohort had SBI on imaging using a classification system informed by characteristics found in pathology studies.20 The presence of SBI doubled the annual decline in functional status due to aging. Hence, whereas an individual may be expected to lose one ADL every 5 years due to aging, the presence of SBI was associated with the loss of 2 ADLs every 5 years.
Functional decline was seen with SBI using both mobility and non-mobility domains of the BI as separate outcomes. This pattern of association was seen with MRI imaging markers believed to be caused by ischemic damage (SBI and lacunar infarcts) but not with other MRI structural findings, such as SPVS and LPVS, which are less believed to represent structural damage to the brain parenchyma. Also, associations were unaffected by additional adjustment for WMHV. There was a greater decline in functional status with increasing number of SBI and lacunar SBI, reflecting a dose-response relationship that lends biological plausibility to the association. Also, these associations were seen even among those with no baseline disability (BI of 95 or 100), which emphasizes the “subclinical” nature of these predictors, and yet their strong predictive power for downward functional trajectories. These findings support the concept of “vascular functional impairment,” whereby subclinical lesions presumed to be caused by vascular dysfunction have an independent association with functional impairment and decline.22
We found evidence for a relationship between SBI location and accelerated functional decline over time. Using the original classification of SBI, subcortical location was associated with accelerated decline over time, and both cortical and subcortical SBI was associated with the most decline. SBI in both locations likely represents a greater overall or cumulative burden of subclinical ischemic lesions, which has a greater effect on ongoing functional decline. With the pathology-informed classification system, the magnitude of additional decline over time with cortical and subcortical SBI was similar, and presence of SBI in both locations was not associated with any incremental decline over time. The differences between SBI and pSBI may reflect more sensitive discrimination between infarct and PVS with the revised pSBI readings, but more research is needed to clarify these differences.
SBI have been associated with vascular events, cognitive impairment,23 and reduced functional status.6 Asymptomatic brain MRI abnormalities, including WMH and infarcts, have been associated with functional impairment up to 4 years of follow-up.6, 8, 24Among 267 men aged 74–95 years,25 those with white matter lesions at baseline had twofold higher adjusted odds of having a 1 SD drop in cognitive performance at 5 years. 2450 individuals were followed for a mean of 4 years, and WMHV and brain infarcts were associated with higher incidence of disability and accelerated decline in gait speed.6 Adjustment for incident stroke, dementia and mini-mental status score did not attenuate associations. In distinction to these prior studies examining relationships between SBI and cognition and functional status, we estimated associations with both baseline functional status and change over time, using multiple repeated measures, and adjusted for the occurrence of stroke and MI, ensuring that associations were independent of clinical events. One novel finding was that SBI was associated not with baseline BI but with accelerated decline over time.
Also, we found no significant associations between PVS and functional status. The underlying mechanism(s) of which perivascular spaces may be a marker are not well delineated at this time. Although some studies have shown associations between PVS and vascular risk factors and outcomes,26–28 the mechanisms underlying PVS may rather involve neurodegenerative processes29–31 that may have a differential effect on functional trajectories compared to cerebrovascular processes.
There are several brain structural changes associated with cerebrovascular disease that may impact cognition and functional status. For example, brain infarcts have been associated with smaller hippocampal volumes,32 reduced gray matter volume33 declines in gait speed, cadence of gait, and length of steps,34–36 and gait variability, all of which can impact cognition and mobility.37 Both subcortical SBI and WMH are in many cases manifestations of small vessel damage, and they often overlap. Another possible mechanism for steeper functional decline over time is the accumulation of SBI, which we were not able to assess with one MRI per individual.
Strengths of this study include the large population-based cohort, accurate assessment of events during follow-up, minimal loss to follow-up, state-of-the-art imaging and measurement of subclinical brain vascular disease, and repeated measures of functional outcomes that allow trajectory analysis. With reliable surveillance and tracking of events and regular and repeated measurements of functional status, this research highlights the likely central role that “subclinical” disease plays in functional ability and health over time. One limitation of the study is that we slightly modified the STRIVE criteria for reading of PVS, which we intended to better discriminate PVS from infarcts, but this may preclude direct comparison to other studies with differing methods.
Better imaging might facilitate the design of studies to better evaluate relationships between SBI and functional decline, and to test interventions that target SBI to delay functional decline.38
Supplementary Material
Supplementary Table 1: Model adjusting for white matter hyperintensity volume
Supplementary Table 2: Unadjusted and adjusted models of the association between silent brain infarcts and functional status, stratified by mobility vs. non-mobility domains
Impact:
1) We certify that this work is novel because we examined disability not only at fixed time points but rather trajectories of disability change over time. We found that subclinical brain infarcts, but not perivascular spaces, were associated with decline trajectories.
Acknowledgments:
None
Sponsor’s Role: None
Footnotes
Conflict of Interest: None
References
- 1.Vermeer SE, Longstreth WT Jr., Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 2.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim GM, Park KY, Avery R, Helenius J, Rost N, Rosand J, et al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke. 2014;45:479–485 [DOI] [PubMed] [Google Scholar]
- 4.Windham BG, Deere B, Griswold ME, Wang W, Bezerra DC, Shibata D, et al. Small brain lesions and incident stroke and mortalitya cohort studysmall brain lesions and incident stroke and mortality. Annals of Internal Medicine. 2015;163:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuller LH, Arnold AM, Longstreth WT Jr., Manolio TA, O’Leary DH, Burke GL, et al. White matter grade and ventricular volume on brain mri as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315 [DOI] [PubMed] [Google Scholar]
- 6.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr., Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654 [DOI] [PubMed] [Google Scholar]
- 7.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and mri-defined brain infarct in an elderly general population. J Geriatr Psychiatry Neurol. 2009;22:266–273 [DOI] [PubMed] [Google Scholar]
- 8.Pohjasvaara TI, Jokinen H, Ylikoski R, Kalska H, Mantyla R, Kaste M, et al. White matter lesions are related to impaired instrumental activities of daily living poststroke. J Stroke Cerebrovasc Dis. 2007;16:251–258 [DOI] [PubMed] [Google Scholar]
- 9.Black S, Gao F, Bilbao J. Understanding white matter disease. Stroke. 2009;40:S48–S52 [DOI] [PubMed] [Google Scholar]
- 10.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, et al. The behavioral risk factor surveys: Ii. Design, methods, and estimates from combined state data. American journal of preventive medicine. 1985;1:9–14 [PubMed] [Google Scholar]
- 11.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: The northern manhattan study. Stroke. 2004;35:2263–2269 [DOI] [PubMed] [Google Scholar]
- 12.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788 [DOI] [PubMed] [Google Scholar]
- 13.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid research clinics coronary primary prevention trial. Jama. 1994;271:999–1003 [DOI] [PubMed] [Google Scholar]
- 14.Mahoney FI, Barthel DW. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–65 [PubMed] [Google Scholar]
- 15.Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: Analysis of repeated barthel index measures. Arch Phys Med Rehabil. 1979;60:14–17 [PubMed] [Google Scholar]
- 16.Shinar D, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, et al. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. 1987;68:723–728 [PubMed] [Google Scholar]
- 17.Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke. 2007;38:1911–1915 [DOI] [PubMed] [Google Scholar]
- 18.Saver JL. Optimal end points for acute stroke therapy trials: Best ways to measure treatment effects of drugs and devices. Stroke. 2011;42:2356–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F, Jerosch-Herold C, Holland R, Drachler Mde L, Mares K, Harvey I. Statistical methods for analysing barthel scores in trials of poststroke interventions: A review and computer simulations. Clin Rehabil. 2006;20:347–356 [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The northern manhattan study. Journal of hypertension. 2015;33:2115–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: The northern manhattan study. Neurology. 2015;85:441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhamoon MS, Dong C, Elkind MS, Sacco RL. Ideal cardiovascular health predicts functional status independently of vascular events: The northern manhattan study. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longstreth WT Jr., Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ Jr., O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382 [DOI] [PubMed] [Google Scholar]
- 24.Longstreth WT Jr., Diehr PH, Yee LM, Newman AB, Beauchamp NJ. Brain imaging findings in elderly adults and years of life, healthy life, and able life over the ensuing 16 years: The cardiovascular health study. J Am Geriatr Soc. 2014;62:1838–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba M, White L, Bell C, Chen R, Petrovitch H, Launer L, et al. White matter lesions on brain magnetic resonance imaging scan and 5-year cognitive decline: The honolulu-asia aging study. J Am Geriatr Soc. 2011;59:1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrobot OA, Attems J, Esiri M, Hortobagyi T, Ironside JW, Kalaria RN, et al. Vascular cognitive impairment neuropathology guidelines (vcing): The contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016;139:2957–2969 [DOI] [PubMed] [Google Scholar]
- 27.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duperron MG, Tzourio C, Sargurupremraj M, Mazoyer B, Soumare A, Schilling S, et al. Burden of dilated perivascular spaces, an emerging marker of cerebral small vessel disease, is highly heritable. Stroke. 2018;49:282–287 [DOI] [PubMed] [Google Scholar]
- 29.Yao M, Herve D, Jouvent E, Duering M, Reyes S, Godin O, et al. Dilated perivascular spaces in small-vessel disease: A study in cadasil. Cerebrovasc Dis. 2014;37:155–163 [DOI] [PubMed] [Google Scholar]
- 30.Feldman RE, Rutland JW, Fields MC, Marcuse LV, Pawha PS, Delman BN, et al. Quantification of perivascular spaces at 7t: A potential mri biomarker for epilepsy. Seizure. 2018;54:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavallari M, Egorova S, Healy BC, Palotai M, Prieto JC, Polgar-Turcsanyi M, et al. Evaluating the association between enlarged perivascular spaces and disease worsening in multiple sclerosis. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2017 [DOI] [PubMed] [Google Scholar]
- 32.Blum S, Luchsinger JA, Manly JJ, Schupf N, Stern Y, Brown TR, et al. Memory after silent stroke: Hippocampus and infarcts both matter. Neurology. 2012;78:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo W, Jiang X, Wei X, Li S, Li M. A study on cognitive impairment and gray matter volume abnormalities in silent cerebral infarction patients. Neuroradiology. 2015;57:783–789 [DOI] [PubMed] [Google Scholar]
- 34.Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, et al. Brain structural change and gait decline: A longitudinal population-based study. J Am Geriatr Soc. 2013;61:1074–1079 [DOI] [PubMed] [Google Scholar]
- 35.Rosano C, Brach J, Longstreth WT Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60 [DOI] [PubMed] [Google Scholar]
- 36.Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, et al. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: Population-based study. Stroke. 2012;43:1505–1510 [DOI] [PubMed] [Google Scholar]
- 37.Rosano C, Brach J, Studenski S, Longstreth WT Jr., Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid CM, Storey E, Wong TY, Woods R, Tonkin A, Wang JJ, et al. Aspirin for the prevention of cognitive decline in the elderly: Rationale and design of a neuro-vascular imaging study (envis-ion). BMC neurology. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Model adjusting for white matter hyperintensity volume
Supplementary Table 2: Unadjusted and adjusted models of the association between silent brain infarcts and functional status, stratified by mobility vs. non-mobility domains