Abstract
Keratoconus (KC) is the most common ectatic corneal disease, with clinical findings that include discomfort, visual disturbance and possible blindness if left untreated. KC affects approximately 1:400 to 1:2000 people worldwide, including both males and females. The aetiology and onset of KC remains a puzzle and as a result, the ability to treat or reverse the disease is hampered. Sex hormones are known to play a role in the maintenance of the structure and integrity of the human cornea. Hormone levels have been reported to alter corneal thickness, curvature, and sensitivity during different times of menstrual cycle. Surprisingly, the role of sex hormones in corneal diseases and KC has been largely neglected. Prolactin-induced protein, known to be regulated by sex hormones, is a new KC biomarker that has been recently proposed. Studies herein discuss the role of sex hormones as a control mechanism for KC onset and progression and evidence supporting the view that prolactin-induced protein is an important hormonally regulated biomarker in KC is discussed.
Keywords: Human cornea, Keratoconus, Sex hormones, Prolactin-induced protein, Bodily fluids
1. The human cornea
The cornea is the outermost avascular and transparent part of the human eye. It serves as a key component in maintaining the shape of the eyeball, as well as its transparency and refractive power. The cornea consists of 5 distinct layers (Fig. 1) and contributes 2/3 (43 diopters or 43D) of the eye’s total refractive power: The outer stratified squamous non-keratinized epithelium, the acellular Bowman’s layer, the stroma with connective tissue and resident cells commonly termed “keratocytes”, the acellular Descemet’s membrane, and the endothelium. The transparency of the cornea is achieved by virtue of being highly aqeous, through its avascularity as well as the size and orientation of the collagen fibrils located in the stroma layer (Benedek, 1971; Hart and Farrell, 1969; Maurice, 1957). The stroma accounts for 90% of the corneal thickness andconsist of collagen, proteoglycans and resident keratocytes (Hassell and Birk, 2010).
Fig. 1.

The layers of the human cornea. Illustrative image of the five human corneal layers, from top to bottom: corneal epithelium, Bowman’s layer, corneal stroma, Descemet’s membrane, and corneal endothelium. Image proportions not to scale.
The corneal stroma collagen fibrils primarily consist of type I and type V collagen (Ihanamaki et al., 2004; Tseng et al., 1982). The fibrils are stacked in lamellae that run like belts across the cornea (Komai and Ushiki, 1991; Meek and Knupp, 2015; Polack, 1961). A distinct difference between the anterior and the posterior part of the cornea exists. In the anterior, the lamellae frequently bifurcate and interweave considerably (Radner et al., 1998), as opposed to the posterior where the lamellae are more hydrated and stacked like plywood in a predominantly vertical and horizontal orientation (Daxer and Fratzl, 1997; Meek et al., 1987). Keratocytes are the main cells of the stroma, though other cells including monocytes and dendritic cells (Hay, 1980) are also present (Birk, 2001). The keratocytes are considered to be quiescent, and maintain a slow turnover of the connective tissue matrix (Hay, 1980; West-Mays and Dwivedi, 2006). Keratocytes can be activated upon injury/trauma/infection to the corneal tissue, differentiating into myofibroblasts. Myofibroblasts characteristically express smooth muscle actin (SMA) and produce high levels of collagen and hyaluronan. Myofibroblasts are also known for the secretion and assembly of a disorganized and an opaque corneal extracellular matrix (ECM), leading to scarring or fibrosis. Over time, myofibroblasts may turn into wound fibroblast capable of creating a more organized ECM which may result in partial restoration of transparency (Hassell and Birk, 2010). In human, this process is very slow and occurs over decades.
2. Keratoconus
The word keratoconus (KC) originates from the Greek words kerato (cornea) and konos (cone). KC is the most common ectatic disease of the cornea and was first described and named by Nottingham in 1854 (Nottingham, 1854). The disease is bilateral though asymmetric, characterized by progressive thinning and steepening of the cornea. KC was previously also characterized as a non-inflammatory disease, however recent studies have indicated that there may be an in-flammatory component (Fan Gaskin et al., 2014; Galvis et al., 2015). Initial presentation typically occurs during adolescence followed by 10–20 years of progression before a stable phase is reached in the third or fourth decade of life (Tuft et al., 1994). In general, the progression of KC is very heterogeneous but ultimately 15–20% of patients will require a corneal transplant (Wagner et al., 2007).
The prevalence of KC is often cited to be 1 in 2000 (Kennedy et al., 1986). However, large geographic variation has been reported with the highest reported prevalence in Israel (2.34%) and Iran (2.53%) (Hashemi et al., 2014; Millodot et al., 2011), versus the lowest prevalence in Denmark (0.084%), Russia (0.0003%), and Finland (0.03%) (Gorskova et al., 1998; Ihalainen, 1986; Nielsen et al., 2007). One of the most recent population-based studies reported a prevalence of 375 per 100,000 (0.375%) in the Netherlands (Godefrooij et al., 2017). It is now believed that there is a significant increase in prevalence, possibly attributed to increased incidences, more advanced diagnostic tools, thorough clinical examination, and registration of KC patients. The observed geographical variation, however, in prevalence remains an intriguing unresolved question.
The geographic component may also affect the gender distribution. Most clinical studies, from around the globe have reported a male predominance in their KC populations. Pearson et al. reported a male/female ratio (M/F) of 3.34 in Caucasians and 1.63 in Asians in 382 KC patients from a catch-population of 900,000 in the UK (Pearson et al., 2000). The US-based Collaborative Longituginal Evaluation of Keratoconus (CLEK)-study further reported a M/F of 1.33 in their multicentre study of 1209 KC patients (Wagner et al., 2007). Recently, Woodward et al. reported a registry-based study of 16,053 KC patients in the United States with an M/F of 1.43 (Woodward et al., 2016). However, some studies primarily from the Middle East and Asia have reported female KC predominance. Jonas et al. reported a M/F of 0.29 in 128 KC patients from a population study of 4711 people (Jonas et al., 2009). The diagnosis of KC in this particular study was determined by a curvature of only > 48 diopters which gives rise to substantial risk of misclassification. Hashemi et al. also used the population sample approach with 4688 persons having Pentacam imaging preformed resulting in 35 KC patients diagnosed with a M/F of 0.58 (Hashemi et al., 2013a,b). Clearly, gender bias may vary with geographic location.
The aetiology of KC is multifactorial (Gordon-Shaag et al., 2015) and remains unresolved. The majority of cases are sporadic, but 10–20% have a family history of KC (Rabinowitz, 1998) (Lass et al., 1990). Monozygotic twins have been shown to develop KC to different degrees though concordantly, which is evidence of both the genetic and the environmental aetiology of KC. (Owens and Gamble, 2003; Tuft et al., 2012). The genetic component may also be linked with ethnicity. Pearson et al. showed a four times higher prevalence of KC in Asians compared to Caucasians living in the same area of the UK (Pearson et al., 2000). Woodward et al., on the other hand, found Asian Americans to have 39% lower odds of being diagnosed with KC compared to Caucasian Americans. The same group also reported that African Americans had 57% and Latino Americans 43% increased odds of developing KC (Woodward et al., 2016). For a long time now, it has been suggested that KC is not a single disease, but a phenotypic presentation of a number of different diseases. This would explain some of the variation observed in the population, but still does not explain the variation in phenotype observed among monozygotic twins (Weed et al., 2006).
Myopia, atopy, eye rubbing, eczema, Down syndrome, parental consanguinity, and connective tissue diseases among others, have been shown to have a positive association with KC (Gordon-Shaag et al., 2015; Patel and McGhee, 2013). A negative association has been reported between KC and diabetes (Kosker and Gurdal, 2016; Naderan et al., 2016), leading to the hypothesis that chronic higher levels of blood glucose results in glycosylation of the corneal fibrils which induces natural collagen cross-linking (CXL) and thereby stiffening the cornea, reducing the risk of KC development (Goldich et al., 2009; Seiler and Quurke, 1998).
2.1. Socioeconomic burden and quality of life
Because KC usually develops during adolescence it often affects the patients and the societies they live in during the most productive years of their life span. Studies have reported that the disease has a significant impact on the patient vision-related quality of life (VRQoL) when compared to healthy individuals, and a considerable number of KC patients report a continued decline in VRQoL over time (Kymes et al., 2008; Kymes et al., 2004).
Studies related to age and life expectancy have been conflicting. Some studies have found significantly fewer KC patients over the age of 50 years in their population (Pobelle-Frasson et al., 2004; Ertan and Muftuoglu, 2008). In fact, one study found that 40% of their KC patients were over 50 years of age. In contrast, others observed mortality rates of KC patients in their clinic to be similar to the general public, however only partially corrected for social class (Moodaley et al., 1992; Yildiz et al., 2009).
From an economic point of view, KC has been shown to cost north of $25,000 more than providing care for an individual over a 37-year period in the United States. Penetrating Keratoplasty (PK) and potential postsurgical complications are responsible for more than half of these costs (Rebenitsch et al., 2011). Advances in treatment options may reduce the cost in the future. CXL has been shown to have a favourable cost-effectiveness ratio compared to PK (Leung et al., 2017; Godefrooij et al., 2017). The cost-effectiveness of DALK compared to PK has also been studied. Though DALK has fewer complications it is also more expensive, and therefore clinical decisions are made based on cost rather than possible long term benefits. (Koo et al., 2011, van den Biggelaar et al., 2011).
2.2. Manifestations and early signs
The symptoms and signs of KC development are highly variable though their prevalence increases with progression. The first symptom noted by patients is reduced uncorrected visual acuity in the sense that objects become blurred and distorted in one direction, resulting in frequent change of glasses or contact lens refraction. Glare is often noted at relatively early stages, especially taillights display glare when driving at night. At later KC stages, glare may also appear in daylight. In addition, photophobia and monocular diplopia are cardinal signs of KC (Rabinowitz, 1998). Slit lamp examination may reveal several characteristic signs: stromal thinning at the apex of the cone (Fig. 2A), Munson’s sign; a V-shaped conformation of the lower eyelid produced by the ectatic cornea in downgaze (Fig. 2B), Paracentral stromal scars (Fig. 2C), Fleischer’s ring: iron deposits partially or completely surrounding the corneal cone (Fig. 2D), Vogt’s striae; fine vertical lines in the deep stroma and Descemet’s membrane that parallel the axis of the cone (Fig. 2E), and breaks in Descemet’s membrane with stromal permeation of aqueous through these breaks, a severe condition known as corneal hydrops (Fig. 2F). It’s very important to note that absence of slit lamp signs of KC does not exclude absence of the disease.
Fig. 2.
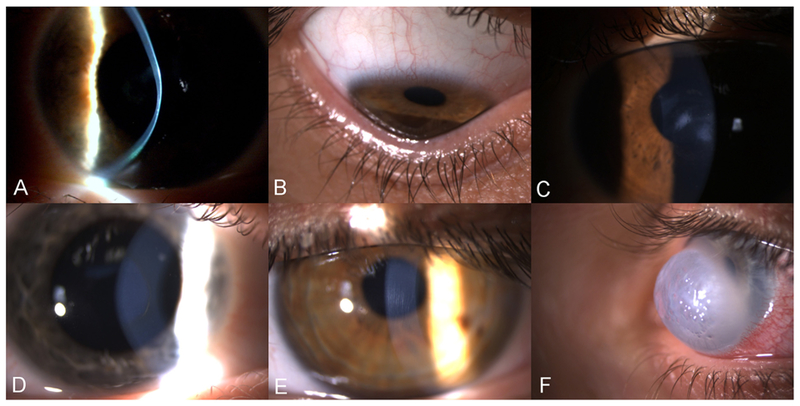
Slit lamp images of KC signs. A: Corneal thinning at the apex of the cone. B: Corneal ectasia, with indentation of the inferior eyelid upon down gazing C: Paracentral stromal scars. D: Fleischer’s ring, a pigmented, often incomplete line of iron deposits running around the base of the cone. E: Vogt’s stria: fine vertical lines, which are breaks in the deep stroma and Descemet’s membrane. F: Corneal hydrops, the most acute presentation of KC. Diffuse stromal opacity and edema, caused by breaks in the Descemet’s membrane leading to influx of fluid in the stroma.
2.3. Clinical diagnosis
It is relatively easy to diagnose KC in advanced stages, but difficult in the initial phase where the cornea looks relatively healthy. Most clinicians agree that KC should be suspected in patients with higher degrees of astigmatism, astigmatism with oblique axis, as well as when progression in astigmatism and/or spherical refraction occurs (Karimian et al., 2008; Serdarogullari et al., 2013) Steep corneal curvature and a lack of 20/20 visual acuity in a non-amblyopic eye with optimal refraction, should also raise suspicion.
To-date, several methods exist for detecting early KC. The characteristic scissor movement upon retinoscopy has been used to diagnose KC. In 1938, Amsler used the simple photographic placido disk to show corneal topographic changes in early KC (Amsler, 1946). Almost 40 years later, placido-based corneal topography digitalized the placido technology, which is based on the reflection of concentric rings off the corneal surface (Klyce, 1984). More recently, advanced tomographic analysis of the cornea has become available. Tomographic analysis constitutes a three-dimensional re-creation of the anterior segment including: anterior and posterior cornea surfaces, corneal thickness analysis, anterior iris and lens. In contrast, corneal topography is restricted to measuring only the anterior corneal surface. Tomography can be obtained as; anterior segment optical coherence tomography (Fig. 3); comparing healthy human cornea (Fig. 3A) and KC cornea (Fig. 3B), Scheimpflug optical cross-sectional analysis (Pentacam; Fig. 4); comparing healthy human cornea (Fig. 4A) and KC cornea (Fig. 4B), or anterior segment ultrasonic bio microscopy (Belin and Khachikian, 2006).
Fig. 3.
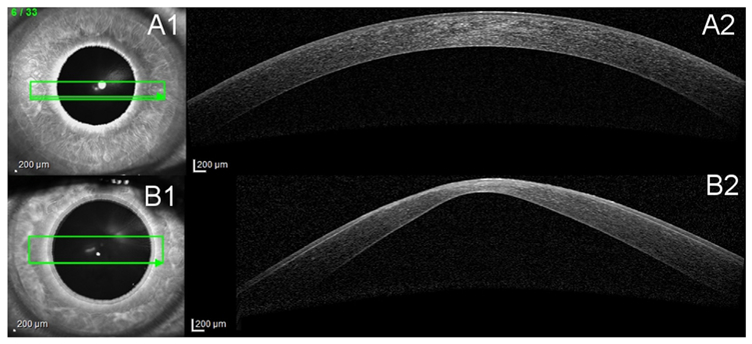
Anterior cross-section Optical Coherence Tomography (OCT). A1 and B1 show the scan location on the cornea. A2 and B2 show the cross-section view of the cornea. A: Healthy cornea. B: Severe KC cornea, characteristic protrusion and thinning as well as scarring at the top of the cone.
Fig. 4.
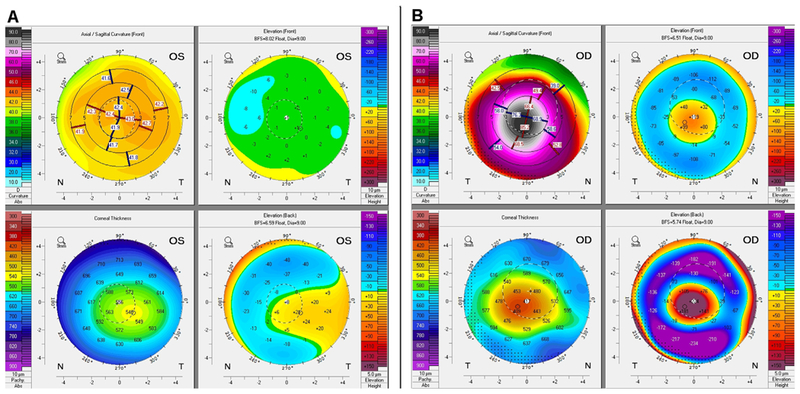
Four image tomography map from Pentacam® HR. A: Healthy cornea B: KC with severe ectasia and thinning, sagittal curvature (upper right), anterior elevation subtraction maps (upper left), corneal thickness (lower right) and posterior elevation subtraction map (lower left) are shown.
The 2015 Global Consensus on Keratoconus and Ectatic Diseases involving 45 KC clinical experts from around the world agreed that abnormal posterior elevation, abnormal corneal thickness distribution, and clinical non-inflammatory corneal thinning are required to diagnose KC. In terms of progression, the Consensus agreed that a change above the imprecision of the testing system in at least two of the three parameters (steepening of the anterior corneal surface, steepening of the posterior corneal surface or thinning and/or an increase in the rate of corneal thickness change from the periphery to the thinnest point) was required to document KC progression. It was also noted that a decrease in best spectacle visual acuity was not a requirement to document progression. Interestingly, the Consensus further concluded that currently no adequate classification system for KC exists (Gomes et al., 2015). Several classification systems have been proposed over the years. The historical Amsler-Krumeich classification divides KC into 4 stages based on: Spectacle refraction, central keratometry, slit-lamp examination for corneal scarring and central corneal thickness (Table 1) (Amsler, 1946; Rabinowitz, 1998). A major argument against this classification is its lack of specificity in early stages as well as lack of inclusion of the posterior curvature (Gomes et al., 2015). The large US-based CLEK study proposed the Keratoconus Severity Score. The KSS is based on slit-lamp signs, topography pattern, corneal scarring, average corneal power and higher-order corneal surface wave front root mean square error (Table 2) (McMahon et al., 2006). The newest severity index, the ABCD grading system, is connected to the Oculus Pentacam tomographic measurements. Unlike earlier proposed classification systems the ABCD incorporates data from the posterior corneal curvature as well as analysis of the thinnest point of the cornea as opposed to central cornea thickness (Table 3) (Duncan et al., 2016).
Table 1.
The Amsler-Krumeich classification system.
| STAGE | Mean keratometry reading | Thickness | Spectacle refraction | Slit lamp |
|---|---|---|---|---|
| 1 | Mean central K readings < 48 D | Myopia, induced astigmatism, or both < 5.00 D | Eccentric steepening | |
| 2 | Mean central K readings < 53.00 D | Corneal thickness > 400 micron | Myopia, induced astigmatism, or both from 5.00–8.00 D | Absence of scarring |
| 3 | Mean central K readings > 53.00 D | Corneal thickness 300–400 micron | Myopia, induced astigmatism, or both from 8.00–10.00 D | Absence of scarring |
| 4 | Mean central K readings > 55.00 D | Corneal thickness < 200 micron | Refraction not measurable | Central corneal scarring |
Table 2.
Keratoconus Severity Score. Instructions for use: (**higher-order first corneal surface wave front root mean square error). For grades 0–1, all of the parameters in a category must be met. For all grades, the required features must be met. The worst of the additional features is then assessed, with the “worst” of the features carrying the greater weight (as long as the required features are met).
| Grade | Stage | Corneal scarring consistent with KC | Slit-lamp signs consistent with KC | Axial Pattern | Other Features |
|---|---|---|---|---|---|
| 0 | Normal topography | None | None | Typical | Average corneal power (ACP) ≤ 47.75 D, Higher-order RMS error** ≤ 0.65 |
| 1 | Atypical Topography | None | None | Atypical:
|
ACP ≤ 48.00 D Higher-order RMS error ≤ 1.00 |
| 2 | Suspect Topography | None | None | Isolated area of steepening:
|
Additional features: ACP ≤ 49.00 D or Higher-order RMS error > 1.00, ≤ 1.50 |
| 3 | Mild disease | None | Possible | Consistent with KC | Additional features: ACP ≤ 52.00 D or Higher-order RMS error > 1.50, ≤ 3.50 |
| 4 | Moderate disease | Add features: Corneal scarring and overall CLEK grade up to 3.0 | Must have | Consistent with KC | Additional features: ACP > 52.00 D, ≤ 56.00 D or Higher-order RMS error > 3.50, ≤ 5.75 |
| 5 | Severe disease | Add features: Corneal scarring CLEK grade 3.5 or greater overall | Must have | Consistent with KC | Additional features: ACP > 56.00 D or Higher-order RMS error > 5.75″ |
Table 3.
The ABCD grading system; Scarring – clear, no scarring (−), scarring, iris details visible (+), scarring, iris obscured (++).
| ABCD criteria | A | B | C | D | Scarring |
|---|---|---|---|---|---|
| Anterior Radius of Curvature (ARC) 3.0 mm zone | Posterior Radius of Curvature (PRC) 3.0 mm zone | Thinnest Pachymetry | Best Corrected Distance Visual Acuity (BDVA) | ||
| 0 | > 7.25 mm (< 46.5 D) | > 5.90 mm | > 490 | ≥20/20 (≥1.0) | - |
| 1 | > 7.05 mm (< 48.0 D) | > 5.70 mm | > 450 | < 20/20 (< 1.0) | −, +, ++ |
| 2 | > 6.35 mm (< 53.0) | > 5.15 mm | > 400 | < 20/40 (< 0.5) | −, +, ++ |
| 3 | > 6.15 mm (< 55.0 D) | > 4.95 mm | > 300 | < 20/100 (< 0.2) | −, +, ++ |
| 4 | < 6.15 mm (> 55.0 D) | > 4.95 | ≤ 300 | < 20/400 (< 0.05) | −, +, ++ |
2.4. Management and treatment options
2.4.1. Treatment according to severity
The treatment of KC depends on the severity and the rate of progression. The initial treatment is spectacles, but with progressive disease spectacles rapidly become insufficient. The next treatment level is contact lenses. With the right choice of contact lense and correct fitting, contact lenses can often provide good visual acuity in the early stages before central scarring. Rigid Gas-Permeable (RGP) contact lenses are the most widely used (Fig. 5A, Fig. 5D). The comfort of RGP lenses can however be a challenge, especially in eyes with steep corneal curvatures. This has led to the development of several other types of contact lenses. Hybrid contact lenses with a rigid center and a softer periphery (Fig. 5B, Fig. 5D), Scleral contact lenses (Fig. 5C, Fig. 5D), and soft contact lenses designed specifically for KC (Visser et al., 2016).
Fig. 5.
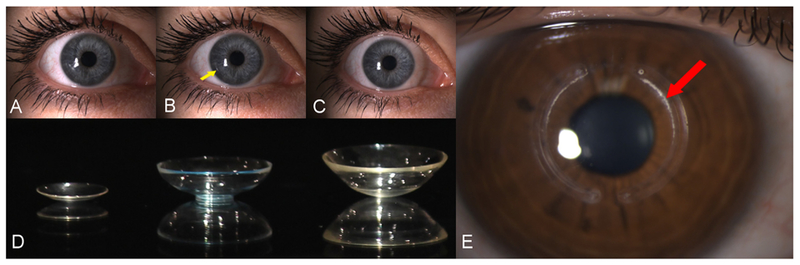
Contact lenses and Intra-corneal ring segments (ICRS). A: Rigid-gas permeable lens B: Hybrid lens with rigid center and soft periphery. Notice transition between rigid and soft (arrow) C: Scleral contact lens. D: Lenses A-C out of eye. E: ICRS. Notice deposit on inner arc (arrow).
If contact lenses are not tolerated and the disease is stable, intracorneal ring segments (ICRS) (Fig. 5E) are a surgical option to improve visual acuity (Vega-Estrada et al., 2015). ICRS consists of one or two semi-circle segments, operated into a surgically created tunnel in the mid corneal stroma. The ICRS are typically placed at the site of the steepest curvature and are designed to take up space in the stroma, thereby contributing to a decrease in curvature (Colin et al., 2000). Other means of improving visual acuity in contact lenses intolerant patients include Topography Supported Custom Ablation Photo-refractive keratotectomy. Studies have shown TOSCA PRK to be a viable option in selected patients (Guedj et al., 2013). Furthermore, TOSCA PRK has been successfully combined with CXL to treat both progression and refractive error (Grentzelos et al., 2017; Kanellopoulos, 2009).
If KC progresses to the most severe stage with excessive ectasia, thinning, scarring, and thereby decreased visual acuity refractive to the above mentioned refractive measures, corneal transplantation is the last resort. Corneal transplantation can be performed as either deep lamellar anterior keratoplasty (DALK) or conventional PK (Castroviejo, 1949; Melles et al., 1999; Anwar & Teichmann, 2002). Sparing of the patients endothelial cells is an important advantage of DALK technique; however, major side effects of keratoplasty are a risk for high degree of astigmatism and a long recovery time. Keratoplasty is intervening in the patient’s daily life and is a very expensive treatment from the society’s point of view (Roussy et al., 2009). A way of avoiding the long recovery time following DALK or PK may be the transplantation of a Bowman’s layer into a deep stromal pocket. Van Dijk et al. showed promising results with this procedure in 20 eyes with progressive KC. Maximum keratometry was significantly reduced and acceptable contact lens corrected vision was preserved in all 20 eyes in the average 20-month follow-up period though only a marginal improvement in visual acuity was observed (Van Dijk et al., 2015).
2.4.2. Treatment of progression
In the past, progression of KC was considered unavoidable and patients were left with nothing more than to wait and see the level of progression and the associated decrease in visual acuity. With today’s technological advancements, a lot has changed. CXL treatment was introduced by Spoerl et al., in 1998, and was FDA-approved in the United States in 2016 (Spoerl et al., 1998).
CXL is a photochemical treatment of KC, consisting of loading of the corneal tissue with the photosensitizer riboflavin followed by irradiation with UVA-light. CXL has been shown to stiffen the cornea and halt the progression of KC (Caporossi et al., 2010; Wollensak et al., 2003). Riboflavin is excited by UVA-light resulting in the formation of free oxygen radicals, and the free oxygen radicals react with collagen molecules and induce covalent bindings. The nature of the covalent bindings is still not fully understood (Sharif et al., 2017), but they have been shown to occur within the collagen molecules, between molecules in the fibril, between collagen and proteoglycans, as well as between two proteoglycan molecules (McCall et al., 2010). Furthermore, CXL was shown to induce resistance against collagenase digestion (Spoerl et al., 2004) which may be of importance in KC patients as their corneas have been shown to have increased expression of collagenolytic matrix metalloproteinases (Mackiewicz et al., 2006).
Clinically, the main objective of the treatment is to stop the progression of KC, preserving the patient’s present visual acuity, and avoiding corneal transplantation in the future (Godefrooij et al., 2017). Several studies have reported improvements in visual acuity following CXL (Ivarsen & Hjortdal, 2013; Hersh et al., 2017). Direct illumination of UVA-light towards the eye has raised concerns with regards to the safety of CXL. Several studies have now shown that CXL is a safe procedure, when certain limits are applied (Caporossi et al., 2010; Hashemi et al., 2013a,b). The diffusion of riboflavin is a key safety component, as riboflavin limits the reach of the UVA irradiation to approximately 200 μm (Sondergaard et al., 2010). Studies of corneas fully loaded with riboflavin have shown that with a minimum central corneal thickness of 400 μm, the endothelium and other posterior structures are not damaged (Spoerl et al., 2011). Although the published literature demonstrates the efficacy of CXL, prospective studies that establish a cause-effect relationship are lacking and the molecular basis of the disease has yet to be determined.
In 2012, our group developed an in vitro model using human corneal fibroblasts (HCFs) and compared them to human keratoconus fibroblasts (HKCs) cultured in a 3-dimensional (3D) model (Fig. 6). This model helps compare the expression and secretion of specific extra-cellular matrix (ECM) components between HKCs and HCFs. For four weeks the cells are stimulated with a stable Vitamin C (VitC) and allowed to secrete and assemble their own ECM. This model has since been spearheading the KC scientific field. More recently, we utilized this 3-D in vitro model to determine the CXL cellular and molecular effects (Sharif et al., 2017). Data from our study revealed that corneal fibrosis markers Collagen III and α-SMA were significantly down-regulated in HKCs post CXL. Furthermore, a significant downregulation was seen in phosphorylated SMADs −2 and −3 expression in HKCs post-CXL, contrary to a significant upregulation in Lysyl oxidase (LOX) expression compared to HCFs. These findings suggest positive impact of CXL on the corneal stroma at the cellular level, which is critical to sustain corneal structure and visual acuity. One of the limitations of this model, and therefore this study, is that only the stromal layer was investigated. Ongoing studies are utilizing co-cultures to include the epithelial layer as well. An in vitro model is useful for investigating KC-related cellular and molecular mechanisms, especially in absence of a suitable animal model. Howeverthis model is not a substitute for human/clinical studies.
Fig. 6.
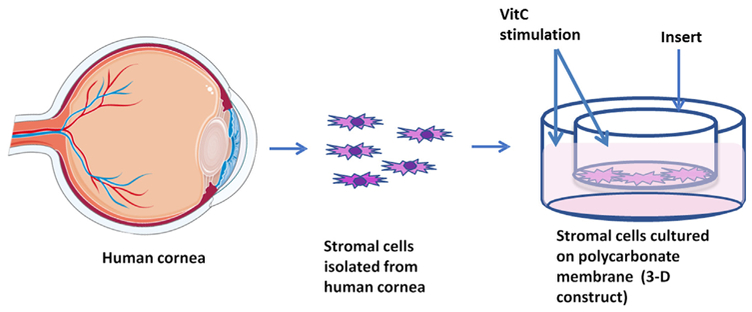
Illustrative image of our 3-D in vitro model. Cells are harvested from donor corneas and grown on polycarbonate membranes with vitamin C stimulation.
3. Pathobiology of Keratoconus
3.1. Structural changes in KC cornea
One of the first theories for the onset of KC was that a defect in the Bowman’s layer of the cornea served as the primary insult, resulting in the release of proteolytic enzymes by epithelial cells, leading to degradation of stromal collagen and eventual weakening of the cornea (Kennedy et al., 1986). The origin of the primary insult was and remains unknown, making this theory hard to prove. However, involvement of epithelial cells in the disease process is supported by structural abnormalities observed by light microscopy (Krachmer et al., 1984).
Stromal changes in the KC cornea have been extensively reported in the literature and whether the corneal stroma is responsible for KC is an ongoing debate. Several studies have shown that stromal thinning occurring in the KC cornea is related to loss of stromal tissue (Shapiro et al., 1986); (Rabinowitz et al., 2005); (Rabinowitz, 1998); (Morishige et al., 2007); (Mathew et al., 2011). However, the mechanisms attributing to corneal structural loss and corneal thinning are still obscure.
3.2. KC associated proteome and extracellular matrix remodeling
Analyses of tears and corneal tissues from KC patients have identi-fied differential expression of several proteins compared with healthy controls. These studies have highlighted the involvement of scarring and apoptosis in the disease process. However, it is unclear whether the pathways are modified as a primary or secondary phenomenon. Evaluation of tear fluid has shown > 1500 proteins present in the tears (Zhou et al., 2012). Proteomic studies of tears from KC patients have established the presence of numerous elevated inflammatory markers, including TNF-α, interleukin-6 (IL6), interleukin-17 (IL17), and inter-cellular adhesion molecule-1 (ICAM-1) (Maertzdorf et al., 2002), implicating inflammatory processes in the pathogenesis of KC (Jun et al., 2011; Lema et al., 2009). Stromal degradation and thinning is one of the most important aspects of KC. Multiple studies relate the thinning to increased levels of proteolytic enzymes and decreased levels of their inhibitors (Wisse et al., 2015). Various studies suggest that there may be an imbalance between pro-inflammatory and anti-inflammatory cytokine leading to altered epithelial and stromal functions (Jun et al., 2011; Wisse et al., 2015).
Proteomic studies of the KC epithelial and stromal layers have demonstrated that structural remodeling and metabolic stress occur in both layers (Chaerkady et al., 2013). In addition to evidence from tear proteomics, tissue studies have highlighted biochemical abnormalities in KC, with reports of decreased, increased, or even normal levels of proteoglycans (Critchfield et al., 1988; Garcia et al., 2016; Khaled et al., 2017; Sawaguchi et al., 1994). These studies indicate a possible disruption in the molecular mechanisms regulating ECM homeostasis. This might be initiated by an increase in proteases, or a decrease in proteinase inhibitors such as α1-antiprotease and tissue inhibitors of metalloproteinases (TIMPs) (Pescosolido et al., 2014). Kenney and co-authors (Kenney et al., 1997) reported dysregulation of ECM and basement membrane components in KC corneas using immuno-fluorescence. The authors concluded that abnormal expression of specific proteins in the KC cornea were not uniform. The study interestingly split the corneas into three distinct regions: nonscarred anterior cornea, scarred anterior and posterior cornea, and Bowman’s layer. Amongst others, nonscarred regions showed decreased staining of entactin/nidogen, fibronectin, alpha 3-alpha 5 chains of type IV collagen. Scarred regions showed increased laminin-5 and perlecan, where the Bowman’s layer showed type VIII collagen, fibrillin-1, and tenascin-C expression.
3.3. KC associated biomechanics
Several studies suggest that thinning of the KC cornea might be occurring due to a defect of collagen lamellae formation (Chen et al., 2015; Mathew et al., 2015; White et al., 2017). The different distribution of stromal lamellae in KC compared to normal corneas has been suggested as a precursor for corneal thinning and KC development (Khaled et al., 2017).
Oxidative damage has been described as a co-factor in KC progression. The level of reactive oxidative species (ROS) in normal corneas is regulated by antioxidant defence mechanisms, and some studies suggest that antioxidant enzyme activity levels are altered in KC (Atilano et al., 2005; Wojcik et al., 2013). A decreased activity of extracellular superoxide dismutase (SOD) in KC corneas was found when compared to healthy controls (Behndig et al., 2001; Olofsson et al., 2007), most likely leading to an increased amount of superoxide radicals (Zelko et al., 2002). Furthermore, KC corneas exhibited a reduced level of aldehyde dehydrogenase Class 3 (ALDH3) that detoxifies reactive aldehydes produced by UV-induced lipid peroxidation (Wojcik et al., 2013).
The important factors related to increased oxidative damage are atopy, ultraviolet radiation and mechanical trauma; the latter could occur as a result of chronic eye rubbing and contact lens wear (Gordon-Shaag et al., 2015). Contrary results have been reported in the literature with regards to whether or not atopy is associated with KC pathogenesis (Lowell and Carroll, 1970), as KC individuals appear to rub their eyes much more frequently than healthy individuals (Galvis et al., 2017). Previous studies have reported differences in topographic measurements (Shajari et al., 2016) as well as differences in progression between KC patients, with and without atopy (King et al., 1999). Interestingly, the latter study found that KC patients with atopy tend to have faster KC progression and more frequent refractive and immunologic complications leading to an earlier need for keratoplasty. Contact lens wear has also been associated with KC progression (Romero-Jiménez et al., 2015). Various studies have shown that both KC patients and otherwise healthy contact lens wearers both have significantly reduced corneal thickness compared to healthy controls and non-contact lens wearers (Pflugfelder et al., 2002). Contact lenses are also known to reduce the amount of oxygen that reaches the cornea leading to a hypoxic environment and potential corneal edema (Holden and Mertz, 1984). This is sharply contrasted in studies by Wagner et al., and Zadnik et al., who observed no association between low patient-reported rigid contact lens comfort and severe disease (Wagner et al., 2007; Zadnik et al., 1998). Acute hypoxia was shown to influence ECM structure in corneal fibroblasts with modulation of MMP expression and collagen secretion (McKay et al., 2017); however, whether contact lens wear could trigger KC development, or not, remains unclear.
3.4. Genetics and KC
KC has been associated with a wide range of ocular, systemic, and genetic conditions including anterior polar cataract, Down syndrome, Ehlers Danlos syndrome, osteogenesis imperfecta and mitral valve prolapse (Wheeler et al., 2012).
Genetic risk factors and specific genes for KC (Table .4) have proven challenging to identify because of the complicated nature of the disease (Nielsen et al., 2013). Various genetic approaches have been utilized including genome-wide association studies (GWAS) and linkage studies in families with suspected dominant inheritance of KC to identify risk loci. The first genetic study attempting to reveal the aetiology of KC dates back to 1992 (Rabinowitz et al., 1992). Rabinowitz et al. selected COL6A1 as a segregation marker for KC to determine whether a mutation in COL6A1, mapped to locus 21q22.3, contributes to the development of KC. Based on the observation that KC has higher prevalence (0.6–16%) in patients with Down syndrome (trisomy 21), this study did not show linkage to the most telomeric region of chromosome 21. However, linkage to another locus on chromosome 21 was documented seven years later in the studied family (Kim et al., 1999). Another candidate gene is the superoxide dismutase isoenzyme 1 (SOD1), located on chromosome 21. This gene was considered a possible candidate gene because oxidative stress is hypothesized to have a role in the aetiology of KC and is also linked to an increased prevalence of KC in patients with Downs syndrome (trisomy 21) (Bykhovskaya et al., 2016). Similarly, other studies have investigated the transcription factor visual system homeobox 1 (VSX1), a gene implicated in posterior polymorphous corneal dystrophy (PPCD). PPCD patients have localized steepening of the anterior cornea similar to KC, however, pathogenic mutations of this gene have been identified in less than 2% of KC patients suggesting that this gene does not have a major role in the molecular pathology of KC. In addition, a mutation in the micro-RNA (miRNA) gene MIR184 (OMIM 613146) was considered to play a role in KC. MIR184 is abundantly expressed in the cornea and lens epithelia, and mutation in the seed region of MIR184 potentially affects its function (Abu-Amero et al., 2015). Moreover, subsequent studies identified this same mutation in families with EDICT syndrome (endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning), and dominant congenital cataract associated with corneal phenotype > (Hughes et al., 2011; Luo et al., 2017). However, a study of 780 KC patients identified MIR184 variants in only 0.25% patients suggesting that variants in MIR184 gene may not account for isolated KC (Abu-Amero et al., 2015; Lechner et al., 2013). Common polymorphic variants upstream of Zinc finger protein gene 469 (ZNF469) have also been associated with central corneal thickness. Rare ZNF469 variants have also been shown in KC patients (Lucas et al., 2017).
Table 4.
Genetic studies and genes involved in KC.
| Gene | SNP | SNP locus | Modality | Reference |
|---|---|---|---|---|
| LOX | rs10519694 | Intron | GWAS | Bykhovskaya et al., 2012 |
| rs2956540 | Intron | GWAS | Dudakova et al., 2015 | |
| HGF | rs3735520 | Dudakova et al., 2015 | ||
| WNT10a | rs121908120 | mis-sense F228I | GWAS | Cuellar-Partida et al., 2015 |
| MPDZ-NF1B | rs1324183 | Li et al., 2013 | ||
| ZNF469 | Multiple | Exon sequencing of ZNF469 |
Vincent et al., 2014 Yildiz et al., 2017 Valgaeren et al., 2018 |
|
| IL1a | rs2071376 | Intron | Kim et al., 2008 | |
| COL5A1 | rs1536482 | Fine-Mapping | Li et al., 2013 | |
| FOXO1 | rs2721051 | GWAS | Lu et al., 2013 | |
| PDGFRA | rs2114039 | GWAS | Mishra et al., 2012 | |
| BANP | rs9938149 | Intergenic | Allele specific | Sahebjada et al., 2013 |
| IL1b | rs16944 | Promoter | PCR | Kim et al., 2008 |
| rs1143627 | Promoter | |||
| VSX1 | R166W, L159M | Heon et al., 2002 | ||
| MIR184 | A (r.57 c > u) mutation in the micro-RNA (miRNA) gene MIR184 (OMIM 613146) |
Abu-Amero et al., 2015 Valgaeren et al., 2018 |
||
| Mitochondrial complex I genes, (MT-ND1, 2, 3, 4, 4L, 5, and 6), especially MT-ND5 | mitochondrial dna sequencing | Pathak et al., 2011 | ||
| SOD1 | 7-base deletion | Intron | Udar et al., 2006 |
TIMP3, transforming growth factor-β1 (TGFβ1), and several other collagen genes (Davidson et al., 2014) have also been studied for their role in KC. Despite these efforts, potentially pathogenic variants have only been identified in a very small number of individuals with KC (Bykhovskaya et al., 2016). Finally, the association of genetic variants in lysyl oxidase (LOX) with KC have also been reported (Bykhovskaya et al., 2012). However, the results remain inconclusive wih great heterogeneity (Zhang et al., 2016b). A comprehensive overview of genetics in KC is available by Valgaeren and co-authors (Valgaeren et al., 2018).
4. Sex hormones
Regulation of hormonal activity, in humans, is critical for a wide range of developmental and physiological processes, particularly sexual development and maturation. Sex hormones, such as progesterone, estrogen, and androgen, are major modulators of activity of the brain, gonadal tissue, and skin (Zhu and Cai, 2006). Clinical changes occur in men and women who experience an excess or depletion of sex hormones. The immune system is likewise significantly regulated by sex hormones both in normal maturation and disease. For example, Lupus flares in patients with Systemic Lupus Erythematosus (SLE), caused by use of oral contraceptives and administration of estrogen have been reported (Rojas-Villarraga et al., 2014). Furthermore, the association of SLE with Klinefelter’s syndrome, and its improvement following testosterone administration, also imply that sex hormones modulate the incidence or severity of SLE (Mok et al., 2001).
In terms of the ocular system, sex hormones are recognized for their critical role in regulating important body functions that affect the eyes, and when they change, so can visual acuity. From childhood to old age, everyone experiences hormone fluctuations. Once hormones stabilize, visual acuity is known to stabilize as well. Women of child-bearing age commonly have changes in visual acuity due to birth, contraceptive pills, or pregnancy (Gupta et al., 2005; Spoerl et al., 2007). In addition to birth control pills, other medications such as antidepressants, anti-anxiety medications, and antihistamines, can cause changes in visual acuity.
The two main classes of sex hormones are androgens and estrogens, of which the most important human derivatives are testosterone and estradiol, respectively. In general, androgens are considered “male sex hormones”, since they have masculinizing effects, while estrogens are considered “female sex hormones” although all types are present in each sex at different levels (Annibalini et al., 2014).
4.1. Androgens
Androgen is a natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors (AR) (Horstman et al., 2012). Gonadotropin-releasing hormone synthesized and released by the hypothalamus, stimulates the production and release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the anterior pituitary (Horstman et al., 2012). FSH is primarily involved in sperm production and LH in testosterone secretion. LH enters the circulation and is transported to the gonads where it activates the synthesis and secretion of testosterone. The main production of androgen occurs in the Leydig cells of the testes, and men have a 25-fold higher testosterone production when compared with women (Yeh et al., 1998). The physiological effects of testosterone are induced by its binding to the intracellular AR, which is then translocated to the nucleus where the AR–testosterone complex induces transcription of specific genes. Testosterone imparts multiple physiological effects including involvement in testicular function, spermatogenesis, hair growth, muscle mass and distribution, bone density, libido, and secondary sexual characteristics.
4.2. Estrogen
Estrogens are a specific category of steroid molecules secreted mainly by the ovaries, but also by peripheral steroidogenic conversion, the placenta and by the testes in men. Women have a higher amount of estrogens compared to men. Estrogens promote the development of female genital organs, and growth of the endometrium, but also, inhibit the secretion of FSH by the pituitary. The important actions of the endogenous estrogens are mediated by estrogen receptors (ERs) (Ogueta et al., 1999). ERs are synthesized in many cell types in two protein forms, ERα and ERβ, functioning as transcription factors once bound to their ligand. ERα is expressed in various tissues including uterus, ovary, testes, bone, and breast, and ERβ is expressed in testes, colon, salivary gland, bone marrow. Moreover, estrogens have also been shown to induce a wide variety of biological effects in many physiological systems in both men and women (Gupta et al., 2005).
Estrogens diminish post-injury disruption and inflammatory responses and may play a protective role against inflammation and oxidative stress. Moreover, myosin function is affected by age and by estradiol level in women. Estrogens and ERs in the skeletal muscle cells of women regulate carbohydrate and lipid metabolism (Horstman et al., 2012). Estradiol also promotes cell activation and proliferation, thereby enhancing the growth and recovery potential of cells (Horstman et al., 2012; Yeh et al., 1998).
4.3. Sex hormones and the human eye
The last two decades have seen remarkable advancements in endocrinology. Yet, a wide gap still lingers in our knowledge regarding the gender-based differences and hormonal receptors in the ocular tissue. The anatomical, developmental, and physiological ocular parameters differ in males and females. Sex hormones may be related to various ocular pathologies, as they can act through sex hormone receptors present in the ocular tissue (Gupta et al., 2005). Any pathology affecting the levels of these sex hormones will most probably affect the ocular tissues as well.
Various studies have reported a significant association between hormone levels and eye diseases. As an example, it has been shown that androgen controls the various physiological and biochemical aspects of the lacrimal apparatus and has been shown to be altered in both males and females. The observations have been further strengthened by the increased association of dry eye during pregnancy and lactation (Horstman et al., 2012). Dry eye is defined as a multifactorial disease, which affects the tears and ocular surface, resulting in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface (Schein et al., 1997). Individuals aged 65–84 years often report symptoms of dry eye (Chia et al., 2003). Moss et al. reported the prevalence of dry eye to be 14.4% in 3722 subjects aged 48–91 years and noted that the prevalence of the condition increased after the age of 59 (Moss et al., 2000). Schein et al. in contrast, found no correlation between dry eye and age or sex, while other researchers have reported such associations to exist (Schein et al., 1997).
During menstruation and menopause, the conjunctival tissue shows cyclic variations in conjunctival epithelium. Even the maturity of the conjunctival tissue strongly correlates with the levels of estrogen. Various studies have observed a significant relationship between altered corneal functions, sex hormones, and topography under different physiological conditions (Goodman-Gruen and Barrett-Connor, 2000). The changes in the corneal curvature and thickness during pregnancy, premenstrual phase, and lactation can result in visual changes and disturbances (Raiskup-Wolf et al., 2008).
4.4. Sex hormones in the human cornea
Corneal thickness alterations influenced by hormonal changes during the menstrual cycle have been reported in women. Manchester et al., first reported a change in corneal hydration during the menstrual cycle in ovulating women (Manchester, 1970). Kiely et al., found cyclic change in corneal thickness during the menstrual cycle, and thickening of the cornea at ovulation (Kiely et al., 1982). Soni et al. revealed that female corneas attained minimal thickness just before ovulation and maximal thickness at the beginning or end of the menstrual cycle (Soni, 1980). In addition, Weinreb et al. provided evidence that corneal thickness increases during pregnancy (Weinreb et al., 1988). Taken together, these reports indicate a strong association between corneal thickness and female hormonal regulation, in particular estrogens (Wang et al., 2017). However, it is not clear whether hormonal influence is exerted through direct interaction within the cornea, or via secondary effects such as systemic water retention by estrogen-induced upregulation of the renin-aldosterone system (Choudhary et al., 2017; Goldich et al., 2011). Interestingly, estrogen deficiency is also associated with increased incidence of macular degeneration in post-menopausal women as has been observed by the Eye Disease Case–Control Study Group.
4.5. Sex hormones and Keratoconus
Clinical information related to sex hormones for patients with KC is often scarce, presenting a significant limitation to further investigations. Recent studies have started highlighting the effects of hormonal changes on corneal structure and KC. In 2010, Fink et al. studied the effects of hormone status and gender on KC severity and progression in both men and women over a 3-year period (Fink et al., 2010). Results from this study revealed that KC progressed in both men and women, aged 48–59 years; however, there were no differences among the groups in progression. Various studies have reported that, during the gestational period, women have experienced a significant progression in KC, as indicated by reversible fluctuations in corneal topography and a decrease in corrected distance visual acuity (Hoogewoud et al., 2013; Pizzarello, 2003). This has led to the suggestion that pregnancy may be considered a risk factor for KC (Bilgihan et al., 2011; Khaled et al., 2017), and has implicated the role of hormone changes. Furthermore, hormonal alterations that occur during pregnancy have a negative impact on corneal biomechanics. It is suggested that the stabilization in the corneal biomechanics during the last half of a normal pregnancy may be due to the action of progesterone, which is known inhibit collagenases via prostaglandin inhibition. This would suggest a protective role for progesterone during pregnancy (Koob et al., 1980), but no studies have delineate this mechanism.
Similarly, in vitro studies have identified a significant elevation in salivary dehydroepiandrosterone sulfate (DHEA-S, a common precursor to other androgens) levels and a decrease in estrone level in KC patients independent of gender (McKay et al., 2016). However, no correlation has been detected between the increased salivary DHEA-S level and increased severity of KC. These hormonal variations may induce significant alterations of the corneal bio stability and worsen underlying corneal ectasia (McKay et al., 2017; McKay et al., 2016).
The effects of sex hormones rely on the receptors present in specific tissues and organs. Corneal tissues express ERs, progesterone receptors, and ARs. We recently investigated the presence of these receptors in KC-derived cells, HKCs, for the first time. Preliminary data shows significant upregulation in ER (ESR1; Fig. 7A; p < 0.05) and a down-regulation of AR (AR; Fig.7B; p < 0.05) in HKCs, compared to HCFs. Interestingly, the mechanism through which hormones regulate corneal homeostasis still remains unclear. The knowledge about links between KC and hormones or hormone receptors is even more limited, and we hope to unravel that interplay.
Fig. 7.
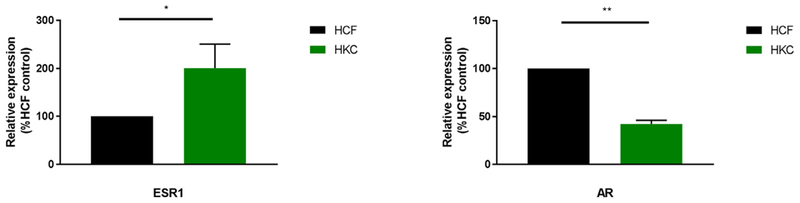
Quantitative Polymerase Chain Reaction (QT-PCR) showing significant upregulation in ER (ESR1; Fig. 7A; p < 0.05) and a downregulation of AR (AR; Fig. 7B; p < 0.05) in HKCs when compared to HCFs. Data shown is preliminary.
Based on the expression patterns of androgen and ERs in corneal tissue, it has been postulated that estrogens are supplied through tears and aqueous humour at concentrations that are approximately half the concentrations found in plasma. The proposed mode of action of these sex hormones is via the regulation of gene expression of resident cor-neal cells, leading to changes in the concentration of ECM proteins, which are critical to the maintenance of corneal integrity. It is plausible that estrogens are responsible for weakening the cornea via the stimulation of matrix metalloproteinases and the release of pros-taglandins, causing activation of proteolytic enzymes for collagen, disruption of collagen ECM, and reduction in corneal stiffness.
4.6. Exogenous hormone stimulation
Clinical data related to sex hormones for patients with KC is often limited and mechanisms through which hormones regulate corneal homeostasis still remains unclear. Based on the hypothesis that there is a critical link between altered hormone levels and KC, we recently reported the effects of exogenous hormones, such as androgens and estrogens, in HKC metabolism using our 3-D model. Our data showed that exogenous DHEA significantly downregulated the pentose phosphate pathway in HKCs, whereas 17β-estradiol led to a significant upregulation in pentose phosphate pathway and glycolytic intermediates in HCFs. These results highlight altered metabolism in HKCs, when compared to HCFs, that can be modulated by specific hormones and ultimately provide a path to novel therapeutics (McKay et al., 2017; McKay et al., 2016).
The effects of hormones in regulating genes associated with ECM deposition are also unknown. Our initial studies, where we stimulated the HCFs and HKCs with 2.5 ng/mL DHEA and 2.5 ng/mL estrone, investigated the expression of fibrotic Collagen type III (Col III) and cellular Fibronectin (cFN). Exogenous DHEA led to significant upregulation of Col III (Fig. 8A; p < 0.05) and downregulation of cFN (Fig. 8B; p < 0.05) in HKCs, while no significant modulation was noted in HCFs. Exogenous Estrone, on the other hand, led to significant down-regulation of Col III in HCFs but not in HKCs (Fig. 8A; p < 0.01), cFN was also downregulated in both HCFs and HKCs (Fig. 8B; p < 0.01). These preliminary studies suggest that DHEA may drive HKCs towards a fibrotic phenotype, whereas exogenous estrone seems to rescue them.
Fig. 8.
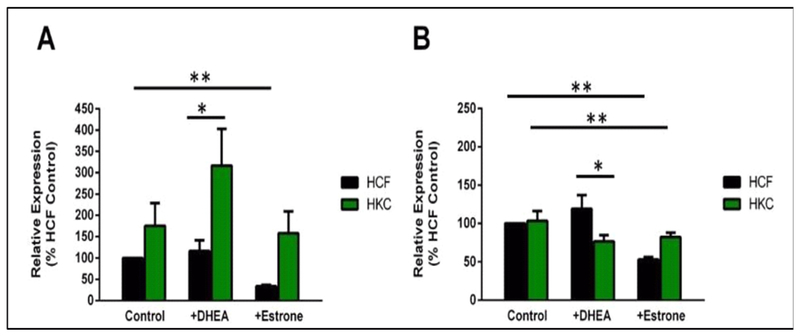
Quantification of Collagen type III (Col III) and cellular Fibronectin (cFN) protein levels in HCF and HKC 3-D constructs. (A) Col III was significantly upregulated in HKCs following exogenous DHEA (p < 0.05). Col III was significantly downregulated in HCFs following exogenous estrone (p < 0.01). (B) cFN was significantly downregulated in HKCs following exogenous DHEA (p < 0.05). cFN was significantly downregulated in both HCFs and HKCs following exogenous Estrone (p < 0.01). Values normalized to HCFs (n = 4). Data shown is preliminary.
Anticipating the hormone-KC connection, we recently utilized a whole-transcriptome high-throughput sequencing (RNA-seq), known to provide the most extensive evaluation with a wide dynamic range and higher sensitivity (Kukurba and Montgomery, 2015). During our preliminary studies we used RNA-seq to assess gene expression profiles of HKCs and HCFs. The objective of this study was to determine the key pathways altered in HKCs as compared to HCFs and begin to delineate their role in the disease. Fig. 9 shows a mechanistic network derived from gene expression profiles from three male and three female, age-matched, donors for HCFs (n = 6) and HKCs (n = 6). This network consists of genes (nodes) connected by lines (edges) indicating a known relationship/interaction in the IPA Knowledge Base. Genes more highly expressed in HKCs are coloured red; genes showing low expression in HKCs are coloured green. Upstream regulators and intermediate nodes (ERK, 17β-estradiol, EGF, SRC) were predicted to be inhibited (blue). The direction and nature of the relationship is also indicated. Based upon the observed gene expression profile, estrogen receptor was predicted to be a key upstream regulator of more than 1000 expressed genes. The actions of estrogen receptor were predicted to be activated in HKCs, whereas expression of downstream nodes STAT3, NFkB, RELA, and ESR1 were predicted to be inhibited in HKCs. While this network is only based on six healthy and six KC donors, it is a good starting point with regards to identifying the key pathways and the downstream players involved in the hormone-KC interplay. It also strengthens the hypothesis that sex hormones may be useful targets for KC treatments.
Fig. 9.

Mechanistic network derived from differential gene expression profile for three male and three female donors for HCFs (n = 6) and HKCs (n = 6), using whole-transcriptome high-throughput sequencing (RNA-Seq). Network shows genes (nodes) connected by lines (edges) indicating a known relationship/interaction in the IPA Knowledge Base. Data shown is preliminary.
5. Prolactin induced protein
Prolactin-induced protein (PIP) is a 17-kDa glycoprotein that was originally identified as gross cystic disease fluid protein 15 and a major component of human milk, breast cyst fluid, and saliva (Haagensen et al., 1980; Haagensen et al., 1979; Murphy et al., 1987). This secre-tory acinar glycoprotein typically localizes in the cytosol of apocrine epithelia in all major organs. PIP is expressed in the glandular epithelium of seminal vesicles, and as an extra parotid glycoprotein in the sublingual and submandibular glands (Caputo et al., 2003).
Several studies have shown that PIP is overexpressed in both primary and metastatic breast tumours, labelling it as a breast tumour marker (Caputo et al., 2000; Darb-Esfahani et al., 2014). PIP is only found to be expressed in apocrine metaplasia of the breast, ductal carcinoma in situ, and not secreted in normal ductal or lobular epithelium (Darb-Esfahani et al., 2014). Despite these findings, the exact functional capacity of PIP in breast cancer has remained obscure. Besides breast cancer, only few tumours, such as carcinomas of the skin appendages and prostate cancer express PIP (Caputo et al., 2003; Caputo et al., 2000). Thus, it is highly specific for mammary differentiation in females, and is frequently used as a prognostic marker for the evaluation of a potential mammary origin of metastatic carcinoma of unknown primary site (Wick et al., 1989).
Baniwal et al. revealed the requirement of PIP for the proliferation of tamoxifen-resistant breast cancer cells suggesting that PIP may be targeted in the treatment of breast cancer patients refractive to hormonal therapy. They observed that PIP silencing by siRNA, inhibited the proliferation of the tamoxifen-resistant T47D breast cancer cells (Baniwal et al., 2013). Furthermore, treatment of human breast cancer cell lines with exogenous recombinant PIP enhanced their proliferation, whereas PIP silencing in ER+ and ER-breast cancer cell lines inhibited cell proliferation and invasion through the ECM (Naderi and Meyer, 2012). Naderi et al. also demonstrated that PIP expression was associated with cell cycle genes and concluded that PIP is required for cell cycle progression in breast cancer (Naderi and Vanneste, 2014b).
PIP is known to be regulated by hormones such as androgens and estrogens (Naderi and Vanneste, 2014). Sex hormones are known to play a role in the maintenance of the structure and integrity of the human cornea (Zhang et al., 2017). Corneal thickness, curvature, and sensitivity were altered throughout the menstrual cycle (Ghahfarokhi et al., 2015). A suggestive link between hormones and corneal structure is suggested by the presence of hormones in the human tear film (Nebbioso et al., 2017). Surprisingly, the role of sex hormones in corneal diseases has been neglected. Our recent findings suggest an intriguing link between KC, PIP, and sex hormones, as discussed below.
5.1. PIP structure, regulation and biological role
PIP gene is located on chromosome 7q32–36, has four exons but only one 900 base mRNA transcript has been described (Naderi, 2015b). PIP is a 146-amino acid long polypeptide that is found in mammary gland, salivary and lacrimal glands, prostate, and other organs (Lonze and Ginty, 2002). Determination of PIP’s crystal structure (Fig. 10) revealed an immunoglobulin fold composed of seven anti-parallel beta-strands and seven loops (Hassan et al., 2009). PIP is shown to have aspartyl protease activity, which signifies the role of PIP as a secreted protein able to mediate ECM degradation (Naderi and Meyer, 2012).
Fig. 10.
Structure of the Prolactin-Induced Protein based PyMOL software (Hassan et al., 2008).
A positive feedback loop between PIP and ERK-Akt signalling was demonstrated by Naderi et al. in murine apocrine cells (Naderi, 2015). Following the induction of PIP expression by CREB1, secreted PIP mediates protease degradation of fibronectin into fragments, which results in the activation of integrin-β1 signalling (Naderi and Meyer, 2012b). Importantly, in the absence of PIP, there is a marked reduction of integrin-β1 binding to ErbB2 that can be reversed by the addition of fibronectin fragments. It is therefore clear that PIP expression plays a major role on cell invasion and the viability of apocrine cells (Naderi, 2015). PIP has also been shown to play an important role in immunoregulation. PIP binds to CD4 in T cells, and inhibits T cell apoptosis, suggesting an ability to modify the immune responses during tumours progression (Autiero et al., 1995; Wick et al., 1989). The abundance of PIP in mucosa, saliva, tears, submucosal glands of the bronchi, and apocrine glands of the skin, suggests that PIP has an important role in mucosal immunity (Hassan et al., 2009).
In both normal and diseased states, PIP expression is known to be regulated by androgen and prolactin hormones. Haagensen et al. (Haagensen et al., 1980) reported a ten fold upregulation of PIP levels in maternal plasma during the third trimester of pregnancy when compared to non-pregnant women. The expression of PIP in human breast cancer cell lines was shown to be up-regulated by prolactin, glucocorticosteroids and androgens, whereas, estrogens inhibited PIP expression (Carsol et al., 2002; Murphy et al., 1987), and has also been shown to be influenced by the cytokines IL-4/IL-13 (Blais et al., 1996). The production of PIP by breast cancer cell lines in response to androgen and glucocorticosteroids could be further differentially regulated by immune factors such as IL-1 and IL-6.
On the subcellular level, the signal transducer and activator of transcription 5 (Stat5) and Runx2 works through AR to regulate PIP expression (Debily et al., 2009; Haagensen et al., 1990; Naderi et al., 2012). However, further studies are required to understand the molecular mechanisms of PIP action and its translational implications in various tissues, specifically in breast tumours.
In addition, various studies revealed that the expression of PIP in neonatal and adult submandibular gland, parotid and sublingual gland is similar to that of salivary peroxidase (Bodner et al., 1983; Kruse et al., 1998; Moriguchi et al., 1995; Yamashina and Barka, 1973).
Observations in mouse models for Sjögren’s syndrome have shown that PIP may also bind to the C-terminal portion of aquaporin-5 (AQP5) leading to its physiological translocation from cytoplasm to the apical membrane of lacrimal glands (De Amicis et al., 2013). Thus, a deviant expression of PIP might negatively affect the trafficking of AQP5 from the cytoplasm to the membrane of the acinar cells, ultimately interfering with the glandular secretions from the salivary and lacrimal glands (Konttinen et al., 2005). Gallo et al. suggested that the production of PIP is significantly reduced in Sjögren’s syndrome shedding new light on the possible pathophysiological role of PIP in primary Sjögren’s syndrome exocrinopathy (Gallo et al., 2013).
5.2. Prolactin-induced protein and the human cornea
The human cornea plays an important role in maintaining the balance of ocular surface microenvironment (Nishtala et al., 2016). Complex array of biomolecules are secreted within the tear fluid, which are potential targets of prognostic value in ocular diseases. Tear fluid as a pool of biomarkers, in ocular and systemic conditions, has been well studied and shown to have translational potential (Hagan et al., 2016; von Thun Und Hohenstein-Blaul et al., 2013). Tear fluid can be collected in a painless, non-invasive fashion, making it an optimal biological fluid to analyse with minimal discomfort to the patient. Studies have been performed and several biomarkers have been suggested including PIP, zinc-alpha-2-glycoprotein (AZGP1) and lipocalin (LCN) (Lema et al., 2010; Nishtala et al., 2016).
In 2014, we identified PIP as a novel biomarker for KC based on our study on human tear samples from 17 healthy donors and 36 KC patients. Protein levels of PIP and AZGP1 have been found to be down-regulated in tears of KC patients by proteomic analysis, suggesting their application as prognostic markers for KC (Nishtala et al., 2016; Priyadarsini et al., 2014). Our proteomics data also showed that regulation of PIP is closely related to AZGPI glycoprotein of 35–44 kDa (Hassan et al., 2008). It has been reported that PIP and AZGPI form a complex in some body fluids, they both localize in close proximity in the same chromosome, and are both regulated by androgens (Murphy et al., 1987; Ueyama et al., 1994). These studies lead us to believe that PIP may important for KC, and a potential therapeutic target. PIP has also been reported to be decreased in studies of Sjögren’s syndrome–associated dry eye (De Amicis et al., 2013). Using mass spec-trometric analysis, tears of KC patients were compared with healthy controls and identified the elevation of matrix metalloproteinase 1 (MMP1), cytokeratins, and precursors to prolactin (Pertovaara et al., 2004). Moreover, Balasubramanium et al. revealed a reduction in total tear protein in KC patients (Balasubramanian et al., 2012). It has also been reported that PIP influences keratin production of stromal and epithelial cells of the cornea and could potentially result in changes of corneal structural stability (Stachon et al., 2017).
It is intriguing to think of PIP as a potential biomarker for KC, given how tightly it is regulated by hormones and how hormones affect KC. In order to further our investigation on this protein, we utilized STRING database to determine the predicted protein-protein interactions using functional clustering. The correlations of these over-expressed membrane proteins are shown in Fig. 11. Several proteins involved in protein binding, peptidase activity, and metallo-endopeptidase activity as derived from the STRING database. Interestingly, but not surprisingly, AZGP1, PGR, ESR1, and AR, were within the top ten highest protein-interaction scores (0.98, 0.96, 0.95, 0.95, and 0.8 respectively). These findings highlight the strong link that exist between PIP, hormones, human cornea, and KC.
Fig. 11.
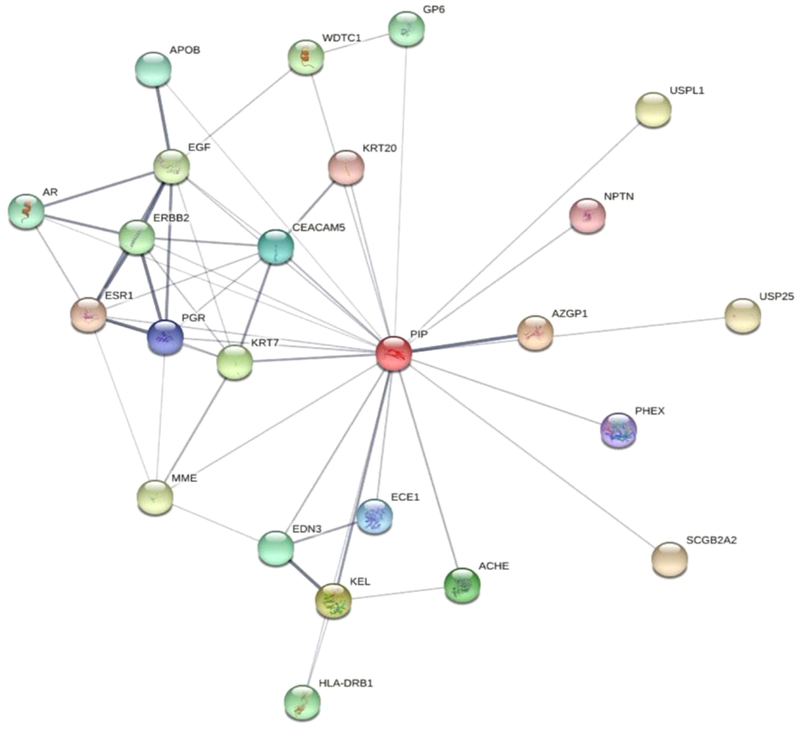
Network view of the predicted interactors of Prolactin-Induced Protein: STRING database, illustrates the display of Protein-Protein interactions through a Java applet.
5.3. Prolactin-induced protein: diagnostic value of saliva in KC
Early disease detection is necessary for early intervention, which increases the chance of successful treatment. This is especially critical for KC given the limitation on diagnosing it at onset. Saliva is a fluid of high prognostic value with many advantageous properties. Collecting saliva is a simple, non-invasive, and low-cost method for disease screening and detection. Saliva is currently used as a body fluid for screening for various local and systemic diseases. Like serum, saliva also contains enzymes, antibodies, and hormones.
Saliva is secreted from 3 paired major salivary glands (parotid, submandibular, and sublingual) (Zhang et al., 2016a). A number of salivary epithelial and inflammatory proteins have been seen as potential biomarkers for diabetes, cardiovascular, oral, and autoimmune diseases such as Sjögren’s syndrome (Baldini et al., 2011). Since saliva is the product of salivary glands, the primary targets of the autoimmune response in Sjögren’s syndrome, it is believed that this secreted fluid can directly mirror the gland’s pathophysiology. Amid various proteomic salivary biomarkers for Sjögren’s syndrome, PIP is expressed in the saliva of these patients (De Amicis et al., 2013).
Prevention of disease morbidity due to KC requires the development non-invasive tools to aid in early screening, diagnosis, and treatment of this disease. Despite significant efforts, a specific biomarker for KC has not been identified. In studies from our group, saliva proteome analysis from patients with KC revealed a unique PIP expression profile, correlating metabolic changes occurring in saliva with clinical findings in KC. Our results reveal a downregulation in PIP expression in saliva samples from KC patients correlating with our finding in tears and cells obtained from KC patients in comparison with healthy controls (Priyadarsini et al., 2014). Regulation of all three systems is shown on Fig. 12.
Fig. 12.

Quantification of PIP protein levels. (A) Human tear samples from healthy and KC donors (n = 32). (B) Human saliva samples from healthy and KC donors (n = 64). (C) HCFs and HKCs in 3-D constructs (n = 6). PIP was significantly downregulated in KC samples, compared to healthy controls, in all three systems; Tears (p < 0.0001), Saliva (p < 0.001), and Cells (p < 0.005).
5.4. Prolactin-induced protein: a novel KC biomarker
We have shown that KC has a systemic component driven by altered hormones contributing to stromal thinning in the KC cornea. Our results suggest that hormonal regulation may play a critical role in the KC pathology. We found significant reduction in salivary estrone levels and a significant increase in DHEA levels in KC patients independent of gender (McKay et al., 2017). Given these discoveries, we believe that KC has a strong systemic component to its onset and progression that is tightly connected to PIP modulation and hormonal stability.
Various studies have demonstrated that the levels of prolactin, as well as androgen and estrogen hormones are essential for ocular surface homeostasis and structural organization (McKay et al., 2016; Stachon et al., 2017). Studies showed that prolactin exhibits an effect on collagen organization in 3-D tissue culture models (Speroni et al., 2014). Additional studies demonstrated that besides being present in lacrimal glands, prolactin is connected with cell proliferation and is a known inducer of PIP, which is involved in the maintenance control of metabolic function. Prolactin also acts as a neuroendocrine regulator of keratin transcription and protein production (Stachon et al., 2017). Studies have shown that prolactin concentration is decreased in aqueous humour of KC patients, which might be important evidence that altering keratin expression within the human cornea could have an impact on KC pathology (Ramot et al., 2010). Our findings that PIP is significantly downregulated in vitro and in vivo (Fig. 12) in KC patients compared to healthy controls (McKay et al., 2017; Priyadarsini et al., 2014) strongly suggest that PIP holds great promise as a novel prognostic biomarker for KC and can aid in providing hints for various stages of KC progression.
6. Conclusions and future directions
In summary, we are intrigued by the versatile function of PIP and the fact that PIP is regulated so profoundly in KC patients. Importantly, our recent studies along with the findings shown here provide a rationale for the tantalizing possibility of exploring PIP as a therapeutic target in KC. Our studies on the human saliva from KC and healthy donors, incredibly, showed that PIP regulation was identical in modulation as it was in tears and cells. Our studies show that PIP is regulated both in situ and systemically in KC patients. Before our ground-breaking studies, saliva sampling has never been analyzed in KC patients despite the fact it is a noninvasive bodily fluid that has provided clinical clues to diseases such as cancer and diabetes. Furthermore, the reported hormonal imbalances in KC are tightly linked to PIP regulation and could provide key mechanistic information. A good example is DHEA hormone. In our studies, DHEA was significantly upregulated in KC patients when compared to controls. Fig. 13 shows DHEA levels over our lifetime. DHEA levels are constantly increasing at the fetus stage and take a dive at birth almost down to zero. By the age of 7 years old, DHEA reaches its lowest levels where it starts going up again. DHEA levels peak around the same time KC defects are known to appear (~15–20 years old). From then on, and for the rest of our lives, DHEA levels continue to decline. Is it possible that the abnormal, high level of DHEA in KC patients that we have reported are a trigger for KC onset and that PIP can be a biomarker for this event? We certainly have strong evidence of this and future studies are designed so that we can delineate this mechanism.
Fig. 13.
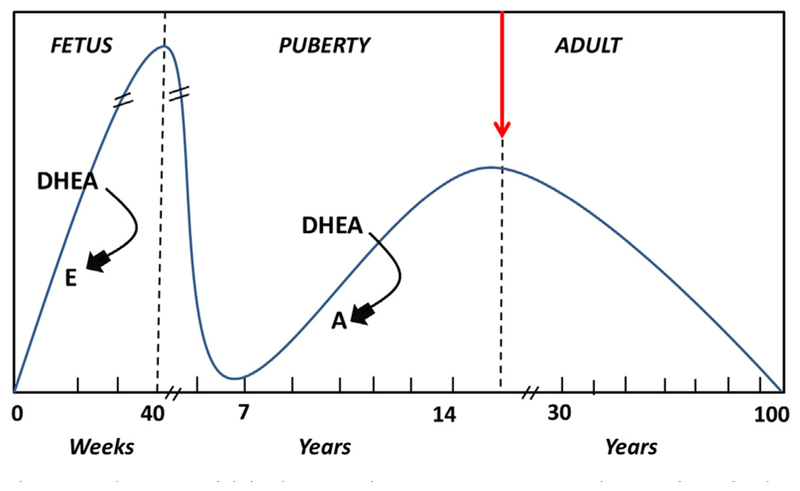
Schematic of dehydroepiandrosterone (DHEA) production from fetal to adult life. The arrows indicate temporal changes in sex hormones controlling DHEA (E: estrogen, A: androgen).
It is possible that PIP could interact with other unknown factors. Papoulidis and co-authors (Papoulidis et al., 2014) published a case study of a patient that developed KC, with partial trisomy 21 and 7q deletion. Prior to this, Pinsard and co-authors (Pinsard et al., 2010) reported a KC association wth Williams-Beuren syndrome (WBS). WBS is known to be an abnormal systemic development caused by micro-deletion of contiguous genes in chromosome 7q11.23. This may implicate chromosome 7 for the development of KC. Because PIP is located on chromosome 7, this intriguing story may reveal more connections between PIP and KC in the near future.
The clinical significance of these observations are enormous, and targeting PIP directly or indirectly may prove effective not only in early disease stages, but also in advanced KC stages. If PIP were to be used as a KC biomarker and/or a therapeutic target, this would be a paradigm shift in KC research, and a huge step towards eliminating KC and all vision threats that come with it.
Future directions will attempt to delineate the roles of PIP in both hormone-responsive and unresponsive KC corneal cells/tissues, thereby extending the importance of PIP from a mere clinical marker to a promising therapeutic target. Investigating biomolecules with “omics” approaches will assist us as we explore this intriguing protein. Finally, the design of any PIP-based therapeutic targets will have to take into consideration these recent findings.
Acknowledgements
This work was supported by the National Institutes of Health and the National Eye Institute [EY028888], and an unrestricted grant (Dean McGee Eye Institute) from the Research to Prevent Blindness. The authors thank Sarah Nicholas for the useful discussions and advice. The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- KC
Keratoconus
- PIP
Prolactin-induced protein
- ECM
Extracellular matrix
- SMA
Smooth muscle actin
- VRQOL
Vision-related quality of life
- PK
Penetrating keratoplasty
- DALK
Deep anterior lamellar keratoplasty
- CXL
Collagen cross-linking
- RGP
Rigid gas-permeable
- TIMPS
Tissue inhibitors of metalloproteinases
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- ICRS
Intra-corneal ring segments
- TGFB1
Transforming growth factor-B1
- SLE
Systemic lupus erythematosus
- LH
Luteinizing hormone
- FSH
Follicle-stimulating hormone
- AR
Androgen receptor
- ER
Estrogen receptor
- HKC
Human keratoconus cells
- HCF
Human corneal fibroblasts
- CLEK
Collaborative longitudinal evaluation of keratoconus
Footnotes
Conflicts of interest
Authors declare no conflict of interest.
References
- Abu-Amero, Helwa, Al-Muammar, Strickland, Hauser, Allingham, Liu, 2015. Screening of the seed region of Mir184 in keratoconus patients from Saudi Arabia. BioMed. Res. Int 2015, 604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsler M, 1946. Kératocône classique et kératocône fruste; arguments unitaires. Ophthalmologica 111, 96–101. [DOI] [PubMed] [Google Scholar]
- Annibalini G, Agostini D, Calcabrini C, Martinelli C, Colombo E, Guescini M, Tibollo P, Stocchi V, Sestili P, 2014. Effects of sex hormones on inflammatory response in male and female vascular endothelial cells. J. Endocrinol. Invest 37, 861–869. [DOI] [PubMed] [Google Scholar]
- Anwar M, Teichmann KD, 2002. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea 21, 374–383. [DOI] [PubMed] [Google Scholar]
- Atilano SR, Coskun P, Chwa M, Jordan N, Reddy V, Le K, Wallace DC, Kenney MC, 2005. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest. Ophthalmol. Vis. Sci 46, 1256–1263. [DOI] [PubMed] [Google Scholar]
- Autiero M, Cammarota G, Friedlein A, Zulauf M, Chiappetta G, Dragone V, Guardiola J, 1995. A 17-kDa CD4-binding glycoprotein present in human seminal plasma and in breast tumor cells. Eur. J. Immunol 25, 1461–1464. [DOI] [PubMed] [Google Scholar]
- Balasubramanian SA, Mohan S, Pye DC, Willcox MD, 2012. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol 90, e303–309. [DOI] [PubMed] [Google Scholar]
- Baldini C, Giusti L, Ciregia F, Da Valle Y, Giacomelli C, Donadio E, Sernissi F, Bazzichi L, Giannaccini G, Bombardieri S, Lucacchini A, 2011. Proteomic analysis of saliva: a unique tool to distinguish primary Sjögren’s syndrome from secondary Sjögren’s syndrome and other sicca syndromes. Arthritis Res. Ther 13, R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Chimge N-O, Jordan VC, Tripathy D, Frenkel B, 2013. Prolactin-Induced protein (PIP) regulates proliferation of luminal a type breast cancer cells in an estrogen-independent manner. PLoS One 8, e62361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behndig A, Karlsson K, Johansson BO, Brannstrom T, Marklund SL, 2001. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Invest. Ophthalmol. Vis. Sci 42, 2293–2296. [PubMed] [Google Scholar]
- Belin MW, Khachikian SS, 2006. New devices and clinical implications for measuring corneal thickness. Clin. Exp. Ophthalmol 34, 729–731. [DOI] [PubMed] [Google Scholar]
- Benedek GB, 1971. Theory of transparency of the eye. Appl. Optic 10, 459–473. [DOI] [PubMed] [Google Scholar]
- Bilgihan K, Hondur A, Sul S, Ozturk S, 2011. Pregnancy-induced Progression of Keratoconus. [DOI] [PubMed]
- Birk DE, 2001. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron (Oxford, England : 1993) 32, 223–237. [DOI] [PubMed] [Google Scholar]
- Blais Y, Gingras S, Haagensen DE, Labrie F, Simard J, 1996. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol. Cell. Endocrinol 121, 11–18. [DOI] [PubMed] [Google Scholar]
- Bodner L, Qwarnstrom E, Omnell K-A, Hand AR, Baum BJ, 1983. Rat submandibular gland secretion: a bilateral and longitudinal comparative study. Comp. Biochem. Physiol. Physiol 74, 829–831. [DOI] [PubMed] [Google Scholar]
- Bykhovskaya Y, Li X, Epifantseva I, Haritunians T, Siscovick D, Aldave A, Szczotka-Flynn L, Iyengar SK, Taylor KD, Rotter JI, Rabinowitz YS, 2012. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest. Ophthalmol. Vis. Sci 53, 4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya Y, Margines B, Rabinowitz YS, 2016. Genetics in Keratoconus: where are we? Eye and Vision 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, 2010. Long-term results of ribo-flavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am. J. Ophthalmol 149, 585–593. [DOI] [PubMed] [Google Scholar]
- Caputo E, Camarca A, Moharram R, Tornatore P, Thatcher B, Guardiola J, Martin BM, 2003. Structural study of GCDFP-15/gp17 in disease versus physiological conditions using a proteomic approach. Biochemistry 42, 6169–6178. [DOI] [PubMed] [Google Scholar]
- Caputo E, Manco G, Mandrich L, Guardiola J, 2000. A novel aspartyl proteinase from apocrine epithelia and breast tumors. J. Biol. Chem 275, 7935–7941. [DOI] [PubMed] [Google Scholar]
- Carsol JL, Gingras S, Simard J, 2002. Synergistic action of prolactin (PRL) and androgen on PRL-inducible protein gene expression in human breast cancer cells: a unique model for functional cooperation between signal transducer and activator of transcription-5 and androgen receptor. Mol. Endocrinol 16, 1696–1710. [DOI] [PubMed] [Google Scholar]
- Castroviejo R, 1949. Keratoplasty in treatment of keratoconus. Arch. Ophthalmol 42, 776–800. [DOI] [PubMed] [Google Scholar]
- Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S, 2013. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J. Proteomics 87, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Stojanovic A, Eidet JR, Utheim TP, 2015. Corneal collagen cross-linking (CXL) in thin corneas. Eye and Vision 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ, 2003. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin. Exp. Ophthalmol 31, 229–232. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Kapoor MS, Singh A, Bodakhe SH, 2017. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthal 29, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin J, Cochener B, Savary G, Malet F, 2000. Correcting keratoconus with intracorneal rings. J. Cataract Refract. Surg 26, 1117–1122. [DOI] [PubMed] [Google Scholar]
- Critchfield JW, Calandra AJ, Nesburn AB, Kenney MC, 1988. Keratoconus: I. Biochemical studies. Exp. Eye Res 46, 953–963. [DOI] [PubMed] [Google Scholar]
- Cuellar-Partida, Springelkamp, Lucas, et al. , 2015. Wnt10a exonic variant increases the risk of keratoconus by decreasing corneal thickness. Hum. Mol. Genet 24, 5060–5068. [DOI] [PubMed] [Google Scholar]
- Darb-Esfahani S, von Minckwitz G, Denkert C, Ataseven B, Hogel B, Mehta K, Kaltenecker G, Rudiger T, Pfitzner B, Kittel K, Fiedler B, Baumann K, Moll R, Dietel M, Eidtmann H, Thomssen C, Loibl S, 2014. Gross cystic disease fluid protein 15 (GCDFP-15) expression in breast cancer subtypes. BMC Canc 14, 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ, 2014. The pathogenesis of keratoconus. Eye 28, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxer A, Fratzl P, 1997. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest. Ophthalmol. Vis. Sci 38, 121–129. [PubMed] [Google Scholar]
- De Amicis F, Russo A, Avena P, Santoro M, Vivacqua A, Bonofiglio D, Mauro L, Aquila S, Tramontano D, Fuqua SA, Ando S, 2013. In vitro mechanism for downregulation of ER-alpha expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res 57, 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debily MA, Marhomy SE, Boulanger V, Eveno E, Mariage-Samson R, Camarca A, Auffray C, Piatier-Tonneau D, Imbeaud S, 2009. A functional and regulatory network associated with PIP expression in human breast cancer. PLoS One 4, e4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakova, Palos, Jirsova, et al. , 2015. Validation of Rs2956540:G > C and Rs3735520:G > a association with keratoconus in a population of European descent. Eur. J. Hum. Genet 23, 1581–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JK, Belin MW, Borgstrom M, 2016. Assessing progression of keratoconus: novel tomographic determinants. Eye and Vision 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertan A, Muftuoglu O, 2008. Keratoconus clinical findings according to different age and gender groups. Cornea 27, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Fan Gaskin JC, Patel DV, McGhee CN, 2014. Acute corneal hydrops in keratoconus - new perspectives. Am. J. Ophthalmol 157, 921–928. [DOI] [PubMed] [Google Scholar]
- Fink BA, Sinnott LT, Wagner H, Friedman C, Zadnik K, 2010. The influence of gender and hormone status on the severity and progression of keratoconus. Cornea 29, 65–72. [DOI] [PubMed] [Google Scholar]
- Gallo A, Martini D, Sernissi F, Giacomelli C, Pepe P, Rossi C, Riveros PP, Mosca M, Alevizos I, Baldini C, 2013. Gross cystic disease fluid protein-15(GCDFP-15)/prolactin-inducible protein (PIP) as functional salivary biomarker for primary Sjögren’s syndrome. J. Genet. Syndr. Gene Ther 4 10.4172/2157-7412.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A, 2015. Keratoconus: an inflammatory disorder? Eye 29, 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvis V, Tello A, Carreño NI, Berrospi RD, Niño CA, 2017. Risk factors for keratoconus: atopy and eye rubbing. Cornea 36, e1. [DOI] [PubMed] [Google Scholar]
- Garcia B, Garcia-Suarez O, Merayo-Lloves J, Alcalde I, Alfonso JF, Fernandez-Vega Cueto L, Meana A, Vazquez F, Quiros LM, 2016. Differential expression of proteoglycans by corneal stromal cells in keratoconus. Invest. Ophthalmol. Vis. Sci 57, 2618–2628. [DOI] [PubMed] [Google Scholar]
- Ghahfarokhi NA, Vaseghi A, Ghahfarokhi NA, Ghoreishi M, Peyman A, Dehghani A, 2015. Evaluation of corneal thickness alterations during menstrual cycle in productive age women. Indian J. Ophthalmol 63, 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP, 2017b. Agespecific incidence and prevalence of keratoconus: a nationwide registration study. Am. J. Ophthalmol 175, 169–172. [DOI] [PubMed] [Google Scholar]
- Goldich Y, Barkana Y, Gerber Y, Rasko A, Morad Y, Harstein M, Avni I, Zadok D, 2009. Effect of diabetes mellitus on biomechanical parameters of the cornea. J. Cataract Refract. Surg 35, 715–719. [DOI] [PubMed] [Google Scholar]
- Goldich Y, Barkana Y, Pras E, Fish A, Mandel Y, Hirsh A, Tsur N, Morad Y, Avni I, Zadok D, 2011. Variations in corneal biomechanical parameters and central corneal thickness during the menstrual cycle. J. Cataract Refract. Surg 37, 1507–1511. [DOI] [PubMed] [Google Scholar]
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrosio R Jr., Guell JL, Malecaze F, Nishida K, Sangwan VS, 2015. Global consensus on keratoconus and ectatic diseases. Cornea 34, 359–369. [DOI] [PubMed] [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E, 2000. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 23, 912–918. [DOI] [PubMed] [Google Scholar]
- Gordon-Shaag A, Millodot M, Shneor E, Liu Y, 2015. The genetic and environmental factors for keratoconus. BioMed Res. Int 2015, 795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorskova EN, Sevost’ianov EN, Baturin NA, 1998. Results of psychological testing of patients with keratoconus. Vestn. Oftalmol 114, 44–45. [PubMed] [Google Scholar]
- Grentzelos MA, Kounis GA, Diakonis VF, Siganos CS, Tsilimbaris MK, Pallikaris IG, Kymionis GD, 2017. Combined transepithelial phototherapeutic keratectomy and conventional photorefractive keratectomy followed simultaneously by corneal crosslinking for keratoconus: cretan protocol plus. J. Cataract Refract. Surg 43, 1257–1262. [DOI] [PubMed] [Google Scholar]
- Guedj M, Saad A, Audureau E, Gatinel D, 2013. Photorefractive keratectomy in patients with suspected keratoconus: five-year follow-up. J. Cataract Refract. Surg 39, 66–73. [DOI] [PubMed] [Google Scholar]
- Gupta P, Johar K, Nagpal K, Vasavada A, 2005. Sex hormone receptors in the human eye. Surv. Ophthalmol 50, 274–284. [DOI] [PubMed] [Google Scholar]
- Haagensen DE Jr., Dilley WG, Mazoujian G, Wells SA Jr., 1990. Review of GCDFP-15. An apocrine marker protein. Ann. N. Y. Acad. Sci 586, 161–173. [DOI] [PubMed] [Google Scholar]
- Haagensen DE Jr., Gall SA, Brazy JE, Giannola J, Wells SA Jr., 1980. Analysis of amniotic fluid, maternal plasma, and cord blood for a human breast gross cystic disease fluid protein. Am. J. Obstet. Gynecol 138, 25–32. [DOI] [PubMed] [Google Scholar]
- Haagensen DE Jr., Mazoujian G, Dilley WG, Pedersen CE, Kister SJ, Wells SA Jr., 1979. Breast gross cystic disease fluid analysis. I. Isolation and radio-immunoassay for a major component protein. J. Natl. Cancer Inst 62, 239–247. [PubMed] [Google Scholar]
- Hagan, Martin, Enríquez-de-Salamanca, 2016. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RW, Farrell RA, 1969. Light scattering in the cornea. J. Opt. Soc. Am 59,766–774. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Beiranvand A, Khabazkhoob M, Asgari S, Emamian MH, Shariati M, Fotouhi A, 2013a. Prevalence of keratoconus in a population-based study in shahroud. Cornea 32, 1441–1445. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Khabazkhoob M, Yazdani N, Ostadimoghaddam H, Norouzirad R,Amanzadeh K, Miraftab M, Derakhshan A, Yekta A, 2014. The prevalence of keratoconus in a young population in Mashhad, Iran. Ophthalmic Physiol. Optic 34, 519–527. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S, 2013b. Corneal collagen cross-linking with riboflavin and ultraviolet a irradiation for keratoconus: long-term results. Ophthalmology 120, 1515–1520. [DOI] [PubMed] [Google Scholar]
- Hassan MI, Kumar V, Singh TP, Yadav S, 2008. Purification and characterization of zinc alpha2-glycoprotein-prolactin inducible protein complex from human seminal plasma. J. Separ. Sci 31, 2318–2324. [DOI] [PubMed] [Google Scholar]
- Hassan, Waheed, Yadav, Singh, Ahmad, 2009. Prolactin inducible protein in cancer, fertility and immunoregulation: structure, function and its clinical implications. Cell. Mol. Life Sci 66, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp. Eye Res 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heon, Greenberg, Kopp, et al. , 2002. Vsx1: a gene for posterior polymorphous dystrophy and keratoconus. Hum. Mol. Genet 11, 1029–1036. [DOI] [PubMed] [Google Scholar]
- Hay ED, 1980. Development of the vertebrate cornea. Int. Rev. Cytol 63, 263–322. [DOI] [PubMed] [Google Scholar]
- Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK, 2017. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 124, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Holden BA, Mertz GW, 1984. Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Invest. Ophthalmol. Vis. Sci 25, 1161–1167. [PubMed] [Google Scholar]
- Hoogewoud F, Gatzioufas Z, Hafezi F, 2013. Transitory topographical variations in keratoconus during pregnancy. J. Refract. Surg 29, 144–146. [DOI] [PubMed] [Google Scholar]
- Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M, 2012. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A: Biol. Sci. Med. Sci 67, 1140–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes Anne E., Bradley Declan T., Campbell M, Lechner J, Dash Durga P., Simpson David A., Willoughby Colin E., 2011. Mutation altering the Mir-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet 89, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen A, 1986. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol (Supplement 178), 1–64. [PubMed] [Google Scholar]
- Ihanamaki T, Pelliniemi LJ, Vuorio E, 2004. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res 23, 403–434. [DOI] [PubMed] [Google Scholar]
- Ivarsen A, Hjortdal J, 2013. Collagen cross-linking for advanced progressive keratoconus. Cornea 32, 903–906. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K, 2009. Prevalence and associations of keratoconus in rural Maharashtra in central India: the central India eye and medical study. Am. J. Ophthalmol 148, 760–765. [DOI] [PubMed] [Google Scholar]
- Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, Hamad A, Chakravarti S, 2011. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One 6, e16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulos AJ, 2009. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J. Refract. Surg. (Thorofare, N.J.: 1995) 25, S812–S818. [DOI] [PubMed] [Google Scholar]
- Karimian F, Aramesh S, Rabei HM, Javadi MA, Rafati N, 2008. Topographic evaluation of relatives of patients with keratoconus. Cornea 27, 874–878. [DOI] [PubMed] [Google Scholar]
- Kennedy RH, Bourne WM, Dyer JA, 1986. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol 101, 267–273. [DOI] [PubMed] [Google Scholar]
- Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV, 1997. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea 16, 345–351. [PubMed] [Google Scholar]
- Khaled ML, Helwa I, Drewry M, Seremwe M, Estes A, Liu Y, 2017. Molecular and histopathological changes associated with keratoconus. BioMed Res. Int 2017, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely PM, Carney LG, Smith G, 1982. Diurnal variations of corneal topography and thickness. Am. J. Optom. Physiol. Opt 59, 976–982. [DOI] [PubMed] [Google Scholar]
- Kim, Mok, Kim, et al. , 2008. Association of −31t > C and −511 C > T polymorphisms in the interleukin 1 beta (Il1b) promoter in Korean keratoconus patients. Mol. Vis 14, 2109–2116. [PMC free article] [PubMed] [Google Scholar]
- Kim W-J, Rabinowitz YS, Meisler DM, Wilson SE, 1999. Keratocyte apoptosis associated with keratoconus. Exp. Eye Res 69, 475–481. [DOI] [PubMed] [Google Scholar]
- King P, Peacock I, Donnelly R, 1999. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol 48, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyce SD, 1984. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest. Ophthalmol. Vis. Sci 25, 1426–1435. [PubMed] [Google Scholar]
- Komai Y, Ushiki T, 1991. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci 32, 2244–2258. [PubMed] [Google Scholar]
- Konttinen YT, Tensing EK, Laine M, Porola P, Tornwall J, Hukkanen M, 2005. Abnormal distribution of aquaporin-5 in salivary glands in the NOD mouse model for Sjogren’s syndrome. J. Rheumatol 32, 1071–1075. [PubMed] [Google Scholar]
- Koo TS, Finkelstein E, Tan D, Mehta JS, 2011. Incremental cost-utility analysis of deep anterior lamellar keratoplasty compared with penetrating keratoplasty for the treatment of keratoconus. Am. J. Ophthalmol 152, 40–47 e42. [DOI] [PubMed] [Google Scholar]
- Koob TJ, Jeffrey JJ, Eisen AZ, Bauer EA, 1980. Hormonal interactions in mammalian collagenase regulation: comparative studies in human skin and rat uterus. Biochim. Biophys. Acta Gen. Subj 629, 13–23. [DOI] [PubMed] [Google Scholar]
- Kosker M, Gurdal C, 2016. Re: Woodward et al.: the association between socio-demographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database (Ophthalmology 2016;123:457–65). Ophthalmology 123, e45–46. [DOI] [PubMed] [Google Scholar]
- Krachmer JH, Feder RS, Belin MW, 1984. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol 28, 293–322. [DOI] [PubMed] [Google Scholar]
- Kruse D, Redman R, Mansson-Rahemtulla B, 1998. Salivary peroxidase immunohistochemistry in the developing rat submandibular gland. J. Dent. Res 232 Amer Assoc dental Research 1619 Duke St, Alexandria, VA 22314 USA. [Google Scholar]
- Kukurba KR, Montgomery SB, 2015. RNA Sequencing and Analysis 2015. Cold Spring Harbor protocols, pp. 951–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kymes S, Walline J, Zadnik K, Sterling J, O Gordon M, 2008. Changes in the Quality-of-life of People with Keratoconus. [DOI] [PMC free article] [PubMed]
- Kymes SM, Walline JJ, Zadnik K, Gordon MO, 2004. Quality of life in keratoconus. Am. J. Ophthalmol 138, 527–535. [DOI] [PubMed] [Google Scholar]
- Lass JH, Lembach RG, Park SB, Hom DL, Fritz ME, Svilar GM, Nuamah IF, Reinhart WJ, Stocker EG, Keates RH, et al. , 1990. Clinical management of keratoconus. A multicenter analysis. Ophthalmology 97, 433–445. [DOI] [PubMed] [Google Scholar]
- Lechner J, Bae HA, Guduric-Fuchs J, Rice A, Govindarajan G, Siddiqui S, Abi Farraj L, Yip SP, Yap M, Das M, Souzeau E, Coster D, Mills RA, Lindsay R, Phillips T, Mitchell P, Ali M, Inglehearn CF, Sundaresan P, Craig JE, Simpson DA, Burdon KP, Willoughby CE, 2013. Mutational analysis of MIR184 in sporadic keratoconus and Myopia. Invest. Ophthalmol. Vis. Sci 54, 5266–5272. [DOI] [PubMed] [Google Scholar]
- Lema I, Brea D, Rodriguez-Gonzalez R, Diez-Feijoo E, Sobrino T, 2010. Proteomic analysis of the tear film in patients with keratoconus. Mol. Vis 16, 2055–2061. [PMC free article] [PubMed] [Google Scholar]
- Lema I, Sobrino T, Duran JA, Brea D, Diez-Feijoo E, 2009. Subclinical keratoconus and inflammatory molecules from tears. Br. J. Ophthalmol 93, 820–824. [DOI] [PubMed] [Google Scholar]
- Li, Bykhovskaya, Canedo, et al. , 2013. Genetic association of Col5a1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest. Ophthalmol. Vis. Sci 54, 2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung VC, Pechlivanoglou P, Chew HF, Hatch W, 2017. Corneal collagen cross-linking in the management of keratoconus in Canada: a cost-effectiveness analysis. Ophthalmology 124, 1108–1119. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD, 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. [DOI] [PubMed] [Google Scholar]
- Lowell FC, Carroll JM, 1970. A study of the occurrence of atopic traits in patients with keratoconus. J. Allergy 46, 32–39. [DOI] [PubMed] [Google Scholar]
- Lu, Vitart, Burdon, et al. , 2013. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet 45, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SEM, Zhou T, Blackburn NB, Mills RA, Ellis J, Leo P, Souzeau E, Ridge B, Charlesworth JC, Brown MA, Lindsay R, Craig JE, Burdon KP, 2017. Rare, potentially pathogenic variants in ZNF469 are not enriched in keratoconus in a large australian cohort of european descent. Invest. Ophthalmol. Vis. Sci 58, 6248–6256. [DOI] [PubMed] [Google Scholar]
- Luo Y, Liu S, Yao K, 2017. Transcriptome-wide investigation of mRNA/circRNA in miR-184 and its r.57c > u mutant type treatment of human lens epithelial cells. Mol. Ther. Nucleic Acids 7, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz Z, Määttä M, Stenman M, Konttinen L, Tervo T, Konttinen YT, 2006. Collagenolytic proteinases in keratoconus. Cornea 25, 603–610. [DOI] [PubMed] [Google Scholar]
- Maertzdorf J, Osterhaus AD, Verjans GM, 2002. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol 169, 5897–5903. [DOI] [PubMed] [Google Scholar]
- Manchester PT Jr., 1970. Hydration of the cornea. Trans. Am. Ophthalmol. Soc 68, 425–461. [PMC free article] [PubMed] [Google Scholar]
- Mathew JH, Goosey JD, Bergmanson JP, 2011. Quantified histopathology of the keratoconic cornea. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom 88, 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew JH, Goosey JD, Soderberg PG, Bergmanson JP, 2015. Lamellar changes in the keratoconic cornea. Acta Ophthalmol 93, 767–773. [DOI] [PubMed] [Google Scholar]
- Maurice DM, 1957. The structure and transparency of the cornea. J. Physiol 136,263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, Dedeic Z, Dionne MJC, Clement EM, Conrad GW, 2010. Mechanisms of cor-neal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest. Ophthalmol. Vis. Sci 51, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Hjortdal J, Priyadarsini S, Karamichos D, 2017. Acute hypoxia influences collagen and matrix metalloproteinase expression by human keratoconus cells in vitro. PLoS One 12, e0176017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Hjortdal J, Sejersen H, Asara JM, Wu J, Karamichos D, 2016. Endocrine and metabolic pathways linked to keratoconus: implications for the role of hormones in the stromal microenvironment. Sci. Rep 6, 25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TT, Szczotka-Flynn L, Barr JT, Anderson RJ, Slaughter ME, Lass JH, Iyengar SK, Group atCS, 2006. A new method for grading the severity of keratoconus: the keratoconus severity score (KSS). Cornea 25, 794–800. [DOI] [PubMed] [Google Scholar]
- Meek KM, Blamires T, Elliott GF, Gyi TJ, Nave C, 1987. The organisation of collagen fibrils in the human corneal stroma: a synchrotron X-ray diffraction study. Curr. Eye Res 6, 841–846. [DOI] [PubMed] [Google Scholar]
- Meek KM, Knupp C, 2015. Corneal structure and transparency. Prog. Retin. Eye Res 49, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS, 1999. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br. J. Ophthalmol 83, 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millodot M, Shneor E, Albou S, Atlani E, Gordon-Shaag A, 2011. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol 18, 91–97. [DOI] [PubMed] [Google Scholar]
- Mishra, Yazar, Hewitt, et al. , 2012. Genetic variants near pdgfra are associated with corneal curvature in Australians. Invest. Ophthalmol. Vis. Sci 53, 7131–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok CC, Lau CS, Wong RW, 2001. Use of exogenous estrogens in systemic lupus erythematosus. Semin. Arthritis Rheum 30, 426–435. [DOI] [PubMed] [Google Scholar]
- Moodaley LC, Woodward EG, Liu CS, Buckley RJ, 1992. Life expectancy in keratoconus. Br. J. Ophthalmol 76, 590–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi K, Yamamoto M, Asano T, Shibata T, 1995. Peroxidase activity and cell differentiation in developing salivary glands of the rats. Okajimas Folia Anat. Jpn 72, 13–28. [DOI] [PubMed] [Google Scholar]
- Morishige N, Wahlert AJ, Kenney MC, Brown DJ, Kawamoto K, Chikama T, Nishida T, Jester JV, 2007. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest. Ophthalmol. Vis. Sci 48, 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SE, Klein R, Klein BE, 2000. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. (Chicago, Ill. : 1960) 118, 1264–1268. [DOI] [PubMed] [Google Scholar]
- Murphy LC, Tsuyuki D, Myal Y, Shiu RP, 1987. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J. Biol. Chem 262, 15236–15241. [PubMed] [Google Scholar]
- Naderan M, Rajabi MT, Zarrinbakhsh P, Naderan M, Bakhshi A, 2016. Association between family history and keratoconus severity. Curr. Eye Res 41, 1414–1418. [DOI] [PubMed] [Google Scholar]
- Naderi A, 2015. Prolactin-Induced protein in breast cancer In: Diakonova PM (Ed.), Recent Advances in Prolactin Research. Springer International Publishing, Cham, pp. 189–200. [Google Scholar]
- Naderi A, Meyer M, 2012. Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res 14, R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi A, Meyer M, Dowhan DH, 2012. Cross-regulation between FOXA1 and ErbB2 signaling in estrogen receptor-negative breast cancer. Neoplasia (New York, N.Y.) 14, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi A, Vanneste M, 2014. Prolactin-Induced protein is required for cell cycle progression in breast cancer. Neoplasia (New York, N.Y.) 16 329–342.e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso M, Del Regno P, Gharbiya M, Sacchetti M, Plateroti R, Lambiase A, 2017. Analysis of the pathogenic factors and management of dry eye in ocular surface disorders. Int. J. Mol. Sci 18, 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Hjortdal J, Pihlmann M, Corydon TJ, 2013. Update on the keratoconus genetics. Acta Ophthalmol 91, 106–113. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Hellmann I, Hubisz M, Bustamante C, Clark AG, 2007. Recent and ongoing selection in the human genome. Nat. Rev. Genet 8, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishtala K, Pahuja N, Shetty R, Nuijts RMMA, Ghosh A, 2016. Tear biomarkers for keratoconus. Eye and Vision 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham J, 1854. Practical Observations on Conical Cornea: and on the Short Sight, and Other Defects of Vision Connected with it. John Churchill.
- Ogueta SB, Schwartz SD, Yamashita CK, Farber DB, 1999. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest. Ophthalmol. Vis. Sci 40, 1906–1911. [PubMed] [Google Scholar]
- Olofsson EM, Marklund SL, Pedrosa-Domellof F, Behndig A, 2007. Interleukin-1alpha downregulates extracellular-superoxide dismutase in human corneal keratoconus stromal cells. Mol. Vis 13, 1285–1290. [PubMed] [Google Scholar]
- Owens H, Gamble G, 2003. A profile of keratoconus in New Zealand. Cornea 22, 122–125. [DOI] [PubMed] [Google Scholar]
- Papoulidis I, Papageorgiou E, Siomou E, Oikonomidou E, Thomaidis L, Vetro A, Zuffardi O, Liehr T, Manolakos E, Vassilis P, 2014. A patient with partial trisomy 21 and 7q deletion expresses mild Down syndrome phenotype. Gene 536, 441–443. [DOI] [PubMed] [Google Scholar]
- Patel DV, McGhee CN, 2013. Quantitative analysis of in vivo confocal microscopy images: a review. Surv. Ophthalmol 58, 466–475. [DOI] [PubMed] [Google Scholar]
- Pathak, Nayak, Singh, et al. , 2011. Mitochondrial complex 1 gene analysis in keratoconus.Mol. Vis 17, 1514–1525. [PMC free article] [PubMed] [Google Scholar]
- Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH, 2000. Does ethnic origin influence the incidence or severity of keratoconus? Eye 14 (Pt 4), 625–628. [DOI] [PubMed] [Google Scholar]
- Pertovaara M, Hulkkonen J, Hurme M, Lehtimaki T, Pasternack A, 2004. Urinary matrix metalloproteinase-9 and interleukin-6 and renal manifestations of primary Sjogren’s syndrome. Rheumatology 43, 807–808. [DOI] [PubMed] [Google Scholar]
- Pescosolido N, Barbato A, Pascarella A, Giannotti R, Genzano M, Nebbioso M,2014. Role of protease-inhibitors in ocular diseases. Molecules 19, 20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Liu Z, Feuer W, Verm A, 2002. Corneal thickness indices discriminate between keratoconus and contact lens-induced corneal thinning. Ophthalmology 109, 2336–2341. [DOI] [PubMed] [Google Scholar]
- Pinsard L, Touboul D, Vu Y, Lacombe D, Leger F, Colin J, 2010. Keratoconus associated with Williams-Beuren syndrome: first case reports. Ophthalmic Genet 31, 252–256. [DOI] [PubMed] [Google Scholar]
- Pizzarello LD, 2003. Refractive changes in pregnancy. Graefe’s Arch. Clin. Exp. Ophthalmol 241, 484–488. [DOI] [PubMed] [Google Scholar]
- Pobelle-Frasson C, Velou S, Huslin V, Massicault B, Colin J, 2004. Keratoconus: what happens with older patients? J. Fr. Ophtalmol 27, 779–782. [DOI] [PubMed] [Google Scholar]
- Polack FM, 1961. Morphology of the cornea. I. Study with silver stains. Am. J. Ophthalmol 51, 1051–1056. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, Hjortdal J, Sarker-Nag A, Sejersen H, Asara JM, Karamichos D, 2014. Gross cystic disease fluid protein-15/prolactin-inducible protein as a biomarker for keratoconus disease. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz YS, 1998. Keratoconus. Surv. Ophthalmol 42, 297–319. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS, Dong L, Wistow G, 2005. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest. Ophthalmol. Vis. Sci 46, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS, Maumenee IH, Lundergan MK, Puffenberger E, Zhu D, Antonarakis S, Francomano CA, 1992. Molecular genetic analysis in autosomal dominant keratoconus. Cornea 11, 302–308. [DOI] [PubMed] [Google Scholar]
- Radner W, Zehetmayer M, Aufreiter R, Mallinger R, 1998. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea 17, 537–543. [DOI] [PubMed] [Google Scholar]
- Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE, 2008. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J. Cataract Refract. Surg 34. [DOI] [PubMed] [Google Scholar]
- Ramot Y, Biro T, Tiede S, Toth BI, Langan EA, Sugawara K, Foitzik K, Ingber A, Goffin V, Langbein L, Paus R, 2010. Prolactin–a novel neuroendocrine regulator of human keratin expression in situ. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 24, 1768–1779. [DOI] [PubMed] [Google Scholar]
- Rebenitsch RL, Kymes SM, Walline JJ, Gordon MO, 2011. The lifetime economic burden of keratoconus: a decision analysis using a markov model. Am. J. Ophthalmol 151 768–773.e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Villarraga A, Torres-Gonzalez J-V, Ruiz-Sternberg Á-M, 2014. Safety of hormonal replacement therapy and oral contraceptives in systemic lupus erythematosus: a systematic review and meta-analysis. PLoS One 9, e104303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Jiménez M, Santodomingo-Rubido J, Flores-Rodríguez P, González-Méijome J-M, 2015. Short-term corneal changes with gas-permeable contact lens wear in keratoconus subjects: a comparison of two fitting approaches. J. Optom 8, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussy J-PF, Aubin M-J, Brunette I, Lachaine J, 2009. Cost of corneal transplantation for the Quebec health care system. Can. J. Ophthalmol 44, 36–41. [DOI] [PubMed] [Google Scholar]
- Sahebjada, Schache, Richardson, et al. , 2013. Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Investig. Ophthalmol. Vis. Sci 54, 8224–8228. [DOI] [PubMed] [Google Scholar]
- Sawaguchi S, Twining SS, Yue BY, Chang SH, Zhou X, Loushin G, Sugar J, Feder RS, 1994. Alpha 2-macroglobulin levels in normal human and keratoconus corneas. Invest. Ophthalmol. Vis. Sci 35, 4008–4014. [PubMed] [Google Scholar]
- Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S, 1997. Prevalence of dry eye among the elderly. Am. J. Ophthalmol 124, 723–728. [DOI] [PubMed] [Google Scholar]
- Seiler T, Quurke AW, 1998. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J. Cataract Refract. Surg 24, 1007–1009. [DOI] [PubMed] [Google Scholar]
- Serdarogullari H, Tetikoglu M, Karahan H, Altin F, Elcioglu M, 2013. Prevalence of keratoconus and subclinical keratoconus in subjects with astigmatism using pentacam derived parameters. J. Ophthalmic Vis. Res 8, 213–219. [PMC free article] [PubMed] [Google Scholar]
- Shajari M, Eberhardt E, Muller M, Al Khateeb G, Friderich S, Remy M, Kohnen T,2016. Effects of atopic syndrome on keratoconus. Cornea 35, 1416–1420. [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Rodrigues MM, Mandel MR, Krachmer JH, 1986. Anterior clear spaces in keratoconus. Ophthalmology 93, 1316–1319. [DOI] [PubMed] [Google Scholar]
- Sharif R, Hjortdal J, Sejersen H, Frank G, Karamichos D, 2017. Human in vitro model reveals the effects of collagen cross-linking on keratoconus pathogenesis. Sci. Rep 7, 12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard AP, Hjortdal J, Breitenbach T, Ivarsen A, 2010. Corneal distribution of riboflavin prior to collagen cross-linking. Curr. Eye Res 35, 116–121. [DOI] [PubMed] [Google Scholar]
- Soni PS, 1980. Effects of oral contraceptive steroids on the thickness of human cornea. Am. J. Optom. Physiol. Opt 57, 825–834. [DOI] [PubMed] [Google Scholar]
- Speroni L, Whitt GS, Xylas J, Quinn KP, Jondeau-Cabaton A, Barnes C, Georgakoudi I, Sonnenschein C, Soto AM, 2014. Hormonal regulation of epithelial organization in a three-dimensional breast tissue culture model. Tissue Eng. C Meth 20, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerl E, Huhle M, Seiler T, 1998. Induction of cross-links in corneal tissue. Exp. Eye Res 66, 97–103. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Terai N, Scholz F, Raiskup F, Pillunat LE, 2011. Detection of bio-mechanical changes after corneal cross-linking using Ocular Response Analyzer software. J. Refract. Surg. (Thorofare, N.J.: 1995) 27, 452–457. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Wollensak G, Dittert DD, Seiler T, 2004. Thermomechanical behavior of collagen-cross-linked porcine cornea. Ophthalmologica 218, 136–140. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Zubaty V, Raiskup-Wolf F, Pillunat LE, 2007. Oestrogen-induced changes in biomechanics in the cornea as a possible reason for keratectasia. Br. J. Ophthalmol 91, 1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachon T, Stachon A, Hartmann U, Seitz B, Langenbucher A, Szentmáry N, 2017. Urea, uric acid, prolactin and fT4 concentrations in aqueous humor of keratoconus patients. Curr. Eye Res 42, 842–846. [DOI] [PubMed] [Google Scholar]
- Tseng SCG, Smuckler D, Stern R, 1982. Comparison of collagen types in adult and fetal bovine corneas. J. Biol. Chem 257, 2627–2633. [PubMed] [Google Scholar]
- Tuft SJ, Hassan H, George S, Frazer DG, Willoughby CE, Liskova P, 2012. Keratoconus in 18 pairs of twins. Acta Ophthalmol 90, e482–e486. [DOI] [PubMed] [Google Scholar]
- Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ, 1994. Prognostic factors for the progression of keratoconus. Ophthalmology 101, 439–447. [DOI] [PubMed] [Google Scholar]
- Udar, Atilano, Brown, et al. , 2006. Sod1: a candidate gene for keratoconus. Invest. Ophthalmol. Vis. Sci 47, 3345–3351. [DOI] [PubMed] [Google Scholar]
- Ueyama H, Naitoh H, Ohkubo I, 1994. Structure and expression of rat and mouse mRNAs for Zn-alpha 2-glycoprotein. J. Biochem 116, 677–681. [DOI] [PubMed] [Google Scholar]
- Valgaeren H, Koppen C, Van Camp G, 2018. A new perspective on the genetics of keratoconus: why have we not been more successful? Ophthalmic Genet 39, 158–174. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar FJHM, Cheng YYY, Nuijts RMMA, Schouten JS, Wijdh R-J, Pels E, van Cleynenbreugel H, Eggink CA, Zaal MJW, Rijneveld WJ, Dirksen CD, 2011. Economic evaluation of deep anterior lamellar keratoplasty versus penetrating keratoplasty in The Netherlands. Am. J. Ophthalmol 151 449–459.e442. [DOI] [PubMed] [Google Scholar]
- van Dijk K, Liarakos VS, Parker J, Ham L, Lie JT, Groeneveld-van Beek EA, Melles GR, 2015. Bowman layer transplantation to reduce and stabilize progressive, advanced keratoconus. Ophthalmology 122, 909–917. [DOI] [PubMed] [Google Scholar]
- Vega-Estrada A, Alio JL, Plaza-Puche AB, 2015. Keratoconus progression after intrastromal corneal ring segment implantation in young patients: five-year follow-up. J. Cataract Refract. Surg 41, 1145–1152. [DOI] [PubMed] [Google Scholar]
- Vincent, Jordan, Cadzow, et al. , 2014. Mutations in the zinc finger protein gene, Znf469, contribute to the pathogenesis of keratoconus. Invest. Ophthalmol. Vis. Sci 55, 5629–5635. [DOI] [PubMed] [Google Scholar]
- Visser ES, Wisse RP, Soeters N, Imhof SM, Van der Lelij A, 2016. Objective and subjective evaluation of the performance of medical contact lenses fitted using a contact lens selection algorithm. Contact Lens Anterior Eye 39, 298–306. [DOI] [PubMed] [Google Scholar]
- von Thun Und Hohenstein-Blaul N, Funke S, Grus FH, 2013. Tears as a source of biomarkers for ocular and systemic diseases. Exp. Eye Res 117, 126–137. [DOI] [PubMed] [Google Scholar]
- Wagner H, Barr JT, Zadnik K, the Collaborative Longitudinal Evaluation of Keratoconus Study G, 2007. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Contact Lens Anterior Eye: J. Br. Contact Lens Assoc 30, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li A-L, Pang Y, Lei Y-Q, Yu L, 2017. Changes in intraocular pressure and central corneal thickness during pregnancy: a systematic review and Meta-analysis. Int. J. Ophthalmol 10, 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed KH, MacEwen CJ, McGhee CN, 2006. The variable expression of keratoconus within monozygotic twins: dundee University Scottish Keratoconus Study (DUSKS). Contact Lens Anterior Eye 29, 123–126. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Lu A, Beeson C, 1988. Maternal corneal thickness during pregnancy. Am. J. Ophthalmol 105, 258–260. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ, 2006. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol 38, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J, Hauser MA, Afshari NA, Allingham RR, Liu Y, 2012. The genetics of keratoconus: a review. Microscopy 001. [DOI] [PMC free article] [PubMed]
- White TL, Lewis PN, Young RD, Kitazawa K, Inatomi T, Kinoshita S, Meek KM, 2017. Elastic microfibril distribution in the cornea: differences between normal and keratoconic stroma. Exp. Eye Res 159, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT, 1989. Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum. Pathol 20, 281–287. [DOI] [PubMed] [Google Scholar]
- Wisse RP, Kuiper JJ, Gans R, Imhof S, Radstake TR, Van der Lelij A, 2015. Cytokine expression in keratoconus and its corneal microenvironment: a systematic review. Ocul. Surf 13, 272–283. [DOI] [PubMed] [Google Scholar]
- Wojcik KA, Kaminska A, Blasiak J, Szaflik J, Szaflik JP, 2013. Oxidative stress in the pathogenesis of keratoconus and fuchs endothelial corneal dystrophy. Int. J. Mol. Sci 14, 19294–19308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T, 2003. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol 135, 620–627. [DOI] [PubMed] [Google Scholar]
- Woodward MA, Blachley TS, Stein JD, 2016. The association between socio-demographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology 123 457–465.e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina S, Barka T, 1973. Development of endogenous peroxidase in fetal rat submandibular gland. J. Histochem. Cytochem 21, 42–50. [DOI] [PubMed] [Google Scholar]
- Yeh S, Miyamoto H, Shima H, Chang C, 1998. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc. Natl. Acad. Sci. U. S. A 95, 5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz, Bardak, Gunay, et al. , 2017. Novel zinc finger protein gene 469 (Znf469) variants in advanced keratoconus. Curr. Eye Res 42, 1396–1400. [DOI] [PubMed] [Google Scholar]
- Yildiz EH, Diehl GF, Cohen EJ, Hammersmith KM, Laibson PR, Rapuano CJ, 2009. Demographics of patients older than 50 Years with keratoconus. Eye Contact Lens 35, 309–311. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO, 1998. Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest. Ophthalmol. Vis. Sci 39, 2537–2546. [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ, 2002. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biol. Med 33, 337–349. [DOI] [PubMed] [Google Scholar]
- Zhang, Cheng, Li, Zhang, Yi, Xu, Zhou, 2016a. Saliva in the diagnosis of diseases. Int. J. Oral Sci 8, 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Hong J, Wu D, Xu J, 2016b. Association of common variants in LOX with keratoconus: a meta-analysis. PLoS One 10, e0145815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jeyalatha MV, Qu Y, He X, Ou S, Bu J, Jia C, Wang J, Wu H, Liu Z, Li W, 2017. Dry eye management: targeting the ocular surface microenvironment. Int. J. Mol. Sci 18, 1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, 2012. In-depth analysis of the human tear proteome. J. Proteomics 75, 3877–3885. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Cai LQ, 2006. Effects of male sex hormones on gender identity, sexual behavior, and cognitive function. Zhong nan da xue xue bao. Yi xue ban = J. Cent. S. Univ. Med. Sci 31, 149–161. [PubMed] [Google Scholar]


