Abstract
Purpose:
Based on improvement in pathologic complete response (pCR) in NeoSphere and TRYPHAENA studies, the FDA approved neoadjuvant pertuzumab for HER2+ localized breast cancer. These studies demonstrated high pCR rates with THP (docetaxel+HP), FEC (5-fluorouracil, epirubicin and cyclophosphamide)-THP, and TCHP (docetaxel, carboplatin+HP). However, in the United States, doxorubicin/cyclophosphamide (AC) is favored over FEC despite no data comparing neoadjuvant AC-THP with AC-TH or TCHP. Here we report outcomes for patients with localized HER2+ breast cancer treated with pertuzumab-containing neoadjuvant regimens and AC-TH.
Methods:
We reviewed clinicopathological characteristics of patients with HER2+ breast cancer (Stage I-III) treated with either a neoadjuvant pertuzumab-containing regimen or dosedense (dd) AC-TH, from 2011 to 2016 at a large academic medical institution and two affiliated community sites. pCR was defined as ypT0/is ypN0. Fisher’s exact test and logistic regression analysis were used for statistical analysis.
Results:
In this study (N = 121), pCR was numerically higher with pertuzumab based regimens, including ddAC-THP (60%), TCHP (63%), THP (55%), as compared with ddAC-TH (46%). THP resulted in significantly less cycle delays due to toxicity compared to the other regimens (p=0.02). THP also resulted in the least dose reductions, lowest rate of hospitalization, and lowest rate of treatment discontinuation.
Conclusions:
Pertuzumab based regimens, including THP, resulted in higher pCR rates as compared to ddAC-TH, with the THP regimen associated with the best tolerability among patients with localized HER2+ breast cancer. Given the various neoadjuvant regimens, additional studies are needed to determine optimal treatment sequencing and escalation/de-escalation strategies to personalize neoadjuvant regimens for localized HER2+ breast cancer.
Keywords: Neoadjuvant, HER2, pathologic complete response, pertuzumab, trastuzumab
INTRODUCTION:
Human epidermal growth factor receptor-2 positive (HER2+) breast cancer is defined by amplification of the HER2/neu oncogene and represents an aggressive subtype of breast cancer [1]. Approximately 20% of breast cancers overexpress the HER2 protein [2]. HER2-targeted therapies, beginning with the anti–HER2 humanized monoclonal antibody trastuzumab, have greatly improved the prognosis of this disease [3]. In the localized setting, the addition of trastuzumab to adjuvant chemotherapy resulted in a relative reduction in the risk of relapse by 50% and mortality by 30% [4–7]. Despite improved outcomes, recurrence and resulting morbidity and mortality in the metastatic setting remains a clinical challenge. Improving therapeutic options in the localized setting to reduce recurrence risk remains an active area of interest.
Neoadjuvant therapy (NAT) is increasingly used in the management of localized breast cancer as an alternative to adjuvant chemotherapy, with studies demonstrating similar long-term outcomes in either setting [8, 9]. However, the use of NAT offers several additional advantages from both a clinical and research perspective. For patients with larger tumors, the use of NAT may reduce tumor size resulting in improved rates of breast conservation surgery (BCS) and less extensive axillary surgery [10]. In addition, neoadjuvant treatment allows for monitoring of treatment response, providing potentially important prognostic information. It also allows for discontinuation of inactive therapy in the setting of disease progression, thereby reducing exposure to ineffective and potentially toxic therapy. From a research perspective, the neoadjuvant platform serves as a human in vivo system to explore surrogate endpoints, predictive biomarkers, and the efficacy of novel therapies [11]. Pathologic complete response (pCR) following neoadjuvant therapy has been shown to be a surrogate marker for disease free-survival and overall survival, particularly for HER2+ breast cancer, and has been utilized by the U.S. Food and Drug administration [12–14].
In 2013, the FDA granted accelerated approval to pertuzumab for use in the neoadjuvant setting with trastuzumab and chemotherapy for HER2+ locally advanced, inflammatory, or early-stage breast cancer (either greater than 2 cm in diameter or node positive), based on improvement in pCR in the NeoSphere (Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation) and TRYPHAENA (Trastuzumab plus Pertuzumab in Neoadjuvant HER2-positive Breast Cancer) studies [15, 16]. Full approval of pertuzumab in the localized setting for patients at high risk of recurrence was recently received based on results of the adjuvant APHINITY study [17]. NeoSphere demonstrated a higher breast pCR rate with THP (docetaxel, trastuzumab, pertuzumab) as compared to TH (breast pCR 45.8 vs. 29%), and TRYPHAENA demonstrated high total pCR rates with FEC (5-fluorouracil, epirubicin and cyclophosphamide)-THP (pCR 54.7%) and TCHP (docetaxel, carboplatin, HP, pCR 63.6%) [15, 16]. However, in the US, doxorubicin and cyclophosphamide (AC), often given in a dose-dense fashion, is favored over FEC, which led to the clinical utilization of AC-THP, despite no data comparing AC-THP with AC-TH or TCHP in the neoadjuvant setting. To address this unmet need, we evaluated the effectiveness and tolerability of pertuzumab-containing neoadjuvant regimens and AC-TH.
METHODS:
Patient Selection
We performed an IRB-approved retrospective review of HER2+ patients treated at a large academic medical institution and two affiliated community sites from 2011–2016 with one of the following neoadjuvant regimens 1) dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab (AC-THP), 2) paclitaxel or docetaxel plus pertuzumab and trastuzumab (THP), 3) docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP), or 4) dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab (AC-TH). Growth factor support was utilized for the dose-dense AC portion of regimens and with the TCHP regimen. The list of patients receiving each regimen was generated from searching pharmacy treatment plan records. Patients were excluded if part of their care was received elsewhere and sufficient details were unavailable.
Data collection and Endpoints
Electronic medical records were examined for patient demographics, breast cancer stage, pathology results, surgical outcomes, treatment details, and echocardiogram results. HER2 positivity was defined as 3+ by immunohistochemistry and/or HER2/CEP17 ratio 2.0 or greater by fluorescence in situ hybridization (FISH) and/or HER2 gene copy number greater than 6.0 as per ASCO/CAP guidelines [18]. The cutoff values for estrogen receptor (ER) positivity and progesterone receptor (PR) positivity were 1% or greater positive nuclei as per ASCO/CAP guidelines [19]. A pCR was defined as no residual invasive disease in the breast and axilla, with non-invasive residuals, including ductal carcinoma in situ (DCIS), permitted (ypT0/is ypN0), as per FDA guidance [14]. Tolerability was defined as the percentage of intended cycles received, dose reduction due to toxicity, dose delay due to toxicity, treatment discontinuation (defined as changing regimens or permanently dropping one of the agents), and hospitalizations from treatment-related toxicity. Cardiac outcomes based on changes in left ventricular ejection fraction (LVEF) were also evaluated.
Statistical Analysis
Descriptive statistics were used to describe baseline characteristics. Fisher’s exact test was used to compare groups, though the study was not powered to detect a statistically significant difference in pCR rates among the different regimens. Given the small sample size, 50% exact binomial confidence intervals (CI) were calculated for pCR rate to provide probability estimate with computational stability. Logistic regression was used to evaluate association of clinicopathological factors with pCR and other outcomes. Statistical significance was defined as a p value less than 0.05. Stata (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) was used to perform analyses.
RESULTS
A total of 121 women treated with a neoadjuvant pertuzumab-containing regimen or AC-TH for HER2+ invasive breast cancer from 2011 through 2016 were included in the analytical dataset. Baseline patient characteristics overall and by regimen are shown in table 1. The majority of patients had grade 3 tumors (62.8%), clinical stage II disease (76.9%), and invasive ductal carcinoma histology (90.1%). Over half the patients had ER+ disease (62%). The median HER2/CEP17 copy number ratio by FISH was 6. Over half the patients underwent a mastectomy (60.3%). The majority of patients received adjuvant radiation (81%). None of the patients had disease progression on initially assigned neoadjuvant regimen.
Table 1.
Patient characteristics (N = 121).
| Overall (%) N = 121 | AC-THP (%)N = 45 | THP* (%) N = 29 | TCHP (%) N = 19 | AC-TH (%)N = 28 | |
|---|---|---|---|---|---|
| Median age at diagnosis (range) | 48 (41–57) | 50 (28–72) | 43 (27–67) | 48 (34–72) | 48 (29–72) |
| Clinical anatomic stage I II III |
3 (2.5) 93 (76.9) 25 (20.7) |
2 (4.4) 37 (82.2) 6 (13.3) |
1 (3.4) 23 (79.3) 5 (17.2) |
0 15 (78.9) 4 (21.1) |
0 18 (64.3) 10 (35.7) |
| Tumor histology Invasive ductal carcinoma (IDC) Invasive lobular carcinoma (ILC) Mixed IDC/ILC Other |
109 (90.1) 5 (4.1) 6 (5.0) 1 (0.8) |
40 (88.9) 1 (2.2) 4 (8.9) 0 |
24 (82.8) 3 (10.3) 1 (3.4) 1 (3.4) |
17 (89.8) 1 (5.3) 1 (5.3) 0 |
28 (100.0) 0 0 0 |
| Grade 1 2 2–3 3 |
3 (2.5) 27 (22.3) 15 (12.4) |
2 (4.4) 9 (20.0) 4 (8.9) |
1 (3.4) 6 (20.7) 6 (20.7) |
0 9 (47.4) 1 (5.3) |
0 3 (10.7) 4 (14.3) |
| 76 (62.8) | 30 (66.7) | 16 (55.2) | 9 (47.4) | 21 (75.0) | |
| Estrogen receptor status ER+ ER- |
75 (62.0) 46 (38.0) |
27 (60.0) 18 (40.0) |
19 (65.5) 10 (34.5) |
15 (78.9) 4 (21.1) |
14 (50.0) 14 (50.0) |
| Progesterone receptor status PR+ PR- |
51 (42.1) 70 (57.9) |
20 (44.4) 25 (55.6) |
10 (34.5) 19 (65.5) |
11 (57.9) 8 (42.1) |
10 (35.7) 18 (64.3) |
| Menopausal status Premenopausal Postmenopausal |
69 (57.0) 52 (43.0) |
27 (60.0) 18 (40.0) |
18 (62.1) 11 (37.9) |
10 (52.6) 9 (47.4) |
14 (50.0) 14 (50.0) |
| HER2 IHC 2+ 3+ Unavailable (FISH only performed) |
24 (19.8) 90 (74.4) 7 (5.79) |
8 (17.8) 35 (77.8) 2 (4.4) |
7 (24.1) 21 (72.4) 1 (3.4) |
6 (31.5) 13 (68.4) 0 |
3 (10.7) 21 (75.0) 4 (14.3) |
| Median HER2/CEP17 ratio by FISH** | 6.2 | 6.5 | 4.95 | 5.1 | 6.7 |
| Median HER2 copy number** | 13 | 14.9 | 12.8 | 12.65 | 9.8 |
| Type of taxane Paclitaxel Docetaxel |
39 (32.2) 82 (67.8) |
21 (46.7) 24 (53.3) |
18 (62.1) 11 (38.0) |
0 19 (100.0) |
0 28 (100.0) |
| Type of surgery Breast conserving surgery Mastectomy |
48 (39.7) 73 (60.3) |
18 (40.0) 27 (60.0) |
8 (27.6) 21 (72.4) |
9 (47.4) 10 (52.6) |
13 (46.4) 15 (53.6) |
| Adjuvant radiation Yes No |
97 (80.2) 24 (19.8) |
39 (86.7) 6 (13.3) |
22 (75.9) 7 (24.1) |
15 (78.9) 4 (21.1) |
21 (75.0) 7 (25.0) |
Legend: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2; CEP17, centromeric probe for chromosome 17; FISH, fluorescence in situ hybridization; AC-THP, dosedense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab; THP, paclitaxel or docetaxel plus pertuzumab and trastuzumab; TCHP, docetaxel/carboplatin/trastuzumab/pertuzumab; AC-TH, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab
*26 (90%) of the neoadjuvant THP patients received adjuvant dose-dense AC
23 (19%) patients did not have FISH performed
Rates of pathologic complete response by regimen
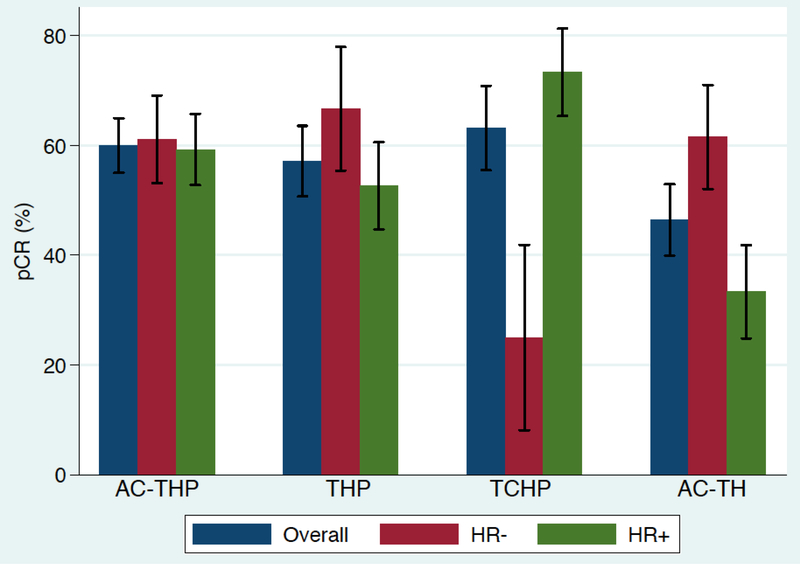
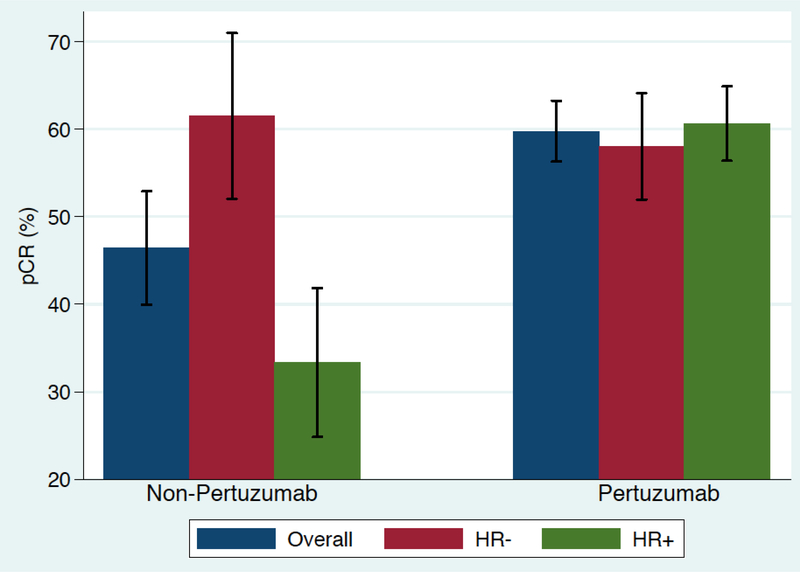
The pCR rate was numerically higher with pertuzumab based regimens, including AC-THP (60%; CI 0.56–0.64), TCHP (63%; CI 0.56–0.69), THP (55%; CI 0.50–0.60), as compared with AC-TH (46%; CI 0.41–0.52). The pCR results stratified by hormone receptor (HR) status are shown in figure 1a. Among HR-positive patients, the pCR rate was highest for TCHP at 73.3% (CI 0.66–0.80) and lowest for AC-TH at 33.3% CI 0.26–0.41), and for HR-negative patients, the pCR rate was highest with THP at 66.7% (CI 0.56–0.76). Overall, patients receiving pertuzumab as part of a neoadjuvant regimen had a pCR rate of 60% (CI 0.56–0.62) while those who did not receive pertuzumab had a pCR rate of 46% (CI 0.41–0.52) as shown in figure 1b. After controlling for age, stage, and HR status, the addition of pertuzumab resulted in an odds ratio of 5.25 favoring pCR compared to regimens without pertuzumab (p = 0.095).
Figure 1a. Pathologic complete response rate (pCR) based on regimen (N = 121).
AC-THP, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab; THP, paclitaxel or docetaxel plus pertuzumab and trastuzumab; TCHP, docetaxel/carboplatin/trastuzumab/pertuzumab; AC-TH, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab
Figure 1b. Pathologic complete response rate (pCR) based on regimen and hormone receptor status (N = 121).
AC-THP, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab; THP, paclitaxel or docetaxel plus pertuzumab and trastuzumab; TCHP, docetaxel/carboplatin/trastuzumab/pertuzumab; AC-TH, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab
Predictors of pathologic complete response
In a univariate and multivariate analysis (table 2), HER2 IHC 3+ staining compared to less than 3+ was significantly associated with achievement of pCR (univariate: OR 3.56, 95% CI 1.37–9.24, p = 0.01; multivariate: OR 3.71, 95% CI 1.1312.22, p = 0.03). Consistent with the literature, other factors with an indication of association with pCR in both the univariate and multivariate models were lower clinical stage, HR negative status, higher HER2 FISH ratio, and the use of pertuzumab, though small sample size limits interpretation. Receiving an anthracyline-based regimen compared to a non-anthracycline-based regimen was not associated with higher odds of pCR (OR 0.82, 95% CI 0.39–1.73, p = 0.61).
Table 2.
Association of clinicopathological factors with pathologic complete response.
| Factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age at diagnosis | 0.97 | 0.94–1.00 | 0.09 | 0.98 | 0.94–1.02 | 0.34 |
| Clinical stage III vs. II |
0.79 | 0.33–1.91 | 0.60 | 0.73 | 0.24–2.20 | 0.57 |
| Grade 3 vs. less than grade 3 | 0.99 | 0.47–2.10 | 0.98 | 0.97 | 0.37–2.52 | 0.94 |
| HR+ vs. HR- | 0.86 | 0.40–1.81 | 0.68 | 0.77 | 0.28–2.10 | 0.60 |
| HER2 FISH ratio > 8 vs. < 8 |
2.06 | 0.84–5.06 | 0.11 | 1.47 | 0.50–4.33 | 0.48 |
| HER2 IHC 3+ vs. less than 3+ |
3.56 | 1.37–9.24 | 0.01 | 3.71 | 1.13- 12.22 |
0.03 |
| Pertuzumabcontaining regimen vs. not | 1.72 | 0.73–4.02 | 0.21 | 2.75 | 0.85–8.88 | 0.09 |
| Anthracyclinecontaining regimen vs. not | 0.82 | 0.39–1.73 | 0.61 | 0.98 | 0.32–2.93 | 0.97 |
Legend: HR+, hormone receptor positive; HER2, human epidermal growth factor 2; FISH, fluorescence in situ hybridization; IHC = immunohistochemistry
Tolerability by regimen
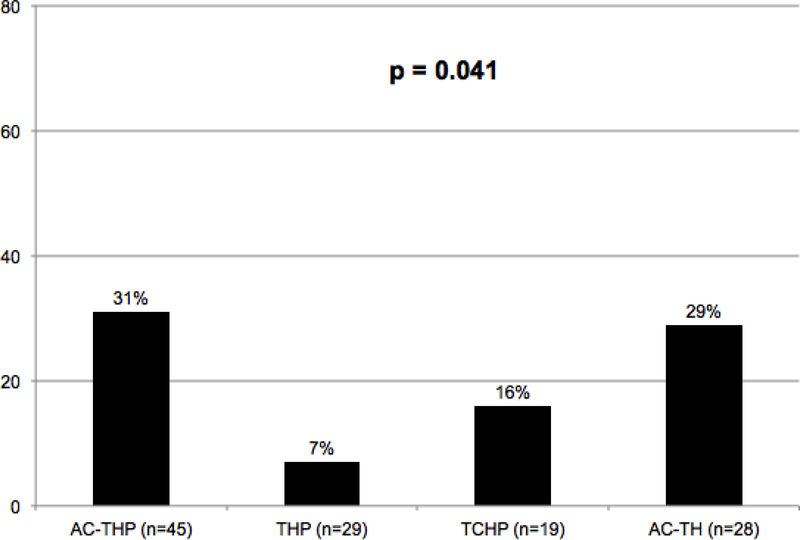
Among the four regimens, those receiving AC-THP, THP, or AC-TH ultimately received at least 95% of the intended total number of cycles of therapy, while those receiving TCHP received 89% of the intended number of cycles. A cycle delay due to toxicity occurred in 31% of the patients receiving AC-THP, 7% for THP, 16% for TCHP, and 29% for AC-TH (p = 0.041; figure 2). THP resulted in fewer dose delays due to toxicity compared to the other regimens combined (p = 0.022). Patients receiving THP also had fewer dose reduction, with 13.8% of THP patients requiring a dose reduction compared to 24.4% for AC-THP, 31.6% for TCHP, and 35.7% for AC-TH. At least one hospitalization from treatment-related toxicity occurred in 18% of the patients receiving AC-THP, 10% for THP, 11% for TCHP, and 11% for AC-TH (p = 0.74). Reasons for hospitalization broken down by regimen, including receipt of paclitaxel vs. docetaxel, are show in table 3. The rate of treatment discontinuation occurred in 11.5% of patients overall, including 16% of patients receiving AC-THP, 16% for TCHP, 3% for THP, and 11% for AC-TH (p = 0.38).
Figure 2. Frequency of cycle delay due to toxicity based on regimen (N=121).
AC-THP, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab; THP, paclitaxel or docetaxel plus pertuzumab and trastuzumab; TCHP, docetaxel/carboplatin/trastuzumab/pertuzumab; AC-TH, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab
Table 3.
Causes of hospitalization by regimen
|
Regimen |
Reasons for Hospitalization | |||||
|---|---|---|---|---|---|---|
| Febrile Neutropenia | Fever without Neutropenia | Venous thromboembolism | Syncope | Septic shock | Congestive heart failure | |
| AC-THP -Taxol -Taxotere |
5 0 5* |
2 0 2 |
0 | 1 1 0 |
0 | 1 1 0 |
| THP -Taxol -Taxotere |
2 0 2 |
0 | 1 1 0 |
0 | 0 | 0 |
| TCHP | 0 | 0 | 0 | 1 | 1 | 0 |
| AC-TH | 3* | 1 | 0 | 0 | 0 | 0 |
Legend: AC-THP, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab; THP, paclitaxel or docetaxel plus pertuzumab and trastuzumab; TCHP, docetaxel/carboplatin/trastuzumab/pertuzumab; AC-TH, dose-dense doxorubicin/cyclophosphamide followed by paclitaxel or docetaxel plus trastuzumab
One patient hospitalized twice
Cardiac outcomes by regimen
After a median follow-up time of 60 months, symptomatic cardiac left ventricular ejection fraction (LVEF) dysfunction during neoadjuvant/adjuvant treatment through the completion of trastuzumab was experienced by two (4%) patients receiving AC-THP, one (3%) patient receiving THP (90% of the THP patients received adjuvant ddAC), one (5%) patient receiving TCHP, and no patients receiving AC-TH (p = 0.82). A summary of changes in LVEF from the start of treatment through the completion of trastuzumab is summarized in supplemental table 1. In considering a decrease in LVEF of less than 50% with an absolute reduction of at least 10% from baseline, as described in the HERA trial [4], there were no statistically significant differences between the groups, with the highest rates numerically being observed in the AC-THP group (AC-THP: 7%, THP: 4%, TCHP: 5%, AC-TH: 4%; p = 1.00).
DISCUSSION:
Our results suggest neoadjuvant pertuzumab based regimens confer superior pCR rates with similar tolerability compared to AC-TH. The observed pCR rates align with results observed in TRYPHAENA. In TRYPHAENA, the pCR rate for FEC-THP was 54.7%, while we observed a pCR rate of 60% for dose-dense AC-THP. For TCHP, TRYPHAENA reported a pCR rate of 63.6% and we observed a pCR rate of 63%. Our results revealed similar tolerability between AC-THP and TCHP, while THP was the best tolerated overall with the least dose reductions, least cycle delays for toxicity, lowest rate of hospitalization, and lowest rate of treatment discontinuation.
Rates of symptomatic LVEF dysfunction were low overall among all regimens, though rates of asymptomatic decreases in LVEF were lower among those receiving the non-anthracycline regimen TCHP. However, given small sample size, assessment of cardiac outcomes is best determined in large registration trials. In TRYPHAENA, symptomatic LVEF dysfunction was noted in 2.7% of patients, and average LVEF decrease was lowest in the TCHP arm (3%). In considering FEC vs. AC, while it was initially suggested that epirubicin was less cardiotoxic than doxorubicin, a Cochrane database review of 5 randomized trials comparing the agents did not find a statistically significant difference in the incidence of heart failure between the two agents [20, 21]. The BERENICE trial demonstrated low rates of symptomatic LVEF dysfunction with both FEC-THP and AC-THP [22].
Similar to the lack of trial data comparing neoadjuvant AC-THP to AC-TH, there is also a lack of trial data comparing neoadjuvant AC-THP and TCHP. Both regimens are considered acceptable pertuzumab-containing regimens according to the National Comprehensive Cancer Clinical Practice Guidelines [22]. However, AC-TH has been compared with TCH in the adjuvant setting [6]. Results of BCIRG 006 trial demonstrated no significant difference in disease-free survival between AC-TH and TCH, though numerically AC-TH was superior (84% vs. 81%) [6]. The lack of significant difference in efficacy outcomes, combined with the modestly increased rates of cardiac dysfunction and leukemia in the anthracycline-containing groups has led some providers to favor TCHP even in the absence of a known contraindication to anthracylines. However, others have expressed concern that that AC was not given in a dose-dense fashion in BCIRG 006, which could account for some of the lack of difference in efficacy given the known benefit of the dose-dense approach in other settings [23]. In the adjuvant APHINITY trial, invasive-disease-free survival was similar between the anthracycline and non-anthracycline regimens, though the study was not designed to compare these regimens head to head and AC was not given in a dose-dense fashion [17]. There is variability in how AC is administered in HER2+ breast cancer, with both every three-week dosing and dose-dense dosing utilized. Our results revealed similar pCR rates between neoadjuvant dose-dense AC-THP and TCHP. A retrospective study of 57 patients receiving neoadjuvant dose-dense AC-THP by Singh et al. demonstrated a pCR rate of 72%.[24] Larger studies are needed to understand the impact of the dose-dense approach on pCR rates.
Compared to NeoSphere, where the breast pCR rate for THP was 45.8%, our study demonstrated a total pCR rate of 55%, though the confidence interval was wide and this higher than expected pCR rate may be due to small sample size. In NeoSphere, patients received FEC in the adjuvant setting and, in the US, most patients who receive neoadjuvant THP will receive adjuvant AC. However, the high pCR rates and favorable tolerability with THP raises the question of whether the achievement of pCR could be used to scale back adjuvant therapy given the prognostic significance of pCR. Studies such as APHINITY, which examined a year of pertuzumab in addition to the standard year of trastuzumab, and ExteNET, which evaluated neratanib after completion of standard trastuzumab-based adjuvant therapy, have focused on adding additional therapy [17, 25]. Importantly, both studies were adjuvant studies and therefore the impact of pCR could not be considered. Given that neoadjuvant therapy is commonly used for HER2+ breast cancer, clinicians are increasingly faced with challenges regarding how to best use and sequence the multitude of therapies available. For example, if a patient has a pCR following neoadjuvant pertuzumab-based therapy, pertuzumab could be continued to complete a year of therapy along with trastuzumab in the adjuvant setting, or one could consider scaling back therapy and omitting further pertuzumab given the prognostic significance of pCR. Similarly, if a patient has pCR after THP, one could question the need for adjuvant AC. In our study, 90% of patients received adjuvant AC after neoadjuvant THP, including the ones who achieved pCR with THP alone. Given the prognostic significance of pCR in HER2+ disease, novel trial designs featuring deescalation of therapy following pCR, and escalation of therapy when pCR is not achieved, are warranted. The planned DAPHNe study will use neoadjuvant THP only (personal communication with PI) and those who achieve a pCR will receive adjuvant HP only to see if anthracyclines can be avoided in this population.
This study has several limitations, in large part due to its retrospective nature. First, given the relatively small sample size for each respective regimen, the study was not powered to compare individual groups and subset analysis, including HR+ status. In addition, the arms were not entirely balanced, including variability in HR+ status, which is a key determinant of pCR. We calculated that to evaluate for 15% improvement in pCR with adequate power (≥ 80%), the sample size needed would be close to 400, and would need 282 AC-THP cases and 170 AC-TH cases (with alpha 0.05 and power 0.8) for a two-sided test, and 222 AC-THP cases and 134 AC-TH cases for a one-sided test. The primary intent of this study was to provide description of real-world clinical outcomes with different pertuzumab based regimens, including AC-THP, as it is frequently used in clinical practice. Second, tolerability was defined using objective measures, as standard grading of toxicities was not available. Important side effects, such as diarrhea seen with pertuzumab, could therefore not be directly compared among the regimens. The type of taxane was also not specifically considered and may have played a role in tolerability, as demonstrated by the hospitalization data showing more frequent hospitalizations with docetaxel than paclitaxel among the same regimen. Finally, given short follow-up time, long-term outcome measures such as disease-free survival and overall survival could not be evaluated.
In summary, pertuzumab based regimens resulted in high pCR rates that were broadly similar between different pertuzumab based regimens. Patients receiving THP exhibited the best tolerability, and additional studies are needed to determine optimal treatment sequencing and escalation/de-escalation strategies, potentially with THP backbone, to personalize neoadjuvant regimens for localized HER2+ breast cancer.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH Grant KL2 TR001100 (to L.S), and Susan G Komen CCR15224703 (to A.B.).
Footnotes
Compliance with Ethical Standards:
Conflict of Interest:
Laura Spring declares that she has no relevant conflict of interest.
Andrzej Niemierko declares that he has no relevant conflict of interest.
Stephanie Haddad declares that she has no relevant conflict of interest.
Megan Yuen declares that she has no relevant conflict of interest.
Amy Comander declares that she has no relevant conflict of interest.
Kerry Reynolds declares that she has no relevant conflict of interest.
Jennifer Shin declares that she has no relevant conflict of interest.
Atul Bahn declares that he has no relevant conflict of interest.
Elena Brachtel declares that she has no relevant conflict of interest.
Michele Specht declares that she has no relevant conflict of interest.
Barbara L. Smith declares that she has no relevant conflict of interest.
Alphonse Taghian declares that he has no relevant conflict of interest.
Rachel Jimenez declares that she has no relevant conflict of interest.
Jeffrey Peppercorn declares that he has no relevant conflict of interest.
Steven J. Isakoff declares that he has no relevant conflict of interest.
Beverly Moy declares that she has no relevant conflict of interest.
Aditya Bardia declares that he has no relevant conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182. doi: 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 2.Elster N, Collins DM, Toomey S, et al. (2015) HER2-family signalling mechanisms, clinical implications and targeting in breast cancer. Breast Cancer Res Treat 149:5–15. doi: 10.1007/s10549-014-3250-x [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. (2001) Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med 344:783–792. doi: 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. (2005) Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med 353:1659–1672. doi: 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. (2005) Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N Engl J Med 353:1673–1684. doi: 10.1056/NEJMoa052122 [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Eiermann W, Robert N, et al. (2011) Adjuvant Trastuzumab in HER2Positive Breast Cancer. N Engl J Med 365:1273–1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, et al. (2006) Adjuvant Docetaxel or Vinorelbine with or without Trastuzumab for Breast Cancer. N Engl J Med 354:809–820. doi: 10.1056/NEJMoa053028 [DOI] [PubMed] [Google Scholar]
- 8.Mauri D, Pavlidis N, Ioannidis JPA (2005) Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. J Natl Cancer Inst 97:188–194. doi: 10.1093/jnci/dji021 [DOI] [PubMed] [Google Scholar]
- 9.Rastogi P, Anderson SJ, Bear HD, et al. (2008) Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–785. doi: 10.1200/JCO.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- 10.King TA, Morrow M (2015) Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol 12:335–343. doi: 10.1038/nrclinonc.2015.63 [DOI] [PubMed] [Google Scholar]
- 11.Bardia A, Baselga J (2013) Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res 19:6360–6370. doi: 10.1158/10780432.CCR-13-0916 [DOI] [PubMed] [Google Scholar]
- 12.Broglio KR, Quintana M, Foster M, et al. (2016) Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol 2:751–760. doi: 10.1001/jamaoncol.2015.6113 [DOI] [PubMed] [Google Scholar]
- 13.Cortazar P, Zhang L, Untch M, et al. (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384:164–172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry: Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. Accessed 13 Jul 2016
- 15.Gianni L, Pienkowski T, Im Y-H, et al. (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, openlabel, phase 2 trial. Lancet Oncol 13:25–32. doi: 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss A, Chia S, Hickish T, et al. (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracyclinefree chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284. doi: 10.1093/annonc/mdt182 [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Procter M, de Azambuja E, et al. (2017) Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 377:122–131. doi: 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond MEH, Hicks DG, et al. (2013) Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol 31:3997–4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 19.Singh JC, Mamtani A, Barrio A, et al. (2017) Pathologic Complete Response with Neoadjuvant Doxorubicin and Cyclophosphamide Followed by Paclitaxel with Trastuzumab and Pertuzumab in Patients with HER2-Positive Early Stage Breast Cancer: A Single Center Experience. The Oncologist 22:139–143. doi: 10.1634/theoncologist.2016-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LA, Cornelius VR, Plummer CJ, et al. (2010) Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10:337. doi: 10.1186/1471-2407-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dalen EC, Michiels EM, Caron HN, Kremer LC (2010) Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev CD005006. doi: 10.1002/14651858.CD005006.pub4 [DOI] [PubMed] [Google Scholar]
- 22.Gradishar WJ, Anderson BO, Balassanian R, et al. (2016) Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN 14:324–354 [DOI] [PubMed] [Google Scholar]
- 23.Citron ML, Berry DA, Cirrincione C, et al. (2003) Randomized Trial of Dose-Dense Versus Conventionally Scheduled and Sequential Versus Concurrent Combination Chemotherapy as Postoperative Adjuvant Treatment of Node-Positive Primary Breast Cancer: First Report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439. doi: 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 24.Singh JC, Mamtani A, Barrio A, et al. (2017) Pathologic Complete Response with Neoadjuvant Doxorubicin and Cyclophosphamide Followed by Paclitaxel with Trastuzumab and Pertuzumab in Patients with HER2-Positive Early Stage Breast Cancer: A Single Center Experience. The Oncologist 22:139–143. doi: 10.1634/theoncologist.2016-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan A, Delaloge S, Holmes FA, et al. (2016) Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:367–377. doi: 10.1016/S1470-2045(15)00551-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.