Abstract
This paper investigates the safety of a novel ‘magnetic injection’ method of delivering therapy to the cochlea, in a rodent model. In this method of administration, a magnetic field is employed to actively transport drug-eluting superparamagnetic iron-oxide core nanoparticles into the cochlea, where they then release their drug payload (we delivered the steroid prednisolone). Our study design and selection of control groups was based on published regulatory guidance for safety studies that involve local drug delivery. We tested for both single and multiple delivery doses to the cochlea, and found that magnetic delivery did not harm hearing. There was no statistical difference in hearing between magnetically treated ears versus ears that received intra-tympanic steroid (a mimic of a standard-of-care for sudden sensorineural hearing loss), both 2 and 30 days after treatment. Since our treatment is local to the ear, the levels of steroid and iron circulating systemically after our treatment were low, below mass-spectrometry detection limits for the steroid and no different from normal for iron. No adverse findings were observed in ear tissue histopathology or in animal gross behavior. At 2 and 30 days after treatment, inflammatory changes examined in the ear were limited to the middle ear, were very mild in severity, and by day 90 there was ongoing and almost complete reversibility of these changes. There were no ear tissue scarring or hemorrhage trends associated with magnetic delivery. In summary, after conducting a pre-clinical safety study, no adverse safety issues were observed.
Keywords: Magnetic nanoparticles, Magnetic injection, Rat cochlea, Auditory brainstem response (ABR), Ototoxicity, Hearing loss, Prednisolone
Chemical compounds studied in this article: Prednisolone, Methyl-prednisolone sodium succinate [Solu-Medrol], Prednisolone sodium phosphate, Iron oxide, Chitosan
1. Introduction
The inner ear is located in the hardest bone in the body, making its direct access difficult. It is also isolated from systemic circulation by a blood-labyrinth barrier, similar to the blood-brain barrier, making it difficult to treat via systemic administration (Borenstein, 2011; Inamura and Salt, 1992; Juhn et al., 2001; Radeloff et al., 2007; Salt and Plontke, 2005). Although it is believed that effective drugs exist to treat conditions of the cochlea and the vestibular system, conditions such as hearing loss, tinnitus, and Ménière’s disease, these drugs do not seem to reach the inner ear in sufficient quantities to be efficacious. For instance, published data indicates that in some cases only 1 out of every 107 drug molecules administered systemically reaches the cochlea (Parnes et al., 1999). For intra-tympanic administration of steroid whereby the drug is deposited into the middle ear next to the window membranes that separate the middle ear from the inner ear, then in human patients < 0.01% reaches the cochlea (Bird et al., 2007; Bird et al., 2011). As indicated in the review article by Salt and Plontke, there is a need for a drug delivery method that can deliver sufficient drug doses to the cochlear safely (Salt and Plontke, 2009).
Our long term goal is to develop and demonstrate an effective and safe method for drug delivery to the cochlea and vestibular system. In order to deliver higher doses of therapy to the inner ear, we have developed a magnetic push (magnetic injection) system that uses magnetic fields to transport therapy from the middle ear, through the window membranes, and into the cochlea (Fig. 1). A formulation of bio-compatible magnetic nanoparticles loaded with drug (e.g. steroid) is first placed in the middle ear by intra-tympanic injection. Delivery of steroids to the cochlea by intra-tympanic administration is a current standard-of-care for treatment of sudden sensorineural hearing loss (SSNHL) (Bielefeld et al., 2010; Hu and Parnes, 2009; Rauch et al., 2011). Steroid delivery to the cochlea can also potentially treat noise-induced hearing loss, suppress tinnitus, or protect hearing from chemotherapy regimens (Bird et al., 2007; Filipo et al., 2010; Li et al., 2013; Martin-Saldana et al., 2016; Parnes et al., 1999; Spear and Schwartz, 2011). In our case, to increase drug delivery to the cochlea, our magnetic injector device is then placed near the ear, and applies a magnetic push force on the nanoparticles to transport them from the middle ear, through the window membranes, and into the cochlea. Once in the cochlea, the particles release their therapeutic payload over time.
Fig. 1.
Magnetic injection to the cochlea. A) Approximate size and placement of a proposed magnet device (shown in dark blue) intended for human clinical use. B) In this type of administration, magnetic nanoparticles with encapsulated drug would be placed in the middle ear by intra-tympanic injection. Then the magnet injector would be applied to magnetically deliver the particles from the middle ear, through the window membranes, and into the cochlea, where they would then release their therapeutic payload. (The ear anatomy figure is from Wikipedia: https://en.wikipedia.org/wiki/Auditory_system). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Any single magnet can only attract (pull in) magnetic particles (Shapiro et al., 2014). In contrast, magnetic injection is achieved by arranging the magnetizations of two or more permanent magnets in such a way that a magnetic field minimum is created behind the nanoparticles, and this magnetic field node acts to push the particles away (to inject them) through the window membranes and into the cochlea (Depireux et al., 2017; Shapiro et al., 2013; Shapiro et al., 2010; Shapiro and Rutel, 2009). Magnetic push uses a magnet placed next to the to-be-treated ear, and is preferred over a magnet system pulling from the other side of the head. For adult human head dimensions, magnetic push (magnetic injection) allows a compact device to act over a short distance from the magnet to the cochlea. A pull magnet placed at the contralateral ear would have to act over a much longer distance, it would have to pull across the entire width of the human head. The closer push magnet placement allows use of a much smaller and hence safer magnetic field (approximately 20 times smaller than would be needed for a pull magnet (Sarwar et al., 2013; Shapiro et al., 2014)). The push magnet device in this study uses permanent magnets with 1.45 Tesla internal magnetization. These magnets generate a maximum external magnetic field strength of about 1 Tesla, which is substantially less than the 3 Tesla and higher magnetic field strengths safely administered to patients by clinical grade MRI machines (Allen and Burdette, 2001).
Delivery and efficacy of magnetic injection have been addressed in prior studies, and are being optimized further in ongoing work. Initial invention, design and validation of a magnetic injection system was initially patented (Shapiro et al., 2013; Shapiro and Rutel, 2009) and then published in Shapiro, Dormer and Rutel (Shapiro et al., 2010). Then magnetic injection to the cochlea in-vivo was demonstrated first in rats, in Sarwar et al. (2013). In these first rat studies, and in subsequent animal studies, the magnet was applied at a magnet-to-cochlea distance anticipated for adult human patients. Subsequently, the therapeutic effect of magnetically delivering prednisolone to the cochlea was studied in recognized animal models for noise induced hearing loss, for tinnitus, and for protection of hearing from cisplatin chemotherapy regimens (Depireux et al., 2017; Ramaswamy et al., 2017; Sarwar et al., 2012; Shapiro et al., 2014). Magnetic delivery to the cochlea was compared to no-treatment, to intra-tympanic steroid and saline injections, and to magnetic delivery of nanoparticles without any attached therapy. Ongoing work is now focused on validating efficacy in large animals and on optimizing the dose delivered to the cochlea in human cadavers.
Since prior and ongoing work has focused on delivery and treatment efficacy, this paper is focused on assessing treatment safety for magnetic delivery to the cochlea. To do so, the current study was designed based on published FDA regulatory guidance for safety studies that involve local drug delivery (FDA, 1995, 2010). This guidance from the FDA (FDA, 2010) is based on and is almost identical to the European standard for pre-clinical safety studies to enable entry into human clinical trials (EMA, 2013). Thus alignment of our safety study with published FDA guidance is anticipated to also align it with EU standards. Since this is a preclinical safety study, it was conducted in normal healthy animals without hearing loss. The study was conducted in rats, which are a recognized animal model in the field of hearing (Fetoni et al., 2011; Li et al., 2015; Lobarinas et al., 2013). Rats were selected over guinea pigs (another common animal model in the field of hearing) because the rat’s ear anatomy is more representative of a human ear anatomy from a local drug delivery point of view. In guinea pigs, the bone at the apex of the cochlea is so thin that drugs can enter the cochlea from the middle ear via multiple locations: via the window membranes at the base of the cochlea, and also by diffusion through the thin bone at the apex of the cochlea (Mikulec et al., 2009). In the human, and in the rat, the bone at the apex of the cochlea is thicker and drugs can only enter at the base of the cochlea through the window membranes. For this ear anatomy reason, we selected rats over guinea pigs for this study.
Since in this instance magnetic injection is intended to deliver therapy into the cochlea, a key concern to address is whether our treatment does or does not damage hearing. Hearing was therefore tested in all groups before any treatment, and 2 and 30 days after treatment. Hearing was tested by ABR (auditory brainstem response), which is a quantitative measure of hearing that is recognized and recommended by the FDA (FDA, 2012; Glasscock et al., 1991). ABR is also recognized by European regulatory agencies, for example in screening the hearing of newborns in the United Kingdom (NSC, 2013).
In our study, the test groups (groups D and F, see Tables 1 and 2) received single dose and multi dose magnetic nanoparticles loaded with prednisolone to one ear; the other ear was left untreated as a same animal control. Control groups included intra-tympanic saline (groups A and E), intra-tympanic methyl-prednisolone (group B: a mimic of a current standard of care for sudden sensorineural hearing loss, no particles or magnet), and an everything-but-the-drug control group (group C, magnet + bare particles without attached drug). The last group was included based on regulatory guidance to have a group that includes all aspects of the drug delivery method, but without any confounds due to the action of a drug (for instance, any inflammation caused by the magnetic delivery of nanoparticles could be suppressed and masked by the presence of an anti-inflammatory steroid). For all groups, for test and control groups, one ear was treated and the other ear was left as a same-animal untreated control ear. Timing of ABRs and overall study design is shown below in the Materials and Methods section, in Tables 1 and 2.
Table 1.
Study design for the rat single dose safety study.
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| Saline | Standard-of-care | Particles + magnet | Particles + prednisolone + magnet | |
| Ears to be treated | in one ear | in one ear | in one ear | in one ear |
| Number of animals | N =12 | N =12 | N =12 | N =12 |
| Day of treatment | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |||
| Treatment | Intra-tympanic saline | Intra-tympanic prednisolone | Intra-tympanic drug-free particles, then apply magnet | Intra-tympanic particles with prednisolone, then apply magnet |
| 2 days after treatment | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |||
| Terminate 50% (N = 6) animals per group. Measure iron, drug in ear and major organs. Ear histology. | ||||
| 1 month after treatment | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |||
| Terminate remaining 50% (N = 6) animals per group. Measure iron, drug in ear, major organs. Ear histology. | ||||
Table 2.
Study design for the rat multi-dose study.
| Group E | Group F | |
|---|---|---|
| Saline (4×) | Particles + prednisolone + magnet | |
| Ears to be treated | In one ear | In one ear |
| Number of animals | N =4 | N = 4 |
| Day of treatment | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |
| 1st treatment | Intra-tympanic saline | Intra-tympanic particles with prednisolone, then apply magnet |
| 1 week after After | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |
| 2nd treatment | Intra-tympanic saline | Intra-tympanic particles with prednisolone, then apply magnet |
| 1 week after After | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |
| 3rd treatment | Intra-tympanic saline | Intra-tympanic particles with prednisolone, then apply magnet |
| 1 week after after | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |
| 4th treatment | Intra-tympanic saline | Intra-tympanic particles with prednisolone, then apply magnet |
| 1 month after | Measure hearing by ABR at rat hearing frequencies (8, 12, 16, 24, 32 kHz) | |
| treatment | Terminate all (N = 8) animals per group. Measure iron, drug in ear, major organs. Ear histology. | |
In addition to testing hearing, our small animal safety study also evaluated ear histopathology at 2, 30, and 90 days after treatment. Histopathological evaluation was conducted by a board-certified veterinary pathologist. He was blinded to group type in treated ears in test groups B-F, but was informed which sections corresponded to ears that received only saline (group A) and which sections corresponded to ears that did not receive any treatment (control ears) so that a baseline for normal/untreated ears could be identified. The histopathology evaluations included an assessment of any signs of toxicity to ear structures. In this first safety study, we did not yet include an evaluation of cochlear hair cell viability, since that would entail a much larger study design. The presence of hemorrhage and inflammation was characterized and localized in detail, and scored on a semi-quantitative scale (from 1-minimal to 5-severe). We also measured the amount of drug and iron systemically, in blood and major organs (brain, heart, liver, spleen, adrenal glands, and kidneys), to assess drug and iron amounts present systemically after our local magnetic delivery. To quantify systemic inflammatory response, ELISA (enzyme-linked immunosorbent assay) was also conducted for three major inflammation markers (for interleukin 1 (IL-1), for interleukin 6 (IL-6), and for tumor necrosis factor alpha (TNF-α)), in animal blood plasma samples 2 and 30 days after intra-tympanic saline and magnetic administration of prednisolone.
2. Materials and methods
We conducted a small-animal (rat) preclinical safety assessment study for magnetic injection of the steroid prednisolone to the cochlea. The groups and the safety and dose aspects tested were selected based on the standard design of preclinical local-administration studies typically submitted to the FDA, and based on available FDA guidance documents (FDA, 1995, 2010). This FDA guidance is based on and is almost identical to European guidance (EMA, 2013), hence alignment of our study with FDA guidance is anticipated to also align it with EU standards. Specifically, since our treatment is local to the ear, we evaluated both the safety of the treatment with respect to hearing and the histopathology of ear tissues, and we also assessed systemic iron and prednisolone levels after our local administration. We conducted both single-dose and multi-dose (4× treatments) experiments, and included a naïve (no treatment) control group to control for any age-related hearing loss. We also included a longer duration (90 day) group to verify particle clearance and lack of chronic inflammation.
2.1. Single dose study design
Our single-dose study was selected to have the four arms shown in Table 1. Group A was the saline control group. Saline was administered intra-tympanically to one ear while the other ear remained as an untreated same-animal control. Group B mimicked a current standard-of-care for sudden sensorineural hearing loss (SSNHL) where patients receive intra-tympanic steroid administration (Bird et al., 2007; Lamm and Arnold, 1999; Okano, 2014; Rauch et al., 2011; Spear and Schwartz, 2011). In this group one ear received intra-tympanic methylprednisolone (the water soluble form of prednisolone), and the other ear remained as an untreated control ear. Group C was an ‘everything-but-the-drug’ group, as is often suggested by the FDA to evaluate the safety of the method of administration without any confounding factors due to the action of a drug (FDA, 1995, 2010). In particular, since prednisolone has a well-known anti-inflammatory effect, the inclusion of group C allowed assessment of whether magnetic nanoparticle alone caused any inflammatory effects, without the presence of any masking by an anti-inflammatory drug (by prednisolone). Thus group C included particles without attached steroid, deposited intra-tympanically to one ear and magnet application to the same ear for 1 h. The other ear received no treatment and acted as a same-animal control ear. Group D was the test group. In this group, each animal received magnetic nanoparticles with attached prednisolone deposited intra-tympanically to one ear, followed by magnet application for 1 h. The other ear acted as an untreated same-animal control ear.
Each group contained N = 12 animals. Half of the animals per group were terminated 2 days after the treatment, and the remaining half was terminated 30 days after treatment. Hearing was measured by ABR immediately before treatment in half the ears that were about to be treated (to establish an ABR baseline), and again 2 and 30 days after the treatment in all the animals immediately before animal termination.
To verify continued particle clearance after magnetic administration, an additional group of rats not shown in Table 1 was terminated 90 days after treatment. These rats received intra-tympanic magnetic nanoparticles with a 1 h magnet application (N = 3) in one ear. In this group, remaining inflammatory changes and iron presence was assessed by histopathology examination of slide sections from untreated and treated ears (see panels E and F in Fig. 10).
Fig. 10.
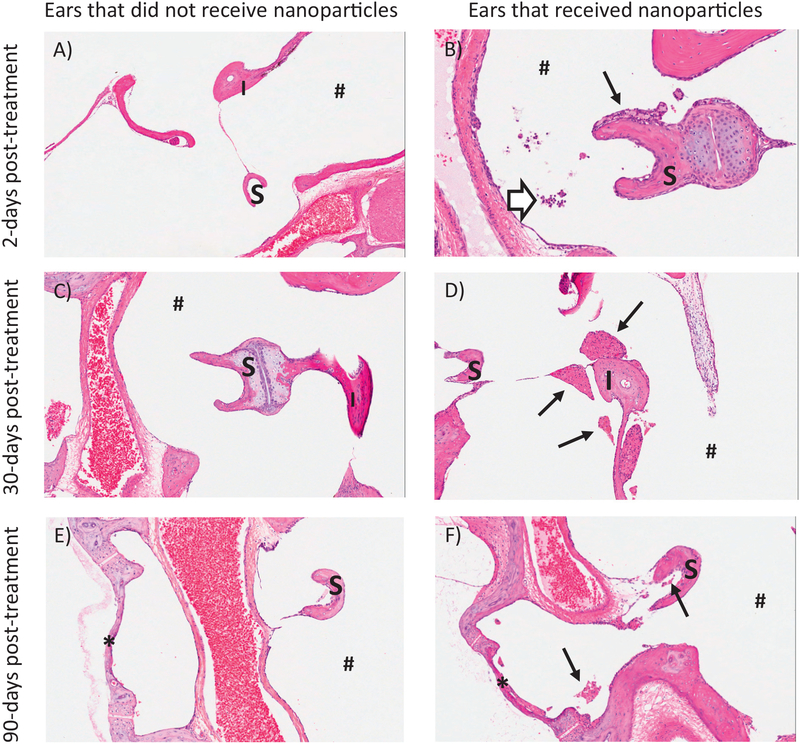
Representative inflammatory changes observed in ears of animals that did and did not receive nanoparticles, at 2, 30, and 90 days after treatment. All examined and observed inflammatory changes were limited to the middle ear of the treated animals. At day 2 after treatment (first row, panels A, B), ears that received nanoparticles (panel B) had low numbers of neutrophils and foamy macrophages in the lumen that adhered to the stapes. At day 30 after treatment (second row, panels C, D), ears that received nanoparticles (panel D) had clumps of lightly pigmented macrophages adhered to ossicles with rare neutrophils. By day 90 after treatment (last row, panels E, F), reversibility of the inflammatory changes was ongoing and almost complete as only few macrophages and no neutrophils remained in the nanoparticle ears (panel F). H&E. S = stapes, I = incus, * = oval window, # = tympanic cavity, thin arrows = macrophages, and thick open arrow = neutrophils. (A similar figure to this figure has previously appeared in a prior invited publication, in reference Lafond et al., 2018.)
2.2. Multi dose study design
In addition to the single dose study summarized above, we also conducted multi-dose testing as summarized in Table 2. Two groups (N = 4 each group) were given either 20 μL of saline to one ear intratympanically (group E), or an equal volume of drug-loaded magnetic particles followed by application of a magnet to the same ear for one hour (group F). These treatments were given once a week for 4 weeks. Hearing of the animals was assessed every week prior to the weekly treatment and 30 days after the last (fourth) treatment. Since this was a relatively lengthy study of 2 months duration total, and since the collected data led us to suspect age related hearing loss in Long Evans rats (as in other commonly used laboratory animals (Bielefeld et al., 2010; Fetoni et al., 2011; Seidman et al., 2004)), we therefore added a group to control for hearing loss due to age. Group G (N = 6 rats) did not receive any treatment, but their hearing was monitored approximately bi-weekly by ABR for 4 months, as they aged from 63 to 184 days old. This age range was chosen to include the age of the youngest rat on the first day of treatment (63 days old) and to monitor the change in hearing with ageing for a 4 month period in order to include and go beyond the oldest (116 days old at termination) rat age in the multi-dose study.
2.3. Animals
Adult male Long Evans rats (7 to 8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in pairs in a climate-controlled facility with a 12 h light/dark cycle. Food and water were provided ad libitum, except during hearing testing. All the rats were tattooed with their ID number on their tail for identification. All the animal studies were conducted in accordance with the policies and recommendations of the National Institute of Health Guide for the Care and Use of Laboratory Animals, and under approval from the Institutional Animal Care and Use Committee of the University of Maryland at College Park.
2.4. Drugs and magnetic nanoparticles
Prescription grade methyl-prednisolone sodium succinate, ‘Solu-Medrol,’ (40 mg/mL, Pharmacia Upjohn, LLC, Kalamazoo, MI, USA) was used for the “standard-of-care” control treatment group. Methylprednisolone succinate has been employed for clinical treatment of hearing problems in a variety of cochlear disorders (Harris and Ryan, 1995; Kanzaki and Ouchi, 1981; Okano, 2014; Trune and Kempton, 2001).
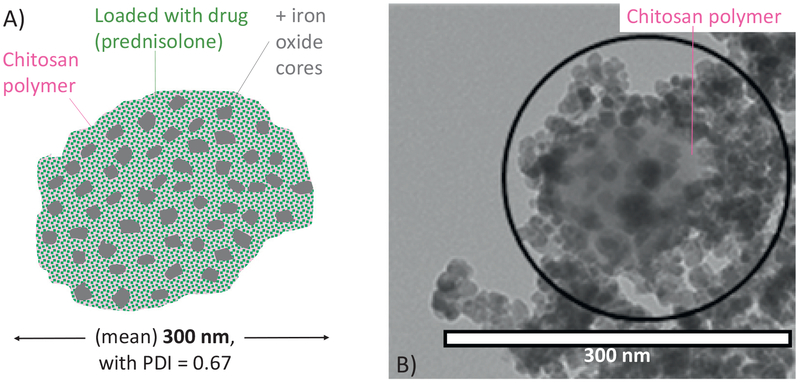
The biocompatible superparamagnetic nano-screenMAG/RChitosan particles we used were produced by chemicell GmbH (Berlin, Germany), they have been tested extensively in animal studies, and are similar to particles that were previously systemically administered to advanced cancer patients with inoperable tumors in phase 1 trials (de la Fuente et al., 2010; El-Kamary et al., 2010; Lubbe et al., 2001; Lubbe et al., 1996a; Lubbe et al., 1996b; Pittler et al., 1999; Wang, 2011). These particles can be made in a variety of sizes by chemicell. We elected to use 300 nm diameter particles since that size was effective for magnetically injecting therapy to the inner ear and has shown therapeutic benefit for noise induced hearing loss, noise induced tinnitus, and cisplatin otoprotection in our prior small-animal studies (Depireux et al., 2017; Ramaswamy et al., 2017; Sarwar et al., 2012; Shapiro et al., 2014). The makeup of these particles is illustrated in Fig. 2. Iron-oxide cores, which makes the particles magnetic, are encased in a porous chitosan matrix. Chemicell associates drug electrostatically to the chitosan matrix. For negative control (group C) animals, the chemicell particles remained unloaded with drug.
Fig. 2.
A) Particle schematic. The nano-screenMAG/R-Chitosan superparamagnetic nanoparticles are composed of multiple iron oxide (magnetite) cores (shown in gray) inside a chitosan polymer matrix (pink background, with light green dots representing the drug). Average particle size is around 300 nm with a polydispersity index (PDI) measured to be 0.67 by dynamic light scattering. The drug (prednisolone sodium phosphate, illustrated as green dots) is associated electrostatically with the porous chitosan matrix and elutes out from the chitosan with a 30 min half-life. B) Sample transmission electron microscopy (TEM) image of the nanoscreenMAG/R particles. The chitosan matrix was pre-stained with 1% Uranyl acetate (negative staining) to enable visualization of the chitosan in addition to the iron-oxide cores. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In terms of the safety of the constituent materials, iron oxide has been approved by the FDA in contrast agents (FDA, 1996a, 1996b; Wang, 2011) and for systemic nanoparticle anemia treatments (FDA, 2009); while chitosan is generally recognized as safe by the FDA (FDA, 2014; Kean and Thanou, 2010) and is under evaluation in human clinical trials (Anraku et al., 2014; de la Fuente et al., 2010; El-Kamary et al., 2010; Hernandez-Gonzaleza et al., 2010; Kim et al., 2014; Neimert-Andersson et al., 2014; Pittler et al., 1999; Smith et al., 2014). Magnetic nanoparticles have also been approved for use in Europe, specifically Endomag’s Sienna + magnetic nanoparticles to help detect breast cancer (http://www.endomagnetics.com/) and MagForce AG’s NanoTherm particles to treat brain tumors (http://www.magforce.de/en/home.html).
For magnetic delivery test animals (groups D and F), chemicell provided us with nano-screenMAG/R-Chitosan particles that had been pre-loaded with prednisolone sodium phosphate. This version of prednisolone was chosen for its water solubility and negative charge. The negative charge on the phosphate group interacts with the positively charged chitosan to form electrostatic bonds allowing the drug to be loaded onto the magnetic chitosan particles. The particles were provided in sterile water at a concentration of 25 mg of particles per 1 mL of solution. Chemicell reported 25 mg of particles per mL of stock solution, with ~6.56 × 1013 particles per gram, and that the prednisolone loaded particles contained about 0.04 g of prednisolone per gram of particles.
2.5. Treatment administration
Before receiving any treatments, all rats were anesthetized with ketamine/xylazine (100/10 mg/kg). The depth of anesthesia of each animal was confirmed by observation of the general condition of the animal and absence of the reflex to a toe pinch.
For all the experiments, the formulation (saline, drug, unloaded magnetic particles, or magnetic particles with attached drug) was administered to one ear by intra-tympanic injection. The same volume of formulation was used for the different groups, either a 30 μL volume for single-dose ears (which maximally filled the middle ears of the animals) or a slightly smaller 20 μL volume for multi-dose ears. This was done so that the safety of the maximally achievable dose, for each type of formulation (saline, drug, or nanoparticles), could be tested in the single dose groups. Using a surgical microscope, the injection side of the tympanic membrane of each animal was visualized (typically in the left ear), and the opening from the bulla into the middle ear was observed through the mostly transparent tympanic membrane. Then a 31G needle (Becton-Dickson, OH) was directed through the pars tensa of the tympanic membrane. By facing the bevel caudally, the ejected solution was directed toward the round window membrane and oval window. When the injection was complete, the needle was carefully withdrawn. One animal was given the injection to the right ear due to an abnormality in its left ear tympanic membrane appearance.
Group A rats received 30 μL of sterile saline (0.9% NaCl). Group B rats, received 30 μL of prescription grade methyl-prednisolone-succi-nate, ‘Solu-Medrol.’ In both groups, the other ear remained untreated and served as a no treatment control.
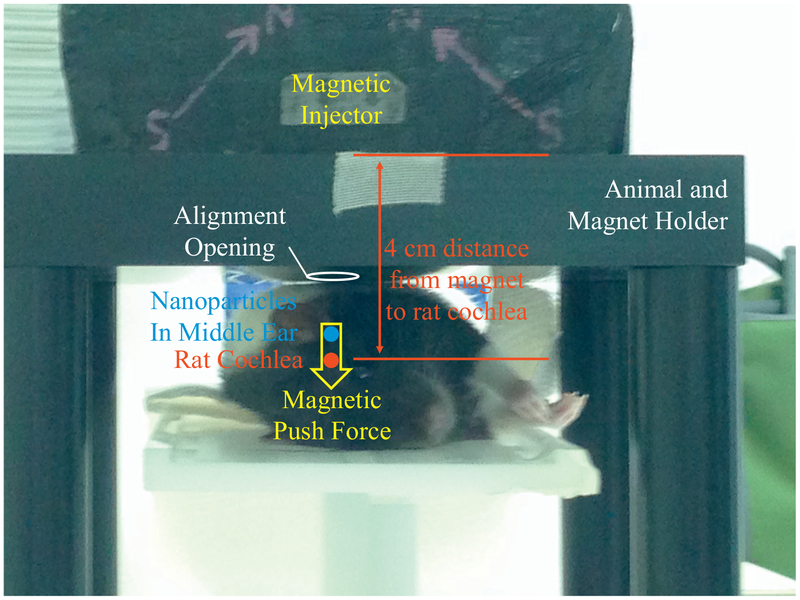
Group C rats received 300 nm diameter magnetic nanoparticles without any attached drug. These rats received 30 μL of nanoscreenMAG/R-Chitosan particles intra-tympanically to one ear (25 mg particles/mL of solution; ~6.56 × 1013 particles/g). After particle administration, our magnetic injector device was applied at a 4 cm distance from the rat’s inner ear to mimic the magnet to particle distance anticipated in adult human patients (Fig. 3). The duration of the magnet application was 1 h.
Fig. 3.
Experimental set up for the rat. The rat is placed laterally on the platform of our holder and the rat’s inferior aspect of the auditory meatus is aligned with an opening in the magnet holder (marked by the white circle). Next our magnetic injector is placed into a slot in the holder above the rat, which ensures that the magnetic force (yellow arrow) is aligned with the location of the magnetic nanoparticles in the rat’s middle ear (blue dot) and the rat’s cochlea (red dot). The magnetic push force then acts to transport the particles from the middle ear, through the window membranes, into the cochlea. The above device was designed to achieve push forces over the magnet to cochlea distance that will be needed in adult patients, and so the push force starts at 3 cm from the magnet face and extends out to 5.5 cm away from the magnet (Sarwar et al., 2013). In our animal studies, we place the magnet at approximately a 4 cm distance from the rat’s cochlear, meaning, the magnet is placed so that the distance from the magnet to the rat cochlea matches the distance from magnet to cochlea that is anticipated for human patients. The magnet is composed of four NdFeB grade N52 permanent magnets bonded together, with a maximum internal magnetization of 1.45 Tesla, thus producing an external field with a maximum strength of about 1 Tesla near the magnet surface (Sarwar et al., 2013; Shapiro et al., 2014). This field strength is substantially less than is administered to patients in clinical grade MRI (magnetic resonance imaging) systems (Allen and Burdette, 2001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Group D rats were treated the same as group C animals, except we used 300 nm diameter magnetic particles with electrostatically associated prednisolone sodium phosphate. Similar to as above, the rats received 30 μL of nano-screenMAG/R/Chitosan-Prednisolone particles (25 mg particles/mL solution; ~6.56 × 1013 particles/g; 0.04 g prednisolone/g particles). The magnet was again applied immediately after the intra-tympanic particle administration, and was held for 1 h at a 4 cm distance from the rat’s cochlea. The nano-screenMAG/R/Chitosan-Prednisolone particles used in group D were supplied by chemicell GmbH already pre-loaded with prednisolone sodium phosphate.
Animals in the multi-dose test groups E and F were administered 20 μL of nano-screenMAG/R/Chitosan-Prednisolone intra-tympanically (25 mg particles/mL; ~6.56 × 1013 particles/g; 0.04 g prednisolone/g particles) into one ear once a week for 4 weeks. Animals in the saline control group received an equal volume (20 μL) of saline into one ear via intra-tympanic injection once a week for 4 weeks.
2.6. Auditory brainstem response (ABR) recordings
ABRs were recorded immediately before treatment, and 2 days and 30 days after treatment. Hearing thresholds were measured at 8, 12, 16, 24, and 32 kHz to bracket the frequency range of rat hearing, using an ABR recording system (Tucker-Davis Technologies, Alachua, FL). Recording needle electrodes were inserted subcutaneously at: (a) a reference along the dorsal midline close to the nuchal crest, (b) behind the left pinna, level with the bulla, (c) behind the right pinna, level with the bulla, and (d) a ground was placed in the skin over the lumbar area. With the sound field calibrated at the level of the ears, 600 sweeps of 5 ms long tone bursts (shaped with 1 ms onset and offset sinusoidal ramps) were presented to the rat at decreasing levels beginning at 94 dB sound pressure level (SPL) and proceeding in 5 dB decrements down to a 14 dB SPL. Electrophysiological signals were recorded for 10 ms. The hearing threshold for each frequency was determined as the lowest intensity at which a definite wave I/II ABR response pattern could be identified.
2.7. Sample collection and ear histopathology
Whole blood samples were collected from animals via cardiac punch under anesthesia immediately before termination and at 2 or 30 days after the treatment. An average of 8–9 mL of total blood per animal was collected with a 20% EDTA coated 25G butterfly blood collection needle (Becton Dickson Co., Franklin Lakes, NJ. USA) attached to a 10 mL syringe. To then prepare plasma samples, whole blood was centrifuged at 2000g for 20 min. The upper aqueous phase containing plasma was carefully removed by a micro pipet and transferred to a new tube. To prepare serum samples, the whole blood sample was left to clot at room temperature for 1 h or more and then centrifuged at 2000g for 20 min. The upper aqueous phase containing serum was carefully removed by a micro pipet and transferred to a new tube. Plasma and serum samples were aliquoted and stored at −20 °C until use.
Following blood collection, animals were terminated by CO2 asphyxiation. Brain, heart, liver, spleen, adrenals, and kidneys were quickly removed by dissection from connective tissues and blood vessels. Tissue samples were briefly washed in ice-cold 1× PBS, divided into halves, weighed, snap frozen in a methanol bath, and stored at −80 °C until use.
Temporal bones were collected from half the ears for rats in the single dose groups (groups A–D), and from 6 ears in the multi-dose groups E and F, to allow histological sectioning and histopathology examination of ear tissues. After decapitation and removal of the jaw, the head was hemisected and the right half placed in ice-cold saline. The temporal bone was isolated, connective tissues and muscles were removed, and a small hole was made in the ventral aspect of the bulla to allow the middle ear to fill with fixative. The temporal bone and bulla were then placed in 4% paraformaldehyde for later sectioning. After the temporal bones were collected, they were sectioned (HistoServ, Germantown, MD). Two series of 10 sections, 10 μm thick, were cut with one section used for H&E staining and the adjoining section used for Prussian Blue staining for iron. A spacing of 100 μm was used to separate pairs of sections. The cutting was made so that at least one section contained the round window membrane, the organ of Corti and the stria vascularis.
One H&E section from each ear sample was evaluated for histo-pathology. Histopathological evaluation was performed by a board-certified veterinary pathologist. One H&E section from each ear sample was examined for evaluation of ear structures, and to assess and quantitatively score for any signs of inflammation. The pathologist was blinded to group type for treated ears in test groups (groups B–F), but was informed which sections corresponded to ears that received only saline (group A) and which sections corresponded to ears that did not receive any treatment at all (right control ears), so that the baseline for normal/untreated ears could be identified. Histopathological evaluation was performed to assess the integrity of and possible signs of toxicity to ear structures. The presence of hemorrhage and inflammation was semi-quantitatively evaluated and inflammatory responses were characterized and localized in detail. A 5-step semi-quantitative scale was used (1-minimal, 2-slight, 3-moderate, 4-marked, and 5-severe).
2.8. Drug and iron concentration measurements
Major organs from groups A–F were analyzed for drug and for iron (Fe) concentration by mass-spectrometry (MS) and by inductively coupled plasma-mass spectrometry (ICP-MS) respectively. Blood samples were analyzed for drug concentration (by mass spectrometry) but not for iron content. Blood naturally contains iron (bound to hemoglobin) which masks any iron that might be added to blood circulation by administration of nanoparticles to the ear.
Quantification of prednisolone levels in blood plasma and rat tissues by mass-spectrometry was performed by Molecular Mass Spectrometry and Diagnostics (MMSD; Warwick, RI., USA). Following shipping of frozen blood plasma and tissue (liver, kidney, spleen, heart, adrenal glands, and brain) samples to MMSD, all sample preparations for the MS assay were carried out by MMSD. Briefly, at MMSD, tissue samples were first homogenized in 1× PBS. From the plasma and tissue homogenates, sample extraction of prednisolone was performed by protein precipitation with addition of a 1:5 volume of neat acetonitrile with 0.1% formic acid (v/v). The samples were centrifuged at 3000 rpm for 10 min at 10 °C, then the sample supernatants were aliquoted into another 96-well plate for high-performance liquid chromatography (HPLC)-MS/MS assays. The injection volume for electrospray was 10 μL, and the mobile phases used were DI water with acetonitrile and 0.1% formic acid, with a mobile phase flow rate of 300 μL/min.
The effective lower limit of mass spectrometry quantification of prednisolone was 100 pg/mL, and the lower limit of detection was 30 pg/mL. A linear standards curve was generated from 100 pg/mL to 500 ng/mL to assess prednisolone concentration in tissue and blood samples. Quality control samples of low, medium, and high concentrations (5 ng/mL, 50 ng/mL, and 500 ng/mL respectively) were run in between every 15 samples throughout the analysis. The quality control sample variability indicated that the sample-to-sample variation was within an acceptable range of 4.5% to 7.2%.
The nanoparticles contain iron-oxide cores to make them magnetic. Two and thirty days after treatment, iron concentrations in tissue samples were quantified by inductively coupled plasma-mass spectroscopy (ICP-MS), following the measurement protocol provided by PerkinElmer and in line with prior published protocols (Kut et al., 2012; Nixon et al., 2000; Thomas, 2013; USDA Food Safety and Inspection Service, 2013; Wegst-Uhrich et al., 2015). Tissue samples were dried in an oven at 93 °C for 4–6 h, and the dried samples were weighed. Then the dried tissue samples were acid digested with 70% nitric acid for 24 to 48 h at 37 °C. In order to achieve complete digestion, 30% hydrogen peroxide (1:1 with nitric acid v/v) was added to fatty tissues such as brain, liver and kidneys. Digested samples were then diluted with 18.2 Ω UV ultra-purified water, and filtered with 0.22 μm PVDF Millex-GV 13 mm filters (Millipore, Cork, Ireland) for the ICP-MS assays.
The tissue concentrations of iron were analyzed by a NexIon300D ICP-MS (PerkinElmer, USA) instrument at the Molecular Characteristics Analysis Complex at the University of Maryland Baltimore County (Baltimore, MD). Inductively coupled plasma mass spectrometry is an analytical technique used for elemental determinations. Compared to other methods such as ICP-AES (ICP with atomic emission spectroscopy) and GFAAS (graphite furnace atomic absorption), ICP-MS has both a lower limit of detection and a higher maximal detection limit, as well as an ability to analyze a wider variety of elements (Thomas, 2013). Using ICP-MS, the amount of iron in tissue samples was determined using a standards curve generated by known amounts of iron in certified iron standards (Fluka/Sigma-Aldrich, Buchs, Switzerland). The iron limit of detection was 10 ng (iron)/g (tissue). Iron concentration was measured in major organs (brain, heart, liver, spleen, adrenal glands, and kidneys) two and thirty days after magnetic administration to the ear.
2.9. Enzyme-linked immunosorbent assay (ELISA)
In addition to an assessment of local inflammation via examination of ear tissues histology, we also assessed the presence of any systemic inflammation 2 and 30 days after our magnetic delivery treatment. Systemic inflammation was examined by enzyme-linked immunosorbent assay (ELISA) of three major inflammation markers in animal blood plasma samples: interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) (deLuca and Gommerman, 2012; Kariya et al., 2013; Opal and DePalo, 2000; Venihaki et al., 2001; Zhang and An, 2007). ELISA IL-1 and TNF- α (R&D systems, Minneapolis, MN) and ELISA IL-6 (Boster Biological Technology, Pleasanton, CA) assay kits were used according to the manufacturers’ specifications. All values were normalized with total protein content determined by BCA protein assays (Thermo Scientific, Rockford, IL). The manufacture stated limit of detections were 31.3, 62.5, and 12.5 pg/mL for IL-1, IL-6 and TNF-α respectively.
3. Results
The treatment we evaluated was magnetic delivery of a corticosteroid (prednisolone) to the cochlea. Increased delivery of a corticosteroid to the cochlea is anticipated to be able to improve treatment of noise induced and sudden hearing loss, to suppress tinnitus, and to protect hearing from cisplatin and carboplatin chemotherapy regimens. Our goal in this paper was to evaluate the safety of magnetic delivery of prednisolone to the cochlear, hence data was collected in healthy animals without any ear trauma that received one of the four treatment types shown in Table 1 (single dose testing) or one of the two treatment types shown in Table 2 (multi dose testing). Below, results are presented for quantitatively assessing hearing and systemic drug and iron loading both 2 and 30 days after local administration of therapy to rat ears, as well as histopathology results for 2, 30, and 90 days after treatment.
3.1. Auditory brainstem response (ABR) results
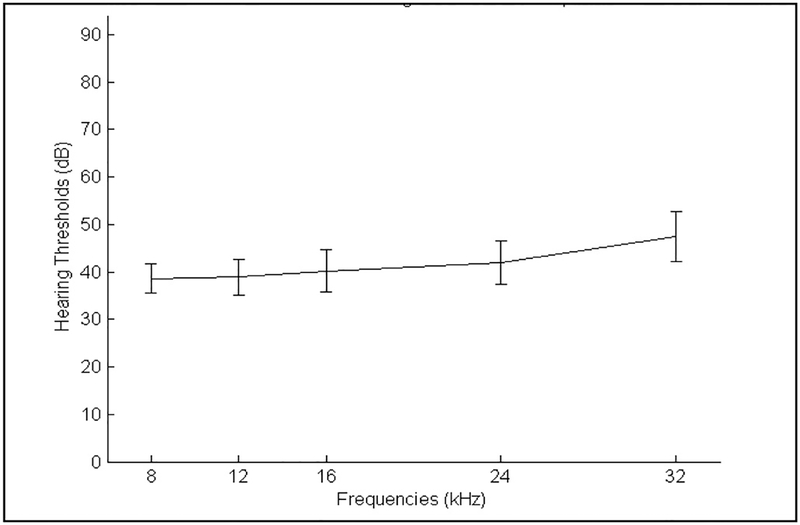
The measured baseline rat hearing thresholds, before any treatment, are shown in Fig. 4. As expected, and consistent with prior literature on open-field ABR measurements, naïve (untreated) rats had hearing thresholds of 40 dB SPL or slightly less at low and mid frequencies in open field, and with modestly higher thresholds at high frequencies, representing healthy hearing (Alvarado et al., 2012; Brozoski et al., 2012; Heffner and Heffner, 2007). With 12 animals per group over 4 groups in the single-dose study, and with half those animals randomly selected for pre-treatment ABRs (N = 24 total), we achieved the standard deviations shown in Fig. 4.
Fig. 4.
Rat hearing ABR baseline before any treatment. Hearing was measured at 8, 12, 16, 24 and 32 kHz in the to-be-treated ear for N = 24 rats. Standard deviations are marked by the vertical confidence intervals in the graph.
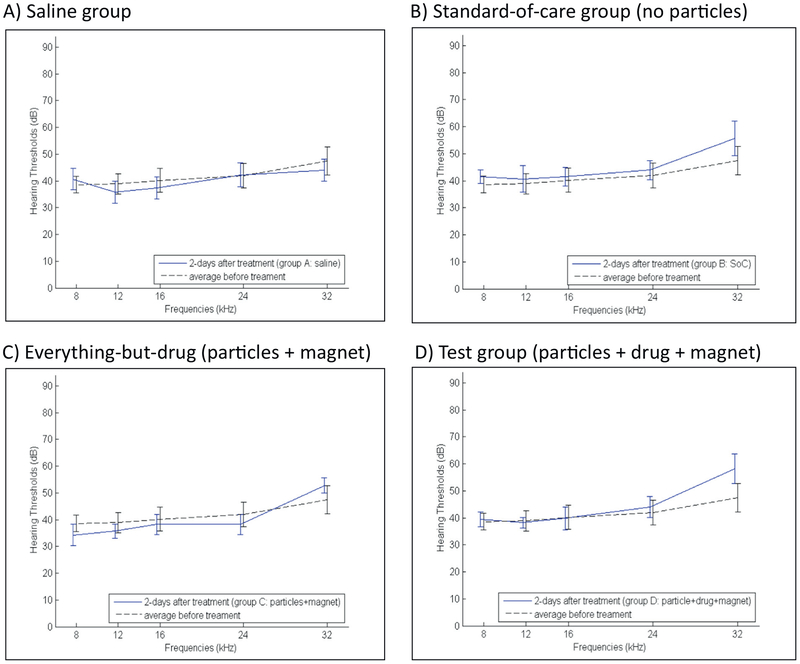
ABR hearing thresholds 2 days after the single-dose treatment, for groups A–D listed in Table 1, are shown in Fig. 5. Panel A shows ABR thresholds 2 days after intra-tympanic saline (group A), panel B shows thresholds 2 days after intra-tympanic prescription grade methylprednisolone (group B, to mimic a standard-of-care for SSNHL), and panels C and D show ABR thresholds after magnetic administration of particles without prednisolone (control group C) and particles with prednisolone (test group D).
Fig. 5.
Rat hearing 2 days after treatment, for all four single-dose groups (Table 1). A) Saline group, B) Standard-of-care group, C) Everything-but-the-drug (particles + magnet) group, and D) Test group (particles with prednisolone + applied magnet). Treated ear ABR thresholds 2 days after each treatment are marked by the solid blue lines versus the pre-treatment ABR thresholds that are reproduced from Fig. 4 are marked by the dashed black line. Vertical bars denote one standard deviation (SD). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
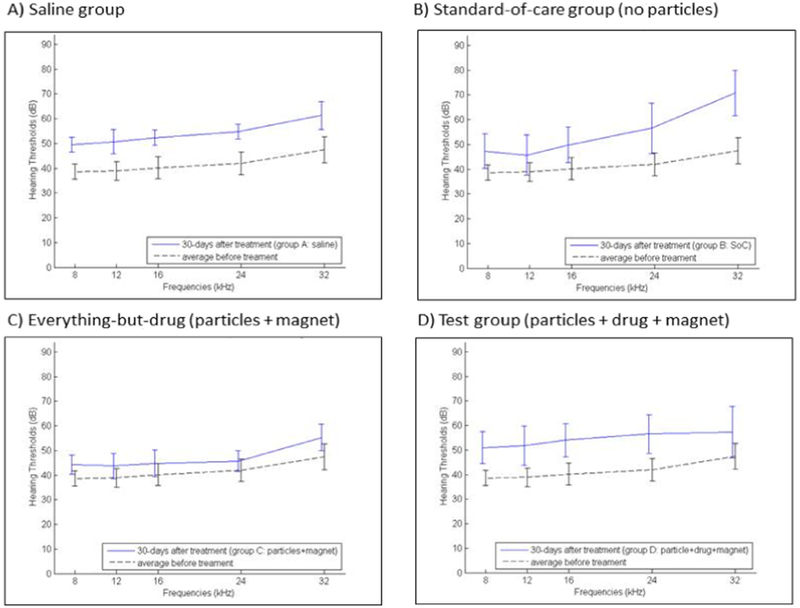
Measurement of ABR hearing thresholds was repeated 30 days after treatment for all four single-dose groups. Fig. 6 shows ABR thresholds 30 days after the single treatment, again with each group shown in its own panel in the figure. The observed rise in hearing thresholds across all groups for 30 days post treatment, as compared to for 2 days post treatment (Fig. 5), is most likely due to rat hearing loss with age, as discussed below.
Fig. 6.
Rat hearing 30 days after treatment, for all four single dose groups (Table 1). A) Saline group, B) Standard-of-care group, C) Everything-but-the-drug (particles + magnet) group, and D) Test group (particles with prednisolone + applied magnet). Treated ear ABR thresholds 30 days after each treatment are marked by the solid blue lines versus the pre-treatment ABR thresholds that are reproduced from Fig. 4 and are marked by the dashed black line. Vertical bars denote one standard deviation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Two-sided t-tests were conducted to assess if there was any statistically-significant difference in ABR thresholds between the four single-dose treatment groups, for both 2 and 30 days after treatment. Groups A and B (the no-particles saline and standard-of-care groups) were t-tested against groups C and D (that had particles without and with attached prednisolone). Thus we carried out four pair-wise t-tests per hearing frequency. To 95% statistical confidence, we found no difference in hearing thresholds in groups C and D compared to the standard-of-care group B, across all frequencies, at both 2 and 30 days after treatment. Group C and D results were also favorable when compared to group A animals that received intra-tympanic saline. With the exception of hearing 2 days after treatment at the highest frequency of 32 kHz, there was no statistical difference in ABRs between magnetic particle groups C or D and group A (saline), at 2 and 30 days after treatment, across all frequencies. The lone statistically-significant (p < 0.05) rise in ABR threshold for group D versus group A at 32 kHz at 2 days after treatment was absent 28 days later, at the 30-day post-treatment time point (see Fig. 6), by which time there were no statistically-significant differences between particle groups C and D and the saline and standard-of-care groups A and B, at any frequency. The most likely reason for the rise of ABR thresholds at high frequency (at 32 kHz) at 2 days post-treatment is the presence of nanoparticles on the round window membrane, which would add weight to that membrane and change the impedance of the cochlea at high frequency (a conductive hearing loss). By 28 days later, clearance of the majority of the nanoparticles from the round window membrane would eliminate this conductive hearing loss.
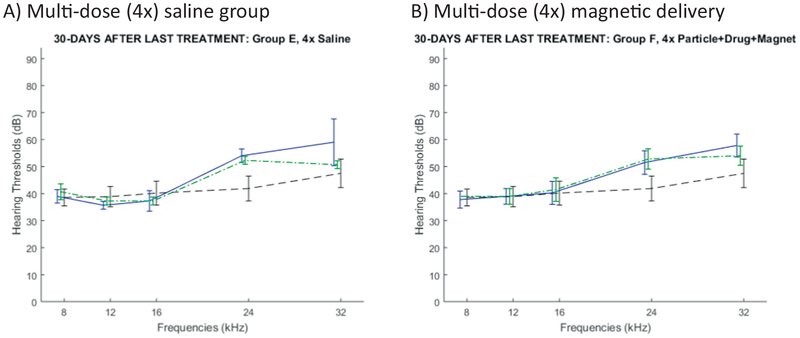
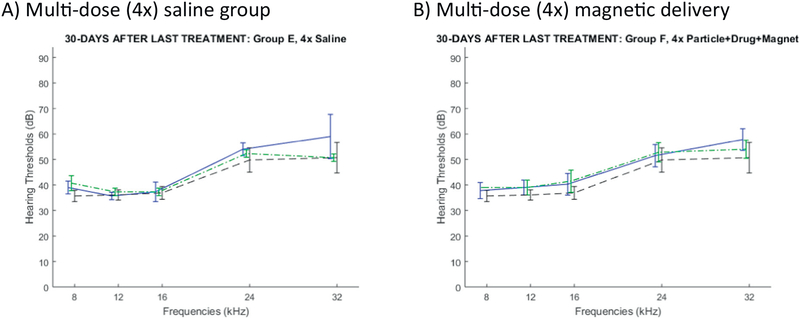
To assess the effect of multiple ear treatments, we further conducted a multi-dose study. As shown in Table 2, four animals received saline intra-tympanically in one ear once a week for 4 weeks (control group E). Another four animals received four treatments of magnetic particles loaded with prednisolone and an applied magnet for 1 h, also once a week for 4 weeks (multi-dose treatment group F). To maximize the data collected from this small additional study, we carried out ABRs for both ears, although as previously only one ear was treated. Fig. 7 shows the ABR thresholds for each frequency, 30 days after the final (fourth) treatment. Although this small four-animals per group study was not powered to statistical significance, we saw no evidence that multiple magnetic treatments led to any hearing loss as compared to the same number of saline administrations (compare the solid blue curve in Fig. 7B versus in Fig. 7A).
Fig. 7.
Rat hearing 30 days after the final dose of treatment administered every week for 4 weeks. A) Multi-dose saline (group E: 3 rats) versus B) Multi-dose magnetic administration (particles with prednisolone + applied magnet, group F: 4 rats). ABR thresholds are shown in solid blue for left treated ears and in dashed green for same-animal untreated ears. The dashed black curve is a repeat of the baseline hearing curve from Fig. 4 (24 rats, 24 ears). (One of the rats in the saline group E died, hence the data for that group has N = 3 rats only.) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
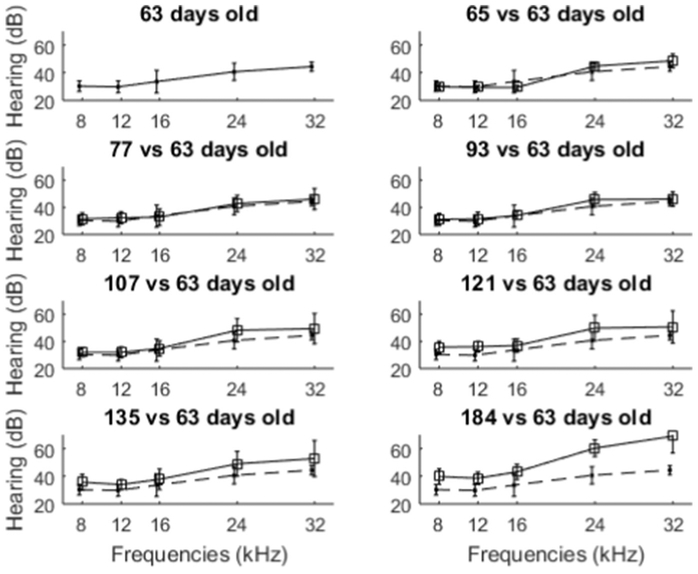
In both the single and multi-dose studies, we observed hearing loss over time even for the saline and the standard-of-care treatment groups, especially at higher hearing frequencies (after 30 days as evident in Fig. 6A, B and after 2 months as in Fig. 7A). To understand whether this hearing loss might be due to rat ageing, we monitored hearing with age for six Long Evans male rats over a four month period. These rats received no treatment of any kind, and ABR measurements were conducted on both ears starting at 63 days of age (to match the youngest rat at its earliest ABR measurement in our studies above) and progressing till 184 days of age. The resulting ABR thresholds are shown in Fig. 8. A trend of hearing loss, especially at higher frequencies, is apparent with rat age. Although we found no prior publications on Long Evans rat hearing change with ageing and hence believe this may be the first reported data on Long Evans age related hearing loss, such observed age related hearing loss is a common feature among rats and other laboratory animals (Bielefeld et al., 2010; Fetoni et al., 2011; Seidman et al., 2004).
Fig. 8.
Rat hearing with age. ABRs were conducted on both ears for N = 6 rats, at the ages indicated in the panel titles. ABR hearing at 63 days old (first panel), is repeated in the 7 other panels as a baseline (dashed curve). These six rats received no treatment of any kind. Vertical bars denote one standard deviation.
Based on this age related hearing loss in Long Evans rats, we replot the data of Fig. 7 (30 days after a month-long multi-dose regimen), but now compared the hearing after multiple treatments to hearing of untreated rats at a similar age. Specifically, Fig. 9 compares hearing 30 days after the multi-dose regimen (average rat age was 116 days old) to hearing of untreated 121 day old rats, the closest matching age in the ageing data of Fig. 8.
Fig. 9.
Repeat of ABR rat hearing data 30 days after the final dose of the multi-dose treatment, but now compared against a no-treatment baseline at about the same average age. A) Multi-dose saline (group E) and B) Multi-dose magnetic administration (group F). ABR thresholds are again shown in solid blue for left treated ears and in dashed green for same-animal untreated ears, but in contrast to Fig. 7, the dashed black curve graphs the average ABR thresholds over both ears for six untreated older (121 day old) rats. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In summary, by 30 days after treatment, there was no difference in hearing between magnetically treated ears (groups C, D for single dose administration and group F for multi dose) compared to ears that received saline (groups A and E) and compared to intra-tympanic prednisolone (group B, a mimic of a standard-of-care for sudden sensor-ineural hearing loss), as shown in Figs. 6 and 7. However, there was a modest rise in hearing thresholds across all groups especially at higher frequencies. Based on the collected ageing data, most of this modest 10 dB loss in hearing can be attributed to normal age-related hearing loss, as shown in Fig. 9 which corrects for rat age.
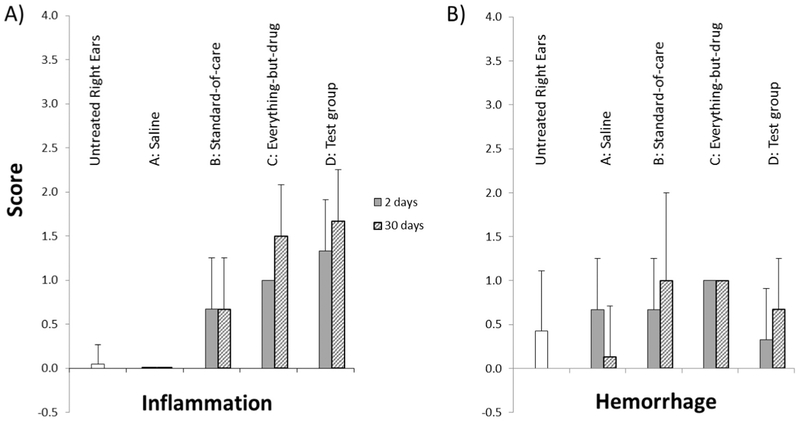
3.2. Ear histopathology assessments and scores
Histology sections were assessed for any treatment-related toxicity. Animals given saline once (group A) and four times (group E) had none and minimal inflammation respectively. Treatment-related in-flammatory changes were noted for all non-saline groups B, C, D, and F, including the prednisolone only group B animals. The incidence and severity of the inflammatory changes appeared slightly increased in animals administered nanoparticles (with or without prednisolone, groups C, D, and F) when compared to animals administered prednisolone alone (group B). The animals administered multiple doses of nanoparticles (group F) had inflammation that was not distinguishable from animals which received a single dose of nanoparticles (groups C and D).
The inflammatory changes examined in this study were limited to the middle ear. Fig. 10 shows sample H&E stained middle ear sections for ears that did not receive particles (left column) versus ears that did (right column). In animals terminated 2 days after treatment (top row), inflammatory changes were characterized by the presence of relatively low numbers of neutrophils and lesser numbers of mostly foamy macrophages in various locations of the middle ear (e.g. tympanic cavity, Eustachian tube, footplate of stapes, malleus, malleal ligament, oval window). In ears that were administered nanoparticles, some macrophages contained golden to brownish pigment (right column) consistent with iron-based pigment. No pigment was observed in ears that did not receive nanoparticles. In animals terminated 30 days after treatment, the inflammatory changes for ears that had received nanoparticles persisted and were of comparable incidence and severity. These changes showed a shift toward a more chronic reaction and were characterized by the presence of mainly macrophages (foamy to pigment-laden and often clumped), with lymphocytes in 2 animals and rare neutrophils in a single animal.
By day 90 after treatment, minimal to slight numbers of macrophages were still present in the middle ear, mainly close to the head of the malleus and next to the round window (Fig. 10F). In 2 out of 3 magnetically-treated ears that were examined, macrophages were present in minimal numbers and did not appear to contain any pigment on H&E. However, the macrophages seen in one of these two ears contained minimal amounts of small granules that stained positive on a Prussian Blue stain (consistent with iron-based pigment, likely from nanoparticles). In the third magnetically-treated ear that was examined, there was a relatively large clump of macrophages close to the head of the malleus. On H&E, these macrophages contained large vacuoles filled with gold to brownish pigment that stained positive on Prussian Blue stain and a few fibroblasts were present interspersed between the macrophages. Although it cannot be excluded that the nanoparticles participated in the findings noted in this ear, the nature and scope of these changes appeared more likely to be secondary to hemorrhage. The pigment noted was most probably hemosiderin (a degradation product of hemoglobin following erythrocyte phagocytosis) rather than nanoparticles. No neutrophils or lymphocytes were noted in the middle ear of any of the animals terminated at study day 90. Thus, it was interpreted that there was partial reversibility of the findings noted in animals terminated on study day 2 and 30. Overall by 90 days after the treatment, there was ongoing and almost complete reversibility of the inflammatory changes (only a few macrophages and no neutrophils remained in ears that received nanoparticles).
Minimal to slight middle ear hemorrhage was noted in several ears representing all treatment groups, with no trend suggesting an effect of the nanoparticles (Fig. 11). This presence of hemorrhage in the examined sections is a common artifact secondary to blood pooling at necropsy. This was corroborated by fresh erythrocytes that were observed in the tissues of animals terminated 30 days after treatment as well as in saline control and untreated ears.
Fig. 11.
Mean inflammation and hemorrhage histopathology scores across all groups. The scoring scale was 1-minimal, 2-slight, 3-moderate, 4-marked, and 5-severe.
All the inflammatory changes noted in this study were very mild in severity and were present in the lumen of the middle ear. No structural damage, degeneration, necrosis, fibrosis or otosclerosis was noted in any of the ears examined and all the changes described were considered non-adverse. There were no observed differences for inflammation scores between group D ears (the test group, nanoparticles loaded with prednisolone) and group C ears (nanoparticles without prednisolone), at both 2 and 30 days after treatment. Hence there was no evidence that the anti-inflammatory properties of prednisolone are masking any in-flammatory response due to the nanoparticles, since the inflammatory scores in group C ears (nanoparticles without prednisolone) were no higher than in group D ears (nanoparticles with prednisolone).
3.3. Systemic drug and iron concentration after local ear delivery
As described in Methods (Section 2.8: Drug and Iron Concentration Measurements), the amount of prednisolone in blood and major organs (liver, kidney, spleen, heart, adrenal glands, and brain) after treatment was quantified by HPLC and mass-spectrometry at MMSD (Molecular Mass Spectrometry and Diagnostics, Warwick, RI). We only quantified systemic drug amounts for samples from the single-dose groups (Table 1). A total of 386 blood and tissue samples were analyzed by MMSD. The effective lower limit of prednisolone quantification was 100 pg/mL. It was found that both 2 and 30 days after treatment, the amount of prednisolone in all blood and tissue samples, across all groups, was below the MMSD 100 pg/mL limit of quantification. As a comparison, in humans the amount of prednisolone measured in the blood plasma following daily oral administration of 20–60 mg prednisolone was reported at ≈ 80 ng/mL (Shibasaki et al., 2008). The amount of endogenous prednisolone in human urine has been reported at 440–470 pg/mL (Fidani et al., 2013). In animals studies, the amount of endogenous prednisolone in equine urine was reported as 50 pg/mL (Fidani et al., 2012) and in bovine urine was reported as 690 pg/mL (Pompa et al., 2011). The observed low systemic level of prednisolone in blood and major organs (< 100 pg/mL, below the mass spectrometry detection limit) is consistent with our local to-ear-only administration of the steroid.
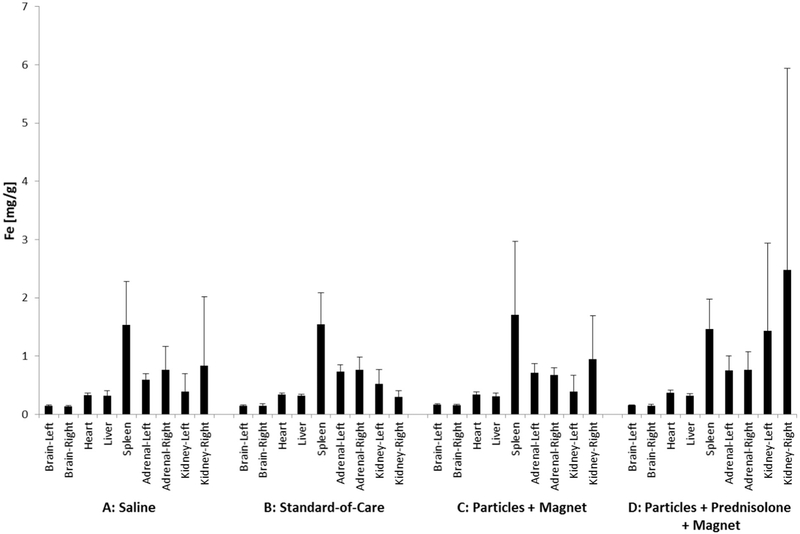
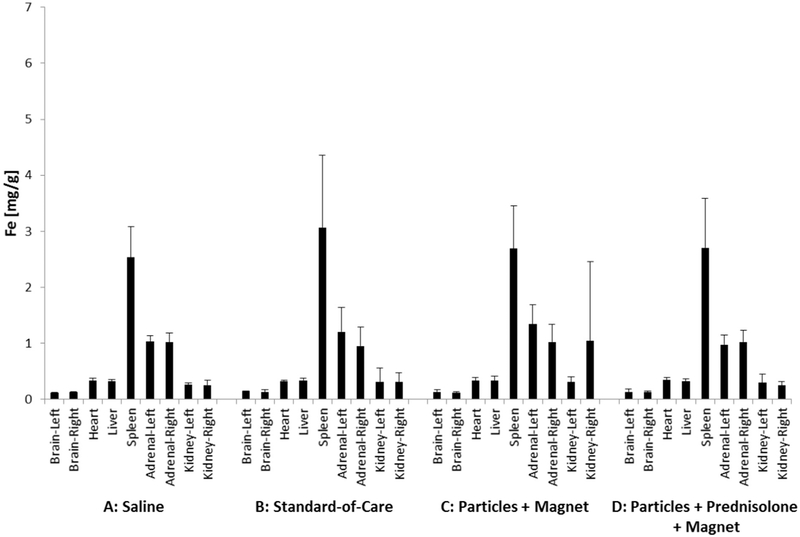
ICP-MS was used to quantify iron oxide levels in major organs after our administration to the ear. The amount of iron measured in major organ samples is shown in Fig. 12 for 2 days and in Fig. 13 for 30 days after a single ear administration, across all samples and for all four single-dose groups. The concentrations of iron measured in organ samples (0.1–2.7 mg/g) was consistent with the concentration of iron oxide naturally found in the major organs (e.g. brain, liver and kidneys) of humans, bovines, rats and mice (Fiorito et al., 2012; Gellein et al., 2003; Varga et al., 2005; Wegst-Uhrich et al., 2015). There was no statistically significant difference in iron concentrations between organ samples from group C and D animals that received magnetic nano-particles versus organ samples from group A and B animals that did not receive any iron oxide particles. Thus iron nanoparticles administered topically to the ear did not change iron levels systemically, there was no statistical difference in iron levels in major organs between groups.
Fig. 12.
Iron concentration in major organs 2 days after treatment, for the four single-dose groups of Table 1. As previously, the groups are marked by A, B, C, and D (with N = 6 rats per group for heart, liver, spleen, left and right adrenal glands and kidneys, and N = 3 rats per group for each side of the brain). Vertical bars denote one standard deviation. There were no statistically significant differences between test groups, adding iron topically to the ear did not change iron levels systemically in major organs.
Fig. 13.
Iron concentration in major organs 30 days after treatment, for the four single-dose groups of Table 1. As previously, the groups are marked by A, B, C, and D (with N = 6 rats per group for heart, liver, spleen, left and right adrenal glands and kidneys, and N = 3 rats per group for each side of the brain). Vertical bars denote one standard deviation. There were no statistically significant differences between test groups, adding iron topically to the ear did not change iron levels systemically in major organs.
In summary, after magnetic administration of prednisolone-loaded magnetic nanoparticles to the ear, it was found that the concentration of prednisolone systemically (in blood and major organs) was below mass spectrometry detection limits (< 100 pg/mL), both 2 and 30 days after single dose treatment. Likewise, 2 and 30 days after single dose ear treatment, the amount of iron was measured in major organs (brain, heart, liver, spleen, adrenal glands, and kidneys) and it was found that iron levels in major organs for animals that received nanoparticles to the ear were no different than for animals that were not administered any magnetic particles (Figs. 12 and 13).
3.4. Systemic inflammation
As described in Methods (Section 2.9: Enzyme-linked immunosorbent assay (ELISA)), we also measured the level of three major inflammation markers (interleukin 1 [IL-1], interleukin 6 [IL-6], and tumor necrosis factor alpha [TNF-α]) in blood plasma samples to assess if there was any systemic inflammation after our ear treatment. All inflammatory cytokines in blood plasma were below detection limits (the manufacture stated limits of detection are: IL-1: 31.3 pg/mL, IL-6: 62.5 pg/mL, and TNF-α: 12.5 pg/mL). Lack of inflammatory response as measured by cytokine detection indicated no systemic inflammation following either single or multi-dose intra-tympanic injection or magnetic delivery for all formulations, at 2 days or 30 days post treatments. No detection of inflammatory cytokines after local magnetic administration to the ear is consistent with undetectable levels of systemic prednisolone, with unchanged levels of systemic iron in major organs (Section 3.3), and with a lack of any adverse histopathology findings in ear tissues (Section 3.2).
4. Discussion
Magnetic nanoparticles have been approved for human use in both the United States and in Europe. In the United States, there are three magnetic nanoparticles that have been approved for use in patients. Two of these particles (Feridex and GastroMark) have been approved by the FDA as imaging contrast agents for magnetic resonance imaging (MRI) (FDA, 1996a, 1996b). The third FDA-approved magnetic nano-particle agent is Feraheme, which is approved for treatment of iron-deficiency anemia (FDA, 2009). None of these three particles carry a drug. In Feraheme, the iron-oxide that makes the nanoparticle magnetic is itself the therapeutic agent. In Europe there are two magnetic nanoparticles that have received CE mark approval: Endomag’s Sienna + and MagForce AG’s NanoTherm particles. Neither one of these two particles carries a drug payload either. Sienna + is a tracer particle that is transported by the lymphatic system and can be used to detect sentinel nodes for breast cancer. NanoTherm magnetic particles convert an alternating magnetic field into heat to thermally treat brain tumors. Thus, in contrast to the Chemicell nanoparticles used in this study, none of the five particles above carry a drug payload.
Magnetic delivery to the inner ear is being developed in response to an unmet clinical need. It is difficult to get drugs into the cochlea with sufficient dose to attain a therapeutic effect. The inner ear is isolated from systemic circulation by a blood-labyrinth barrier similar to the blood-brain barrier (Borenstein, 2011; Inamura and Salt, 1992; Juhn et al., 2001; Radeloff et al., 2007; Salt and Plontke, 2005). As little as one out of every 107 drug molecules administered systemically reaches the cochlea (Parnes et al., 1999). Even when drugs are placed into the middle ear intra-tympanically, the literature indicates that for example < 0.01% (< 1 in every 10,000 molecules) reaches the cochlea in human patients (Bird et al., 2007; Bird et al., 2011). Hence even though it is believed that drugs exist (and are being developed) that could treat hearing loss, tinnitus, and vertigo; it is thought that those drugs do not enter the cochlea with sufficient dose to have a therapeutic effect.
Magnetic forces can be used to transport drug-eluting iron-oxide nanoparticles into the cochlea, to increase drug dose. In contrast to a magnet pulling nanoparticles into the cochlea, which requires placing a large magnet on the side of the head contralateral to the treated ear, in magnetic injection the magnet acts over a short distance from magnet to cochlea. This enables a compact magnet push device to effectively inject into the cochlea (as schematized in Fig. 1). In live animal and in initial human cadaver studies, magnetic injection forces have delivered substantially more drug into the cochlea and have shown a strong therapeutic effect as compared to controls without a magnet or magnetic particles (Depireux et al., 2017; Ramaswamy et al., 2017; Sarwar et al., 2012; Shapiro et al., 2014).
Since our prior and ongoing work has focused on magnetic delivery and efficacy, this study was focused solely on assessing the safety of magnetic drug injection to the cochlea. Our envisioned treatment is topical and we administered a small amount of particles, only about 750 μg of particles total, to the middle ear. Hence the systemic level of drug and nanoparticles after our ear treatment was anticipated to be low. The animal data confirmed that this was indeed the case. The systemic level of drug after topical ear administration was below mass-spectrometry detection limits. The systemic level of iron was no different in animals that received nanoparticles to the ear than in animals that did not. And there was no detection of systemic inflammatory response (no detection of inflammatory cytokines) after administration of nanoparticles to animal ears.
To assess safety for the ear and its continued normal function, rat hearing was measured by ABR (auditory brainstem recordings) before and 2 and 30 days after treatment in normal (no hearing loss) animals. Two-sided t-tests indicated no difference in hearing thresholds 30 days after treatment in ears that received nanoparticles compared to ears that did not. At 2 days after treatment, there was a lone statistically-significant change in hearing at the highest measured frequency between group D ears (drug loaded nanoparticles) and group A ears (intra-tympanic saline), at 32 kHz, but this difference was absent 28 days later. This change in hearing at high frequency is likely due to the mass of nanoparticles on the round window membrane (which would slow its response at higher frequencies, a conductive hearing loss). By 28 days later, this difference in hearing at high frequency was absent, likely due to the clearance of nanoparticles from the round window membrane.
Ear histopathology evaluation by a board-certified veterinary pathologist noted no structural damage, degeneration, necrosis, fibrosis or otosclerosis in any of the ears examined. This ear histology is shown in Fig. 10, which we note has also appeared in a previous invited publication that was focused on middle ear histopathology (Lafond et al., 2018). All observed histopathology changes were very mild in severity and were considered non-adverse. By 90 days after treatment there was ongoing and almost complete reversibility even of these mild changes.
Overall there is an unmet need to better deliver medications to the cochlea. Magnetic injection has the potential to minimally invasively deliver high doses of drug to the cochlea. In our animal studies we have observed that magnetic delivery even of generic drugs (e.g. common anti-inflammatory steroids) to the cochlea can produce a strong therapeutic effect (Depireux et al., 2017; Ramaswamy et al., 2017; Sarwar et al., 2012; Shapiro et al., 2014). Magnetic delivery of new and novel drugs, proteins, and genes is anticipated to provide an even stronger therapeutic benefit. Conversely, even new and novel drugs will not have any effect for treating hearing loss, suppressing tinnitus, or relieving vertigo if they do not reach a therapeutic dose in the cochlea. Hence magnetic injection is being developed to enable safe and effective delivery of therapeutic compounds to the inner ear, to treat hearing loss, tinnitus, and vertigo.
5. Conclusion
A safety study was conducted in rodents for magnetic delivery of drug to the cochlea. In this proposed novel delivery method, a magnetic device applies forces on drug-loaded bio-compatible iron-oxide nano-particles that have been placed in the middle ear by intra-tympanic needle injection. The magnetic force pushes the particles away from the magnet and transports the particles from the middle ear, through the window membranes, and into the cochlea where they then release their therapeutic payload (in this case the steroid prednisolone).
The test arms of this preclinical study included single and multiple magnetic doses, and control arms included intra-tympanic saline and intra-tympanic prednisolone. It was found that there was no statistical difference in hearing between magnetically treated ears versus ears that received intra-tympanic steroid, both 2 and 30 days after treatment. There were no adverse histopathology findings for ear tissues. All ear inflammatory changes were very mild in severity and by 90 days after treatment there was ongoing and almost complete reversibility of all changes. No ear tissue scarring or hemorrhage trends were associated with magnetic delivery. Additionally, the systemic levels of drug and iron were low after magnetic delivery to the ear. Systemic drug levels were below mass-spectrometry detection limits, and systemic iron levels were no different in animals that received nanoparticles to their ears than in animals that did not. In summary, after conducting a rodent safety study for magnetic injection of therapy to the cochlea, no adverse safety findings were observed.
Acknowledgements and disclosures
Funding is gratefully acknowledged from Action on Hearing Loss (TRIH 2013) in the UK, from the Congressionally Directed Medical Research Program (CDMRP) (W81XWH-09-1-0368), from the National Institutes of Health (NIH) (HHSN261201700023C and 2R44DC013534-02A1), from the Maryland Innovation Initiative at the Maryland Technology Development Corporation (TEDCO) (1212-004) and the Maryland Industrial Partnerships (MIPS) (5021) program in the State of Maryland, as well as from the Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s National Medical Center (30003062), in Washington DC. We would also like to thank Mr. Josh Wilhide at the Molecular Characterization and Analysis Complex at UMBC in Baltimore for his help with ICP-MS protocols, Dr Yue Li from Biochemistry at the University of Maryland College Parkith who helped with HPLC measurements, and Dr. Jerome Lewis for his reviews of the manuscript.
INW, BS, and DAD disclose that they have a financial equity stake in Otomagnetics.
References
- Allen ED, Burdette JH, 2001. Questions and Answers in MRI, 2 ed. Mosby, St. Louis, MO. [Google Scholar]
- Alvarado JC, Fuentes-Santamaria V, Janeno-Flores T, Blanco JL, Juiz JM, 2012. Normal variations in the morphology of autidotry brain stem response (ABR) waveforms: a study in Wistar rats. Neurosci. Res 73, 302–311. [DOI] [PubMed] [Google Scholar]
- Anraku M, Tanaka M, Hiraga A, Nagumo K, Imafuku T, Maezaki Y, Iohara D, Uekama K, Watanabe H, Hirayama F, Maruyama T, Otagiri M, 2014. Effects of chitosan on oxidative stress and related factors in hemodialysis patients. Carbohydr. Polym 112, 152–157. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, Tanaka C, Chen GD, Henderson D, 2010. Age-related hearing loss: is it a preventable condition? Hear. Res 264, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ, 2007. Intratympanic versus intravenous delivery of methylprednisolone to cochlear peri-lymph. Otology Neurotol 28, 1124–1130. [DOI] [PubMed] [Google Scholar]
- Bird PA, Murray DP, Zhang M, Begg EJ, 2011. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to Cochlear peri-lymph. Otology Neurotol 32, 933–936. [DOI] [PubMed] [Google Scholar]
- Borenstein JT, 2011. Intracochlear drug delivery systems. Expert. Opin. Drug Del 8, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T, Odintsov B, Bauer C, 2012. Gamma-aminobutyric acid and glutamic acid levels in the auditory pathway of rats with chronic tinnitus: a direct determination using high resolution point-resolved proton magnetic resonance spectroscopy (1HMRS). Front. Syst. Neurosci 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depireux DA, Shimoji M, Shukoor M, Ramaswamy B, Benhal P, Shapiro B, 2017. Magnetic delivery of therapy to the cochlea. Hear. J 14–20. [Google Scholar]
- El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO, 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis 202, 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA, E.M.A.S.M, 2013. ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pahrmacetuicals Step5 In: Health European Medicines Agency, London, UK, pp. 1–26. [Google Scholar]
- FDA, 1995. In: Adminsitration F.A.D. (Ed.), Guidance for Industry: Content and Format of Investigational New Drug Applications (INDs) for Phase I Studies of Drugs, Including Well-characterized, Therapeutic, Biotechnology-derived Products
- FDA, 1996a. In: Adminsitration F.A.D. (Ed.), Feridex (Ferumoxides) I.V. NDA020416. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=020416.
- FDA, 1996b. In: Adminsitration F.A.D. (Ed.), Gastromark (Ferumoxsil) Oral NDA 020410. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&Applno=020410.
- FDA, 2009. In: Adminsitration F.A.D. (Ed.), Feraheme (Ferumoxytol) Injection. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022180.
- FDA, 2010. Guidance for Industry: M3 (R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Markety Authorization for Pharmaceuticals [PubMed]
- FDA, F, 2012. Guidance for industry: acute Bacterial otitis media: developing drugs for treatment In: Office of Communiations FDA, Silber Spring, MD. [Google Scholar]
- FDA, 2014. In: Adminsitration F.A.D. (Ed.), GRAS Substances (SCOGS) Database
- Fetoni AR, Picciotti PM, Paludetti G, Troiani D, 2011. Pathogenesis of presbycusis in animal models: a review. Exp. Gerontol 46, 413–425. [DOI] [PubMed] [Google Scholar]
- Fidani M, Pompa G, Mungiguerra F, Casati A, Fracchiolla ML, Arioli F, 2012. Investigation of the presence of endogenous prednisolone in equine urine by high-performance liquid chromatography mass spectrometry and high-resolution mass spectrometry. Rapid Commun. Mass Spectrom 26, 879–886. [DOI] [PubMed] [Google Scholar]
- Fidani M, Gamberini MC, Pompa G, Mungiguerra FM, Casati A, Arioli F, 2013. Presence of endogenous prednisolone in human urine. Steroids 78, 121–126. [DOI] [PubMed] [Google Scholar]
- Filipo R, Covelli E, Balsamo G, Attanasio G, 2010. Intratympanic prednisolone therapy for sudden sensorineural hearing loss: a new protocol. Acta Otolaryngol 130, 1209–1213. [DOI] [PubMed] [Google Scholar]
- Fiorito V, Crich SG, Silengo L, Altruda F, Aime S, Tolosano E, 2012. Assessment of iron absorption in mice by ICP-MS measurements of Fe-57 levels. Eur. J. Nutr 51, 783–789. [DOI] [PubMed] [Google Scholar]
- Gellein K, Garruto RM, Syversen T, Sjobakk TE, Flaten TP, 2003. Concentrations of Cd, Co, Cu, Fe, Mn, Rb, V, and Zn in Formalin-fixed Brain Tissue in Amyotrophic Lateral Sclerosis and Parkinsonism-dementia Complex of Guam Determined by High-resolution ICP-MS, Biological Trace Element Research Humana Press Inc., pp. 39–60. [DOI] [PubMed] [Google Scholar]
- Glasscock M, Jackson C, Josey A, 1991. The ABR Handbook: Auditory Brainstem Response, 2nd ed. Thieme Medical Pub, Stuttgart, Germany. [Google Scholar]
- Harris JP, Ryan AF, 1995. Fundamental immune-mechanisms of the brain and inner-ear. Otolaryngol. Head Neck Surg 112, 639–653. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, 2007. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci 46, 20–22. [PubMed] [Google Scholar]
- Hernandez-Gonzaleza SO, Gonzalez-Ortiza M, Martinez-Abundisa E, Robles-Cervantes JA, 2010. Chitosan improves insulin sensitivity as determined by the euglycemic-hyperinsulinemic clamp technique in obese subjects. Nutr. Res 30, 392–395. [DOI] [PubMed] [Google Scholar]
- Hu A, Parnes LS, 2009. Intratympanic steroids for inner ear disorders: a review. Audiol. Neurootol 14, 373–382. [DOI] [PubMed] [Google Scholar]
- Inamura N, Salt AN, 1992. Permeability changes of the blood-labyrinth barrier measured in vivo during experimental treatments. Hear. Res 61, 12–18. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Hunter BA, Odland RM, 2001. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int. Tinnitus J 7, 72–83. [PubMed] [Google Scholar]
- Kanzaki J, Ouchi T, 1981. Steroid-responsive bilateral sensorineural hearing-loss and immune-complexes. In: Archives of Oto-Rhino-Laryngology-Archiv Fur Ohren-Nasen-Und Kehlkopfheilkunde 230 pp. 5–9. [DOI] [PubMed] [Google Scholar]
- Kariya S, Okano M, Higaki T, Makihara S, Haruna T, Eguchi M, Nishizaki K, 2013. Neutralizing antibody against granulocyte/macrophage colony-stimulating factor inhibits inflammatory response in experimental otitis media. Laryngoscope 123, 1514–1518. [DOI] [PubMed] [Google Scholar]
- Kean T, Thanou M, 2010. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev 62, 3–11. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ahn HY, Kwak JH, Shin DY, Kwon YI, Oh CG, Lee JH, 2014. The effects of chitosan oligosaccharide (GO2KA1) supplementation on glucose control in subjects with prediabetes. Food Funct 5, 2662–2669. [DOI] [PubMed] [Google Scholar]
- Kut C, Zhang Y, Hedayati M, Zhou H, Cornejo C, Bordelon D, Mihalic J, Wabler M, Burghardt E, Gruettner C, Geyh A, Brayton C, Deweese TL, Ivkov R, 2012. Preliminary study of injury from heating systemically delivered, nontargeted dextransuperparamagnetic iron oxide nanoparticles in mice. Nanomedicine 7, 1697–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente M, Ravina M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ, 2010. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev 62, 100–117. [DOI] [PubMed] [Google Scholar]
- Lafond J-F, Shimoji M, Ramaswamy B, Shukoor M, Malik P, Shapiro B, Depireux D, 2018. Middle ear histopathology following magnetic delivery to the cochlea or prednisolone-loaded Iron oxide nanoparticles in rats. Toxicol. Pathol 46, 101–106. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W, 1999. How useful is corticosteroid treatment in cochlear disorders? In: Oto-Rhino-Laryngologia Nova 9 pp. 203–215. [Google Scholar]
- Li ML, Lee LC, Cheng YR, Kuo CH, Chou YF, Chen YS, Yao CM, Chen PR, Hsu CJ, Song YL, Lee CF, 2013. A novel aerosol-mediated drug delivery system for inner ear therapy: Intratympanic aerosol methylprednisolone can attenuate acoustic trauma. IEEE Trans. Biomed. Eng 60, 2450–2460. [DOI] [PubMed] [Google Scholar]
- Li P, Gao K, Ding D, Salvi R, 2015. Characteristic anatomical structures of rat temporal bone. J. Otolaryngol 10, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL, 2013. The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear. Res 295, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbe AS, Bergemann C, Huhnt W, Fricke T, Riess H, Brock JW, Huhn D, 1996a. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res 56, 4694–4701. [PubMed] [Google Scholar]
- Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dorken B, Harrmann F, Gurtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke A-J, Ohlendorf D, Huhnt W, Huhn D, 1996b. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 56, 4686–4693. [PubMed] [Google Scholar]
- Lubbe AS, Alexiou C, Bergemann C, 2001. Clinical applications of magnetic drug targeting. J. Surg. Res 95, 200–206. [DOI] [PubMed] [Google Scholar]
- deLuca LS, Gommerman JL, 2012. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat. Rev. Immunol 12, 339–351. [DOI] [PubMed] [Google Scholar]
- Martin-Saldana S, Palao-Suay R, Trinidad A, Aguilar MR, Ramirez-Camacho R, San Roman J, 2016. Otoprotective properties of 6 alpha-methylprednisolone-loaded nanoparticles against cisplatin: in vitro and in vivo correlation. Nanomed. Nanotechnol 12, 965–976. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Plontke SK, Hartsock JJ, Salt AN, 2009. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in Guinea pigs: implications for local drug delivery in humans. Otol. Neurotol 30, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimert-Andersson T, Binnmyr J, Enoksson M, Langeback J, Zettergren L, Hallgren A-C, Franzen H, Singer P, Gronlund H, Gafvelin G, 2014. Evaluation of safety and efficacy as an adjuvant for the chitosan-based vaccine delivery vehicle ViscoGel in a single-blind randomized Phase I/IIa clinical trial. Vaccine 32, 5967–5974. [DOI] [PubMed] [Google Scholar]
- Nixon DE, Butz J, Eckdahl SJ, Burritt MF, 2000. Determination of Copper and Iron in Liver Tissue Using the ELAN DRC ICP-MS Perkinelmer Application Note, ICP Mass Spectrometry Perkinelmer. [Google Scholar]
- NSC, U.N.S.C, 2013. In: England, P.H. (Ed.), Guidance Newborn Hearing Screening: Programme Overview NHS Population Screening Londan, England. [Google Scholar]
- Okano T, 2014. Immune system of the inner ear as a novel therapeutic target for sensorineural hearing loss. Front. Pharmacol 5, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, DePalo VA, 2000. Anti-inflammatory cytokines. Chest 117, 1162–1172. [DOI] [PubMed] [Google Scholar]
- Parnes LS, Sun A-H, Freeman DJ, 1999. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope 109, 1–17. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Abbot NC, Harkness EF, Ernst E, 1999. Randomized, double-blind trial of chitosan for body weight reduction. Eur. J. Clin. Nutr 53, 379–381. [DOI] [PubMed] [Google Scholar]
- Pompa G, Arioli F, Casati A, Fidani M, Bertocchi L, Dusi G, 2011. Investigation of the origin of prednisolone in cow urine. Steroids 76, 104–110. [DOI] [PubMed] [Google Scholar]
- Radeloff A, Unkelbach MH, Tillein J, Braun S, Helbig S, Gstottner W, Adunka OF, 2007. Impact of intrascalar blood on hearing. Laryngoscope 117, 58–62. [DOI] [PubMed] [Google Scholar]
- Ramaswamy B, Roy S, Apolo AB, Shapiro B, Depireux DA, 2017. Magnetic nanoparticle mediated steroid delivery mitigates cisplatin induced hearing loss. Front. Cell. Neurosci 11, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, Goebel JA, Hammerschlag PE, Harris JP, Isaacson B, Lee D, Linstrom CJ, Parnes LS, Shi H, Slattery WH, Telian SA, Vrabec JT, Reda DJ, 2011. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 305, 2071–2079. [DOI] [PubMed] [Google Scholar]
- Salt AN, Plontke SK, 2005. Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today 10, 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Plontke SK, 2009. Principles of local drug delivery to the inner ear. Audiol. Neurootol 14, 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar A, Lee R, Depireux DA, Shapiro B, 2012. Treating Tinnitus by Magnetic Pushing of Therapy: Rat Experiments, 9th International Conference on the Scientific and Clinical Applications of Magnetic Carriers Minneapolis, MN USA. [Google Scholar]
- Sarwar A, Lee R, Depireux DA, Shapiro B, 2013. Magnetic injection of nanoparticles into rat inner ears at a human head working distance. IEEE Trans. Magn 49, 440–452. [Google Scholar]
- Seidman MD, Ahmad N, Joshi D, Seidman J, Thawani S, Quirk WS, 2004. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Otolaryngol 124, 16–24. [DOI] [PubMed] [Google Scholar]
- Shapiro B, Rutel I, 2009. In: USTPO (Ed.), Devices, Systems, and Methods for Magnetic-assisted Therapeutic Agent Delivery USTPO, USA. [Google Scholar]
- Shapiro B, Dormer K, Rutel IB, 2010. A two-magnet system to push therapeutic nanoparticles. AIP Conf. Proc. J 72, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B, Depireux DA, Rutel I, Dormer K, 2013. In: USPTO (Ed.), Devices and Methods for Directing Agents to the Middle Ear and the Inner Ear (Continuation in Part) USPTO, USA. [Google Scholar]
- Shapiro B, Kulkarni S, Nacev A, Sarwar A, Depireux D, 2014. Shaping magnetic fields to direct therapy to ears and eyes. Annu. Rev. Biomed. Eng 16, 455–481. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Nakayama H, Furuta T, Kasuya Y, Tsuchiya M, Soejima A, Yamada A, Nagasawa T, 2008. Simultaneous determination of prednisolone, prednisone, cortisol, and cortisone in plasma by GC-MS: estimating unbound prednisolone concentration in patients with nephrotic syndrome during oral prednisolone therapy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 870, 164–169. [DOI] [PubMed] [Google Scholar]
- Smith A, Perelman M, Hinchcliffe M, 2014. Chitosan a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccin. Immunother 10, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear SA, Schwartz SR, 2011. Intratympanic steroids for sudden sensorineural hearing loss: a systematic review. Otolaryngol. Head Neck Surg 145, 534–543. [DOI] [PubMed] [Google Scholar]
- Thomas R, 2013. Practical Guide to ICP-MS A Tutorial for Beginners, 3 ed. CRC Press Taylor & Francis Group, New York. [Google Scholar]
- Trune DR, Kempton JB, 2001. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Fas(lpr) autoimmune mice. Hear. Res 155, 9–20. [DOI] [PubMed] [Google Scholar]
- USDA Food Safety and Inspection Service, O.O.P.H.S, 2013. In: USDA (Ed.), Determination of Metals by ICP-MS and ICP-OES (Optical Emssion Spectrometry)
- Varga I, Szebeni A, Szoboszlai N, Kovacs B, 2005. Determination of trace elements in human liver biopsy samples by ICP-MS and TXRF: hepatic steatosis and nickel accumulation. Anal. Bioanal. Chem 383, 476–482. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Dikkes P, Carrigan A, Karalis KP, 2001. Corticotrophin-releasing hormone regulates IL-6 expression during inflammation. J. Clin. Investig 108, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-XJ, 2011. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant. Imag. Med. Surg 1, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegst-Uhrich SR, Mullin EJ, Ding DL, Manohar S, Salvi R, Aga DS, Roth JA, 2015. Endogenous concentrations of biologically relevant metals in rat brain and cochlea determined by inductively coupled plasma mass spectrometry. Biometals 28, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, An J, 2007. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin 45, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]