Abstract
We previously described an in vitro model in which serous ovarian cystadenomas were transfected with SV40 large T antigen, resulting in loss of RB and P53 functions and thus mimicking genetic defects present in early high-grade serous extra-uterine Müllerian (traditionally called high-grade serous ovarian) carcinomas including those associated with the BRCA1 mutation carrier state. We showed that replicative aging in this cell culture model leads to a mitotic arrest at the spindle assembly checkpoint. Here we show that this arrest is due to a reduction in microtubule anchoring that coincides with decreased expression of the BUB1 kinase and of the phosphorylated form of its substrate, BUB3. The ensuing prolonged mitotic arrest leads to cohesion fatigue resulting in cell death or, in cells that recover from this arrest, in cytokinesis failure and polyploidy. Down-regulation of BRCA1 to levels similar to those present in BRCA1 mutation carriers leads to increased and uncontrolled microtubule anchoring to the kinetochore resulting in overcoming the spindle assembly checkpoint. Progression to anaphase under those conditions is associated with formation of chromatin bridges between chromosomal plates due to abnormal attachments to the kinetochore, significantly increasing the risk of cytokinesis failure. The dependence of this scenario on accelerated replicative aging can, at least in part, account for the site specificity of the cancers associated with the BRCA1 mutation carrier state, as epithelia of the mammary gland and of the reproductive tract are targets of cell-nonautonomous consequences of this carrier state on cellular proliferation associated with menstrual cycle progressions.
Keywords: ovarian carcinoma, aneuploidy, BRCA1, cytokinesis failure, P53
INTRODUCTION
Mitosis is a highly orchestrated process regulated by multiple checkpoints. The spindle assembly checkpoint, which takes place during prometaphase, ensures proper chromosome segregation by preventing progression from metaphase to anaphase until each of the following is completed: (i) spindle/microtubule formation, (ii) attachment of the microtubules to the kinetochore, (iii) generation of tension on the centromeres of sister chromatids by motor proteins, and (iv) dissociation of the mitotic checkpoint complex 1. This multiprotein complex, present at the kinetochores of sister chromatids, sequesters CDC20, the activating component of the anaphase promoting complex 2. Premature exit from this checkpoint can lead to cytokinesis failure due to the presence of lagging chromosomes or abnormal spindle anchoring 3. This results in polyploidy and subsequent aneuploidy, one of the hallmarks of cancer. Several cancer types, particularly those associated with the BRCA1 mutation carrier state, are characterized by an aneuploid state that is near polyploid, suggesting a role for this scenario in their development 4.
Women harboring germline mutations in BRCA1 or BRCA2 are predisposed to triple negative breast carcinomas and high-grade serous carcinomas of the ovary, which will be referred to as high-grade serous extra-uterine Müllerian carcinomas in this manuscript because, as explained earlier, this nomenclature more accurately conveys the true site of origin of these cancers 5. Indeed, the notion that these tumors originate in the ovarian surface epithelium has been challenged and is no longer favored by the scientific community 6, 7. Numerical chromosomal abnormalities characterized by a near polyploid aneuploid state, together with the presence of a P53 mutation, are the only clonal abnormalities commonly associated with these cancers, hence the idea that they are primarily a disease of chromosomes as opposed to being driven by alterations in specific signaling pathways 8. We previously developed an in vitro cell culture model where serous extra-uterine Müllerian cystadenomas, which are benign tumors of the same differentiation lineage as high-grade serous extra-uterine Müllerian carcinomas, were transfected with SV40 large T antigen to extend their in vitro life span 9, 10. This antigen, by inhibiting the P53 and RB proteins, mimics alterations typically present in early cancers associated with the BRCA1 mutation carrier state. Indeed, clonal P53 mutations are regarded as a molecular signature for cancer precursor lesions in BRCA1 mutation carriers in the absence of any morphological evidence of cancer 11. Forced expression of SV40 large T antigen was also used in cell culture and transgenic models of high-grade serous extra-uterine Müllerian carcinomas used successfully in other laboratories 12, 13.
We reported earlier that this in vitro culture model is characterized by a cell cycle arrest at the spindle assembly checkpoint as the cells reach high replicative ages in vitro, which can be overcome by down-regulation of BRCA1 to levels comparable to those present in human BRCA1 mutation carriers 14. Such premature recovery from the spindle assembly checkpoint is not without cost, as it can lead to cytokinesis failure resulting in polyploidy and subsequent aneuploidy. An association between decreased BRCA1 levels and risk of polyploidy was also reported by Wang et al. 15 in a slightly different in vitro model. This chain of events can lead to spontaneous malignant transformation and, in fact, led to the establishment of immortal cell lines in our laboratory 10. We hypothesize, based on observations not only with this in vitro model, but also with mice carrying a conditional Brca1 knockout 16 as well as observations with human populations 17, 18, that the molecular events mediating predisposition to triple negative breast carcinoma and high-grade serous extra-uterine Müllerian carcinoma in BRCA1 mutation carriers entail an interplay between cell-nonautonomous consequences of this carrier state in ovarian granulosa cells resulting in increased cellular proliferation and, thus, premature aging in mammary and extra-uterine Müllerian epithelia, and cell-autonomous consequences leading to numerical chromosomal abnormalities in these epithelia due to cytokinesis failure associated with advanced replicative ages 14.
We sought to better understand the mechanism underlying the prolonged arrest at the spindle assembly checkpoint associated with increased replicative age, which is a central element of this proposed interplay between cell-autonomous and -nonautonomous mechanisms of cancer predisposition, in an effort to gain insights into the molecular events associated with early cancer development in individuals with germline BRCA1 mutations.
MATERIALS AND METHODS
Cell strains and culture conditions
The isolation and characterization of ML10 cells was previously described 9. ML10 cells were grown in Minimal Essential Medium (MEM) supplemented with 10% Fetal Bovine Serum (FBS) and 50U Penicillin, 50 µg Streptomycin per ml. Cells were incubated at 37°C under atmospheric conditions containing 95% humidity and 5% carbon dioxide.
Confocal immunofluorescence imaging
Cells were cultured on LabTek II cell culture chamber slides (VWR, cat # 62407-290). The slides were placed in phosphate buffered saline (PBS) containing 4% paraformaldehyde and 0.2% Triton X-100 for 30 minutes at 37°C for visualization of nuclear proteins, or at room temperature for visualization of total cellular proteins, followed by three 5-minute washes in PBS and incubation for 1 hour at room temperature in PBS containing 1% bovine serum albumin used as blocking agent. For studies entailing imaging of DNA only, the cells were mounted in SlowFade Gold Antifade Mountant with DAPI (ThermoFisher Scientific, Cat # S36938) and cover slipped. For protein stains, the cells were probed overnight with primary antibody, washed 3 times for 5 minutes with PBS, hybridized to appropriate fluorescent-labelled secondary antibodies for 2 hours at room temperature in the absence of light, and washed 3 times for 5 minutes. The slides were cover slipped and visualized using a PerkinElmer Spinning Disk Confocal Microscope with 63X objective and captured and analyzed using the Volocity system.
Protein extraction procedures
For nuclear and cytoplasmic protein separation and extraction, tissue culture dishes were briefly rinsed with ice cold PBS, lysed in Buffer A [0.1% Triton X, 20mM N-ethylmaleimide (NEM), 10mM HEPES pH 7.9, 10mM potassium chloride, 10mM beta-glycerophosphate 1mM sodium vanadate, 0.5mM phenylmethanesulfonylfluoride (PMSF), 2ug/ml aprotinin, 1.5mM magnesium chloride, 1ug/ml leupeptin, 1ug/ml pepstatin A, and 0.5mM dithiothreitol (DTT)], and harvested from the dish bottom by scraping. The cell lysates were rocked for 20 minutes at 4°C and centrifuged at 1150 × g for 10 min at the same temperature. The supernatant, containing the cytoplasmic proteins, was recovered and the cell pellet was resuspended in Buffer C [0.42mM sodium chloride, 6.25% glycerol, 20mM NEM, 20mM HEPES pH 7.9, 1mM sodium vanadate, 10mM beta-glycerophosphate, 2ug/ml aprotinin, 1.5mM magnesium chloride, 1ug/ml leupeptin, 1um/ml pepstatin A, 0.5mM DTT, 0.2mM ethylenediaminetetraacetic acid (EDTA), and 0.5mM PMSF]. The resuspended pellets were rocked for 30 minutes at 4°C and centrifuged at 4°C for 30 min at 15871 × g. The supernatants containing the nuclear proteins were collected and stored at −80°C until use.
For total protein extraction the cells were lysed in Triton lysis buffer, pH 8.0, containing 25mM sodium phosphate, 150 mM sodium chloride, 1% Triton X-100, 5mM EDTA, 50mM sodium fluoride, freshly supplemented with 1mM PMSF, 1mM sodium vanadate, 10ug/ml aprotinin, 10ug/ml leupeptin, 5uM pepstatin A, 25mM phenylarsine oxide (PAO); the preparation was rocked for 30 min at 4°C and centrifuged at 4°C for 30 min at 15871 × g. The supernatants, containing the total cellular proteins, were stored at −80°C.
Western blotting
Proteins larger than 100kDa and those between 37-100kDa were electrophoresed on 8% and 10% polyacrylamide gels, respectively, while those smaller than 37kDa were separated on 12% polyacrylamide gels. The protein samples were dissolved in 6X Laemmli buffer (0.416M sodium dodecyl sulfate, 0.896mM Bromophenol Blue, 47%/6.44M glycerol, 85mM Tris pH 6.8, 0.6M dithiothreitol), placed in boiling water baths for 5 min, loaded onto gels, and electrophoresed at 80V for 30 min, followed by 150V for 60-70 minutes. Wet transfer to PVDF membranes (BioRad, Cat # 1620177) was performed at 40V for at least 24 h at 4°C. The membranes were briefly washed with Tris buffered saline (pH 8.1) containing 0.1% Tween 20 and blocked for 1 hour at room temperature in the same buffer supplemented with 5% blocking grade milk proteins, Bio-Rad, Cat # 1706404). The membranes were probed overnight at 4°C with primary antibody diluted in Tris buffered saline containing 0.1% Tween 20 and blocking proteins, washed, and hybridized to secondary antibody for 1 hour at room temperature followed by a final wash in a similar solution. The membranes were treated with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, Cat # 34077), and exposed to X-ray films.
Transfection with siRNA
The siRNA sequences used for BRCA1 down-regulation were published 14. The siRNA sequence against Green Fluorescence Protein used as control was 5’-GACGUAAACGGCCACAAGU-3’. Transfection was achieved using Lipofectamine 2000 Transfection Reagent (ThermoFisher Scientific, Cat # 11668019) according to manufacturer’s protocol.
Time lapse microscopy
The cells were cultured on glass chamber glass slides and treated with siRNA against either GFP or BRCA1 for 24 hours before being transferred to a Perkin Elmer Spinning Disk Confocal microscope fitted with an incubator under controlled temperature and atmospheric conditions. Photographs were taken every minute over a 20-hour period using the 10X objective and captured and analyzed using the Volocity system.
Source and dilutions of antibodies
For confocal microscopy, the following antibodies were used at a dilution of 1 µg/mL: EB1 (BD BioScience, Cat# 610535) and BUB3 (Santa Cruz Biotechnology, sc-136217). Secondary antibodies included goat anti-mouse IgG (H+L) Alexa Fluor 555 conjugate (Thermo Fisher, Cat #A21422) and goat-anti-rabbit IgG (H+L). Alexa Fluor 647 conjugate (Thermo Fisher Cat # A-21244), used at a dilution of 1µg/mL. For western blotting, dilutions of 1:1000 were used for antibodies against Ku70 (Santa Cruz Biotechnology, Cat# sc-12729), GAPDH (Santa Cruz Biotechnology, Cat# sc-25778) and BUB3 and dilutions of 1:500 were used for antibodies against EB1, phospho-BUB3 (Thermo Fisher, Cat# PA5-37772) and BUB1 (Santa Cruz Biotechnology, Cat# sc-28257).
Statistical analyses
We used 2-tailed Fisher’s exact test to compare proportions of the different parameters of interest between different cell populations.
RESULTS
Mechanism of metaphase arrest in aging ovarian cystadenomas
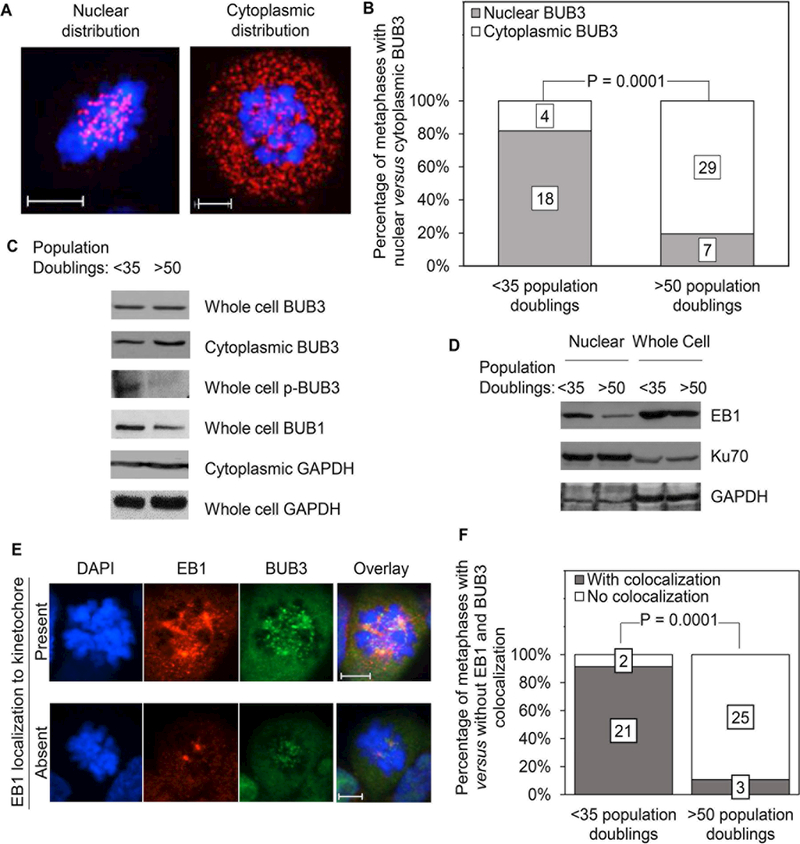
We reported earlier that in vitro cultures of extra-uterine Müllerian serous cystadenomas, which are of the same differentiation lineage as the cancers of the female reproductive tract that are typically associated with the BRCA1 mutation carrier state, show increased predisposition to cell cycle arrest at the spindle assembly checkpoint when they reach advanced replicative age 14. We sought to better understand the mechanisms underlying this arrest by investigating changes in the intracellular distribution of BUB3, a component of the metaphase checkpoint complex also involved in proper microtubule attachments, as a function of replicative aging. Representative examples of cells with predominantly nuclear and cytoplasmic distribution of BUB3 during mitosis are shown in Fig. 1A. Eighteen of 22 (82%) mitotic cells with a replicative age of less than 35 population doublings (low replicative age) showed a primarily nuclear distribution of BUB3 compared to 7 of 36 (19%) mitotic cells with a replicative age of more than 50 population doublings (high replicative age), an age at which most cells are arrested at the spindle assembly checkpoint (Fig. 1B). The majority of the cells in the latter age group showed a predominant cytoplasmic distribution of BUB3. This difference in the intra-cellular distribution of BUB3 between cells of low versus high replicative age is further supported by the demonstration of increased cytoplasmic BUB3 in the absence of a corresponding increase in total cellular BUB3 in aging populations by western blotting analyses (Fig. 1C). The presence of cytoplasmic BUB3 in cells of low replicative age in these studies performed on heterogeneous cell populations not synchronized in their cell cycle is explained by the fact that BUB3 is a multi-functional protein not expressed solely during mitosis
Figure 1: Reduced microtubule anchoring in aging ovarian cystadenomas.
A: Confocal images of ovarian cystadenomas in prometaphase/metaphase stained with a fluorescent antibody against BUB3 (red) and counterstained with DAPI (blue) showing representative examples of predominantly nuclear (left) and cytoplasmic (right) distribution of BUB3. B: Cystadenomas of low (<35 population doublings) versus high (>50 population doublings) replicative ages were examined by confocal microscopy as in (A); mitotic cells were scored based on presence of predominantly nuclear versus cytoplasmic distribution of BUB3; the numbers on the stacked bars represent the total number of cells for each parameter. C: Western blots of either whole cellular protein extracts or of proteins extracted from the cytoplasmic fraction only of cultured cystadenomas with less than 35 versus more than 50 population doublings were probed with antibodies against the indicated proteins; GAPDH was used as loading control. D: Western blots of nuclear and whole cell protein extracts of cystadenomas of low and high replicative ages probed with an antibody against EB1; Ku70 and GAPDH are used as loading controls for nuclear and cytoplasmic proteins, respectively. E: Confocal microscopy images showing examples of ovarian cystadenomas undergoing mitosis with and without EB1 localization to the kinetochore; immunopositivity for BUB3 is used as a surrogate marker for the kinetochore. F: Cystadenomas of low versus high replicative ages were examined by confocal microscopy as in (E) and the mitotic cells were scored based on presence versus absence of colocalization of EB1 and BUB3 to the kinetochore; the numbers on the stacked bars represent the total number of cells for each parameter. Magnification bars: 6 microns.
BUB3 is a target of phosphorylation by the BUB1 kinase, known as the master regulator of the metaphase checkpoint complex formation 19. The phosphorylated form of BUB3 recruits microtubules to the kinetochores to promote anchoring 20. We hypothesized that failure of BUB3, a mitotic checkpoint complex protein, to associate with chromosomes in cells of higher replicative age, which can readily account for the observed prolonged mitotic arrest in this cell population, might reflect a decrease in BUB3 phosphorylation. Indeed, a substantial relative decrease in the phosphorylated form of BUB3 was seen by western blotting analyses in cell populations with more than 50 population doublings compared to populations with less than 35 population doublings (Fig. 1C). This decrease coincided with a decrease in total cellular BUB1 (Fig. 1C).
Such decrease in BUB3 phosphorylation supports the idea that recruitment of microtubules to the kinetochore is hindered in aging cell populations. We investigated this possibility further by examining the intracellular distribution of End Binding Protein 1 (EB1), a protein that accumulates at the kinetochores upon microtubule anchoring and stabilizes their attachment to centromeres 21. Western blots probed with an antibody against EB1 showed decreased levels of this protein in cells of high replicative age. These differences were most prominent in nuclear extracts compared to whole cell extracts (Fig. 1D). This conclusion was supported by comparing the intracellular localization of EB1 to that of nuclear BUB3 (used as surrogate marker of kinetochore localization) in mitotic cells from the 2 age groups by confocal microscopy (Fig. 1E). Quantification of the confocal observations showed that EB1 was not only primarily localized to the DNA, but also showed strong colocalization with BUB3 in the younger age group as expected while it was primarily localized to the centrosomes, the site of microtubule nucleation, in cells from the older age group (Fig. 1E-F). We conclude that the mitotic arrest at the spindle assembly checkpoint that characterizes aging serous ovarian cystadenomas is due, at least in part, to a defect in microtubule anchoring.
Fate of arrested cells
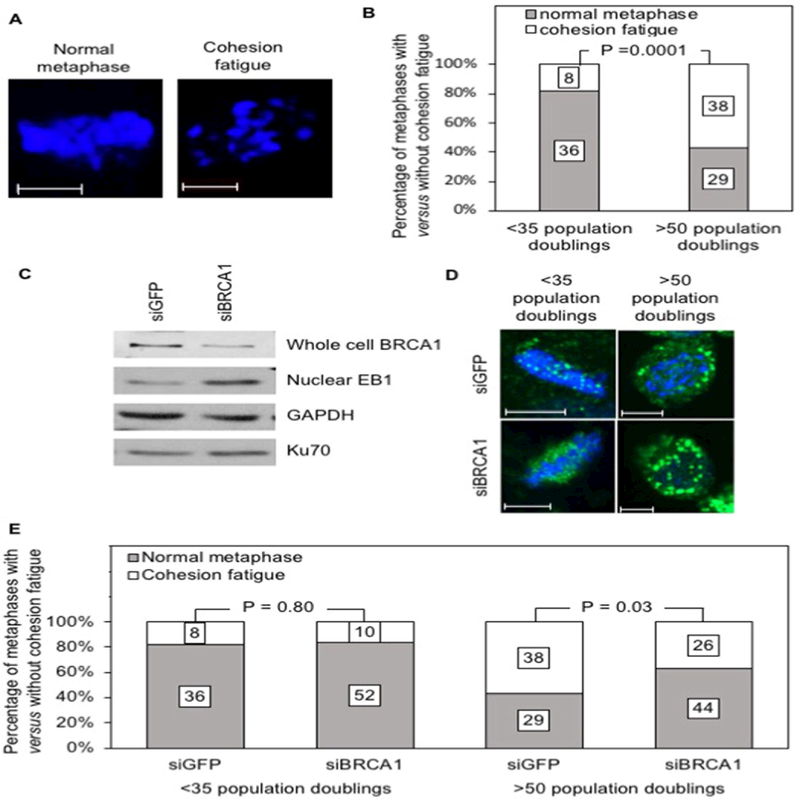
Cohesion fatigue is a possible consequence of a prolonged mitotic arrest such as that associated with replicative aging in our cell culture model. This phenomenon is manifested by asynchronous migration of chromatids from the metaphase plate in cells that are arrested or delayed in metaphase 22. It leads to either cell death or, less commonly, to mitotic slippage and cytokinesis failure, as typically observed in aging populations of cultured ovarian cystadenomas 14. Fig. 2A shows 2 different cells examined by confocal microscopy after visualization of the metaphase plates using DAPI staining: one (left) with an intact metaphase plate associated with a normal metaphase and the other (right) illustrating migration of chromatids from the metaphase plate indicative of cohesion fatigue. Fifty seven percent (38 of 67) of mitotic cells with a replicative age higher than 50 population doublings showed evidence of cohesion fatigue when examined by confocal microscopy compared to 18% (8 of 44) of mitotic of cells in the younger replicative age group (Fig. 2B). We conclude that the increased rate of cell death and of cytokinesis failure associated with a prolonged cell cycle arrest in our cell culture model are mediated, at least in part, by cohesion fatigue.
Figure 2: Mechanism of escape from the spindle assembly checkpoint in cells with reduced BRCA1 expression.
A: Confocal images of cells stained with DAPI showing a normal metaphase (left) and a metaphase undergoing cohesion fatigue (right). B: Cystadenomas of low (<35 population doublings) versus high (>50 population doublings) replicative ages were examined by confocal microscopy and the mitotic cells were scored based on presence of either normal metaphases versus metaphases showing evidence of cohesion fatigue; the numbers on the stacked bars represent the total number of cells for each parameter. C: Western blot of nuclear or total protein extracts obtained from cystadenomas treated with siRNA directed against either BRCA1 or GFP (control) and probed with an antibody against EB1; a probe for Ku70 was used to evaluate loading of nuclear proteins while a probe for GAPDH was used to evaluate loading of cytoplasmic protein extracts. D: Cells stained with an antibody against EB1 (green) and counterstained with DAPI (blue) were examined by confocal microscopy and photographed under constant exposure; these representative images illustrate the increase in the size of EB1 signals in cells treated with siRNA directed against BRCA1 compared to cells treated with siRNA directed against GFP. E: Cystadenomas of low versus high replicative ages were treated with siRNA directed against either BRCA1 or GFP and examined by confocal microscopy; the mitotic cells were scored based on presence of either normal metaphases versus metaphases showing evidence of cohesion fatigue; the numbers on the stacked bars represent the total number of cells for each parameter. Magnification bars: 6 microns in (A) and 8 microns in (D).
Consequences of BRCA1 down-regulation
Premature recovery from the arrest at the spindle assembly checkpoint associated with replicative aging in cultures of ovarian cystadenomas can be achieved by down-regulation of BRCA1, a protein controlling familial predisposition to triple-negative breast and high-grade serous extra-uterine Müllerian carcinomas 14. Given our current data suggesting a role for defective microtubule anchoring in causing this arrest, we hypothesized that decreased BRCA1 expression could somehow overcome this defect. Western blot analysis of nuclear protein extracts derived from cultured serous cystadenomas showed increased EB1 levels following treatment with siRNA against BRCA1 (siBRCA1) in support of this hypothesis (Fig. 2C). This conclusion was further supported by confocal microscopy, which typically showed doubling of the size of EB1 signals following down-regulation of BRCA1 (Fig. 2D). This was associated with a decrease in the proportion of aging cells undergoing cohesion fatigue, as 38 of 67 (57%) cells treated with siRNA against GFP (siGFP) showed this abnormality compared to 26 of 70 (37%) cells treated with siBRCA1 (Fig. 2E). This is consistent with the notion that increased microtubule anchoring triggered by BRCA1 down-regulation relieves the cells from the stress associated with a prolonged mitotic arrest. Such down-regulation did not lead to a significant reduction in cohesion fatigue in the younger cell population, as this mitotic abnormality was seen in 8 of 44 (18%) cells treated with siRNA against GFP and in 10 out of 62 (16%) cells treated with siRNA against BRCA1 (Fig. 2E). This was expected because prolonged arrests at the spindle assembly checkpoint due to defective microtubule anchoring are infrequent in this population.
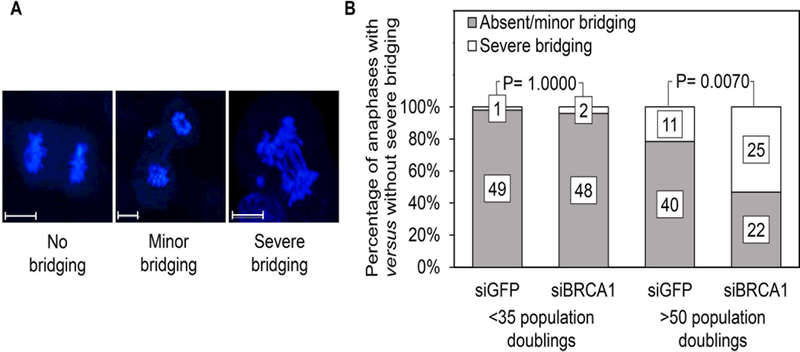
Our previous results showed that down-regulation of BRCA1 leads to a marked increase in cytokinesis failure resulting in polyploidy 14. Dysregulated microtubule attachment can lead to abnormal attachment to the kinetochore such as, for example, merotelic attachments, preventing normal cytokinesis 23. Such attachments are seen when the kinetochore of a sister chromatid is attached to microtubules from both spindle poles, generating opposing pulling forces. The condensed chromatid strands unravel under such conditions as they are being pulled to opposite poles resulting in chromatid strings or bridges between the two chromosome plates. Minor bridging (2 or fewer bridges) is compatible with normal cytokinesis because it can be overcome by the force of cleavage furrow ingression. More severe bridging (more than 2 bridges), however, cannot be overcome by similar forces resulting in cytokinesis failure and the generation of binucleated, tetraploid cells 24. Examples of cells showing either no bridging, minor bridging, or severe bridging in our cell culture model are shown in Fig. 3A. We hypothesized that the resumption of mitotic progression associated with cytokinesis failure observed after BRCA1 down-regulation in aging cell populations might be accounted for by a chain of event triggered by increased microtubule anchoring resulting in abnormal attachment to the kinetochore and formation of bridges between spindle poles, thus interfering with cleavage furrow ingression. Severe bridging was rarely present in cells of low replicative age regardless of their state of BRCA1 down-regulation (present in 2% of cells treated with siRNA against GFP compared to 4% of cells treated with siRNA against BRCA1) (Fig. 3B). This is consistent with the fact that a prolonged mitotic arrest due to defective microtubule anchoring is rarely present in this age group, thus negating the effect of BRCA1 down-regulation. In contrast, the rate of severe bridging was increased substantially in aging cells following treatment with siRNA against BRCA1 (53% of cells treated with siRNA against BRCA1 versus 22% of controls) (Fig. 3B), likely due to unsuccessful attempts at correcting anchoring deficiencies. We conclude that reduced BRCA1 levels lead to uncontrolled anchoring of microtubules to the kinetochore and subsequent chromosome bridging and cytokinesis failure, accounting for the increased rate of polyploidy reported earlier in aging cell populations following BRCA1 down-regulation 14. The fact that 22% of aging cells treated with siGFP showed severe bridging suggests that some degree of abnormal microtubule attachment also occurs spontaneously in cells undergoing a prolonged arrest.
Figure 3: Consequences of BRCA1 down-regulation on formation of bridges between chromosome plates.
A: Examples of mitotic cells in anaphase showing varying degrees of chromosome bridging seen by confocal microscopy after staining with DAPI; magnification bars: 8 microns. B: Cystadenomas of low (<35 population doublings) versus high (>50 population doublings) replicative ages were examined by confocal microscopy as in (A); cells undergoing anaphase were scored based on presence of either absent/minor or severe chromosome bridging; the numbers on the stacked bars represent the total number of cells for each parameter.
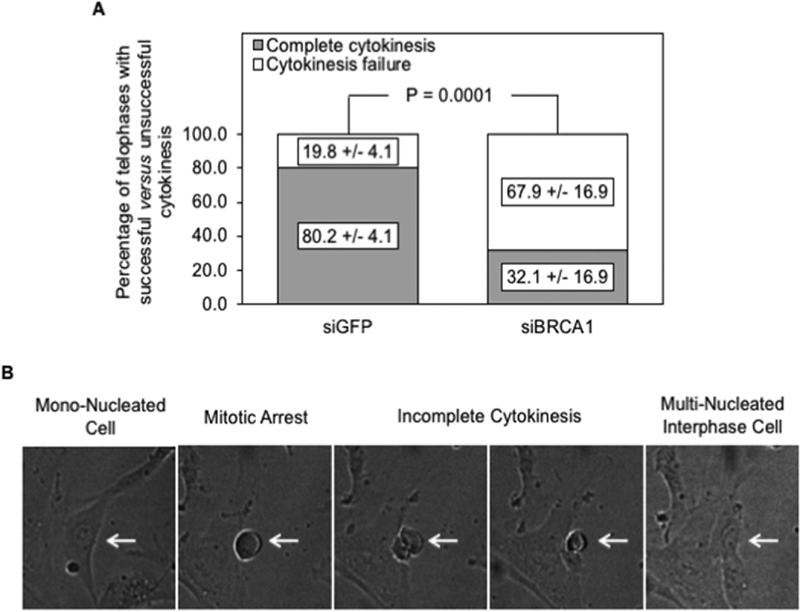
We examined the mitotic fate of aging cells treated with either control siRNA (siGFP) or siRNA directed against BRCA1 by time-lapse photography to further support our conclusions. All cells that attempted telophase in these two treatment groups were examined by 3 independent observers in a time-lapse video covering a period of 20 hours. Those that demonstrated cleavage furrow ingression followed by complete cytoplasmic separation were categorized as achieving complete cytokinesis. Cells that entered mitosis but did not resolve into 2 nascent daughter cells, regardless of attempt at cleavage furrow ingression, were categorized as showing cytokinesis failure. As shown in Fig. 4A, the rate of cytokinesis failure was significantly higher in cells treated with siRNA targeted against BRCA1 (67.9%) compared to the control cells treated with siGFP (19.8%) (P = 0.0001). This experiment was repeated a second time with a different movie and showed similar results. Fig. 4B shows snapshots of a representative cell entering mitosis and subsequently failing cytokinesis. The entire movie can be seen in Suppl. Fig. 1.
Figure 4: Consequences of BRCA1 down-regulation on cytokinesis failure.
A: Cystadenomas of high replicative ages were treated with siRNA directed against either BRCA1 or GFP and examined by time-lapse photography over a 20-hour period; all cells undergoing mitosis were followed and scored as showing either completion of cytokinesis or cytokinesis failure; the numbers on the stacked bars represent averages of the total number of cells for each parameter; the error bars represent variation in independent observations of the entire 20-hour video by 3 observers. Selected frames from the time-lapse microscopy video used are shown in panel (B), illustrating a mononuclear cell subsequently undergoing a mitotic arrest followed by cytokinesis failure resulting in a multi-nucleated interphase cell. The entire video is submitted as supplemental data.
DISCUSSION
Our results clearly show that sustained cellular proliferation leading to increased replicative age, in the absence of a normal P53 function, impairs normal anchoring of microtubules to the kinetochore in in vitro cultures of serous extra-uterine Müllerian cystadenomas undergoing mitosis. This coincides with a decrease in expression of the BUB1 kinase, which is the master regulator of metaphase checkpoint complex formation, and in a concomitant decrease in phosphorylation of its substrate, BUB3. The ensuing prolonged mitotic arrest eventually leads to uncoordinated migration of chromosomes to the mitotic spindle poles due to cohesion fatigue. Although cell death is the most likely outcome, mitotic slippage occasionally leads cells to survive at the expense of cytokinesis failure and polyploidy due to interference of lagging chromosomes with cleavage furrow ingression. The likelihood of cytokinesis failure is magnified if BRCA1 expression is decreased to levels approximating those present in human BRCA1 mutation carriers because such reduction leads to increased and uncoordinated attachments of microtubules to the kinetochore. This results not only in sufficient tension to overcome the aforementioned failure of microtubule anchoring, but also in abnormal attachments to the kinetochore, such as merotelic attachments, which are defined as anchoring of microtubules from opposite poles to the same kinetochore. Evidence for increased occurrence of such attachments in cells with decreased BRCA1 levels comes from higher incidence of chromatid bridges between chromosomal plates, indicating the presence of forces pulling individual chromosomes in opposite directions. This results in cytokinesis failure due to interference with cleavage furrow ingression.
Aneuploidy associated with a near polyploid state, which is a consequence of reduced BRCA1 expression 14, is often the only genetic abnormality present in high-grade serous extra-uterine Müllerian carcinomas in addition to abnormalities in P53 8. Our findings shed light not only on the molecular events predisposing to aneuploidy, but also on mechanisms that magnify its frequency in BRCA1 mutation carriers. The results also provide a possible explanation for the restricted site specificity of the cancers associated with the BRCA1 mutation carrier state, which are limited to the breast and to the reproductive tract in spite of the fact that BRCA1 is widely expressed in most tissues. Indeed, our data suggest that these consequences of reduced BRCA1 expression on premature progression to anaphase resulting in cytokinesis failure are only significant in cells subjected to accelerated replicative aging, a condition that can be triggered in mammary and in serous extra-uterine Müllerian epithelia due to cell-nonautonomous consequences of the BRCA1 mutation carrier state 18, 25–27. Evidence from observations with animal models as well as with human studies 18, 25–27 suggest that the BRCA1 mutation carrier state leads to changes in the hormonal fluctuations associated with the menstrual cycle, which are the most important risk factor for the sporadic (non-familial) forms high-grade serous extra-uterine Müllerian and breast carcinomas 28, 29. Such hormonal fluctuations can influence mitogenic pathways such as, for example, the RANK pathway in targeted tissues 17, 30, resulting in premature replicative aging due to increased cellular proliferation. Our working hypothesis for high-grade serous extra-uterine Müllerian and triple negative breast carcinoma predisposition based in this scenario is summarized in Fig. 5. This model readily accounts for the well-established notion that interruption of the menstrual cycle via either pregnancy or oral contraceptive use has profound protective effects against high-grade serous extra-uterine Müllerian and breast carcinomas not only in the general population 28, 29, but also in BRCA1 mutation carriers 31–33.
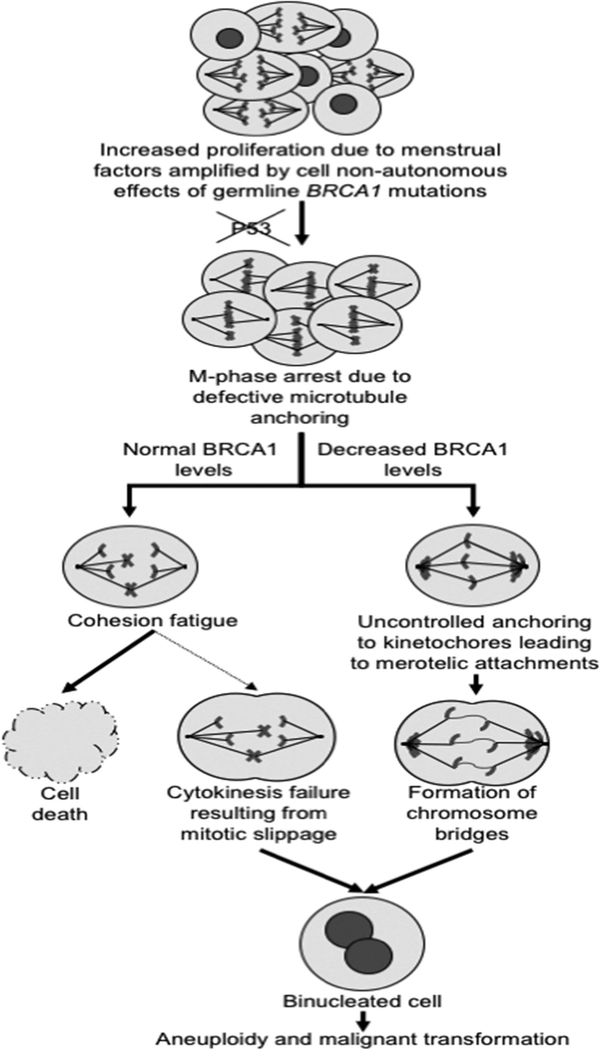
Figure 5: Working model for interplay between cell-nonautonomous and cell-autonomous mechanisms of cancer predisposition in BRCA1 mutation carriers.
The menstrual cycle, under the influence of ovarian granulosa cells and cells from the anterior pituitary, control the proliferation of tissues that have an elevated cancer risk in BRCA1 mutation carriers 11. These cell-nonautonomous signals are amplified in such carriers resulting in accelerated replicative aging. P53 alterations, which are frequent in these tissues in BRCA1 mutation carriers, eventually lead to mitotic arrest in aging cells due to defective microtubule anchoring. Cohesion fatigue is the most likely outcome in tissues with premature replicative aging due to increased menstrual cycle activity in the absence of a germline BRCA1 mutation, but some cells abruptly exit the arrest due to mitotic slippage resulting in cytokinesis failure and binucleation due to interference of lagging chromosomes or microtubules at the site of cell cleavage. The presence of a germline BRCA1 mutation not only intensifies accelerated replicative aging, but also leads to recovery from the mitotic arrest due to uncontrolled kinetochore attachments resulting in the formation of chromosome bridges; this significantly increases the rate of cytokinesis failure, leading to polyploidy and, subsequently, aneuploidy and malignant transformation.
The molecular events leading to cytokinesis failure and polyploidy, which are discussed here in the context of their role in predisposition to malignant transformation in BRCA1 mutation carriers, most likely continue to operate after malignant transformation has taken where they might contribute to the generation of polyploid giant cancer cells, recently shown in Jinsong Liu’s laboratory 34, 35 to express normal and cancer stem cell markers and to have the ability to increase their viability by reducing their DNA content via asymmetric divisions, budding, or bursting. This group suggested that these events, which are related to an early embryological mechanism common in the blastomere stage of preimplantation embryos, may represent important mechanisms for generating cancer stem-like cells and may also contribute to tumor heterogeneity 36.
The exact mechanisms whereby decreased BRCA1 expression can lead to uncontrolled anchoring of microtubules to the kinetochore are still unclear. The consequences of these abnormalities on the normal events needed to ensure proper homeostasis during mitotic progression are also incompletely understood. BRCA1 acts as a ubiquitin ligase when bound to BARD1 37, causing degradation of gamma-tubulin, the nucleating component for microtubule polymerization. Loss of this function leads to an increase in microtubule nucleation as demonstrated by increased formation of astral microtubules 38. It is possible that reduced availability of BRCA1 interferes with the control of this process, leading to excessive and poorly regulated microtubule anchoring. Another possibility is that the Chromosomal Passenger Complex, which is involved in regulating chromosome bi-orientation in metaphase, requires an intact BRCA1 function. Aurora B, a component of this complex, might be especially affected by the dysregulation in microtubule anchoring associated with deficiencies in BRCA1, as one of its functions includes correction of abnormal attachments to the kinetochores 39.
A better understanding of the mitotic events triggered by BRCA1 deficiencies might lead to the identification of biomarkers associated with these events that could be evaluated in biopsies of breast or fallopian tube before malignant transformation and provide important tools in the clinical management and follow up of BRCA1 mutation carriers. For example, they could be used as intermediate surrogate biomarkers of response in cancer risk reduction trials. This knowledge could also facilitate the development of non-surgical means of cancer risk reduction based on targeting mediators of the events leading to mitotic errors in BRCA1 mutation carriers. The only effective means of cancer risk reduction in BRCA1 mutation carriers currently entails surgical removal of breast and reproductive organs, with important consequences including infertility, premature menopause, and social/emotional side effects 40. This leads to many individuals to postpone their surgery and thus increase their risk of cancer, underscoring the need for such non-surgical approaches to cancer risk reduction in this population. Agents targeting Poly(ADP ribose) Polymerase (PARP) proteins might have merit in this context, as PARP inhibition appears to recapitulate phenotypic features associated with replicative aging observed in our present study, namely pre-anaphase arrest, cytokinesis failure, and multinucleation. Indeed, there is increasing evidence supporting a role for poly(ADP ribosyl)ating BUB3 and other centromeric proteins in mitotic progression, including for proper spindle assembly and function 41–47. While PARP inhibitors are often used for the treatment of the cancers developing in the context of the BRCA1 mutation carrier state given the dependence of these cancers on PARP proteins for DNA repair functions 48, their potential effects on cancer predisposition due to interference with the role of PARP proteins in mediating the mitotic events influenced by this carrier state, such as those reported here, merit further investigation. In addition, the idea that PARP inhibitors are effective in the treatment of BRCA1-deficient cancers due primarily to accumulation of double stranded DNA breaks secondary to deficient homologous recombination has been challenged 49. Thus, exploring the function of PARP proteins in the regulation of the mitotic events altered in BRCA1-deficient cells including those addressed in our study may provide important insights into the mechanism through which PARP inhibition induces cell death in such cancers.
Supplementary Material
Novelty:
The data provide novel insights into the mechanisms leading to aneuploidy in high-grade serous extra-uterine Müllerian carcinomas and on the role of BRCA1 on mitotic homeostasis
Impact:
By shedding light into the early events associated with their development, our study contributes to progress toward better prevention of important cancers of women and toward better understanding of their precursor lesions, which in turn should facilitate their early detection
ACKNOWLEDGEMENTS
This work was aided by grant number R01 CA133117 from the US National Institutes of Health to L Dubeau, by The Eve Appeal (www.eveappeal.org.uk) and UCLH/UCL, which received a proportion of its funding from the Department of Health NIHR Biomedical Research Centers funding scheme, and by the Cell and Tissue Imaging Core Facility of the USC/Norris Comprehensive Cancer Center (P30 CA14089).
REFERENCES
- 1.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint Curr Biol, vol. 22, 2012:R966–R80. [DOI] [PubMed] [Google Scholar]
- 2.Chao WC, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex Nature, vol. 484, 2012:208–13. [DOI] [PubMed] [Google Scholar]
- 3.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer Curr Opin Genet Dev, vol. 17, 2007:157–62. [DOI] [PubMed] [Google Scholar]
- 4.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol, vol. 18, 2006:658–67. [DOI] [PubMed] [Google Scholar]
- 5.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol 2008;9:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubeau L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol. Oncol 1999;72:437–42. [DOI] [PubMed] [Google Scholar]
- 7.Dubeau L, Drapkin R. Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol 2013;24 (Suppl 8):viii28–viii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo MP, Gomperts B, Imren S, DeClerck YA, Ito M, Velicescu M, Felix JC, Dubeau L. Establishment of long-term in vitro cultures of human ovarian cystadenomas and LMP tumors and examination of their spectrum of expression of matrix-degrading proteinases. Gynecol Oncol 1997;67:277–84. [DOI] [PubMed] [Google Scholar]
- 10.Velicescu M, Yu J, Herbert BS, Shay JW, Granada E, Dubeau L. Aneuploidy and telomere attrition are independent determinants of crisis in SV40-transformed epithelial cells Cancer Res, vol. 63, 2003:5813–20. [PubMed] [Google Scholar]
- 11.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube J Pathol, vol. 211, 2007:26–35. [DOI] [PubMed] [Google Scholar]
- 12.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Female Mice Chimeric for Expression of the Simian Virus 40 TAg under Control of the MISIIR Promoter Develop Epithelial Ovarian Cancer. Cancer es 2003;63:1389–97. [PubMed] [Google Scholar]
- 13.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, Mills GB, Bast RC Jr. A Genetically Defined Model for Human Ovarian Cancer. Cancer Res 2004;64:1655–63. [DOI] [PubMed] [Google Scholar]
- 14.Yu VM, Marion CM, Austria TM, Yeh J, Schonthal AH, Dubeau L. Role of BRCA1 in controlling mitotic arrest in ovarian cystadenoma cells. Int J Cancer 2012;130:2495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wang Z, Qi Z, Yin S, Zhang N, Liu Y, Liu M, Meng J, Zang R, Zhang Z, Yang G. The negative interplay between Aurora A/B and BRCA1/2 controls cancer cell growth and tumorigenesis via distinct regulation of cell cycle progression, cytokinesis, and tetraploidy. Mol Cancer 2014;13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Yen HY, Austria T, Pettersson J, Peti-Peterdi J, Maxson R, Widschwendter M, Dubeau L. A mouse model that reproduces the developmental pathways and site specificity of the cancers associated with the human BRCA1 mutation carrier state. EBioMedicine 2015;2:1318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widschwendter M, Burnell M, Fraser L, Rosenthal AN, Philpott S, Reisel D, Dubeau L, Cline M, Pan Y, Yi PC, Gareth Evans D, Jacobs IJ, et al. Osteoprotegerin (OPG), The Endogenous Inhibitor of Receptor Activator of NF-kappaB Ligand (RANKL), is Dysregulated in BRCA Mutation Carriers. EBioMedicine 2015;2:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widschwendter M, Rosenthal AN, Philpott S, Rizzuto I, Fraser L, Hayward J, Intermaggio MP, Edlund CK, Ramus SJ, Gayther SA, Dubeau L, Fourkala EO, et al. The sex hormone system in carriers of BRCA1/2 mutations: a case-control study. Lancet Oncol 2013;14:1226–32. [DOI] [PubMed] [Google Scholar]
- 19.Roberts BT, Farr KA, Hoyt MA. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase Mol Cell Biol, vol. 14, 1994:8282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logarinho E, Bousbaa H. Kinetochore-microtubule interactions “in check” by Bub1, Bub3 and BubR1: The dual task of attaching and signalling Cell Cycle, vol. 7, 2008:1763–8. [DOI] [PubMed] [Google Scholar]
- 21.Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol 2000;149:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daum JR, Potapova TA, Sivakumar S, Daniel JJ, Flynn JN, Rankin S, Gorbsky GJ. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr Biol 2011;21:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregan J, Polakova S, Zhang L, Tolic-Norrelykke IM, Cimini D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol 2011;21:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pampalona J, Roscioli E, Silkworth WT, Bowden B, Genesca A, Tusell L, Cimini D. Chromosome bridges maintain kinetochore-microtubule attachment throughout mitosis and rarely break during anaphase. PLoS One 2016;11:e0147420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong H, Yen HY, Brockmeyer A, Liu Y, Chodankar R, Pike MC, Stanczyk FZ, Maxson R, Dubeau L. Changes in the mouse estrus cycle in response to Brca inactivation suggest a potential link between risk factors for familial and sporadic ovarian cancer. Cancer Res 2010;70:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Pike MC, Wu N, Lin YG, Mucowski S., Punj V, Tang Y, Yen H-Y, Stanczyk FZ, Enbom E, Austria T, Widschwendter M, et al. Brca1 mutations enhance mouse reproductive functions by increasing responsiveness to male-derived scent. PLoS ONE 2015;10:e01139013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen HY, Gabet Y, Liu Y, Martin A, Wu NL, Pike MC, Frenkel B, Maxson R, Dubeau L. Alterations in Brca1 expression in mouse ovarian granulosa cells have short-term and long-term consequences on estrogen-responsive organs. Lab Invest 2012;92:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pike MC, Pearce CL, Peters R, Cozen W., Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril 2004;83:186–95. [DOI] [PubMed] [Google Scholar]
- 29.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am. J. Epidemiol 1992;136:1184–203. [DOI] [PubMed] [Google Scholar]
- 30.Nolan E, Vaillant F, Branstetter D, Pal B, Giner G, Whitehead L, Lok S, Mann G, kConFab., Rohrbach K, Huang L-Y, Soriano R, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nature Med 2016;22:933–9. [DOI] [PubMed] [Google Scholar]
- 31.Kramer JL, Velazquez IA, Chen BE, Rosenberg PS, Struewing JP, Greene MH. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-ip of BRCA1 mutation carriers. J Clin Oncol 2005;23:8629–35. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin JR, Risch HA, Lubinski J, Moller P, Ghadirian P, Lynch H, Karlan B, Fishman D, Rosen B, Neuhausen S, Offit K, Kauff N, et al. Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet Oncol 2007;8:26–34. [DOI] [PubMed] [Google Scholar]
- 33.Narod SA, Risch HA, Moslehi R, Dorum A, Neuhausen S, Olsson H, Provencher D, Radice P, Evans G, Bishop S, Brunet J-S, Ponder BAJ. Oral contraceptives and the risk of hereditary ovarian cancer. New Engl J Med 1998:424–8. [DOI] [PubMed] [Google Scholar]
- 34.Niu N, Zhang J, Mercado-Uribe I, Tao F, Han Z, Pathak S, Multani AS, Kuang J, Yao J, Bast RC, Sood AK, Hung M-C, et al. Linking genomic reorganization to tumor initiation via giant cell cycle. Oncogenesis 2016;5:e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014;33:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene 2017;36:4887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baer R, Ludwig T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr Opin Genet Dev 2002;12:86–91. [DOI] [PubMed] [Google Scholar]
- 38.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol Cell Biol 2005;25:8656–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krenn V, Musacchio A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front Oncol 2015;5:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oostrom I, Meijers-Heijboer H, Lodder LN, Duivenvoorden HJ, van Gool AR, Seynaeve C, van der Meer CA, Klijn JG, van Geel BN, Burger CW, Wladimiroff JW, Tibben A. Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol 2003;21:3867–74. [DOI] [PubMed] [Google Scholar]
- 41.Boehler C, Gauthier LR, Mortusewicz O, Biard DS, Saliou J-M, Bresson A, Sanglier-Cianferani S, Smith S, Schreiber V, Boussin F, Dantzer F. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc Natl Acad Sci USA 2011;108:2783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nature Cell Biol 2005;7:1133–9. [DOI] [PubMed] [Google Scholar]
- 43.Kashima L, Idogawa M, Mita H, Shitashige M, Yamada T, Ogi K, Suzuki H, Toyota M, Ariga H, Sasaki Y, Tokino T. CHFR Protein Regulates Mitotic Checkpoint by Targeting PARP-1 Protein for Ubiquitination and Degradation*. J Biol Chem 2012;287:12975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena A, Saffery R, Wong LH, Kalitsis P, Choo KHA. Centromere Proteins Cenpa, Cenpb, and Bub3 Interact with Poly(ADP-ribose) Polymerase-1 Protein and Are Poly(ADP-ribosyl)ated*. J Biol Chem 2002;277:26921–6. [DOI] [PubMed] [Google Scholar]
- 45.Saxena A, Wong LH, Kalitsis P, Earle E, Shaffer LG, Choo KHA. Poly(ADP-ribose) polymerase 2 localizes to mammalian active centromeres and interacts with PARP-1, Cenpa, Cenpb and Bub3, but not Cenpc. Hum Molec Genet 2002;11:2319–29. [DOI] [PubMed] [Google Scholar]
- 46.Schoonen PM, Talens F, Stok C, Gogola E, Heijink AM, Bouwman P, Foijer F, Tarsounas M, Blatter S, Jonkers J, Rottenberg S, van Vugt MATM. Progression through mitosis promotes PARP inhibitor-induced cytotoxicity in homologous recombination-deficient cancer cells. Nature Communications 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang F, Baumann C, De La Fuente R. Persistence of histone H2AX phosphorylation after meiotic chromosome synapsis and abnormal centromere cohesion in poly (ADP-ribose) polymerase (Parp-1) null oocytes. Devlop Biol 2009;331:326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meehan RS, Chen AP. Gynecologic Oncology Researc and Practice 2016;3:3:DOI 10.1186/s40661-016-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Molec Oncol 2011;5:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.