Abstract
Substance use disorders (SUDs) remain problematic as many individuals are untreated or do not benefit from the currently available interventions. Thus, there is an urgent need to develop novel pharmacological interventions to treat SUDs. Evidence suggests that the female sex hormone, progesterone, attenuates the craving for and the euphoric effects of drugs of abuse. Research to date has demonstrated that progesterone may modulate responses to drugs of abuse and may have utility as a novel treatment for SUDs. A literature search was conducted to identify and examine studies that administered exogenous progesterone. Sixteen publications were identified, exploring the utility of exogenous progesterone or its metabolite, allopregnanolone, among a range of substances, including amphetamines (one study), benzodiazepines (one study), cocaine (nine studies) and tobacco/nicotine (five studies). Results indicated that exogenous progesterone and, its metabolite allopregnanolone, demonstrated preliminary efficacy as a treatment for substance use in both men and women. Notably, progesterone appears to target negative affect and augment cognitive functioning, especially among female substance users. Additional research is needed to explore the potential use of exogenous progesterone and allopregnanolone in the treatment of SUDs, including that associated with alcohol and opioids, but considering the current promising findings, exogenous progesterone and allopregnanolone may have utility as novel pharmacological treatments for SUDs.
1. INTRODUCTION
Substance use disorders (SUDs) are a widespread global problem among adults, with recent estimates that over 29 million individuals engage in problematic alcohol and drug use worldwide [1]. SUDs accounted for over 300,000 deaths globally in 2016, likely due to increased risks of cardiovascular, pulmonary and liver diseases, cancer, mental illness, drug overdose risk, infectious diseases and other short and long-term health consequences [1–3]. Despite several developments in evidence-based pharmacological and behavioral interventions, many individuals with SUDs remain untreated or do not benefit from the currently available treatments [4]. Therefore, development of novel treatment approaches for SUDs remains an important goal.
Studies examining the impact of sex and cycle phase on SUDs demonstrate that the female sex hormone, progesterone, modulates responses to drugs of abuse and may serve as a novel intervention to treat SUDs. Most notably, evidence suggests that progesterone may attenuate craving and euphoric effects from some drugs of abuse and improve response inhibition function [5–9]. These findings led to the possibility that progesterone and its metabolites may have utility in the development of novel SUD treatment approaches. The purpose of the present paper is to identify and review the current literature surrounding the potential utility of exogenous progesterone administration for the treatment of SUDs. The paper will begin with a brief review of the sex differences (male versus female) associated with the development and treatment of SUDs and then explore the underlying mechanisms associated with these differences. We then review the neurobiological effects of female sex hormones, cycle phase effects, and the clinical/pre-clinical studies examining the impact of cycle phase on addictive behaviors in female only samples. Following this, we review studies that have examined the use of exogenous progesterone as an effective treatment for SUDs. We conclude with a discussion of the future directions of progesterone treatment as a pharmacological intervention for SUDs. Of note, the paper primarily focuses on progesterone, given the current evidence that it may attenuate craving and subjective positive drug responses [5–9]. However, the review will briefly provide background information on estradiol, another female sex hormone, to provide context for the information presented in this review.
1.1 Sex Differences in SUD
1.1.1 Preclinical evidence of sex differences in acquisition, maintenance and extinction of drug use
The sex differences observed in pre-clinical studies are seen across all phases of substance use, including in the acquisition of drug taking behavior, as well as during the maintenance and extinction of drug use [10–31]. These findings indicate that there are unique biological and behavioral differences associated with SUDs among males and females.
In animal models, self-administration paradigms are used to model aspects of SUDs associated with binge drug taking, intoxication and escalation of drug use. Generally, animal models have shown that females acquire self-administration of drugs of abuse including alcohol, cocaine, nicotine and opioids, more rapidly than males [10–12]. Female rats have been shown to voluntarily consume more alcohol than male rats and they generally acquire self-administration of cocaine and heroin more rapidly than males on the same reinforcement schedule [13–15]. Females, compared to males, also have higher levels of responding in both fixed and progressive-ratio schedules (i.e., work harder to self-administer) for nicotine, cocaine, methamphetamine and opioid self-administration, suggesting greater motivation to obtain drugs [10,11,16–18].
In preclinical models of relapse, female rats have been shown to have a higher rate of drug-seeking behavior than males during a drug induced-reinstatement procedure for cocaine [19,20]. Overall, females exhibit increased sensitivity for drug-, cue-, and stress-induced reinstatement across substances, including alcohol, cocaine and methamphetamine [10,21–24], suggesting that females are at increased risk for relapse. Sex differences have also been observed in preclinical models of withdrawal, as females consuming cocaine, cannabinoids and nicotine exhibit more pronounced physical and behavioral symptoms than males [e.g., 25–27]. Notably, higher rates of toxicity (e.g., rapid weight loss or death) have been observed among female rodents during an initial withdrawal period from intravenous cocaine [20]; however, studies have also demonstrated that males when compared to females, have shown marked symptoms when withdrawing from alcohol, opioids and other psychostimulants [e.g., 28–31].
Overall in animal models of drug dependence, females generally demonstrate quicker acquisition, increased consumption and greater sensitivity to reinforcing effects in various drugs of abuse including alcohol, cocaine, nicotine and opioids than males. Thus, the present evidence suggests a greater vulnerability to addiction in females [10–15,19–24]. Given this highlighted increase in the susceptibility towards addiction, unique mechanisms may account for these observed sex differences and these mechanisms may present potential treatment targets for SUDs.
1.1.2 Clinical and epidemiological evidence of sex differences in SUD prevalence
Current reports indicate that while men use substances at a higher rate than women, the gender gap is narrowing [32]. Lifetime prevalence for a SUD is approximately 12.3% for men and 7.7% for women in the United States; however, 12-month prevalence rates illustrate this narrowing gap, with 4.9% of men and 3.0% of women meeting diagnostic criteria for a SUD [4]. For example, marginal differences are observed for 12-month prevalence rate for cocaine use disorder (men 0.5%; women 0.2%) and current cigarette use (men 16.7%; women 13.6%) [4,33]. Additionally, data suggest increasing rates of alcohol consumption among women, with 47.7% of women reporting drinking at least 5 drinks per day over the past year from 2012–2013, while 33.5% of women reported the same drinking rate in 2001–2002 [34].
These rates are especially problematic as recent evidence demonstrates that women escalate to problematic drug use faster than men in a telescoping course [11]. Women exhibit shorter durations from onset of substance use, to regular use and treatment entry, across substances including alcohol, cannabis, cocaine, and opioids [35,36]. Women also encounter more significant medical, psychiatric and social consequences related to their drug use than men [32]. For instance, women are more likely develop organ damage and are at increased risk of cardiovascular disease, stroke and lung cancer than men [37,38]. Furthermore, substance use significantly impacts gynecological health, including experience of amenorrhea, irregular menses, and cramping [38].
Despite these severe health consequences, less than 20 percent of women receive treatment for SUDs [39,40]. Overall substance users experience high rates of relapse, with estimates ranging from 50–90% [41]. Women exhibit increased rates of relapse and are more likely to utilize substances to relieve negative affect than men [42]; thus, there are likely additional barriers to abstinence among women, including increased susceptibility to craving, as well as negative affect and stress-reactivity [43–45]. Targeting these potential barriers may prove to be beneficial when developing novel pharmacological interventions for SUDs.
1.1.3 Sex differences in the response to subjective effects of drugs of abuse
A large body of evidence supports unique sex differences in response to drugs of abuse. Across various drugs of abuse, it has been postulated that men use drugs for their reinforcing effects, whereas women are more likely to use drugs for affect and stress regulation [46,47]. For instance, it is well established that female tobacco smokers are more likely than males to smoke for the alleviation of negative affect and relapse rates in women are associated more significantly with higher levels of stress than males [48–51]. Female smokers have been found to experience greater subjective negative affect, craving, arousal and stress-reactivity in response to stress cues and they smoke more quickly following negative mood induction than males [48–51]. Furthermore, men endorse motivation to ameliorate craving as a predictor in tobacco relapse; however, in women, depressive mood, anxiety, anger and perceived stress have been found to be associated with relapse [49]. Female tobacco smokers have been found to be less sensitive to the pharmacological effects of smoking than males, but are more sensitive to non-pharmacological, as well as subjective stimuli implicating that these areas are important when developing novel cessation therapeutics[52].
These sex differences are also observed in abuse of other substances. For example, among alcohol-dependent individuals, women demonstrated a greater sensitivity stress-induced alcohol craving and anxiety compared to men [53]. Similarly, women compared to men, have diminished euphoria in response to intravenous nicotine and smoked cocaine [54,55]. These findings highlight unique sex differences that may inform, sex-specific pharmacological and behavioral interventions for SUDs.
1.2 Overview of Female Sex Hormones
Before reviewing the effects of progesterone on SUD, it is important to summarize the physiological functions of both progesterone and estradiol, to provide context for the present review.
1.2.1 Progesterone
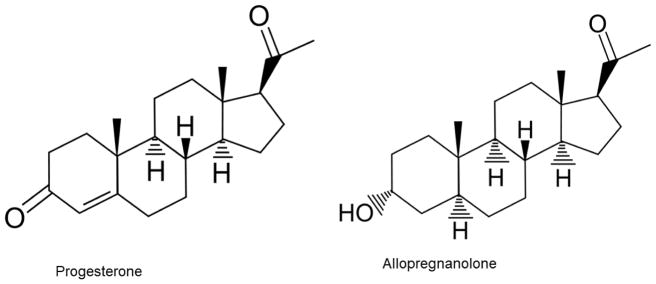
Progesterone is a 21-carbon hormone, derived from cholesterol, that is synthesized in the gonads, as well as adrenal glands. See Figure 1. Progesterone is well known for its reproductive functions in women, especially in maintenance of pregnancy, while its functions in men are less clear [56]. Progesterone and its metabolites, allopregnanolone and pregnanolone, are also called “neurosteroids” as they are synthesized in the brain de novo from cholesterol [57]. These hormones are highly lipophilic and easily cross the blood-brain barrier [58]. As demonstrated in multiple studies over the past decades, progesterone and its metabolites, regulate neuronal signaling and function through genomic and nongenomic actions [9, 57]. The genomic effects are mediated by two isoform progesterone receptors (PR): PRA and PRB. Similar to other steroid hormones, progesterone regulates gene transcription through binding of intracellular PR. Once activated, PR interact with a nuclear transcription factor through the progesterone response element (PRE), which regulates transcription of multiple genes [59,60]. The non-genomic effects of progesterone are likely mediated through interaction with multiple neurotransmitter receptors including sigma, glutamate, GABAA and nicotinic receptors (α4β2, α5) [9,61].
Figure 1.
Progesterone and allopregnanolone chemical structures.
Allopregnanolone has positive modulatory effects on GABAA receptors and thereby enhances GABA transmission. GABAergic transmission is the main inhibitory neurotransmitter system in the brain and has been proposed to result in diminish drug reward [9]. In addition, two other types of PR that are distinct from classical PRs have also been identified: membrane PRs (mPRs) and progesterone membrane receptor component (PGMRC). The function of these two receptor types remains to be determined [65].
1.2.2 Estradiol
Estradiol is another female sex hormone produced primarily in the gonads, with other tissues producing smaller amounts, including the adrenal glands, kidney, adipose tissue, and the brain. Derived from cholesterol, estradiol is a naturally occurring form of estrogen, and is the most potent form present during the reproductive years [66]. With specific receptor sites throughout the central nervous system, estradiol has been shown to be associated with alterations with several neurotransmitter systems. For instance, the binding of estrogen is associated with an increase of density in the serotonergic and dopamine receptors [67]. These alternations in neurotransmitter systems have implicated estradiol as a mediator for addictive behaviors and reward responses.
1.2.3 Menstrual and estrous cycle descriptions
The human menstrual cycle lasts between 25 and 35 days, with a median cycle length of 28 days [68]. The cycle is typically divided into the follicular and luteal phases, separated by ovulation, marked by a preovulatory surge in luteinizing hormone (LH). The cycle begins with menses, marking the onset of the follicular phase. The follicular phase is characterized by elevated levels of estradiol, with levels ranging from 20 to 80 pg/mL during the early to midfollicular phase. Estradiol levels peak at 200 to 500 pg/mL during the preovulatory LH surge before decreasing, with levels ranging from 60 to 200 pg/mL during the midluteal phase. Conversely, progesterone is at its nadir during the follicular phase, with levels less than 1.5 ng/mL. Levels begin to increase prior to the preovulatory LH surge, peaking during the midluteal phase, where progesterone is typically higher than 7 ng/mL. Of note, during pregnancy, serum progesterone raises to 40 ng/mL by the end of the first trimester, reaching a peak, approximately 150 ng/mL, at full-term pregnancy [69].
The rodent estrous cycle typically occurs over four days and is divided into the proestrus, estrus, metestrus and diestrus phases, with ovulation occurring between proestrus and estrus. Progesterone levels peak twice, during the evenings of proestrus and metestrus phases, with levels ranging from 65–200 nM at their peak [70,71]. Lower stable levels of progesterone range from 10–20 nM during the remainder of the cycle. Serum estradiol levels peak during the afternoons (beginning at noon) of the metestrus and proestrus, with peak levels ranging from 130–325 pM. However, estradiol levels fall to less than 10 pM by ovulation [70,71].
1.3 Preclinical and Clinical Studies of the Effect of Female Sex Hormones on Drug Use
Preclinical and clinical studies exploring the underlying mechanisms of sex differences in SUD have demonstrated that female sex hormones impact substance use and subjective substance use behaviors (e.g., withdrawal, response, and craving). The impact of fluctuations of female sex hormone levels across the menstrual/estrous cycles on substance use continue to be an active area of research. Overall, it appears that estradiol augments drug reward, while progesterone attenuates such effects [9].
1.3.1 Preclinical Evidence
Preclinical studies in female samples have demonstrated the cycle phase effect on substance use, reward and withdrawal, as well as the importance of actual female sex hormone levels regarding substance-use variables. It appears that estrogen may augment drug-seeking behavior and drug-reinforced responding, as previous studies have demonstrated that female rats with chemically or surgically blocked estrogen, responded less for cocaine [72]. Furthermore, estradiol has been shown to augment cocaine acquisition in female rats, but when progesterone is administered concurrently with estradiol, this increase in responding is inhibited [73]. Administration of exogenous progesterone to female animals has been shown to decrease cocaine self-administration, resulting in a right-shift of the cocaine dose-effect curve in primates and attenuate the escalation of cocaine self-administration in rodents [74,75]. Furthermore, exogenous progesterone has been shown to decrease impulsive choice for cocaine, but not food among female rodents [76]. A review of cocaine extinction demonstrated that female rats in the estrous phase respond more during cocaine extinction than other phases, but exogenous estrogen enhanced cocaine-reinstatement [77]. Overall, evidence suggests that exogenous progesterone displays anti-addictive effects in preclinical studies, as it attenuates effects in drug use across the phases of substance use, including acquisition, self-administration, and reinstatement/ extinction.
1.3.2 Clinical Evidence
Clinical studies indicate that elevated levels of progesterone, as observed in the luteal phase (progesterone dominant) may decrease abuse-related effects of drugs of abuse, as well as increase odds of abstinence from smoking cigarettes. Smoking cessation and subsequent relapse has been shown to vary by menstrual cycle phase, with several studies in female samples demonstrating that when non-pharmacological interventions are utilized, the luteal phase, compared to the follicular phase (estradiol dominant), is associated with better outcomes [78–80]. Further, higher progesterone levels during the luteal phase have been associated with decreased urge to smoke and attenuated ratings of euphoria in female smokers [54,80–82]. When looking at actual hormone levels in female smokers, as opposed to dichotomous menstrual cycle categorizations, increasing progesterone is associated with a 23% increase in odds of abstinence among women tobacco smokers receiving medication treatment [83]. Preliminary evidence suggests that higher allopregnanolone concentrations are associated with a decrease in subjective negative affect following nicotine nasal spray in premenopausal women [84].
The association between progesterone and decreases in positive subjective ratings also extends to other drugs of abuse. In two previous studies, women cocaine users, who were in the luteal phase (progesterone dominant) of their menstrual cycle, demonstrated diminished responses to the subjective effects of cocaine (e.g., “good drug effect”) when compared to those women in the follicular phase (estradiol dominant) of their menstrual cycle or men [5,8]. Furthermore, among cocaine-dependent women, high endogenous progesterone levels were associated with attenuated drug craving and anxiety responses [85]. Similarly, healthy women had diminished subjective responses to oral d-amphetamine during the luteal phase, compared to the follicular phase [86].
Additionally, research demonstrates that progesterone may attenuate stress-response. Less susceptibility to stress and cue-related craving has been observed during the luteal phase, when progesterone levels are high, while reports of greater stress and arousal, as well as greater orbitofrontal cortex activation in response to smoking cues (e.g., increased relapse vulnerability), have been found during the follicular phase of the menstrual cycle [48,84,87–89].
Preliminary evidence also suggests that progesterone positively impacts cognitive functioning, which would be of significant clinical importance in the treatment of SUDs, as impairments in cognitive control and other executive functions have been associated with poor treatment outcomes, including treatment dropout in both genders [90,91]. It is postulated that progesterone has cognitive enhancing effects, as it has been shown to improve verbal working memory, as well as augment measures of sustained attention during the luteal phase, when progesterone levels are higher than the follicular phase [85,92]. Together, these studies provide convincing evidence that high endogenous progesterone levels are associated with attenuated craving to smoke cigarettes, self-report positive subjective responses to stimulant drugs, and stress reactivity in females, as well as preliminary evidence of cognitive enhancing effects within the context of SUDs.
1.4 Review of studies of progesterone administration in the context of SUDs
Beginning with the identification of sex differences in substance use, both preclinical and clinical literature consistently identify that females have a greater vulnerability to addiction and are more sensitive to the reinforcing effects of substance use (particularly negative reinforcement) than males [10–31,46–55]. Based upon animal and human literature to date, there is evidence to suggest an inverse relationship between endogenous progesterone levels and substance use behavior in females [9,72–80,85,91,92]. Further, higher endogenous progesterone levels in the luteal phase are also associated with attenuation of positive subjective effects of drugs and improved negative affect and cognitive functioning in women [5,8,48,54,78–87].
Given the current evidence, exogenous progesterone may prove to be an effective intervention for SUDs. The present review examines the current literature surrounding administration of exogenous progesterone for licit and illicit substance use and explores its potential utility as a novel treatment for SUD.
1.4.1 Narrative Review Search
The present narrative review includes articles identified in a PubMed search from December 2016 through November 2017 to locate studies which administered exogenous progesterone within the context of substance use (e.g., to attenuate drug effects). Search terms included: “progesterone,” “exogenous progesterone,” “progesterone treatment,” and “progesterone administration” AND at least one of the following: “amphetamines,” “alcohol,” “cannabis,” “cocaine,” “drug use,” “heroin,” “marijuana,” “methamphetamine,” “nicotine,” “opioids,” tobacco,” “smoking,” “substance use,” and “substance use treatment.” Results were restricted to clinical trials. Additional manuscripts were identified by examining reference lists.
The search yield 137 relevant abstracts; each abstract was then reviewed to evaluate if the article met the following inclusion criteria: (1) published in English, (2) administered exogenous, natural progesterone at least once, (3) included administration of a drug of abuse or conducted within a substance-using population. Excluded publications included those that described impact of hormone levels (e.g., progesterone levels) on drug effects and outcomes without administration of exogenous, natural progesterone. Additionally, commentaries on the utility and side effects of administering progesterone in healthcare settings were excluded. This resulted in a full article review of 17 manuscripts. The identified publications’ full manuscripts were then reviewed by both authors for sample description, study paradigms, and drug/progesterone administration. These criteria resulted in a pool of sixteen manuscripts, spanning investigations of amphetamines (one study), benzodiazepines (one study), cocaine (nine studies) and tobacco/nicotine (five studies); among these reports all included a placebo-controlled condition. One report was eliminated from the present narrative review, as it detailed the administration of oral contraceptives, not exogenous progesterone. These reports included measures of drug use (i.e., ad lib/self-administration, abstinence, withdrawal symptoms), subjective/ affect effects, physiological, stress (i.e., hypothalamic–pituitary–adrenal axis), and cognitive tasks. Studies are presented in Table 1.
Table 1.
| Drug Type | Participants | Study Type | Treatment | Results | Authors |
|---|---|---|---|---|---|
| Amphetamine | 18 females | Randomized, placebo- controlled, within- subjects study | Chronic treatment; oral micronized PROG (200mg/day) vs. placebo administered over 3 days in the F phase | PROG pretreatment increased positive subjective ratings of drug and choice of drug over money in study paradigm. | Reed et al., 2010 [97] |
| Benzodiazepine | 35 daily benzodiazepine users (gender not reported) | Randomized, double-blind, between- subjects study | Chronic treatment; Oral micronized PROG titrated to a mean dose of 1,983mg/day in 3 weeks and given for an additional 4 weeks vs. placebo. | PROG treatment did not have a significant effect on severity of withdrawal symptoms or in the discontinuation of benzodiazepines. | Schweize et al., 1995 [105] |
| Cocaine | 46 treatment- seeking cocaine dependent participants (17 females; 29 males) | Randomized, double-blind, within- subjects study; three cued stress conditions: stressed, drug and relaxed | Chronic treatment; 400mg/day micronized PROG vs. placebo for 7 days | Participants’ results were group utilizing a median spilt based upon ALLO level (high vs. low). The high ALLO group reported lower post- stress imagery cocaine craving scores on all three study sessions. High ALLO reported higher positive emotion at baseline and overall lower peak cocaine craving. Increased levels of ALLO were associated with reduced cortisol levels at pre-imagery. | Milivojevic et al., 2016 [99] |
| 28 participants (19 males; 9 females) | Randomize | Chronic treatment; 400mg/day PROG vs. placebo for 7 days | PROG improved scores on the Thought Facilitation Task scale. | Milivojevic et al., 2014 [106] | |
| 50 postpartum females with cocaine use disorders | Randomized, double- blinded, placebo controlled study | Chronic treatment; 200mg PROG/day vs. placebo for 12 weeks | Placebo group had more self-reported cocaine use during the study; there were no significant differences between groups at 3- month follow-up. There were no group differences between positive urine drug screens. The placebo group relapsed more quickly and among participants who did not reported drug use, the placebo group had more positive urine samples. | Yonkers et al., 2014 [101] | |
| 42 early abstinent, treatment- seeking cocaine users (24 males; 18 females) | Randomized, double-blind, placebo- controlled study; three cued stress conditions: stressed, drug and relaxed | Chronic treatment; 400mg PROG vs. placebo for 7 days | PROG group selectively demonstrated efficacy to attenuate drug craving and stress arousal in early abstinent cocaine dependent individuals; this effect was more robust in females. | Fox et al., 2013 [98] | |
| 10 non- treatment seeking female cocaine smokers | Randomized, placebo controlled study | Chronic treatment; 300mg/day PROG vs. placebo for three days | Smoked cocaine administration did not differ across treatment groups and PROG did not attenuate administration or subjective ratings | Reed et al., 2011 [101] | |
| 45 male cocaine users, maintained on methadone | Randomized, double-blind, placebo- controlled study | Chronic treatment; 200 increased to 600mg/day over 4 weeks PROG vs. placebo for 6 additional weeks | PROG group demonstrated a slight reduction in probability of UDS positive for cocaine, while the placebo group showed a slight increase. No medication differences were observed for opioid drug screens. | Sofuoglu et al., 2007 [102] | |
| 21 smoked cocaine participants (11 females in F/L phases; 10 males) | Randomized study | Chronic treatment; 150mg/day PROG vs. placebo for 4 days | PROG attenuated positive subjective effects in females. There were no group or gender differences in terms of cocaine use within the study paradigm. | Evans et al., 2006 [94] | |
| 10 female cocaine users (early F phase) | Double-blind, placebo- controlled crossover study | Single dose; 400mg/study session PROG vs. placebo | Subjective ratings of “high” and “feel the effects of the last dose” were attenuated in the PROG group. | Sofuoglu et al., 2004 [96] | |
| 5 smoked- cocaine female users | Randomized, within- subjects | Single dose; 200mg/study session micronized PROG vs. placebo | Reduced subjective responses in PROG group. | Sofuoglu et al., 2002 [95] | |
| Tobacco/Nicotine | 41 postpartum female smokers | Randomized, double-blind, between- subjects, placebo controlled study | Chronic treatment; 400mg/day micronized PROG vs. placebo for 8 weeks | PROG group had higher proportion of participants achieving abstinence at week 8 and a slower rate of relapse*; PROG group also endorsed lower craving scores. | Forray et al., 2017 [100] |
| 46 nicotine abstinent, postpartum women | Randomized, within- subjects | Chronic treatment; 400mg/day micronized PROG vs. placebo for 4 weeks | PROG group exhibited higher rates of abstinence at weeks 4 and 7*. These participants also exhibited more median days until relapse*. | Allen et al., 2016 [104] | |
| 64 smokers (30 females; 34 males) | Randomized, within- subjects study | Chronic treatment; 200mg/day micronized PROG vs. 400mg/day micronized PROG vs. placebo over 4 days | No PROG treatment differences were observed for adlib smoking; 200mg groups had more correct responses on DSST. PROG groups demonstrated decreased positive subjective ratings and increased negative ratings. | Sofuoglu et al., 2011 [7] | |
| 12 participants (6 males; 6 females in early F phase) | Randomized; within- subjects, placebo- controlled study | Single dose; 200mg/study session micronized PROG vs. placebo over two study sessions | PROG treatment increased negative subjective ratings and decreased urges to smoke when compared to placebo condition. | Sofuoglu et al., 2009 [55] | |
| 12 female smokers | Double-blind, within- subjects, placebo- controlled study | Single dose; 200mg/study session micronized PROG vs. placebo | PROG treatment diminished subjective ratings of good effects and decreased craving for cigarettes. Progesterone decreased smoking*. | Sofuoglu et al., 2001 [93] |
ALLO= allopregnanolone; DSST= Digit Symbol Substitution Test; F=follicular phase; HPW= high progesterone week; LPW= low progesterone week; L= luteal phase; PROG= progesterone; UDS= urine drug screen; *= not statistically significant
2. Use of Progesterone as Treatment for SUDs
2.1 Drug Effects
2.1.1 Positive and Negative Drug Effects
Overall, evidence suggests that exogenous progesterone diminishes positive subjective drug effects. One study of both men and women (in the early follicular phase) smokers demonstrated that administration of progesterone (200mg) was associated with lower ratings of “drug liking” than placebo [7]. Additionally, 200mg and 400mg doses of progesterone demonstrated lower ratings of “drug strength” regarding smoking [7]. Similarly, two additional studies of men and women (in the early follicular phase) smokers showed that a single dose of 200mg progesterone significantly diminished ratings on measures of “good” drug effects and “drug liking,” as well as increased “bad” drug effects, following nicotine administration (smoked self-administration or IV injection), when compared to placebo [55,93].
Regarding the subjective effects in response to cocaine administration in normally menstruating women in the follicular phase (scheduled 6–10 days following onset of menses), administration of progesterone (150mg/day) over four days attenuated “good drug” and “drug quality” ratings when compared to men and women in the follicular phase, receiving placebo [94]. A single dose of progesterone (200mg) in females in the early follicular phase was related to a diminished rating of “feel the effect of last dose” after smoked cocaine administration when compared to placebo treatment [95]. Finally, both male and female cocaine users reported attenuated subjective ratings of “high” and “effect of last dose,” in response to intravenous cocaine in comparison to placebo after receiving two doses of oral progesterone (200mg) [96].
Conversely, in the one study of amphetamines to date, chronic administration of progesterone (200mg/day over 3 days) enhanced positive subjective effects, as well as reports of “good drug effect” and “drug liking,” when compared to the placebo condition [97].
Thus, evidence indicates that progesterone pretreatment reduces the positive subjective effects from nicotine and cocaine use among males and females. Given the limited research to date in amphetamines (one study), it is not yet clear if progesterone administration would be an effective means to attenuate subjective responses to these drugs. The effect of exogenous progesterone on subjective drug effects has not been studied for other substances of abuse.
2.1.2 Drug-Induced Urges
Across clinical studies for cocaine and nicotine, administration of exogenous progesterone appears to attenuate craving ratings. Among a group of early abstinent, treatment-seeking cocaine users, those receiving daily doses of 400mg (for seven days) progesterone reported lower levels of cocaine craving following a drug-cue when compared to those receiving placebo treatment [98]. Furthermore, once the same sample was grouped based upon plasma levels of allopregnanolone, those women with increased levels of allopregnanolone reported an overall lower peak cocaine craving across drug-, stress- and relaxing-cue conditions [99]. These findings also are consistent with studies conducted in cigarette smokers, where both male and female smokers receiving progesterone treatment reported attenuated craving for and/or urges to smoke, when compared to placebo treatment [7,55,93,100]. Overall, evidence suggests that exogenous progesterone treatment reduces drug-induced cravings and urges.
2.2 Drug Use
2.2.1 Human Laboratory Studies
Only one study to date has shown a trend in decreased smoking behavior within a laboratory setting. In this study, following a single dose of progesterone (200mg), women smokers (in the early follicular phase) demonstrated attenuated smoking, compared to placebo, during a self- administration smoking paradigm, where they exchanged tokens valued at $1–2 dollars for two puffs of a cigarette [93].
Another study demonstrated that progesterone treatment (200mg/day administered over 3 days) compared to placebo, was related to an increase in amphetamine choices over various monetary amounts (ranging from $0.25–64), as measured over nine discrete choices between drug and money during an established multiple-choice procedure [97]. Further, research among cocaine human laboratory studies provides no evidence that progesterone pretreatment (both chronic and single dose administration ranging from 150–400mg/session) as compared to placebo, affected cocaine use (measured via self-administration), plasma cocaine concentration, or pharmacokinetics [94–96,101]. Laboratory results to date demonstrate that progesterone has a limited, if any, effect on reducing drug use or altering pharmacokinetics of drugs in both men and women.
2.2.2 Clinical Trials
Among trials of cocaine users, chronic administration of progesterone (ranging from 10 weeks to 12 weeks of 100–300mg/day progesterone) when compared to placebo, resulted in a slight reduction in probability of positive urine drug screen for cocaine in women, but not in men, who were using cocaine while maintained on methadone [102,103]. Two trials of postpartum tobacco smokers who were administered 400mg/day progesterone (duration of 4 or 8 weeks, respectively) showed increased rates of abstinence compared to those administered placebo medication [100,104]. In another study, no differences in drug abstinence were observed following progesterone treatment (titrated to mean daily dose of 1,983 mg) versus placebo treatment among male and post-menopausal benzodiazepine users, in benzodiazepine use [105]. The limited evidence available supports that exogenous progesterone may assist in increasing abstinence among postpartum women who are either cocaine users or tobacco smokers, but continued studies are needed among other drugs of abuse in various participant populations.
2.3 Effects on Drug Withdrawal, Urges and Affect
2.3.1 Withdrawal and Urges
Across studies of benzodiazepines and tobacco, exogenous progesterone (ranging from a single 200mg day to mean daily dose of 1,983mg) as compared to placebo, did not attenuate withdrawal symptoms in women or men [55,93,105]. Conversely, progesterone treatment (either in a single dose of 200mg or 400mg/day treatment over 12 weeks) versus placebo treatment decreased craving and urge to smoke in a range of populations, including men, women and postpartum women [55,93,100]. However, this finding was not replicated in a randomized control study of cocaine use disorder, following 12 weeks of 100mg/day progesterone versus placebo in postpartum women [103]. Thus, progesterone treatment may have utility in attenuating symptoms of withdrawal in men and women tobacco smokers, but additional research is needed in other substances of abuse.
2.3.2 Affect
There appears to be limited evidence to suggest that progesterone treatment changes measures of negative affect, depression or anxiety. Several studies did not demonstrate significant changes in negative affect, depression or anxiety from baseline, following progesterone treatment. There were no differences in progesterone treatment (single dose of 200mg) versus placebo in measures of depressive symptoms (e.g., the Profile of Mood States) in men and women tobacco or cocaine users [55,96]. Additionally, there was no difference between 200 or 400mg/day progesterone treatment and placebo in two 12-weeks trials on the Edinburgh Postnatal Depression Scale in postpartum cocaine users or tobacco smokers [103,104].
However, one study demonstrated a relationship between high allopregnanolone and high positive emotion [99]. Further, another study demonstrated that progesterone pretreatment (200mg/day for 3 days) when combined with amphetamine (10–20mg doses), decreased state anxiety ratings [97]. Given this information, there is mixed evidence to date that exogenous progesterone, administered within a substance use context, augments negative affect, depression or anxiety.
2.4 Stress Response
2.4.1 Hypothalamic–pituitary–adrenal axis (HPA) hormones
In one study of male and female treatment-seeking cocaine users, those receiving progesterone (400mg/day for seven days) demonstrated decreased cue-induced cortisol responses and increased cue-induced ACTH responses versus placebo treatment [98]. Further, when these participants were grouped based upon their plasma allopregnanolone levels, individuals with higher levels of allopregnanolone exhibited lower baseline levels of cortisol than those with lower levels; however, this group also exhibited a greater change in cortisol level when presented with stress-inducing guided imagery [99]. These limited results demonstrated mixed evidence for the effect of exogenous progesterone on HPA hormones.
2.4.3 Stress-induced Affect
In terms of stress-induced affect changes, cocaine- dependent women receiving placebo at the start of their menstrual cycle, reported lower ratings of positive mood and decreased negative emotion following stress-cued imagery than women receiving progesterone (400mg/day for seven days) [98]. This, albeit, limited evidence suggests that progesterone may be protective against stress-induced affect.
2.4.3 Cardiovascular
In one study of oral amphetamine in healthy women, chronic administration of 200mg/day of progesterone over three days attenuated both systolic and diastolic amphetamine-increased blood pressure with no observed treatment group differences in heart rate [97]. One study of intravenous cocaine demonstrated a decrease in diastolic blood pressure among men and early follicular phase women receiving progesterone (400mg/session) versus placebo, with no effect on systolic blood pressure or heart rate after intravenous cocaine administration [96]. Women in the follicular phase who were chronically administered 150mg/day exogenous progesterone for four days versus placebo and then smoked cocaine, demonstrated decreased diastolic pressure following 25mg of cocaine, but diastolic pressure increased following 12mg of cocaine [94]. This study also illustrated that cocaine-induced increases in heart rate were attenuated in the group receiving progesterone compared to placebo treatment [94]. Similarly, a single dose of 400mg, but not 200mg of progesterone or placebo attenuated diastolic blood pressure in women tobacco smokers in the early follicular phase compared to men smokers; however, systolic blood pressure was not significantly affected in either genders [7]. No significant treatment differences were observed in blood pressure (diastolic or systolic) or heart rate after a period of nicotine self-administration (intravenous nicotine and smoked nicotine) following a single dose of 200/mg progesterone versus placebo in the two remaining nicotine studies which reported cardiovascular measures [55,93]. Thus, progesterone administration demonstrated mixed evidence on its ability to attenuate cardiovascular effects of substances of abuse.
2.5 Cognitive Function
Progesterone has been shown to have positive cognitive inhibitory effects. In one study, following seven days of 400mg/day progesterone administration, women and men abstaining cocaine use had improved inhibitory performances, as measured by the Stroop Color Word Task [98]. Additionally, among these abstinent treatment-seeking cocaine users, those with higher levels of allopregnanolone, also demonstrated higher Stroop Color/Word scores following both drug- and stress-cued imagery relative to pre-imagery scores when compared to participants with low levels of allopregnanolone [99]. Women tobacco smokers in the early follicular phase receiving a single dose of 200mg progesterone versus placebo also exhibited improved Stroop performance, but no improvement was observed in men [7].
Progesterone treatment (a single dose of 200mg) has been shown to improve cognitive performance on the Digit Symbol Substitution Test (DSST) in both men and women (in the early follicular phase) abstinent tobacco smokers when compared to placebo or 400mg of progesterone [7]. Conversely, both DSST and delayed memory task scores were impaired following 200mg/day progesterone pretreatment versus placebo over three days, in a study of follicular phase women who were administered oral amphetamine; no other impulsivity measures (i.e., Immediate Memory Task; GoStop Task; Delay Discounting Task) were impaired [97].
Additionally, there is preliminary evidence that exogenous progesterone may improve regulation of some emotional processing mechanisms. One study has demonstrated that chronic administration of 400mg/day for seven days versus placebo, improved scores on the Thought Facilitation Task scale on the Mayer-Salovey-Caruso Emotional Intelligence Test, in early abstinent cocaine users who also abused alcohol; there was no observed sex difference between males and females [106].
3. DISCUSSION
Overall, findings to date suggest that progesterone has utility as a novel pharmacological treatment for SUDs. While little evidence suggests that exogenous progesterone reduces drug use in laboratory studies, there is evidence to suggest that progesterone may prevent relapse and improves abstinence from cocaine and cigarette smoking [7,102]. Most striking is the available clinical evidence to demonstrate that exogenous progesterone increases abstinence among postpartum women abusing cocaine or tobacco cigarettes. Yonkers and colleagues (2014) reported that women receiving placebo medication had more self-reported cocaine use when compared to those receiving progesterone [103]. Among tobacco smokers, two studies have demonstrated that those receiving progesterone had higher rates of abstinence, with one study reporting a slower rate of relapse at the 3-month follow-up among those taking exogenous progesterone [100,104]. These findings suggest that progesterone may be a potential treatment option to improve abstinence and prevent relapse especially in postpartum females abusing cocaine and nicotine. Whether progesterone may be useful for the treatment of other drugs of abuse remains to be determined in future studies.
While future studies are needed to investigate the utility of exogenous progesterone to treat a range of substances, the present review shows that progesterone may have the potential to treat SUDs, given the promising findings to date in reducing subjective drug ratings and craving in cocaine and nicotine. Progesterone treatment in both males and females generally decreased positive subjective ratings of both nicotine and cocaine craving and urges to smoke [7,55,93–96,98–100]. These results are consistent with preclinical and clinical evidence demonstrating that endogenous progesterone attenuates reports of drug craving, urges, and euphoric drug effects [6,10]. These findings highlight the potential utility of exogenous progesterone in the pharmacological treatment of SUDs. Additionally, while these positive findings appear across the treatment of both men and women, given the difficulty women have in successful SUD treatment outcomes, the results of exogenous progesterone are especially promising in women and need to be studied further [42–45].
Despite these promising results, the underlying mechanisms for these observed effects have yet to be elucidated. One postulation may include progesterone’s role in attenuating negative affect and stress-reactivity. Substance use literature consistently postulates that women are more likely to use substances to alleviate negative affect, including stress, than men [32,42,107,108]. One study demonstrated that women receiving placebo in comparison to progesterone treatment reported lower ratings of positive mood and higher ratings of negative emotion following stress-induction [98]. Thus, it may be possible that progesterone dampens such reactivity and offers a potential treatment target for individuals abusing substances
Another possible underlying mechanism is progesterone’s effects on cognitive functioning. The current literature indicates progesterone has positive cognitive inhibitory effects on drug- and stress-cued response among individuals abusing tobacco and cocaine [7,98]. Both clinical and preclinical literature illustrate that progesterone and its metabolites augment learning and memory through neuronal activity [109]. This suggests that progesterone may attenuate deficits in learning and memory processes, thus assisting in the treatment of SUDs [58]. However, we are not aware of any studies demonstrating that progesterone’s beneficial effects on drug use is mediated by either improvement of negative affect or cognitive function.
3.1 Future Directions
Given the current promising results, future research needs to focus on elucidating the underlying mechanisms, including the neurobiological underpinnings of progesterone’s effects. This will assist in the identification of the patient populations that will most benefit from its use. For instance, the attenuating effect on negative affect and stress-reactivity may allow exogenous progesterone to be further developed as a pharmacological intervention to target affect/stress and augment existing substance use interventions in women. Preliminary evidence suggests that exogenous progesterone and a psychosocial intervention had therapeutic promise among women, notably postpartum women and the present results illustrate that administration of progesterone may augment response to current SUD interventions [103]. However, additional research with larger sample sizes and in other licit and illicit substances including alcohol and opioids is needed. More research is also needed to evaluate the role of progesterone in treating males with SUDs. Additionally, given the overlapping mechanisms, including reward pathways, the utility of exogenous progesterone for the treatment of behavioral and food addictions should also be explored [110].
Furthermore, while progesterone has been linked with attenuating drug craving, reward and negative affect in both the clinical and preclinical literature, these effects have not been consistently robust. While the present results are promising and illustrate beneficial effects of progesterone in both genders, it is likely that exogenous progesterone functions differently in men and women. Thus, there are many additional questions that need to be answered as exogenous progesterone is developed as a potential pharmacological intervention. For instance, both pre-clinical and clinical work suggests that progesterone decreases drug reward and positive subjective drug effects while also attenuating negative affect in females, with effects surpassing those seen in males [94]. It is unclear what may account for these observed differences. Future research should explore the potential impact of reward processing in males, considering potential modulating factors, including impulsivity and stimulation. These studies would lead to better informed and targeted, gender-sensitive pharmacological interventions.
Research is also needed to examine the utility of progesterone’s metabolite, allopregnanolone as a treatment for SUDs among women, given that it has demonstrated some effectiveness in improving negative affect in this population. For instance, one recent study demonstrated that among pregnant women, low serum levels of allopregnanolone were associated with increased self-rated depression scores, suggesting that high serum concentrations of allopregnanolone may be protective against depression [111]. In previous studies, allopregnanolone has been associated with decreased negative affect and this effect is likely associated with this metabolite’s modulation of GABAA receptor [9]. A recent study demonstrated that intravenous allopregnanolone significantly improved depression ratings among women with postpartum depression [112]. Additionally, higher allopregnanolone levels were associated with lower levels of baseline cortisol and positive inhibitory effects in drug- and stress-cues [106]. It appears that allopregnanolone may also have utility in targeting negative affect and stress-reactivity among women substance users as a pharmacological strategy to augment current substance use treatments. Thus, additional research is needed to investigate allopregnanolone’s role in treatment of SUDs.
4. Conclusion
The present narrative review details evidence that progesterone has utility in the treatment of SUDs in both men and women. The findings presented here support further investigation of progesterone as a novel pharmacological intervention to improve the treatment for SUDs. Exogenous progesterone has demonstrated preliminary utility to potentially target negative affect, which may most benefit women with SUDs, as this population shows vulnerability to the effects of negative affect and stress-reactivity on substance use. Furthermore, exogenous progesterone was shown to augment cognitive functioning, in both men and women which offers the opportunity to enhance current treatment intervention outcomes. Additional research on hormonal therapy for SUDs is needed to expand the generalizability of the present results across substances of abuse and to test the utility of exogenous allopregnanolone among various populations.
Key Points.
The present narrative review indicates that progesterone and its metabolite, allopregnanolone, may have utility in the treatment of substance use disorders among both men and women.
Cumulating evidence suggests that progesterone may act through the attenuation of drug cravings and positive drug effects, as well as the augmentation of cognitive functioning.
Further research is warranted to explore the generalizability of these results and investigate the use of progesterone and allopregnanolone in treatment across substances of abuse.
Acknowledgments
Funding: Article preparation was supported by VA New England Mental Illness Research, Education and Clinical Center (MIRECC), as well as National Institute of Drug Abuse (NIDA) training grant T32-DA007238.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest: MacKenzie Peltier, PhD and Mehmet Sofuoglu, MD, PhD declare that they have no conflicts of interest.
References
- 1.United Nations Office on Drugs and Crime (UNODC) [Accessed on 27, Dec 2017];World drug report 2017. 2017 http://www.unodc.org/wdr2017/
- 2.Naghavi M, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Drug Abuse (NIDA) [Accessed 06 October 2017];Health consequences of drug misuse. 2017 https://www.drugabuse.gov/related-topics/health-consequences-drug-misuse.
- 4.Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS. Epidemiology of DSM-5 drug use disorder: Results from the national epidemiologic survey on alcohol and related conditions–III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans S, Haney M, Foltin R. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 6.Moran-Santa Maria MM, Flanagan J, Brady K. Ovarian hormones and drug abuse. Curr Psychiatry Rep. 2014;16:511. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36:123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharm. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 9.Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: Evidence from initiation to relapse. Exp Clin Psychopharm. 2010;18:451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker JB, Hu M. Sex differences in drug Abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley AL, Hempel BJ, Clasen MM. Sex as a biological variable: Drug use and abuse. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 14.Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav. 2017;152:6–67. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall PA, Stewart RT, Besheer J. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol Biochem Behav. 2017;156:1–9. doi: 10.1016/j.pbb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- 17.Donny E, Caggiula A, Rowell P, Gharib M, Maldovan V, Booth S, Mielke MM, Hoffman A, Mccallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 18.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- 20.Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–51. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- 21.Bertholomey ML, Nagarajan V, Torregrossa MM. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 2016;233:2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2004;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- 24.Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2012;120:233–237. doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentile NE, Andrewkanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic–pituitary–adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;85:145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety. Behav Brain Res. 2012;231:48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- 28.Bisagno V, Fergunson D, Luine VN. Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav. 2003;73:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 29.Cicero TJ, Nock B, Meyer ER. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav. 2002;72:691–697. doi: 10.1016/s0091-3057(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 30.Reilly W, Koirala B, Devaud LL. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol and Alcoholism. 2008;44:561–566. doi: 10.1093/alcalc/agp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varlinskay E, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 32.McHugh RK, Wigderson S, Greenfield SF. Epidemiology of substance use in reproductive-age women. Obstet Gynecol Clin North Am. 2014;41:177–189. doi: 10.1016/j.ogc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Current cigarette smoking among adults—United States, 2005–2015. Morbidity and Mortality Weekly Report. 2016;65:1205–11. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 34.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis B, Hoffman LA, Nixon SJ. Sex differences in drug use among polysubstance users. Drug Alcohol Depend. 2014;145:127–133. doi: 10.1016/j.drugalcdep.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis B, Nixon SJ. Characterizing gender differences in treatment seekers. Alcoholism Clin Exp Res. 2014;38:275–284. doi: 10.1111/acer.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins KA. Smoking cessation in women. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 38.Substance Abuse and Mental Health Service Administration (SAMSHA) Physiological effects of alcohol, drugs, and tobacco on women. Substance Abuse Treatment: Addressing the specific needs of women (Treatment Improvement Protocol, No. 5) 2009 https://www.ncbi.nlm.nih.gov/books/NBK83244/ [PubMed]
- 39.National Institute of Drug Abuse (NIDA) [Accessed 09 Oct 2017];Substance use in women. 2016 https://www.drugabuse.gov/publications/research-reports/substance-use-in-women.
- 40.Terplan M, McNamara EJ, Chisolm MS. Pregnant and non-pregnant women with substance use disorders: The gap between treatment need and receipt. J Addict Dis. 2012;31:342–349. doi: 10.1080/10550887.2012.735566. [DOI] [PubMed] [Google Scholar]
- 41.Hudson A, Stamp JA. Ovarian hormones and propensity to drug relapse: A review. Neurosci Biobehav Rev. 2011;35:427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharm. 1996;4:166–177. [Google Scholar]
- 43.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, Mazure CM, McKee SA. Targeting the Noradrenergic System for Gender-Sensitive Medication Development for Tobacco Dependence. Nicotine Tob Res. 2015;17:486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger AH, McKee SA, George TP. Smoking cue reactivity in adult smokers with and without depression: A pilot study. Am J Addict. 2012;21:136–144. doi: 10.1111/j.1521-0391.2011.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosgrove KP, Wang S, Kim S, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED. Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci. 2014;34:16851–16855. doi: 10.1523/JNEUROSCI.3661-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender Differences in Craving and Cue Reactivity to Smoking and Negative Affect/Stress Cues. Am J Addict. 2012;21(3):210–220. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima M, al’ Absi M. Predictors of Risk for Smoking Relapse in Men and Women: A Prospective Examination. Psychol Addict Behave. 2012;26(3):633–637. doi: 10.1037/a0027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husky MM, Mazure CM, Paliwal P, McKee SA. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93(1–2):176–179. doi: 10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberger AH, McKee SA. Gender Differences in Smoking Following an Implicit Mood Induction. Nicotine Tob Res. 2012;14(5):621–625. doi: 10.1093/ntr/ntr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins KA, Karlitz JL, Kunkle N. Sex differences in subjective responses to moderate versus very low nicotine content cigarettes. Nicotine Tob Res. 2017 doi: 10.1093/ntr/ntx205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartwell EE, Ray LA. Sex moderates stress reactivity in heavy drinkers. Addict Behav. 2013;38:2643–6. doi: 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 54.DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, Physiological, and Cognitive Responses to Intravenous Nicotine: Effects of Sex and Menstrual Cycle Phase. Neuropsychopharmacology. 2014;39:1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Human Psychopharmacology (Berl) 2009;24:559–564. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? Aging Male. 2004;7:236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 57.Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48–61. doi: 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh M, Su C, Ng S. Non-genomic mechanisms of progesterone action in the brain. Front Neurosci. 2013;7:159. doi: 10.3389/fnins.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schumacher M, Coirini H, Robert F, Guennoun R, El-Etr M. Genomic and membrane actions of progesterone: Implications for reproductive physiology and behavior. Behav Brain Res. 1999;1:37–52. doi: 10.1016/s0166-4328(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 61.Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson I. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br J Pharmacol. 2011;162:311–327. doi: 10.1111/j.1476-5381.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–63. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of α5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8:398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pandya A, Yakel JL. Allosteric modulators of the α4β2 subtype of Neuronal Nicotinic Acetylcholine Receptors. Biochem Pharmacol. 2011;82:952–958. doi: 10.1016/j.bcp.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Novel progesterone receptors: neural localization and possible functions. Front Neurosci. 2013;7:164. doi: 10.3389/fnins.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol Med. 2013;19:197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pigott TA, Walker MH, Teitelbaum SA, Lu C. Sex differences and neurotransmitter systems in addiction. In: Brady KT, Back SE, Greenfield SF, editors. Women & Addiction: A Comprehensive Handbook. 242–256. New York: The Guilford Press; 2009. pp. 99–117. [Google Scholar]
- 68.Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology, Physiology, Pathology, and Clinical Management. 6. Philadelphia: Sauders Elsevier; 2009. pp. 139–154. [Google Scholar]
- 69.Carmina E, Lobo RA. Evaluation of hormonal status. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology, Physiology, Pathology, and Clinical Management. 6. Philadelphia: Sauders Elsevier; 2009. pp. 801–824. [Google Scholar]
- 70.Knobil E, Neill JD. Knobil and Neill’s Physiology of Reproduction. 3. Boston: Elsevier; 2006. [Google Scholar]
- 71.Sun J, Walker AJ, Dean B, Van den Buuse M, Gogos A. Progesterone: The neglected hormone in schizophrenia? A focus on progesterone-dopamine interactions. Psychoneuroendocrinology. 2016;74:126–140. doi: 10.1016/j.psyneuen.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 72.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 73.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2005;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 74.Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharm. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- 75.Mello NK. Hormones, nicotine, and cocaine: Clinical studies. Horm Behav. 2010;58:57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smethells JR, Swalve NL, Eberly LE, Carroll ME. Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology (Berl) 2016;233:2999–3008. doi: 10.1007/s00213-016-4345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carpenter MJ, Saladin ME, Leinbach AS, Larowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: A pilot feasibility study. Res J Womens Health. 2008;17:293–301. doi: 10.1089/jwh.2007.0415. [DOI] [PubMed] [Google Scholar]
- 80.Franklin TR, Allen SS. Influence of menstrual cycle phase on smoking cessation treatment outcome: A hypothesis regarding the discordant findings in the literature. Addict. 2009;104:1941–1942. doi: 10.1111/j.1360-0443.2009.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allen AM, Allen SS, Lunos S, Pomerleau CS. Severity of withdrawal symptomatology in follicular versus luteal quitters: The combined effects of menstrual phase and withdrawal on smoking cessation outcome. Addict Behav. 2010;35:549–552. doi: 10.1016/j.addbeh.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen AM, al’ Absi M, Lando H, Allen SS. Allopregnanolone association with psychophysiological and cognitive functions during acute smoking abstinence in premenopausal women. Exp Clin Psychopharm. 2015;23:22–28. doi: 10.1037/a0038747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res. 2015;17:398–406. doi: 10.1093/ntr/ntu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allen AM, Lunos S, Heishman SJ, al’ Absi M, Hatsukami D, Allen SS. Subjective response to nicotine by menstrual phase. Addict Behav. 2015;43:50–53. doi: 10.1016/j.addbeh.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moran-Santa Maria MM, Sherman BJ, Brady K, Baker NL, Hyer JM, Ferland C, McRae-Clark AL. Impact of endogenous progesterone on reactivity to yohimbine and cocaine cues in cocaine-dependent women. Pharmacol Biochem Behav. 2017 doi: 10.1016/j.pbb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White TL, Justice AJ, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 87.Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR. Influence of Menstrual Cycle Phase on Neural and Craving Responses to Appetitive Smoking Cues in Naturally Cycling Females. Nicotine Tob Res. 2015;17:390–397. doi: 10.1093/ntr/ntu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox H, Sinha R. Women & Addiction: A Comprehensive Handbook. New York, NY: The Guilford Press; 2009. Stress, neuroendocrine response, and addiction in women; pp. 65–83. [Google Scholar]
- 89.Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: What we know and what we need to know. Ann Behav Med. 2009;37:117–125. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- 90.Streeter CC, Terhune DB, Whitfield TH, et al. Performance on the Stroop Predicts Treatment Compliance in Cocaine-Dependent Individuals. Neuropsychopharmacology. 2007;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- 91.Berent-Spillson A, Briceno E, Pinsky A, et al. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology. 2015;59:25–36. doi: 10.1016/j.psyneuen.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solís-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology. 2008;33:989–998. doi: 10.1016/j.psyneuen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: Effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 94.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 95.Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- 96.Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Reed SC, Levin FR, Evans SM. The effects of progesterone pretreatment on the response to oral d-amphetamine in women. Horm Behav. 2010;58:533–543. doi: 10.1016/j.yhbeh.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Forray A, Gilstad-Hayden K, Suppies C, Bogen D, Sofuoglu M, Yonkers KA. Progesterone for smoking relapse prevention following delivery: A pilot, randomized, double-blind study. Psychoneuroendocrinology. 2017;86:96–103. doi: 10.1016/j.psyneuen.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav. 2011;59:227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sofuoglu M, Poling J, Gonzale G, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Psychopharmacology (Berl) 2007;15:453–60. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- 103.Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, Shaw H, Shaw J, Sofuoglu M. Progesterone reduces cocaine use in postpartum women with a cocaine use disorder: A randomized, double-blind study. Lancet Psychiatry. 2014;1:360–367. doi: 10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allen SS, Allen AM, Lunos S, Tosun N. Progesterone and postpartum smoking relapse: A pilot double-blind placebo-controlled randomized trial. Nicotine Tob Res. 2016;18:2145–2153. doi: 10.1093/ntr/ntw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schweize E, Case WG, Garcia-Espana F, Greenblatt DJ, Rickels K. Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology (Berl) 1995;117:424–9. doi: 10.1007/BF02246214. [DOI] [PubMed] [Google Scholar]
- 106.Milivojevic V, Sinha R, Morgan P, Sofuoglu M, Fox H. Effects of endogenous and exogenous progesterone on emotional intelligence in cocaine dependent men and women who also abuse alcohol. Hum Psychopharmacol. 2014;29:589–598. doi: 10.1002/hup.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McHugh RK, DeVito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Connery HS, Weiss RD. Gender differences in a clinical trial for prescription opioid dependence. J Subst Abuse Treat. 2013;45:38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–220. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weng J, Chung B. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids. 2016;111:54–59. doi: 10.1016/j.steroids.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 110.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73(9):827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hellgren C, Akerud H, Skalkidou A, Backstrom T, Sundstrom-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69:147–153. doi: 10.1159/000358838. [DOI] [PubMed] [Google Scholar]
- 112.Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S. Brexanolone (SAGE-547 injection) in post-partum depression: a randomized controlled trial. 2017;390:480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]