Abstract
Our understanding of how membrane trafficking pathways function to direct morphogenetic movements and the planar polarization of developing tissues is a new and emerging field. While a central focus of developmental biology has been on how protein asymmetries and cytoskeletal force generation direct cell shaping, the role of membrane trafficking in these processes has been less clear. Here, we review recent advances in Drosophila and vertebrate systems in our understanding of how trafficking events are coordinated with planar cytoskeletal function to drive lasting changes in cell and tissue topologies. We additionally explore the function of trafficking pathways in guiding the complex interactions that initiate and maintain core PCP (Planar Cell Polarity) asymmetries and drive the generation of systemically oriented cellular projections during development.
Keywords: gastrulation, planar polarity, apical constriction, PCP signaling, Rab proteins, exocyst, Rab11, morphogenesis, Rab35, Dynamin, Clathrin, cell intercalation, Rab5
1 Introduction
Vesicle-directed membrane trafficking is an efficient mechanism for the delivery of discrete packets of membrane lipids, protein and lumenal components to different regions of the cell. Both exocytic and endocytic mechanisms contribute to the generation of polarized axes of information and membrane domains of specialized functions. It has long been appreciated that apical-basal polarity enforces directional membrane trafficking in epithelial cells, and that, in turn, polarized trafficking has an essential role in the establishment of apical-basal polarity.1,2,3 However, a new emerging role for trafficking pathways is their effects on processes of planar polarity and development. Planar polarity is a system(s) of information that provides orientations within the plane of an epithelium or tissue. This can lead to the common orientation of projecting hairs, cilia, and bristles, as well as guide developmental processes that lead to rearrangements of tissue dimensions and cell topologies. In this review, we will discuss several important planar polarized processes with a focus on recent Drosophila and vertebrate literature in which membrane trafficking events contribute to the generation of planar polarized protein distributions and asymmetric force generation. We apologize in advance to all colleagues whose relevant work was not cited here because of space limitations and decisions on manuscript organization.
Basics of Planar Polarity
Planar polarity pathways direct asymmetric enrichments of protein populations at cell boundaries with certain, systemic orientations. These protein populations can then interact in agonistic or antagonistic interactions to promote further planar polarization of cells and boundary elements. Once established, systems of planar polarity direct morphogenetic events that lead to the creation of oriented structures. In adult organisms, examples of these final outcomes of planar polarity are the common projections of hairs in the mammalian skin or bristles in the adult fly epidermis and wing.4,5 However, planar polarized processes also actively guide major developmental events.6 Gastrulation in both vertebrates and invertebrates is dependent on planar polarity, and organ systems such as the patterning of the inner ear or elongation of the gut primordia are similarly dependent.4
Different morphogenetic events during animal development do not necessarily re-utilize identical planar polarized proteins and pathways. Instead, several different systems of planar polarity exist. The first discovered system of planar polarity is the classic PCP pathway, which is centered around core planar distributions of Frizzled (Fz), Dishevelled (Dsh), Diego (Dgo), Prickle (Pk), Flamingo (Fmi), and Van Gogh (Vang).7,8,9,10 The PCP pathway has additional contributions from the Fat-Dachsous module of protocadherin signaling. However, the PCP pathway is not the only planar cell pathway. Other systems of planar polarity are less consistent in their composition, but often involve actomyosin contractile components as well as Par proteins, which were first discovered as partitioning mutants in C. elegans, such as Par-3/Bazooka and their binding partners aPKC and Par-6.11,12,13
Planar polarity and membrane trafficking
Planar polarity directs the development of a diverse array of tissues, but how membrane trafficking pathways guide planar polarized processes has been less clear. In general, the effects of membrane trafficking on planar polarized processes can largely be broken down into two categories: 1) trafficking events that direct cell topology altering and/or force-generating mechanisms that directly guide rearrangements of tissues and their dimensions, and 2) trafficking events that direct the generation of informational asymmetric domains of formal planar polarity patterning protein (largely PCP proteins). We will discuss each of these in separate sections, although these two categories of interactions can each influence the other.
2 Development, planar force generation and morphogenesis
Some of the best-known morphogenetic processes that are directed by planar polarity are those involving convergent extension movements. These movements involve the coordinated convergence of cells along one tissue axis, which results in the extension of the tissue in a perpendicular axis. Interestingly, convergent extension movements can direct tissue shaping in both mesenchymal as well as epithelial contexts, and requires planar polarized networks in most circumstances.14 The ability of tissues to elongate is essential to the shaping of many different organ systems, as well as the entire organismal anterior-posterior axis. Indeed, tissues as diverse as germband extension in the Drosophila embryo, primitive streak formation in the chick embryo,15 neural tube closure in Xenopus,16 and palate fusion17 and limb bud elongation in the mouse,18 all depend on tissue elongation to help attain their final tissue dimensions. A central mechanism that drives convergent extension movements is cell intercalation. During cell intercalation, the oriented insertion of cells between preexisting neighboring cells occurs. This insertion of new cells pushes the previously neighboring cells apart, which results in a lengthening of the tissue in the opposing dimension (Figure 1A).
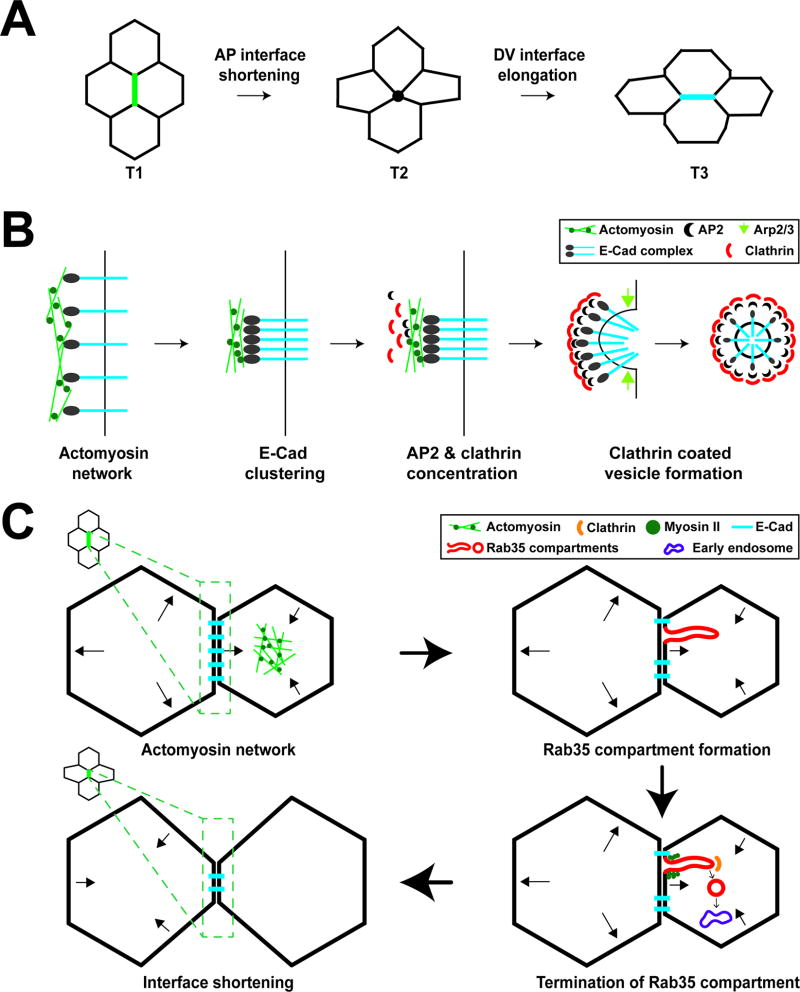
FIGURE 1.
Membrane trafficking directs changes in cell shape during early Drosophila embryogenesis. (A) Diagram of cell intercalation and T1–T3 transitions during Drosophila germband extension. At T1 interfaces, the anterior-posterior (AP) interface shrinks to a four-cell vertex (T2), followed by new interface formation and elongation of a dorsal-ventral interface (T3). (B) E-cadherin uptake during AP interface shortening. Actomyosin contraction drives E-cadherin clustering. AP2 and Clathrin are enriched at AP interfaces and endocytose clustered E-cadherin. The scission of vesicles from AP interfaces is driven by Arp2/3 complex function. (C) Membrane dynamics and interface ratcheting during cell intercalation. Cell areas oscillate through apical Myosin II function. During area oscillations, Rab35 compartments form to efficiently take up plasma membrane and cargo proteins, leading to processively shortened AP interfaces. Myosin II and Clathrin-mediated endocytosis are required to terminate Rab35 compartments and direct endocytosed material to Rab5 early endosomes leading to irreversible interface contraction.
Cell intercalation in Drosophila
In Drosophila, the embryonic de novo generation of a cellular epithelial sheet is rapidly followed by a dramatic reorganization and elongation of the new epithelium along the Anterior-Posterior (AP) axis.11,19 A conserved convergent extension program operates through oriented cell intercalation, which causes the tissue to narrow in one dimension (along the dorsal-ventral axis, DV) and lengthen in a perpendicular AP dimension.20,21,22,23,24 In 45 minutes of development, and largely in the absence of cell division, the Drosophila embryonic epithelium will more than double in length.
The underlying mechanism that directs cell intercalation are changes in the topological relationships between cells. The oriented contraction of T1 interfaces (adhesive cell surfaces between AP neighboring cells) is followed by the growth of T3 interfaces (surfaces between DV neighboring cells) and leads to tissue narrowing and extension (Figure 1A). This cellular reshaping requires the function of apical and junctional proteins.25,26,27 Contractile Myosin II proteins are found in several different populations in the early embryonic epithelium – one population is present in the apical/medial cap of intercalating cells where it mediates oscillations in cell area,28,29 while another population is present at AP interfaces (Figure 1B,C).26,27 Conversely, by mid-germband extension (GBE), the adherens junction proteins E-cadherin and Armadillo/β-catenin are concentrated in interfaces located between dorsal and ventral neighbors (DV interfaces). This combination of tension-producing actomyosin networks and cadherin-dependent adhesion complexes are believed to be the key determinants directing early morphogenesis in the Drosophila embryo.11,30,31,32 Given these planar polarized protein distributions, one outstanding question in the field has been if membrane trafficking events are planar polarized and contribute to these morphogenetic processes. As E-cadherin is a transmembrane protein and is the major adhesive protein in the Drosophila embryo at these stages, a reasonable hypothesis would be that changes in cell-sidedness are driven by the turnover and targeted trafficking of E-cadherin. Two recent papers have addressed this question (Figure 1B,C).33,34
Trafficking in the generation of planar polarized cell rearrangements
An important first finding on the effects of membrane trafficking during cell intercalation was that elements of clathrin-mediated endocytosis (CME) are planar polarized.33 The adaptor protein αAP2 and the light chain of Clathrin (Clc) are asymmetrically enriched at plasma membranes between Anterior-Posterior (AP) neighboring cells, a majority of which are contractile T1 interfaces (Figure 1B). Additionally, an unbiased marker for extracellular uptake, fluorescently labelled dextran, shows a planar polarized enrichment at AP interfaces. Inhibiting endocytosis through either small molecule (chlorpromazine, PitStop-2) or genetic (Shibire/Dynamints alleles) methods disrupted planar polarized distributions of E-cadherin, and halted topological transitions and T1 interface contraction.33,34 These results demonstrated that endocytic turnover of E-cadherin drives neighbor exchange and changes in cell-cell relationships.
Further work examined the role of the cytoskeleton in directing cell intercalation as well as the trafficking dynamics of these processes. Two different filamentous actin networks are present at cell junctions during tissue elongation in Drosophila and possess distinct functions in planar polarized endocytosis. The forced localization of the Arp2/3 complex activator Scar at the plasma membrane led to enhanced dextran uptake during cell intercalation, and, conversely, reducing Scar function decreased the number of endocytic events. Interestingly, the formin Diaphanous did not function by directly driving endocytosis, but instead in allowing Myosin II to cluster E-cadherin proteins through contractile force generation. This clustering appears to trigger the clathrin- and dynamin-mediated planar polarized endocytosis of E-cadherin, which in turn causes T1 interface shortening and cell intercalation by reducing local cell-cell adhesions. Thus, branched actin Arp2/3 complex function is likely involved in E-cadherin-containing vesicle scission, while Diaphanous/formin linear actin networks direct contractile function and E-cadherin clustering.33
The above data indicates that planar polarized vesicle formation occurs during tissue remodeling and is essential for cell intercalation, but the involvement of Rab trafficking pathways in these processes has not been clear. Rab GTPase family proteins are key mediators of membrane trafficking and cytoskeletal dynamics. When in an active GTP-bound state, Rab proteins regulate membrane compartment behaviors through their association with tethering and trafficking effectors.35,36,37 A localization screen of the 31 Drosophila Rab proteins has been performed during cell intercalation, and a planar polarized Rab protein was identified.34 Rab35 compartments form at AP interfaces and display dynamic behaviors, with compartments forming and then resolving on the order of ~140 seconds. Interestingly, this is on a similar time-scale as pulsed oscillations of the actomyosin networks described above, suggesting a possible link between the two processes. Indeed, Rab35 compartments form during periods of active interface contraction and represent transient infoldings of the cell surface. A potential relationship between cytoskeletal force generation and membrane trafficking pathways was further explored by inhibiting Myosin II contractility through the injection of a Rok inhibitor. In these experiments, Rab35 compartments could initiate, but did not terminate, suggesting a model in which Myosin II function is required for compartmental scission and endocytic uptake of plasma membrane components. Consistent with previous results,33 inhibition of CME also produced elongated Rab35 tubules that failed to terminate, demonstrating that Rab35 compartments are used as efficiency platforms that permit the enhanced uptake of plasma membrane. Finally, Rab5 endosomes were shown to associate with Rab35 compartments, and appeared to grow in size and assist in the termination process of Rab35 compartments. It is interesting to note that prior work in Drosophila has shown that E-cadherin traffics through early (Rab5) and recycling (Rab11) endosomes and requires exocyst complex function. Further, the activity of these endosomal structures has been shown to be essential to morphogenesis.38,39,40 These results would be consistent with trafficking networks driving essential changes in E-cadherin mediated cell adhesion that direct cell intercalation.
The above results also address a larger issue in that changes in cell shape in many systems are driven by pulsatile processes that initiate directed movement before cycling through periods in which the force generating network reforms.41,42,43,44 This pulsed behavior requires a consolidation, or ratcheting mechanism, that prevents the reversal of cell shape changes when pulsed contractile networks dissipate. It appears that trafficking networks are well positioned to ensure the progressive nature of interface contractions. For example, oscillations in cell area as well as interface lengths produce cycles of high and low tension, periods during which trafficking networks could use these areas of slackened cell surface to internalize plasma membrane and integral proteins. This in turn would prevent interfaces from rebounding in length when contractile force generation terminates. Indeed, when Rab35 function was disrupted interfaces showed a wobble phenotype in which interface contractions occurred, but were rapidly followed by reversals and cells exhibited a loss of progressive, sustained changes in cell shape. These results indicate the complex nature of interactions between cytoskeletal and trafficking networks during cell intercalation. Not surprisingly, to effect long term changes in cell shape, cells coordinately exploit a variety of cell biological mechanisms that include membrane trafficking and actomyosin function. However, our understanding of how this coordination of mechanisms happens is still in its beginning stages.
3 Polarized trafficking events that direct vertebrate gastrulation
The bending of epithelial sheets is a common morphogenetic process that drives tissue invaginations in a diverse array of species.45,46,47,48 While not typically planar polarized, these events represent a similar remodeling of specific cell surfaces, and also show a similar combination of cytoskeletal and membrane trafficking events that lead to changes in cell shape. Such epithelial bending, or furrow formation, is primarily initiated through the contraction of apical cell areas of specific cell populations. For instance, amphibian bottle cell formation and avian neural tube formation exhibit the constriction of cell apices in defined regions of the epithelium.47,49,50,51 This cell shape change, termed apical constriction, is defined by the contraction of the apical surface of epithelial cells. This results in a fundamental change in cell geometry and drives the generation of wedge or pyramidal shaped cells. The mechanical processes that drive apical constriction appear to be a core set of F-actin, Myosin II, and adhesion proteins, which generate and transmit forces across the tissue to promote cell shape change. Different tissues regulate contractility and adhesion function in unique ways, but endocytic-driven membrane remodeling has been increasingly shown to be essential in the regulation of actomyosin networks, cell adhesion, and cell shape.
The function of endocytic mechanisms during invagination of Xenopus laevis bottle cells has been examined by following the uptake of biotinylated plasma membrane. Biotinylated membrane accumulated in internal puncta specifically in apical constricting cells, and these structures were also shown to be positive for EEA1 and Rab5, demonstrating that plasma membrane was transiting to endosomal compartments.52 Additionally, embryos expressing dominant negative Dynamin, which weakly binds to GTP, or morpholinos against the two Xenopus Dynamin homologs dnm-like and dnm-2, inhibited apical constriction. Finally, embryos expressing dominant negative Rab5, in which internalized biotinylated vesicles were almost undetectable, possessed inefficient apical constriction and a failure in the cell shape changes typical of bottle cells during gastrulation. These results indicate the importance of endocytic trafficking during apical constriction.
However, given the above results, one outstanding question concerns the nature of the relationship between endocytic pathways and actomyosin contractility. Results from the planar polarized movements of cell intercalation in Drosophila have shown a reciprocal relationship between trafficking pathways and actomyosin networks, in which the disruption of endocytosis produces a concomitant decrease in Myosin II localization.33,34 By contrast, in bottle cells it appears that Myosin II localization is upstream of early endocytic machinery. This has suggested a model in which actomyosin contractility generates an initial decrease in apical area that drives the folding of apical membrane into microvilli. The excess membrane stored in these microvilli is then removed by endocytosis. This is supported by evidence demonstrating that the initial constriction rate is indistinguishable in wild type and dominant negative dynamin cells.52 It was only as apical constriction continued that dynamin disrupted cells began to possess slower rates of apical contraction and eventually stalled. One physical interpretation of these results is that excess membrane begins to fold into extensive microvilli that disrupt the productive engagement of force generating F-actin networks. It is interesting to note that there are significant parallels in this model to the function of Rab5 and Dynamin during Drosophila cellularization in which endocytic uptake of microvilli fuels furrow ingression.53,54
Additional work has examined apical constriction and elements of the recycling trafficking pathway during neural tube closure in Xenopus. A dominant negative version of the recycling endosomal protein Rab11 has been shown to interfere with apical constriction required for neural folding, neural tube closure, and bottle cell formation.55,56 Intriguingly, during neural tube folding Rab11 endosomes become enriched at apical surfaces in a planar polarized fashion. This planar polarized recruitment of Rab11 requires the functioning of the PCP proteins Diversin/Diego, Vangl2, and Dishevelled, and demonstrates that systems of planar polarity can contribute to a common apical constriction and trafficking pathway. As opposed to previous reports on bottle cell formation and early endocytic machinery, planar polarized Rab11 is required for activated Myosin II localization. In embryos expressing dominant negative Rab11 or injected with Rab11 morpholinos, the accumulation of phosphorylated Myosin light chain II during neural tube closure was undetectable. In addition, the planar polarized apical localization of Rab11 is accompanied by a similar asymmetric distribution of the Rab11-binding exocyst component Sec15. These results suggest that exocytic trafficking through Rab11 and exocyst dependent pathways direct actomyosin contractility, and further reveal that PCP proteins can impose planar polarity on Rab11 endosomal pathways.
It also appears that the relationship between endosomal trafficking and planar signaling is not always unidirectional, and tissues may possess a positive-regulatory loop between PCP and trafficking proteins. While, as discussed above, Rab11 apical accumulation requires Vangl2 function during Xenopus gastrulation, it also appears that Rab11 vesicles deliver Vangl2 as a cargo protein.56 When Rab11 function was depleted through the use of antisense morpholino oligonucleotides, Vangl2 localization was lost from the plasma membrane and accumulated in the cytoplasm in constricting gastrula cells. This discovery supports a positive-feedback loop, in which the Rab11 endosomal pathway targets PCP proteins to their specific localization, and the accumulation of these cargo proteins, in turn, regulate Rab11 distributions. Thus, Rab11-mediated endocytic recycling and Vangl2 planar polarity are essential for successful apical constriction and effective changes in cell shape.
Early endosomal function and morphogenesis in zebrafish
In addition to the work from Xenopus, data from another widely used vertebrate model, zebrafish, suggests that the early endocytic pathway plays an important role during cell migration and gastrulation. In zebrafish gastrulation, proper formation of the tissue layers requires cell movements of mesodermal and endodermal (mesendodermal) progenitors. During cell migration at these stages, Slb/Wnt11 controls the endocytosis of E-cadherin through the functioning of the Rab5 GTPase.57 In slb/wnt11 compromised cells, E-cadherin displays an aberrant distribution in which it becomes primarily enriched at the cell surface and relatively few cytoplasmic puncta are found. When slb/wnt11 function is provided back to mesendodermal cells, E-cadherin distributions at the plasma membrane become more varied and a number of cytoplasmic E-cadherin puncta are observed. Compromising slb/wnt11 function also causes the abnormal migration and aggregation of mesendodermal cells and defects in tissue movement towards the animal pole. To examine this in greater detail, embryos that expressed a constitutively active version of Rab5c were compared to slb/wnt11 expressing embryos and, again, a greater number of E-cadherin positive cytoplasmic puncta were identified. These puncta also possessed Rab5c, and suggested that E-cadherin must be dynamically regulated through endocytic processes for efficient cell migration. Supporting this, the overexpression of Rab5c in slb/wnt11 defective embryos partially rescues the mutant phenotype. These data indicate that Wnt11 functions upstream of Rab5-dependent trafficking pathway to properly balance cell cohesion and migration during zebrafish gastrulation.57
Trafficking of E-cadherin by Rab GTPases is also critical during the initiation of the gastrulation movements of epiboly and radial cell intercalation.58 Canonically, Rab GTPases play different roles in regulating endocytic trafficking, with Rab4 involved in rapid recycling to the plasma membrane, Rab5c implicated in early endosomal formation, and Rab11 regulating recycling endosomal trafficking. During epiboly, Rab4, Rab5c and Rab11 were observed to colocalize with endosomal E-cadherin. Interestingly, E-cadherin colocalization with Rab5 or Rab11 was found in puncta that were near the plasma membrane as well as at larger endosomes, suggesting that E-cadherin could be transported via early Rab5c-positive endosomes, and then recycled to the plasma membrane in either a direct Rab11 dependent fashion, or an indirect Rab4 dependent process. Indeed, disrupting Rab5 function through either the injection of Rab5 morpholinos or a Rab5 GAP protein, RN-tre, led to impaired E-cadherin internalization defects in cells undergoing epiboly. These studies additionally suggested that EGF-mediated phosphorylation of p120 catenin may trigger endocytosis, possibly through a caveolin system that could bias E-cadherin uptake towards a recycling, rather than degradative, pathway. These findings support a model in which Rab-mediated membrane trafficking pathways regulate morphogenesis and cell migration by controlling cell adhesion through the endocytosis of E-cadherin.
4 PCP and trafficking pathways in Drosophila wing development
We now turn our attention to PCP proteins and the establishment of informational asymmetric planar domains that guide hair and bristle formation. The Drosophila wing is the most thoroughly studied planar polarized tissue and has a rich history of key planar polarity findings. Each wing epithelial cell produces a single actin-rich hair (a trichome), which is oriented distally and in parallel to the wing veins. In the larval epidermis, the wing imaginal disc consists of 30 cells corresponding to anterior and posterior clonal compartments. At the end of the first larval instar, cell proliferation resumes and continues through pupariation. A series of morphological changes occur in the wing imaginal disc in the early pupal stages, including evagination, eversion, and elongation, which depend on cell rearrangements and mechanical forces that direct the emergence of the wing blade from the imaginal disc.59,60 During this time, the cells of the wing become increasingly ordered and pack hexagonally as they polarize along the proximal-distal axis.61 By late pupal development, cell division is largely complete, and the core PCP genes determine the oriented formation of individual trichomes, which act to guide the flow of air over the wing surface during flight (Figure 2A,C).7,59,60,62
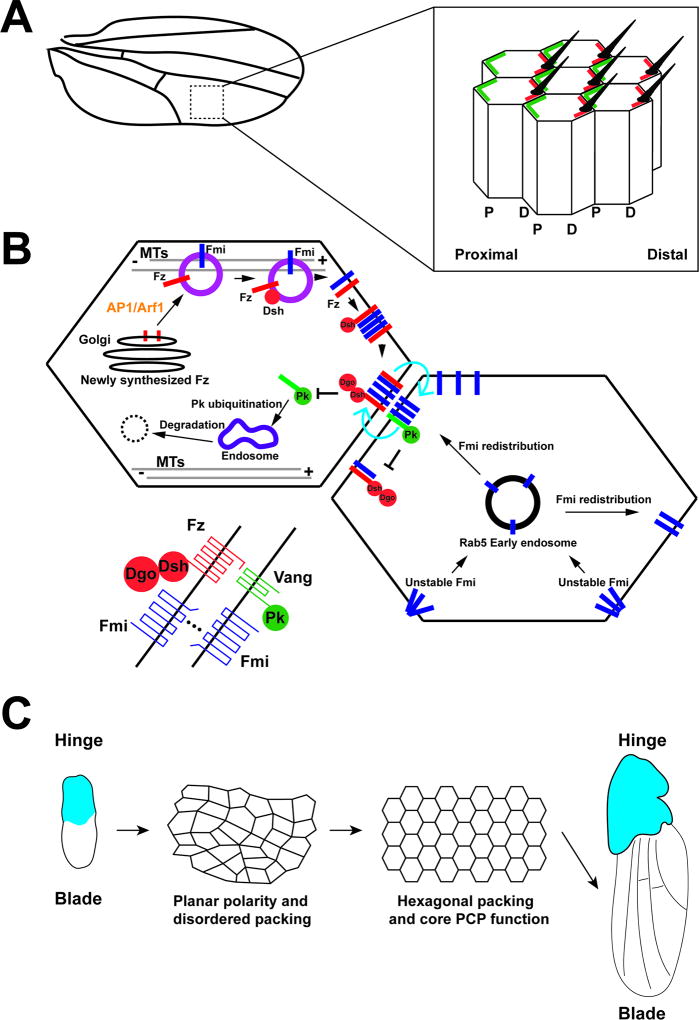
FIGURE 2.
Illustrative representation of membrane trafficking and PCP function in Drosophila wing development. (A) Diagram of the Drosophila wing and close up of planar polarized wing epithelial cells. Apically projecting trichomes (hairs) are present and oriented distally, and proximal (P) and distal (D) PCP domains are shown. (B) Diagram of trafficking pathways affects PCP pathways and Drosophila wing development. Newly synthesized Fz vesicles are generated through AP1/Arf1 function and transported, along with Fmi and Dsh, through a planar polarized microtubule network to the distal cell surface. The localization of the Fmi-Fz-Dsh complex at distal cell edges recruits Dgo. Distal Fmi clustering recruits neighboring cell Fmi through homophilic interactions producing Fmi localization at both proximal and distal surfaces. Proximal Fmi accumulation recruits PCP molecules Vang and Pk. The established distal and proximal machineries mutually exclude each other, with Fz-Dsh-Dgo complexes mediating Pk ubiquitination that results in Pk turnover at distal regions. The Pk-Vang complex inhibits Fz-Dsh-Dgo complex formation at proximal cell surface. Unengaged Fmi proteins are unstable and recycled and redistributed through Rab5 compartments. (C) Hexagonal packing occurs during wing development, and the wing epithelium becomes progressively more ordered. The core PCP proteins direct interface remodeling that leads to cell rearrangements that produce an ordered, hexagonal array.
The canonical Planar Cell Polarity (PCP) system
Six proteins form the core PCP system and display asymmetric distributions along the AP axis in planar polarized wing epithelial cells. Frizzled (Fz), Dishevelled (Dsh) and Diego (Dgo) localize to distal adherens junctions, while Van Gogh (Vang, also known as Strabismus) and Prickle (Pk) enrich at proximal cell edges (Figure 2B). Flamingo (Fmi), an atypical cadherin, is on both the proximal and distal cell surfaces. Of the six core PCP molecules, three are cytoplasmic proteins (Dsh, Pk, and Diego), and the other three are transmembrane proteins (Fz, Vang, and Fmi). Fz is a seven-pass transmembrane protein that recruits Dsh and Dgo to cell membranes, and Vang is a four-pass transmembrane protein that can bind to Pk, Dsh and Dgo, and recruits Pk to cell interfaces. The atypical cadherin Fmi is a seven-pass transmembrane protein that shows homology to G-protein-coupled receptors and can form homophilic cell adhesions.4,8,9,10 These proximal and distal complexes have antagonistic interactions that amplify initial asymmetric distributions, but it is becoming increasingly clear that membrane trafficking pathways and polarized transport assist in the establishment of these planar polarized distributions.5,6
In addition to the core PCP proteins, the Fat/Dachsous/Four-jointed system also assists in the establishment of global cell orientations and mediates long range effects of PCP signaling. Fat (Ft) and Dachsous (Ds) are atypical cadherins, while Four-jointed (Fj) is a type II transmembrane kinase that can phosphorylate Ft and Ds, and regulates PCP signaling. While it has been proposed that Ft-Ds signaling acts upstream of core PCP proteins, the relationship of these two systems during development has been contentious. Ft-Ds proteins polarize to form proximal and distal domains that are tightly coupled to each other and precede hair formation.63,64,65,66 The final outcome of defects in PCP function is the generation of ectopic single or multiple hairs that are not properly aligned with the wing axis.
Planar microtubules arrays are required for polarized transport of PCP molecules
During pupal wing development, apical non-centrosomal microtubules are enriched at the level of the adherens junctions and become oriented along the proximal-distal axis of the wing.67,68,69,70,71 This is consistent with the generation of a planar polarized cytoskeleton, and suggests that trafficking pathways could utilize this oriented microtubule network (Figure 2B). Indeed, Fmi-containing vesicles are observed to be associated with proximal-distal microtubules, and disruption of microtubule networks perturbs Fz and Fmi distribution. This then results in the mislocalization of F-actin during prehair formation. It is interesting to note that E-cadherin appears to be trafficked in different vesicles and did not associate with the proximal-distal microtubule array, suggesting that distinct PCP cargo vesicles are generated and capable of associating with a dedicated microtubule network. Based on this data, it has been proposed that intracellular Fmi and Fz-containing vesicles are preferentially transported to distal cell interfaces along these polarized microtubule arrays.70 Additionally, Dsh has also been shown to be transcytosed on these microtubules, at least partially in vesicles distinct from Fz vesicles.71 Two patterns of Dsh trafficking have been observed: 1) A majority of Dsh-containing vesicles emerge from one side of the cell and are directly transported to the membrane of an opposing interface and, 2) a small portion of the vesicles lacked directional bias and were frequently observed to move away from apical plane, which may reflect a more classical recycling pathway. On the other hand, no proximal PCP proteins were observed to engage in directional trafficking. Only distal PCP molecules such as Fz and Dsh demonstrated biased trafficking through microtubule networks, suggesting a possible function for plus-end directed motor proteins in planar polarity. Taken together, these results show that microtubule polarization is essential for guiding core PCP localizations during wing development.
How then is the initial symmetry breaking achieved, and how do cells of the wing establish a PCP system? This is an unsolved question; however, the Ft/Ds/Fj system has been proposed to act as a global PCP patterning module which transduces tissue level directional cues to orient the core PCP proteins. Previous studies have shown that, throughout wing development, microtubule orientation correlates with the direction of Ds and Fj gradients and does not depend on core PCP protein asymmetry. Additionally, when local cell areas of mutant ft function (mutant clonal populations) were created, the microtubule networks in wild-type cells polarized towards the Ft-deficient area.71 Finally, in wings that have been manipulated to express an ectopic Ds gradient, the microtubule network is found to reverse its orientation.72 This suggests that PCP vesicles traffic along a planar polarized microtubule network which is oriented by global polarity cues to direct initial asymmetries of core PCP proteins.
One complication that arises from this data is that the majority of Fmi-containing vesicles are transported distally, and yet Fmi maintains a bipolar localization that is enriched at both proximal and distal interfaces. Recent work has shown that newly synthesized Fz is transported to the membrane through an AP-1/Arf1 pathway. This trafficking network promotes Fz levels at cell-cell contacts and the formation of stabilized Fmi-Fz and Fmi-Vang complexes.73 The observation that Arf1 directs Fz trafficking from Golgi immediately after its synthesis supports a model in which stable PCP complexes originate mainly from newly produced proteins. Additional stabilization of proximal Fmi populations likely also occurs through homophilic binding of counterpart Fmi protein on the opposing distal interface.74 The formation of Fmi-Fmi interface-bridging complexes helps recruit Fz-Dsh complex localization at the distal interface which, along with proximal protein function, results in the local clustering of Fz-Dsh. However, it is interesting to note that Dsh and Dgo are not required for Fmi or Fz localization.74,75,76,77,78 Fz and Vang cooperate to stably localize Fmi to junctions, forming as clustered puncta in membrane subdomains. Membrane trafficking through Rab5- and Dynamin-dependent processes then removes unstable Fmi,79 and Fmi is redistributed by Rab5 and its effector Rabenosyn-5 through early endosomes.80 Dsh has also been shown to concentrate in puncta through limits on its lateral diffusion.79
The above largely discusses how initial asymmetries in distal-localizing PCP populations are biased by planar trafficking and transport pathways, but how do proximal PCP proteins like Vang and Pk become localized? The establishment of proximal Vang and Pk populations does not appear to be analogous to how distal polarities are first generated – i.e., there are not observable asymmetries in microtubule transport of Vang and Pk to proximal interfaces. Instead, proximal recruitment appears to be driven by mutual exclusion mechanisms coupled to endocytic processes. The distal clustering of Fz through interactions with Fmi has been shown to initiate a polarized distribution of Vang on the opposing proximal interface81. As a part of the PCP mutual exclusion mechanism, Fz complexes possess an antagonizing activity towards the accumulation of Pk-Vang-Fmi complexes by a Cullin1-E3 ligase-mediated ubiquitination of Pk. The subsequent internalization of Pk leads to the removal of Pk-Vang from distal regions, and an indirect accumulation at proximal interfaces.82 Additionally, local clustering through Fmi-Fmi intercellular bridges also likely limits the lateral diffusion of Vang and Pk. Thus, trafficking-dependent mechanisms are essential mediators of PCP turnover and permit competition between proximal and distal complexes to amplify initial planar asymmetries. These results also show the importance of both exocytic and endocytic trafficking events in guiding discrete domains of PCP signaling complexes.
Membrane trafficking pathways in pre-hair and hair formation
In late wing development, membrane trafficking pathways are also active during the planar polarized formation of hairs. Specifically, PCP effectors are necessary to restrict trichome formation, both in terms of the numbers of trichomes as well as their location at the cell surface.63 Gilgamesh (Gish, or Drosophila casein kinase 1 γ) has been found to direct a membrane trafficking pathway for the refinement of hair formation.83 Rab11 and its effector, nuclear fallout (nuf, a Rab11-FIP3 homolog), function together with the exocyst complex subunit Sec15 to direct recycling endosome localization through microtubule-dependent trafficking.84 With this apical localization established, Rab11 then guides a targeted membrane recycling pathway that is essential for polarized hair formation, and Gish, in turn, regulates the localization of Rab11 machinery. The loss of gish function causes ectopic trichome formation and failures in the proper nucleation of filamentous actin required for polarized trichome formation.83 These data demonstrate that Gish directs the polarized trafficking and enrichment of Rab11 endosomes that restrict actin nucleation to a single site in the distal domain by vesicle recycling.
PCP signaling and the regulation of cell topologies
As demonstrated by the above discussion on PCP function in the wing, PCP signaling is often thought of foremost as a regulator of informational domains that direct oriented projections of hairs and bristles. However, even in the wing, PCP proteins also direct the organization of epithelial cell topologies. Throughout most of wing development, epithelial cells demonstrate a high degree of disorder, with a majority of cells varying from a low energy 6-sided packing array. However, as the wing begins final differentiation movements, the epithelial cells become progressively packed into a honeycomb-like, regular hexagonal array that will ensure an even distribution of hairs across the wing surface. During this process of tissue ordering, the percent of hexagonal cells increases from less than 40% to nearly 80%, and intercellular junction lengths are remodeled to produce hexagonal repacking.61,70 In this process, PCP proteins such as Fz play an important informational role in guiding hexagonal packing and membrane trafficking. The endocytic uptake and redelivery of E-cadherin to the cell surface is an essential part of hexagonal packing. Specifically, E-cadherin is endocytosed through a Dynamin-mediated pathway. E-cadherin is subsequently recycled through Rab11 endosomes during repacking, and PCP proteins polarize exocyst/Sec5-dependent vesicle trafficking that lead to junctional remodeling.61 These cycles of interface contraction and growth drive hexagonal packing in a manner that has homologies to cell intercalation in the early Drosophila embryo, although the final outcomes on tissue topologies are dissimilar.
A further role for membrane trafficking during PCP and hexagonal packing was revealed when it was shown that that the ATP6AP2/VhaPRR, accessory subunit of the vacuolar (V)-ATPase plays a role in regulating Fz complex degradation. Specifically, VhaPRR is recruited by the Fmi complex to PCP domains, and participates in vesicular acidification and endolysosomal degradation. Lack of VhaPRR function leads to increasing internalization of Fz-Fmi PCP proteins, and the failure of E-cadherin lysosomal degradation. These changes in E-cadherin levels produce altered cell packing as extra adherens junction proteins recycle back to the cell interface.85 These results illustrate how both core PCP proteins as well as force-generating adhesion proteins are highly dependent on membrane trafficking networks.
In summary, membrane trafficking pathways play multiple roles in canonical PCP signaling in the wing. Membrane trafficking is essential to the initiation, amplification, and final differentiation of planar polarized events during wing development. While membrane trafficking pathways direct the formation of informational PCP domains, they are also important in guiding the final topological relationships that are established late in wing development. These studies in the wing also demonstrate the close relationship between microtubule networks and planar polarized trafficking events.
5 Conclusions and future perspectives
Although our understanding of the interplay between membrane trafficking networks and planar polarity systems is just beginning, it is clear that powerful relationships exist between these two systems (Table 1). Further work will be needed to tease apart the relative contributions of exocytic and endocytic pathways in directing planar polarized distributions of proteins and cell topologies. However, it is perhaps not surprising that trafficking events that can directly remodel the quantity and composition of the plasma membrane are essential cell biological determinants of cell shape. The studies cited in this review demonstrate how internalization pathways that function through classic endocytic mechanisms can lead to the degradation, recycling, or transcytotic events required for the establishment of planar polarity and developmental morphogenesis. Although the requirement for trafficking pathways in planar polarity is clear, the molecular mechanisms that enforce polarity remain to be identified in many systems. It will be interesting to see if new molecular links between informational PCP proteins and trafficking pathways emerge, or if planar modifications in membrane identity (for example, the regulation of phosphoinositide levels) lead to biases in endocytic and/or exocytic pathways.
Table 1.
Summary of trafficking protein function during morphogenesis and planar polarity.
| Protein | Organism | Subcellular structure |
Polarity | Process | Function |
|---|---|---|---|---|---|
|
| |||||
| Rab4 | Zebrafish | Endosome | Epiboly cell migration | E-cad recycling58 | |
|
| |||||
| Rab5 | Drosophila embryo | Early endosome | Apical cytoplasm | Epithelial formation | Microvilli uptake54 |
| Germband extension | Associates with Rab3534 | ||||
|
|
|
||||
| Drosophila wing | Wing development | Fmi redistribution80 | |||
|
|
|
||||
| Zebrafish | Epiboly cell migration | E-cad recycling58 | |||
|
|
|
||||
| Xenopus | Apical | Bottle cell formation | Apical constriction52 | ||
|
| |||||
| Rab11 | Drosophila embryo | Recycling endosome | Apical cytoplasm | Epithelium formation | Membrane trafficking53 |
|
|
|
||||
| Drosophila wing | Apical cytoplasm | Wing development | PCP trafficking84 | ||
| Cell packing61 | |||||
|
|
|
||||
| Xenopus | Neural tube folding | Apical constriction56 | |||
| Myosin II activation55 | |||||
| PCP trafficking55,56 | |||||
|
|
|
||||
| Zebrafish | Epiboly cell migration | E-cad recycling58 | |||
|
| |||||
| Rab35 | Drosophila embryo | Endocytic compartments | AP interface | Germband extension | Interface contraction34 |
|
| |||||
| AP2 | Drosophila embryo | Endocytosis | AP interface | Germband extension | E-cad uptake33 |
|
| |||||
| Clathrin | Drosophila embryo | Endocytosis | AP interface | Germband extension | E-cad uptake33 |
| Rab35 compartment termination34 | |||||
|
| |||||
| Dynamin | Drosophila embryo | Endocytosis | Apical | Epithelium formation | Microvilli uptake53,54 |
|
| |||||
| AP interface | Germband extension | E-cad uptake33 | |||
| Rab35 compartment termination34 | |||||
|
| |||||
| Arf1 | Drosophila wing | Transport vesicles | Proximal to distal | Wing development | PCP trafficking73 |
|
| |||||
| Sec5/Sec15/exocyst | Drosophila embryo | Exocyst complex, plasma membrane | Apical | Epithelium formation | Exocytic trafficking38,39 |
|
|
|||||
| Drosophila wing | Wing development | Exocytic trafficking61 | |||
| Cell packing61 | |||||
|
|
|||||
| Xenopus | Apical surface | Neural tube folding | Apical constriction55 | ||
It will also be important to explore these relationships in both vertebrate and invertebrate systems, and to see if certain trafficking regimes dominate depending on the degree to which developing tissues are dedicated to certain outcomes. For example, some tissues may undergo limited cell rearrangements and planar polarity may be largely focused on the creation of planar polarized cell projections such as hairs or cilia. Other tissues may be highly dynamic and require rapid morphogenetic cell-cell rearrangements, which may have profound effects on the trafficking pathways that function in these cells. Indeed, although the length of this review precluded further discussion, membrane trafficking pathways have further layers of rich interactions in guiding PCP function in vertebrate development. Membrane trafficking is essential in the generation of planar arrays of cilia that direct cell signaling, convergent extension movements, notochord development, and the generation of left-right asymmetry.86,87,88 For example, the function of a Rab8-dependent exocytic trafficking pathway required for primary ciliogenesis has been particularly well established.89,90,91 Additionally, membrane trafficking pathways can ensure equal partitioning of PCP proteins and aid in the restoration of planar polarities after cells round up and temporarily lose planar polarity during cell division.92 These are just a few further examples of the intimate connections between trafficking networks and planar polarity. As our cell biological understanding of these processes advances, it will be interesting to compare their final outcomes to those that occur in model systems of planar polarity such as the Drosophila embryo and wing.
Brief synopsis statement.
Our understanding of how membrane trafficking pathways function to direct morphogenetic movements and the planar polarization of developing tissues is a new and emerging field. While a central focus of developmental biology has been on how protein asymmetries and cytoskeletal force generation direct cell shaping, the role of membrane trafficking in these processes has been less clear. Here, we review recent advances in our understanding of how trafficking events are coordinated with planar polarity and cytoskeletal function to drive lasting changes in cell and tissue topologies.
Acknowledgments
We thank members of the Blankenship for critical reading and constructive comments on the manuscript. This study was supported by grants from the NIH NIGMS (grant numbers R01 GM090065 and R15 GM126422).
Footnotes
Conflict of Interest
None declared.
References
- 1.Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nature cell biology. 2012;14(12):1235. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X, Surma MA, Simons K. Polarized sorting and trafficking in epithelial cells. Cell research. 2012;22(5):793. doi: 10.1038/cr.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasky AJ, Mangan A, Prekeris R. Polarized protein transport and lumen formation during epithelial tissue morphogenesis. Annual review of cell and developmental biology. 2015;31:575–591. doi: 10.1146/annurev-cellbio-100814-125323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207(2):171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129(6):1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Maung SMTW, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7(3):165–179. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Developmental biology. 2007;302(1):181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annual review of genetics. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Seminars in cell & developmental biology. 2009;20(8):964–971. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Seminars in cell & developmental biology. 2008;19(3):263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang CF, Munro E. The PAR proteins: from molecular circuits to dynamic self-stabilizing cell polarity. Development. 2017;144(19):3405–3416. doi: 10.1242/dev.139063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nance J. Getting to know your neighbor: cell polarization in early embryos. J Cell Biol. 2014;206(7):823–832. doi: 10.1083/jcb.201407064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson LA, Joshi SD, Kim HY, Von Dassow M, Zhang L, Zhou J. Emergent morphogenesis: elastic mechanics of a self-deforming tissue. Journal of biomechanics. 2010;43(1):63–70. doi: 10.1016/j.jbiomech.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449(7165):1049. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 16.Davidson L, Keller R. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126(20):4547–4556. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Lewis AE, Singh V, Ma X, Adelstein R, Bush JO. Convergence and extrusion are required for normal fusion of the mammalian secondary palate. PLoS biology. 2015;13(4):e1002122. doi: 10.1371/journal.pbio.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau K, Tao H, Liu H, et al. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nature cell biology. 2015;17(5):569. doi: 10.1038/ncb3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annual review of genetics. 2001;35(1):747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 20.Irvine KD, Wieschaus E. fringe, a Boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell. 1994;79(4):595–606. doi: 10.1016/0092-8674(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 21.Keller R, Davidson L, Edlund A, et al. Mechanisms of convergence and extension by cell intercalation. Philosophical Transactions of the Royal Society B. Biological Sciences. 2000;355(1399):897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Developmental cell. 2002;2(6):695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 23.Nikolaidou KK, Barrett K. Getting to know your neighbours; a new mechanism for cell intercalation. Trends in Genetics. 2005;21(2):70–73. doi: 10.1016/j.tig.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Current biology. 2005;15(6):R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Developmental cell. 2004;6(3):343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 26.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429(6992):667. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 27.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Developmental cell. 2006;11(4):459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Rauzi M, Lenne P-F, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468(7327):1110. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Gonzalez R, Zallen JA. Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Physical biology. 2011;8(4):045005. doi: 10.1088/1478-3975/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vichas A, Zallen JA. Translating cell polarity into tissue elongation. Seminars in cell & developmental biology. 2011;22(8):858–864. doi: 10.1016/j.semcdb.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecuit T, Lenne P-F, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annual review of cell and developmental biology. 2011;27:157–184. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- 32.Kong D, Wolf F, Großhans J. Forces directing germ-band extension in Drosophila embryos. Mechanisms of development. 2017;144:11–22. doi: 10.1016/j.mod.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nature cell biology. 2011;13(5):529. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]
- 34.Jewett CE, Vanderleest TE, Miao H, et al. Planar polarized Rab35 functions as an oscillatory ratchet during cell intercalation in the Drosophila epithelium. Nature communications. 2017;8(1):476. doi: 10.1038/s41467-017-00553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeffer SR. Structural clues to Rab GTPase functional diversity. Journal of Biological Chemistry. 2005;280(16):15485–15488. doi: 10.1074/jbc.R500003200. [DOI] [PubMed] [Google Scholar]
- 36.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proceedings of the National Academy of Sciences. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly EE, Horgan CP, Goud B, McCaffrey MW. The Rab family of proteins: 25 years on. Portland Press Limited; 2012. [DOI] [PubMed] [Google Scholar]
- 38.Langevin J, Morgan MJ, Rossé C, et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Developmental cell. 2005;9(3):365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Blankenship JT, Fuller MT, Zallen JA. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. Journal of Cell Science. 2007;120(17):3099–3110. doi: 10.1242/jcs.004770. [DOI] [PubMed] [Google Scholar]
- 40.Roeth JF, Sawyer JK, Wilner DA, Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS One. 2009;4(10):e7634. doi: 10.1371/journal.pone.0007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin–myosin network drive apical constriction. Nature. 2009;457(7228):495. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauzi M, Verant P, Lecuit T, Lenne P-F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature cell biology. 2008;10(12):1401. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 43.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137(7):1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 44.Sawyer JK, Choi W, Jung K-C, He L, Harris NJ, Peifer M. A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Molecular biology of the cell. 2011;22(14):2491–2508. doi: 10.1091/mbc.E11-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson LA. No strings attached: new insights into epithelial morphogenesis. BMC biology. 2012;10(1):105. doi: 10.1186/1741-7007-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annual review of cell and developmental biology. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 47.Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141(10):1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heer NC, Martin AC. Tension, contraction and tissue morphogenesis. Development. 2017;144(23):4249–4260. doi: 10.1242/dev.151282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Developmental biology. 1984;105(2):257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- 50.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71(3):171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 51.Shook D, Keller R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mechanisms of development. 2003;120(11):1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Lee J-Y, Harland RM. Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Current Biology. 2010;20(3):253–258. doi: 10.1016/j.cub.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelissier A, Chauvin J-P, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Current biology. 2003;13(21):1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 54.Fabrowski P, Necakov AS, Mumbauer S, et al. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nature communications. 2013;4:2244. doi: 10.1038/ncomms3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ossipova O, Kim K, Lake BB, Itoh K, Ioannou A, Sokol SY. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nature communications. 2014;5:3734. doi: 10.1038/ncomms4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ossipova O, Chuykin I, Chu C-W, Sokol SY. Vangl2 cooperates with Rab11 and Myosin V to regulate apical constriction during vertebrate gastrulation. Development. 2015;142(1):99–107. doi: 10.1242/dev.111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich F, Krieg M, Schötz E-M, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Developmental cell. 2005;9(4):555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Song S, Eckerle S, Onichtchouk D, Marrs JA, Nitschke R, Driever W. Pou5f1-dependent EGF expression controls E-cadherin endocytosis, cell adhesion, and zebrafish epiboly movements. Developmental cell. 2013;24(5):486–501. doi: 10.1016/j.devcel.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milán M, Campuzano S, García-Bellido A. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proceedings of the National Academy of Sciences. 1996;93(2):640–645. doi: 10.1073/pnas.93.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milán M, Campuzano S, García-Bellido A. Cell cycling and patterned cell proliferation in the Drosophila wing during metamorphosis. Proceedings of the National Academy of Sciences. 1996;93(21):11687–11692. doi: 10.1073/pnas.93.21.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Classen A-K, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Developmental cell. 2005;9(6):805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Wootton RJ. Functional morphology of insect wings. Annual review of entomology. 1992;37(1):113–140. [Google Scholar]
- 63.Adler PN. Planar signaling and morphogenesis in Drosophila. Developmental cell. 2002;2(5):525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 64.Eaton S. Cell biology of planar polarity transmission in the Drosophila wing. Mechanisms of development. 2003;120(11):1257–1264. doi: 10.1016/j.mod.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Developmental cell. 2002;3(6):851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 66.Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Seminars in cell & developmental biology. 2002;13(3):217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 67.Fristrom D, Fristrom JW. The mechanism of evagination of imaginal discs of Drosophila melanogaster: I. General considerations. Developmental biology. 1975;43(1):1–23. doi: 10.1016/0012-1606(75)90127-x. [DOI] [PubMed] [Google Scholar]
- 68.Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. The Journal of cell biology. 1996;135(5):1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mechanisms of development. 1998;70(1–2):181–192. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- 70.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Developmental cell. 2006;10(2):209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. Elife. 2014;3 doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harumoto T, Ito M, Shimada Y, et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Developmental cell. 2010;19(3):389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvajal-Gonzalez JM, Balmer S, Mendoza M, et al. The clathrin adaptor AP-1 complex and Arf1 regulate planar cell polarity in vivo. Nature communications. 2015;6:6751. doi: 10.1038/ncomms7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Usui T, Shima Y, Shimada Y, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98(5):585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 75.Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Developmental cell. 2001;1(1):93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 76.Shimada Y, Usui T, Yanagawa S-i, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Current Biology. 2001;11(11):859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 77.Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Molecular cell. 2001;7(2):367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 78.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130(13):3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 79.Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Developmental cell. 2011;20(4):511–525. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mottola G, Classen A-K, González-Gaitán M, Eaton S, Zerial M. A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development. 2010;137(14):2353–2364. doi: 10.1242/dev.048413. [DOI] [PubMed] [Google Scholar]
- 81.Chen W-S, Antic D, Matis M, et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133(6):1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho B, Pierre-Louis G, Sagner A, Eaton S, Axelrod JD. Clustering and negative feedback by endocytosis in planar cell polarity signaling is modulated by ubiquitinylation of prickle. PLoS genetics. 2015;11(5):e1005259. doi: 10.1371/journal.pgen.1005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gault WJ, Olguin P, Weber U, Mlodzik M. Drosophila CK1-γ, gilgamesh, controls PCP-mediated morphogenesis through regulation of vesicle trafficking. J Cell Biol. 2012;196(5):605–621. doi: 10.1083/jcb.201107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riggs B, Rothwell W, Mische S, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163(1):143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermle T, Guida MC, Beck S, Helmstädter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. The EMBO journal. 2013;32(2):245–259. doi: 10.1038/emboj.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adler PN, Wallingford JB. From planar cell polarity to ciliogenesis and back: the curious tale of the PPE and CPLANE proteins. Trends in cell biology. 2017;27(5):379–390. doi: 10.1016/j.tcb.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahaffey JP, Grego-Bessa J, Liem KF, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140(6):1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Molecular biology of the cell. 2013;24(5):555–565. doi: 10.1091/mbc.E12-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshimura S-i, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. The Journal of cell biology. 2007;178(3):363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 91.Knödler A, Feng S, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proceedings of the National Academy of Sciences. 2010;107(14):6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nature cell biology. 2011;13(8):893. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]