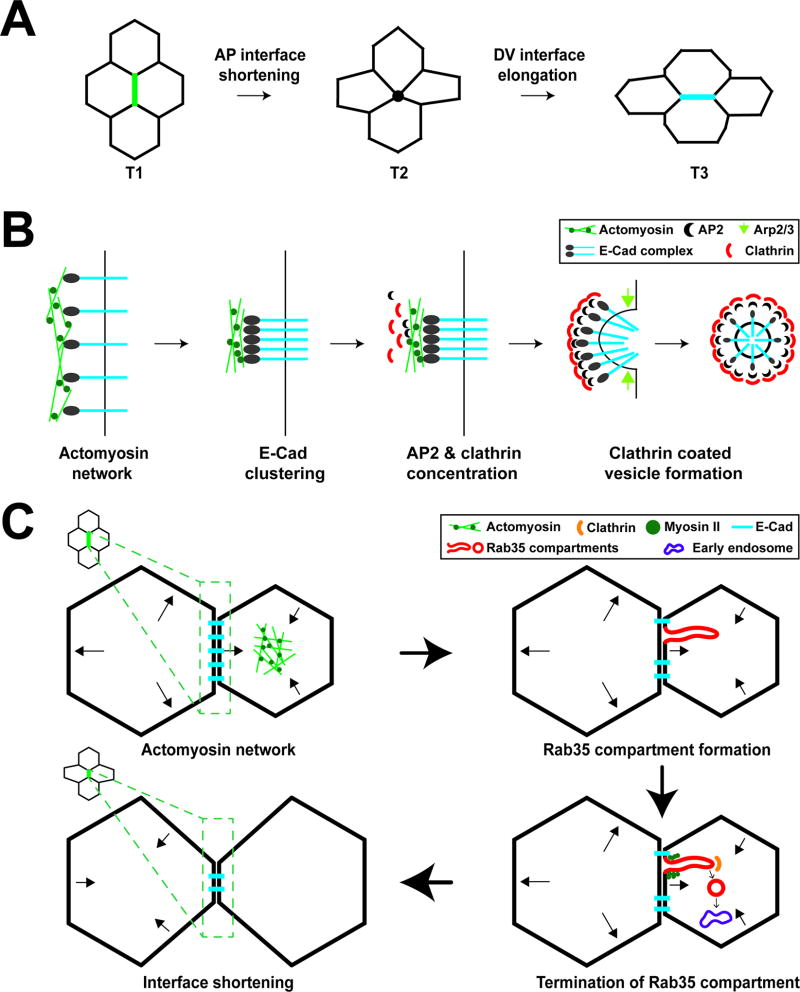
FIGURE 1.
Membrane trafficking directs changes in cell shape during early Drosophila embryogenesis. (A) Diagram of cell intercalation and T1–T3 transitions during Drosophila germband extension. At T1 interfaces, the anterior-posterior (AP) interface shrinks to a four-cell vertex (T2), followed by new interface formation and elongation of a dorsal-ventral interface (T3). (B) E-cadherin uptake during AP interface shortening. Actomyosin contraction drives E-cadherin clustering. AP2 and Clathrin are enriched at AP interfaces and endocytose clustered E-cadherin. The scission of vesicles from AP interfaces is driven by Arp2/3 complex function. (C) Membrane dynamics and interface ratcheting during cell intercalation. Cell areas oscillate through apical Myosin II function. During area oscillations, Rab35 compartments form to efficiently take up plasma membrane and cargo proteins, leading to processively shortened AP interfaces. Myosin II and Clathrin-mediated endocytosis are required to terminate Rab35 compartments and direct endocytosed material to Rab5 early endosomes leading to irreversible interface contraction.