Abstract
Phagocytosis is an essential step in the innate immune response to invasive fungal infections. This process is carried out by a proverbial “village” of professional phagocytic cells, which have evolved efficient machinery to recognize and ingest pathogens, namely macrophages, neutrophils and dendritic cells. These innate immune cells drive early cytokine production, fungicidal activity, antigen presentation and activation of the adaptive immune system. Despite the development of antifungal agents with potent activity, the biological activity of professional phagocytic innate immune cells has proven indispensable in protecting a host from invasive fungal infections. Additionally, an emerging body of evidence suggests non-professional phagocytes, such as airway epithelial cells, carry out phagocytosis and may play a critical role in the elimination of fungal pathogens. Here, we review recent advances of phagocytosis by both professional and non-professional phagocytes in response to fungal pathogens, with a focus on invasive aspergillosis as a model disease.
Introduction
Fungal diseases cause a tremendous burden of morbidity and mortality. Invasive fungal infections (IFI) account for more than one million deaths annually, and more than 50% of deaths in patients with HIV/AIDS.1–3 The burden of fungal infections remains significant in the setting of HIV/AIDS, and continues to rise with the use of immunosuppression in cancer chemotherapy, treatment of rheumatologic diseases, solid organ and hematologic transplants. Fungal infections have been increasingly recognized as a complicating factor following other insults such as trauma or influenza virus infection.4–6 Despite the availability of diagnostic tools and antifungal medications, the mortality of IFI often remains in excess of 50%.3 In addition to antifungal therapy, there is clear evidence that an appropriately functioning immune system is a prerequisite for a favorable clinical outcome.7,8 Furthermore, fungal infections are being recognized as significant contributors to a growing burden of morbidity through non-invasive processes such as keratitis and allergic diseases such as asthma and allergic bronchopulmonary aspergillosis.3,9
Professional phagocytic cells (macrophages, neutrophils and dendritic cells), as well as less traditional phagocytic cells, such as airway epithelium, play a vital role in protecting the host from daily fungal exposure through the direct act of engulfment or phagocytosis. The interactions of phagocytic cells with fungal pathogens has been previously reviewed elsewhere for both typical phagocytes10–12 and airway epithelial cells.13–15 Here, we will provide an updated review on advances in phagocyte-fungal interactions with a focus on the most common pathogens, Candida and Aspergillus. We will also review our expanding understanding of the role of airway epithelium as a potential phagocyte in the host-pathogen interactions with invasive pulmonary fungal pathogens.
Macrophages
Macrophages play a critical role in the early response to fungal infections, helping both contain pathogenic organisms and recruit additional immune cells for further response. These phagocytic cells are present in many tissues of the body. Macrophages utilize pattern recognition receptors to identify the presence of conserved pathogen-associated molecular patterns (PAMPs) exposed on microbial surfaces. Fungal cell wall carbohydrates are the dominant PAMPs recognized by macrophage-associated pattern recognition receptors (PRRs).16,17 This observation has been borne out in several animal models including zebrafish, where macrophages were crucial to the early response to A. fumigatus, with neutrophil recruitment occurring later.18,19
While phagocytosis by macrophages is a critical step in the response to some fungal pathogens, this process is not a uniform feature of macrophage responses to all fungal pathogens. Despite participating in Dectin-1 mediated cytokine production, RAW 264.7 macrophages and C57BL/6 primary bone marrow derived macrophages are unable to phagocytose Exserohilum spores or hyphae owing to their large size.20 In this study, Dectin-1 KO mice and wild-type mice injected with Exserohilum spores had similar neutrophilic tissue infiltration and granuloma formation with multinucleated giant cells; no difference in hyphal growth was observed. Macrophages interacting with Exserohilum demonstrated robust recruitment of Dectin-1 to the site of interaction with fungal hyphae, and were associated with high levels of TNF-α production consistent with frustrated phagocytosis. The molecular mechanisms contributing to enhanced signaling and consequently, increased cytokine production is, in part, due to β-1,3-glucan clustering of Dectin-1 and the exclusion of the phosphatases, CD45 and CD148, that allows for downstream macrophage signaling.21 Similar to the failure of ingesting a large fungal pathogen, the inability to phagocytose β-1,3-glucan particles by chemical inhibition results in increased levels of TNF-α production.22,23
These data support a model where phagocytosis can act as a modulator of immune activation, variably promoting or terminating inflammatory signaling depending on the host-pathogen interactions and conditions.23
Multiple pathways contribute to activation and phagocytosis of macrophages in response to fungal pathogens. Signaling through Dectin-1 via spleen tyrosine kinase (syk) promotes macrophage TNF-α production. Maximal production of TNF-α by macrophages in response to A. fumigatus, however, requires phagocytosis dependent signaling through the TLR9-BTK-calcineurin-NFAT pathway.19 Human macrophages also activate the calcineurin-NFAT signaling pathway in response to A. fumigatus stimulation.24 Emerging evidence also suggests that components of the ubiquitin-ligase pathway contribute to modulation of the Dectin-1/2 signaling pathway in macrophages. The RING-finger-type E3 ubiquitin ligase Casitas B lymphoma-b (CBLB) provides negative regulation of Dectin-1/2 signaling. CBLB−/− mice were relatively protected in a model of disseminated candidiasis.25 CBLB was found to regulate Dectin-1 expression and ROS production without impacting phagocytosis, suggesting additional fungal phagocytic receptors are regulated independent of CBLB.
Interestingly, when macrophages fail to eliminate phagocytosed fungal pathogens, the fungi can undergo transfer to adjacent host cells. Macrophages that phagocytosed A. fumigatus conidia but were unable to eliminate the cargo can undergo necroptosis and laterally transfer conidia to another macrophage; this conidial lateral transfer is calcineurin-dependent and likely mediated by actin polymerization by vasodilator-stimulated phosphoprotein (VASP).24 Taken together, these findings suggest that independent pathways exist for coordinating the macrophage response to fungal pathogens depending on the capacity to phagocytose fungi.
In order to achieve efficient phagocytosis and processing of fungal pathogens, macrophages mature their phagosomes through the recruitment of organelles including lysosomes and additional host protein machinery essential for the elimination of captured fungal cargo. The nature of the cargo appears to influence recruitment of specific host phagosome proteins (MKM and JMV, unpublished observations). This ability to tailor the phagosomal host repertoire relies, in part, on the PRR engaged at the initial encounter of the fungal cargo. Dectin-1-syk-dependent signaling is responsible for phagosomal maturation, and blockage of this axis results in phagolysosmal maturation arrest.26 More recently, macrophages have been shown to utilize proteins related to the autophagy pathway.27 Dectin-1 activation is linked to LC3 recruitment to fungal phagosomes via syk activation.28 Recruitment of LC3 by NADPH-ROS generation in primary bone marrow macrophages promotes killing of C. albicans.29 LC3 is also recruited to fungal phagosomes in human monocytes containing live, but not killed, A. fumigatus conidia indicating that macrophages are capable of sensing and continually modifying specific responses well after cargo has been introduced into a phagosome.30
Fungal pathogens have evolved mechanisms to escape macrophage phagocytosis. Remodeling of the C. albicans fungal cell wall following phagocytosis exposes chitin, which induces host-arginase-1 production leading to decreased nitric oxide production through iNOS.31 C. albicans was found to actively alkalinize acidic nutrient-poor environments through an Stp2p transcription factor mediated pathway which in turn promotes hyphal morphogenesis.32 Additionally, the Candida Ahr1p transcription factor is involved in controlling alkalization of macrophage phagosomes, which, in turn, is important for driving macrophage IL-1β expression and pyroptosis.33 Direct manipulation of epithelial cell membrane integrity was recently identified through a secreted C. albicans protein, candidalysin.34 Emerging evidence indicates that candidalysin may also alter macrophage membrane integrity.35 The impact of C. glabrata is capable of efficient survival and replication within macrophage phagosomes, in part through maintenance of a neutral pH despite strong recruitment of VTPases to the phagosomal membrane.36 Recent deletion libraries demonstrate that the mutants lacking in mannosyltransferases are unable to resist acidification suggesting pathogen-specific post-translational medications may play a role in shaping the phagosomal milieu.37,38 Upregulation of key nutrient transporters are essential for survival in phagocytes.39–42 These pathogen-driven maneuvers can allow phagosomal escape and evasion of the fungicidal mechanisms employed by macrophages. The clinical significance of these resulting latent fungal reservoirs is yet to be determined.
Neutrophils
Neutrophils are the most abundant phagocyte of the innate immune system, and serve a primary role in the recognition and elimination of many fungal pathogens. In addition, they possess a wide breadth of functions now recognized to play critical roles in the fungal defense. They generally lack the ability to serve as efficient antigen presenting cells, although observation of neutrophil subpopulations capable of expressing major histocompatibility complex (MHC) have been made.43–47 Additionally, it was recently observed that activated neutrophils can present antigen to cognate memory CD4+ T cells through MHC class II.44 That said, the lack of sufficient neutrophil numbers or neutropenia is a widely recognized risk factor for the acquisition of invasive fungal infections. Furthermore, defects in neutrophil phagosome function including defects in NADPH oxidase, such as chronic granulomatous disease, are also risk factors for fungal colonization and disease. A variety of animal models demonstrate efficient neutrophil chemotaxis with rapid extravasation to the site of fungal infections. 48–51 To correct deep neutropenia multiple trials using granulocyte transfusions have been attempted. Despite these efforts, the results have been largely disappointing without a clear signal of protection. A recent trial of high-dose granulocyte transfusions in patients with severe neutropenia demonstrated no improvement in microbial response with the addition of these transfusions to standard antimicrobial therapy.52 The absence of a therapeutic indication is thought to be related to rapid apoptosis and loss of antifungal activity when neutrophils are collected ex vivo.53,54 One novel approach to improve the fungicidal efficacy of neutrophils is to use them as a drug delivery tool. HL-60 cells, a leukemic neutrophil-like cell, loaded with posaconazole, an extended-spectrum azole, were able to limit A. fumigatus hyphal growth in vitro, and could reduce fungal burden in an in vivo murine model of neutropenic invasive pulmonary aspergillosis in comparison to untreated cells.55 This approach of cell-directed chemotherapy has the potential to improve antifungal treatment while minimizing drug toxicity. Neutrophil killing of A. fumigatus is mediated, in part, by GM-CSFRβ signaling, and addition of recombinant GM-CSF can promote increased fungal clearance from the lungs of WT mice.56 IL-15 produced by Ly6Chigh monocytes in response to type 1 interferons and Dectin-1 signaling promotes NK-cell production of GM-CSF, in turn, activating neutrophils phagocytic and fungicidal activity against C. albicans.57
Neutrophils utilize various pathways to target their cytotoxic effects in response to distinct fungal forms. Furthermore, the signaling pathways and receptors utilized by neutrophils to promote killing can be distinct than those utilized by dendritic cells (DCs) and macrophages. For example, human neutrophils utilize FcγRII receptors to recognize IgG-opsonized A. fumigatus hyphal cell wall for fungicidal activity.58 Following recognition, neutrophil killing activity relied critically on NADPH oxidase and myeloperoxidase (MPO) for hyphal elimination. In contrast, the germination of conidia in human neutrophils appeared primarily to be related to iron starvation via lactoferrin, was independent of MPO activity, and A. fumigatus hyphal killing was reliant on a PKCα/β-dependent, Dectin-1-independent pathway.58 In stark contrast, neutrophil killing of C. albicans is predominantly through ROS generation, and the degranulation response is dependent on Dectin-1 and Mac-1-activation of downstream PKC-δ signaling.59 Response pathways to both fungal species is through CARD9-independent signaling, which stands in contrast to the β-1,3-glucan-recognition pathways described in DCs and macrophages. Additionally, engagement of the chemokine receptor, CXCR1, is also important for neutrophil degranulation following C. albicans exposure in both humans and mice.51 Contact to neutrophil chemoattractants such as fMLP, LTB4 and IL-8 increases neutrophil fungicidal activity.60
In addition to killing fungi via phagocytosis and nutrient sequestration, neutrophils can create neutrophil extracellular traps (NET or NETosis) as a final hyphal control mechanism, which will be reviewed in this special issue.61,62 Interestingly, a neutrophil commitment to engage a target by phagocytosis vs NETosis appears to be driven by a size discrimination.63 In this study, phagocytosis of smaller fungal particles recruited neutrophil elastase to the phagolysosome.
Neutrophil interactions with larger hyphal components failed to recruit neutrophil elastase to the phagolysosome and, instead, shows elastase localizing with the nucleus where chromatin decondensation and histones undergo proteolytic cleavage. The contribution of NETs to fungal control remains controversial; some studies have suggested minimal contribution to A. fumigatus hyphal killing while other studies suggest NETs may play a role in fungal commensal maintenance. NET formation in the mucosal lumen has been shown to assist in control of C. albicans invasion of zebrafish swimbladders, suggesting this process contributes to commensal relationship between host and pathogen and may have implications for diseases such as vulvovaginal candidiasis.64 Mice deficient in the oxidase enzyme required for NET formation are more susceptible to mucosal candidiasis.63,65,66 Additionally, Aspergillus strains that produced large amounts of cell wall galactosaminogalactan (GAG) had increased virulence and increased resistance to the direct damage caused by the neutrophil contents within NETs.67 Finally, immunomodulating agents directly impact neutrophil function. In neutrophils isolated from patients following hematopoietic stem cell transplantation, impairment of Aspergillus growth correlated with calcineurin-inhibitor levels and decreased NET formation.68
Beyond their action in killing fungal pathogens, neutrophils are capable of inhibiting fungal growth through the deprivation of essential nutrients. Neutrophil-derived calprotectin contained in NETs was shown to contribute to the control of C. albicans abscesses in mice.69 Neutrophil-derived calprotectin, a heterodimer of S100A8 and S100A9, limits A. fumigatus hyphal growth through the chelation of extracellular zinc and manganese in a corneal infection model. Interestingly, S100A9 −/− neutrophils show no difference in A. fumigatus conidial killing and bone marrow chimeric mice with this mutation had no difference in response to invasive pulmonary aspergillosis suggesting fungi either possess countermeasures to these specific host nutrient deprivation mechanisms, or that the contributions of these mechanisms are dependent on the site of infection within the host.70
Neutrophil clearance of fungi is likely a balance between unique intracellular signaling pathways, relative contributions of selected fungicidal killing mechanisms, all of which are dictated by the organism, fungal morphotype encountered, and host context.
Dendritic Cells
DCs serve as a bridge between the innate and adaptive immune systems. Conventional dendritic cells (cDCs) are highly-efficient antigen-presentation cells. DCs display a large array of PRR including the Toll-Like receptors (TLRs) and C-type lectin receptors (CLR). Locally activated DCs are highly capable phagocytic cells. Following phagocytosis at peripheral sites of infection, DC then migrate to lymphoid organs and, subsequently activate cognate T-cells directing T-cell commitment linage towards T-helper (TH) subsets including TH1, TH2 and TH17 cells.
A TH17 cell response is critical in the response to invasive pulmonary aspergillosis.71 It was recently shown that CD103+ murine DCs are able to regulate TH17 differentiation in response to A. fumigatus.71 DC are capable of increasing IL-2 production in response to stimulation with whole-β-1,3-glucan particles, resting conidia, swollen conidia and Aspergillus hyphae.71,72 This process can be interrupted through inhibition of phagocytosis with cytochalasin D implicating phagocytosis as a necessary prerequisite for cytokine production. DC-specific IL-2−/− mice displayed an increase in TH17 cells and an increased mortality compared to wild type mice suggesting that interaction and phagocytosis of fungal targets serves as TH17 compartment modulator. The mechanism of IL-2-dependent modulation was found to be related to production of IL-23 with subsequent overexpansion of the TH17 population. Notably, the increase in production of IL-2 appears to be driven by Dectin-1 signaling through a MyD88 independent manner.71
Calcineurin-dependent signaling through the Nuclear Factor of Activated T-cells (NFAT), a transcription factor family implicated in innate immunity, appears to be critical in the dectin-1 mediated response of DCs to fungal pathogens. Calcineurin inhibitors commonly used for immunosuppression following hematopoietic stem cell transplant (HSCT) have been linked to an increased risk of invasive fungal disease independent of neutropenia.18,73 Similarly to Aspergillus, calcineurin signaling is critical in the murine response to Candida.74 In addition to the whole pathogen, fungal cell wall components, including β-1,3-glucan, binding to Dectin-1 is capable of calcineurin-dependent NFAT signaling in DC and macrophages.75 Likewise, CD11c-conditional knockout of the calcineurin B1 subunit (cnb1) in murine bone marrow derived DC did not upregulate IL-2 or long-pentraxin 3 proteins in response to whole-β-1,3-glucan particles. Cnb1 conditional knockout mice had increased mortality to A. fumigatus infection.76 These findings suggest that suppression of DC signaling by calcineurin inhibitors such as tacrolimus (FK506) may be a pathway-specific risk factor resulting in the increased risk of fungal infection in following HSCT. Interestingly, human plasmacytoid DC (pDC), utilize Dectin-2 as the primary c-type lectin receptor triggered in response to A. fumigatus hyphae. Blocking antibodies to Dectin-1 had minimal effect on the production interferon-α and TNF-α by human pDCs following stimulation by A. fumigatus hyphae; whereas anti-Dectin-2 antibody suppressed production of cytokines.77 Ligation of dectin-2 activates NFAT through Syk-CARD9.77,78 While human pDCs also express TLR9, cytokine response to A. fumigatus hyphae appears to occur in a TLR9-independent manner.79 These observations reinforce the concept that the primary roles of pDCs is in regulating T-cell response towards fungal pathogens.
pDCs are capable of directly inhibiting the growth of multiple fungal organisms. When stimulated by Aspergillus hyphae, DCs can participate in extracellular trap formation in a manner usually associated with neutrophils.77 Murine pDCs can directly inhibit the growth of Cryptococcus neoformans via ROS production in a Dectin-3-dependent manner.80 Despite the Dectin-3-mediated fungicidal activity, the physiological significance of direct fungal inhibition by pDC is unclear. pDCs are relatively rare, representing only 0.2% to 0.8% of the peripheral blood mononuclear cells in circulation.81 In addition, Dectin-3 knockout mice had no change in survival compared to wild-type C57/Bl6 mice in a pulmonary C. neoformans challenge model.80 pDCs express a number of different mannan receptors including Dectin-2, DC-SIGN, Dectin-3 and CD206 that could contribute to fungal recognition and elimination. Dectin-2 knockout mice demonstrate increased levels of the TH2-related cytokines IL-4, IL-5 and IL-13 in response to C. neoformans suggesting that Dectin-2 may be important for tuning the TH1/TH2 response axis.82 CCR2+ monocytes and monocyte derived-DC were shown to promote neutrophil-mediated killing of A. fumigatus conidia; these CCR2+ monocytes and monocyte derived-DC played an important direct role in NADPH-oxidase mediated clearance of Aspergillus in a mouse model of invasive pulmonary aspergillosis.83
Functional genomic studies revealed that the type 1 interferon pathway plays a critical role in the host response to Candida infection.84 pDCs are capable of producing up to 1,000-fold more type I interferon than other cell types and potentially serve as the primary cell source.85,86 Single-cell RNA sequencing (scRNA-seq) is a next-generation sequencing approach that allows for gene expression profiling and clustering analysis of subpopulations within a heterogeneous cluster of cells. Using scRNA-seq it was recently identified that the population of cells previously reported as pDCs is likely a mixed population of phenotypically similar but functionally distinct cells. While a population of CD11C−/CD123+ pDCs was identified, a separate subset population of AXL+SIGLEC6+CD123+CD11C−/lo cells (AS DCs) was also noted.87 This re-identified pDC population produced significant amounts of interferon-α in response to TLR9 stimulation with CpG, however the AS DCs produced minimal interferon-α in response to the same stimulus suggesting this population, while sharing some features with pDC, serves a yet to be defined function. Notably, the T-cell stimulating role of pDCs appeared to be primarily the role of AS DCs and not of the general pool of pDCs.87
Airway epithelial cells
The airway epithelium is a complex tissue consisting of multiple cell types, where the relative populations of various cells differs with position in the airway tree. The bronchial airways are lined with a diverse pseudostratified epithelium including ciliated, club and secretory, goblet, other less common cell types such as brush, and airway basal cells that serve as stem cell populations in the conducting airways. The alveolar epithelium consists of type I and type II pneumocytes, with type II pneumocytes serving as the regenerative stem cell following airway injury.88,89 Airway epithelial cells are immunologically active and contribute to the initiation of TH1, TH17 and TH2 mediated immune responses.90 The airway epithelium of the small airway and alveoli are the first point of contact for inhaled Aspergillus conidia. The role of airway epithelium as an immunologically active tissue in response to A. fumigatus has been recently reviewed 13 so here we will focus particularly on the potential role of airway epithelial cells as non-traditional phagocytes.
The ability of Aspergillus to specifically adhere to the airways contributes to invasive infection (reviewed in 91). A. fumigatus, the major cause of invasive pulmonary aspergillosis, has an increased ability to bind to the airway basal lamina and fibronectin.92 Aspergillus conidia are also capable of adhering to A549 cells, a hypotriploid lung adenocarcinoma cell line used as a model for alveolar epithelium. 93 Viruses such as influenza can damage the airway epithelium and expose the basement membrane for binding by pathogens.94 Interferon-γ has been shown to promote Aspergillus adherence to A549 cells.95 Negatively-charged carbohydrates, fucose-specific Lectin A (FleA), and the protein AfCalAp have been implicated in Aspergillus conidial adherence to airways. 92,96,97 The Aspergillus fungal cell wall component, galactosaminogalactan, also contributes to fungal adherence to A549 cells.98,99 Sialic acid-containing moieties promote adherence of Aspergillus to airway epithelial cells while reducing basal lamina binding.100–102 Opsonization by secreted proteins such as, H-ficolin, a soluble lectin-like opsonin produced by airway epithelial cells can bind to Aspergillus conidia resulting in adherence to respiratory epithelium.103 Additional work is required to determine if a role exists for β-glucan-recognizing receptors recently described in oral epithelial cells, such as EphA2 104, in the bronchopulmonary system.
A number of in vitro models have demonstrated that airway epithelial cells are capable of phagocytosing A. fumigatus conidia. However, the phagocytic activity of airway epithelium in vivo remains controversial; differences between in vitro and in vivo observations may be due in part to recapitulating and modifying normal airway epithelium in culture. It has been hypothesized that airway epithelial cells may serve as a space where Aspergillus conidia are protected from patrolling immune cells allowing for hyphal germination.105–107 Phagocytosis by non-ciliated rabbit tracheoepithelial cells and rat alveolar type II cells has been observed in liquid culture.106 A549 cells and 16HBE14o-cell monolayers (a transformed cell line model of human bronchial epithelium) are capable of phagocytosing Aspergillus conidia through an actin-dependent polymerization process.107–109 A number of genes involving in actin cytoskeleton rearrangement contribute to conidial phagocytosis as demonstrated by gene microarrays and bulk RNAseq.109,110 Stimulation with β-1,3-glucan alters the ratio between non-phosphorylated and phosphorylated phospholipase D activation states suggesting Dectin-1 also plays a role in conidial internalization.111 The Aspergillus transcription factor PacC, which plays a role in cell wall remodeling, contributes to conidial internalization by airway epithelium as pacCΔ A. fumigatus mutant conidia demonstrated decreased Dectin-1 dependent internalization into A549 cells.112 Cofilin-1 is an actin modulating protein involved in the maintenance and regulation of apical junctional complexes in epithelial barriers.113 Phosphorylation of cofilin-1 contributes to internalization of Aspergillus conidia. Cofilin phosphorylation were not reduced by pre-treatment with a blocking anti-dectin-1 antibody pointing towards engagement of alternative phagocytic mechanism.114 Aspergillus conidia phagocytosed by A549 cells or primary human nasal epithelium are passed through to mature phagolysosomes marked by LAMP-1.107,115
In vivo, there remains no clear evidence of phagocytosis of Aspergillus conidia by airway epithelium. Using transmission electron microscopy of bronchial epithelial cells in immunosuppressed mice, conidia localized to epithelial cell junctions, but internalization was not observed.116 Airway epithelial cells express a range of PRR, which could likely facilitate the phagocytosis of Aspergillus conidia. Signaling through TLR-2 increases Dectin-1 expression in airway epithelial cells.117 Activation of TLR-3, which recognizes dsRNA, results in the release of interferon-β and IP-10/CXCL-10 from airway epithelial cells in response to resting conidia through a pathway that is partially dependent on NFκB-signalling.118 Recently, it was recognized that Dectin-1 binding promotes TLR9 recruitment to the phagosome during the recognition of Aspergillus conidia.119 The role of TLR9 in airway epithelial cells has yet to be defined.
Ultimately, our understanding of the role of airway epithelial cells and alveolar epithelial cells is limited by the availability of airway models. Research in this area has primarily been conducted in the A549 adenocarcinoma cell line. It is unclear how these findings translate to primary human cells who may be at risk for invasive fungal disease. The lack of conidial phagocytosis in vivo suggests that findings in an in vitro immortalized cell line may not completely recapitulated true disease. Studies in primary human airway epithelium are complicated by a number of factors including 1) challenges in obtaining primary cells, 2) the complex interplay of multiple cell types in the tissue, and 3) the functional limitations of working in air-liquid interface. Additionally, recapitulating and manipulating the alveolar organoid in vitro represents an additional set of challenges. Overcoming these challenges to experiment directly in primary human airway epithelium will be critical in understanding the early steps in establishing fungal lung diseases.
Conclusions
Phagocytosis is a basic tenant of innate immune cells that play a fundamental role in the detection of and defense from invasive fungal diseases. Professional phagocytes, such as macrophages, neutrophils and DC are effector arms of the innate immune system clearing fungal pathogens. They are necessary for the production of key cytokines to support the innate immune response and possess potent fungicidal activity. Despite serving as the first-line tissue defense to inhaled fungal pathogens, the role of non-professional phagocytes, such as epithelial cells, is less clear. Aspergillus conidia interactions with the airway epithelium may also contribute to immune surveillance evasion, thus promoting infection. Our understanding of the airway epithelium-fungal pathogenesis has been limited by appropriate tissue systems. As advanced models are developed, we will gain key insights into the epithelium as a bridge between the host, pathogen and environment.
There are a number of key open questions that remain in the field: What are the relative contributions to receptors other than Dectin-1 in driving and modulating the response to fungal pathogens? Does phagocytosis of inhaled Aspergillus conidia occur under physiological conditions with completely recapitulated airway epithelium? Are all cells capable of forming extracellular traps, or is this a neutrophil and pDC specific process? Are the immune responses needed to contain and eradicate fungal pathogens specific to the site of infection? What remains clear is that a deeper understanding of the host-pathogen interactions between fungi and humans will be critical in developing new therapeutic strategies.
Figure 1.
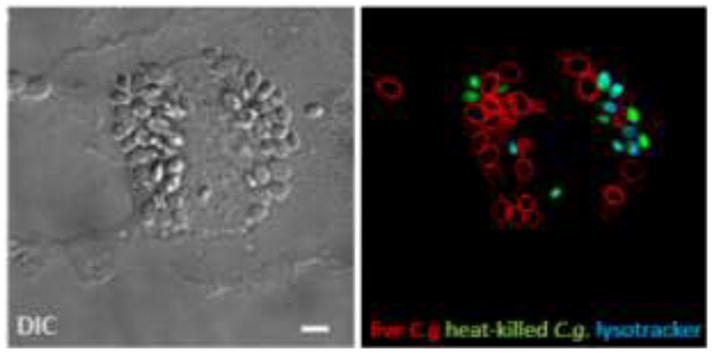
Phagocytosed C. glabrata by a macrophage. Live (red) and heat-killed C. glabrata (green) were incubated with a mouse primary bone-marrow derived macrophage. Lysotracker (blue) delineates the acidified compartments containing only dead yeast, whereas live C. glabrata evades phagosomal acidification remaining in a neutral compartment. Bar = 5μm.
Figure 2.
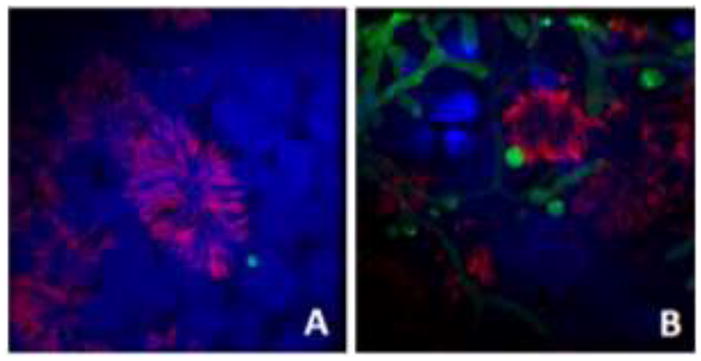
A. fumigatus conidia interacting with fully differentiated human airway epithelium. Live, swollen FLARE-strain A. fumigatus conidia were incubated with fully differentiated adult human small airway epithelium derived from basal stem cells, grown at air-liquid interface. Conidia were allowed to incubate for (A) 6 hours or (B) 18 hours. Samples were stained for acetylated tubulin, a ciliated cell marker (red) and DAPI (nucleus, blue). Live conidia and hyphae express DSred (green). The FLARE strain was kindly provided by Dr. Tobias Hohl (MSKCC, New York, NY USA).
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grants NIAID K08 AI110655 (to M.K.M), RO1 AI097519 and RO1 AI136529 (to J.M.V.) and a Ruth L. Kirschstein National Research Service Award T32 HL116275 from the NIH National Heart, Lung, and Blood Institute (to M.B.F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong-James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 2014;22:120–127. doi: 10.1016/j.tim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Lionakis MS, Iliev ID, Hohl TM. Immunity against fungi. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GD, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.van de Veerdonk FL, et al. Influenza-Associated Aspergillosis in Critically Ill Patients. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 5.Nulens EF, Bourgeois MJ, Reynders MB. Post-influenza aspergillosis, do not underestimate influenza B. Infect Drug Resist. 2017;10:61–67. doi: 10.2147/IDR.S122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crum-Cianflone NF. Invasive Aspergillosis Associated With Severe Influenza Infections. Open Forum Infect Dis. 2016;3:ofw171. doi: 10.1093/ofid/ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansour MK, Tam JM, Vyas JM. The cell biology of the innate immune response to Aspergillus fumigatus. Ann N Y Acad Sci. 2012;1273:78–84. doi: 10.1111/j.1749-6632.2012.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuehler C, et al. Immune Reconstitution After Allogeneic Hematopoietic Stem Cell Transplantation and Association With Occurrence and Outcome of Invasive Aspergillosis. J Infect Dis. 2015;212:959–967. doi: 10.1093/infdis/jiv143. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner DL, Klein BS. Lung epithelium: barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma. Curr Opin Microbiol. 2017;40:8–13. doi: 10.1016/j.mib.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour MK, Reedy JL, Tam JM, Vyas JM. Macrophage Cryptococcus interactions: an update. Curr Fungal Infect Rep. 2014;8:109–115. doi: 10.1007/s12281-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour MK, Levitz SM. Interactions of fungi with phagocytes. Curr Opin Microbiol. 2002;5:359–365. doi: 10.1016/s1369-5274(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 12.Erwig LP, Gow NA. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 13.Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus Conidia with Airway Epithelial Cells: A Critical Review. Front Microbiol. 2016;7:472. doi: 10.3389/fmicb.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margalit A, Kavanagh K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev. 2015;39:670–687. doi: 10.1093/femsre/fuv018. fuv018 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Latge JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12:863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 17.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 18.Herbst S, et al. A new and clinically relevant murine model of solid-organ transplant aspergillosis. Dis Model Mech. 2013;6:643–651. doi: 10.1242/dmm.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst S, et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7:240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reedy JL, et al. The Carbohydrate Lectin Receptor Dectin-1 Mediates the Immune Response to Exserohilum rostratum. Infect Immun. 2017;85 doi: 10.1128/IAI.00903-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodridge HS, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann F, Carmona E, Puri V, Pagano RE, Limper AH. Macrophage internalization of fungal beta-glucans is not necessary for initiation of related inflammatory responses. Infect Immun. 2005;73:6340–6349. doi: 10.1128/IAI.73.10.6340-6349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas M, et al. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol. 2008;181:3549–3557. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, et al. Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death. Am J Respir Crit Care Med. 2016;194:1127–1139. doi: 10.1164/rccm.201601-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, et al. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat Med. 2016;22:906–914. doi: 10.1038/nm.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansour MK, et al. Dectin-1 activation controls maturation of beta-1,3-glucan-containing phagosomes. J Biol Chem. 2013;288:16043–16054. doi: 10.1074/jbc.M113.473223. M113.473223 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam JM, et al. The Role of Autophagy-Related Proteins in Candida albicans Infections. Pathogens. 2016;5 doi: 10.3390/pathogens5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol (Camb) 2012;4:220–227. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam JM, et al. Dectin-1-dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages. J Infect Dis. 2014;210:1844–1854. doi: 10.1093/infdis/jiu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrmizi I, et al. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol. 2013;191:1287–1299. doi: 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagener J, MacCallum DM, Brown GD, Gow NA. Candida albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. MBio. 2017;8 doi: 10.1128/mBio.01820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vylkova S, et al. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio. 2011;2:e00055–00011. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vylkova S, Lorenz MC. Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis. Infect Immun. 2017;85 doi: 10.1128/IAI.00832-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyes DL, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasper LFA, Mogavero S, Höfs S, Martin R, Wilson D, Hube B. Role of the fungal peptide toxin Candidalysin in macrophage damage and inflammatory response. Mycoses. 2016;59:15. doi: 10.1111/myc.12546. [DOI] [Google Scholar]
- 36.Seider K, et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol. 2011;187:3072–3086. doi: 10.4049/jimmunol.1003730. [DOI] [PubMed] [Google Scholar]
- 37.Seider K, et al. Immune evasion, stress resistance, and efficient nutrient acquisition are crucial for intracellular survival of Candida glabrata within macrophages. Eukaryot Cell. 2014;13:170–183. doi: 10.1128/EC.00262-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasper L, et al. Identification of Candida glabrata genes involved in pH modulation and modification of the phagosomal environment in macrophages. PLoS One. 2014;9:e96015. doi: 10.1371/journal.pone.0096015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dade J, et al. HcZrt2, a zinc responsive gene, is indispensable for the survival of Histoplasma capsulatum in vivo. Med Mycol. 2016;54:865–875. doi: 10.1093/mmy/myw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miramon P, Lorenz MC. The SPS amino acid sensor mediates nutrient acquisition and immune evasion in Candida albicans. Cell Microbiol. 2016;18:1611–1624. doi: 10.1111/cmi.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kretschmer M, et al. Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect Immun. 2014;82:2697–2712. doi: 10.1128/IAI.01607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oehler L, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vono M, et al. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood. 2017;129:1991–2001. doi: 10.1182/blood-2016-10-744441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith WB, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–3944. [PubMed] [Google Scholar]
- 46.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 47.Fanger NA, et al. Activation of human T cells by major histocompatability complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–4135. [PubMed] [Google Scholar]
- 48.Lin L, et al. Safety and efficacy of activated transfected killer cells for neutropenic fungal infections. J Infect Dis. 2010;201:1708–1717. doi: 10.1086/652496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L, Ibrahim AS, Baquir B, Palosaari A, Spellberg B. Luminescent-activated transfected killer cells to monitor leukocyte trafficking during systemic bacterial and fungal infection. J Infect Dis. 2012;205:337–347. doi: 10.1093/infdis/jir725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lilly EA, Ikeh M, Nash EE, Fidel PL, Jr, Noverr MC. Immune Protection against Lethal Fungal-Bacterial Intra-Abdominal Infections. MBio. 2018;9 doi: 10.1128/mBio.01472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swamydas M, et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci Transl Med. 2016;8:322ra310. doi: 10.1126/scitranslmed.aac7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price TH, et al. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection. Blood. 2015;126:2153–2161. doi: 10.1182/blood-2015-05-645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glasser L. Effect of storage on normal neutrophils collected by discontinuous-flow centrifugation leukapheresis. Blood. 1977;50:1145–1150. [PubMed] [Google Scholar]
- 54.Huestis DW, Glasser L. The neutrophil in transfusion medicine. Transfusion. 1994;34:630–646. doi: 10.1046/j.1537-2995.1994.34794330020.x. [DOI] [PubMed] [Google Scholar]
- 55.Baistrocchi SR, et al. Posaconazole-Loaded Leukocytes as a Novel Treatment Strategy Targeting Invasive Pulmonary Aspergillosis. J Infect Dis. 2016 doi: 10.1093/infdis/jiw513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasahara S, et al. Role of Granulocyte-Macrophage Colony-Stimulating Factor Signaling in Regulating Neutrophil Antifungal Activity and the Oxidative Burst During Respiratory Fungal Challenge. J Infect Dis. 2016;213:1289–1298. doi: 10.1093/infdis/jiw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez-Andres J, et al. Inflammatory Ly6Chigh Monocytes Protect against Candidiasis through IL-15-Driven NK Cell/Neutrophil Activation. Immunity. 2017;46:1059–1072e1054. doi: 10.1016/j.immuni.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Gazendam RP, et al. Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects. J Immunol. 2016;196:1272–1283. doi: 10.4049/jimmunol.1501811. [DOI] [PubMed] [Google Scholar]
- 59.Li X, et al. PKC-delta activation in neutrophils promotes fungal clearance. J Leukoc Biol. 2016;100:581–588. doi: 10.1189/jlb.4A0915-405R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones CN, et al. Human Neutrophils Are Primed by Chemoattractant Gradients for Blocking the Growth of Aspergillus fumigatus. J Infect Dis. 2016;213:465–475. doi: 10.1093/infdis/jiv419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 62.Metzler KD, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Branzk N, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gratacap RL, Scherer AK, Seman BG, Wheeler RT. Control of mucosal candidiasis in the zebrafish swimbladder depends on neutrophils that block filament invasion and drive extracellular trap production. Infect Immun. 2017 doi: 10.1128/IAI.00276-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015;8:221–231. doi: 10.1038/mi.2014.57. [DOI] [PubMed] [Google Scholar]
- 66.Ermert D, et al. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee MJ, et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog. 2015;11:e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imbert S, et al. Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol. 2016;138:860–868. doi: 10.1016/j.jaci.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 69.Urban CF, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark HL, et al. Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. J Immunol. 2016;196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zelante T, et al. CD103(+) Dendritic Cells Control Th17 Cell Function in the Lung. Cell Rep. 2015;12:1789–1801. doi: 10.1016/j.celrep.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 72.Zelante T, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun. 2012;3:683. doi: 10.1038/ncomms1685. [DOI] [PubMed] [Google Scholar]
- 73.Crassard N, et al. Invasive aspergillosis and allogeneic hematopoietic stem cell transplantation in children: a 15-year experience. Transpl Infect Dis. 2008;10:177–183. doi: 10.1111/j.1399-3062.2008.00304.x. [DOI] [PubMed] [Google Scholar]
- 74.Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med. 2010;207:923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 76.Zelante T, et al. Impaired calcineurin signaling in myeloid cells results in downregulation of pentraxin-3 and increased susceptibility to aspergillosis. Mucosal Immunol. 2017;10:470–480. doi: 10.1038/mi.2016.52. [DOI] [PubMed] [Google Scholar]
- 77.Loures FV, et al. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramirez-Ortiz ZG, et al. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe. 2011;9:415–424. doi: 10.1016/j.chom.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hole CR, et al. Antifungal Activity of Plasmacytoid Dendritic Cells against Cryptococcus neoformans In Vitro Requires Expression of Dectin-3 (CLEC4D) and Reactive Oxygen Species. Infect Immun. 2016;84:2493–2504. doi: 10.1128/IAI.00103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt B, Fujimura SH, Martin JN, Levy JA. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006;26:55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura Y, et al. Dectin-2 deficiency promotes Th2 response and mucin production in the lungs after pulmonary infection with Cryptococcus neoformans. Infect Immun. 2015;83:671–681. doi: 10.1128/IAI.02835-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Espinosa V, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smeekens SP, et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat Commun. 2013;4:1342. doi: 10.1038/ncomms2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 86.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villani AC, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017:356. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donne ML, Lechner AJ, Rock JR. Evidence for lung epithelial stem cell niches. BMC Dev Biol. 2015;15:32. doi: 10.1186/s12861-015-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwasaki A, Foxman EF, Molony RD. Early local immune defences in the respiratory tract. Nat Rev Immunol. 2017;17:7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheppard DC. Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr Opin Microbiol. 2011;14:375–379. doi: 10.1016/j.mib.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wasylnka JA, Moore MM. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect Immun. 2000;68:3377–3384. doi: 10.1128/iai.68.6.3377-3384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeHart DJ, Agwu DE, Julian NC, Washburn RG. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infect Dis. 1997;175:146–150. doi: 10.1093/infdis/175.1.146. [DOI] [PubMed] [Google Scholar]
- 94.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134:1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 95.Bromley IM, Donaldson K. Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: relevance to the asthmatic lung. Thorax. 1996;51:1203–1209. doi: 10.1136/thx.51.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Houser J, et al. A soluble fucose-specific lectin from Aspergillus fumigatus conidia--structure, specificity and possible role in fungal pathogenicity. PLoS One. 2013;8:e83077. doi: 10.1371/journal.pone.0083077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Upadhyay SK, et al. Identification and characterization of a laminin-binding protein of Aspergillus fumigatus: extracellular thaumatin domain protein (AfCalAp) J Med Microbiol. 2009;58:714–722. doi: 10.1099/jmm.0.005991-0. [DOI] [PubMed] [Google Scholar]
- 98.Gravelat FN, et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog. 2013;9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee MJ, et al. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J Biol Chem. 2014;289:1243–1256. doi: 10.1074/jbc.M113.522516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiralongo J, Wohlschlager T, Tiralongo E, Kiefel MJ. Inhibition of Aspergillus fumigatus conidia binding to extracellular matrix proteins by sialic acids: a pH effect? Microbiology. 2009;155:3100–3109. doi: 10.1099/mic.0.026997-0. [DOI] [PubMed] [Google Scholar]
- 101.Warwas ML, Watson JN, Bennet AJ, Moore MM. Structure and role of sialic acids on the surface of Aspergillus fumigatus conidiospores. Glycobiology. 2007;17:401–410. doi: 10.1093/glycob/cwl085. [DOI] [PubMed] [Google Scholar]
- 102.Wasylnka JA, Simmer MI, Moore MM. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology. 2001;147:869–877. doi: 10.1099/00221287-147-4-869. [DOI] [PubMed] [Google Scholar]
- 103.Bidula S, et al. H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses. Immunology. 2015;146:281–291. doi: 10.1111/imm.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swidergall M, Solis NV, Lionakis MS, Filler SG. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat Microbiol. 2018;3:53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fliesser M, et al. Hypoxia-inducible factor 1alpha modulates metabolic activity and cytokine release in anti-Aspergillus fumigatus immune responses initiated by human dendritic cells. Int J Med Microbiol. 2015;305:865–873. doi: 10.1016/j.ijmm.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 106.Paris S, et al. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect Immun. 1997;65:1510–1514. doi: 10.1128/iai.65.4.1510-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasylnka JA, Moore MM. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Cell Sci. 2003;116:1579–1587. doi: 10.1242/jcs.00329. [DOI] [PubMed] [Google Scholar]
- 108.Wasylnka JA, Moore MM. Uptake of Aspergillus fumigatus Conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect Immun. 2002;70:3156–3163. doi: 10.1128/IAI.70.6.3156-3163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez P, Hackett TL, Moore MM, Knight DA, Tebbutt SJ. Functional genomics of human bronchial epithelial cells directly interacting with conidia of Aspergillus fumigatus. BMC Genomics. 2010;11:358. doi: 10.1186/1471-2164-11-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen F, et al. Transcriptome Profiles of Human Lung Epithelial Cells A549 Interacting with Aspergillus fumigatus by RNA-Seq. PLoS One. 2015;10:e0135720. doi: 10.1371/journal.pone.0135720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han X, et al. beta-1,3-Glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells. PLoS One. 2011;6:e21468. doi: 10.1371/journal.pone.0021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bertuzzi M, et al. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog. 2014;10:e1004413. doi: 10.1371/journal.ppat.1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bao Z, et al. Evidence for the involvement of cofilin in Aspergillus fumigatus internalization into type II alveolar epithelial cells. BMC Microbiol. 2015;15:161. doi: 10.1186/s12866-015-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Botterel F, et al. Phagocytosis of Aspergillus fumigatus conidia by primary nasal epithelial cells in vitro. BMC Microbiol. 2008;8:97. doi: 10.1186/1471-2180-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rammaert B, et al. Absence of Fungal Spore Internalization by Bronchial Epithelium in Mouse Models Evidenced by a New Bioimaging Approach and Transmission Electronic Microscopy. Am J Pathol. 2015;185:2421–2430. doi: 10.1016/j.ajpath.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 117.Sun WK, et al. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur J Clin Microbiol Infect Dis. 2012;31:2755–2764. doi: 10.1007/s10096-012-1624-8. [DOI] [PubMed] [Google Scholar]
- 118.Beisswenger C, Hess C, Bals R. Aspergillus fumigatus conidia induce interferon-beta signalling in respiratory epithelial cells. Eur Respir J. 2012;39:411–418. doi: 10.1183/09031936.00096110. [DOI] [PubMed] [Google Scholar]
- 119.Khan NS, et al. Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan. The Journal of Immunology. 2016;196:2249–2261. doi: 10.4049/jimmunol.1401545. [DOI] [PMC free article] [PubMed] [Google Scholar]


