Abstract
Albinism, typically characterized by decrease melanin synthesis, is associated with significant visual deficits owing to developmental changes during neurosensory retina development. All albinism is caused by genetic mutations in a group of diverse genes including enzymes, transporters, G-protein coupled receptor (GPCR). Interestingly, these genes are not expressed in the neurosensory retina. Further, regardless of cause of albinism, all forms of albinism have the same retinal pathology, the extent of which is variable. In this review, we explore the possibility that this similarity in retinal phenotype is because all forms of albinism funnel through the same final common pathway. There are currently 7 known genes linked to the 7 forms of ocular cutaneous albinism. These types of albinism are the most common, and result in changes to all pigmented tissues (hair, skin, eyes). We will discuss the incidence and mechanism, where known, to develop a picture as to how the mutations cause albinism. Next, we will examine the one form of albinism which causes tissue-specific pathology, ocular albinism, where the eye exhibits the retinal albinism phenotype despite near normal melanin synthesis. We will discuss a potential way to treat the disease and restore normal retinal development Finally, we will briefly discuss the possibility that this same pathway may intersect with the most common cause of permanent vision loss in the elderly.
Keywords: Albinism, Retinal pigment epithelium, GPR143, OA1, Melanin, PEDF
Melanogenesis and Albinism
Albinism is a group of several rare genetic diseases that affects approximately 1 in 20,000 people, and is frequently but not always, accompanied by decreased melanin accumulation within melanocytes and retinal pigment epithelium cells (Jeffery, 1998; King & Summers, 1988; William S. Oetting & King, 1999; R A Spritz & Hearing Jr., 1994; R A Sturm, Box, & Ramsay, 1998). More specifically, pigmentation of our hair, skin, and eyes is attributable to the deposition of melanin within a specific organelle called the melanosome or pigment granule. The apparent darkness of a tissue relates to the number and density of melanosomes, and the amount of melanin deposited in the organelle.
The accumulation of melanosomes in a tissue is largely based on genotype, and in particular the melanocortin 1 gene (MCR1) (Rees, 2003; Richard A Sturm & Duffy, 2012). MCR1 exhibits many polymorphisms and these small changes are responsible for much of the variation in pigmentation that occurs among humans. Melanosomes are complex organelles that are at least partially derived from the endosomal/lysosomal pathway. They begin as pre-melanosomes (stage 1) and as they accumulate proteins they proceed through stages 2 and 3 until they ultimately become mature stage 4 melanosomes (Marks & Seabra, 2001; M. V. Schiaffino, 2010; Setty et al., 2007). In melanocytes derived from neural crest cells, which function in cutaneous pigmentation (eg. skin and hair), mature melanosomes are typically released. In the retinal pigment epithelium (RPE), derived from neuroectoderm rather than neural crest, mature melanosomes are produced and retained (Boulton, Docchio, Dayhaw-Barker, Ramponi, & Cubeddu, 1990; Sarna et al., 2003).
Several forms of albinism are caused by alterations in the melanosome maturation process. Genetic alterations that impact the synthesis of melanin, or melanosome biogenesis and trafficking, cause most forms of albinism. These mutations generally have a broad impact in all pigmented tissues. Genetic alterations that cause lysosomal storage defects, such as in Hermansky-Pudlak Syndrome (April & Barsh, 2006; Clark & Griffiths, 2003; Olkkonen & Ikonen, 2006; Setty et al., 2007; Shotelersuk & Gahl, 1998), Griscelli syndrome (Bahadoran et al., 2001; Chen, Samaraweera, Sun, Kreibich, & Orlow, 2002; Kasai et al., 2005; Manuscript, 2010), and Chediak-Higashi syndrome (Dell’Angelica, Mullins, Caplan, & Bonifacino, 2000; Holt, Gallo, & Griffiths, 2006; M. V. Schiaffino, 2010; Shiflett, Kaplan, & Ward, 2002) include albinism as part of a larger syndromic pathology.
Nontraditional ‘albinism’ phenotypes include examples where the migration of neural crest derived melanocytes fails (Waardenburg syndrome) (Saito et al., 2003; Shibahara et al., 2001; Watanabe et al., 2002), or when melanocytes are destroyed as part of an autoimmune disease, such as occurs in vitiligo (Engelhard, Bullock, Colella, Sheasley, & Mullins, 2002; Silverberg, 2014; Richard A. Spritz, 2013). Another atypical cause of albinism includes defects in the gene encoding GPR143, which causes ocular albinism with phenotypic changes which primarily manifest in the eye, while other tissues are affected minimally, if at all (Oetting, Summers, & King, 1994; Rosenberg & Schwartz, 1998; Struck, 5). The ear also contains pigmented cells, and deafness can be associated with albinism, Waardenburg Syndrome for example, see (Beighton et al., 1991; Tak et al., 2004; Winship, Gericke, & Beighton, 1984).
Albinism is typically broken down according to the genetic change causing the disease. Ocular cutaneous albinism (OCA) has seven known genes associated with it, OCA 1-7, listed in table 1. OCA is inherited in an autosomal recessive manner, and due to founder effects, is not manifested evenly throughout the human population. Rather, isolated populations can vary dramatically in the form and incidence of OCA (Braathen & Ingstad, 2006; Cruz-Inigo, Ladizinski, & Sethi, 2011; Hedrick, 2003; Hong, Zeeb, & Repacholi, 2006; Lund, Maluleke, Gaigher, & Gaigher, 2007; Mártinez-García & Montoliu, 2013). OCA to a varied extent affects all pigmented tissues, skin, hair, iris and retina. OCA1-7 are associated with defects in the ability to produce or accumulate melanin from tyrosine, as summarized in Figure 1. Thus, OCA affects all pigmented tissues because the enzymatic process is the same in all cells.
Table 1.
Current Albinism Genetics
| Albinism type | Protein | Gene location | Protein function | Reference |
|---|---|---|---|---|
| OCA1 | Tyrosinase | 11q14.3 | enzyme | (W S Oetting, Fryer, Shriram, & King, 2003) |
| OCA2 | ‘P’ gene | 15q12-q13.1 | transporter | (M H Brilliant, 2001) |
| OCA3 | TRP1 | 9p23 | enzyme | (Manga et al., 1997) |
| OCA4 | MATP (SLC45A2) | 5p13.2 | transporter | (Newton et al., 2001) |
| OCA5 | 4q24 | (Kausar, Bhatti, Ali, Shaikh, & Ahmed, 2013) | ||
| OCA6 | SLC24A5 | 15q21.1 | transporter | (Montoliu et al., 2014) |
| OCA7 | LRMDA | 10q22.2-q22.3 | Leucine rich repeat | |
| OA1 | GPR143 | Xp22.2 | GPCR |
For Albinism genetics review see (Montoliu et al., 2014)
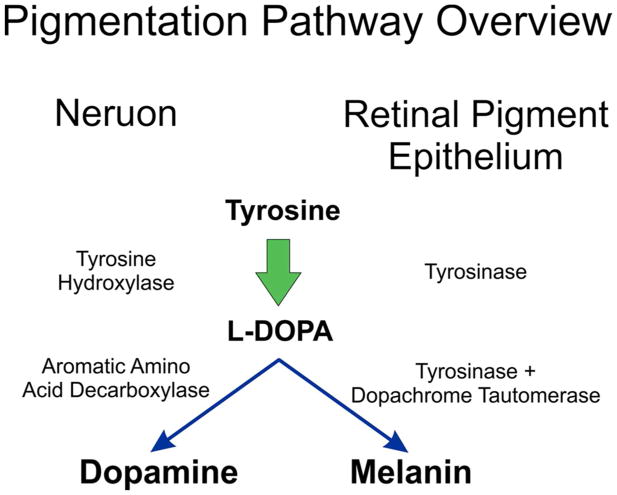
Figure 1.
Overview of Melanin synthesis compared to neuronal dopamine synthesis. Both L-DOPA and dopamine are derived from tyrosine, with an intermediate production of L-DOPA. In neurons, L-DOPA is decarboxylated to form dopamine. In RPE, L-DOPA is further reacted with tyrosinase to create an indole, which is then polymerized to form insoluble melanin
Dopaminergic neurons also produce L-DOPA from tyrosine, but use a separate enzymatic pathway (Figure 1.). A completely different form of albinism, ocular albinism type 1 (OCA1) is caused in mutations GPR143 which is a G-protein coupled receptor (GPCR) (Oetting, 2002; Schiaffino et al., 1995). GPR143 is expressed in melanocytes and RPE but does not appear to directly affect the melanogenic pathway as loss of GPR143 signaling activity has little effect on melanin accumulation. The gene encoding GPR143 is on the X chromosome, so OA1 primarily affect males. Heterozygous females exhibit some mosaicism due to X linked chromosome inactivation (Lam et al., 1997).
Ocular Cutaneous Albinism (OCA)
OCA1 is caused by mutations in tyrosinase, the rate limiting enzyme of melanogenesis, Figure 1. Tyrosinase catalyzes several steps through which tyrosine is modified to L-DOPA, then L-DOPA is the substrate used to produce melanin. In this complex chemistry, L-DOPA is the reactive product, the substrate, and a co-factor regulating tyrosinase activity (Ramsden & Riley, 2014; Sánchez-Ferrer, Neptuno Rodríguez-López, García-Cánovas, & García-Carmona, 1995). OCA1 is the most prevalent form of albinism world-wide, accounting for 50% of OCA, but this is not uniform in all populations. OCA1 is estimated at approximately 1 in 40,000 in the American population (Hutton & Spritz, 2008) provided we exclude those of African descent, in which OCA1 is very uncommon (Gronskov et al., 2007). In general, the level of residual activity of tyrosinase or melanin accumulation correlates with disease severity.
OCA2 is caused by mutations in the ‘p’ gene which encodes a 12-transmembrane spanning domain protein with homology to membrane transporters. One function of the ‘p’ protein relates to regulation of melanosome pH (M H Brilliant, 2001). Melanosomal pH is of importance because tyrosinase activity is pH dependent, as is tyrosinase binding of Cu, an important cofactor for the enzyme (Ramsden & Riley, 2014; Sánchez-Ferrer et al., 1995). Overall OCA2 accounts for 30% of OCA worldwide but as is common for OCA types, the incidence is not uniform. OCA2 is the most common form of OCA in Africans (Simeonov et al., 2013). The incidence of OCA2 in the southern part of Africa is as high as 1 in 3,900 (Puri et al., 1997), and is approximately 1 in 10,000 for people of African descent living in America.
OCA3 is caused by mutations in the gene encoding tyrosinase-related protein (TYRP1). The function of TRP1 is in the biosynthesis of melanin from tyrosine, TRP1 catalyzes the oxidation of 5–6-dihydroxyindole-2-carboxylic acid (DHICA) to an indole that participates in the polymerization of melanin (Hearing & Tsukamoto, 1991; Ito, 2003), depicted in Figure 1. and it has an incidence rate of 1:8,500 in Africa (J. G R Kromberg et al., 2012; Manga, Kerr, Ramsay, & Kromberg, 2013). OCA3 has a less severe phenotype than other forms of OCA.
OCA4 is caused by mutations in the Membrane Associated Transporter Protein (MATP) gene now known as SLC45A2. This gene encodes a protein with 12-transmembrane spanning domains and shares homology with a sugar transporter (Simeonov et al., 2013). OCA4 incidence world-wide is low, estimated at 1:100,000. However, OCA4 is much more common in the Japanese population where it accounts for 27% of OCA incidence (Suzuki & Tomita, 2008). The function of MATP is as a transporter protein in support of melanogenesis and is thought to regulate melanosomal pH. Interestingly however, is that both OCA2 and OCA4 are both caused by mutations in genes encoding 12-transmembrane spanning transport proteins. Further, both OCA2 and 4 are characterized by mistrafficking tyrosinase suggesting organelle biogenesis underlies both OCA2 and 4 (Costin, Valencia, Vieira, Lamoreux, & Hearing, 2003).
OCA5 has been linked to chromosome 4q24 in one family, further study is necessary to examine the mechanism of pathobiology but the incidence appears quite low (Montoliu et al., 2014).
OCA6 is linked to a mutation in a transporter protein, SLC24A5 which is likely to support melanin synthesis. SLC24A5 is also known as sodium/potassium/channel exchanger 5 and disruption of this gene causes a significant reduction in melanin synthesis (Montoliu et al., 2014). Genetic variation in this gene is responsible for a significant portion of skin color variation in humans, especially comparing Europeans to Africans (Lamason, 2005). The incidence of OCA6 is very low.
OCA7 has been mapped to 4q24, the function of this gene remains unknown (Montoliu et al., 2014).
A remaining mystery regarding albinism is the missing genetic correlation between genotype and phenotype. For OCA, as a recessive genetic disease, patients should have two copies of the mutant gene based on clinical presentation. For example, if a patient has OCA1, they should have two copies of mutant tyrosinase genes. However, genetic analysis does not always show this. For example, genetic screening showed 25% of patients lacked 2 mutations in the same OCA gene (Simeonov et al., 2013). Ideas to compensate for missing genetic heritability include missing OCA genes, promotor variants, or genetic interaction (Simeonov et al., 2013).
Albinism Pathobiology
Despite the fact that significant advances have been made regarding the genetics of albinism, and that we have increased knowledge with regard to how the genetic changes that cause OCA1-4 alter melanin accumulation, our understanding of how this reduction in melanin relates to the cardinal symptoms of albinism pathology remain limited. Loss of skin pigmentation is linked to an increased incidence of carcinoma (Murray H. Brilliant, 2015; Jennifer G.R. Kromberg, Castle, Zwane, & Jenkins, 1989; Lekalakala et al., 2015). This is frequently regarded as evidence that cutaneous pigmentation protects from sun damage. Sensitivity to sun damage, is not however, the main pathologic outcome in albinism, low vision is. Clinically, the cardinal symptom of those with albinism are visual problems that occur from abnormal retinal development prior to birth, or during foveal development after birth (Summers, 1996, 2009). Albinism frequently causes low vision, with many exhibiting best corrected visual acuity as low as 20:400. The average best corrected visual acuity in those with albinism is about 20:100. These visual defects are variable but generally can be equated to the residual level of melanin accumulation, with the important exception of individuals with OA1.
Visual Deficits Common to All Forms of Albinism
Foveal hypoplasia
Nystagmus
Iris Transluminance
Reduction in the Uncrossed retinal projection.
Reduced photoreceptors and ganglion cells.
Strabismus
Photophobia
The albinism retinal phenotype is complex and recent advances in imaging techniques such as Spectral Domain Ocular Coherence Tomography and adaptive optics have illustrated a disconnect between anatomical features and measured visual acuity (McAllister et al., 2010; McCafferty et al., 2015; Provis, Dubis, Maddess, & Carroll, 2013). Features such as foveal pit development and photoreceptor numbers are not tightly linked to visual ability, in fact the foveal pit may add little to visual performance (Provis et al., 2013).
Ocular Albinism (OA1): RPE and its Pigmentation Contribute to Retinal Development, but How?
Mutations in GPR143 that cause OA1 yield all of the visual consequences of OCA, but do so with normal or near normal levels of melanin synthesis and accumulation (M. V. Schiaffino, 2010). In cells lacking GPR143, the melanosomes are misshapen and become enlarged ‘macromelanosomes’, but the melanin synthesis machinery is intact, expressed and functional. This important observation allows us to separate melanin accumulation and synthesis from the retinal development deficit that occurs as a consequence of albinism. It’s not the lack of melanin responsible for improper retinal development. OA1 is likely to be the cornerstone from which we understand the relationship between RPE pigmentation and vision. Ocular development fails in the absence of GPR143 signaling - despite normal or near normal pigmentation (M. V. Schiaffino, 2010). This suggests that GPR143 signaling activity underlies the ocular defects associated with albinism, because regardless of RPE pigmentation, loss of GPR143 yields the same result. Thus, we have a disease characterized by absent or reduced melanin accumulation (OCA), tied to a gene that does not participate in melanin synthesis (OA1), and a disease whose primary clinical problem occurs in neurons that never express genes involved.
The RPE is the pigmented layer of the retina, and is the most likely tissue impacted directly by OCA and OA1, and RPE expresses all of the albinism causing genes. The RPE is a simple epithelial monolayer that functions in close support of the outer retina and is in tight physical association with the photoreceptor outer segments. The RPE is the first tissue to express the melanogenic genes in the embryo (Bharti, Nguyen, Skuntz, Bertuzzi, & Arnheiter, 2006; Surace, Angeletti, Ballabio, & Marigo, 2000), such that the RPE is the first pigmented tissue during development. This simplifies the situation greatly.
Natures Experiment
Observational data:
Albinism from any cause, be it an enzymatic dysfunction in melanin synthesis, a lysosomal storage defect, or loss of a GPCR not directly related to melanin synthesis, the retinal phenotype is the same, low vision due to developmental changes listed.
At the developmental stage the retinal deficits commence, the RPE is the only pigmented tissue in the embryo.
The retinal deficits that occur when GPR143 signaling activity is lost are the same as those in OCA, but the RPE accumulates melanin – so it’s not melanin.
The effect of albinism on retinal development must be paracrine from the RPE as the neurons in the retina do not express any of the albinism genes.
Interventional Data:
Genetic restoration of RPE pigmentation in albino animal models rescues retinal development (Gimenez et al., 2004).
Retinal development can be saved in albino animals when the RPE is made to express tyrosine hydroxylase which produces L-DOPA but not melanin, see Figure 1 (Lavado, Jeffery, Tovar, de la Villa, & Montoliu, 1999).
Conclusion
There is an intersection between RPE pigmentation and retinal development, but it’s not melanin.
Hypothesis
Pigmentation is upstream of GPR143 signaling activity, and anything that perturbs melanin production concomitantly reduces GPR143 signaling activity. GPR143 is the final common pathway of diverse forms of albinism.
GPR143
We discovered that the ligand for GPR143 is L-DOPA (Lopez, Decatur, Stamer, Lynch, & McKay, 2008), an intermediate compound produced during melanin synthesis (Figure 1). RPE make and release L-DOPA when they synthesize and accumulate melanin (Roffler-Tarlov, Liu, Naumova, Bernal-Ayala, & Mason, 2013). Tyrosinase reacts with tyrosine, creating L-DOPA, and then reacts with L-DOPA in subsequent steps to produce melanin (Figure 1). Albino animals that lack tyrosinase lack production of both L-DOPA and melanin. In contrast, tyrosine hydroxylase reacts with tyrosine creating L-DOPA, but then stops. Tyrosine hydroxylase does not produce melanin, only L-DOPA. Since tyrosine hydroxylase rescues albino retinal development in animals lacking tyrosinase (Lavado et al., 1999), L-DOPA must be the key. The development of the albino retinal phenotype is associated with loss of L-DOPA, not melanin, and loss of the L-DOPA receptor results in the same phenotype as loss of L-DOPA. Thus, our view is that all forms of albinism have the same retinal defects because they lack GPR143 signaling, either the receptor is lost or the ligand is missing.
GPR143 binding to L-DOPA causes an immediate increase in intracellular calcium (Ca++i). L-DOPA is an agonist for GPR143, signaling through the Gαq pathway, causing Ca++I to increase. cAMP levels were measured and did not increase or decrease in response to GPR143 activation. We demonstrated this in native RPE expressing the endogenous receptor at normal levels, as well as in commonly used models to study GPCR pharmacology such as transfected COS and CHO cells overexpressing the receptor.
The related molecule dopamine, competes with L-DOPA for the same binding site in GPR143, but inactivates the receptor. Thus, dopamine functions as an antagonist of GPR143. Tyrosine, from which both L-DOPA and dopamine are derived (Figure 1), binds to GPR143 but with low affinity. Tyrosine at high concentrations, such as those found in normal culture media, for example DMEM with 400uM tyrosine, causes GPR143 internalization. Normal serum levels of tyrosine are under 100uM for comparison (Geisler et al., 2015). It is likely therefore that excessive tyrosine in the culture medium underlies the mislocalization of GPR143 to the cytoplasm in some previous studies (M. V Schiaffino et al., 1999). Using eye cup preparations from human donor tissue, GPR143 is localized to the apical surface of the RPE in situ (Lopez et al., 2008). The plasma membrane localization of GPR143 in RPE makes GPR143 similar to all other GPCRs studied to date, which comprise the largest family of cell surface receptors. High concentrations of tyrosine present in typical culture media may either activate the receptor or compete with agonists, which is why custom low tyrosine medium is recommended to study GPR143 activity. Our data illustrate GPR143 signaling is directly linked to the melanin synthesis machinery in an autocrine loop (depicted in Figure 2), despite GPR143 not actually participating in melanin synthesis (Gross, 2008).
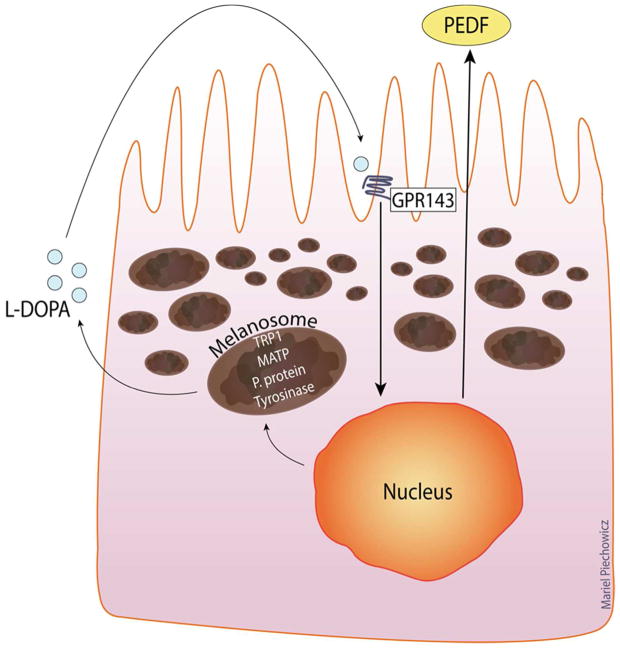
Figure 2.
RPE express the full complement of melanogenic proteins, including GPR143. This figure illustrates the autocrine loop created in the melanogenic system when the cells synthesize melanin, they release LDOPA as a byproduct, L-DOPA then activates GPR143, stimulating the secretion of PEDF.
The question remains, how does a gene and pathway expressed by the RPE shape retinal development? Since the genes responsible for albinism are not expressed in the retinal neurons, the effect from RPE to the retina must be paracrine.
Several studies have investigated the contribution of L-DOPA to retina development (Donatien & Jeffery, 2002; Eisenhofer et al., 2003; Esteve & Jeffery, 1998; Kralj-Hans, Tibber, Jeffery, & Mobbs, 2006; Rachel et al., 2002) including the rescue of normal retinal development in albino animals by RPE expression of tyrosine hydroxylase (Lavado et al., 1999). All of these studies were conducted prior to discovery of a L-DOPA receptor in the RPE, GPR143. To that end we investigated downstream effectors controlled by GPR143 signaling in response to L-DOPA with the potential to alter retinal development.
Pigment epithelial derived factor (PEDF) is a very potent neurotrophic factor originally discovered as the protein released by pigmented fetal human RPE that fostered neuron survival (Jablonski, Tombran-Tink, Mrazek, & Iannaccone, 2000; Steele, Chader, Johnson, & Tombran-Tink, 1993; Tombran-Tink, Shivaram, Chader, Johnson, & Bok, 1995). Since the original discovery, multiple studies have shown that PEDF is a potent neurotrophic factor, but also that PEDF strongly inhibits angiogenesis. PEDF is a noncatalytic member of the SERPIN family of proteins, and pigmented RPE secrete abundant levels of the protein (Tombran-Tink et al., 1995). Using pigmented fetal human RPE cultures that express endogenous GPR143 we demonstrated that GPR143 signaling activity regulates RPE PEDF secretion (Falk et al., 2012; Lopez et al., 2008). One significant difficulty in studying endogenous GPR143 is that its expression is driven by the same transcription factor as tyrosinase, MITF-m (M. V. Schiaffino, 2010). Thus, any cell making GPR143 is also making tyrosinase, the source of its ligand, creating an active autocrine loop. Experimentally, the advantage is that we can see which cells are expressing GPR143, because they’re brown. However, this also means we have an active autocrine loop, and the cells are making and responding to the ligand. To short circuit this loop, we use phenylthiourea (PTU) which inhibits tyrosinase by covalently inactivating the enzyme. Treating pigmented RPE with PTU drastically reduces their PEDF secretion, and this can be restored with exogenous L-DOPA (Lopez et al., 2008). Together, L-DOPA is the ligand for GPR143, and GPR143 signaling controls the downstream RPE secretion of PEDF.
GPR143 signaling in RPE also controls of release of apical exosomes (Locke et al., 2014). Exosomes are small extracellular vesicles, 40–150nM in diameter, produced and accumulated in the multivesicular body (MVB), for review (Pant, Hilton, & Burczynski, 2012). Exosomes are released from cells when the MVB fuses with the plasma membrane. Exosomes are thought to function in inter-tissue communication as they carry mRNA, miRNA, proteins, and bioactive lipids. RPE are known to release both apical and basal exosomes (Biasutto, Chiechi, Couch, Liotta, & Espina, 2013; Kannan, Sreekumar, & Hinton, 2012; Locke et al., 2014; Sreekumar et al., 2010; Wang et al., 2009a, 2009b). Apical exosomes have the capacity to function as carriers from the RPE to the retina. Using human eyes cups from donor eyes we demonstrated that, first, RPE are constitutively releasing a large quantity of exosomes, and second, L-DOPA treatment of the RPE immediately halts exosome release. Others have suggested that GPR143 plays a role in endosomal trafficking (M. V. Schiaffino, 2010; Shen, Samaraweera, Rosenberg, & Orlow, 2001), and suggested the presence of macromelanosomes in OA1 indicate a function in melanosome biogenesis. The observation that L-DOPA causes an immediate change in exosome release provides further evidence that GPR143 functions at some point in endosomal trafficking.
How might halting exosome release change retinal development though? It is possible that exosomes are part of a communication pathway from RPE to the retina, but at this point we do not have enough information. We know from our analysis of over 30 sets of human donor eyes, we reproducibly found 3 populations of exosomes based on size characteristics (Locke et al., 2014). They are likely to represent ‘RPE specific’ exosomes, but what information they carry and how the retina responds to them is unknown. It must also be noted that our study in human eyes only used aged donor tissue, with most of our donors being over 75 years old. We have no data from the developing eye and retina, which may differ. A distinct possibility is that the constitutive release of exosomes from aged RPE tissue represents a symptom of the overloaded lysosomal/endosomal system in aged RPE, not reflective of developmental RPE biology.
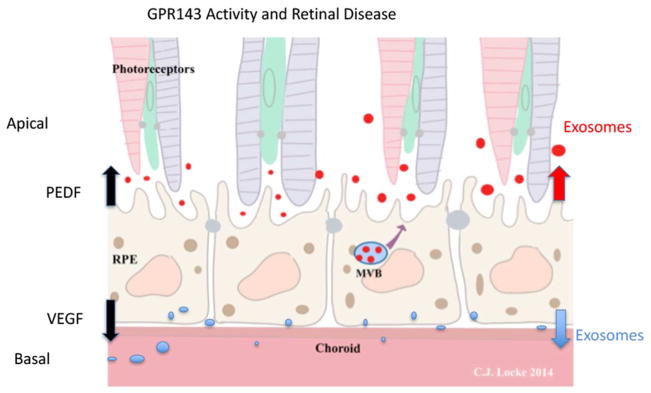
In Figure 3, we summarize our findings regarding GPR143 signaling activity in response to L-DOPA. GPR143 upregulates RPE secretion of PEDF while down regulating VEGF. Both of these activities are likely to have significant implications for retinal development, and may serve as a point of RPE control over both retina and choroid development. GPR143 signaling also has an immediate effect on apical exosome release using in situ human RPE. Our eye cup model does not allow for collection of basal RPE exosomes. There is insufficient information currently to suggest how this GPR143 activity may alter retinal development, but the activity was robust, and reproduced in every eye cup studied. The observation strongly supports the data suggesting GPR143 signaling regulates endosomal trafficking.
Figure 3.
Activation of GPR143 controls several RPE activities that could contribute to retinal health and disease. L-DOPA activation of GPR143 upregulates RPE secretion of PEDF while simultaneously down regulating VEGF. GPR143 signaling halts apical exosome release, and could alter basal exosome release, which has not been tested.
A clinical trial was developed to determine whether L-DOPA could correct or treat the visual deficits in those with albinism (Summers et al., 2014). 45 people were tested in a prospective randomized clinical trial to determine whether L-DOPA was safe and increased visual acuity in those with albinism. Results demonstrated L-DOPA was safe but produced no change in visual acuity at 20 weeks. However, the retinal deficit encountered in albinism are developmental in nature, and the study participants ranged in age from 3.5–57.8 years of age, with a mean age of 14.5 years old. The data illustrated that this strategy to combat albinism visual deficits is safe, but may need to be conducted on primarily very young patients, or perhaps followed longer. This study could be redesigned and is still the best hope for helping children with albinism.
GPR143 Role in the Aging Retina
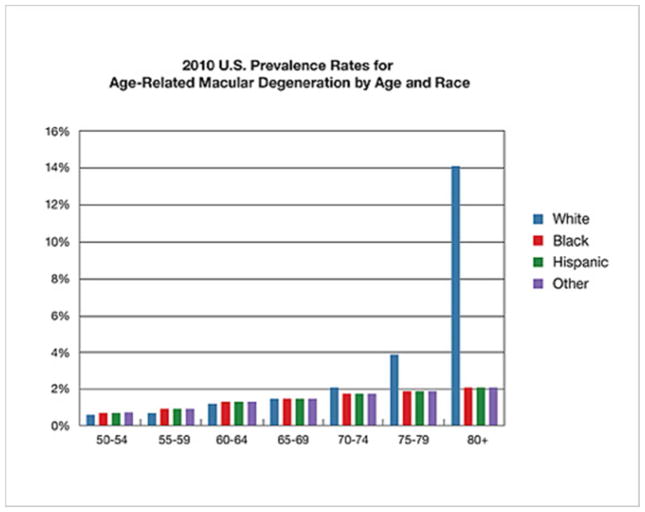
GPR143 signaling may play a role in retinal disease long past embryogenesis. The most common cause of irreversible blindness is Age-related Macular Degeneration (AMD). Unlike albinism discussed above, this is not a rare disorder. Rather, AMD affects greater than 30% of individuals over the age of 75 (Klein et al., 2011). The incidence of AMD has a very strong racial bias (Figure 4) suggesting that basal pigmentation may play a role in AMD susceptibility. Greater melanin production and accumulation appears protective from AMD development (Murray H. Brilliant et al., 2016; McKay & Schwartz, 2016).
Figure 4.
Data illustrates percentage of individuals with AMD segregated by Age and Race. Source: https://nei.nih.gov/eyedata/amd
Does GPR143 signaling and greater melanin protect from AMD? In normal ageing RPE, melanin content decreases and PEDF in the retina decreases (Sarna et al., 2003). Pigmentation is greatest in the macula, which loses 35% of its melanin during aging (Boulton & Dayhaw-Barker, 2001; Sarna et al., 2003). Importantly, the region with severe development defects in albinism, the fovea, is at the center of the macula. Together, these observations suggest that GPR143 signaling may decrease with age, and perhaps contribute to the progression of AMD in the aging population.
To investigate this, we used 3 separate medical record databases across the country, encompassing over 87,000,000 unique individuals, approximately 1/4th of the population of America. Our only manipulation of the data set was to limit our analysis to only those we could show had access to ophthalmology care, bringing the test population to 15,215,458. We asked the question, are individuals taking L-DOPA (primarily for movement disorders such as Parkinson’s Disease) protected from AMD? The answer was a resounding yes (Murray H. Brilliant et al., 2016).
Our data illustrate that those taking L-DOPA were significantly less likely to develop AMD, and if they do, the onset of the disease was significantly delayed. The level of L-DOPA in the blood stream of patients taking L-DOPA for movement disorders is sufficient to activate GPR143 (Pilling, Baker, Iversen, Iversen, & Robbins, 1975; Satoh, Kuzuu, Doi, Masaka, & Sakakibara, 2015) as we have previously used 1μM L-DOPA to activate the receptor (Lopez et al., 2008). GPR143 is the only L-DOPA receptor known, and L-DOPA has no disease modifying effect in Parkinson’s disease, it only provides symptomatic relief. Together, this suggests GPR143 activity may protect from AMD and could underlie the racial bias in AMD pathology. Note here, we do not propose GPR143 signaling activity or lack thereof as a cause of AMD. Rather, that increased GPR143 as related to melanin content of the RPE, may cause increased PEDF and decreased VEGF, both of which may protect from AMD. This protection would be linked to the basal pigmentation of the RPE and age of the individual. As RPE melanin decreases, reduced protection from GPR143 signaling activity is expected. Just as is the case for the racial incidence, all races get AMD, just that the incidence is notably much higher in the white population, as well as the aging population as RPE melanin is lost. This was a retrospective chart review, a big one, but caution is taken in our interpretation (Lopez et al., 2008; McKay & Schwartz, 2016). A double-blind, prospective clinical trial is warranted by these results.
Concluding Remarks
In summary, visual function, development, and retention are exquisitely linked to RPE pigmentation. The final common pathway appears to go through GPR143 signaling activity. In albinism, there is either a loss of the ligand, L-DOPA, or loss of the GPR143 receptor itself. During development, the only tissue pigmented when retina deficits occur is the RPE, allowing us to conclude that RPE melanin biogenesis is critical to visual system development. Regardless of the mutation or gene affected, anything that decreases GPR143 signaling results in the same retinal deficits and low vision. With respect to the aging retina, we suggest that below some critical level, GPR143 signaling is insufficient to support aging retinal neurons. Importantly, augmentation of GPR143 signaling with supplemental L-DOPA appears to forestall AMD development and reduces the likelihood of ever developing the disease. GPR143 signaling activity appears at the cornerstone of retinal health and development.
With regard to future directions, we have explored only two protein agents (PEDF and VEGF) for response from GPR143 activity, and one biological pathway, exosome communication. Future areas of exploration include, identification of the gene repertoire that responds to GPR143 signaling activity, and how GPR 143 signaling affects endosomal trafficking. Since daily phagocytosis and degradation/recycling of photoreceptor outer segments is a primary role for RPE in support of the retina, understanding how GPR143 interacts and perhaps controls that process is of upmost importance. How might the whole picture relate to development and preservation of retinal visual function? GPCRs are remarkable diverse receptors, with wide ranging control over complex biological systems. We are just at the beginning of investigating GPR143 signaling activity.
Significance Statement.
This review of the literature makes the novel case for a final common pathway for all forms of albinism, which is not currently established. We put together the evidence that such a common pathway does in fact, exist We provide a rational basis and mechanism for treating retinal defects associated with albinism. Finally, we broaden the discussion to include the most common form of irreversible blindness in the elderly where the pathways collide.
Acknowledgments
Sponsor: NIH/NEI grant number: R01EY026544 (BSM)
The Author wishes to thank Christi Locke and Mariel Piechowicz for their original artwork
Footnotes
Conflict Statement. Dr. McKay has a patent issued for the use of L-DOPA to treat AMD, but has received no financial compensation related to the patent or for this manuscript
References
- April CS, Barsh GS. Skin layer-specific transcriptional profiles in normal and recessive yellow (Mc1re/Mc1re) mice. Pigment Cell Research/Sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2006;19(3):194–205. doi: 10.1111/j.1600-0749.2006.00305.x. https://doi.org/10.1111/j.1600-0749.2006.00305.x [DOI] [PubMed] [Google Scholar]
- Bahadoran P, Aberdam E, Mantoux F, Busca R, Bille K, Yalman N, … Ballotti R. Rab27a: A key to melanosome transport in human melanocytes. J Cell Biol. 2001;152(4):843–850. doi: 10.1083/jcb.152.4.843. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11266474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P, Ramesar R, Winship I, Viljoen D, Greenberg J, Young K, … Sellars S. Hearing impairment and pigmentary disturbance. Ann N Y Acad Sci. 1991;630:152–166. doi: 10.1111/j.1749-6632.1991.tb19584.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1952586. [DOI] [PubMed] [Google Scholar]
- Bharti K, Nguyen MTT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: Specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Research. 2006 doi: 10.1111/j.1600-0749.2006.00318.x. https://doi.org/10.1111/j.1600-0749.2006.00318.x [DOI] [PMC free article] [PubMed]
- Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319(13):2113–2123. doi: 10.1016/j.yexcr.2013.05.005. https://doi.org/10.1016/j.yexcr.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15(Pt 3):384–389. doi: 10.1038/eye.2001.141. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11450762. [DOI] [PubMed] [Google Scholar]
- Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 1990;30(9):1291–1303. doi: 10.1016/0042-6989(90)90003-4. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2219746. [DOI] [PubMed] [Google Scholar]
- Braathen SH, Ingstad B. Albinism in Malawi: Knowledge and beliefs from an African setting. Disability and Society. 2006;21(6):599–611. https://doi.org/10.1080/09687590600918081 [Google Scholar]
- Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14(2):86–93. doi: 10.1034/j.1600-0749.2001.140203.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11310796. [DOI] [PubMed] [Google Scholar]
- Brilliant MH. Albinism in Africa: A medical and social emergency. International Health. 2015;7(4):223–225. doi: 10.1093/inthealth/ihv039. https://doi.org/10.1093/inthealth/ihv039 [DOI] [PubMed] [Google Scholar]
- Brilliant MH, Vaziri K, Connor TB, Schwartz SG, Carroll JJ, McCarty CA, … McKay BS. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. American Journal of Medicine. 2016;129(3):292–298. doi: 10.1016/j.amjmed.2015.10.015. https://doi.org/10.1016/j.amjmed.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Samaraweera P, Sun TT, Kreibich G, Orlow SJ. Rab27b association with melanosomes: dominant negative mutants disrupt melanosomal movement. J Invest Dermatol. 2002;118(6):933–940. doi: 10.1046/j.1523-1747.2002.01754.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12060386. [DOI] [PubMed] [Google Scholar]
- Clark R, Griffiths GM. Lytic granules, secretory lysosomes and disease. Curr Opin Immunol. 2003;15(5):516–521. doi: 10.1016/s0952-7915(03)00113-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14499259. [DOI] [PubMed] [Google Scholar]
- Costin GE, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4. J Cell Sci. 2003;116(Pt 15):3203–3212. doi: 10.1242/jcs.00598. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12829739. [DOI] [PubMed] [Google Scholar]
- Cruz-Inigo AE, Ladizinski B, Sethi A. Albinism in Africa: stigma, slaughter and awareness campaigns. Dermatol Clin. 2011;29(1):79–87. doi: 10.1016/j.det.2010.08.015. https://doi.org/10.1016/j.det.2010.08.015 [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. Faseb J. 2000;14(10):1265–1278. doi: 10.1096/fj.14.10.1265. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10877819. [DOI] [PubMed] [Google Scholar]
- Donatien P, Jeffery G. Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Investigative Ophthalmology & Visual Science. 2002;43(4):1198–1203. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11923266. [PubMed] [Google Scholar]
- Eisenhofer G, Tian H, Holmes C, Matsunaga J, Roffler-Tarlov S, Hearing VJ. Tyrosinase: a developmentally specific major determinant of peripheral dopamine. Faseb J. 2003;17(10):1248–1255. doi: 10.1096/fj.02-0736com. https://doi.org/10.1096/fj.02-0736com [DOI] [PubMed] [Google Scholar]
- Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol Rev. 2002;188:136–146. doi: 10.1034/j.1600-065x.2002.18812.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12445287. [DOI] [PubMed] [Google Scholar]
- Esteve JV, Jeffery G. Reduced retinal deficits in an albino mammal with a cone rich retina: a study of the ganglion cell layer at the area centralis of pigmented and albino grey squirrels. Vision Res. 1998;38(6):937–940. doi: 10.1016/s0042-6989(97)00229-0. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9624442. [DOI] [PubMed] [Google Scholar]
- Falk T, Congrove NR, Zhang S, McCourt AD, Sherman SJ, McKay BS. PEDF and VEGF-A output from human retinal pigment epithelial cells grown on novel microcarriers. J Biomed Biotechnol. 2012;2012:278932. doi: 10.1155/2012/278932. https://doi.org/10.1155/2012/278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Mayersbach P, Becker K, Schennach H, Fuchs D, Gostner JM. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines. 2015;26(1):31–36. https://doi.org/10.1515/pterid-2014-0015 [Google Scholar]
- Gimenez E, Lavado A, Giraldo P, Cozar P, Jeffery G, Montoliu L. A transgenic mouse model with inducible Tyrosinase gene expression using the tetracycline (Tet-on) system allows regulated rescue of abnormal chiasmatic projections found in albinism.[erratum appears in Pigment Cell Res. 2005 Jun;18(3):224] Pigment Cell Research. 2004;17(4):363–370. doi: 10.1111/j.1600-0749.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Gronskov K, Ek J, Brondum-Nielsen K, Grønskov K, Ek J, Brondum-Nielsen K, … Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007;2(1):43. doi: 10.1186/1750-1172-2-43. https://doi.org/10.1186/1750-1172-2-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L. A molecular link between albinism and visual deficits. PLoS Biology. 2008;6(9):e248. doi: 10.1371/journal.pbio.0060248. https://doi.org/10.1371/journal.pbio.0060248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. Faseb J. 1991;5(14):2902–2909. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1752358. [PubMed] [Google Scholar]
- Hedrick PW. Hopi Indians, “cultural” selection, and albinism. American Journal of Physical Anthropology. 2003;121(2):151–156. doi: 10.1002/ajpa.10180. https://doi.org/10.1002/ajpa.10180 [DOI] [PubMed] [Google Scholar]
- Holt OJ, Gallo F, Griffiths GM. Regulating secretory lysosomes. J Biochem. 2006;140(1):7–12. doi: 10.1093/jb/mvj126. https://doi.org/10.1093/jb/mvj126 [DOI] [PubMed] [Google Scholar]
- Hong ES, Zeeb H, Repacholi MH. Albinism in Africa as a public health issue. BMC Public Health. 2006;6(1):212. doi: 10.1186/1471-2458-6-212. https://doi.org/10.1186/1471-2458-6-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SM, Spritz RA. Comprehensive analysis of oculocutaneous albinism among non-hispanic caucasians shows that OCA1 Is the most prevalent OCA type. Journal of Investigative Dermatology. 2008;128(10):2442–2450. doi: 10.1038/jid.2008.109. https://doi.org/10.1038/jid.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. A Chemist’s View of Melanogenesis. Pigment Cell Research. 2003;16(3):230–236. doi: 10.1034/j.1600-0749.2003.00037.x. https://doi.org/10.1034/j.1600-0749.2003.00037.x [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20(19):7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G. The retinal pigment epithelium as a developmental regulator of the neural retina. Eye. 1998;12(Pt 3b):499–503. doi: 10.1038/eye.1998.137. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9775209. [DOI] [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR. Novel roles for alpha-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31(6):576–604. doi: 10.1016/j.preteyeres.2012.06.001. https://doi.org/10.1016/j.preteyeres.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, … Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation.[see comment] Journal of Clinical Investigation. 2005;115(2):388–396. doi: 10.1172/JCI22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RA, Summers CG. Albinism. Dermatol Clin. 1988;6(2):217–228. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3288382. [PubMed] [Google Scholar]
- Klein R, Chou CFF, Klein BEK, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. https://doi.org/10.1001/archophthalmol.2010.318 [DOI] [PubMed] [Google Scholar]
- Kralj-Hans I, Tibber M, Jeffery G, Mobbs P. Differential effect of dopamine on mitosis in early postnatal albino and pigmented rat retinae. Journal of Neurobiology. 2006;66(1):47–55. doi: 10.1002/neu.20200. https://doi.org/10.1002/neu.20200 [DOI] [PubMed] [Google Scholar]
- Kromberg JGR, Bothwell J, Kidson SH, Manga P, Kerr R, Jenkins T. TYPES OF ALBINISM IN THE BLACK SOUTHERN AFRICA POPULATION. East African Medical Journal. 2012;89(1):20–27. [PubMed] [Google Scholar]
- Kromberg JGR, Castle D, Zwane EM, Jenkins T. Albinism and skin cancer in Southern Africa. Clinical Genetics. 1989;36(1):43–52. doi: 10.1111/j.1399-0004.1989.tb03365.x. https://doi.org/10.1111/j.1399-0004.1989.tb03365.x [DOI] [PubMed] [Google Scholar]
- Lam BL, Fingert JH, Shutt BC, Singleton EM, Merin LM, Brown HH, … Stone EM. Clinical and molecular characterization of a family affected with X-linked ocular albinism (OA1) Ophthalmic Genet. 1997;18(4):175–184. doi: 10.3109/13816819709041432. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9457748. [DOI] [PubMed] [Google Scholar]
- Lamason RL. SLC24A5, a Putative Cation Exchanger, Affects Pigmentation in Zebrafish and Humans. Science. 2005;310(5755):1782–1786. doi: 10.1126/science.1116238. https://doi.org/10.1126/science.1116238 [DOI] [PubMed] [Google Scholar]
- Lavado A, Jeffery G, Tovar V, de la Villa P, Montoliu L. Ectopic expression of tyrosine hydroxylase in the pigmented epithelium rescues the retinal abnormalities and visual function common in albinos in the absence of melanin. Journal of Neurochemistry. 1999;96(4):1201–1211. doi: 10.1111/j.1471-4159.2006.03657.x. [DOI] [PubMed] [Google Scholar]
- Lekalakala PT, Khammissa RAG, Kramer B, Ayo-Yusuf OA, Lemmer J, Feller L. Oculocutaneous Albinism and Squamous Cell Carcinoma of the Skin of the Head and Neck in Sub-Saharan Africa. Journal of Skin Cancer. 2015;2015:167847. doi: 10.1155/2015/167847. https://doi.org/10.1155/2015/167847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke CJ, Congrove NR, Dismuke WM, Bowen TJ, Stamer WD, McKay BS. Controlled exosome release from the retinal pigment epithelium insitu. Experimental Eye Research. 2014;129(0):1–4. doi: 10.1016/j.exer.2014.10.010. https://doi.org/10.1016/j.exer.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biology. 2008;6(9):1861–1869. doi: 10.1371/journal.pbio.0060236. https://doi.org/10.1371/journal.pbio.0060236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PM, Maluleke TG, Gaigher I, Gaigher MJ. Oculocutaneous albinism in a rural community of South Africa: A population genetic study. Annals of Human Biology. 2007;34(4):493–497. doi: 10.1080/03014460701401261. https://doi.org/10.1080/03014460701401261 [DOI] [PubMed] [Google Scholar]
- Manga P, Kerr R, Ramsay M, Kromberg JGR. Biology and genetics of oculocutaneous albinism and vitiligo - Common pigmentation disorders in southern Africa. South African Medical Journal. 2013;103(SUPPL 1):984–988. doi: 10.7196/samj.7046. https://doi.org/10.7196/SAMJ.7046 [DOI] [PubMed] [Google Scholar]
- Manuscript A. Physiological factors that regulate skin pigmentation. 2010;35(2):193–199. doi: 10.1002/biof.29. https://doi.org/10.1002/biof.29.Physiological [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: Membrane dynamics in black and white. Nature Reviews Molecular Cell Biology. 2001 doi: 10.1038/35096009. https://doi.org/10.1038/35096009 [DOI] [PubMed]
- Mártinez-García M, Montoliu L. Albinism in Europe, 40. The Journal of Dermatology §. 2013 doi: 10.1111/1346-8138.12170. https://doi.org/10.1111/1346-8138.12170 [DOI] [PubMed]
- McAllister JT, Dubis AM, Tait DM, Ostler S, Rha J, Stepien KE, … Carroll J. Arrested development: high-resolution imaging of foveal morphology in albinism. Vision Res. 2010;50(8):810–817. doi: 10.1016/j.visres.2010.02.003. https://doi.org/10.1016/j.visres.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty BK, Wilk MA, McAllister JT, Stepien KE, Dubis AM, Brilliant MH, … Summers CG. Clinical Insights Into Foveal Morphology in Albinism. Journal of Pediatric Ophthalmology and Strabismus. 2015;52(3):167–172. doi: 10.3928/01913913-20150427-06. https://doi.org/10.3928/01913913-20150427-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BS, Schwartz SG. Pigmentation and Macular Degeneration: Is There a Role for GPR143? Journal of Ocular Pharmacology and Therapeutics. 2016;32(1):3–4. doi: 10.1089/jop.2016.29007.bsm. https://doi.org/10.1089/jop.2016.29007.bsm [DOI] [PubMed] [Google Scholar]
- Montoliu L, Grønskov K, Wei AH, Martínez-García M, Fernández A, Arveiler B, … Li W. Increasing the complexity: new genes and new types of albinism. Pigment Cell & Melanoma Research. 2014;27(1):11–18. doi: 10.1111/pcmr.12167. https://doi.org/10.1111/pcmr.12167 [DOI] [PubMed] [Google Scholar]
- Oetting WS. New insights into ocular albinism type 1 (OA1): Mutations and polymorphisms of the OA1 gene. Hum Mutat. 2002;19(2):85–92. doi: 10.1002/humu.10034. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11793467. [DOI] [PubMed] [Google Scholar]
- Oetting WS, King RA. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 1999;13(2):99–115. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. https://doi.org/10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Oetting WS, Summers CG, King RA. Albinism and the associated ocular defects. Metab Pediatr Syst Ophthalmol. 1994;17(1–4):5–9. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8719278. [PubMed] [Google Scholar]
- Olkkonen VM, Ikonen E. When intracellular logistics fails--genetic defects in membrane trafficking. J Cell Sci. 2006;119(Pt 24):5031–5045. doi: 10.1242/jcs.03303. https://doi.org/10.1242/jcs.03303 [DOI] [PubMed] [Google Scholar]
- Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83(11):1484–1494. doi: 10.1016/j.bcp.2011.12.037. https://doi.org/10.1016/j.bcp.2011.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling JB, Baker J, Iversen LL, Iversen SD, Robbins T. Plasma concentrations of L-dopa and 3-methoxydopa and improvement in clinical ratings and motor performance in patients with Parkinsonism treated with L-dopa alone or in combination with amantadine. Journal of Neurology Neurosurgery, and Psychiatry. 1975;38:129–135. doi: 10.1136/jnnp.38.2.129. Retrieved from http://jnnp.bmj.com/content/jnnp/38/2/129.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: Cones, the fovea and the a vascular zone. Progress in Retinal and Eye Research. 2013 doi: 10.1016/j.preteyeres.2013.01.005. https://doi.org/10.1016/j.preteyeres.2013.01.005 [DOI] [PMC free article] [PubMed]
- Puri N, Durham-Pierre D, Aquaron R, Lund PM, King RA, Brilliant MH. Type 2 oculocutaneous albinism (OCA2) in Zimbabwe and Cameroon: Distribution of the 2.7-kb deletion allele of the P gene. Human Genetics. 1997;100(5–6):651–656. doi: 10.1007/s004390050568. https://doi.org/10.1007/s004390050568 [DOI] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci. 2002;22(11):4249–4263. doi: 10.1523/JNEUROSCI.22-11-04249.2002. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CA, Riley PA. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorganic and Medicinal Chemistry. 2014 doi: 10.1016/j.bmc.2014.02.048. https://doi.org/10.1016/j.bmc.2014.02.048 [DOI] [PubMed]
- Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. https://doi.org/10.1146/annurev.genet.37.110801.143233 [DOI] [PubMed] [Google Scholar]
- Roffler-Tarlov S, Liu JH, Naumova EN, Bernal-Ayala MM, Mason CA. L-Dopa and the albino riddle: content of L-Dopa in the developing retina of pigmented and albino mice. PLoS One. 2013;8(3):e57184. doi: 10.1371/journal.pone.0057184. https://doi.org/10.1371/journal.pone.0057184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg T, Schwartz M. X-linked ocular albinism: prevalence and mutations--a national study. Eur J Hum Genet. 1998;6(6):570–577. doi: 10.1038/sj.ejhg.5200226. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9887374. [DOI] [PubMed] [Google Scholar]
- Saito H, Yasumoto K, Takeda K, Takahashi K, Yamamoto H, Shibahara S. Microphthalmia-associated transcription factor in the wnt signaling pathway. Pigment Cell Res. 2003;16(3):261–265. doi: 10.1034/j.1600-0749.2003.00039.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12753399. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer Á, Neptuno Rodríguez-López J, García-Cánovas F, García-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochimica et Biophysica Acta (BBA)/Protein Structure and Molecular. 1995;1247(1):1–11. doi: 10.1016/0167-4838(94)00204-t. https://doi.org/10.1016/0167-4838(94)00204-T [DOI] [PubMed] [Google Scholar]
- Sarna T, Burke JM, Korytowski W, Rozanowska M, Skumatz CM, Zareba A, Zareba M. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res. 2003;76(1):89–98. doi: 10.1016/s0014-4835(02)00247-6. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12589778. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuzuu A, Doi H, Masaka T, Sakakibara R. Monitoring Of Blood L-DOPA And L-DOPA Metabolite Concentrations And Adverse Events In Patients With Advanced Parkinsons Disease Receiving L-DOPA And Amantadine Combination Therapy: A Clinical Study. Research & Reviews: Journal of Pharmacy and Pharmaceutical Sciences. 2015;4(2) Retrieved from http://www.rroij.com/open-access/monitoring-of-blood-ldopa-and-ldopametabolite-concentrations-and-adverse-events-in-patients-with-advanced-parkinsons-disease-receiving-ldopa-and-amantadine-combination-therapy-a-clinical-study.php?aid=60941. [Google Scholar]
- Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol. 2010;42(7):1094–1104. doi: 10.1016/j.biocel.2010.03.023. https://doi.org/10.1016/j.biocel.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV, Bassi MT, Galli L, Renieri A, Bruttini M, … De Nigris F, et al. Analysis of the OA1 gene reveals mutations in only one-third of patients with X-linked ocular albinism. Hum Mol Genet. 1995;4(12):2319–2325. doi: 10.1093/hmg/4.12.2319. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8634705. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, D’Addio M, Alloni A, Baschirotto C, Valetti C, Cortese K, … Ballabio A. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet. 1999;23(1):108–112. doi: 10.1038/12715. https://doi.org/10.1038/12715 [DOI] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, … Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18(3):768–780. doi: 10.1091/mbc.E06-12-1066. https://doi.org/10.1091/mbc.E06-12-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Samaraweera P, Rosenberg B, Orlow SJ. Ocular albinism type 1: more than meets the eye. Pigment Cell Res. 2001;14(4):243–248. doi: 10.1034/j.1600-0749.2001.140403.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11549106. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function, and regulation. J Investig Dermatol Symp Proc. 2001;6(1):99–104. doi: 10.1046/j.0022-202x.2001.00010.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11764295. [DOI] [PubMed] [Google Scholar]
- Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi Syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15(4):251–257. doi: 10.1034/j.1600-0749.2002.02038.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12100490. [DOI] [PubMed] [Google Scholar]
- Shotelersuk V, Gahl WA. Hermansky-Pudlak syndrome: models for intracellular vesicle formation. Mol Genet Metab. 1998;65(2):85–96. doi: 10.1006/mgme.1998.2729. https://doi.org/10.1006/mgme.1998.2729 [DOI] [PubMed] [Google Scholar]
- Silverberg NB. Pediatric Vitiligo. Pediatric Clinics of North America. 2014 doi: 10.1016/j.pcl.2013.11.008. https://doi.org/10.1016/j.pcl.2013.11.008 [DOI] [PubMed]
- Simeonov DR, Wang X, Wang C, Sergeev Y, Dolinska M, Bower M, … Adams DR. DNA Variations in Oculocutaneous Albinism: An Updated Mutation List and Current Outstanding Issues in Molecular Diagnostics. Human Mutation. 2013;34(6):827–835. doi: 10.1002/humu.22315. https://doi.org/10.1002/humu.22315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. Journal of Dermatology. 2013 doi: 10.1111/1346-8138.12147. https://doi.org/10.1111/1346-8138.12147 [DOI] [PMC free article] [PubMed]
- Spritz RA, Hearing VJ., Jr Genetic disorders of pigmentation. Adv Hum Genet. 1994;22:1–45. doi: 10.1007/978-1-4757-9062-7_1. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7539206. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5(10):e12578. doi: 10.1371/journal.pone.0012578. https://doi.org/10.1371/journal.pone.0012578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90(4):1526–1530. doi: 10.1073/pnas.90.4.1526. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8434014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck MC. Albinism: Update on Ocular Features. Current Ophthalmology Reports. 2015;3(4):232–237. https://doi.org/10.1007/s40135-015-0083-7 [Google Scholar]
- Sturm RA, Box NF, Ramsay M. Human pigmentation genetics: the difference is only skin deep. Bioessays. 1998;20(9):712–721. doi: 10.1002/(SICI)1521-1878(199809)20:9<712::AID-BIES4>3.0.CO;2-I. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9819560. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL. Human pigmentation genes under environmental selection. Genome Biology. 2012;13(9):248. doi: 10.1186/gb-2012-13-9-248. https://doi.org/10.1186/gb-2012-13-9-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CG. Vision in albinism. Trans Am Ophthalmol Soc. 1996;94:1095. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8981720. [PMC free article] [PubMed] [Google Scholar]
- Summers CG. Albinism: classification, clinical characteristics, and recent findings. Optom Vis Sci. 2009;86(6):659–662. doi: 10.1097/OPX.0b013e3181a5254c. https://doi.org/10.1097/OPX.0b013e3181a5254c [DOI] [PubMed] [Google Scholar]
- Summers CG, Connett JE, Holleschau AM, Anderson JL, De Becker I, McKay BS, Brilliant MH. Does levodopa improve vision in albinism? Results of a randomized, controlled clinical trial. Clin Experiment Ophthalmol. 2014 doi: 10.1111/ceo.12325. https://doi.org/10.1111/ceo.12325 [DOI] [PMC free article] [PubMed]
- Surace EM, Angeletti B, Ballabio A, Marigo V. Expression pattern of the ocular albinism type 1 (Oa1) gene in the murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2000;41(13):4333–4337. Retrieved from http://www.iovs.org/cgi/content/full/41/13/4333. [PubMed] [Google Scholar]
- Suzuki T, Tomita Y. Recent advances in genetic analyses of oculocutaneous albinism types 2 and 4. J Dermatol Sci. 2008;51(1):1–9. doi: 10.1016/j.jdermsci.2007.12.008. https://doi.org/10.1016/j.jdermsci.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Tak WJ, Kim MN, Hong CK, Ro BI, Song KY, Seo SJ. Ocular albinism with sensorineural deafness. International Journal of Dermatology. 2004;43(4):290–292. doi: 10.1111/j.1365-4632.2004.01857.x. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995;15(7 Pt 1):4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7623128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009a;5(4):563–564. doi: 10.4161/auto.5.4.8163. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19270489. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009b;4(1):e4160. doi: 10.1371/journal.pone.0004160. https://doi.org/10.1371/journal.pone.0004160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Takeda K, Yasumoto K, Udono T, Saito H, Ikeda K, … Shibahara S. Identification of a distal enhancer for the melanocyte-specific promoter of the MITF gene. Pigment Cell Res. 2002;15(3):201–211. doi: 10.1034/j.1600-0749.2002.01080.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12028584. [DOI] [PubMed] [Google Scholar]
- Winship I, Gericke G, Beighton P. X-linked inheritance of ocular albinism with late-onset sensorineural deafness. Am J Med Genet. 1984;19(4):797–803. doi: 10.1002/ajmg.1320190421. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6542750. [DOI] [PubMed] [Google Scholar]