Abstract
The current study’s aim is to prepare lipid based sustained release tablets via a twin-screw granulation technique and compare those dosage forms with conventional techniques, namely wet granulation and direct compression. The granules were successfully manufactured in a single-step, continuous twin-screw granulation process with a low proportion of binder (Klucel™ EF, HPC SSL) using Compritol® 888 ATO, Precirol® ATO 5 and Geleol™ as sustained release agents. The granules prepared showed good flow characteristics and compaction properties. DSC and XRD studies were conducted to characterize the granules prepared via a twin-screw granulation method and the results demonstrated the crystalline nature of lipids within the granules. FTIR data indicated that there were no interactions with the formulation components investigated. The formulations developed by all three methods were compressed into tablets with a mechanical strength of 14–16 KP. The tablets formulated were characterized for physicochemical properties, in vitro drug release studies, water uptake and erosion studies. These results showed that the drug was not completely released after 24h for tablets developed by the wet granulation process using all three lipids. The tablets prepared by the direct compression method demonstrated a burst release within 8 to 10h from Precirol ATO 5® and Geleol™ formulations compared to Compritol® 888 ATO. However, tablets prepared using twin-screw granulation exhibited sustained release of the drug over 24h and the water uptake and erosion results were in accordance with dissolution data. Stability data for 45 days at accelerated conditions (40 °C/75% RH) showed similar release profiles with f2 values above 50 for all of the twin screw granulation formulations, indicating the suitability of the process for formulating sustained release tablets. These findings of a single-step, continuous twin-screw granulation process are novel and demonstrate new opportunities for development of sustained release tablets.
Keywords: Twin-screw granulation, Sustained release, Lipid, Wet granulation, Binder
Graphics abstract
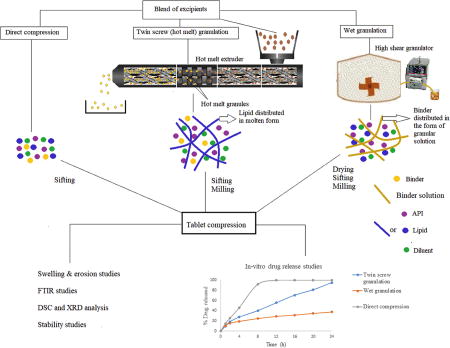
1. Introduction
In the formulation of sustained release (SR) tablet dosage forms, granulation is one of the most significant unit operations, which enables size enlargement of particles by agglomeration. These granules ensure uniform distribution of API within the dosage form. In addition, they enhance uniform flow, as well as compaction and compression properties, which makes the production process much easier and consistent (Shanmugam, 2015).
Recently, many novel granulation techniques like ultra-spray congealing (Passerini et al., 2006) and foam granulation (Thompson et al., 2012) were reported as techniques that utilize solvents in the granulation process (Hamdani et al., 2006). One of the most viable options for solvent free granulation is melt granulation. It generally utilizes molten binder, which melts or softens, aiding the agglomeration of fine particles to form granules. Since melt granulation is a solvent free method, it further eliminates the drying step. Thus it is considered more economical in terms of time and energy compared to the conventional wet granulation method (Dalziel et al., 2013). However, melt granulation has disadvantages such as the use of molten binder in high proportion (Hamdani et al., 2003) and the possibility of physico-chemical interactions between molten binder and the other formulation ingredients. These constraints limit the melt granulation process with respect to the formulation of dosage form and can compromise the quality of the final product (Maderuelo et al., 2011). Hot melt extrusion is a modern, versatile green technology with the capability of commercial scalability (Repka et al., 2007; Van den Mooter, 2012). Twin-screw granulation is a continuous process that is cost effective and reduces the processing time compared to the conventional granulation process. Furthermore, optimization of process parameters and screw configuration enables granules to be produced with desired strength and compressibility (Tan et al., 2014).
Theophylline, a water soluble and high melting point (M.P of 272–274 °C) drug, was used in this study to assess the application of twin-screw (hot melt) granulation with high melting point drugs. Solid lipid agents are one of the most feasible options to modify the drug release from the dosage form. The glycerides with relatively high melting points (at range of 60–80 °C) are chemically inert and control the water influx into the tablet matrix in all pH media. This property along with their applicability to the melt state and in the compression process made their role crucial in the formulation of modified release dosage forms (Fetih, 2010; Rao et al., 2009). In this study, three different lipids with variable physico-chemical properties were selected as functional sustained release agents. Compritol® 888 ATO, glyceryl behenate (National Formulary) (glyceryl dibehenate (European Pharmacopoeia), is a derivative of esterification of glycerin by behenic acid (C22 fatty acid) with a HLB value of 1. This is a hydrophobic mixture (%w/w) of mono (12 – 18%), di- (45 –54%) and tri- (28 – 32%) behenate of glycerol with a melting point range of 69 – 74 °C. Precirol® ATO 5 is glyceryl palmito-stearate, which consists of esters of palmitic (C16) and stearic (C18) acids. It has a HLB of 2 and a melting point of 50 – 60 °C. Geleol™ consists of 40 – 55% monoacylglycerols, 30 – 45% diacylglycerols, and 5 – 15% triacylglycerols of palmitic (C16) and stearic (C18) acids with HLB of 3 and a melting point of 54 – 64 °C (Aburahma and Badr-Eldin, 2014; Hamdani et al., 2003). There are very few reports published about the production of granules through twin-screw granulation with the relatively higher drug load. Therefore, there is a need of extensive study in this area of formulation development (Krause et al., 2009; Lakshman et al., 2011; Patil et al., 2015).
This study is mainly focused on the development of sustained release tablets using different processing techniques and the investigation of the impact of different formulation and process variables on granulation and tablet properties. This novel study differs from previous studies on twin-screw granulation because the process employed here is melting the solid lipid agent with softening of binder with less than 10% concentration to produce the granules with relatively higher drug load and good compression characteristics. The lowest possible processing temperature with shear in twin-screw granulation was studied to formulate the granules to produce tablets with desired sustained release characteristics. The process employed in this study is not a regular granulation process as it utilizes lipid in molten state, which was distributed between remaining ingredients with the assistance of shear generated by the twin-screw granulation process. Additionally, the formulations were also prepared via two other conventional techniques such as direct compression and wet granulation with suitable grades of excipients. The feasibility of all the processes to produce sustained release tablets were investigated. The effect of the excipients on process feasibility and effect of processing technique on the quality of the tablets was investigated.
2. Materials and methods
2.1. Materials
Theophylline anhydrous was purchased from Sigma Aldrich. Compritol® 888 ATO, Precirol® ATO 5 and Geleol™ were gift samples from Gattefosse (NJ, USA). The binders HPC SL and HPC SSL (hydroxy propyl cellulose) were purchased from Nisso America Inc. (Nippon soda, NY, USA). Klucel™ EF (hydroxy propyl cellulose) was supplied by Ashland (DE, USA). Kollidon® VA 64 was supplied by BASF (USA). The diluents Avicel PH® 102 and Avicel® DG (microcrystalline cellulose) were gift samples from FMC Biopolymer (USA). Magnesium stearate was purchased from Alfa Aesar (USA). All the other chemicals employed in this study were of analytical grade.
2.2. Selection of excipients and preparation of physical blend
The lipids with different lipophilicities, crystalline nature and compactness were utilized in the formulation of SR tablets. The binders of lower viscosity grade (M.W 40000 – 80000) were selected to prepare SR tablets by different techniques. The main interest of employing binder in these formulations was to provide the required mechanical strength to the formulation to execute the process properly without interfering with the drug release pattern. Different grades of water insoluble diluent microcrystalline cellulose (MCC) were selected based on their suitability to the technique employed. The formulation compositions for the preparation of SR tablets by wet granulation (WG) (F1 –F6), direct compression (DC) (F7–F12) and twin screw granulation (TSG) (F13 – F18 & F19 –F30), with different lipids and binders proportions are in Table 1 and Table 2. The required ingredients except lubricant in each formulation were weighed accurately and sifted through #30 ASTM mesh. The physical mixture was blended in a V-shell blender (Maxiblend®, Globe Pharma) for 15 min at 20 RPM. The moisture content of the dry blend was determined by using a moisture balance (MB45, Ohaus).
Table 1.
Compositions of wet granulation, direct compression and twin screw granulation formulations.
| Formulation Ingredients* |
F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Theophylline | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 |
| Compritol® ATO 888 | 20.0 | - | - | 10.0 | 10.0 | - | 20.0 | - | - | 10.0 | 10.0 | - | 20.0 | - | - | 10.0 | 10.0 | - |
| Precirol® ATO 5 | - | 20.0 | - | 10.0 | - | 10.0 | - | 20.0 | - | 10.0 | - | 10.0 | - | 20.0 | - | 10.0 | - | 10.0 |
| Geleol™ | - | - | 20.0 | - | 10.0 | 10.0 | - | - | 20.0 | - | 10.0 | 10.0 | - | - | 20.0 | - | 10.0 | 10.0 |
| Avicel® 102 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | - | - | - | - | - | - |
| Avicel® DG | - | - | - | - | - | - | - | - | - | - | - | - | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 |
| HPC SL | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| HPC SSL | - | - | - | - | - | - | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | - | - | - | - | - | - |
| Klucel™ EF | - | - | - | - | - | - | - | - | - | - | - | - | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Magnesium stearate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
All the amounts are expressed as % w/w
Table 2.
Compositions of twin screw granulation formulations with different binders and different methods of binder incorporation.
| Formulation Ingredients* | F19 | F20 | F21 | F22 | F23 | F24 | F25 | F26 | F27 | F28 | F29 | F30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Theophylline | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 |
| Compritol® 888 ATO | 20.0 | 20.0 | 20.0 | - | - | - | - | - | - | 10.0 | 10.0 | - |
| Precirol® ATO 5 | - | - | - | 20.0 | 20.0 | 20.0 | - | - | - | 10.0 | - | 10.0 |
| Geleol™ | - | - | - | - | - | - | 20.0 | 20.0 | 20.0 | - | 10.0 | 10.0 |
| Avicel® DG | 39.5 | 39.5 | 38.5 | 39.5 | 39.5 | 38.5 | 39.5 | 39.5 | 38.5 | 39.5 | 39.5 | 39.5 |
| Klucel™ EF IG | - | 3.0 | 4.0 | - | 3.0 | 4.0 | - | 3.0 | 4.0 | 3.0 | 3.0 | 3.0 |
| HPC SSL (IG) | 7.0 | - | - | 7.0 | - | - | 7.0 | - | - | - | - | - |
| HPC SSL (EG) | - | 4.0 | 4.0 | - | 4.0 | 4.0 | - | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Magnesium stearate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
All the amounts are expressed as % w/w
2.3. Wet granulation
Wet granulation of batch size 200 g was performed on GMX-LAB Micro® (Freund Vector) using a 1 L bowl. Before the granulation process, the rate of binder addition was calibrated by the peristaltic pump. Dry mixing was performed for 5 min at 250 RPM, and granulation was performed by adding aqueous binder (HPC SL) solution at a constant rate (20 – 22 g/min) with an impeller speed of 250 RPM and kneading time of 1 min after granulation at a chopper speed of 450 RPM. The water uptake capacity of the blend was calculated by the following equation:
| (1) |
The granules obtained were dried in an oven (Precision, Econotherm) at 45° C, and loss of drying was recorded by using moisture analyzer (MB 45, Ohaus). The granules were collected and milled in a laboratory mixer and sifted through #25 ASTM mesh. The fractions of #25 ASTM retained and #120 ASTM passed granules were rejected. The fraction of granules collected was lubricated with required amount of accurately weighed magnesium stearate (sifted through #60 ASTM mesh) using V-shell blender (Maxiblend®, Globe Pharma) for 3 min.
2.4. Direct compression
The physical blend was lubricated with accurately weighed amount of magnesium stearate (previously sifted through #60 ASTM mesh) for 3 min using V-shell blender (Maxiblend®, Globe Pharma).
2.5. Twin screw granulation
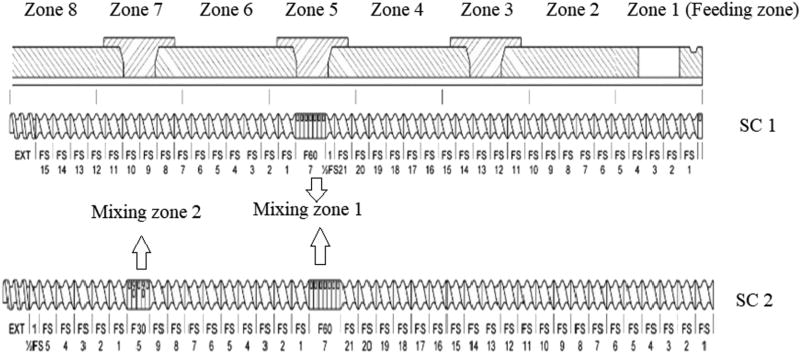
Twin-screw granulation was performed on 11mm twin screw extruder (Process 11 Thermo Fisher Scientific), and the feed rate was calibrated before granulation. Based on preliminary studies the temperature from Zone 2 to Zone 8 were maintained at 95, 110, 120, 120, 105, 85 and 75°C, respectively, for Compritol® 888 ATO formulations; 80, 95, 110, 110, 90, 75 and 65°C, respectively, for Precirol® ATO 5 and Geleol™ formulations. Zone 1 (feeding zone) was at ambient temperature. For formulations with combination of lipids, the temperature range was selected based on the lipid with the higher M.P. The screw configuration employed is represented schematically in Fig. 1. A modified screw configuration of one mixing zone at Zone 5 (with 7 mixing elements making an angle of 60° with each other) was tried initially. Later, based on the results of granulation, the configuration was altered to two mixing zones with the first mixing zone at Zone 5 (with 7 mixing elements making an angle of 60° with each other) and an additional mixing zone at Zone 7 (with 5 mixing elements making an angle of 30 ° with each other). The temperature was set at the maximum at Zone 5 (first mixing zone) at about 40 – 45°C above the M.P. of the lipid employed. The die plate was removed to collect the granules. The residence time was calculated by introducing the food color, blue (powder), into the feeding zone after the barrel was completely filled with blend and the time point of the first appearance of color in the granules was recorded. The granule formation, torque at the point of granulation and the appearance of the granules were recorded, and granules obtained were allowed to cool at room temperature. The granules were milled by laboratory mixer, sifted through #25 ASTM mesh and lubricated with magnesium stearate (previously sifted through #60 ASTM mesh) using maxiblender at 20 RPM for 3 min.
Figure 1.
Different screw configurations of twin screw granulation process
2.6. Preparation of SR Tablets
The SR tablets were prepared by three methods: wet granulation, direct compression and twin-screw granulation. The lubricated physical blend, granules of the wet granulation method and granules of twin screw granulation were compressed into tablets (600±10 mg) using 11mm round flat punches with hardness of 14 – 16 KP on Piccola 10 station tablet compression machine equipped with a gravimetric hopper at turret speed of 20 RPM.
2.7. High performance liquid chromatography Analysis
Quantitative high performance liquid chromatography (HPLC) was performed as reported in USP 38 on an isocratic high performance liquid chromatography (Waters Corp., Milford, MA, USA) equipped with an auto sampler, UV/VIS detector and Empower software. The analytical column Phenomenex Luna C18 (5µm, 250 mm × 4.6 mm) was used to analyze theophylline at a detection wavelength of 280 nm. The composition of the mobile phase employed was 92:7:1 (% v/v/v) of 0.01 M sodium acetate trihydrate buffer: acetonitrile: glacial acetic acid. The mobile phase was pumped from the solvent reservoir to the column at a flow rate of 1 mL/min. The samples were filtered through 0.22µ filter (Millex® GV, Durapore® PVDF) before being injected into the column, and the injection volume was 10µL.
2.8. Pre-compression characteristics
The flow properties of the physical blend for direct compression, granules of wet granulation and twin screw granulation were assessed by the angle of repose and Carr’s index (Gaber et al., 2015; Patil et al., 2015).
| (2) |
| (3) |
2.9. Characterization of the tablets
The compressed tablets were characterized for physical properties such as hardness, friability and weight variation as per compendial procedures. The friability was assessed using FT2 friability tester (Schleuniger® Pharmatron), and the mechanical strength of tablets was determined using hardness tester (Schleuniger® Pharmatron). The compressed tablets were evaluated for assay and content uniformity.
2.10. Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) studies were conducted on an Agilent Cary 660 FTIR Spectrometer (Agilent Technologies, Santa Clara, CA) in the range of 4000 – 650 cm−1 to study the interaction between the theophylline and the lipid components in the physical mixture and in granules. The bench was equipped with an ATR (Pike Technologies MIRacle ATR, Madison, WI), which was fitted with a single-bounce, diamond-coated ZnSe internal reflection element.
2.11. Differential scanning calorimetry
Differential scanning calorimetry (DSC) analysis was performed using Diamond DSC, PerkinElmer with Pyris manager software (PerkinElmer Life and Analytical Sciences, CT, USA). Approximately 5 mg of sample was weighed and hermetically sealed in an aluminum pan. An empty aluminum pan was used for reference. The heating rate was set at 10°C/min from 25°C to 150°C under an inert atmosphere of nitrogen at a flow rate of 20 mL/min.
2.12. X-ray diffraction analysis
The X-ray diffraction (XRD) analysis was carried out on a Rigaku X-ray equipment (D/MAX-2500PC, Rigaku Corporation, Tokyo, Japan) equipped with a copper tube anode using a step width of 0.02°/s over a range from 5° to 40° at room temperature on a 2θ scale. The operating conditions for the experiment were voltage at 40 kV, current at 100 mA and scanning speed at 10°/min.
2.13. Water uptake studies
Swelling and erosion studies were conducted according to the slightly modified reported method (Sriamornsak et al., 2007). The accurately weighed tablets were placed in the dissolution medium with a pH of 1.2 for 2 hours followed by a pH 6.8 phosphate buffer. The tablets were gently removed at regular time points as specified in the dissolution study and placed on a preweighed sieve ASTM #120. The tablets were gently blotted with a soft tissue paper to remove excess test medium and accurately weighed on analytical balance (XSE 204, Mettler Toledo). Each study was carried out in triplicates, and fresh samples were studied for each time point. With the equation below, the percentage of weight change was calculated for each formulation.
| (4) |
2.14. Matrix erosion studies
Matrix erosion studies were performed as per the method reported (Roy and Rohera, 2002). The samples from the water uptake studies were placed in an oven at 50 °C for 24 hours for complete drying and then cooled to ambient temperature. The final dry weight was recorded. The study was performed in triplicates. The tablet erosion at each time point was determined by employing the following equation:
| (5) |
2.15. In vitro drug release studies
The release of theophylline from the SR tablets was studied using USP apparatus type II (SR8-plus™, Hanson), maintained at 37 ± 0.5 °C and 50 RPM (n = 6). The study was conducted in 300 mL of 0.1 N HCl with pH 1.2 for the first 2 hours, followed by phosphate buffer pH 6.8 with a total media volume of 1000 mL over a period of 24 hours. Aliquots of 2 mL were collected at predetermined time intervals (1, 2, 4, 6, 8, 10, 12, 16, 20 and 24 h) and replenished with an equivalent volume of fresh medium. The samples were filtered through a 0.45 µm membrane filter and estimated for theophylline content by HPLC analysis.
2.16. Drug release kinetics
In vitro drug release data of selected formulations was subjected to different mathematical models (first order, zero order, Higuchi, Korsmeyer-Peppas and Hixon-Crowell models) to assess the rate and mechanism of drug release (Costa and Lobo, 2001; Higuchi, 1963; Korsmeyer et al., 1983).
| (6) |
Where Q is the amount of drug released in time t, Q0 denotes the initial drug amount and k stands for the rate constant.
| (7) |
Where Q is the denotes of drug released in time t, Q0 represents the initial drug amount and k stands for the rate constant
| (8) |
Where Q represents the amount of drug released in time t, and k denotes the rate constant.
| (9) |
Where Q represents the amount of drug released in time t, k denotes the rate constant and n is the diffusion exponent, which designates the mechanism of drug release.
| (10) |
Where Qt represents the amount of drug released in time t, Q0 denotes the initial drug amount and k stands for the rate constant.
2.17. Stability studies
The stability studies were carried out at 40°C and 75% RH for 45 days for the selected formulations (F1, F2, F3, F7, F8, F9, F20, F23 and F26) with higher lipid proportion in all techniques. Thirty tablets from each of the formulations were placed in a HDPE container and placed in a stability chamber (Caron, 6030) for 45 days. Samples were removed from the chamber and tested for drug content and dissolution. The results were recorded, and the similarity factor (f2) was calculated as per the implementations of U.S. FDA. Dissolution profiles between the initial and stability samples of formulations were compared (Pillay and Fassihi, 1998; U.S.FDA, 1997). The f2 is a logarithmic transformation of the sum-squared error of differences between the initial and stability samples. It was calculated using the following equation:
| (11) |
Where Rt represents cumulative drug release rate of the initial sample, Tt is cumulative release rates of stability sample at the predetermined time point and n denotes the number of time points. The f2 value ≥50 infers the similarity between the dissolution profiles of initial and stability samples.
2.18. Statistical analysis
The statistical analysis of the in vitro drug release profiles was performed using Prism software (GraphPad Prism 7.03, GraphPad Software Inc., La Jolla, CA, USA). Two-way ANOVA followed by Bonferroni post-hoc test was performed to compare the drug release profiles of different formulations up to 24 hours. Difference of p-value below 0.05 was considered statistically significant.
3. Results & Discussion
3.1. Process parameters of wet granulation
The bulk densities (B.D) and occupancy of the physical blends of different formulations were in the range of 0.40 – 0.50 g/ml and 40 – 60%, respectively. The bulk densities were observed to be 0.379, 0.392 and 0.414 g/ml for Compritol® 888 ATO, Precirol® ATO 5 and Geleol™ formulations, respectively. The water uptake for successful granulation was assessed based on preliminary trials. The water uptake was in the range of 47.8 – 55.46% with all lipids studied and was optimum to produce granules for tableting. The granules obtained were free from lumps and dead spots with similar granulation time (2.5 to 3 min) for all lipids studied. However, there were reports of poor granulation using the hydrophobic material because of the hindrance of formation of liquid bridges between the particles of the blend because of high contact angle between the blend and the binder solution (Simons and Fairbrother, 2000). In this study, the homogenous granulation was observed and may be due to the presence of water absorbable diluent, water soluble drug and binder in the formulation. The total wettability of the formulation was improved by HPC SL binder followed by the destructive nucleation of hydrophobic portion by shear force generated by the impeller (Vonk et al., 1997). After drying, final moisture content, determined by moisture analyzer (MB 45, Ohaus), showed values in the range of 2.11 – 2.46 % depending on the moisture content of the respective physical mixture.
3.2. Screening of binder for twin screw granulation
The preliminary studies of twin-screw granulation were conducted with similar binder (HPC SL) and diluent grade (Avicel® 102PH) used in wet granulation and produced poor granules, which was in contrast with the results of wet granulation. This suggests the need for selecting appropriate binder and diluent for twin-screw granulation. As an alternative, Klucel™ EF (M.W 80,000) and Kollidon® VA 64 (M.W 45000–70000) were selected as binders initially with Avicel DG® as the diluent in twin-screw granulation. These lower molecular weight binders were selected to avoid the possible interference of high molecular weight binders on the drug release pattern. Both of the binders employed produced good quality granules, which was evident from a low percentage of fines. Finally, Klucel™ EF at concentrations of 2.5 to 7.5 % was used in further studies because of its ability to form granules at lower concentrations with all lipids. The granule forming ability varied with the type and proportion of lipid in the formulation based on physical nature and compactness of the lipid.
3.3. Process parameters in twin screw granulation
The various process parameters of twin-screw granulation are shown in Table 3. The processable torque values were observed with employed feed rate and this suggests the suitability of granulation with desired characteristics using twin-screw extruder.
Table 3.
Process parameters of twin screw granulation.
| Formulation Code |
% Feed rate (kg/h) | RPM | Torque (N-m) (Maximum torque is equivalent to 12 N-m) |
|---|---|---|---|
| F19 | 0.262± 0.007 | 40 | 1.20–1.45 |
| F20 | 0.258± 0.011 | 40 | 1.24–1.52 |
| F21 | 0.260± 0.006 | 40 | 1.31–1.62 |
| F22 | 0.268± 0.009 | 40 | 1.19–1.51 |
| F23 | 0.271± 0.010 | 40 | 1.15–1.48 |
| F24 | 0.264± 0.006 | 40 | 1.19–1.46 |
| F25 | 0.274± 0.008 | 40 | 1.37–1.58 |
| F26 | 0.266± 0.010 | 40 | 1.39–1.61 |
| F27 | 0.269± 0.005 | 40 | 1.42–1.66 |
| F28 | 0.256± 0.009 | 40 | 1.36–1.53 |
| F29 | 0.260± 0.006 | 40 | 1.41–1.59 |
| F30 | 0.263± 0.012 | 40 | 1.42–1.62 |
3.3.1. Effect of feed rate and screw speed
The effect of feed rate was studied in the range of 4 –7 g/min and screw speed of 40 – 100 RPM, which showed substantial impact on the formation of granules. The formulations with feed rate of 5 – 6 g/min and screw speed of 40 RPM showed the formation of granules with lower percent (less than 10% of particles below 125 µm) of fines. With increased feed rate (>7 g/min), the granules were not completely formed, which may be due to insufficient shear in barrel because of overload of feed. However, with very low feed rate (1 – 2 g/min) more than 40% fines were obtained. This may be due to crushing of granules between elements because of high shear in the barrel. The residence time was in the range of 2 – 3 min.
3.3.2. Effect of Screw configuration
Initial screw configuration with a single mixing zone at Zone 5, containing 7 mixing elements with angle of 60° with each other, produced partial granules due to less shear in the extruder barrel. However, introduction of an additional mixing zone at Zone 7 with 5 mixing elements with angle 30° with each other produced desirable granules. This granule formation was due to an additional mixing zone and the first mixing zone at Zone 5 which assisted the molten lipid distribution and aided granulation followed by consolidation of granules by the additional shear generated at mixing Zone 7.
3.4. Process of Direct Compression
Initially, tablets were formulated using HPC-SL (d90:155µ) binder which showed poor compressibility and as a result capping and lamination issues were observed. Eventually, the binder was replaced with HPC SSL, which comprises a lower particle size (d90: 85µ) and hence resulted in good compressibility and tableting properties. The reason for this improvement is the distribution of finer binder particles throughout the blend leading to greater cohesion properties of the blend via enhanced Vander Waals forces. (Mangal et al., 2016). Thus, in this study the tablets prepared using HPC SSL showed desirable mechanical strength of 14–16 KP with good tableting characteristics.
3.5. Pre-compressional and compressional characteristics
The pre-compressional and compressional characteristics of highest lipid concentration formulations prepared by all three methods were shown in table 4. The flow characteristics determined using the angle of repose values for the formulations using twin screw granulation and wet granulation were excellent compared to direct compression blend, whereas, Carr’s Index values for the formulations were in the increasing order of melt granulation, wet granulation and direct compression methods. All formulations showed a hardness between 14–16 KP. Weight variation (within ±2%), assay (97.4±1.04 −102±2.01%) and content uniformity values of formulations are within the pharmacopeial limits indicating the suitability of the methods.
Table 4.
Physical properties and assay values of wet granulation, direct compression and twin screw granulation formulations.
| Formulation code |
Flow properties | Physical properties of tablets | ||||
|---|---|---|---|---|---|---|
| Angle of repose |
Carr's Index (%) |
Hardness (KP) |
%Friability | Thickness(mm) | Assay (%) | |
| F1 | 28.3± 0.75 | 12.7± 1.75 | 16.3±1.22 | 0.16±0.01 | 5.98±0.05 | 98.8±1.14 |
| F2 | 27.5±0.93 | 14.3± 0.85 | 15.9±0.99 | 0.13±0.04 | 5.89±0.04 | 97.1±1.53 |
| F3 | 26.2± 1.11 | 13.9± 0.64 | 15.8±1.41 | 0.12±0.02 | 5.86±0.07 | 101±2.11 |
| F7 | 34.4± 0.39 | 21.6± 0.91 | 14.4±1.47 | 0.25±0.04 | 6.05±0.03 | 99.5±2.31 |
| F8 | 33.8± 1.12 | 20.3± 1.55 | 15.1±0.93 | 0.38±0.09 | 6.09±0.04 | 101±1.24 |
| F9 | 33.1± 2.12 | 19.8± 0.67 | 14.6±0.56 | 0.42±0.06 | 6.11±0.06 | 97.9±0.94 |
| F20 | 25.9± 1.63 | 12.3± 0.39 | 15.8±0.58 | 0.12±0.03 | 5.49±0.02 | 102±1.26 |
| F23 | 26.2± 1.85 | 13.1± 0.92 | 16.2±1.25 | 0.14±0.06 | 5.79±0.06 | 98.1±1.28 |
| F26 | 27.3± 1.47 | 11.9± 0.43 | 15.4±1.49 | 0.11±0.03 | 5.55±0.04 | 100.3±2.34 |
3.6. FTIR analysis
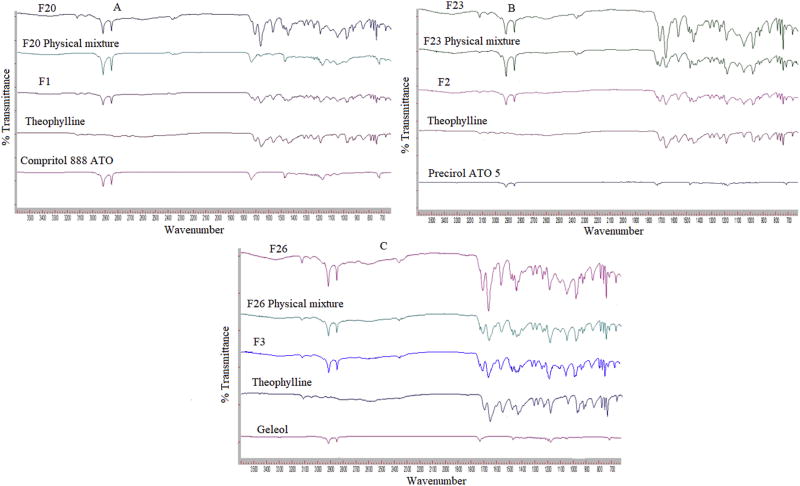
The FTIR spectra of theophylline, physical mixture and formulations for all lipids are represented in Fig. 2. The infrared spectra of theophylline revealed characteristic peak at 1660 cm−1 representing the imide group of heterocyclic ring, C-N stretching vibration at 1239 cm−1, NH stretching vibration at 3119 cm−1, C-N stretching at 1285 cm−1 and N-H bending vibration at 1561 cm−1 (Bashir et al., 2016; Gunasekaran et al., 2005). The FT-IR spectra for Compritol® 888 ATO shown characteristic peaks at 2916, 1737 and 1471 cm−1 due to the C-H stretching, C=O stretching and C-H bending respectively (Shah et al., 2016). Precirol ATO 5 spectra showed peaks at 1730 cm−1 due to C=O stretching of carbonyl group and 2913 cm−1 of C-H stretching, which are characteristic of glyceryl palmitostearate (Deore et al., 2010). Geleol™ showed characteristic bands of C-H stretching at 1200–1000 cm−1 and C-H bending at 717 cm−1 (Gardouh et al., 2013).The FTIR spectra of all the formulations prepared showed the respective characteristic peaks of theophylline and respective lipid components indicating no interaction between the components of the formulation even after processing at different increased temperatures. This suggests suitability of these methods to formulate the sustained release formulation of theophylline with these lipids.
Figure 2.
FTIR spectra of physical mixture, wet granulation and twin screw granulation formulations of different lipids: A) Compritol®888 ATO; B) Precirol®ATO 5; C) Geleol™
3.7. Solid state characterization
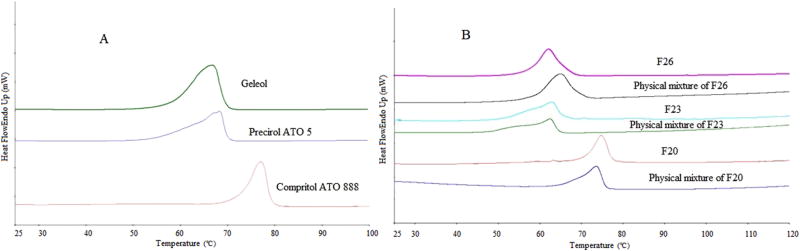
DSC thermograms of theophylline, lipids, physical mixtures and formulations are represented in Fig. 3. The thermograms of API and lipids showed characteristic melt endotherms at respective melting points. The DSC studies of the formulations F20, F23, F26 showed the characteristic peaks of the respective lipids at 74.9, 62.8 and 62.1°C, which would most likely be due to recrystallization of lipids during the event of cooling to room temperature. This also suggests that there is no interaction of the lipids with other components of the formulation (Small, 1984). These observations are in accordance with the results observed by Hamdani et al. 2003, which suggested that the recrystallized state of distributed lipid and the single peak of lipid indicated a stable form (β) of the lipid within the formulations. The absence of other crystalline peaks in the thermograms may be attributed due to normal cooling, i.e. allowing to cool to room temperature (Bhalekar et al., 2009). There have been similar investigations reported in the literature on lipid crystallization behavior (Malode et al., 2015; Windbergs et al., 2009).
Figure 3.
DSC thermograms of A) Compritol®888 ATO, Precirol®ATO 5, Geleol™; B) Physical mixtures and formulations of twin screw granulation (F20, F23 and F26)
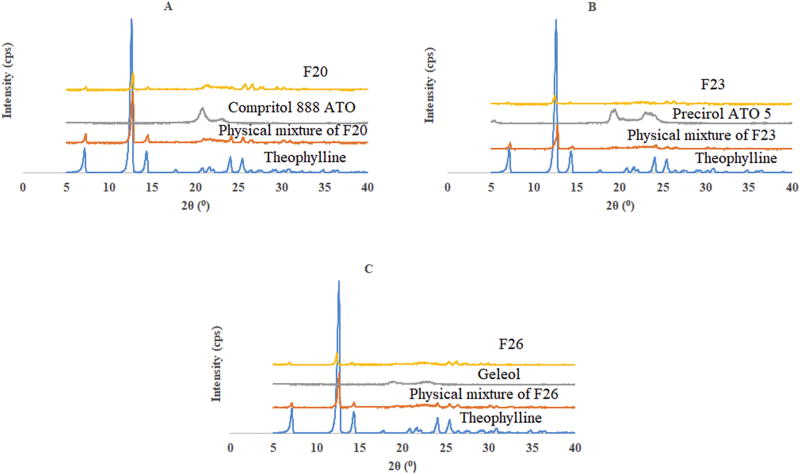
XRD spectra of theophylline, lipids, physical mixtures and formulations are represented in Fig. 4. These results confirm the recrystallization properties of lipids on cooling to room temperature. Lipid based dosage forms exhibit complex behavior with respect to their structural aspects based on the difference in lateral packing of fatty acid chains. Generally they exhibit 3 different 3-D structures: high energy α form, intermediate β1 form and the thermodynamically stable β form (Sato et al., 1999). In the theophylline sample at 2θ 12.6, 7.1 had greatest intensities followed by low intense peaks at 14.3, 24.0, 25.5 and 26.1. For Compritol® 888 ATO intense peaks were observed in the range 20.8 (2θ) and comparatively low intense peaks at 23.3 and 19.9 (2θ). Precirol® ATO 5 showed the high intensity peaks at 19.5, 22.9 and 23.4 and for Geleol™ at 18.9, 19.3, and 22.4 (2θ). In the granules of F20 the peaks at 12.8, 21.6, and 25.8 correspond to the API and peaks observed at 20.7 and 24.5 (2θ) indicate the presence of crystalline Compritol® 888 ATO. In the spectra of F23 intense peaks at 22.5 and 23.3 (2θ) indicate the crystalline Precirol® ATO 5 and 12.5, 7.0, 14.2, 25.5 and 26.4 (2θ) represent the theophylline peaks. Similarly, the characteristic peaks in F26 at 12.4, 6.9, 14.4, 25.3, 26.2 (2θ) are evident for API and 22.7 and 19.1 (2θ) represent the crystalline Geleol™. These results indicate the recrystallization of the stable lipid form and crystalline state of theophylline. This crystallinity of theophylline was due to lower processing temperatures employed (well below the theophylline M.P) during granulation processing followed by compression into tablets. The reason for the recrystallization of lipids may be attributed to distribution of the molten lipids with the solid components of the formulation in which it existed as an immiscible phase with rest of the ingredients. Additionally, the change in the temperature (from the last extrusion zone to room temperature) was sudden rather than a slow progressive cooling of granules. Furthermore, the application of shear in the granulation process promotes dynamic interactions of meta stable forms of the lipids and eventually leads to crystallization of the lipid into its stable form (β) (Sato, 2001).
Figure 4.
XRD spectra of theophylline, physical mixture and corresponding lipid of formulations A) F20; B) F23; C) F26
3.8. Water uptake and matrix erosion studies
The water uptake studies and erosion studies are shown in Fig. 5 and Fig. 6. The water uptake values were represented up to a point where the tablet weight did not fall below the initial tablet weight. The formulated systems contain one-third of the amount of the water soluble drug load in the matrix and the drug release is controlled by the hydrophobic lipid material. These systems differ from systems which contain hydrophilic polymers in that the drug release is controlled by the amount of aqueous phase entering the matrix and how much drug is dissolved in the aqueous phase and diffuses out (Nokhodchi et al., 2012; Rosiaux et al., 2014). The lipids employed have different affinities towards the aqueous environment and the mechanism of drug release differ from one to another as the formulations were prepared by different manufacturing techniques. The amount of water uptake and the stability of the matrix during the dissolution are the crucial factors to be considered and to investigate that water uptake and erosion studies were performed. The formulations with high single lipid content were selected for these water uptake and erosion studies in direct compression and twin-screw granulation methods, whereas all of the formulations of the wet granulation method were selected for the study.
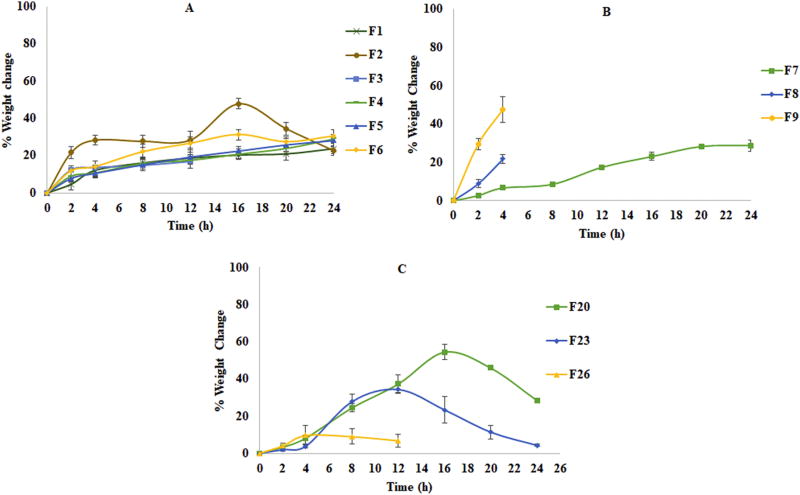
Figure 5.
Water uptake studies of formulations (mean ± SD; n=3) A) Wet granulation (F1–F6); B) Direct compression (F7–F9); C) Twin screw granulation (F20, F23 and F26)
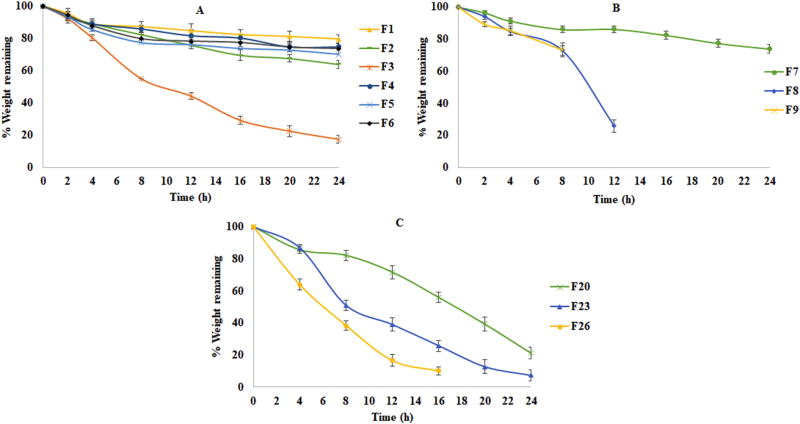
Figure 6.
Erosion studies of formulations (mean ± SD; n=3). A) Wet granulation (F1–F6); B) Direct compression (F7–F9); C) Twin screw granulation (F20, F23 and F26)
The DC formulations demonstrated water uptake values in accordance with the HLB value of the lipid components within the matrix system: Compritol® 888 ATO (HLB-1), Precirol® ATO 5 (HLB-2) and Geleol™ (HLB-3). The water uptake of the formulations was found to be in the order of F7> F8> F9 (Fig. 5B and Fig. 6B). The physical observations made during the study indicated that the Compritol® 888 ATO based formulation F7 was mostly intact and non-disintegrating until the end of the 24h study period with 28.8% uptake. In contrast, Precirol® ATO 5 and Geleol™ based formulations lost their integrity at 12h and 10h, respectively. These observations may be attributed to the longer lipid chain (C22) of Compritol® 888 ATO compared to Precirol® ATO 5 and Geleol™ (C16 – C18). The disintegration of the Geleol™ formulations was faster than the Precirol® ATO 5 formulations and this could be explained by the high proportion of the distearate portion in Precirol® ATO 5 (Aburahma and Badr-Eldin, 2014; Rosiaux et al., 2014). At the end of 4h, the water uptake was very high in the case of the Geleol™ formulation (45.6%) compared to the Precirol® ATO 5 formulation (24.3%) and Compritol® 888 ATO formation (6.7%). These results demonstrate that the affinity of the formulation for aqueous media, which is dependent on the HLB of the lipid components, influences the stability of the tablet matrix. As the API is water soluble the amount of medium entering into the matrix will dissolve the API, producing empty spaces within the matrix, thus subsequently affecting the matrix integrity with time (Kreye et al., 2011). These results were also supported by the data from erosion studies where the maximum erosion of F9 (27.6%) occurred at 8h into the study followed by disintegration of the tablet. In contrast, F8 maintained its integrity up to 12h. F7 essentially demonstrated a non-disintegrating matrix where after 24h showed only a 26.1% reduction in weight of the dry matrix. This attests to the integrity of Compritol® 888 ATO formulations. These results obtained can be correlated with the dissolution profiles.
The formulations of WG technique showed better matrix integrity compared to the formulations of direct compression. This may be attributed due to the binder solution which forms the liquid bridges between the particles and strengthens the bond by formation of solid bridges upon evaporation of the solvent when the granules are dried (Cai et al., 2013). In the water uptake studies (Fig. 5A and Fig. 6A), the minimum increment in percentage of weight was shown by formulation F1 that consists of Compritol® 888 ATO with relatively low erosion behavior. The Precirol® ATO 5 and Geleol™ formulations showed disintegration behavior with the time and Geleol™ formulations shown comparatively more erosion over the time. The % water uptake in these formulations (single lipid component and lipid combinations) was affected by lipid components employed and their HLB values. The erosion behavior also suggests that the increase in matrix weight depends on water uptake and the disintegration behavior of the tablet matrix. Minimum erosion was observed in formulations F1 that might be due to very less water intake due to lower HLB of the lipid agent, which is providing more integrity to the matrix. The erosion levels of the lipid combination formulations were less in Compritol® 888 ATO formulations compared to Geleol™ formulations. This might be due to the comparatively more hydrophilic nature of Geleol™. These results indicate that in these formulations the erosion was controlled by the HLB value of the lipids in combination and the type of lipid.
In TSG formulations (Fig. 5C and Fig. 6C), Compritol® 888 ATO formulations showed non-disintegrating behavior up to 16 h and relatively less erosion behavior about 79% matrix eroded at end of 24 h. While the Precirol® ATO 5 formulations showed comparatively more water uptake and erosion behavior and shown 100% erosion of matrix at 24 h. Geleol™ based formulations showed mainly erosion based behavior over the time period of test and the tablets disintegrated after 16 h. A balance between water uptake and erosion was reflecting the HLBs of the lipid components used. The TSG tablets showed more stable tablet matrix compared to the directly compressed tablets. The reason for the stability or integrity might be due to granular form of the formulation compressed into tablet which makes the matrix behave more accordingly with HLB of the lipid and the increased tortuosity of lipid matrix due to melt granulation might be promoting relatively less water uptake and greater integrity of matrix (Nguyen et al., 2010).
3.9. In vitro drug release studies
The dissolution profiles of the formulations prepared by different techniques showed different (Fig. 7 and Fig. 8) release patterns based on their composition. Physical observations made during the dissolution studies of all formulations and the stability or integrity of the matrix was observed. The formulations shown variability in matrix stability during dissolution studies and that was reflected in the drug release profiles. The factors that played crucial role in the release behavior are the lipids used in the formulation and the integrity of the compressed tablet during the dissolution study over 24 h of study. The binder concentration, the mode and stage of incorporation of the binder affected the drug release profiles. The physical stability of the matrix was observed to be directly related to the drug release as more exposure of the surface area of matrix to aqueous media enhances the drug release.
Figure 7.
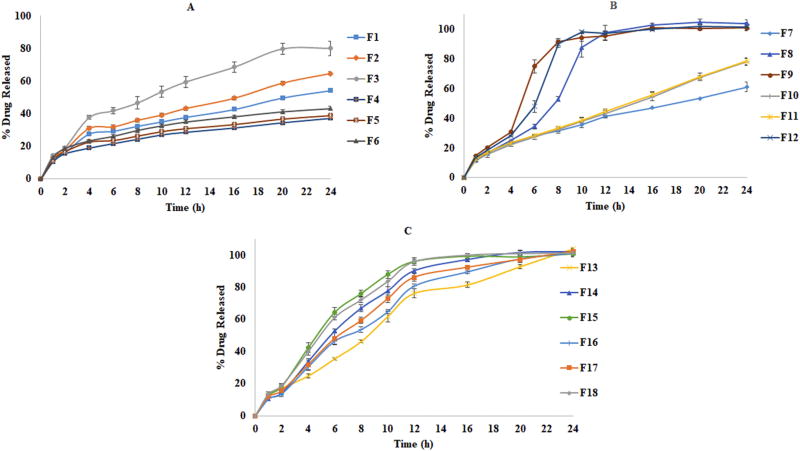
In vitro drug release profiles of formulations (mean ± SD; n=6) A) Wet granulation (F1–F6); B) Direct compression (F7–F12); C) Twin screw granulation (F13–F18)
Figure 8.
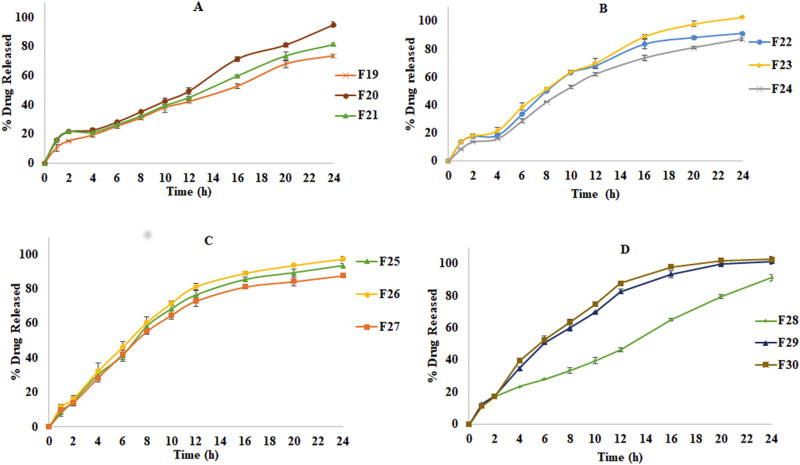
In vitro drug release profiles of formulations of twin screw granulation with different compositions of intra and extra granular binders (mean ± SD; n=6) A) F19, F20 and F21 (Compritol®888 ATO based formulations); B) F22, F23 and F24 (Precirol®ATO 5 based formulations); C) F25,F26 and F27 (Geleol™ based formulations) D) F28,F29 and F30 (formulations based on combination of lipids)
3.9.1. Effect of lipid on drug release in direct compression technique
In the formulations prepared by DC method (Fig. 7B), the formulation F7 with 20% Compritol® 888 ATO shown the highest sustained release profile due to the low HLB value. The tablet matrix was stable and not disintegrated in 24h and shown negligible amount of erosion, which is in correlation with the water uptake and erosion studies. The high lipophilic nature of the Compritol® 888 ATO due to higher carbon chain length (C22) along with its relatively higher melting point might have regulated the water influx and thereby controlled the drug dissolution and diffusion into the media.
The formulations with 20% of Precirol® ATO 5 (F8) and Geleol™ (F9) showed disintegration from 4 h with complete disintegration and drug release in 12 and 10 h, respectively indicating greater affinity of the tablet matrix for dissolution medium. There was a significant difference (p <0.05) in drug release profile between F7 and formulations F8, F9 F12. This difference in release profile is due to relatively high HLB lipids of Precirol® ATO 5 and Geleol™ at higher drug: lipid ratio which resulted greater exposure of drug to dissolution medium, associated with the formation of pores in the matrix due to solubilization of drug particles further affecting the matrix stability (Liu et al., 2001; Schulze and Winter, 2009). This might be the reason for burst release of drug during 4–8 h in F8, F9 and F12 followed by complete disintegration and total release. The release profiles of combination formulations F10 and F11 (approximately 80% release at the end of 24 h) were almost super imposable and showed no statistically significant difference with each other and the without any burst release compared to F8, F9. These release profiles were observed due to more hydrophobic nature of Compritol® 888 ATO part in those formulations. The high melting point of Compritol® 888 ATO, which is approximately 20°C greater than the melting points of Precirol® ATO 5 and Geleol™ could be an additional factor for relatively greater integrity of the matrix in Compritol® 888 ATO formulations. The reason for this is the possibility of relatively more weakening of bonds of triglycerides of lipids with lower melting point at a temperature of dissolution study (37°C) compared to lipids of higher melting point. This loss of structural integrity along with differences in HLB value of the lipids affected the drug release profiles. Similar observations were reported by Abd El-Halim et al. 2010. The combined effect of high melting point of Compritol® 888 ATO along with low HLB value (1) was reflected in drug release profiles.
The formulation F12, which contains Precirol® ATO 5, and Geleol™ which is less lipophilic than combinations involved Compritol® 888 ATO disintegrated and showed maximum release in 10 h. These results were in correlation with water uptake and erosion studies and in accordance with previous reports by Kreye et al. 2011.
3.9.2. Dissolution studies of twin screw granulation
3.9.2.1. Preliminary trials of twin screw granulation
The TSG formulations have demonstrated different release profiles compared to directly compressed formulations (Fig. 7C). The release profiles were more sustained (24 h) compared to direct compressed formulations, except Compritol® 888 ATO based formulations. The reason for greater sustained release might be the dispersion of lipid in the molten form associated with high shear produced by twin screws which makes, the more effective distribution of lipid between the particles and improved drug-lipid bonding assisted by the binder which agglomerates the particles into a granular form (Patel et al., 2007). The compact hydrophobic matrix formed due to redistribution of lipid from hot process is different from DC and WG and this increased matrix tortuosity was retarding the drug release into aqueous media (Zhang and Schwartz, 2003). The greater sustained release obtained in DC for Compritol® 888 ATO formulations might be due to greater integrity of Compritol® 888 ATO based matrices in the dissolution media. The greater integrity of matrices might be due to the relatively higher difference between M.P of Compritol® 888 ATO and temperature of dissolution media (El-Halim et al., 2010). The release patterns were dependent on the integrity of the tablet in media resulted from the granules of particular lipid, concentration of binder in melt granulation, presence of intra granular (IG) and extra granular (EG) binder content along with HLB of the lipid.
3.9.2.2. Effect of intra and extra granular binders on drug release
In preliminary granulation trials (Fig. 7C) all the formulations formulated with 6% Klucel™ EF as intra granular binder produced desired granules but disintegrating matrix and complete drug release between 12–24 h based on the composition. Formulations F13, F14 and F15 had a faster rate of release between 4–10 h and except F13 all formulations disintegrated after 12 h. This disintegrating behavior might be due to presence of Klucel™ EF, a water soluble binder that was promoting erosion and subsequently destabilizing the matrix over the time further leads to faster dissolution. The higher proportion of water soluble drug may be one of the factors for greater exposure of the drug to the medium after disintegration. The formulations with single lipid were further studied to evaluate the binder effect. The trials were designed with intra granular and the extra granular addition of binders Klucel™ EF and HPC SSL. The results suggest that formulations with different lipids shown different release patterns with respect to the procedure of binder incorporation and proportion of binder indicating the suitability of the composition (Fig. 8). Nature of binder and its concentration obviously improved sustained release profile of all the formulations. The balance between the proportion of Klucel™ EF and HPC SSL influenced the physical integrity of the matrix and drug release profiles. In all the formulations (F19, F22 and F25) replacement of intra granular binder Klucel™ EF with HPC SSL showed relatively more sustained release profiles (p <0.05) with reduced drug release between 4–10 h, as HPC SSL was observed to have relatively low erosion characteristics on tablet matrix due to its finer particle size and greater distribution characteristics in the blend. Addition of extra granular HPC SSL improved the tablet integrity, all lipids in a combination of 3% Klucel™ EF (IG) and 4% of HPC SSL (EG) showed sustained release profile up to 24 h. The reason for the improved sustained release characteristics might be due to the lesser erosion behavior promoted by HPC SSL as its smaller particle size (d90: 85µ) and better distribution properties could provide more tensile strength to the matrix (Arndt and Kleinebudde, 2017). When the proportion of intra granular binder (Klucel™ EF) was increased in combination with extra granular HPC SSL (4+4% of IG+EG) the release was further sustained. The reason might be the increase in total binder level that promotes gelling of matrix as the two binders are reported to improve the gelling properties. The increased Klucel™ EF proportion relatively increases intra granular viscosity of the formulation as Klucel™ EF contains more hydroxypropoxy substitutions (Desai et al., 2006). The effect of change of binder to HPC SSL and change of binder incorporation method (IG+EG) produced significantly different release profiles after 12h for Compritol®888 ATO and Precirol®ATO5 (p <0.05) based formulations. This suggests the relation between the physical integrity offered to a tablet by particular lipid and the type of binder and technique employed. The impact of change in concentration of IG and EG binder was observed to be less in Geleol™ formulations as they showed continuous erosion behavior. These results signify the role of physico-chemical properties of lipid and suitability of the binder. Based on these results a combination of 3% Klucel™ EF (IG) and 4% HPC SSL (EG) was chosen for combination formulations as this combination produced complete drug release over 24 h. In formulations with a combination of lipids, the formulation F28 (a combination of Compritol® 888 ATO and Precirol® ATO 5) demonstrated more sustained release than that of Geleol™ formulations, which might be due to the erosion behavior of Geleol™, as well as its higher hydrophilicity that further affected the matrix stability and release. These release profiles were in correlation with the water uptake and erosion behavior results of formulations with individual lipids.
3.9.3. Impact of nature of lipids in Wet granulation
In WG formulations the drug release profiles were well sustained and the stability of the matrix was greater over 24 h compared to TSG and DC formulations (Fig. 7A). The greater stability of the matrix and sustained release might be due to the liquid bridges of the aqueous binder solution formed between the particles of the blend, which provides more binding strength and makes intact granules when the water is evaporated by the drying process (Cai et al., 2013). Another factor is water-soluble theophylline, might be dissolved in the aqueous binder fluid and distributed between the lipid agent particles in the matrix helping in incorporation of drug in the hydrophobic part of the matrix. The dissolution profiles of formulations with a combination of lipids were expected to be intermediary of the release profiles of the formulations with single lipids as observed in the case of direct compression and twin-screw granulation. Remarkably, the formulations with combinations of lipids exhibited a much slower drug release than the formulations in which a single lipid was utilized. In all formulations of wet granulation, the release reflected the lipid’s HLB and drug release was significantly varied (p <0.05) after 4 h between the formulations with single lipids (F1, F2 and F3) compared to their respective combinations (F4, F5 and F6). The reason for the hindrance of drug release in combination formulations might be attributed to the difference in the chemical compositions (% of mono, di and tri glycerides) and chain lengths (Compritol® 888 ATO is a derivative of behenic acid (C22) whereas Precirol® ATO and Geleol™ are derivatives of palmitic acid (C16) and stearic acid (C18)) of the lipids involved in the formulations. Even though the water uptake studies showed that F1 exhibited minimum water uptake and erosion, the drug release was higher than the combination formulations. This suggests that even though the water uptake was more in formulations F4, F5 and F6 compared to F1, drug release was lower. Again, this might be due to variability in chain lengths and HLBs of lipid moieties distributed within the tablet matrices, therefore making drug diffusion through that environment a difficult process. This indicates the complexity in drug diffusion through the matrix due to the synergistic effect observed in formulations with combination of lipids. Thus, the factors observed to control drug release include strength of the bonds within the matrix, as well as uniformity in the lipid portion of the matrix.
These above observations reveal that drug release was more sustained and incomplete in wet granulation. In summary, TSG formulations demonstrated improved controlled-release over 24h compared to DC formulations and WG formulations.
3.10. Release kinetics
The release kinetics data for formulations with a single lipid of all techniques is represented in Table 5. The release kinetics varied with the type of lipid components in formulations and with the processing technique employed. The formulations prepared via WG technique followed zero order release profiles with Fickian diffusion mechanism except Geleol™ formulation, which showed anomalous non-Fickian diffusion. Similarly, TSG based formulations (F13 – F18) showed zero order release profiles. Formulations F14 and F15 with Precirol® ATO 5 and Geleol™ showed case II transport with n > 0.89. F13 with Compritol® 888 ATO based formulations showed non-Fickian diffusion. In formulations F19 – F30, the Compritol® 888 ATO formulations with intra and extra granular binder showed correlation with Hixon-Crowell equation. However, the rest of the formulations showed non-Fickian diffusion except F25 (Geleol™ 20%) which showed case II transport. The DC formulations, F7 with Compritol® 888 ATO, showed Fickian diffusion. Formulations with Geleol™ showed good correlation with Hixon-Crowell model when used alone and in combination with Precirol® ATO 5. These results showed how the nature of the lipid and the impact of the technique employed for the process influenced drug release kinetics. The release kinetics of Compritol® 888 ATO formuations showed non-Fickian anomalous transport with the application of twin -screw granulation due to the different matrix integrity and erosion behavior compared to DC and WG. The reason for this is the higher melting point of Compritol® 888 ATO, which was reflected, in the physical integrity of the tablet and drug release characteristics of DC tablets. However, TSG formulations showed varied matrix integrity and release characteristics compared to DC formulations. This could be attributed to recrystallization of lipid agents Compritol® 888 ATO, Precirol® ATO 5 and Geleol™ in TSG technique. This suggests the impact of processing technique and physico-chemical properties of the lipid agents in the development of sustained release tablets. The WG formulations mostly showed Fickian diffusion which might be due to the more compact nature of the matrix formed by aqueous granulation and the dissolved API in binder solution which makes the drug partitioning form hydrophobic matrix into the media a diffusion based process which is evident from drug release studies (Sudha et al., 2010). The diffusional exponent (n) value of WG formulations with combination of lipids was 0.35 – 0.38, suggesting the inclination to Fickian diffusion from the stable matrix. This might be the reason for the hindrance of drug release observed during in vitro drug release studies.
Table 5.
Release kinetics of formulations of wet granulation, direct compression and twin screw granulation.
| Formulation code |
Zero order |
First order |
Higuchi | Korsmeyer- Peppas |
Diffusional Exponent (n) |
Hixon –Crowell Correlation coefficient (R2) |
|---|---|---|---|---|---|---|
| Correlation coefficient (R2) | ||||||
| F1 | 0.9604 | 0.816 | 0.9858 | 0.9845 | 0.457 | 0.8566 |
| F2 | 0.9586 | 0.8219 | 0.9835 | 0.9795 | 0.478 | 0.8786 |
| F3 | 0.931 | 0.7731 | 0.9865 | 0.977 | 0.585 | 0.8356 |
| F7 | 0.9843 | 0.8832 | 0.9926 | 0.9956 | 0.479 | 0.928 |
| F8 | 0.8300 | 0.7634 | 0.8710 | 0.9573 | 0.681 | 0.7945 |
| F9 | 0.9330 | 0.9201 | 0.9211 | 0.9954 | 0.535 | 0.9286 |
| F13 | 0.9621 | 0.8474 | 0.9658 | 0.9641 | 0.669 | 0.8966 |
| F14 | 0.9012 | 0.7455 | 0.9833 | 0.9395 | 0.939 | 0.8725 |
| F15 | 0.9695 | 0.8659 | 0.9894 | 0.971 | .9314 | 0.9438 |
| F20 | 0.9873 | 0.8652 | 0.9256 | 0.8982 | 0.501 | 0.9819 |
| F23 | 0.9569 | 0.8414 | 0.9761 | 0.9225 | 0.674 | 0.8899 |
| F26 | 0.8816 | 0.7254 | 0.9652 | 0.9849 | 0.813 | 0.7864 |
3.11. Stability studies
The stability samples showed no variation in physical characteristics and assay values were comparable with initial samples and within the acceptable limits as per USP 40 (94 – 106%). The dissolution profiles of all twin-screw granulation formulations were similar to the initial samples with f2 value greater than 50. Only Compritol® 888 ATO based formulations showed similar release profiles in wet granulation and direct compression. This is in accordance to the previous studies where Compritol® 888 ATO is reported to be more stable due to its long carbon chain (C22) compared to Precirol® ATO 5 and Geleol™ (C16 – C18) and has resistance to structural changes (Hamdani et al., 2003). The dissolution profiles suggest the suitability of twin-screw granulation technique for all of the lipids used in this study, and it demonstrates the versatility of twin-screw granulation technique in formulating lipid based sustained release tablets.
4. Conclusion
The twin-screw granulation process was successfully performed for all the formulations with different lipids by varying the formulation and process conditions. The maximum range of binder concentration used in this study was 7–8% and the prepared granules demonstrated good flow and compressibility. The compressed tablets exhibited desired mechanical strength to withstand mechanical shocks. The usage of process conditions for high melting point drugs far below their melting point can play a significant role in the quality of the final product. The drug release profiles indicated the control of active release throughout the period tested in the formulations prepared via twin-screw granulation over conventional techniques. Very few studies of successful granulation have been reported with these low binder concentrations and this study’s processing conditions. Therefore, this novel study could provide valuable insights for formulation and processing conditions in twin screw granulation, as well as and strongly impact its extensive applications in the future.
Acknowledgments
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). The authors would like to thank Inayet Ellis (Gattefosse) for the generous supply of the excipients and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aburahma MH, Badr-Eldin SM. Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin Drug Deliv. 2014;11:1865–1883. doi: 10.1517/17425247.2014.935335. [DOI] [PubMed] [Google Scholar]
- Arndt O-R, Kleinebudde P. Influence of binder properties on dry granules and tablets. Powder Technology. 2017 doi: 10.1016/j.powtec.2017.04.054. [DOI]
- Bashir S, Teo YY, Ramesh S, Ramesh K. Synthesis, characterization, properties of N-succinyl chitosan-g-poly (methacrylic acid) hydrogels and in vitro release of theophylline. Polymer. 2016;92:36–49. [Google Scholar]
- Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharm Sci Tech. 2009;10:289–296. doi: 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Farber L, Zhang D, Li F, Farabaugh J. A new methodology for high drug loading wet granulation formulation development. Int. J. Pharm. 2013;441:790–800. doi: 10.1016/j.ijpharm.2012.09.052. [DOI] [PubMed] [Google Scholar]
- Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Dalziel G, Nauka E, Zhang F, Kothari S, Xie M. Assessment of granulation technologies for an API with poor physical properties. Drug Dev. Ind. Pharm. 2013;39:985–995. doi: 10.3109/03639045.2012.687744. [DOI] [PubMed] [Google Scholar]
- Deore R, Kavitha K, Tamizhmani T. Preparation and evaluation of sustained release matrix tablets of tramadol hydrochloride using glyceryl palmitostearate. Trop J Pharm Res. 2010;9:275–281. [Google Scholar]
- Desai D, Rinaldi F, Kothari S, Paruchuri S, Li D, Lai M, Fung S, Both D. Effect of hydroxypropyl cellulose (HPC) on dissolution rate of hydrochlorothiazide tablets. Int. J. Pharm. 2006;308:40–45. doi: 10.1016/j.ijpharm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- El-Halim A, Shady M, Amin MM, El-Gazayerly ON, Ad El-Gawad NA. Comparative study on the different techniques for the preparation of sustained-release hydrophobic matrices of a highly water-soluble drug. Drug Discov. Ther. 2010;4:484–492. [PubMed] [Google Scholar]
- Fetih GN. Formulation and characterization of Gelucire pellets for sustained release of Ibuprofen. Bulletin of Pharmaceutical Sciences. 2010;33:217–224. [Google Scholar]
- Gaber DM, Nafee N, Abdallah OY. Mini-tablets versus pellets as promising multiparticulate modified release delivery systems for highly soluble drugs. Int. J. Pharm. 2015;488:86–94. doi: 10.1016/j.ijpharm.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Gardouh AR, Gad S, Ghonaim HM, Ghorab MM. Design and Characterization of Glyceryl Monostearate Solid Lipid Nanoparticles Prepared by High Shear Homogenization. British Journal of pharmaceutical research. 2013;3:326–346. [Google Scholar]
- Gunasekaran S, Sankari G, Ponnusamy S. Vibrational spectral investigation on xanthine and its derivatives—theophylline, caffeine and theobromine. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2005;61:117–127. doi: 10.1016/j.saa.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Hamdani J, Moes AJ, Amighi K. Physical and thermal characterisation of Precirol and Compritol as lipophilic glycerides used for the preparation of controlled-release matrix pellets. Int. J. Pharm. 2003;260:47–57. doi: 10.1016/s0378-5173(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Hamdani J, Moës AJ, Amighi K. Development and in vitro evaluation of a novel floating multiple unit dosage form obtained by melt pelletization. Int. J. Pharm. 2006;322:96–103. doi: 10.1016/j.ijpharm.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of sustained- action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Krause J, Thommes M, Breitkreutz J. Immediate release pellets with lipid binders obtained by solvent-free cold extrusion. Eur. J. Pharm. Biopharm. 2009;71:138–144. doi: 10.1016/j.ejpb.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Kreye F, Siepmann F, Siepmann J. Drug release mechanisms of compressed lipid implants. Int. J. Pharm. 2011;404:27–35. doi: 10.1016/j.ijpharm.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Lakshman JP, Kowalski J, Vasanthavada M, Tong WQ, Joshi YM, Serajuddin A. Application of melt granulation technology to enhance tabletting properties of poorly compactible high- dose drugs. J. Pharm. Sci. 2011;100:1553–1565. doi: 10.1002/jps.22369. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2001;52:181–190. doi: 10.1016/s0939-6411(01)00162-x. [DOI] [PubMed] [Google Scholar]
- Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release. 2011;154:2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Malode VN, Paradkar A, Devarajan PV. Controlled release floating multiparticulates of metoprolol succinate by hot melt extrusion. Int. J. Pharm. 2015;491:345–351. doi: 10.1016/j.ijpharm.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Mangal H, Kirsolak M, Kleinebudde P. Roll compaction/dry granulation: Suitability of different binders. Int. J. Pharm. 2016;503:213–219. doi: 10.1016/j.ijpharm.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Shen W, Hapgood K. Effect of formulation hydrophobicity on drug distribution in wet granulation. Chemical Engineering Journal. 2010;164:330–339. [Google Scholar]
- Nokhodchi A, Raja S, Patel P, Asare-Addo K. The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts: BI. 2012;2:175. doi: 10.5681/bi.2012.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini N, Albertini B, Perissutti B, Rodriguez L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int. J. Pharm. 2006;318:92–102. doi: 10.1016/j.ijpharm.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Patel DM, Patel NM, Patel VF, Bhatt DA. Floating granules of ranitidine hydrochloride-gelucire 43/01: formulation optimization using factorial design. AAPS PharmSciTech. 2007;8:E25–E31. doi: 10.1208/pt0802030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H, Tiwari RV, Upadhye SB, Vladyka RS, Repka MA. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process. Int. J. Pharm. 2015;496:33–41. doi: 10.1016/j.ijpharm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J. Control. Release. 1998;55:45–55. doi: 10.1016/s0168-3659(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rao MR, Ranpise AA, Thanki K, Borate S, Parikh G. Effect of processing and sintering on controlled release wax matrix tablets of ketorolac tromethamine. Indian J. Pharm. Sci. 2009;71:538. doi: 10.4103/0250-474X.58188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, Martin C, McGinity JW. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007;33:1043–1057. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- Rosiaux Y, Jannin V, Hughes S, Marchaud D. Solid lipid excipients - matrix agents for sustained drug delivery. J. Control. Release. 2014;188:18–30. doi: 10.1016/j.jconrel.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Roy DS, Rohera BD. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur. J. Pharm. Sci. 2002;16:193–199. doi: 10.1016/s0928-0987(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Sato K. Crystallization behaviour of fats and lipids—a review. Chem. Eng. Sci. 2001;56:2255–2265. [Google Scholar]
- Sato K, Ueno S, Yano J. Molecular interactions and kinetic properties of fats. Prog. Lipid Res. 1999;38:91–116. doi: 10.1016/s0163-7827(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Schulze S, Winter G. Lipid extrudates as novel sustained release systems for pharmaceutical proteins. J. Control. Release. 2009;134:177–185. doi: 10.1016/j.jconrel.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Shah B, Khunt D, Bhatt H, Misra M, Padh H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J Drug Deliv Sci Technol. 2016;33:37–50. [Google Scholar]
- Shanmugam S. Granulation techniques and technologies: recent progresses. BioImpacts: BI. 2015;5:55. doi: 10.15171/bi.2015.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons S, Fairbrother R. Direct observations of liquid binder–particle interactions: the role of wetting behaviour in agglomerate growth. Powder Technol. 2000;110:44–58. [Google Scholar]
- Small DM. Lateral chain packing in lipids and membranes. J. Lipid Res. 1984;25:1490–1500. [PubMed] [Google Scholar]
- Sriamornsak P, Thirawong N, Weerapol Y, Nunthanid J, Sungthongjeen S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007;67:211–219. doi: 10.1016/j.ejpb.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Sudha B, Sridhar B, Srinatha A. Modulation of tramadol release from a hydrophobic matrix: implications of formulations and processing variables. AAPS PharmSciTech. 2010;11:433–440. doi: 10.1208/s12249-010-9400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DCT, Chin WWL, Tan EH, Hong S, Gu W, Gokhale R. Effect of binders on the release rates of direct molded verapamil tablets using twin-screw extruder in melt granulation. Int. J. Pharm. 2014;463:89–97. doi: 10.1016/j.ijpharm.2013.12.053. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weatherley S, Pukadyil R, Sheskey P. Foam granulation: new developments in pharmaceutical solid oral dosage forms using twin screw extrusion machinery. Drug Dev. Ind. Pharm. 2012;38:771–784. doi: 10.3109/03639045.2011.633265. [DOI] [PubMed] [Google Scholar]
- U.S.FDA. Center for Drug Evaluation and Research (CDER) US Department of Health and Human Services; Rockville: 1997. Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms. [Google Scholar]
- Van den Mooter G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol. 2012;9:e79–e85. doi: 10.1016/j.ddtec.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Vonk P, Guillaume C, Ramaker J, Vromans H, Kossen N. Growth mechanisms of high-shear pelletisation. Int. J. Pharm. 1997;157:93–102. [Google Scholar]
- Windbergs M, Strachan CJ, Kleinebudde P. Investigating the principles of recrystallization from glyceride melts. AAPS Pharm Sci Tech. 2009;10:1224–1233. doi: 10.1208/s12249-009-9311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-E, Schwartz JB. Melt granulation and heat treatment for wax matrix-controlled drug release. Drug Dev. Ind. Pharm. 2003;29:131–138. doi: 10.1081/ddc-120016720. [DOI] [PubMed] [Google Scholar]