Highlights
-
•
Lower limb and trunk muscle strength are associated with comfortable/maximum gait speed in patients with sub-acute stroke.
-
•
Non-paretic dorsiflexors and left trunk lateral flexors predict comfortable/maximum gait speed in sub-acute stroke.
-
•
The effects of strengthening in these muscles on post-stroke gait speed need more investigation.
Keywords: Lower extremity, Torso, Muscle strength, Walking speed
Abstract
Objectives
To investigate the association between measures of strength of the lower limb and trunk muscles and gait speed and to identify the muscle group that would best predict gait speed in individuals with sub-acute stroke.
Methods
Using a cross-sectional observational study design, forty-four individuals with sub-acute stroke (62 years, SD = 14; 4 months, SD = 1 post-stroke) were assessed. The evaluations were performed at a university laboratory, participants’ homes, or community-based settings. Bilateral maximum isometric strength (hip, knee, and ankle flexors/extensors, hip abductors, trunk flexors/extensors, and trunk lateral flexors and rotators) was measured using a portable dynamometer. Comfortable and maximum gait speeds were measured using the 10-m walk test.
Results
Weak to moderate associations were found between measures of strength of the lower limb muscles and comfortable (0.36 ≤ r ≤ 0.53; p < 0.05) and maximum (0.37 ≤ r ≤ 0.59; p < 0.05) gait speeds, except for the non-paretic knee flexors and comfortable gait speed (p = 0.06). Weak to moderate associations were also found between measures of strength of the trunk muscles and comfortable (0.39 ≤ r ≤ 0.50; p < 0.05) and maximum (0.39 ≤ r ≤ 0.61; p < 0.05) gait speeds. Stepwise multiple regression analyses revealed that the non-paretic dorsiflexors and the left lateral trunk flexors explained 29% and 42% of the variance in the maximum and comfortable gait speeds, respectively.
Conclusions
The strength of the lower limb and trunk muscles was positively associated with comfortable and maximum gait speeds. The muscle strength of the non-paretic dorsiflexors and the left lateral trunk flexors might have a role to play in determining comfortable and maximum gait speeds of individuals with sub-acute stroke.
Introduction
Stroke is the main cause of adult disability.1 Weakness of the lower limb muscles is one of the main impairments after stroke2, 3 and is associated with limited ability to perform activities of daily living4, 5 and with reduced gait speed in individuals with stroke.6 Community walking ability is a common goal for individuals with stroke; therefore, interventions aiming at increasing gait speed should be considered. Therefore, it is important to understand the magnitude of the associations between muscle groups of the lower limbs and gait speed.
Mentiplay et al.6 conducted a systematic review to assess the associations between the strength of the lower limb muscles and gait speed in individuals with stroke and reported very weak to strong associations.6 However, the included studies had several limitations.6 The majority of these eligible studies only assessed one muscle group, and few assessed the non-paretic lower limb muscles and maximum gait speed. As the evaluation of various muscle groups may assist in the interpretation of the relative importance of each group to gait speed and the non-paretic muscles also show strength impairment,3 bilateral measures of seven lower limb muscle groups were obtained in the present study. Furthermore, maximum gait speed was evaluated in this study as it is an important requirement for the performance of activities in community-based settings and for ensuring individuals’ safety when crossing the street.7, 8
Individuals with stroke are classified according to typical phases of spontaneous motor recovery progress.9 These phases are determined based on the timing of important biological processes of neural repair, which may affect impairment and functional limitations.9 An existing longitudinal study observed significant improvements in gait speed between the evaluations performed at both 1 week (acute phase) and 3 months (sub-acute phase) after stroke.10 Thus, it is important to take the post-stroke phases into account. As none of the studies that were included in a previous review6 took the post-stroke phases into account, the present study assessed only patients with sub-acute stroke.
In addition to strength deficits in the lower limb muscles, patients with stroke also present trunk muscle weakness.11, 12, 13, 14 Previous studies have already reported significant associations between weakness of the trunk flexors/extensors and impaired balance and lower independence in walking and transfers, such as getting up from a sitting position.12, 15 However, none of the studies investigated the associations between measures of strength of the trunk muscles and gait speed after stroke. A better understanding of these associations could clarify which muscle group(s) should be targeted during strength training after stroke.
The aims of this study were to investigate the associations between measures of strength of the lower limb and trunk muscles and comfortable and maximum gait speeds and to identify the muscle group that would best predict gait speed in individuals with sub-acute stroke. This information may help clinicians identify the muscles to target during strength evaluation for planning strengthening programs aimed at increasing gait speed after stroke.
Methods
Design and participants
This cross-sectional observational study was carried out in a university laboratory, participants’ homes, and community-based settings. Study participants were recruited from research groups and healthcare centers. The inclusion criteria were as follows: age ≥20 years, clinical diagnosis of stroke between 3 and 6 months (sub-acute phase),9 and ability to independently walk for 10 m with or without aid.
The exclusion criteria were as follows: (a) cognitive impairment, as determined by the cut-off scores on the Mini-Mental State Examination (illiterate: 13 points; elementary and middle school: 18 points; high school: 26 points),16 and/or receptive aphasia, as assessed by the inability to respond to the command “Lift up your good arm and open your good hand, please”17; (b) reports of any health condition that could compromise the strength of the lower limb and/or trunk muscles; and (c) complaints of pain during the assessment procedures.
All participants provided written consent based on the previous approval of the Research Ethical Committee (Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil).
MedCalc® softwarea was used to determine the sample size, considering a power = 0.80, r = 0.69, and α = 0.05. The correlation coefficient of r = 0.69 was considered as a moderate effect size.18, 19 Since, according to Munro,19 a moderate association is between 0.50 and 0.69, the upper limit (r = 0.69), was chosen, to avoid a high artificially association, but one that would be high enough to be meaningful. The required sample would be 14 individuals. However, to ensure sampling variability (required for association analyses), attempt was made to assess 14 individuals per age group (20–39 years, 40–59 years, and ≥60 years), totaling 42 individuals. Additionally, sampling variability was attempted for sex, comfortable gait speed (<0.4 m/s, household ambulators; 0.4–0.8 m/s, limited community ambulators; and >0.8 m/s, community ambulators),20 and degree of lower limb motor impairment (lower limb section of the Fugl-Meyer scale, 0–34 points: <17 indicate severe impairments, 18–22 moderately severe impairment, 23–28 moderate impairment, and >29 mild impairment).21 However, it is important to highlight that the sample size should be at least 14. In some participants, not all muscle groups were assessed because they were not able to generate isometric contractions. Thus, the sample size for each muscle group assessed varied.
Dependent and independent variables
The dependent variables were comfortable and maximum gait speeds, whereas the independent variables or predictors were the strength of the major muscle groups of the lower limbs and trunk. Bilateral measures of seven lower limb and six trunk muscle groups were obtained, namely the hip flexors/extensors/abductors, knee flexors/extensors, ankle dorsiflexors/plantar flexors, and trunk flexors/extensors/lateral flexors/rotators. These muscle groups were selected because of previous reported associations with gait speed and mobility in individuals with acute and chronic stroke and/or because they had been little or not at all assessed in previous studies.6
Procedures
The data were collected on one day by a trained examiner who was a physical therapist with 1-year experience in muscle strength and gait speed measurements. The training was provided for 1 week by two other physical therapists with previous experience. The eligibility criteria were assessed, and clinical and demographic data, such as age, sex, height, body mass index, paretic side, type of stroke, time elapsed since stroke, lower limb motor recovery (lower limb section of the Fugl-Meyer scale),3, 21 and trunk impairment (trunk impairment scale),3, 22 were subsequently collected. Finally, muscle strength and gait speed were assessed.
Measurement of the strength of the lower limb and trunk muscles was performed using a handheld microFET2® dynamometer,b which is considered a practical standard method for the assessment of isometric strength.23 This device has adequate reliability for assessing the strength of the lower limb and trunk muscles of individuals with sub-acute stroke.23, 24
Lower limbs were assessed alternately, with each muscle group of the non-paretic lower limb evaluated first, followed by that of the paretic lower limb. The participants and their body segments were standardized positioned (Fig. 1).24, 25, 26, 27 Prior to the measurement of strength, the participants were asked to perform one submaximal isometric contraction for the purpose of familiarization and subsequently one maximum isometric contraction for 5 s, and the peak value was recorded. During the tests, they received the following verbal encouragement: “Go, go, go, go…”. Only one trial for each assessed muscle group was performed.26 A 20-s rest interval was allowed between the trials.24, 25
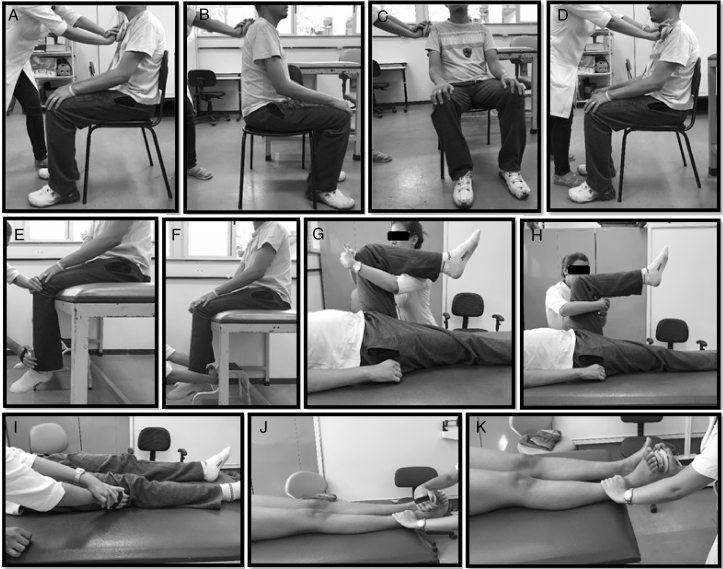
Figure 1.
Measurement of muscle strength with the hand-held dynamometer. (A) Trunk flexors; (B) trunk extensors; (C) lateral trunk flexors; (D) lateral trunk rotators; (E) knee extensors; (F) knee flexors; (G) hip flexors; (H) hip extensors; (I) hip abductors; (J) ankle dorsiflexors; (K) ankle plantarflexors.
Comfortable and maximum gait speeds were measured using the 10-m walk test, which has shown adequate reliability, validity, and clinical applicability for gait speed assessment after stroke.28, 29 To measure comfortable and maximum gait speeds in m/s, the participants walked in a 14-m corridor, and the time required to cover the central 10 m was recorded.28, 29 Comfortable gait speed was first assessed, followed by the measurement of maximum gait speed. Participants could rest between the two assessments if they desired. Verbal commands were standardized, according to the instructions provided by Nascimento et al.29 For the assessment of comfortable gait speed, participants received verbal command to walk in their comfortable and habitual speed.28 For the evaluation of maximum gait speed, participants were requested to walk as fast as possible and safely, but without running, to reach a bus that was about to pull out.28
Statistical analyses
Data analysis was performed by a researcher who was not involved in the data collection. Descriptive statistics were calculated, and tests for normality of data distribution (Shapiro–Wilk tests) was calculated. Pearson correlation coefficients were calculated to investigate the associations between measures of muscle strength and gait speed. The strength of associations was classified as follows: very weak, ≤0.25; weak, 0.26–0.49; moderate, 0.50–0.69; strong, 0.70–0.89; and very strong, 0.90–1.00.19 Stepwise multiple linear regression analyses were performed to investigate the muscle group of the lower limbs and trunk that would predict both comfortable and maximum gait speeds. All analyses were performed using SPSSc (α = 0.05).
Results
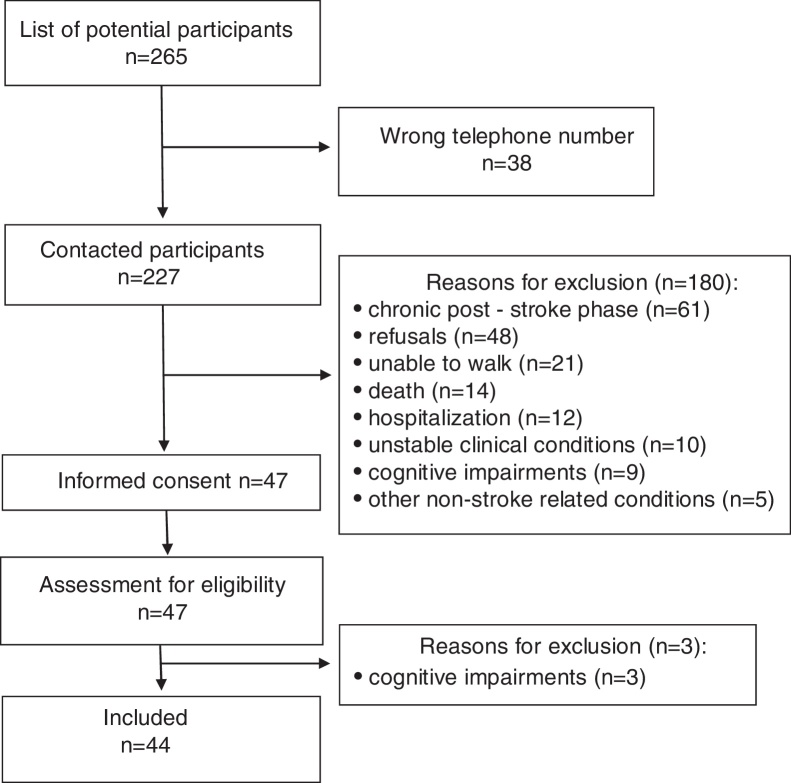
From a list of approximately 265 individuals, 38 could not be contacted (Fig. 2). Forty-four individuals with sub-acute stroke (24 men) were eligible and participated in the study. Their mean age was 62 years, SD = 14, and their mean post-stroke time was 4 months, SD = 1. Their mean comfortable gait speed was 0.8 m/s, SD = 0.38, whereas their maximum gait speed was 1.1 m/s, SD = 0.54. The clinical and demographic characteristics of the participants are given in Table 1.
Figure 2.
Flow diagram through the study.
Table 1.
Clinical and demographic characteristics of the participants (n = 44).
| Characteristics | Results |
|---|---|
| Age, years, mean (SD) [min–max] | 62 (15) [27–89] |
| Time post-stroke, months, mean (SD) [min–max] | 4 (1) [3–6] |
| Body mass index (kg/m2), mean (SD) [min–max] | 25 (4) [15–37] |
| Sex, women, n (%) | 20 (45) |
| Paretic side, Right, n (%) | 19 (43) |
| Type of stroke, n (%) | |
| Ischemic | 42 (96) |
| Hemorrhagic | 2 (4) |
| Comfortable gait speed, 10-m walk test, m/s, mean (SD) [min–max] | 0.8 (0.38) [0.14–1.53] |
| Household ambulators, n (%) | 10 (23) |
| Limited-community ambulators, n (%) | 10 (23) |
| Community ambulators, n (%) | 24 (54) |
| Maximum gait speed, 10-m walk test, m/s, mean (SD) [min–max] | 1.1 (0.54) [0.19–2.17] |
| Lower limb motor impairments (Fugl-Meyer scores: 0–34), n (%) | |
| Mild | 31 (71) |
| Moderate | 5 (11) |
| Moderately severe | 2 (5) |
| Severe | 6 (14) |
| Trunk impairments (TIS scores: 0–23), median (IQR)18 | 17 (6) |
n, number; SD, standard deviation; min, minimum; max, maximum; TIS, Trunk Impairment Scale; IQR, interquartile range.
As shown in Table 2, weak to moderate associations were found between measures of strength of the lower limb muscles and both comfortable (0.36 ≤ r ≤ 0.53; p < 0.05) and maximum (0.37 ≤ r ≤ 0.59; p < 0.05) gait speeds, except for the non-paretic knee flexors and comfortable gait speed (p = 0.06). Weak to moderate associations were also found between measures of strength of the trunk muscles and comfortable gait speed (0.39 ≤ r ≤ 0.52; p < 0.05) in individuals with sub-acute stroke; there were also moderate associations between measures of strength of the trunk muscles and maximum gait speed (0.50 ≤ r ≤ 0.61; p < 0.05) in individuals with sub-acute stroke.
Table 2.
Descriptive statistics and association coefficients (r values) between measures of strength of the lower limb and trunk muscles and comfortable/maximum gait speeds in individuals with sub-acute stroke.
| Muscular group | Mean, SD [min–max] | Comfortable gait speed r (n) |
Maximum gait speed r (n) |
|---|---|---|---|
| Paretic lower limb | |||
| Hip flexors | 7.5, SD 3.7 [2.0–19.5] | 0.49 (41)* | 0.55 (40)* |
| Hip extensors | 10.8, SD 4.3 [1.9–21.4] | 0.38 (39)* | 0.48 (38)* |
| Hip abductors | 8.5, SD 3.8 [1.6–18.5] | 0.53 (42)* | 0.59 (41)* |
| Knee flexors | 6.6, SD 3.6 [1.6–16.6] | 0.43 (40)* | 0.52 (39)* |
| Knee extensors | 9.8, SD 4.4 [1.8–19.1] | 0.40 (41)* | 0.52 (40)* |
| Ankle plantar flexors | 8.9, SD 4.3 [1.9–20.6] | 0.47 (40)* | 0.53 (40)* |
| Ankle dorsiflexors | 5.7, SD 2.4 [1.7–14.1] | 0.37 (39)* | 0.43 (39)* |
| Non-paretic lower limb | |||
| Hip flexors | 8.8, SD 4.07 [2.3–19.4] | 0.47 (41)* | 0.52 (40)* |
| Hip extensors | 10.9, SD 3.5 [4.3–19] | 0.46 (40)* | 0.55 (39)* |
| Hip abductors | 9.5, SD 3.3 [3.0–19.1] | 0.51 (42)* | 0.53 (41)* |
| Knee flexors | 8.2, SD 3.3 [2.0–19.2] | 0.29 (42) (p = 0.06) | 0.40 (41)* |
| Knee extensors | 11.1, SD 4.1 [3.8–20.9] | 0.36 (41)* | 0.49 (40)* |
| Ankle plantar flexors | 9.6, SD 3.8 [4.1–17.4] | 0.47 (42)* | 0.37 (41)* |
| Ankle dorsiflexors | 7.0, SD 2.7 [1.4–16.2] | 0.47 (42)* | 0.40 (41)* |
| Trunk | |||
| Flexors | 10.0, SD 3.4 [3.3–18.1] | 0.50 (43)* | 0.60 (42)* |
| Extensors | 10.6, SD 2.9 [3.6–19.2] | 0.47 (43)* | 0.60 (42)* |
| Right lateral flexors | 8.6, SD 2.9 [3.2–15.2] | 0.48 (40)* | 0.58 (39)* |
| Left lateral flexors | 8.9, SD 3.3 [3.5–19.0] | 0.52 (42)* | 0.61 (41)* |
| Right lateral rotators | 7.6, SD 2.6 [3.4–14.2] | 0.39 (40)* | 0.50 (39)* |
| Left lateral rotators | 7.4, SD 2.5 [2.0–13.1] | 0.41 (41)* | 0.52 (40)* |
n, number; r, Pearson correlation coefficient; SD, standard deviation; min, minimum; max, maximum.
p < 0.05.
The results of the regression analyses indicated that only the non-paretic ankle dorsiflexors and left lateral trunk flexors were retained in the models (Table 3). The non-paretic dorsiflexors and the left lateral trunk flexors explained 29% (B = 0.09, 0.04 ≤ 95% confidence interval for B ≤ 0.14, p = 0.002) and 42% (B = 0.12, 0.07 ≤ 95% confidence interval for B ≤ 0.18, p < 0.001) of the variance in maximum and comfortable gait speeds, respectively (Table 3).
Table 3.
Results of the Step-wise multiple regression analyses regarding the predictors (strength of the lower limb and trunk muscles) of comfortable and maximum gait speeds in individuals with sub-acute stroke (n = 44).
| Muscular group | Comfortable gait speed |
||||
|---|---|---|---|---|---|
| B | 95% confidence interval for B |
R2 | p | ||
| Lower bound | Upper bound | ||||
| Non-paretic dorsiflexors | 0.09 | 0.04 | 0.14 | 0.29 | 0.002 |
| Maximum gait speed | |||||
| Left lateral trunk flexors | 0.12 | 0.07 | 0.18 | 0.42 | <0.001 |
n, number; B, regression coefficient; R2, determination coefficient; p, level of significance; p-value < 0.05: statistically significant.
Discussion
Weak to moderate associations were found between measures of strength of the lower limb and trunk muscles and both comfortable and maximum gait speeds, except for the non-paretic knee flexors, which did not show any association with comfortable gait speed. However, only the non-paretic dorsiflexors and the left lateral trunk flexors remained as predictors of comfortable and maximum gait speeds, respectively.
Muscles transform metabolic energy into mechanical energy to generate force and produce accelerations and decelerations of body segments or of the entire body.2 Gait speed is the rate of linear forward motion of the body; thus, theoretically, muscle strength may be related to gait speed. As muscle strength and gait speed are reduced after stroke,2, 30 it is important to understand the associations between these variables. A review6 that aimed at identifying studies that had investigated the associations between the strength of the lower limb muscles and gait speed in individuals with stroke found significant very weak to strong associations between the strength of the paretic and non-paretic (0.11 ≤ r ≤ 0.83) lower limb muscles and comfortable and maximum gait speed. The results of most of the studies included by Mentiplay et al.6 in individuals after stroke (although not classified according to post-stroke phases) are in line with the findings of this study in individuals with sub-acute stroke, which found significant weak to moderate associations between the strength of the lower limb muscles and comfortable gait speed (0.36 ≤ r ≤ 0.53). However, according to Mentiplay et al.,6 very few studies have investigated the associations between the strength of the lower limb muscles and maximum gait speed in individuals with stroke. Of the 21 studies included in their review, only seven (33.3%) and four (19.1%) assessed the associations between the strength of the paretic and non-paretic lower limbs, respectively, and maximum gait speed in individuals after stroke. However, only the paretic and non-paretic knee extensors and the paretic ankle plantar flexors were assessed.6 The present study assessed the associations between 14 lower limb muscle groups and maximum gait speed in individuals with sub-acute stroke and found significant weak to moderate associations (0.37 ≤ r ≤ 0.59). Therefore, most lower limb muscle groups contribute to comfortable and maximum gait speed in individuals with sub-acute stroke.
The trunk muscles have two main functions during gait: to ensure stability and enable movement.11 The activity of the trunk muscles during walking in healthy individuals, measured by surface electromyography, increases at higher speeds.11, 31 This is probably due to the increased demand for stabilization and the higher need for compensation of the rotational movements.11 However, to our knowledge, no studies have investigated the associations between the strength of the trunk muscles and gait speed in individuals with stroke. This present study found weak to moderate associations between the strength of the trunk muscles and comfortable gait speed (0.39 ≤ r ≤ 0.52) and moderate associations between that of the trunk muscles and maximum gait speed (0.50 ≤ r ≤ 0.61) in individuals with sub-acute stroke. These results emphasize the importance of considering the trunk muscles when assessing gait speed in individuals with stroke. As the impairment of the trunk muscles after stroke may be related to gait speed, these muscles should be considered during evaluations for planning strengthening interventions in individuals with sub-acute stroke.
Previous studies that performed regression analyses to identify the muscle groups that would predict gait speed after stroke only assessed comfortable gait speed in individuals in the chronic phase.6, 32, 33, 34, 35 Among all the lower limb muscles that remained significant in the regression analyses, the paretic ankle dorsiflexors appeared with greater frequency, and explained 30–49% of the variance in comfortable gait speed.32, 33, 34 This could be explained by the fact that weakness of the dorsiflexors prevents foot clearance during gait, resulting in decreased step length and a series of compensations to avoid foot drop,34 which, in turn, may lead to reduced gait speed.34 These results are not in agreement with the findings of the present study, which found that the non-paretic dorsiflexors predicted comfortable gait speed in individuals with sub-acute stroke. The differences could be explained by the fact that none of the studies that performed regression analyses in individuals with stroke assessed individuals in the sub-acute phase. As mentioned before, muscle strength impairment is more pronounced during the early post-stroke phases,2 as spontaneous motor recovery has not stabilized.7 Another possible hypothesis for the divergence in findings is that the mean comfortable gait speed of the participants in the present study (0.8 m/s) was higher than that in other studies (between 0.5 and 0.75 m/s). This finding highlights the importance of evaluating, and possibly strengthening, the non-paretic muscles after stroke.
None of the studies that performed regression analyses in individuals with stroke investigated the associations between the strength of the trunk muscles and maximum gait speed, which limits the comparisons of the results. A trunk kinematic deviation, often observed during post-stroke gait, is characterized by a large lateral displacement toward the non-paretic side.13, 36 It is believed that this augmentation in lateral displacement is due to the weakness of the hip abductors, as trunk lateral displacement in healthy individuals is eccentrically controlled by the hip abductors.36 Thus, a possible explanation for the fact that the left lateral trunk flexors predicted maximum gait speed in this study is that this muscle group attempts to compensate for the weakness of the hip abductors and control lateral trunk displacement. Because the majority of the participants in this study had left hemiparesis, it was expected that there would be a large lateral displacement toward the right side and that the left lateral trunk flexors would be required for control. In addition, the weakness of the left lateral trunk flexors could be associated with hip hiking during gait for foot clearance due to the lack of flexion of the hip, knee, and ankle joints.13 To walk at maximum speeds, foot clearance may be more important; thus, hip hiking could be a compensatory strategy adopted by the participants. To confirm these hypotheses, futures studies should measure trunk kinematics.
The absence of studies that included individuals with sub-acute stroke and also evaluated the trunk muscles limits a deeper discussion regarding the present findings. However, the differences between the present and previous findings in individuals with chronic stroke emphasize the importance of addressing specific phases of spontaneous motor recovery in individuals with stroke.7 A better understanding of the associations between the strength of various muscle groups and gait speed may help clinicians identify the muscle group to target during muscle strength evaluation and possibly during muscle-strengthening interventions aimed at increasing gait speed in individuals with sub-acute stroke.
Study limitations
A possible limitation of this study is that most of the participants had mild motor impairments, which may limit generalization of the results. In addition, more than half of the variance in gait speed remained unexplained by the regression models. This could probably be enhanced by including other variables such as balance ability. Nevertheless, the purpose of this study was to identify the muscles of the lower limbs and trunk that would predict gait speed in individuals with sub-acute stroke, and not to clarify all the variance in gait speed. These results suggest the importance of strengthening the muscles of the lower limbs and trunk as a strategy to improve gait speed in individuals with sub-acute stroke. However, as cross-sectional observational studies do not establish any causal associations, further studies are needed to assess the effects of strengthening interventions for the lower limb and trunk muscles on comfortable and maximum gait speeds after stroke.
Conclusions
Significant and positive moderate associations were found between the strength of the lower limb and trunk muscles and comfortable and maximum gait speeds in individuals with sub-acute stroke. However, only the non-paretic dorsiflexors and left lateral trunk flexors explained the variance in comfortable and maximum gait speeds, respectively.
Acknowledgment of financial support
Financial support provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/UFMG).
Conflicts of interest
The authors report no conflicts of interest.
Footnotes
MedCalc Software, Ostend, Belgium.
Hoggan Health Industries, Inc, Draper, UT, USA.
SPSS Inc., Chicago, IL, USA.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. [Epub 2017 Jan 25] doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohannon R.W. Muscle strength and muscle training after stroke. J Rehabil Med. 2007;39(January (1)):14–20. doi: 10.2340/16501977-0018. [DOI] [PubMed] [Google Scholar]
- 3.Dorsch S., Ada L., Canning C.G. Lower limb strength is significantly impaired in all muscle groups in ambulatory people with chronic stroke: a cross-sectional study. Arch Phys Med Rehabil. 2016;97(4):522–527. doi: 10.1016/j.apmr.2015.10.106. [DOI] [PubMed] [Google Scholar]
- 4.Kim C.M., Eng J.J. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 5.LeBrasseur N.K., Sayers S.P., Ouellette M.M., Fielding R.A. Muscle impairments and behavioral factors mediate functional limitations and disability following stroke. Phys Ther. 2006;86(10):1343–1350. doi: 10.2522/ptj.20050162. [DOI] [PubMed] [Google Scholar]
- 6.Mentiplay B.F., Adair B., Bower K.J., Williams G., Tole G., Clark R.A. Associations between lower limb strength and gait velocity following stroke: a systematic review. Brain Inj. 2014;29(4):409–422. doi: 10.3109/02699052.2014.995231. [DOI] [PubMed] [Google Scholar]
- 7.Kollen B., Kwakkel G., Lindeman E. Hemiplegic gait after stroke: is measurement of maximum speed required? Arch Phys Med Rehabil. 2006;87(3):358–363. doi: 10.1016/j.apmr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Salbach N.M., O’Brien K., Brooks D. Speed and distance requirements for community ambulation: a systematic review. Arch Phys Med Rehabil. 2014;95(1):117–128. doi: 10.1016/j.apmr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt J., Hayward K.S., Kwakkel G. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12(5):444–450. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez M.C., Bussmann J., Janssen W. Accelerometric assessment of different dimensions of natural walking during the first year after stroke: recovery of amount, distribution, quality and speed of walking. J Rehabil Med. 2015;47(8):714–721. doi: 10.2340/16501977-1994. [DOI] [PubMed] [Google Scholar]
- 11.Anders C., Wagner H., Puta C., Grassme R., Petrovitch A., Scholle H.C. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol. 2007;17(2):245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Karatas M., Cetin N., Bayramoglu M., Dilek A. Trunk muscle strength in relation to balance and functional disability in unihemispheric stroke patients. Am J Phys Med Rehabil. 2004;83(2):81–87. doi: 10.1097/01.PHM.0000107486.99756.C7. [DOI] [PubMed] [Google Scholar]
- 13.Van Criekinge T., Saeys W., Hallemans A. Trunk biomechanics during hemiplegic gait after stroke: a systematic review. Gait Posture. 2017;54:133–143. doi: 10.1016/j.gaitpost.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Quintino L.F., Franco J., Gusmão A.F.M., Silva P.F.S., Faria C.D.C.M. Trunk flexor and extensor muscle performance in chronic stroke patients: a case-control study. Braz J Phys Ther. [Epub 2017 Dec 12] doi: 10.1016/j.bjpt.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva P., Franco J., Gusmão A., Moura J., Teixeira-Salmela L., Faria C. Trunk strength is associated with sit-to-stand performance in both stroke and healthy subjects. Eur J Phys Rehabil Med. 2015;51(6):717–724. [PubMed] [Google Scholar]
- 16.Bertolucci P.H., Brucki S.M., Campacci S.R., Juliano Y. The mini-mental state examination in a general population: impact of educational status. Arch Neuropsych. 1994;52:1–7. [PubMed] [Google Scholar]
- 17.Teixeira-Salmela L.F., Devaraj R., Olney S.J. Validation of the human activity profile in stroke: a comparison of observed, proxy and self-reported scores. Disabil Rehabil. 2007;29(19):1518–1524. doi: 10.1080/09638280601055733. [DOI] [PubMed] [Google Scholar]
- 18.Portney L.G., Watkins M.P. 3rd ed. F.A. Davies; Philadelphia, PA, USA: 2015. Foundations of Clinical Research: Applications to Practice. [Google Scholar]
- 19.Munro B. 5th ed. Lippincott Williams & Willkins; Philadelphia: 2005. Statistical Methods for Health Care Research; pp. 239–258. [Google Scholar]
- 20.Perry J., Garrett M., Gronley J.K., Mulroy S.J. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 21.Dutil E. La librairie de l’Université de Montréal; Montreal: 1989. Protocole d’évaluation de La fonction sensori-motrice: Test de Fugl Meyer. 54 pp. [Google Scholar]
- 22.Castellassi C.S., Ribeiro A.F., Fonseca V.C., Beinotti F., Oberg T.D., Lima N.M.F.V. Reliability of Brazilian version trunk impairment scale for stroke patients. Fisioter Mov Curitiba. 2009;22(2):189–199. [Google Scholar]
- 23.Stark T., Walker B., Phillips J.K., Fejer R., Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM&R. 2011;3:472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Aguiar L.T., Martins J.C., Lara E.M., Albuquerque J.A., Teixeira-Salmela L.F., Faria C.D. Dynamometry for the measurement of grip, pinch, and trunk muscles strength in subjects with subacute stroke: reliability and different number of trials. Braz J Phys Ther. 2016;20(5):395–404. doi: 10.1590/bjpt-rbf.2014.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza L.A., Martins J.C., Teixeira-Salmela L.F. Validity and reliability of the modified sphygmomanometer test to assess strength of the lower limbs and trunk muscles after stroke. J Rehabil Med. 2014;46(7):620–628. doi: 10.2340/16501977-1823. [DOI] [PubMed] [Google Scholar]
- 26.Martins J.C., Teixeira-Salmela L.F., Aguiar L.T., Souza L.A.C., Lara E.M., Faria C.D.C.M. Assessment of the strength of the trunk and upper limb muscles in stroke subjects with portable dynamometry: a literature review. Fisioter Mov. 2015;28(1):169–186. [Google Scholar]
- 27.Martins J.C., Aguiar L.T., Lara E.M. Assessment of the strength of the lower limb muscles in subjects with stroke with portable dynamometry: a literature review. Fisioter Mov. 2016;29(1):193–208. [Google Scholar]
- 28.Faria C.D., Teixeira-Salmela L.F., Neto M.G., Rodrigues-de-Paula F. Performance-based tests in subjects with stroke: outcome scores, reliability and measurement errors. Clin Rehabil. 2012;26(5):460–469. doi: 10.1177/0269215511423849. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento L.R., Caetano L.C.G., Freitas D.C.M.A., Morais T.M., Polese J.C., Teixeira-Salmela L.F. Different instructions during the ten-meter walking test determined significant increases in maximum gait speed in individuals with chronic hemiparesis. Braz J Phys Ther. 2012;16(2):122–127. doi: 10.1590/s1413-35552012005000008. [DOI] [PubMed] [Google Scholar]
- 30.Zoffoli L., Lucertini F., Federici A., Ditroilo M. Trunk muscles activation during pole walking vs. walking performed at different speeds and grades. Gait Posture. 2016;46(May):57–62. doi: 10.1016/j.gaitpost.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Dunn A., Marsden D.L., Van Vliet P., Spratt N.J., Callister R. Independently ambulant, community-dwelling stroke survivors have reduced cardiorespiratory fitness, mobility and knee strength compared to an age- and gender-matched cohort. Top Stroke Rehabil. 2017;24:163–169. doi: 10.1080/10749357.2016.1236482. [DOI] [PubMed] [Google Scholar]
- 32.Lin P.Y., Yang Y.R., Cheng S.J., Wang R.Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch Phys Med Rehabil. 2006;87(4):562–568. doi: 10.1016/j.apmr.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Ng S.S., Hui-Chan C.W. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch Phys Med Rehabil. 2012;93:1046–1051. doi: 10.1016/j.apmr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Dorsch S., Ada L., Canning C.G. The strength of the ankle dorsiflexors has a significant contribution to walking speed in people who can walk independently after stroke: an observational study. Arch Phys Med Rehabil. 2012;93(6):1010–1016. doi: 10.1016/j.apmr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Nasciutti-Prudente C., Oliveira F.G., Houri S.F., Neto M.H., Teixeira-Salmela L.F. Relationships between muscular torque and gait speed in chronic hemiparetic subjects. Disabil Rehabil. 2009;31(2):103–108. doi: 10.1080/09638280701818055. [DOI] [PubMed] [Google Scholar]
- 36.Tyson S.F. Trunk kinematics in hemiplegic gait and the effect of walking aids. Clin Rehabil. 1999;13(August (4)):295–300. doi: 10.1191/026921599666307333. [DOI] [PubMed] [Google Scholar]