Highlights
-
•
Children with cerebral palsy have variable degrees of respiratory muscle weakness.
-
•
IMT improves respiratory muscle strength, trunk control and exercise capacity in these children.
-
•
IMT also improves daily living activities and quality of life.
Keywords: Cerebral palsy, Inspiratory muscle training, Trunk control, Exercise capacity, Quality of life
Abstract
Background
Respiratory muscle weakness and its relation to other impairments in children with cerebral palsy (CP) have been shown in the latest studies. The effects of inspiratory muscle training (IMT) in this population have not been comprehensively investigated so far.
Objectives
To investigate the effects of IMT on trunk control, pulmonary functions, respiratory muscle strength, daily living activities, exercise capacity and quality of life in children with CP.
Methods
This was a prospective-randomized controlled trial. Twenty-five children with CP were randomly assigned to the treatment (n = 13) or the control group (n = 12). The treatment group received IMT at 30% of maximal inspiratory pressure (MIP) and the control group received sham therapy (5% of MIP) for 6 weeks. Also, both groups received routine conventional physical therapy (stretching, strengthening, and functional exercises, etc.) for 6 weeks. The primary outcome measure was trunk control. Secondary outcome measures were pulmonary function, respiratory muscle strength, daily living activities, functional exercise capacity and quality of life.
Results
The treatment group had better outcome for trunk control (3.87, 95% CI 3.72–4.02). Also, respiratory muscle strength, daily living activities, functional exercise capacity and quality of life were significantly improved in the treatment group compared with controls. No improvements were observed in the pulmonary function test scores between the groups.
Conclusion
Inspiratory muscle training improves trunk control, respiratory muscle strength, daily living activities, functional exercise capacity and quality of life in children with CP and it can be included in the physiotherapy and rehabilitation programs.
Introduction
Cerebral palsy (CP) is an umbrella term defined as “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain”.1 There is no specific treatment for the brain damage that produces motor dysfunction in patients with CP. Treatments generally focus on impairments related to motor dysfunction such as secondary musculoskeletal problems.2
The musculoskeletal system structures are interconnected and form the biomechanical bases of human movement.3 In CP, abnormalities of muscle strength and tone, contractures, and abnormal bone growth deteriorate the body's biomechanical properties.4, 5 Biomechanical deteriorations affect the body's ‘core’, adversely affecting essential functions in children with CP. The core acts as a stable base that supports the motion of distal segments6 and also controls trunk motion in all planes.7 Without adequate core stabilization, children with CP may have difficulty performing daily living activities such as getting dressed, walking, or playing with friends. These difficulties may in turn reduce quality of life. Thus, optimizing core muscle function is important for meeting the functional demands of daily living activities and maximizing quality of life for children with CP. In addition, respiratory muscles, especially the diaphragm that forms the roof of the inner core, control both respiration and core stabilization.6 Recent studies have elucidated the relationship between respiratory muscle weakness and other impairments in children with CP.8, 9, 10, 11
The beneficial effects of inspiratory muscle training (IMT) have been established in patients with respiratory and neurological disorders.6, 12, 13, 14, 15 However, no studies have investigated the effects of IMT on non-respiratory functions (core stabilization, trunk control, etc.). In addition, we know of only one study that investigated the effects of respiratory muscle training in children with CP.16 Unfortunately, their outcome measure only included body function and structure, and was therefore limited.16 There exists no evidence as to the effects of respiratory muscle training on daily living activities, participation, or quality of life in children with CP. We considered the International Classification of Functioning, Disability and Health, version for children and youth (ICF-CY) based outcome measures while planning the current trial.
This randomized controlled trial aimed to investigate the effects of IMT on respiratory muscle strength, pulmonary functions, trunk control, daily living activities, functional exercise capacity, and quality of life in children with CP.
Methods
Participants
This study was conducted between December 2016 and September 2017. Children with CP (n = 25), between the ages of 7–14 years, referred from Gazi University, Faculty of Medicine, Department of Child Health and Diseases, were recruited on the basis of their willingness to participate in the present study. Inclusion criteria were: diagnosis of CP by a pediatrician or pediatric neurologist; classified of Gross Motor Function Classification System (GMFCS) Level I (walks without restriction) and/or Level II (walks without assistive devices, but with some limitations), and ability to walk for at least six minutes. Exclusion criteria were: orthopedic surgery or botulinum toxin-A (BoNT-A) injections within the past six months, acute or chronic pulmonary diseases, acute medical illnesses, musculoskeletal problems that could affect walking ability, and the present of an intellectual impairment that could influence participation and/or motivation.
The study was approved by the Ethics Committee of the Kecioren Training and Research Hospital, Ankara, Turkey (Protocol ID: 2012-KAEK-l5l 1295) and performed in accordance with the Declaration of Helsinki. After approval by the ethics committee, the study was registered at ClinicalTrials.gov (Registration number: NCT02998281). Written informed consent to participate was obtained from the parents of all children.
Randomization and allocation
This study was designed as prospective, randomized, controlled and assessor-blinded trial. Children with CP were randomly allocated, via computerized random assignment, to either a treatment group or a control group. Allocation was concealed by using sealed and numbered black opaque envelopes prepared by a researcher not involved in the study. The physical therapist that collected the data was unaware of group assignment. Assessments and IMT were performed by different physical therapists. Before and after IMT we evaluated respiratory muscle strength, pulmonary function, trunk control, daily living activities, functional exercise capacity, and quality of life.
Assessment of clinical outcomes
Demographic and clinical variables were recorded for each child, including age, height, weight, gender, and parents’ educational statuses. Topografic classification17 and GMFCS levels18 of children were also determined by the physiotherapist.
Primary outcome
Trunk control was evaluated using the Trunk Control Measurement Scale (TCMS), which is reliable and valid in children with spastic CP.19 This scale consists of 15 items and all items are scored on a 2-, 3-, or 4-point scale. The TMCS is divided into the following subscales: static sitting balance (TMCS-SSB; items 1–5), dynamic sitting balance (TMCS-DSB) and selective movement control (TMCS-DSB-SMC; items 6–12), and dynamic reaching (TMCS-DSB-DR; items 12–15). TMCS total scores range from 0 to 58, with higher scores indicating better trunk control.19
Secondary outcomes
To evaluate pulmonary functions, we performed spirometric measurements using a calibrated spirometer (ZAN 100 flow handy II, ZAN, Oberthulba, Germany), according the guidelines of the American Thoracic Society and European Respiratory Society.20 Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), Forced expiratory flow from 25 to 75% (FEF25–75%), and peak expiratory flow (PEF) were measured and expressed as the percentage of the respective predicted value.21
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were evaluated using an electronic pressure transducer (MicroRPM; Micromedical, Kent, UK) with a rigid flanged mouthpiece. Measurements were done according to American Thoracic Society/European Respiratory Society standards.22 MIP was measured from residual volume to total lung capacity, after maximal exhalation; MEP was measured from total lung capacity to residual volume, after maximal inhalation. The measured pressures were maintained for at least one second prior to recording. A maximum of nine maneuvers were performed.23 Of these, three acceptable maneuvers were obtained; of which at least two were reproducible (there was less than 5% difference between the best values). The highest value was recorded and expressed in cmH2O. If the last maneuver was the highest we obtained an additional measure.22, 23 Normal values as per Domènech-Clar et al.23 were used to determine the percentage of predicted values. A MIP lower than −80 cmH2O was used to define inspiratory muscle weakness.22
The Pediatric Evaluation of Disability Inventory (PEDI) was used to evaluate daily living activities. The PEDI consist of two subscales: the Functional Skill Scales (PEDI-FSS) and the Caregiver Assistance Scale (PEDI-CAS). These subscales measure daily living activities in three content domains: self-care, mobility, and social function. The PEDI-FSS has 73 self-care items, 59 mobility items, and 65 social function items. Each item is scored as 0 (unable) or 1 (capable). The PEDI-CAS consists of 8 self-care items, 7 mobility items, and 5 social function items. The score of each item ranges from 0 to 5. All of the domain scores are transformed to a 0–100 scale (percentage of total possible score achieved). Higher scores indicate better daily living function.24, 25 The reliability and validity of the PEDI in children with CP have been published.25
The 6-minute walk test (6MWT) was used to evaluate functional exercise capacity. Children were instructed to walk for 6 min up and down a 30-m unobstructed corridor. Measurement was done according to American Thoracic Society guidelines.26 During the test, we monitored heart rate using the Polar A300 heart rate monitor (Polar Instruments, Shangai, China). Because none of the children had previously performed the 6MWT, each child repeated the test two times. Children rested for a minimum of 30 min between tests and the highest distance was recorded. The reproducibility and validity of the 6MWT in GMFCS I and II children with CP have been published.27
Quality of life was assessed using the Turkish version of the Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL-Child).28, 29 The questionnaire consists of seven broad domains including social well-being and acceptance, functioning, participation and physical health, emotional well-being, access to services, pain and impact of disability, and family health. The primary caregiver proxy report version of the CP QOL-Child, consisting of 66 items, was used in the present study. The scoring of the questionnaire was performed according to the CP QOL manual.30 The raw scores of each domain are then converted into a 0–100 scale.
Intervention
Inspiratory muscle training was performed using a pressure threshold-loading device (Threshold IMT, Philips-Respironics, Pittsburgh, PA, USA). This device provides a specific, measurable resistance that is constant during each inspiration. Before training began, the children completed a one-week familiarization period with their parents in order to adequately learn diaphragmatic breathing with the device. The treatment group received IMT at 30% of MIP. The MIP was measured during a supervised period each week, and the training load was adjusted weekly to maintain 30% of MIP. The control group received sham IMT at fixed workload of 5% of MIP. Children were instructed to maintain diaphragmatic breathing with the device for 10–15 breaths, try rest for 5–10 s between breaths, and maintain this pattern for 15 min, twice a day. Both groups trained for a total of 30 min per day, 7 days per week, for 6 weeks. At home, parents monitored their children while they performed IMT. The children were checked via phone call 3 times a week to insure they were performing IMT correctly. The children were also given training diaries and told to fill out the diaries with their parents, which were checked once weekly by the investigators. The total minutes spent training was calculated according to the reports written in the diaries. During the IMT/sham IMT period, both groups received routine conventional physiotherapy for 40 min per day, 3 days per week by a physiotherapist with specialization in pediatric neurology. The conventional physiotherapy program included conventional mat activities, specific stretching exercises for tightened muscles, range of motion exercises for joint elasticity, and therapist-guided techniques for facilitating normal movement patterns. No children received any other treatments, such as occupational or speech therapy.
Statistical analysis
All statistical analysis was conducted using SPSS 20.0 (SPSS, Chicago, IL, USA). On the basis of a pilot study, the primary clinical outcome of the current study was TCMS total score that determined to obtain a power of 0.80 with α level of 0.05 with an effect size of 0.85; total sample size estimation would be 12 participants per group using G*Power software. Data normality was tested using the Kolmogorov–Smirnov calculation. Baseline demographic and clinical characteristics of the groups compared using Mann– Whitney U test and differences were reported as median and interquartile range (%25–%75 IQR). Nominal data were compared using a chi-square test. Analysis of covariance was used to determine whether there are any significant differences between pre- and post-test conditions (pulmonary functions, respiratory muscle strength, trunk control, daily living activities, 6MWT and quality of life). Each outcome measure baseline values were used as covariates in the analysis. Manually adjusted post hoc comparisons were done using Bonferroni test. Effect sizes for the vastness of statistically significant group differences were characterized using d. Also, effects sizes were expressed as small (>0.20), moderate (between 0.50 and 0.80), and large (≥0.80).31, 32 Post hoc statistical power was calculated using G*Power software to TCMS total score.33 The analysis was performed per-protocol on the children included in the study. A p value < 0.05 was considered as statistically significant.
Results
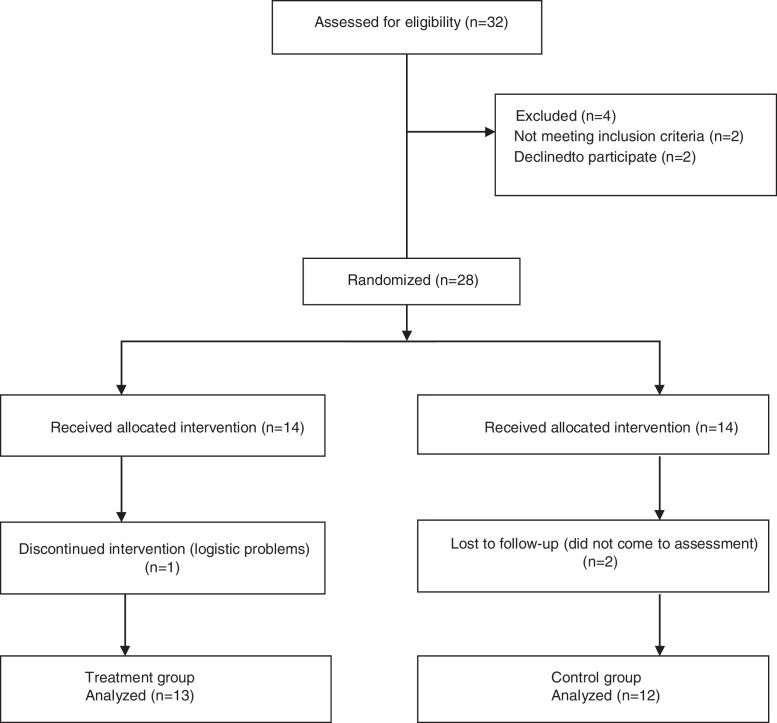
A total of 32 children with CP were assessed for eligibility. Among them, 4 children were excluded (Fig. 1). Twenty-eight children were randomly allocated either treatment or control group. Finally, 13 children in the treatment group (7.14% loss to follow up) and 12 children in the control group (14.28% loss to follow up) completed the study. The IMT was well tolerated by all of the children with CP. Adherence to the IMT program was high in both groups. There was no difference between groups in time spent during IMT; the treatment group spent 1141.66 ± 97.66 min (∼90.5% of expected), and the control group 1086.81 ± 81.06 min (∼86.9% of expected).
Figure 1.
Flow chart.
The baseline demographic characteristics of the groups are presented in Table 1.
Table 1.
Baseline demographic characteristics of treatment and control groups.
| Characteristics | Treatment group | Control group |
|---|---|---|
| Median (%25–75 IQR) | Median (%25–75 IQR) | |
| Age, years | 9.5 (8–10) | 8 (7–12) |
| Male/female, n (%) | 6/7 (46.1%/53.8%) | 6/6 (50%/50%) |
| Weight, kg | 28 (24.25–37) | 30 (28–36) |
| Height, cm | 130 (124–140) | 130 (128–141) |
| BMI, kg/m2 | 16.38 (14.30–18.97) | 18.98 (16.86–20.46) |
| GMFCS I/II, n (%) | 9 (69.2%)/4 (30.7%) | 8 (66.6%)/4 (33.3%) |
| Topographic classification, n(%) | ||
| Hemiplegic/diplegic | 8 (61.5%)/5 (38.4%) | 8 (66.6%)/4 (66.6%) |
| Mother's education, n(%) | ||
| Primary school | 1 (7.6%) | 1 (8.33%) |
| Junior high school | 1 (7.6%) | 1 (8.33%) |
| Senior high school | 5 (38.4%) | 6 (50.0%) |
| University or above | 6 (46.1%) | 4 (33.3%) |
| Father's education, n(%) | ||
| Primary school | 1 (7.6%) | 1 (8.3%) |
| Junior high school | 1 (7.6%) | 1 (8.3%) |
| Senior high school | 4 (30.7%) | 5 (41.6%) |
| University or above | 7 (53.8%) | 5 (41.6%) |
BMI, Body Mass Index; GMFCS, Gross Motor Function Classification System.
There were no significant differences in pulmonary function test scores between groups after inspiratory muscle training (p > 0.05, Table 2).
Table 2.
Effects of inspiratory muscle training on pulmonary function, respiratory muscle strength, trunk control and functional exercise capacity.
| Characteristics | Treatment group |
Control group |
Treatment affect | Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Mean difference %95 CI | Group difference | Before | After | Mean difference %95 CI | Group difference | |||
| Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | p | d | |||
| FEV1, % | 88.30 ± 18.96 | 88.90 ± 20.09 | 0.60 (−4.24 to 5.44) | 0.797 | 91.77 ± 8.42 | 94.00 ± 10.35 | 2.22 (−2.88 to 7.33) | 0.372 | 0.633 | |
| FVC, % | 80.30 ± 17.16 | 81.60 ± 17.84 | 1.30 (−3.37 to 5.97) | 0.565 | 84.77 ± 11.80 | 86.00 ± 12.10 | 1.22 (−3.70 to 6.15) | 0.608 | 0.981 | |
| FEV1/FVC | 105.50 ± 10.20 | 106.70 ± 10.20 | 1.20 (−1.05 to 3.45) | 0.277 | 106.00 ± 9.23 | 105.44 ± 9.76 | −0.55 (−2.93 to 1.81) | 0.628 | 0.274 | |
| FEF25–75, % | 88.10 ± 26.41 | 87.80 ± 24.04 | −0.30 (−5.11 to 4.51) | 0.828 | 87.66 ± 17.46 | 89.44 ± 16.80 | 1.77 (−3.29 to 6.84) | 0.483 | 0.539 | |
| PEF, % | 69.30 ± 17.41 | 77.10 ± 23.19 | 7.80 (4.015 to 11.58) | 0.001* | 69.36 ± 15.12 | 73.18 ± 14.67 | 3.81 (0.21 to 7.42) | 0.049* | 0.127 | |
| MIP, cmH2O | 65.83 ± 15.70 | 94.50 ± 16.07 | 28.66 (23.33 to 34.00) | <0.001* | 51.54 ± 22.54 | 57.36 ± 23.43 | 5.81 (0.24 to 11.39) | 0.041* | <0.001* | 1.20 |
| MIP, % predicted | 89.92 ± 17.21 | 129.65 ± 20.91 | 39.73 (32.20 to 47.25) | <0.001* | 69.03 ± 21.73 | 77.25 ± 23.28 | 8.21 (0.36 to 16.07) | 0.041* | <0.001* | 1.22 |
| MEP, cmH2O | 68.00 ± 23.21 | 82.50 ± 24.34 | 14.50 (8.00 to 20.99) | 0.001* | 52.90 ± 19.27 | 60.90 ± 16.48 | 8.00 (1.22 to 14.78) | 0.023* | 0.165 | 0.42 |
| MEP, % predicted | 74.14 ± 25.81 | 89.23 ± 25.05 | 15.08 (8.26 to 21.90) | <0.001* | 56.20 ± 15.97 | 65.85 ± 15.19 | 9.65 (2.52 to 16.78) | 0.010* | 0.265 | 0.54 |
| TCMS-SSB (0–20) | 19.08 ± 2.31 | 19.41 ± 1.72 | 0.33 (−0.09 to 0.76) | 0.123 | 19.36 ± 1.20 | 19.63 ± 1.50 | 0.27 (−0.17 to 0.72) | 0.222 | 0.842 | |
| TCMS-DSB-SMC (0–28) | 20.58 ± 3.17 | 23.75 ± 2.80 | 3.16 (2.47 to 3.85) | <0.001* | 20.54 ± 4.34 | 20.54 ± 4.41 | −3.55E−015 (−0.72 to 0.72) | 1.000 | <0.001* | 0.82 |
| TCMS-DSB-DR (0–10) | 7.41 ± 1.88 | 8.41 ± 1.56 | 1.00 (0.57 to 1.42) | <0.001* | 7.27 ± 1.48 | 7.63 ± 1.28 | 0.36 (−0.08 to 0.80) | 0.104 | 0.043* | 0.65 |
| TCMS-Total (0–58) | 47.08 ± 6.00 | 51.58 ± 5.24 | 4.50 (3.61 to 5.38) | <0.001* | 47.18 ± 5.58 | 47.81 ± 5.67 | 0.63 (−0.28 to 1.56) | 0.167 | <0.001* | 0.72 |
| 6MWT, m | 500.21 ± 60.61 | 566.60 ± 76.61 | 66.38 (53.53 to 89.23) | <0.001* | 456.69 ± 84.36 | 465.89 ± 91.56 | 9.20 (−4.22 to 22.62) | 0.169 | <0.001* | 0.84 |
| 6MWT, % predicted | 75.86 ± 9.48 | 85.98 ± 12.37 | 10.11 (8.06 to 12.16) | <0.001* | 69.91 ± 11.73 | 71.30 ± 12.78 | 1.39 (−0.74 to 3.52) | 0.190 | <0.001* | 0.86 |
FEV1, Forced Expiratory volume in One Second; FVC, Forced Vital Capacity; FEF25–75%, Forced Expiratory Flow from 25 to 75%; PEF, Peak Expiratory Flow; MIP, Maximal Inspiratory Pressure; MEP, Maximal Expiratory Pressure; TCMS-SSB, Trunk Control Measurement Scale-Static Sitting Balance; TCMS-DSB-SMC, Trunk Control Measurement Scale-Dynamic Sitting Balance-Selective Motor Control; TCMS-DSB-DR, Trunk Control Measurement Scale-Dynamic Sitting Balance-Dynamic Reaching; 6MWT, Six Minute Walk Test.
p ≤ 0.05.
Ten children (76.9%) in the treatment group and 10 children (83.3%) in the control group had inspiratory muscle weakness at baseline (MIP < 80 cmH2O). Improvements in MIP (22.85 cmH2O, 95% CI 12.84–32.86; p < 0.001) and MIP percentage of predicted (31.52%, 95% CI 21.52–41.48; p < 0.001) were significantly greater in the treatment group compared with the control group (Table 2). There were no significant differences in MEP and MEP percentage of predicted between groups (Table 2).
There was no difference in TCMS-SSB score between the groups (Table 2). The TCMS-DSB-SMC (3.16, 95% CI 2.54–3.78; p < 0.001), TCMS-DSB-DR (0.64, 95% CI 0.32–0.96; p < 0.001) and TCMS-Total (3.87, 95% CI 3.72–4.02; p < 0.001) scores were significantly improved in the treatment group compared with the control group (Table 2).
The PEDI-FSS self-care (1.86, 95% CI 0.68–3.04; p = 0.007), PEDI-FSS mobility (2.78, 95% CI 1.72–3.84; p = 0.001) and social function (3.95, 95% CI 3.00–4.90; p = 0.015) domain scores were significantly improved in the treatment group compared with control group (Table 3). No difference was observed between the groups in the PEDI-CAS subscale scores (Table 3).
Table 3.
Effects of inspiratory muscle training on daily living activities and quality of life.
| Characteristics | Treatment group |
Control group |
Treatment affect | Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Mean difference %95 CI | Group difference | Before | After | Mean difference %95 CI | Group difference | |||
| Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | p | d | |||
| PEDI-FSS (scaled score 0–100) | ||||||||||
| Self-care | 90.86 ± 7.18 | 93.15 ± 5.38 | 2.28 (0.95 to 3.61) | 0.002* | 86.30 ± 5.68 | 86.69 ± 5.40 | 0.42 (−0.89 to 1.89) | 0.410 | 0.049* | 0.56 |
| Mobility | 94.63 ± 5.05 | 98.02 ± 3.60 | 3.39 (1.87 to 4.90) | <0.001* | 95.06 ± 4.93 | 95.68 ± 5.93 | 0.61 (−0.96 to 2.19) | 0.427 | 0.016* | 0.58 |
| Social function | 94.10 ± 4.94 | 96.60 ± 4.29 | 2.56 (0.20 to 4.92) | 0.035* | 93.42 ± 5.24 | 92.02 ± 7.01 | −1.39 (−3.86 to 1.07) | 0.252 | 0.025* | 0.78 |
| PEDI-CAS (scaled score 0–100) | ||||||||||
| Self-care | 91.45 ± 6.78 | 92.70 ± 5.78 | 1.25 (−0.91 to 3.41) | 0.243 | 85.68 ± 13.60 | 86.81 ± 12.45 | 1.13 (−1.12 to 3.39) | 0.380 | 0.941 | |
| Mobility | 96.42 ± 6.68 | 97.85 ± 5.85 | 1.42 (−0.54 to 3.39) | 0.147 | 93.24 ± 8.40 | 94.80 ± 7.20 | 1.55 (−0.50 to 3.61) | 0.130 | 0.925 | |
| Social function | 96.33 ± 4.33 | 97.66 ± 3.98 | 1.33 (−0.54 to 3.21) | 0.155 | 93.45 ± 6.75 | 94.54 ± 5.44 | 1.09 (−0.87 to 3.05) | 0.261 | 0.855 | |
| CPQOL-child (scaled score 0–100) | ||||||||||
| Social well-being and acceptance | 78.19 ± 16.16 | 88.63 ± 14.16 | 10.44 (6.18 to 14.69) | <0.001* | 78.03 ± 10.43 | 74.73 ± 9.96 | −3.29 (−7.73 to 1.14) | 0.138 | <0.001* | 0.94 |
| Functioning | 76.56 ± 15.42 | 80.15 ± 14.01 | 3.59 (1.14 to 6.04) | 0.006* | 70.91 ± 11.29 | 69.40 ± 13.36 | −1.50 (−4.06 to 1.04) | 0.234 | 0.007* | 0.76 |
| Participation and physical health | 88.19 ± 21.63 | 92.62 ± 19.52 | 4.42 (−1.26 to 10.10) | 0.121 | 76.62 ± 15.26 | 80.31 ± 20.21 | 3.69 (−0.75 to 10.62) | 0.106 | 0.572 | |
| Emotional well-being | 72.67 ± 20.04 | 80.22 ± 16.22 | 7.55 (1.55 to 13.55) | 0.016* | 60.94 ± 17.25 | 61.43 ± 12.72 | 0.49 (−5.77 to 6.75) | 0.872 | 0.105 | |
| Access to services | 34.79 ± 10.65 | 35.48 ± 10.41 | 6.45(−21.67 to 2.88) | 0.638 | 30.07 ± 10.28 | 33.69 ± 7.67 | 3.61 (−1.16 to 6.06) | 0.064 | 0.073 | |
| Pain and impact of disability | 32.94 ± 22.26 | 24.30 ± 14.17 | −8.64 (−17.63 to 0.34) | 0.059 | 41.85 ± 19.48 | 42.09 ± 16.74 | 0.24 (−9.14 to 9.63) | 0.957 | 0.170 | |
| Family health | 67.18 ± 24.26 | 67.55 ± 22.94 | 0.36 (−3.08 to 3.81) | 0.828 | 60.22 ± 12.50 | 58.98 ± 12.71 | −1.23 (−4.84 to 2.36) | 0.483 | 0.511 | |
PEDI-FSS, Pediatric Evaluation of Disability Inventory-Functional Skill Scale; PEDI-CAS, Pediatric Evaluation of Disability Inventory-Caregiver Assistance Scale; CPQOL, Cerebral Palsy Quality of Life.
p ≤ 0.05.
The distance covered during the 6MWT (57.1 m, 95% CI 37.58–76.62, p < 0.001) and percent of the predicted 6MWT distance (8.72%, 95% CI 5.34–12.10%, p < 0.001) significantly improved in the treatment group compared with the control group (Table 2).
The CPQOL-Child social well-being and acceptance (13.73, 95% CI 12.86–14.60, p < 0.001) and functioning domain (5.09, 95% CI 2.09–8.09; p = 0.004) scores significantly improved in the treatment group compared with control group (Table 3). No difference was observed between the groups in the other domains of the CPQOL-Child (Table 3).
Discussion
This was the first clinical trial to simultaneously examine the effects of IMT on body function, daily living activities, participation, and quality of life in children with CP. The main findings of our study were that IMT improved respiratory muscle strength, trunk control, daily living activities, functional exercise capacity, and quality of life in children with CP. The present study had an adequate sample size, a control group, and an adequate statistical power (96.8%) to support our hypothesis. Compliance with the IMT program was acceptable and the program was well tolerated by all participants.
Similar to previous studies, 76.9% of children with CP in the treatment group and 83.3% in the control group had inspiratory muscle weakness (MIP < 80 cmH2O).22 We applied IMT for 6 weeks, 7 days per week for 30 min per day, using a moderate intensity (30% of MIP). This resulted in MIP improvements with a large treatment effect. Previous studies have also demonstrated a relationship between IMT and improved inspiratory muscle strength in various patient populations,6, 12, 13, 14, 15 similar to our findings. Although MEP significantly improved in both groups, higher IMT workload was not superior to lower workload. Lower workloads may also have therapeutic effect in improving MEP, thus further studies are needed to investigate the effects of IMT on MEP in children with CP.
To date, to our knowledge, there is only one study in the literature has investigated the effects of IMT on pulmonary functions in children with CP.16 In their study, IMT improved FEV1 and FVC. In our study, no improvements were recorded in dynamic lung volumes after IMT. This difference might relate to different techniques of IMT application. Lee et al.,16 used feedback respiratory training, which is also known as voluntary isocapnic hyperpnea. This method generally uses deep and fast breathing at 70–80% of maximum voluntary ventilation in each inspiration for endurance training.34 We used mechanical threshold training technique, the most used form of IMT. The mechanical threshold training technique operates independently of breath volume, working on inhalation against an adjustable (relative to MIP) pressure threshold. This might explain the differences between the dynamic lung volume results after IMT. PEF and MEP both depend on expiratory muscle strength. Thus, improvement in PEF likely relates to improvement in MEP (56% treatment group, 48% control group) after IMT. For better understanding of the effects of IMT on pulmonary function in children with CP, application of higher intensity and/or longer duration training should be investigated. Restrictive lung disease is also a problem in children with CP, thus investigating the effects of the IMT on static lung volumes may be beneficial.
This is the clinical trial in the literature to investigate the effects of IMT on trunk control. We found a significant improvement in selective movement control and dynamic reaching subscales after IMT in children with CP. The improvement in trunk control, especially in dynamic sitting balance subscale scores, might indicate improvement in non-respiratory respiratory muscle function. Inspiratory muscles (especially the diaphragm, which contributes the intra-abdominal pressure modulation), play an important ancillary role in core stability and postural control.35 A number of studies also reported contraction of the diaphragm for trunk stabilization prior to rapid arm movements, flexion of the upper and lower extremities, and trunk extension.7, 36, 37 We thought that inspiratory muscle induced core stabilization might improve after IMT, thereby improving balance control during dynamic situations. There were no improvements in static sitting balance subscale scores after IMT. This might be related to higher baseline scale scores (median score 18 out of 20). To better understand the effects of IMT on static sitting balance, future studies should use different scales. Investigating the effects of IMT on trunk control during gait in children with CP may also be beneficial.
Past exercise and field tests demonstrated reduced exercise capacity in children with CP.38, 39 Consistent with the previous studies,12, 13, 40 we observed marked improvement in the 6MWT distance after IMT. Although there is no determined minimally clinically important difference (MCID) for 6MWT distance in children with CP, the distance covered during the 6MWT (57.5 m) in our study was greater than other studies where the MCID for the 6MWT distance ranged from 11 to 54 m.41 Our findings serve as a valuable addition to the literature to improve exercise capacity in children with CP. Also, IMT might be considered as part of a rehabilitation program to improve exercise capacity, especially in children with GMFCS level III-V, where mobility is limited.
We investigated the effects of IMT on daily living activities and showed that IMT improved functional skills of children with CP relative to daily living activities. This might be related to improvement in other clinical parameters such as trunk control and/or exercise capacity after IMT. Some daily living activities could be complicated for children with CP because of the necessity for simultaneous control of mobility, endurance, trunk stability, and balance. Thus, increased dynamic trunk control and endurance after IMT may affect the performance of daily living activities in children with CP. Performance of daily living activities can be affected by various intrinsic and extrinsic factors such as the child's interest and preferences (intrinsic), or opportunity to participate in age-appropriate activities (extrinsic). Additional studies are needed to show the effects of IMT on daily living activities.
Cerebral palsy is a non-progressive neurologic disorder with no cure. Therefore, children with CP often have to deal with lifelong disabilities that affect quality of life. Assessment of quality of life is also important in children with CP to identify threats to quality of life and adjust treatment plans accordingly. In our study, we found a significant improvement in social well-being, acceptance, and functioning domains of the CPQOL after IMT. Also, the emotional well-being and acceptance domains improved in the treatment group. These improvements may be related to improvement in participation, exercise capacity, and trunk control after IMT, factors that are closely interconnected and greatly affect quality of life. For instance, improvement in exercise capacity may allow a child to play longer with friends. In this way, socialization increases and quality of life improves. Quality of life can be affected by many factors such as nation, economic status, intra- and interpersonal perspective, educational level etc. Therefore, more studies are needed in order to better understand the effects of IMT.
As a conclusion, IMT was a feasible and effective physiotherapy for children with CP and improved respiratory muscle strength, trunk control, daily living activities, functional exercise capacity and quality of life. Our findings demonstrated that IMT is a clinically practical treatment in children with CP. Our study included GMFCS levels I and II children with CP. Future studies should investigate the effects of IMT in GMFCS levels III–V children with CP whose functional limitations are higher.
Although the power of the study was high, which was calculated based on the primary outcome measure; the effect size indicated small and moderate treatment effects for some other outcome measures. Therefore, the study cohort size may be a limitation of the current study, and increasing the sample size might allow us to observe the maximum effects of IMT.
Funding
This research was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) under Student Fellowship Program BIDEB 2211 and Gazi University Project of Scientific Investigation Coordination Unit, Project Code: 47/2017-10.
Authors’ contributions
The role of the authors in this study is as follows: Muserrefe Nur Keles – primary authors, performed data collection, analysis, interpretation and manuscript writing; Bulent Elbasan – management of the study, interpretation of the results; Umut Apaydin – performed data collection; Zeynep Arıbas – manuscript writing, Arzu Bakirtas and Nurdan Kokturk critical discussion.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Clinical Trials Registry ID: NCT02998281 (https://www.clinicaltrials.gov/ct2/show/NCT02998281?term=NCT02998281.&rank=1).
References
- 1.Rosenbaum P., Paneth N., Leviton A. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 2.Bax M., Goldstein M., Rosenbaum P. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 3.Hamill J., Knutzen K.M. Lippincott Williams & Wilkins; 2006. Biomechanical Basis of Human Movement. [Google Scholar]
- 4.Graham H.K., Selber P. Musculoskeletal aspects of cerebral palsy. J Bone Joint Surg Br. 2003;85:157–166. doi: 10.1302/0301-620x.85b2.14066. [DOI] [PubMed] [Google Scholar]
- 5.Almeida K.M., Fonseca S.T., Figueiredo P.R., Aquino A.A., Mancini M.C. Effects of interventions with therapeutic suits (clothing) on impairments and functional limitations of children with cerebral palsy: a systematic review. Braz J Phys Ther. 2017;21:307–320. doi: 10.1016/j.bjpt.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell A. Elsevier Health Sciences; 2013. Respiratory Muscle Training E-Book: Theory and Practice. [Google Scholar]
- 7.Hudson A.L., Butler J.E., Gandevia S.C. Role of the diaphragm in trunk rotation in humans. J Neurophysiol. 2011;106:1622–1628. doi: 10.1152/jn.00155.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H.Y., Chen C.C., Hsiao S.F. Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Res Dev Disabil. 2012;33:1176–1182. doi: 10.1016/j.ridd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Park E.S., Park J.H., Rha D.W. Comparison of the ratio of upper to lower chest wall in children with spastic quadriplegic cerebral palsy and normally developed children. Yonsei Med J. 2006;47:237–242. doi: 10.3349/ymj.2006.47.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon Y.H., Lee H.Y. Differences of respiratory function in children with spastic diplegic and hemiplegic cerebral palsy, compared with normally developed children. J Pediatr Rehabil Med. 2013;6:113–117. doi: 10.3233/PRM-130246. [DOI] [PubMed] [Google Scholar]
- 11.Kwon Y.H., Lee H.Y. Differences of respiratory function according to level of the gross motor function classification system in children with cerebral palsy. J Phys Ther Sci. 2014;26:389–391. doi: 10.1589/jpts.26.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselink R., De Vos J., Van Den Heuvel S. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 13.Silva I.S., Fregonezi G.A., Dias F.A. The Cochrane Library; 2013. Inspiratory Muscle Training for Asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houston B.W., Mills N., Solis-Moya A. The Cochrane Library; 2013. Inspiratory Muscle Training for Cystic Fibrosis. [DOI] [PubMed] [Google Scholar]
- 15.Pollock R.D., Rafferty G.F., Moxham J. Respiratory muscle strength and training in stroke and neurology: a systematic review. Int J Stroke. 2013;8:124–130. doi: 10.1111/j.1747-4949.2012.00811.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.Y., Cha Y.J., Kim K. The effect of feedback respiratory training on pulmonary function of children with cerebral palsy: a randomized controlled preliminary report. Clin Rehabil. 2014;28:965–971. doi: 10.1177/0269215513494876. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R.J., Hanna S.E., Rosenbaum P.L. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther. 2000;80:974–985. [PubMed] [Google Scholar]
- 18.Palisano R.J., Rosenbaum P., Bartlett D. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 19.Heyrman L., Molenaers G., Desloovere K. A clinical tool to measure trunk control in children with cerebral palsy: the Trunk Control Measurement Scale. Res Dev Disabil. 2011;32:2624–2635. doi: 10.1016/j.ridd.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Miller M.R., Crapo R., Hankinson J. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer P.H., Borsboom G., Brunekreef B. Spirometric reference values for white European children and adolescents: Polgar revisited. Pediatr Pulmonol. 1995;19:135–142. doi: 10.1002/ppul.1950190209. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 23.Domènech-Clar R., López-Andreu J., Compte-Torrero L. Maximal static respiratory pressures in children and adolescents. Pediatr Pulmonol. 2003;35:126–132. doi: 10.1002/ppul.10217. [DOI] [PubMed] [Google Scholar]
- 24.Haley S.M. PEDI Resarch Group; 1992. Pediatric Evaluation of Disability Inventory (PEDI): Development, Standardization and Administration Manual. [Google Scholar]
- 25.Erkin G., Elhan A.H., Aybay C. Validity and reliability of the Turkish translation of the Pediatric Evaluation of Disability Inventory (PEDI) Disabil Rehabil. 2007;29:1271–1279. doi: 10.1080/09638280600964307. [DOI] [PubMed] [Google Scholar]
- 26.ATS committee on proficiency standards for clinical pulmonary function laboratories ATS statement: guideline for the six minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Leunkeu A.N., Shephard R.J., Ahmaidi S. Six-minute walk test in children with cerebral palsy gross motor function classification system levels I and II: reproducibility, validity, and training effects. Arch Phys Med Rehabil. 2012;93:2333–2339. doi: 10.1016/j.apmr.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Waters E., Davis E., Mackinnon A. Psychometric properties of the quality of life questionnaire for children with CP. Dev Med Child Neurol. 2007;49:49–55. doi: 10.1017/s0012162207000126.x. [DOI] [PubMed] [Google Scholar]
- 29.Atasavun Uysal S., Duger T., Elbasan B., Karabulut E., Toylan I. Reliability and validity of the cerebral palsy quality of life questionnaire in the Turkish population. Percept Mot Skills. 2016;122:150–164. doi: 10.1177/0031512515625388. [DOI] [PubMed] [Google Scholar]
- 30.Waters E., Davis E., Boyd R. Deakin University; Melbourne: 2006. Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL-Child) Manual. [Google Scholar]
- 31.Morris S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386. [Google Scholar]
- 32.Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. [Google Scholar]
- 33.Erdfelder E., Faul F., Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 34.Scherer T.A., Spengler C.M., Owassapian D. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. Am J Respir Crit Care Med. 2000;162:1709–1714. doi: 10.1164/ajrccm.162.5.9912026. [DOI] [PubMed] [Google Scholar]
- 35.Hodges P.W., Gandevia S.C. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Appl Physiol. 2000;89:967–976. doi: 10.1152/jappl.2000.89.3.967. [DOI] [PubMed] [Google Scholar]
- 36.Hodges P., Cresswell A., Thorstensson A. Preparatory trunk motion accompanies rapid upper limb movement. Exp Brain Res. 1999;124:69–79. doi: 10.1007/s002210050601. [DOI] [PubMed] [Google Scholar]
- 37.Kolar P., Sulc J., Kyncl M. Stabilizing function of the diaphragm: dynamic MRI and synchronized spirometric assessment. J Appl Physiol. 2010;109:1064–1071. doi: 10.1152/japplphysiol.01216.2009. [DOI] [PubMed] [Google Scholar]
- 38.Verschuren O., Takken T. Aerobic capacity in children and adolescents with cerebral palsy. Res Dev Disabil. 2010;31:1352–1357. doi: 10.1016/j.ridd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald D., Hickey C., Delahunt E. Six-minute walk test in children with spastic cerebral palsy and children developing typically. Pediatr Phys Ther. 2016;28:192–199. doi: 10.1097/PEP.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 40.Gomes-Neto M., Saquetto M.B., Silva C.M. Effects of respiratory muscle training on respiratory function, respiratory muscle strength, and exercise tolerance in patients poststroke: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2016;97:1994–2001. doi: 10.1016/j.apmr.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Schrover R., Evans K., Giugliani R. Minimal clinically important difference for the 6-min walk test: literature review and application to Morquio A syndrome. Orphanet J Rare Dis. 2017;12:78. doi: 10.1186/s13023-017-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]