Abstract
Canine disseminated fungal infection by Aspergillus species carries a guarded to grave prognosis as they often rapidly progress and are refractory to treatment with many euthanased soon after diagnosis. This case report describes a 2.5 year old female spayed German Shepherd Dog diagnosed with disseminated Aspergillus deflectus infection for which definitive treatment was declined by the owners. With only palliative management the dog survived three years and two months before succumbing to chronic kidney disease.
Keywords: Aspergillus deflectus, Disseminated aspergillosis, Palliative care, Survival
1. Introduction
Disseminated invasive aspergillosis (DIA) is an uncommon disease in dogs and the German Shepherd Dog (GSD) is overrepresented [1]. The most frequent causative agents are Aspergillus terreus (subgenus Circumdati) and A. deflectus (subgenus Nidulantes) [2], with other species from subgenus Circumdati (A. carneus, A. flavipes, A. niger) [2], from subgenus Nidulantes, (A. versicolor) [3], and subgenus Fumigati (A. fumigatus, A. lentulus, A. felis) [2], [4] being reported less often. Other fungal genera can have similar presentations. Morphological diagnosis of fungal species is often inaccurate and unable to differentiate closely related or so-called “cryptic” species. This might have led to misidentification in earlier studies. Molecular testing methods using polymerase chain reaction and comparative sequence analysis enable definitive identification of most clinical isolates [5].
The clinical signs associated with DIA in dogs vary depending on the location of the infection, with renal impairment, lymphadenopathy and discospondylitis reported commonly [6], [7]. Diagnosis of fungal infection is achieved through identification of fungal hyphae within tissue samples or urine or by detection of the fungal cell wall antigen galactomannan in blood or urine [8]. Serological tests to detect Aspergillus-specific antibodies have not been reliable [6], [9], [10]. Once the mycotic infection is detected, culture and identification is required but can still remain challenging [11].
Treatment with systemic fungal agents has traditionally been poorly successful. Treatment with newer triazole agents has improved the outlook [12], but the cost associated with these treatments in a large dog can be prohibitive for many owners [12]. There is little information on the outcome when palliative measures are the sole treatment.
This case report describes long term survival with a generally good quality of life in a young GSD with DIA given palliative treatment only.
2. Case
A 2.5 year old female spayed GSD was presented for investigation of polyuria, polydipsia and urinary incontinence at night of at least four months duration. The patient had not had any prior illnesses other than some injuries following a dog attack. She was the only survivor from her litter and had to be hand raised, and two subsequent litters from the same dam all died. There were no samples or further information from any of the pups or the dam available.
The dog had been evaluated by the referring veterinarian for nocturnal incontinence and occasional urine dribbling. There were no significant findings on physical examination and oral stilboestrol was prescribed for potential urethral sphincter incompetence. There was improvement initially, which was not sustained. Two months after the initial presentation the owner reported polyuria and polydipsia. No significant physical examination abnormalities were detected. Blood and urine samples were submitted. The urine showed isosthenuria (specific gravity 1.010) and no other abnormalities. The haematology showed a mild lymphocytosis (5.4 × 109/L, ref range 0.9 – 3.5) and eosinophilia (2.1 × 109/L, ref range 0.0 – 1.4); the biochemistry profile showed a mild hypoglycaemia (2.4 mmol/L, ref range 3.3 – 6.8) attributed to delay in analysis, azotaemia (urea 12.7 mmol/L, ref range 2.5 – 10; creatinine 160 μmol/L, ref range 50–150) and hyperglobulinaemia (48 g/L, ref range 25 – 45). The dog was treated empirically with oral cephalexin for suspected urinary tract infection and there was resolution of the incontinence and improvement in the polyuria and polydipsia. The signs recurred with cessation of the antibiotics. Repeat haematology and serum biochemistry after the antibiotics showed similar changes to the previous test with the only new finding being erythrocytosis (8.7 × 1012/L, ref range 4.9 – 8.2). Referral for further evaluation was recommended at this time.
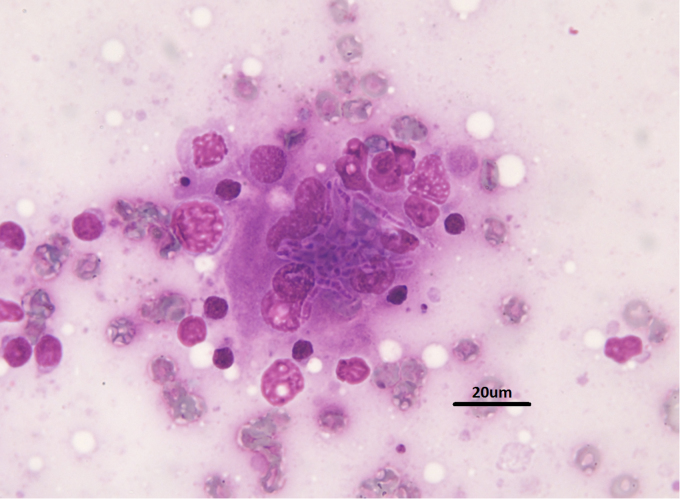
On presentation at the University Veterinary Teaching Hospital Sydney four months after the initial presentation (day 0), the dog was in poor body condition (Body Condition Score 2/5) and had a moderate generalised peripheral lymphadenopathy. The remainder of the examination was unremarkable. Haematology, serum biochemistry, urinalysis, urine culture, fine needle aspirate of lymph nodes and abdominal ultrasonography were performed. The blood results showed a mild lymphocytosis (4.6 × 109/L, ref range 0.9 – 3.5) and marginal azotaemia (urea 12.3 mmol/L, ref range 2.5 – 9.6; creatinine 0.15 mmol/L, ref range 0.05 – 0.15). Urinalysis showed isosthenuria (SG 1.012) and 1 + blood, the latter thought to be the result of cystocentesis. Urine culture was negative. Abdominal ultrasonography revealed a mild bilateral nephropathy characterised by reduced corticomedullary delineation with each kidney containing a single small corticomedullary cyst, a diffusely enlarged spleen with a heterogeneous area in the tail and generalised abdominal lymphadenopathy. Cytology of peripheral lymph node aspiratess all showed macrophages containing thin, branching septate hyphae (Fig. 1). There was only a very mild increase in inflammatory cells in the node with small lymphocytes comprising 82% of nucleated cells, 5% medium lymphocytes, 2% large lymphocytes and 5% plasma cells. Macrophages and neutrophils comprised less than 3% of the nucleated cells. A diagnosis of disseminated invasive fungal disease was made.
Fig. 1.
Photomicrograph of the fine needle aspirate showing the fungal hyphae. (Bar = 20 µm).
The following week (day 8) the dog was returned for samples to be collected for fungal culture and identification. Further imaging, including spinal radiographs was recommended, but declined by the owners. At this presentation there was no change in the physical examination findings and the owner reported that at home the dog was generally well, eating normally and playful. Aspirates from multiple lymph nodes were inoculated onto Sabouraud's dextrose with and without gentamicin and chloramphenicol as well as blood agar plates. Fungal colonies were grown on all, but one plate and their appearance were not typical of Aspergillus sp. The isolate was sent to the National Mycology Reference Centre for molecular identification using internal transcribed spacer ribosomal DNA (ATS1–5.8S-ITS2) sequencing and was identified as Aspergillus deflectus based on 100% homology with A. deflectus NRRL2206 (Genbank Accession NR 135349). Sequencing of the partial beta-tubulin gene was also performed at the University of Sydney and identity of the isolate as A. deflectus was confirmed (98.6% homology with A. deflectus NRRL 2206). The isolate did not sporulate so antifungal susceptibility testing could not be performed.
The results were discussed with the owners and systemic azole antifungal therapy was recommended. Due to concerns about the cost of medicating the patient and potential drug related toxicities, the owners declined treatment. They changed her diet from a commercial diet to a home cooked one as well as seeking the advice of a naturopath.
Over the next 14 months the owner reported intermittently by phone that the dog was stable and generally well other than persistence of mild polyuria and polydipsia. On day 440 after first presentation the owner called to report the dog was reluctant to jump and was quieter than usual. Spinal involvement was suspected, and imaging was recommended. The owners deferred investigations for a month, as there was no overt spinal pain detected by a physiotherapist who examined the patient. However, during the month the pain became more obvious and the dog developed right forelimb lameness and was presented for further evaluation 19 months (day 470) from first presentation.
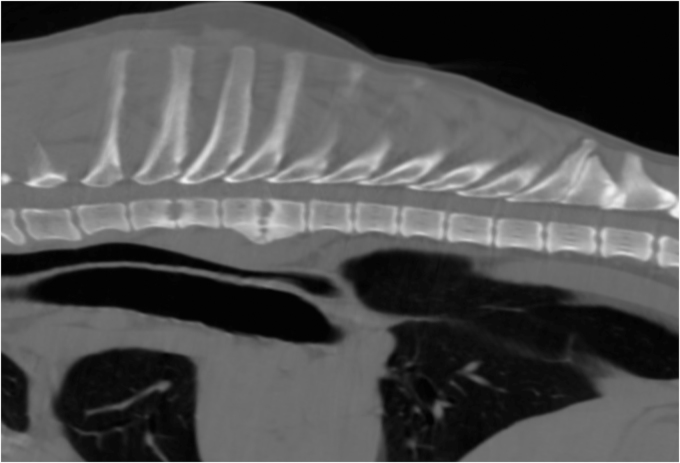
A limited blood profile was performed that showed mild azotaemia (urea 11.3 mmol/L, ref range 2.5 – 9.6; creatinine 231 μmol/L, ref range 45–159) and hyperproteinaemia. Under general anaesthesia a whole body computed tomography scan was performed. This showed lysis of the end plates and irregular end plate margins at T2-T3 and T4-T5. There was an irregular area of calcification in the left kidney associated with a wedge shaped hypovascular area, rounding of the spleen and lymphadenopathy. Sampling of the affected disc spaces to confirm fungal discospondylitis was discussed but declined by the owners (Fig. 2).
Fig. 2.
Sagittal CT image of the thoracic spine showing the lesions consistent with discospondylitis at T2–3 and T4–5.
Systemic antifungal medication was discussed again, but also declined. Pain relief with gabapentin and firocoxib was prescribed with a warning that the firocoxib could worsen renal function. Samples from lymph nodes and urine were submitted for fungal culture and identification. Fungal colonies were grown from both urine and lymph nodes aspirates. Molecular identification targeting the partial beta-tubulin gene revealed these colonies to be Aspergillus deflectus.
The owner elected not to administer any of the pain medications and the dog improved without medication. Over the following 18 months the owner reported that the dog had several intermittent episodes of pain while walking, torticollis and difficulty jumping, which responded to short courses of non-steroidal anti-inflammatory drugs. Her appetite and general behaviour between episodes was considered normal by the owners and by the referring veterinarian.
Thirty seven months after initial presentation (day 1134) the owners reported that the dog was unwell with reduced appetite, intermittent vomiting and lethargy. Serum biochemistry showed severe azotaemia (creatinine 1083 μmol/L. ref range 44 – 159, urea 92.3 mmol/L, ref range 2.5 – 10, SDMA 54 μg/dL, ref range 0 – 14) with minimal response to dietary management. The signs worsened and 38 months after initial presentation (day 1151) she was euthanased. A post mortem had previously been requested but was declined by the owners.
3. Discussion
There are no reports of long term follow up of a dog with confirmed DIA that did not receive definitive treatment. This dog survived 38 months from the time of confirmed diagnosis before succumbing to Stage IV chronic kidney disease. If the renal impairment was due to aspergillosis from the outset, as suspected, then her survival would have been 3.5 y (42 months). The deaths of all the other pups in the litter and of all pups in two subsequent litters from the same dam could be suggestive of transplacental transmission of aspergillosis, as has been previously reported in one dog [13] and even longer survival, but without information on the dam and pups this remains speculative.
The initial relatively mild clinical signs led to a hypothesis that this might have been a systemic mycosis other than aspergillosis, or that the patient's immune response was sufficient to control, but not eliminate the infection. The former was eliminated via fungal identification and testing for the latter was not available. Given the increased risk of DIA in GSDs, as well as a female predisposition, a genetic immune deficiency is considered likely, but to date has not been identified. GSDs are also over-represented for a form of chronic bronchopulmonary aspergillosis, characterised by cavitated lung lesions [14]. Lung lobectomy and antifungal therapy was curative (>6 yr survival) in two GSDs with this form of aspergillosis [14]
Reports show GSDs are over-represented for selective IgA deficiency [15] and some with DIA have been shown to have low levels of IgA. However, whether this is associated with susceptibility to DIA or co-incidence is not known [16]. The immune response to fungi is complex and involves both the innate and adaptive immune systems [17]. The specific deficiencies identified in people with DIA have not been found in dogs [18]. Recent studies have shown that Il-17 and natural killer cells are critical in prevention of systemic infection by fungi, but also bacteria, viruses and tumours [19]. The authors are not aware of studies in this area in GSDs.
In a previous report of four cases of A. deflectus infection in GSDs [20], three dogs were euthanased shortly after diagnosis. One of the dogs presented with front leg lameness of one month duration and a lytic lesion in the left ulna was found along with two lung nodules. Biopsy of the bone lesion showed changes consistent with fungal osteomyelitis. The dog was seen about six weeks later with progression of disease involving additional bone lesions in the left radius, the right distal humerus, left tibia and zygomatic arch. The dog was commenced on ketoconazole. There was progression especially in the left front leg with marked weight loss and amputation was performed three months after initial presentation. Four months after surgery, seven months after initial presentation and eight months after the first signs, the dog was reported to be doing well with weight gain and no further progression of signs. As the duration of ketaconazole therapy after surgery was not stated and the progression of disease had occurred during treatment, this is the longest surviving dog with DIA and without known definitive treatment that is reported prior to the current case.
This is the first report of long term survival in a dog with DIA, including renal involvement and discospondylitis. Palliative care may be a viable option in cases with mild clinical signs where antifungal therapy is not possible.
Acknowledgements
The authors would like to acknowledge the owners of the patient and the local veterinarians who remained in contact and provided follow up information on a regular basis.
Acknowledgments
Conflict of interest
None of the authors have anything to declare.
Ethical form
Please note that this journal requires full disclosure of all sources of funding and potential conflicts of interest. The journal also requires a declaration that the author(s) have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
The statements on funding, conflict of interest and consent need to be submitted via our Ethical Form that can be downloaded from the submission site www.ees.elsevier.com/mmcr. Please note that your manuscript will not be considered for publication until the signed Ethical Form has been received.
References
- 1.Day M.J., Penhale W.J. An Immunohistochemical study of canine disseminated Aspergillosis. Aust. Vet. J. 1991;68(12):383–386. doi: 10.1111/j.1751-0813.1991.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 2.Schultz R.M. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J. Vet. Intern. Med. 2008;22(4):851–859. doi: 10.1111/j.1939-1676.2008.0125.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S.P. Aspergillus versicolor, a new causative agent of canine disseminated aspergillosis. J. Clin. Microbiol. 2012;50(1):187–191. doi: 10.1128/JCM.05388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrs V.R. Aspergillus felis sp. nov., an emerging agent of invasive aspergillosis in humans, cats, and dogs. PLoS One. 2013;8(6):e64871. doi: 10.1371/journal.pone.0064871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meason-Smith C. Panfungal polymerase chain reaction for identification of fungal pathogens in formalin-fixed animal tissues. Vet. Pathol. 2017;54(4):640–648. doi: 10.1177/0300985817698207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day M.J. Immunological study of systemic Aspergillosis in German Shepherd Dogs. Vet. Immunol. Immunopathol. 1985;9(4):335–347. doi: 10.1016/0165-2427(85)90063-7. [DOI] [PubMed] [Google Scholar]
- 7.Watt P.R. Disseminated opportunistic fungal disease in dogs - 10 cases (1982 - 1990) J. Am. Vet. Med. Assoc. 1995;207(1):67–70. [PubMed] [Google Scholar]
- 8.Garcia R.S. Sensitivity and specificity of a blood and urine galactomannan antigen assay for diagnosis of systemic aspergillosis in dogs. J. Vet. Intern. Med. 2012;26(4):911–919. doi: 10.1111/j.1939-1676.2012.00935.x. [DOI] [PubMed] [Google Scholar]
- 9.Day M.J., Penhale W.J. Humoral immunity in disseminated Aspergillus terreus infection in the dog. Vet. Microbiol. 1988;16(3):283–294. doi: 10.1016/0378-1135(88)90032-6. [DOI] [PubMed] [Google Scholar]
- 10.Garcia M.E. The value of the determination of anti-Aspergillus IgG in the serodiagnosis of canine aspergillosis: comparison with galactomannan detection. J. Vet. Med. Ser. B-Infect. Dis. Vet. Public Health. 2001;48(10):743–750. doi: 10.1046/j.1439-0450.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomez B.L. Molecular diagnosis of endemic and invasive mycoses: advances and challenges. Rev. Iberoam. De. Micol. 2014;31(1):35–41. doi: 10.1016/j.riam.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Foy D.S., Trepanier L.A. Antifungal treatment of small animal veterinary patients. Vet. Clin. North Am.-Small Anim. Pract. 2010;40(6) doi: 10.1016/j.cvsm.2010.07.006. (p. 1171-+) [DOI] [PubMed] [Google Scholar]
- 13.Elad D., Lahav D., Blum S. Transuterine transmission of Aspergillus terreus in a case of disseminated canine aspergillosis. Med. Mycol. 2008;46(2):175–178. doi: 10.1080/13693780701658371. [DOI] [PubMed] [Google Scholar]
- 14.Whitley N.T. Long term survival in two German shepherd dogs with Aspergillus-associated cavitary pulmonary lesions. J. Small Anim. Pract. 2010;51(10):561. doi: 10.1111/j.1748-5827.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 15.Tengvall K. Genome-wide analysis in German shepherd dogs reveals association of a locus on CFA 27 with atopic dermatitis. PLoS Genet. 2013;9(5):e1003475. doi: 10.1371/journal.pgen.1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day M.J., Penhale W.J. An immunohistochemical study of canine disseminated aspergillosis. Aust. Vet. J. 1991;68(12):383–386. doi: 10.1111/j.1751-0813.1991.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 17.Underhill D.M., Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity. 2015;43(5):845–858. doi: 10.1016/j.immuni.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco J.L., Garcia M.E. Immune response to fungal infections. Vet. Immunol. Immunopathol. 2008;125(1–2):47–70. doi: 10.1016/j.vetimm.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Bar E. IL-17 regulates systemic fungal immunity by Controlling the functional competence of NK cells. Immunity. 2014;40(1):117–127. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Jang S.S. Aspergillus deflectus infection in 4 dogs. J. Med. Vet. Mycol. 1986;24(2):95–104. [PubMed] [Google Scholar]