Abstract
Rasmussen’s encephalitis (RE) is a rare pediatric neurological disorder, and the exact etiology is not clear. Viral infection may be involved in the pathogenesis of RE, but conflicting results have reported. In this study, we evaluated the expression of both Epstein-Barr virus (EBV) and human herpes virus (HHV) 6 antigens in brain sections from 30 patients with RE and 16 control individuals by immunohistochemistry. In the RE group, EBV and HHV6 antigens were detected in 56.7% (17/30) and 50% (15/30) of individuals, respectively. In contrast, no detectable EBV and HHV6 antigen expression was found in brain tissues of the control group. The co-expression of EBV and HHV6 was detected in 20.0% (6/30) of individuals. In particular, a 4-year-old boy had a typical clinical course, including a medical history of viral encephalitis, intractable epilepsy, and hemispheric atrophy. The co-expression of EBV and HHV6 was detected in neurons and astrocytes in the brain tissue, accompanied by a high frequency of CD8+ T cells. Our results suggest that EBV and HHV6 infection and the activation of CD8+ T cells are involved in the pathogenesis of RE.
Keywords: Rasmussen’s encephalitis (RE), Epstein-Barr virus (EBV), Human herpes virus 6 (HHV6), Seizures, CD8+ T cells
Introduction
Rasmussen’s encephalitis (RE) is a rare syndrome of the nervous system first described by Rasmussen et al. (1958). It usually occurs in children, with an estimated incidence of 2–3 (average of 1.8) per 10 million under 18 years old in Europe, and the mean age of disease onset is approximately 6 years old (Bien et al. 2013). The clinical manifestations mainly include intractable seizures, unilateral hemispheric atrophy, deterioration of neurological function, and cognitive impairment. Hemispherectomy is the only effective treatment for controlling seizures and cognitive deterioration (Luan et al. 2016).
The pathogenesis and etiology of RE are unclear. The histopathological changes in the brain tissue of patients with RE include neuron loss, lymphocyte infiltration, and microglial nodules, which are similar to those observed in viral encephalitis (Pardo et al. 2004). Thus, viral infection may serve as an etiological factor. Viral components, such as herpes simplex virus 1 (HSV-1), Epstein-Barr virus (EBV), enterovirus (EV), human papillomaviruses (HPV), and human cytomegalovirus (HCMV), have been detected in brain tissues from patients with RE (Power et al. 1990; Jay et al. 1995; Chen et al. 2016; Zhang et al. 2017). However, a number of reports have indicated a lack of virus detection in RE brain tissues (Atkins et al. 1995). Therefore, the role of viral infection in RE remains controversial. In this study, we examined the co-expression of EBV and HHV6 antigens in the brain tissues of 30 patients with RE and analyzed the role of infection in RE using a typical case. Our data provide helpful information for understanding the pathogenesis of RE.
Materials and Methods
Patient Cohort and Brain Sample Collection
Between 2008 and 2015, 30 patients with RE were admitted to Sanbo Brain Hospital (Beijing, China) and were enrolled in this study. Clinical diagnosis was made according to the European diagnostic criteria (Bien et al. 2002). Each patient was subjected to magnetic resonance imaging (MRI) examination using an MRI scanner (Siemens 3.0T TIM Trio MRI; Munich, Germany) for diagnosis before undergoing craniotomy. After the operation, at least two blocks (0.5 × 0.5 × 0.5 cm each) of brain tissues from different areas were collected from the patients. The tissues were fixed, embedded in paraffin, sliced (thickness of 6 μm), and then subjected to histopathological analysis by standard hematoxylin and eosin (HE) staining and the detection of EBV and HHV6 antigens by immunohistochemical (IHC) staining.
A total of 16 patients who underwent surgical treatment for cerebral trauma were used as a control group; they were generally matched with the RE group with respect to age. Patients with other nervous system diseases were excluded.
Scoring Criteria of Brain Atrophy Grade
The brain atrophy grade (AG) for each patient was defined according to MRI characteristics and ranged from 0 to 3 (Zhang et al. 2017), with a higher AG indicating more severe atrophy of the brain.
Immunohistochemical Staining (IHC) and Double Immunofluorescence Staining
Paraffin sections were treated according to a routine IHC method (Wang et al. 2017). In brief, paraffin was removed and sections were incubated with 3% peroxide-methanol and 1% bovine serum albumin to block endogenous peroxidase activity and nonspecific antibody binding, respectively. For antigen retrieval, sections were heated to 100 °C for 15 min in sodium citrate-hydrochloric acid buffer solution, followed by incubation with mouse antibodies against EBV Latent Membrane Protein 1 (LMP1) (ab78113; Abcam, Cambridge, MA, USA) or against HHV6 (ab128404; Abcam) at 4 °C overnight. After washing, sections were incubated with horseradish peroxidase (HRP)-labeled secondary antibodies for 60 min at 25 °C. Finally, diaminobenzidine substrate was added for coloration. The sections were counterstained with hematoxylin and images were obtained (Olympus BX61; Tokyo, Japan).
Co-localization of EBV/HHV6 and neuron or astrocytes was detected by double immunofluorescence staining. After washing, each section was incubated with a mouse anti-EBV LMP1 antibody, a mouse anti-HHV6 antibody, a rabbit anti-microtubule-associated protein-2 (MAP2) antibody (sc-20172), or a rabbit anti-glial fibrillary acidic protein (GFAP) antibody (ab7260) at 4 °C overnight. Then, EBV and HHV6 were visualized with anti-mouse IgG H&L (Alexa Fluor® 488, ab150117, 1:1000; Abcam), and MAP-2 and GFAP were visualized with anti-rabbit IgG H&L (Alexa Fluor® 594, ab150080, 1:1000; Abcam). Images were obtained using a confocal microscope (TCS SP8 Leica, Wetzlar, Germany).
Scoring Criteria of IHC
IHC results were evaluated using a scoring methodology described previously (Wang et al., 2017). In brief, yellow- or brown-stained particles in the cytoplasm or nuclei were considered positive signals and analyzed using image analysis software (Image-Pro Plus 6.0; Media Cybernetics Inc., Bethesda, MD, USA). The scoring system combined the percentage of positive cells, categorized as 0 (< 5%), 1 (5%–25%), 2 (26%–50%), 3 (51%–75%), or 4 (> 75%), and a subjective assessment of staining intensity, scored as 0 (colorless), 1 (light yellow), 2 (yellow or brown), or 3 (dark brown). These scores were summed to obtain an overall IHC score of ≤ 1 for negative staining, 2–3 for weakly positive staining, 4–5 for moderately positive staining, and > 6 for strongly positive staining.
Statistical Analysis
Statistical analyses were performed using SPSS 13.0. Fisher’s exact test was used to determine the correlation between the expression levels of EBV and HHV6 antigens and the severity of brain atrophy. P < 0.05 was considered statistically significant.
Results
Detection of EBV and HHV6 in Brain Tissues of 30 Patients with RE
There were 16 males and 14 females in the RE group, with a mean age of seizure onset of 5.6 years and a mean age at surgery of 6.8 years. The 16 trauma patients in the control group were generally matched with the RE patient group. There were 10 males and 6 females with a mean age at surgery of 8.2 years.
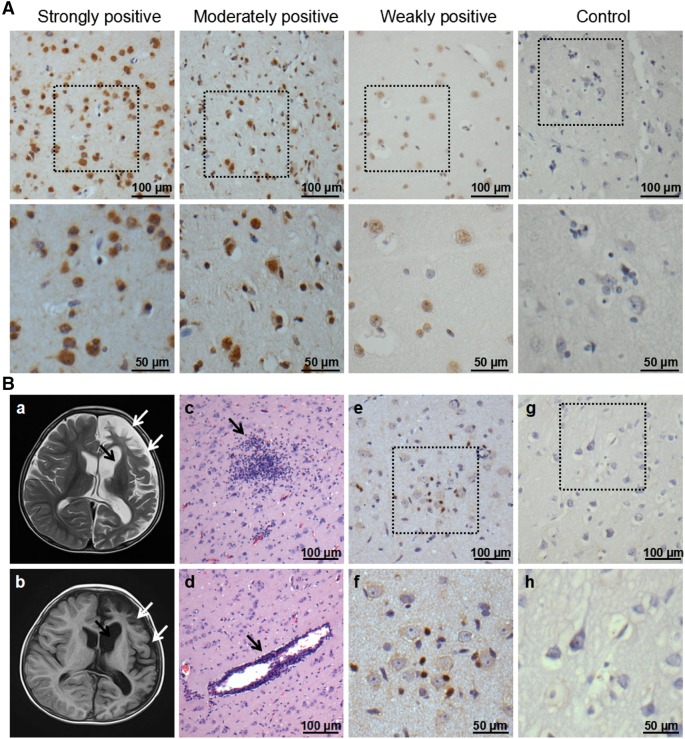
Expression levels of EBV and HHV6 in the brain tissues of patients with RE were analyzed by IHC and recorded according to a scoring methodology described previously (Wang et al. 2017). As shown in Table 1, in the RE group, EBV was detected in 56.7% (17/30) of patients with RE. Strongly and moderately positive staining levels were each found in two cases and thirteen cases showed weakly positive staining. The positive rate and staining intensity were similar to those of our previous report (Wang et al. 2017). For HHV6, 50% (15/30) of patients with RE showed positive staining. Three and four cases exhibited strongly and moderately positive staining, respectively, and eight cases showed weakly positive staining (Table 2, Fig. 1A). In contrast, both EBV and HHV6 were negative in the brain tissues of 16 control individuals. The co-expression of EBV and HHV6 was detected in 20.0% (6/30) of patients. Both EBV and HHV6 antigens were observed in the cytoplasm and/or nuclei of neuron-like cells in lesion areas of RE brains (Fig. 1A, Wang et al. 2017).
Table 1.
EBV antigen expression in brain tissues of 30 patients with RE and 16 control individuals.
| No. of cases (%) | Total | |||
|---|---|---|---|---|
| +++ | ++ | + | ||
| RE (n = 30) | 2 (6.67) | 2 (6.67) | 13 (43.33) | 17 (56.67) |
| Control (n = 16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
+++: Strongly positive; ++: Moderately positive; +: Weakly positive.
Table 2.
HHV6 antigen expression in brain tissues of 30 patients with RE and 16 control individuals.
| No. of cases (%) | Total | |||
|---|---|---|---|---|
| +++ | ++ | + | ||
| RE (n = 30) | 3 (10.00) | 4 (13.33) | 8 (26.67) | 15 (50.00) |
| Control (n = 16) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
+++: Strongly positive; ++: Moderately positive; +: Weakly positive.
Fig. 1.
HHV6 expression in brain tissues of patients with RE and controls, as well as characteristics of magnetic resonance imaging (MRI) and histopathological changes in the RE case. A Representative images of strong, moderate, and weak positive staining and negative staining for the HHV6 antigen under low (scale bar = 100 µm) and high (scale bar = 50 µm) magnification. Neuron-like cells were stained. B Atrophy of the left hemisphere cortex (white arrows) and widening of the caudate nucleus (black arrow) were observed in the patient by T2 (B-a) and FLAIR (B-b) images. HE staining shows microglial nodule formation (B-c), lymphocyte infiltration, and perivascular cuff (B-d) in the temporal lobe cortex of the brain. Activation of CD8+ T cells was detected in the RE (B-e) and (B-f) but not in the brain tissue from the trauma patient (B-g) and (B-h) by IHC under low (scale bar = 100 µm) and high (scale bar = 50 µm) magnification.
According to the MRI results for patients with RE, various grades of brain atrophy were observed. The association between EBV, HHV6, and co-infection with EBV and HHV6 and brain atrophy was analyzed. According to a Fisher’s exact test, RE patients with positive EBV and HHV6 staining exhibited more severe brain atrophy (Tables 3, 4) than that of EBV- and HHV6-negative cases (χ2 = 10.47, P = 0.002; χ2 = 8.89, P = 0.008, respectively). Moreover, all 6 patients with EBV and HHV6 co-infection exhibited a high grade of brain atrophy (3 patients each for AG 2 and 3), indicating a link between co-infection and the severity of brain atrophy. This result suggests that EBV and HHV6 co-infection may lead to more severe neuronal damage and may be involved in disease progression.
Table 3.
Association between brain atrophy and EBV antigen expression in brain tissues of patients with RE.
| Brain atrophy | Non-atrophy | Total | |
|---|---|---|---|
| EBV-positive cases | 15 | 2 | 17 |
| EBV-negative cases | 4 | 9 | 13 |
| Total | 19 | 11 | 30 |
Statistical analysis was performed using Fisher’s test; P = 0.002, odds ratio = 16.9.
Table 4.
Association between brain atrophy and HHV6 antigen expression in brain tissues of patients with RE.
| Brain atrophy | Non-atrophy | Total | |
|---|---|---|---|
| HHV6-positive cases | 13 | 2 | 15 |
| HHV6-negative cases | 5 | 10 | 15 |
| Total | 18 | 12 | 30 |
Statistical analysis was performed using Fisher’s test; P = 0.008, odds ratio = 13.0.
Typical RE Clinical Course in a 4-Year-old Boy
Among 6 cases with EBV and HHV6 co-expression, we focused on a typical RE case. A 4-year-old boy who presented with falling down while walking or nodding more than 20 times a day was admitted to Beijing Sanbo Brain Hospital (Capital Medical University, China) on March 5, 2015. According to his mother, the boy experienced repeated fever and convulsions several times and was diagnosed with “viral encephalitis” at the local hospital during the spring and summer in 2014. After treatment with sodium valproate (20 mg, tid) and clonazepam (0.5 mg, bid) for about 3 months, the clinical signs improved significantly and the frequency of focal seizures was reduced. However, he manifested nodding and limb shaking again in the winter of 2014. Further treatment with valproate, clonazepam, and levetiracetam (0.75 mg, bid) did not result in the expected therapeutic effect and the patient could no longer stand beginning at the end of 2014, mainly due to gradually increased muscle tension. On March 5, 2015, the boy was admitted to our hospital for further treatment. MRI of the brain showed atrophy of the left temporal lobe, a swollen sulcus, and caudate nuclei. Grey and white matter hyperintensities of the left cerebral hemisphere were detected on FLAIR images (Fig. 1B-a, 1B-b). According to the European diagnostic criteria (Varadkar et al. 2014), the boy was diagnosed with RE. Finally, he had a hemispherectomy on March 16, 2015 and the seizures disappeared after the operation.
During the operation, samples from different brain areas were collected from the patient. The brain sections were subjected to a histopathological analysis and HSV1, HSV2, HCMV, EBV, and HHV6 viral antigen detection. By HE staining, pyknosis, loss of neurons, diffuse lymphocyte infiltration, perivascular lymphocyte cuffing, and glial cell proliferation were observed in the temporal lobe cortex of the brain (Fig. 1B-c, 1B-d). Moreover, using a rabbit anti-CD8a polyclonal antibody (cat: ab17335-1-AP, 1:200; Proteintech, Chicago, IL, USA), a large number of CD8+ T cells were detected in the brain tissue of this patient and were located close to neurons (Fig. 1B-e). This elevation in CD8+ T cells was not observed in the control brain samples from a trauma patient (Fig. 1B-g). These results indicate that cytotoxic T cells are activated in the brains of patients with RE.
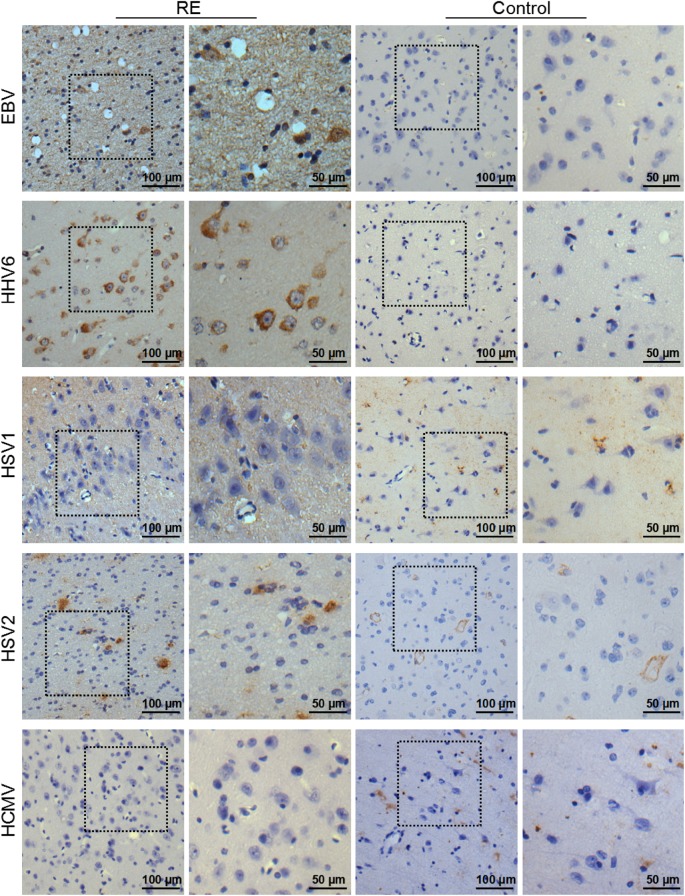
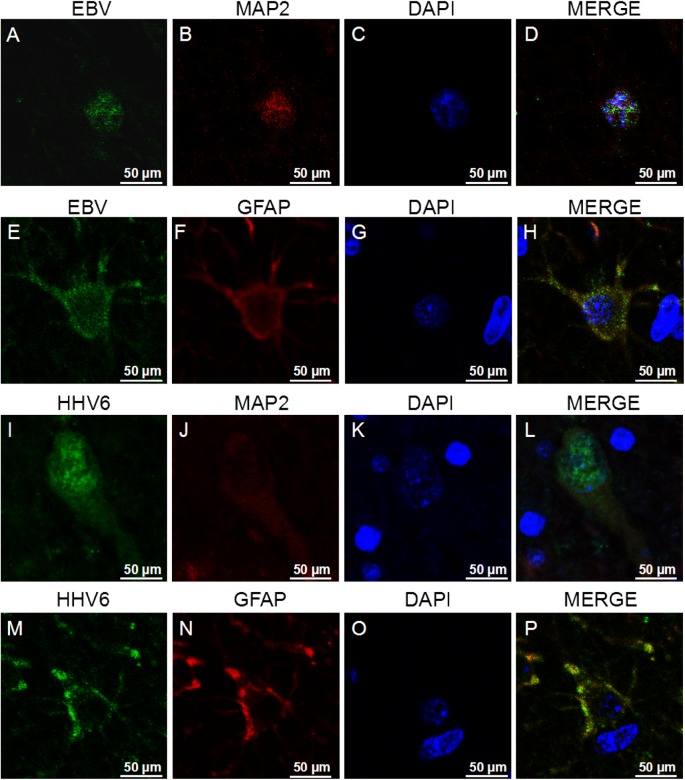
Notably, strongly EBV- and HHV6-positive immune reactions were detected in the cortex of the patient brain using a mouse anti-EBV-LMP1 antibody and anti-HHV6 antibody (Fig. 2). EBV and HHV6 antigens were detected in both the ‘nidus’ and ‘non-nidus’ areas of the brain, with varying staining levels, but not in the control brain tissue. Double immunofluorescence staining showed that the EBV and HHV6 antigens not only co-existed with MAP-2, a neuronal biomarker, but also co-localized with glial fibrillary acidic protein GFAP, an astrocyte biomarker, indicating that EBV and HHV6 could infect both neurons and astrocytes (Fig. 3). However, no HSV1, HSV2, or HCMV was detected in RE brain tissues by IHC. In combination with the detection of a large number of CD8+ T cells in the RE brain, our results suggest that EBV and HHV6 infection may lead to neuronal damage and brain atrophy by activating CD8+ T cells.
Fig. 2.
Representative images showing positive staining for EBV and HHV6 and negative staining for HSV1, HSV2, and HCMV in brain tissue of the RE case. Negative staining was obtained for HSV1, HSV2, EBV, HCMV, and HHV6 in the brain tissue from a trauma patient. Bar = 100 µm or 50 µm.
Fig. 3.
Representative images showing that EBV and HHV6 antigens were detected in neurons and astrocytes in the brain tissue of the patients with RE. Bar = 50 µm.
Discussion
Viral infection is considered as an important factor in the pathogenesis of RE. Previously, antigens or DNA fragments of EBV and HSV-1 have been detected in brain tissues of patients with RE by several methods (Walter and Renella, 1989; Jay et al. 1995). Furthermore, HCMV antigens have been found in neurons, astrocytes, and vascular endothelial cells in brain tissues of patients with RE (Zhang et al. 2017). HPV positivity has been detected in brain tissues of three RE cases (Chen et al. 2016). However, reports of multiple virus types in RE brain tissues are lacking. In this study, the co-expression of EBV and HHV6 was detected in 20% (6/30) of patients with RE and was closely associated with the severity of brain atrophy, indicating that these viruses may contribute to the pathogenesis of RE. In particular, we noted a boy of 4 years old who had a typical clinical course and manifestations of RE, including a medical history of viral encephalitis, progressive epilepsy, and typical histopathological changes in the brain tissue. Both EBV and HHV6 antigens were detected in the brain tissue by IHC and double immunofluorescence staining, indicating that the infection with the two viruses may be associated with the pathogenesis of RE.
EBV, which belongs to the Gamma-herpesvirinae subfamily, was first discovered in children’s lymphoma by Epstein in Africa in 1964. It can be transmitted through the saliva and blood transfusions (Ascenção et al. 2016). A total of 90%–95% of the adult human population carries EBV as a latent infection without clinical signs (Kano et al. 2017). EBV infection is often observed in immunocompromised individuals, such as patients with acquired immune deficiency syndrome (AIDS) caused by human immunodeficiency virus infection (Ning. 2011). However, reports of EBV infection in the central nervous system are rare. HHV6, which belongs to the Beta-herpesvirinae subfamily, was discovered in peripheral blood mononuclear cells from patients with AIDS and lymphoproliferative disorders in 1986 (Salahuddin et al. 2007). HHV6 infection, usually asymptomatic, is associated with the common, self-limited childhood illness roseola infantum and rarely with more severe syndromes. HHV6 consists of HHV6A and HHV6B. HHV6A has been described as more neurovirulent and has been associated with a number of neurological disorders, especially Alzheimer’s disease (Readhead et al. 2018). HHV6B is considered as a possible pathogenic factor in mesial temporal lobe epilepsy (Kawamura et al. 2015). Unfortunately, we could not distinguish between HHV6A and HHV6B by IHC in this study. However, the simultaneous detection of EBV and HHV6 infection in patients with RE has not been reported previously. In this case, EBV and HHV6 antigens were detected in neurons and astrocytes in RE brain tissues, and a large number of CD8+ T cells were detected adjacent to neurons, indicating that EBV/HHV6 infection and the activation of CD8+ T cells contribute to RE. It is speculated that after CD8+ T cells recognized these virus antigens, they can damage EBV- and HHV6-carrying host cells, resulting in a loss of neurons and astrocytes, which may be linked to brain atrophy in RE. The mechanisms underlying the link between viral infection and the occurrence of RE need to be investigated further.
Our results were consistent with those of a previous report (Merkler et al. 2006) suggesting that some viruses act as triggers to induce an immune response and may not exist during the entire course of RE. The viruses were probably cleared by cytotoxic T-lymphocytes after the initial infection; cells would be damaged during this process, which might be associated with neuronal loss and undetected virus antigens in some RE cases.
In summary, both HHV6 and EBV antigens were detected simultaneously in 20% of brain tissues of patients with RE in this study. In particular, a typical RE case showed strongly positive staining for these two viruses, accompanied by a large number of CD8+ T cells. Our results suggest an association between EBV and HHV6 infection and the activation of CD8+ T cells in RE. These results improve our understanding of the pathogenesis of RE, which will be helpful for developing therapeutic strategies for this disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81701992, 81471957, and 81671971), the Beijing Municipal Natural Science Foundation (7144217), the Capital Applied Clinic Research Programs of Science and Technology (Z131107002213171), the Beijing Rising-star Plan of Science and Technology (Z141107001814042), and Scientific Research Common Program of Beijing Municipal Commission of Education (KM201610025001).
Author Contributions
GM and JA conceived and designed the experiments. DL and XW performed the experiments and analyzed the data. YW, CC, PW, DF, YG, and TL contributed reagents/materials/analysis tools. DL wrote the manuscript and prepared the figures and tables. GL and JA revised the manuscript, organized the collaboration, and directed the project. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This study was approved by the Ethics Committee of Sanbo Brain Hospital, Capital Medical University (2013061801), and written informed consent was obtained from all participants or their guardians prior to the study.
Contributor Information
Jing An, Phone: +86-10-83950107, Email: anjing@ccmu.edu.cn.
Guoming Luan, Phone: +86-10-62856916, Email: luangm3@163.com.
References
- Ascenção BB, Gonçalves AC, Luís N, Sá J, Brito AP, Poças JM. Epstein-Barr virus hemorrhagic meningoencephalitis: case report and review of the literature. J Neurovirol. 2016;22:695–698. doi: 10.1007/s13365-016-0430-y. [DOI] [PubMed] [Google Scholar]
- Atkins MR, Terrell W, Hulette CM. Rasmussen’s syndrome: a study of potential viral etiology. Clin Neuropathol. 1995;14:7–12. [PubMed] [Google Scholar]
- Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, Schramm J, Elger CE, Lassmann H. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, von Lehe M, Becker AJ, Bast T, Herkenrath P, Karenfort M, Kruse B, Kurlemann G, Rona S, Schubert-Bast S, Vieker S, Vlaho S, Wilken B, Elger CE. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 2013;54:543–550. doi: 10.1111/epi.12042. [DOI] [PubMed] [Google Scholar]
- Chen S, Chen S, Guan Y, Zhang Y, Qi X, An J, Wang Y, Luan G. Elevated expression of human papillomavirus antigen in brain tissue of patients with Rasmussen’s encephalitis. Epilepsy Res. 2016;126:119–125. doi: 10.1016/j.eplepsyres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Jay V, Becker LE, Otsubo H, Cortez M, Hwang P, Hoffman HJ, Zielenska M. Chronic encephalitis and epilepsy (Rasmussen’s encephalitis): detection of cytomegalovirus and herpes simplex virus 1 by the polymerase chain reaction and in situ hybridization. Neurology. 1995;45:108–117. doi: 10.1212/WNL.45.1.108. [DOI] [PubMed] [Google Scholar]
- Kano K, Katayama T, Takeguchi S, Asanome A, Takahashi K, Saito T, Sawada J, Saito M, Anei R, Kamada K, Miyokawa N, Nishihara H, Hasebe N. Biopsy-proven case of Epstein-Barr virus (EBV)-associated vasculitis of the central nervous system. Neuropathology. 2017;37:259–264. doi: 10.1111/neup.12356. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Nakayama A, Kato T, Miura H, Ishihara N, Ihira M, Takahashi Y, Matsuda K, Yoshikawa T. Pathogenic role of human herpesvirus 6B infection in mesial temporal lobe epilepsy. J Infect Dis. 2015;212:1014–1021. doi: 10.1093/infdis/jiv160. [DOI] [PubMed] [Google Scholar]
- Luan G, Gao Q, Zhai F, Chen Y, Li T. Upregulation of HMGB1, toll-like receptor and RAGE in human Rasmussen’s encephalitis. Epilepsy Res. 2016;123:36–49. doi: 10.1016/j.eplepsyres.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Merkler D, Horvath E, Bruck W, Zinkernagel RM, Del la Torre JC, Pinschewer DD. Viral deja vu elicits organ-specific immune disease independent of reactivity to self. J Clin Invest. 2006;116:1254–1263. doi: 10.1172/JCI27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S. Innate immune modulation in EBV infection. Herpesviridae. 2011;2:1. doi: 10.1186/2042-4280-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, Vining EP, Guo L, Skolasky RL, Carson BS, Freeman JM. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45:516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- Power C, Poland SD, Blume WT, Girvin JP, Rice GP. Cytomegalovirus and Rasmussen’s encephalitis. Lancet. 1990;336:1282–1284. doi: 10.1016/0140-6736(90)92965-K. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/WNL.8.6.435. [DOI] [PubMed] [Google Scholar]
- Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, Reiman EM, Schadt EE, Ehrlich ME, Gandy S, Dudley JT. Multiscale analysis of independent alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64.e7–82.e7. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin SZ, Snyder KA, Godwin A, Grewal R, Prichard JG, Kelley AS, Revie D. The simultaneous presence and expression of human hepatitis C virus (HCV), human herpesvirus-6 (HHV-6), and human immunodeficiency virus-1 (HIV-1) in a single human T-cell. Virol J. 2007;4:106. doi: 10.1186/1743-422X-4-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, Vincent A, Mathern GW, Cross JH. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter GF, Renella RR. Epstein-Barr virus in brain and Rasmussen’s encephalitis. Lancet. 1989;1:279–280. doi: 10.1016/S0140-6736(89)91292-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Liu D, Wang P, Fan D, Guan Y, Li T, Luan G, An J. Elevated expression of EBV and TLRs in the brain is associated with Rasmussen’s encephalitis. Virol Sin. 2017;32:423–430. doi: 10.1007/s12250-017-4058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Chen S, Chen S, Guan Y, Liu C, Li T, Luan G, An J. Expression of human cytomegalovirus components in the brain tissues of patients with Rasmussen’s encephalitis. Virol Sin. 2017;32:115–121. doi: 10.1007/s12250-016-3917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]