Abstract
The monoamine neurotransmitter, 5-Hydroxytryptamine or serotonin, is derived from tryptophan and synthesized both centrally and systemically. Fourteen structurally and functionally distinct receptor subtypes have been identified for serotonin, each of which mediates the neurotransmitter’s effects through a range of downstream signaling molecules and effectors. Although it is most frequently described for its role in the etiology of neuropsychiatric and mood disorders, serotonin has been implicated in a slew of fundamental physiological processes, including apoptosis, mitochondrial biogenesis, cell proliferation and migration. Its roles as the neurotransmitter have also emerged in pathogenic conditions ranging from anorexia nervosa to cancer. This has necessitated the understanding of the signaling mechanisms underlying the serotonergic system, which led us to construct a consolidative pathway map, which will provide as a resource for future biomedical investigation on this pathway. Using a set of stringent NetPath annotation criteria, we manually curated molecular reactions associated with serotonin and its receptors from publicly available literature; the reaction categories included molecular associations, activation/inhibition, post-translation modification, transport, and gene regulation at transcription and translational level. We identified 90 molecules in serotonin-serotonin receptor pathway. We submitted the curated data to NetPath, a publicly available database of human signaling pathways, in order to enable the wider scientific community to readily access data and contribute further to this pathway.
Keywords: NetSlim, Post-translational modification, Protein-protein interaction, Gene expression, Sleep, Serotonylation
Introduction
The neurotransmitter 5-Hydroxytryptamine (5-HT), or serotonin, is most commonly known for its role in the pathophysiology of various neuropsychiatric disorders. However, an abundance of literature based on serotonergic manipulation in animal models has yielded knowledge of the plethora of physiological process directly and indirectly influenced by this biogenic monoamine. This is hardly surprising, given that to date seven 5-HT receptor families (5-HT1–7) have been identified. These include 5-HT1A, B, D, E, F; 5-HT2A, B, C; 5-HT3; 5-HT4; 5-HT5A, B; 5-HT6; and 5-HT7. Barring the 5-HT3 receptor, which functions as a ligand-gated ion channel (LGIC) (Reeves and Lummis 2002), all other serotonergic receptors mediate their actions via G proteins. By coupling to Gαi, Gαq/11, or Gαs, the 5-HT receptors are able to exert their influence on several biochemical pathways that are much further downstream.
Serotonin is synthesized via a two-step metabolic pathway: tryptophan is first hydroxylated to 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase (TPH), which is a rate-limiting step. This is followed by the decarboxylation of 5-HTP by aromatic l-acid decarboxylase to finally form 5-HT (El-Merahbi et al. 2015). Serotonin acts as a neurotransmitter within the central nervous system (CNS), where it is synthesized by raphe neurons in the brain stem; peripherally it can play multiple roles as a hormone, auto- or paracrine factor, and synthesized by both gut neurons and enterochromaffin cells located in the gastrointestinal system (Jenkins et al. 2016). The blood-brain barrier keeps systemic and central serotonin pools distinct.
The serotonin transporter (SERT) is responsible for the reuptake of free 5-HT in the synaptic cleft; this is, in fact, the mechanism of action exploited by selective serotonin reuptake inhibitors (SSRIs), typically used as antidepressants or anxiolytic therapy. By blocking the recapture of 5-HT molecules by SERT, SSRIs increase extracellular 5-HT concentration. The pharmacological focus on the role of 5-HT in the CNS is particularly significant, given that approximately 90–95% of the body’s 5-HT is actually localized to the periphery, thus forming the base for the majority of serotonergic influences (Mohammad-Zadeh et al. 2008). Serotonergic signaling has received particular emphasis within the context of neuropsychiatry since the pharmacological manipulation of 5-HT has provided clinicians with significant gains in the treatment of mood disorders.
Serotonin has also been implicated in the regulation of an array of biological functions both within and outside the CNS. Within the CNS, 5-HT is involved in the regulation of mood and social cognition (Jenkins et al. 2016), neurogenesis (Alenina and Klempin 2015), and memory (Nikoui et al. 2016). Serotonergic dysregulation has been linked to numerous psychological conditions, particularly major depressive disorder (Salomon and Cowan 2013). In the periphery, 5-HT has been routinely implicated in the mitogenesis and proliferation of fibroblast cells (Welsh et al. 2004). It also contributes to metabolic homeostasis by regulating the activity of organs involved in glucose and lipid metabolism (El-Merahbi et al. 2015). Several studies have also demonstrated the robust influence of 5-HT on augmented arterial contraction in cardiac disease and its effects on cardiac hypertrophy (Matsumoto et al. 2010; Vindis et al. 2010). Serotonergic signaling has also been implicated in bronchoconstriction (Hershenson et al. 1995) and the control of rhythmic breathing (Manzke et al. 2010).
While a complete review of the physiological influences of 5-HT and its receptors is beyond the scope of the present study, we attempted to summarize significant and frequently replicated findings. Given the multitude of implications for 5-HT in both physiological and pathogenic processes and its central and systemic presence, a detailed pathway map collating the experimental data available will augment the understanding of this signal transduction network. To the best of our knowledge, an easily accessible and integrative depiction of 5-HT signaling is currently not available, and, therefore, the present study attempted to address this. A systematic effort to catalog the molecules involved in this pathway may help shed new light on the underlying mechanisms involves and add to the translational value of this information, including the accelerated discovery of new therapeutic targets.
Methodology
We conducted an exhaustive literature review of all the studies available on PubMed that examined the downstream effects of both endogenous and synthetic ligand binding to the various 5-HTRs. Only original studies, as opposed to reviews, were considered. Articles were carefully manually screened for ligand-evoked reactions that could be categorized into the following groups: 1) molecular association (protein-protein interactions), 2) post-translational modification (PTM), 3) translocation/transport of proteins between subcellular compartments, 4) protein expression, 5) activation/inhibition, and, finally, 6) gene regulation at the mRNA and/or protein level (both up- and down-regulation). This categorization strictly adhered to the annotation criteria previously outlined in the studies examining BDNF/p75NTR (Sandhya et al. 2013), Oncostatin M (Dey et al. 2013), oxytocin-oxytocin receptor (Chatterjee et al. 2016), interleukin-17 (Sharma et al. 2015), and FGF-1/FGFR (Raju et al. 2014) pathways. Pathbuilder, an in-house annotation tool, was utilized for the annotation of categorized reactions (Kandasamy et al. 2009). Individual molecular reactions were connected via hyperlink to the original PubMed article from which they were sourced.
Our annotation also includes a brief comment about the annotation reaction as well as about the physiological models within which they were observed (e.g., cell lines, transgenic animals, tissue). An instructive pathway map was generated using PathVisio, an open source, free pathway drawing tool (van Iersel et al. 2008). The map provides a pictorial summary of all the reactions annotated, including downstream effectors at both the transcriptional and translational level. Annotated pathway reactions were exported to the NetPath database, an in-house developed pathway resource (Kandasamy et al. 2010). A designated pathway authority (an established expert in subject-matter) carefully reviewed the constructed pathway map, and the annotation reactions upon which it was based.
Results
Our initial search on PubMed retrieved around 7500 articles that met one or more of the parameters stipulated in our query term. Of these around 206 articles were selected for further curation, based on their fulfillment of all our annotation criteria. The annotated articles yielded a total of 23 molecular association reactions, 84 PTM reactions, 43 activation/inhibition reactions, 32 protein translocation reactions, and 112 gene regulation reactions at transcriptional level and 105 at translational level. Each of these reactions was integrated into a representative map of the signaling pathway (Fig. 1). The NetPath database includes all 5-HT: 5-HT receptor-related signaling events and associated statistics and is freely available to members of the scientific community (http://www.netpath.org/pathways?path_id=NetPath_86). All information is presented in standard community exchange formats, namely Biological PAthway eXchange (BioPAX) (Demir et al. 2010), Systems Biology Markup Language (SBML) (Hucka et al. 2003), and Proteomics Standards Initiative Molecular Interaction (PSI-MI) (Orchard and Kerrien 2010).
Fig. 1.
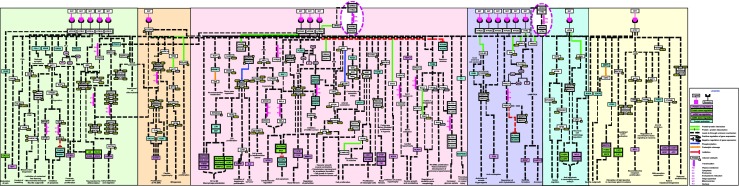
A schematic representation of the NetPath reactions induced by serotonin-serotonin receptor. The pathway reaction map depicts molecular events induced through serotonin-serotonin receptor interaction. Pathway map represents protein-protein associations, enzyme catalysis reactions, translocation events, gene and/or protein regulation induced upon treatment with serotonin or its analogues. Legends describe the reaction events in the map
As stated elsewhere (Millan et al. 2008) and as demonstrated by our map, it is apparent that there are no “absolute” demarcations between the transduction mechanisms employed by different 5-HT receptor subtypes. We also observed that stimulation of 5-HT receptors with 5-HT induces a similar pattern of enrichment of downstream signaling modules, such as MEK/MAPK, PI3K/AKT, Ras/Raf, and RhoA/ROCK, across all the 5-HT receptors. Our map indicates that several biological processes may be influenced by the combinatorial effect of several receptors, as opposed to being controlled by a singular receptor. For instance, we observed that activation of 5-HT1, 5-HT2 or 5-HT6 receptors are involved in suppression of apoptotic proteins, such as CASP3 and CASP9, via MEK/MAPK or PI3K/AKT signaling module (Hsiung et al. 2005; Nebigil et al. 2003; Wang et al. 2014). Similarly, cell proliferation was regulated by the AKT/mTOR and MEK/MAPK pathways by both the 5-HT1 and 5-HT2 receptors. It should be noted that activation of the JAK/STAT pathway was only evident for the 5-HT2 receptor (Banes et al. 2005).
We noted that certain biological processes tended to be regulated by specific subtypes; for instance the 5-HT1 and 5-HT7 receptors was found to be involved in the modulation of ion channel activity, which subsequently affected fundamental events, including respiration and synaptic transmission (Vasefi et al. 2013; Cai et al. 2002; Manzke et al. 2010). The 5-HT2 receptor was predominantly involved in the pathogenesis of cardiac hypertrophy and cardiac growth/remodeling via the secretion of NPPA/NPPB and RCAN1 proteins (Liang et al. 2006; Bush et al. 2004).
Interestingly, we see a complex system of cross-talk emerge between the different receptor subtypes and, curiously, across different neurotransmitter systems as well. For instance, a coherent action of 5-HT2 and 5-HT7 receptor in the macrophage polarization process (de Las and Corbi 2014). Also, 5-HT can functionally compensate for the lesion-mediated loss of striatal dopamine and can mitigate glutamate mediated excitotoxicity (Brown and Gerfen 2006; Wang et al. 2006). The physiological and pathological effects of serotonin reflect this complexity, as the serotonergic system is implicated in a range of conditions, including cancer, anorexia nervosa, atherosclerosis, cardiac hypertrophy, and several mood disorders.
Conclusions
The increasing availability of cell signaling data pertaining to serotonin-serotonin receptor signaling has warranted the systematic cataloging, identification, and characterization of the molecular components involved in the signal transduction of this biogenic amine. Availability of serotonin-serotonin receptor signaling through a centralized system will facilitate understanding of the mammalian serotonergic system. It will also help in understanding regulation and function of this pathway in normal and pathological condition, thereby enabling the formulation of hypotheses that can also be rigorously tested. Researchers can contribute to further characterization of the pathway by sharing their insights in the NetPath comments section (http://www.netpath.org/comments).
Acknowledgements
We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). We thank the Department of Biotechnology (DBT), Government of India for research support to the Institute of Bioinformatics (IOB), Bangalore. We thank the “Infosys Foundation” for research support to IOB. LG, OC and PM are recipients of DST INSPIRE Fellowship from the Department of Science and Technology (DST), Government of India. JA is recipient of Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- 5-HTR
5-Hydroxytryptamine receptor
- CNS
Central nervous system
- GPCR
G-protein coupled receptor
- TPH
Tryptophan hydroxylase
- PTMs
Post-translational modifications
- PPIs
Protein-protein interactions
- BioPAX
Biological pathway exchange
- SBML
Systems biology markup language
- PSI-MI
Proteomics standards initiative for molecular interaction
Compliance with ethical standards
Conflict of interest
The author(s) declare no conflicts of interests.
References
- Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. 2015;277:49–57. doi: 10.1016/j.bbr.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D, Marrero MB. Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin. Am J Physiol Cell Physiol. 2005;288:C805–C812. doi: 10.1152/ajpcell.00385.2004. [DOI] [PubMed] [Google Scholar]
- Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 map kinase pathway. J Comp Neurol. 2006;498:415–430. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush E, Fielitz J, Melvin L, Martinez-Arnold M, McKinsey TA, Plichta R, Olson EN. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci U S A. 2004;101:2870–2875. doi: 10.1073/pnas.0308723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Chatterjee O, Patil K, Sahu A, Gopalakrishnan L, Mol P, Advani J, Mukherjee S, Christopher R, Prasad TS. An overview of the oxytocin-oxytocin receptor signaling network. J Cell Commun Signal. 2016;10:355–360. doi: 10.1007/s12079-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las C-EM, Corbi AL. Serotonin modulation of macrophage polarization: inflammation and beyond. Adv Exp Med Biol. 2014;824:89–115. doi: 10.1007/978-3-319-07320-0_9. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D'Eustachio P, Schaefer C, Luciano J, Schacherer F, Martinez-Flores I, Hu Z, Jimenez-Jacinto V, Joshi-Tope G, Kandasamy K, Lopez-Fuentes AC, Mi H, Pichler E, Rodchenkov I, Splendiani A, Tkachev S, Zucker J, Gopinath G, Rajasimha H, Ramakrishnan R, Shah I, Syed M, Anwar N, Babur O, Blinov M, Brauner E, Corwin D, Donaldson S, Gibbons F, Goldberg R, Hornbeck P, Luna A, Murray-Rust P, Neumann E, Ruebenacker O, Samwald M, van Iersel M, Wimalaratne S, Allen K, Braun B, Whirl-Carrillo M, Cheung KH, Dahlquist K, Finney A, Gillespie M, Glass E, Gong L, Haw R, Honig M, Hubaut O, Kane D, Krupa S, Kutmon M, Leonard J, Marks D, Merberg D, Petri V, Pico A, Ravenscroft D, Ren L, Shah N, Sunshine M, Tang R, Whaley R, Letovksy S, Buetow KH, Rzhetsky A, Schachter V, Sobral BS, Dogrusoz U, McWeeney S, Aladjem M, Birney E, Collado-Vides J, Goto S, Hucka M, Le Novere N, Maltsev N, Pandey A, Thomas P, Wingender E, Karp PD, Sander C, Bader GD. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Radhakrishnan A, Syed N, Thomas JK, Nadig A, Srikumar K, Mathur PP, Pandey A, Lin SK, Raju R, Prasad TS. Signaling network of Oncostatin M pathway. J Cell Commun Signal. 2013;7:103–108. doi: 10.1007/s12079-012-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Merahbi R, Loffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589:1728–1734. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Hershenson MB, Chao TS, Abe MK, Gomes I, Kelleher MD, Solway J, Rosner MR. Histamine antagonizes serotonin and growth factor-induced mitogen-activated protein kinase activation in bovine tracheal smooth muscle cells. J Biol Chem. 1995;270:19908–19913. doi: 10.1074/jbc.270.34.19908. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Tamir H, Franke TF, Liu KP. Roles of extracellular signal-regulated kinase and Akt signaling in coordinating nuclear transcription factor-kappaB-dependent cell survival after serotonin 1A receptor activation. J Neurochem. 2005;95:1653–1666. doi: 10.1111/j.1471-4159.2005.03496.x. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J, Forum S. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP (2016) Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain Axis. Nutrients 8 [DOI] [PMC free article] [PubMed]
- Kandasamy K, Keerthikumar S, Raju R, Keshava Prasad TS, Ramachandra YL, Mohan S, Pandey A. PathBuilder--open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YJ, Lai LP, Wang BW, Juang SJ, Chang CM, Leu JG, Shyu KG. Mechanical stress enhances serotonin 2B receptor modulating brain natriuretic peptide through nuclear factor-kappaB in cardiomyocytes. Cardiovasc Res. 2006;72:303–312. doi: 10.1016/j.cardiores.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hulsmann S, Ponimaskin E, Muller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor alpha3 controls breathing in mice. J Clin Invest. 2010;120:4118–4128. doi: 10.1172/JCI43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Ishida K, Taguchi K, Kamata K. Enhancement of mesenteric artery contraction to 5-HT depends on rho kinase and Src kinase pathways in the Ob/Ob mouse model of type 2 diabetes. Br J Pharmacol. 2010;160:1092–1104. doi: 10.1111/j.1476-5381.2010.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003;17:1373–1375. doi: 10.1096/fj.02-1122fje. [DOI] [PubMed] [Google Scholar]
- Nikoui V, Javadi-Paydar M, Salehi M, Behestani S, Dehpour AR. Protective effects of Lithium on Sumatriptan-induced memory impairment in mice. Acta Med Iran. 2016;54:226–232. [PubMed] [Google Scholar]
- Orchard S, Kerrien S. Molecular interactions and data standardisation. Methods Mol Biol. 2010;604:309–318. doi: 10.1007/978-1-60761-444-9_21. [DOI] [PubMed] [Google Scholar]
- Raju R, Palapetta SM, Sandhya VK, Sahu A, Alipoor A, Balakrishnan L, Advani J, George B, Kini KR, Geetha NP, Prakash HS, Prasad TS, Chang YJ, Chen L, Pandey A, Gowda H. A network map of FGF-1/FGFR signaling system. J Signal Transduct. 2014;2014:962962. doi: 10.1155/2014/962962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves DC, Lummis SC. The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review) Mol Membr Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Cowan RL. Oscillatory serotonin function in depression. Synapse. 2013;67:801–820. doi: 10.1002/syn.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, Nanjappa V, Narayana J, Somani BL, Mukherjee KK, Pandey A, Christopher R, Prasad TS. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal. 2013;7:301–307. doi: 10.1007/s12079-013-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Balakrishnan L, Datta KK, Sahasrabuddhe NA, Khan AA, Sahu A, Singhal A, Getnet D, Raju R, Chatterjee A, Gowda H, Keshava Prasad TS, Shankar S, Pandey A. A knowledgebase resource for interleukin-17 family mediated signaling. J Cell Commun Signal. 2015;9:291–296. doi: 10.1007/s12079-015-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasefi MS, Yang K, Li J, Kruk JS, Heikkila JJ, Jackson MF, MacDonald JF, Beazely MA. Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol Brain. 2013;6:24. doi: 10.1186/1756-6606-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C, D'Angelo R, Mucher E, Negre-Salvayre A, Parini A, Mialet-Perez J. Essential role of TRPC1 channels in cardiomyoblasts hypertrophy mediated by 5-HT2A serotonin receptors. Biochem Biophys Res Commun. 2010;391:979–983. doi: 10.1016/j.bbrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wang KY, Wang WC, Sihra TS. Unexpected inhibitory regulation of glutamate release from rat cerebrocortical nerve terminals by presynaptic 5-hydroxytryptamine-2A receptors. J Neurosci Res. 2006;84:1528–1542. doi: 10.1002/jnr.21060. [DOI] [PubMed] [Google Scholar]
- Wang CC, Man GC, Chu CY, Borchert A, Ugun-Klusek A, Billett EE, Kuhn H, Ufer C. Serotonin receptor 6 mediates defective brain development in monoamine oxidase A-deficient mouse embryos. J Biol Chem. 2014;289:8252–8263. doi: 10.1074/jbc.M113.522094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DJ, Harnett M, MacLean M, Peacock AJ. Proliferation and signaling in fibroblasts: role of 5-hydroxytryptamine2A receptor and transporter. Am J Respir Crit Care Med. 2004;170:252–259. doi: 10.1164/rccm.200302-264OC. [DOI] [PubMed] [Google Scholar]


