Abstract
Thrombopoietin (THPO), also known as megakaryocyte growth and development factor (MGDF), is a cytokine involved in the production of platelets. THPO is a glycoprotein produced by liver and kidney. It regulates the production of platelets by stimulating the differentiation and maturation of megakaryocyte progenitors. It acts as a ligand for MPL receptor, a member of the hematopoietic cytokine receptor superfamily and is essential for megakaryocyte maturation. THPO binding induces homodimerization of the receptor which results in activation of JAKSTAT and MAPK signaling cascades that subsequently control cellular proliferation, differentiation and other signaling events. Despite the importance of THPO signaling in various diseases and biological processes, a detailed signaling network of THPO is not available in any publicly available database. Therefore, in this study, we present a resource of signaling events induced by THPO that was manually curated from published literature on THPO. Our manual curation of thrombopoietin pathway resulted in identification of 48 molecular associations, 66 catalytic reactions, 100 gene regulation events, 19 protein translocation events and 43 activation/inhibition reactions that occur upon activation of thrombopoietin receptor by THPO. THPO signaling pathway is made available on NetPath, a freely available human signaling pathway resource developed previously by our group. We believe this resource will provide a platform for scientific community to accelerate further research in this area on potential therapeutic interventions.
Keywords: BioPAX, Cancer, Hematopoiesis, Thrombocytopenia, Thrombocytosis
Introduction
Thrombopoietin (THPO) is a 332 amino acid-long protein, which is highly glycosylated and has a molecular mass of approximately 70 kDa (Wolber and Jelkmann 2002). Production of THPO predominantly occurs in the liver while other organs including kidney, lung, spleen, bone marrow and brain also secrete the hormone in small amounts (Nomura et al. 1997). The gene encoding THPO protein is located on chromosome 3q26.33-q27 in humans (Suzukawa et al. 1995). THPO is a cytokine that has been reported to play an important role in proliferation and differentiation of megakaryocyte progenitors (Bacon et al. 1995; Kaushansky et al. 1995). It acts in early and late stages of megakaryocyte lineage to promote the proliferation of megakaryocyte progenitors and increases the ploidy of these cells (de Sauvage et al. 1996). Pro-platelet processes are formed from polyploid megakaryocytes, which later fragment into platelets (Kaushansky 2005). It has been shown that THPO and MPL knockout mice have reduced number of platelets and megakaryocytes indicating the importance of THPO signaling in maintaining the high number of platelets in blood (Murone et al. 1998). It also stimulates the growth of other blood cells including granulocytes, erythrocytes and monocytes (Wolber et al. 1999; Kaushansky et al. 1996).
Receptor for THPO is MPL (THPOR), which belongs to the type I hematopoietic cytokine receptor family (Vigon et al. 1992). The MPL gene is located on chromosome 1p34 (Le Coniat et al. 1989). MPL is predominantly expressed on the surface of megakaryocyte progenitors, platelets and hematopoietic stem cells (HSCs), where it plays a major role in maturation of megakaryocytes, regulation of platelet production, maintenance and self -renewal of HSCs (de Sauvage et al. 1996, Debili et al. 1995, Qian et al. 2007).
Thrombopoietin levels in blood are inversely related to number and size of platelets in healthy individuals. Platelet homeostasis is maintained by internalization of THPO from blood by high affinity THPO receptors (MPL) on platelets followed by degradation (Dahlen et al. 2003). Altered plasma THPO levels have been reported in several clinical conditions, including several hematological diseases associated with thrombocytopenia and thrombocytosis (Emmons et al. 1996; Kosugi et al. 1996; Cerutti et al. 1997). Thrombocytopenia, a disorder characterized by low levels of platelets in blood, has been reported in patients suffering from Immune Thrombocytopenia (ITP), HIV, Hepatitis C virus infection and cancer (Emmons et al. 1996; Ballem et al. 1992; Wang et al. 2004; Elting et al. 2001). Cancer patients who undergo chemotherapy have been reported to suffer from thrombocytopenia (Winer et al. 2015). THPO receptor agonists such as romiplostim and eltrombopag are used in thrombocytopenic patients to induce proliferation and differentiation of megakaryocytes (Erickson-Miller et al. 2009; Winer et al. 2015). In physiological conditions such as reactive thrombocytosis or thrombocythemia, platelet numbers as well as THPO levels are abnormally high in blood (Wolber and Jelkmann 2002; Cerutti et al. 1997). Reactive thrombocytosis is associated with inflammatory conditions including diseases such as acute myocardial infarction and unstable angina pectoris (Senaran et al. 2001). Plasma THPO levels have also been found to be high in cigarette smokers as compared to non-smokers and it increases platelet activation including platelet-leukocyte adhesion and P-Selectin expression on platelets in smokers (Lupia et al. 2010). Platelet activation increases the risk of cardiovascular diseases including atherosclerosis and thrombosis in smokers (Lupia et al. 2010).
Upon binding to its receptor, THPO induces various biochemical events to promote proliferation, differentiation and cell survival. THPO binding to the receptor MPL leads to the homodimerization of the receptor, which is followed by JAK2 activation. JAK2 phosphorylates tyrosine residues of receptor and creates docking sites for various signaling molecules including SHC, GRB2, SOS, VAV and CBL to initiate intracellular signaling (Murray 2007; Miyakawa et al. 1996). The major signaling pathways activated by THPO includes JAK/STAT, MAPK/ERK and PIK3/AKT (Bacon et al. 1995, Geddis et al. 2001, de Sauvage et al. 1996, Rojnuckarin et al. 1999). JAK/STAT and MAPK pathways are involved in proliferation and maturation of megakaryocyte progenitors while PI3K/AKT pathway is required for cell cycle progression (Geddis et al. 2001; Nakao et al. 2008). THPO, in combination with other cytokines, also supports survival of hematopoietic stem cells (Qian et al. 2007).
Impact of THPO signaling in cancer development is also reported in a few studies. In acute myeloid leukemia (AML), THPO has been reported to promote survival and proliferation of leukemic cells (Pulikkan et al. 2012). Mutations in MPL receptor have been identified in myeloproliferative neoplasms due to which it remains constitutively active and results in increased proliferation of hematopoietic cells (Defour et al. 2016). One of the characteristic features of myeloproliferative neoplasms (MPN) is formation of spontaneous megakaryocytic colonies. Suppression of MPL in megakaryocytes from patients of MPN in vitro decreases their colony forming ability (Li et al. 1996). In colorectal cancer, THPO facilitates the self-renewal of tumor initiating cells by activation of Wnt signaling pathway and potentiates metastasis of these cells to liver (Wu et al. 2015). In majority of cancers including head and neck cancer, patients are afflicted by thrombocytosis, which has been found to be responsible for the poor prognosis (Rachidi et al. 2014). Higher platelet count in oral squamous cell carcinoma has been shown to have direct correlation with tumor size, metastasis and overall survival rate (Lu et al. 2007). Platelets secrete various cytokines and growth factors, which play an important role in migration, invasion and proliferation of cancer cells (Pilatova et al. 2013; Bambace and Holmes 2011).
THPO plays a vital role in cellular processes such as proliferation, differentiation and cell survival. Therefore, it is necessary to develop a THPO signaling network. In this study, we have curated literature information pertaining to THPO signaling from published literature and developed a pathway map to facilitate better understanding of THPO-induced signaling.
Materials and methods
PubMed search was performed for thrombopoietin pathway using the keywords such as thrombopoietin, thrombopoietin signaling pathway, THPOR, c-MPL and THPO. The articles were manually screened to retrieve the molecular associations, enzyme-substrate reactions, activation/inhibition reactions, gene regulation events and protein transport reactions involved in thrombopoietin pathway. The information was curated using PathBuilder, a web-based in-house tool developed by our group (Kandasamy et al. 2009). A detailed map of thrombopoietin was developed and submitted to NetPath (Kandasamy et al. 2010). Several other signaling pathways were curated which include; oxytocin receptor signaling network (Chatterjee et al. 2016), brain derived neurotropic factor (BDNF) pathway (Sandhya et al. 2013), aryl hydrocarbon receptor (Yelamanchi et al. 2016), macrophage migration inhibitory factor (Subbannayya et al. 2016); and endothelial TEK tyrosine kinase (Khan et al. 2014) signaling pathways, which are available in NetPath (Kandasamy et al. 2010). A map of pathway reactions involved in thrombopoietin pathway was generated using PathVisio (van Iersel et al. 2008). In addition, a confident signaling network of THPO is drawn by following the NetSlim criteria as described previously (http://www.netpath.org/netslim/) (Raju et al. 2011). All curated molecular events were reviewed by a pathway authority (subject expert in the field) to confirm the authenticity of the reactions pertaining to thrombopoietin pathway.
Results and discussion
Data integration and development of thrombopoietin signaling pathway
Literature search of all the key terms related to thrombopoietin signaling in PubMed database fetched more than 4700 research articles. These articles were screened for information related to signaling events induced by THPO. We have catalogued 48 molecular associations, 66 catalytic reactions involving post-translational modifications (PTMs), 19 protein translocation events and 43 activation/inhibition reactions along with information related to the host organism. Under catalytic reactions, PTMs captured through annotation included phosphorylation and ubiquitination. PTMs were assessed on two modes: direct and indirect. In direct reactions, upstream enzyme was curated for specific substrate in the published literature. Indirect reactions include those where the type of modification has been experimentally proven but no information exists about its immediate upstream enzyme. The information about modification, site, residue, position, species involved, type of experiment and PubMed IDs were also captured. Genes regulated by THPO which have been validated using microarray and non-microarray techniques including northern blotting, serial analysis of gene expression and quantitative RT-PCR were also documented. One hundred genes were found to be regulated by THPO-MPL signaling, out of which 77 genes were upregulated and 23 downregulated.
Data curation and availability of data formats
The THPO pathway data is freely available in NetPath (http://www.netpath.org/pathways?path_id=NetPath_140) (Kandasamy et al. 2010). The NetPath page of THPO pathway has description of the pathway along with the number of molecules and reactions involved. A high confidence map of THPO signaling containing molecular events which follow NetSlim criteria has been included (Raju et al. 2011). A brief description of the THPO pathway as well as the pathway map has been provided in the NetSlim page of THPO pathway and can be downloaded from http://www.netpath.org/netslim/THPO_pathway.html. The data in NetPath and NetSlim maps is compatible with various standard data exchange formats including Proteomics Standards Initiative Molecular Interaction XML format (PSI-MI) (Kerrien et al. 2007), a standard format for data representation in proteomics to facilitate data comparison, exchange and verification; BioPAX (http://www.biopax.org/) (Demir et al. 2010). a standard language that enables exchange, visualization and analysis of biological pathway data; and the Systems Biology Markup Language (SBML) (http://sbml.org/), a computer-readable format for representing models of biological processes (Hucka et al. 2003). These pathway maps can be downloaded in GenMAPP, GPML and PDF formats.
Summary of signaling events regulated by the THPO signaling pathway
The various signaling events induced by THPO binding to its receptor MPL is illustrated in Fig. 1). Thrombopoietin binds to the extracellular domain of receptor MPL, which leads to the homodimerization of the receptor to initiate intracellular signaling. Receptor dimerization allows JAK kinases, which are bound to cytoplasmic domains of MPL receptor, to cross-phosphorylate and hence activate each other (Tortolani et al. 1995). Activated JAK2 kinase phosphorylates tyrosine residues on distal portion of MPL receptor which leads to the recruitment of various proteins to the receptor via their Shc homology (SH2) or phosphotyrosine-binding (PTB) motifs (Bacon et al. 1995; Ezumi et al. 1995). JAK2 also phosphorylates and activates STAT family of latent transcription factors (Murray 2007; Bacon et al. 1995). Phosphorylation causes dimerization of STAT proteins allowing their translocation to the nucleus where they stimulate transcription of genes like BCL2L1, cyclin D1 and c-MYC which have been reported to play an important role in cell survival and proliferation (Li 2008). STAT3 and STAT5 are predominantly phosphorylated in response to THPO (Miyakawa et al. 1996; Bacon et al. 1995).
Fig. 1.
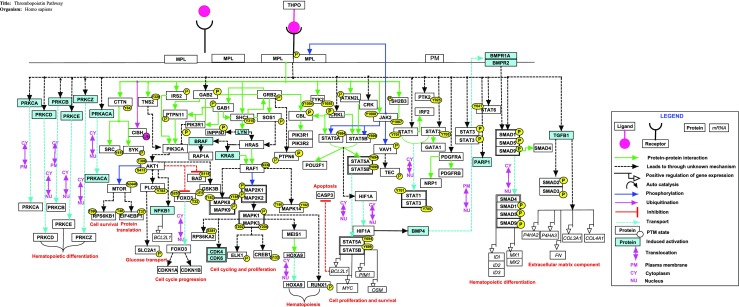
A schematic representation of signaling reactions induced by thrombopoietin. The pathway map was generated using PathVisio tool and is available in NetPath. Different types of reactions in THPO pathway map are distinguished with different colors and arrows as provided in the legend. Solid and indirect arrows represent direct and indirect reactions respectively. The major pathways activated by THPO are JAK/STAT, MAPK/ERK and PIK3/AKT
THPO also activates the phosphatidylinositol-3-kinase (PI3K) pathway and the mitogen activated protein kinase (MAPK) pathways. In PI3K pathway, THPO activates PI3K, which leads to phosphorylation and activation of its downstream effector AKT (Geddis et al. 2001). AKT promotes cell cycle progression by phosphorylating transcription factor FOXO3, preventing its translocation to nucleus, which inhibits expression of cell cycle inhibitor CDKN1B and cell surface death receptor FAS protein (Nakao et al. 2008; Tanaka et al. 2001). Phosphorylation of BAD protein by AKT prevents its inhibitory effects on anti-apoptotic protein BCL2L1 thereby promoting cell survival (del Peso et al. 1997). In MAPK pathway, THPO recruits SOS1 (guanine nucleotide exchange factor) which activates RAS (Rojnuckarin et al. 1999). RAS activates RAF kinase proteins which then phosphorylate and activate cascade of MAP kinases including MAPK1, MAPK3, MAPK8, MAPK9 and MAPK14. These MAP kinases activate cell cycle regulatory genes including CDK4/6, ELK1 and CREB1 that have been known to be involved in proliferation and differentiation of megakaryocytes (Rojnuckarin et al. 1999; Ritchie et al. 1999).
THPO signaling also plays an important role in the development of hematopoietic stem cells. In mouse embryonic stem cells, THPO has been shown to activate BMP4 which will lead to phosphorylation of SMAD proteins (Pramono et al. 2016). Phosphorylated SMAD proteins translocate to nucleus where they associate with coactivators and regulate expression of genes including RUNX1, GATA1, GATA2, MSX and TAL1 which are involved in HSC development (Jeanpierre et al. 2008; Pramono et al. 2016). In human UT7 cells, thrombopoietin increases the expression of MEIS1 and induces its interaction with HOXA9 resulting in the nuclear accumulation of this complex which plays key role in HSC development (Kirito et al. 2004).
THPO has been reported to be involved in development and maintenance of some cancers. PI3K/AKT/mTOR pathway activated by THPO helps in survival and proliferation of MPL expressing cells in acute myelogenous leukemia (Pulikkan et al. 2012). THPO/MPL signaling promotes proliferation and survival of leukemia cells by upregulation of BCL2L1 through activation of JAK/STAT and MEK/ERK pathways (Chou et al. 2012; Nishikawa et al. 2014). In hepatoblastoma cells, THPO induces proliferation, migration, chemo invasion and cell survival by activating PI3K/Akt and NF-KB pathways (Romanelli et al. 2006).
Conclusions
The manually curated and freely available detailed map of THPO pathway developed in this study will facilitate better understanding of thrombopoietin-induced signaling and the impact of thrombopoietin-mediated physiological disorders such as thrombocytopenia and cancer. More molecules and reactions are now under thrombopoietin pathway in public data, which will be exchanged automatically across databases, and will become a part of several gene-set enrichment tools. This will pave the way for discovery of more diseases where this pathway has potential role. All valuable suggestions and critical comments from scientific community are welcome through NetPath http://www.netpath.org/comments.
Acknowledgements
We thank the Department of Biotechnology, Government of India for research support to Institute of Bioinformatics. FAB is a recipient of a Senior Research Fellowship from University Grants Commission (UGC), JA is a recipient of Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India.
Abbreviations
- THPO
Thrombopoietin
- MPL
MPL proto-oncogene, thrombopoietin receptor
- JAK2
Janus kinase 2
- STAT
Signal transducer and activator of transcription protein
- MAPK
Mitogen-activated protein kinase
- PIK3
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- MPN
Myeloproliferative neoplasms
- RT-PCR
Real-time polymerase chain reaction
- BioPAX
Biological Pathway Exchange
- SBML
Systems Biology Markup Language
- PSI-MI
Proteomics Standards Initiative for Molecular Interaction`
- PTM
Post-translational modification
Compliance with ethical standards
Competing interests
The author(s) declare that they have no competing interests.
Contributor Information
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in
Aditi Chatterjee, Phone: 91-80-28416140, Email: aditi@ibioinformatics.org.
References
- Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL, O'Shea JJ. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995;370:63–68. doi: 10.1016/0014-5793(95)00796-C. [DOI] [PubMed] [Google Scholar]
- Ballem PJ, Belzberg A, Devine DV, Lyster D, Spruston B, Chambers H, Doubroff P, Mikulash K. Kinetic studies of the mechanism of thrombocytopenia in patients with human immunodeficiency virus infection. N Engl J Med. 1992;327:1779–1784. doi: 10.1056/NEJM199212173272503. [DOI] [PubMed] [Google Scholar]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Custodi P, Duranti M, Noris P, Balduini CL. Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol. 1997;99:281–284. doi: 10.1046/j.1365-2141.1997.3823196.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee O, Patil K, Sahu A, Gopalakrishnan L, Mol P, Advani J, Mukherjee S, Christopher R, Prasad TS. An overview of the oxytocin-oxytocin receptor signaling network. Journal of Cell Communication and Signaling. 2016;10:355–360. doi: 10.1007/s12079-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou FS, Griesinger A, Wunderlich M, Lin S, Link KA, Shrestha M, Goyama S, Mizukawa B, Shen S, Marcucci G, Mulloy JC. The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO. Blood. 2012;120:709–719. doi: 10.1182/blood-2012-01-403212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen DD, Broudy VC, Drachman JG. Internalization of the thrombopoietin receptor is regulated by 2 cytoplasmic motifs. Blood. 2003;102:102–108. doi: 10.1182/blood-2002-11-3468. [DOI] [PubMed] [Google Scholar]
- de Sauvage FJ, Carver-Moore K, Luoh SM, Ryan A, Dowd M, Eaton DL, Moore MW. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183:651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debili N, Wendling F, Cosman D, Titeux M, Florindo C, Dusanter-Fourt I, Schooley K, Methia N, Charon M, Nador R, et al. The Mpl receptor is expressed in the megakaryocytic lineage from late progenitors to platelets. Blood. 1995;85:391–401. [PubMed] [Google Scholar]
- Defour JP, Chachoua I, Pecquet C, Constantinescu SN. Oncogenic activation of MPL/thrombopoietin receptor by 17 mutations at W515: implications for myeloproliferative neoplasms. Leukemia. 2016;30:1214–1216. doi: 10.1038/leu.2015.271. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D'Eustachio P, Schaefer C, Luciano J, Schacherer F, Martinez-Flores I, Hu Z, Jimenez-Jacinto V, Joshi-Tope G, Kandasamy K, Lopez-Fuentes AC, Mi H, Pichler E, Rodchenkov I, Splendiani A, Tkachev S, Zucker J, Gopinath G, Rajasimha H, Ramakrishnan R, Shah I, Syed M, Anwar N, Babur O, Blinov M, Brauner E, Corwin D, Donaldson S, Gibbons F, Goldberg R, Hornbeck P, Luna A, Murray-Rust P, Neumann E, Ruebenacker O, Samwald M, van Iersel M, Wimalaratne S, Allen K, Braun B, Whirl-Carrillo M, Cheung KH, Dahlquist K, Finney A, Gillespie M, Glass E, Gong L, Haw R, Honig M, Hubaut O, Kane D, Krupa S, Kutmon M, Leonard J, Marks D, Merberg D, Petri V, Pico A, Ravenscroft D, Ren L, Shah N, Sunshine M, Tang R, Whaley R, Letovksy S, Buetow KH, Rzhetsky A, Schachter V, Sobral BS, Dogrusoz U, McWeeney S, Aladjem M, Birney E, Collado-Vides J, Goto S, Hucka M, Le Novere N, Maltsev N, Pandey A, Thomas P, Wingender E, Karp PD, Sander C, Bader GD. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, Kanesan K, Cantor SB, Benjamin RS. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–1146. doi: 10.1200/JCO.2001.19.4.1137. [DOI] [PubMed] [Google Scholar]
- Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, Dunbar CE, Shulman NR. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–4071. [PubMed] [Google Scholar]
- Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, Sellers TS, Rosen J, Miller SG, Luengo JI, Duffy KJ, Jenkins JM. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–430. doi: 10.1634/stemcells.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezumi Y, Takayama H, Okuma M. Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 1995;374:48–52. doi: 10.1016/0014-5793(95)01072-M. [DOI] [PubMed] [Google Scholar]
- Geddis AE, Fox NE, Kaushansky K. Phosphatidylinositol 3-kinase is necessary but not sufficient for thrombopoietin-induced proliferation in engineered Mpl-bearing cell lines as well as in primary megakaryocytic progenitors. J Biol Chem. 2001;276:34473–34479. doi: 10.1074/jbc.M105178200. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J, Forum S. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Jeanpierre S, Nicolini FE, Kaniewski B, Dumontet C, Rimokh R, Puisieux A, Maguer-Satta V. BMP4 regulation of human megakaryocytic differentiation is involved in thrombopoietin signaling. Blood. 2008;112:3154–3163. doi: 10.1182/blood-2008-03-145326. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Keshava Prasad TS, Ramachandra YL, Mohan S, Pandey A. PathBuilder--open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K, Broudy VC, Lin N, Jorgensen MJ, McCarty J, Fox N, Zucker-Franklin D, Lofton-Day C. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci U S A. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K, Lin N, Grossmann A, Humes J, Sprugel KH, Broudy VC. Thrombopoietin expands erythroid, granulocyte-macrophage, and megakaryocytic progenitor cells in normal and myelosuppressed mice. Exp Hematol. 1996;24:265–269. [PubMed] [Google Scholar]
- Kerrien S, Orchard S, Montecchi-Palazzi L, Aranda B, Quinn AF, Vinod N, Bader GD, Xenarios I, Wojcik J, Sherman D, Tyers M, Salama JJ, Moore S, Ceol A, Chatr-Aryamontri A, Oesterheld M, Stumpflen V, Salwinski L, Nerothin J, Cerami E, Cusick ME, Vidal M, Gilson M, Armstrong J, Woollard P, Hogue C, Eisenberg D, Cesareni G, Apweiler R, Hermjakob H. Broadening the horizon--level 2.5 of the HUPO-PSI format for molecular interactions. BMC Biol. 2007;5:44. doi: 10.1186/1741-7007-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Sandhya VK, Singh P, Parthasarathy D, Kumar A, Advani J, Gattu R, Ranjit DV, Vaidyanathan R, Mathur PP, Prasad TS, Mac Gabhann F, Pandey A, Raju R, Gowda H. Signaling network map of endothelial TEK tyrosine kinase. Journal of Signal Transduction. 2014;2014:173026. doi: 10.1155/2014/173026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirito K, Fox N, Kaushansky K. Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol Cell Biol. 2004;24:6751–6762. doi: 10.1128/MCB.24.15.6751-6762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Kurata Y, Tomiyama Y, Tahara T, Kato T, Tadokoro S, Shiraga M, Honda S, Kanakura Y, Matsuzawa Y. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93:704–706. doi: 10.1046/j.1365-2141.1996.d01-1702.x. [DOI] [PubMed] [Google Scholar]
- Le Coniat M, Souyri M, Vigon I, Wendling F, Tambourin P, Berger R. The human homolog of the myeloproliferative virus maps to chromosome band 1p34. Hum Genet. 1989;83:194–196. doi: 10.1007/BF00286717. [DOI] [PubMed] [Google Scholar]
- Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hetet G, Kiladjian JJ, Gardin C, Grandchamp B, Briere J. Proto-oncogene c-mpl is involved in spontaneous megakaryocytopoiesis in myeloproliferative disorders. Br J Haematol. 1996;92:60–66. doi: 10.1046/j.1365-2141.1996.00297.x. [DOI] [PubMed] [Google Scholar]
- Lu CC, Chang KW, Chou FC, Cheng CY, Liu CJ. Association of pretreatment thrombocytosis with disease progression and survival in oral squamous cell carcinoma. Oral Oncol. 2007;43:283–288. doi: 10.1016/j.oraloncology.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lupia E, Bosco O, Goffi A, Poletto C, Locatelli S, Spatola T, Cuccurullo A, Montrucchio G. Thrombopoietin contributes to enhanced platelet activation in cigarette smokers. Atherosclerosis. 2010;210:314–319. doi: 10.1016/j.atherosclerosis.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, Ikeda Y. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood. 1996;87:439–446. [PubMed] [Google Scholar]
- Murone M, Carpenter DA, de Sauvage FJ. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells. 1998;16:1–6. doi: 10.1002/stem.160001. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nakao T, Geddis AE, Fox NE, Kaushansky K. PI3K/Akt/FOXO3a pathway contributes to thrombopoietin-induced proliferation of primary megakaryocytes in vitro and in vivo via modulation of p27(Kip1) Cell Cycle. 2008;7:257–266. doi: 10.4161/cc.7.2.5148. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Arai S, Masamoto Y, Kagoya Y, Toya T, Watanabe-Okochi N, Kurokawa M. Thrombopoietin/MPL signaling confers growth and survival capacity to CD41-positive cells in a mouse model of Evi1 leukemia. Blood. 2014;124:3587–3596. doi: 10.1182/blood-2013-12-546275. [DOI] [PubMed] [Google Scholar]
- Nomura S, Ogami K, Kawamura K, Tsukamoto I, Kudo Y, Kanakura Y, Kitamura Y, Miyazaki H, Kato T. Cellular localization of thrombopoietin mRNA in the liver by in situ hybridization. Exp Hematol. 1997;25:565–572. [PubMed] [Google Scholar]
- Pilatova K, Greplova K, Demlova R, Bencsikova B, Klement GL, Zdrazilova-Dubska L. Role of platelet chemokines, PF-4 and CTAP-III, in cancer biology. J Hematol Oncol. 2013;6:42. doi: 10.1186/1756-8722-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramono A, Zahabi A, Morishima T, Lan D, Welte K, Skokowa J. Thrombopoietin induces hematopoiesis from mouse ES cells via HIF-1alpha-dependent activation of a BMP4 autoregulatory loop. Ann N Y Acad Sci. 2016;1375:38–51. doi: 10.1111/nyas.13138. [DOI] [PubMed] [Google Scholar]
- Pulikkan JA, Madera D, Xue L, Bradley P, Landrette SF, Kuo YH, Abbas S, Zhu LJ, Valk P, Castilla LH. Thrombopoietin/MPL participates in initiating and maintaining RUNX1-ETO acute myeloid leukemia via PI3K/AKT signaling. Blood. 2012;120:868–879. doi: 10.1182/blood-2012-03-414649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z. Lower circulating platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma. J Hematol Oncol. 2014;7:65. doi: 10.1186/s13045-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Nanjappa V, Balakrishnan L, Radhakrishnan A, Thomas JK, Sharma J, Tian M, Palapetta SM, Subbannayya T, Sekhar NR, Muthusamy B, Goel R, Subbannayya Y, Telikicherla D, Bhattacharjee M, Pinto SM, Syed N, Srikanth MS, Sathe GJ, Ahmad S, Chavan SN, Kumar GS, Marimuthu A, Prasad TS, Harsha HC, Rahiman BA, Ohara O, Bader GD, Sujatha Mohan S, Schiemann WP, Pandey A. NetSlim: high-confidence curated signaling maps. Database (Oxford) 2011;2011:bar032. doi: 10.1093/database/bar032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A, Braun SE, He J, Broxmeyer HE. Thrombopoietin-induced conformational change in p53 lies downstream of the p44/p42 mitogen activated protein kinase cascade in the human growth factor-dependent cell line M07e. Oncogene. 1999;18:1465–1477. doi: 10.1038/sj.onc.1202439. [DOI] [PubMed] [Google Scholar]
- Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. 1999;94:1273–1282. [PubMed] [Google Scholar]
- Romanelli RG, Petrai I, Robino G, Efsen E, Novo E, Bonacchi A, Pagliai G, Grossi A, Parola M, Navari N, Delogu W, Vizzutti F, Rombouts K, Gentilini P, Laffi G, Marra F. Thrombopoietin stimulates migration and activates multiple signaling pathways in hepatoblastoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G120–G128. doi: 10.1152/ajpgi.00350.2004. [DOI] [PubMed] [Google Scholar]
- Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, Nanjappa V, Narayana J, Somani BL, Mukherjee KK, Pandey A, Christopher R, Prasad TS. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. Journal of Cell Communication and Signaling. 2013;7:301–307. doi: 10.1007/s12079-013-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaran H, Ileri M, Altinbas A, Kosar A, Yetkin E, Ozturk M, Karaaslan Y, Kirazli S. Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol. 2001;24:405–408. doi: 10.1002/clc.4960240511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya T, Variar P, Advani J, Nair B, Shankar S, Gowda H, Saussez S, Chatterjee A, Prasad TS. An integrated signal transduction network of macrophage migration inhibitory factor. Journal of Cell Communication and Signaling. 2016;10:165–170. doi: 10.1007/s12079-016-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K, Satoh H, Taniwaki M, Yokota J, Morishita K. The human thrombopoietin gene is located on chromosome 3q26.33-q27, but is not transcriptionally activated in leukemia cells with 3q21 and 3q26 abnormalities (3q21q26 syndrome) Leukemia. 1995;9:1328–1331. [PubMed] [Google Scholar]
- Tanaka M, Kirito K, Kashii Y, Uchida M, Watanabe T, Endo H, Endoh T, Sawada K, Ozawa K, Komatsu N. Forkhead family transcription factor FKHRL1 is expressed in human megakaryocytes. Regulation of cell cycling as a downstream molecule of thrombopoietin signaling. J Biol Chem. 2001;276:15082–15089. doi: 10.1074/jbc.M007958200. [DOI] [PubMed] [Google Scholar]
- Tortolani PJ, Johnston JA, Bacon CM, McVicar DW, Shimosaka A, Linnekin D, Longo DL, O'Shea JJ. Thrombopoietin induces tyrosine phosphorylation and activation of the Janus kinase, JAK2. Blood. 1995;85:3444–3451. [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigon I, Mornon JP, Cocault L, Mitjavila MT, Tambourin P, Gisselbrecht S, Souyri M. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992;89:5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CS, Yao WJ, Wang ST, Chang TT, Chou P. Strong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infection. Clin Infect Dis. 2004;39:790–796. doi: 10.1086/423384. [DOI] [PubMed] [Google Scholar]
- Winer ES, Safran H, Karaszewska B, Richards DA, Hartner L, Forget F, Ramlau R, Kumar K, Mayer B, Johnson BM, Messam CA, Mostafa Kamel Y. Eltrombopag with gemcitabine-based chemotherapy in patients with advanced solid tumors: a randomized phase I study. Cancer Med. 2015;4:16–26. doi: 10.1002/cam4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci. 2002;17:6–10. doi: 10.1152/physiologyonline.2002.17.1.6. [DOI] [PubMed] [Google Scholar]
- Wolber EM, Dame C, Fahnenstich H, Hofmann D, Bartmann P, Jelkmann W, Fandrey J. Expression of the thrombopoietin gene in human fetal and neonatal tissues. Blood. 1999;94:97–105. [PubMed] [Google Scholar]
- Wu Z, Wei D, Gao W, Xu Y, Hu Z, Ma Z, Gao C, Zhu X, Li Q. TPO-induced metabolic reprogramming drives liver metastasis of colorectal Cancer CD110+ tumor-initiating cells. Cell Stem Cell. 2015;17:47–59. doi: 10.1016/j.stem.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Yelamanchi SD, Solanki HS, Radhakrishnan A, Balakrishnan L, Advani J, Raja R, Sahasrabuddhe NA, Mathur PP, Dutta P, Prasad TS, Korbonits M, Chatterjee A, Gowda H, Mukherjee KK. Signaling network map of the aryl hydrocarbon receptor. Journal of Cell Communication and Signaling. 2016;10:341–346. doi: 10.1007/s12079-016-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


